Abstract
Acrostalagmus is known for its ability to produce numerous bioactive natural products, making it valuable in drug development. This review provides information on the sources, distribution, chemical structure types, biosynthesis, and biological activities of the compounds isolated from the genus Acrostalagmus in the family Plectosphaerellaceae from 1969 to 2022. The results show that 50% of the compounds isolated from Acrostalagmus are new natural products, and 82% of the natural products derived from this genus are from the marine Acrostalagmus. The compounds isolated from Acrostalagmus exhibit diverse structures, with alkaloids being of particular importance, accounting for 56% of the natural products derived from this genus. Furthermore, within the alkaloid class, 61% belong to the epipolythiodioxopiperazine family, highlighting the significance of epipolythiodioxopiperazine as a key characteristic structure within Acrostalagmus. Seventy-two percent of natural products derived from Acrostalagmus display bioactivities, with 50% of the bioactive compounds exhibiting more significant or comparable activities than their positive controls. Interestingly, 89% of potent active compounds are derived from marine fungi, demonstrating their promising potential for development. These findings underscore Acrostalagmus, particularly the marine-derived genus Acrostalagmusas, a valuable source of new bioactive secondary metabolites, and emphasize the vast resource importance of the ocean.
1. Introduction
Acrostalagmus is a genus of ascomycete fungi in the class Sordariomycetes, order Glomerellales, family Plectosphaerellaceae. There are four species (A. annulatus, A. cf. luteoalbus, A. cf. luteoalbus CK1, A. luteoalbus) of the genus Acrostalagmus in the National Center for Biotechnology Information (NCBI) database (https://www.ncbi.nlm.nih.gov/data-hub/taxonomy/tree/?taxon=461148 (accessed on 27 July 2023)). The colony of Acrostalagmus is brick red, because of its red spores, with white mycelium at edge. The mass production of spores causes the overall colony to present a ring pattern with different shades. With the extension of culture time, the color gradually deepened and darkened, showing rust red [].
Most of the fungi belonging to the genus Acrostalagmus are alkalitolerant [,] or alkalophilic [] fungi, and are widely distributed in different ecological environments, including forest [], sand ridge state [], marine [,] and polar ecosystems []. The genus Acrostalagmus can survive in different circumstances due to its ability to produce kinds of enzymes [,,] and secondary metabolites [] with a variety of bioactivities [,,]. The crude extracts of some Acrostalagmus species exhibited significant brine shrimp lethality, as well as antibacterial, antifungal and DPPH radical scavenging activities [,], meaning they have potential to produce abundant natural products with remarkable activities. Gas chromatography mass (GC-MS) [], high-performance liquid chromatography (HPLC)-electrospray ionization (ESI)-MS [], and ultra-HPLC-MS/MS spectrometry [] have been used to analyze the secondary metabolites of the genus Acrostalagmus and further demonstrate the great ability of this genus to produce bioactive compounds. To date, there has been no summary reviewing the natural products of the Acrostalagmus genus. In consideration of the above-mentioned facts, the chemical structure types, sources, distribution, biological activities, and biological synthesis of the compounds isolated from Acrostalagmus from 1969 to 2022 are comprehensively reviewed in this paper.
2. Terpenoids
The first research on the secondary metabolites isolated from the genus Acrostalagmus was performed in 1969 by George A. Ellestad et al. []. Two norditerpenes, named LL-Z1271α (1) and LL-Z1271γ (2) (Figure 1), were isolated from an unidentified Acrostalagmus sp. NRRL-3481 [,]. In 1971, one norditerpene analogue, LL-Z1271β (3), was discovered from the same species by the same research group []. In 1974, three other analogs 4–6 were obtained from the culture of Acrostalagmus NRRL-3481 []. Terpenoids 1–6 were deduced to be biosynthesized from microbiological degradation of a diterpene, such as labdadienol, through oxidative cleavage between C-12 and C-13 [] (Figure 2). The absolute configuration of 4 at the location of C-8 was deduced to be 8R according to the supposed biosynthesis pathway from compound 6 to 4. Compound 1 displayed remarkable antifungal activity in vitro against kinds of fungi and in vivo against some experimental ringworm infections in guinea pigs []. Additionally, 1 displayed effectiveness against the fungi that cause infection in humans with the minimum inhibitory concentrations (MICs) against Cryptococcus neoformans and Candida albicans of 2 µg/mL and 8 µg/mL, respectively []. Compound 1 was the inhibitor of Pseudogymnoascus destructans, which is the fungus that leads to a devastating disease of hibernating bats named white-nose syndrome (WNS), with an MIC value of 15 μg/mL []. The cytotoxicity of 1 against the murine P388 lymphocytic leukemia cell line and a series of human cancer cell lines were evaluated and IC50 values ranging from 0.14 to 4.1 µg/mL [] were obtained. Compound 3 also showed cytotoxic activity against human cancer cell line HL-60 with an IC50 value of 0.60 µM, with the same level as the positive control epirubicin (IC50 = 0.71 µM) []. Compound 1, as a plant growth regulator, showed significant inhibitory activity on the growth of an Avena coleoptile section comparable to those of structural analogues, inumakilactones, nagilactones, and podolactones, which showed strong inhibitory activity to the expansion and mitosis of plant cell []. At a concentration of 10−4 M, Compound 1 significantly inhibited the germination and growth development of three plant species: two monocotyledons (Allium cepa and Hordeum vulgare) and one dicotyledon (Lactuca sativa), with an inhibition rate of over 80%, which is more active than the commercial herbicide LOGRAN®, indicating that 1 shows potential as a herbicide template and may serve as a next generation of natural agrochemicals []. Compound 1 displayed potent inhibitory activity to the production of IL-1β (interleukin-1β, a proinflammatory cytokine produced primarily by macrophages and monocytes in answer to various stimuli []) in the manner of dose-dependent application with an IC50 value of 0.049 μM in human whole blood [,]. Compound 1 exhibited a much weaker inhibitory effect on leucine uptake than on IL-1β production which suggests that the compound’s action is not a result of general effects on protein synthesis. The inhibition mechanism of 1 is also not because of the ATP-induced release, effects on caspase-1, or a lysosomotrophic effect []. Further research on the target for 1 is in progress, which may identify a mechanistically new approach for the treatment of IL-1β associated diseases [].
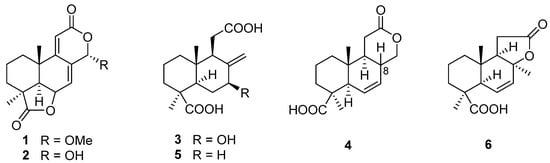
Figure 1.
Chemical structures of compounds 1–6 [,,,].
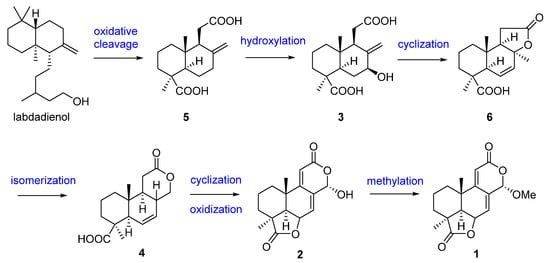
Figure 2.
Presumed biosynthesis pathway of compounds 1–6 [].
3. Alkaloids
Melinacidin, a mixture of at least four closely related compounds obtained from the culture broth of the fungus Acrostalagmus cinnabarinus var. melinacidinus, was first discovered in 1968 and showed antibacterial activity against various of Gram-positive bacteria in vitro []. However, melinacidin was ineffective in protecting mice from the infection of Staphylococcus aureus when administered subcutaneously at the maximum tolerated dose of 1 mg/mL []. The mechanism of antibacterial activity of melinacidin was studied and found to be blocked the synthesis of nicotinic acid and its amide in Bacillus subtilis cells. The biosynthetic pathway leading to nicotinic acid was interfered with by melinacidin before the formation of quinolinic acid []. The antifungal activity of melinacidin was only exhibited on nocardia asteroides and Blastomyces dermatitidis with MIC values of 10 and 1000 μg/mL, respectively. Melinacidin displayed inhibition of the growth of KB cells in tissue cultures with an ID50 (50% inhibition of protein synthesis) value of 0.014 μg/mL and had marginal in vivo activity in mice infected with Herpes virus []. In 1972, melinacidin was separated into three compounds, melinacidins II, III, and IV (7–9), and their structure characterizations were described []. While the certain structures of 7–9 were finally determined in 1977 to be epipolythiodioxopiperazines (ETPs) (Figure 3) []. Compound 9 showed potent cytotoxicity against murine P388 leukemia cells with an IC50 value of 0.05 µM []. Compound 9 also exhibited antibacterial activities against methicillin-resistant S. aureus (MRSA) and vancomycin-resistant Enterococcus faecium (VRE) with the MIC values of 0.7 and 22 μg/mL, respectively. The antibacterial activity of 9 to MRSA exhibited double the activity of the positive control vancomycin (MIC = 1.4 µg/mL) [].
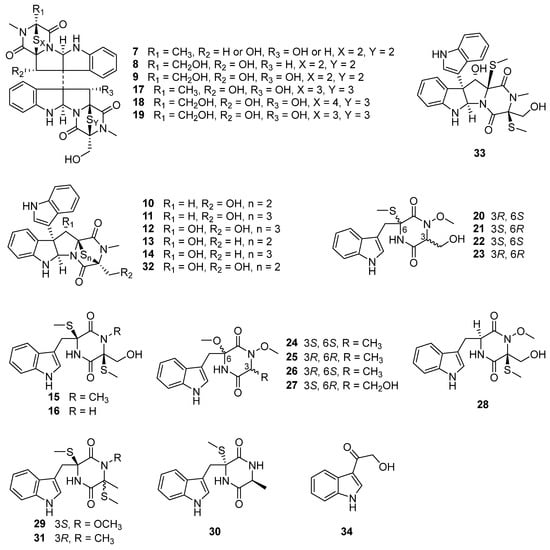
Figure 3.
Chemical structures of compounds 7–34 [,,,,,].
Chemical investigation of the deep-sea sediment-derived fungus A. luteoalbus SCSIO F457 led to the isolation of two new indole diketopiperazines, luteoalbusins A and B (10 and 11), as well as eight known diketopiperazines, T988A (12), gliocladines C and D (13 and 14), chetoseminudins B and C (15 and 16) (Figure 3), cyclo(L-Trp-L-Ser) (35), cyclo(L-Trp-L-Ala) (36), and cyclo(L-Trp-N-methyl-L-Ala) (37) []. The bi-indole diketopiperazines (10–14) exhibited potent cytotoxicity against four cancer cell lines, SF-268, MCF-7, NCI-H460, and HepG-2, with IC50 values ranging from 0.23 to 17.78 µM. The new compounds 10 and 11 showed stronger cytotoxicity against all four tested cancer cell lines than that of the positive control cisplatin []. Compounds 10 and 11 also displayed prominent cytotoxic activities against A549, HeLa, and HCT116 cancer cell lines with IC50 values ranging from 0.52 to 2.33 µM []. Compound 12 was first discovered from a decaying wood derived fungus Tilachlidium sp. CANU-T988, and displayed cytotoxicity to P388 leukemia cells with an IC50 value of 0.25 µM []. Compounds 13 and 14 were first isolated from the submerged wood derived fungus Gliocladium roseum 1A and showed nematicidal activities toward Caenorhabditis elegans and Panagrellus redivivus with ED50 (concentrations causing more than 50% mortality after 24 h) values of 200/250 and 200/250 µg/mL, respectively []. Compounds 10 and 13 were exhibited antimicrobial activities against Canidia albicans and Aeromonas salmonicida with MIC values of 12.5/12.5 (10) and 25/50 (13) µM, respectively []. Compounds 15 and 16 were first found from the fungus Chaetomium seminudum 72-S-204-1, and 15 showed weak immunosuppressive activity with an IC50 value of 24 µg/mL on Con A-induced (T-cells) proliferations of mouse splenic lymphocytes []. Compounds 15 and 16 exhibited potent cytotoxic activities against murine lymphoma L5178Y cell line with EC50 values of 0.26 and 0.82 µM, respectively, which are more potent than that of the positive control kahalalide F (EC50 = 4.3 µM) []. Compound 15 also showed obvious enzyme inhibition against mushroom tyrosinase with an IC50 value of 31.7 ± 0.2 µM, which is stronger than the inhibitory activity of the positive control kojic acid (IC50 = 40.4 ± 0.1 μM) [].
Two new epipolythiodioxopiperazines (ETPs), chetracins E and F (17 and 18), as well as one known congener, chetracin C (19), were isolated from the culture extract of A. luteoalbus HDN13-530, a fungus obtained from the soil of Liaodong Bay []. Compounds 17–19 displayed extensive cytotoxic activities toward a series of cancer cell lines A549, HCT116, K562, H1975 and HL-60 with the IC50 values ranging from 0.2 to 2.1 µM, and 17 even showed stronger cytotoxicity to H1975 cancer cell line with an IC50 value of 0.2 µM than that of positive drug doxorubicin hydrochloride (IC50 = 0.8 µM) []. One of the reasons 17–19 cytotoxicity is possible due their ability to reduce the expressions of Akt, EGFR, and the active forms of Akt, EGFR, Erk, and Stat3 (Hsp90 client oncoproteins) in H1975 cells at the concentration of 0.5 µM, indicating their inhibition to C-terminal Hsp90 []. Compound 19 was first isolated from Antarctic soil derived fungus Oidiodendron truncatum GW3-13 and showed significant cytotoxicity against a panel of the cancer cell lines HCT-8, Bel-7402, BGC-823, and A2780 with IC50 values that ranged 0.49–0.70 µM [].
Three pairs of new N-methoxy-indolediketopiperazines enantiomers, (±)-acrozines A–C (20–25, Figure 3), were isolated from the marine green alga Codium fragile derived endophytic fungus A. luteoalbus TK-43 []. Four new acrozine-type indolediketopiperazines, acrozines D–G (26–29, Figure 3), along with six known analogues, pseudellone D (30), lasiodipline E (31), chetoseminudins B and C (15 and 16), T988 C and B (32 and 33) (Figure 3), were isolated from the culture extract of the same fungal species TK-43 []. Compounds 15, 16, and 20–33 were evaluated for their antimicrobial activities toward 15 plant pathogenic fungi, one human pathogenic bacterium, and 10 aquatic pathogens. Only (–)-acrozine B (23) showed antifungal activity toward the plant pathogen Fusarium solani with an MIC value of 32 μg/mL, which is stronger than the activities of its enantiomer 22 and its epimers 20 and 21 (MIC > 64 μg/mL) []. These results indicate that the absolute configurations of 3R, 6R are the key structures to producing antifungal activity. While compound 25 with the same configurations of 3R, 6R had no antifungal activity, this might suggest the significance of methylene hydroxyl and thiomethyl groups located at C-3 and C-6, respectively, for the antifungal activity. Compounds 30 and 16 showed antibacterial activity against Edwardsiella icataluri with MIC values of 3 and 5 μM, respectively, which are comparable to that of the positive control, chloromycetin (MIC = 2 μM) []. Compound 32 showed broad-spectrum antibacterial activity and demonstrated more potent activity (MIC = 2 μM) against Vibrio parphemolyticus than the positive control chloromycetin (MIC = 12 μM) []. The antimicrobial activity of 32 against Candida albicans (MIC = 6.25 μM) and Aeromonas salmonicida (MIC = 3.125 μM) were comparable to that of positive control ciprofloxacin (MIC = 6.25 μM) []. The results indicate that antibacterial activities are significantly reduced (from 30 and 16 to 20–29, 31, and 15) when there is a methoxy or methyl substitution at N-2. Additionally, antibacterial activity is significantly increased when there is a disulfide bridge (from 33 to 32) [,]. Compound 31 was first discovered from the culture of Illigera rhodantha (a flower belongs to Hernandiaceae) derived endophytic fungus Lasiodiplodia pseudotheobromae F2, and exhibited strong antibacterial activity toward the clinical strains Bacteroides vulgates, Streptococcus sp., Veillonella parvula, and Peptostreptococcus sp., with an MIC value range of 0.12–0.25 μg/mL, comparable or even more significant than that of positive control tinidazole (MIC values range of 0.12–0.5 μg/mL) []. T988 A and C (12 and 32) showed potent antibacterial activities against S. aureus, methicillin-resistant S. aureus, and S. pyogenes with IC50 values of 3.8/5.8, 8.4/5.6, and 1.8/3.1 μM, respectively. It was demonstrated that 12 and 32 exhibited antibacterial synergy in combination with ciprofloxacin, ampicillin, and streptomycin []. The biofilm inhibition caused by 12 and 32 in S. aureus and S. pyogenes was approximately 70% at their MIC and over 60% at one-sixteenth of their MIC, respectively []. The mechanism of antibacterial activity in compounds 12 and 32 was explored and it was found that they have the ability to inhibit bacterial transcription/translation in vitro and inhibit the production of staphyloxanthin in S. aureus [].
Compounds 20–29 were tested for their anti-acetylcholinesterase (AChE) activity. (±)-Acrozines A had medium anti-AChE activity with IC50 value of 9.5 μM, and the chiral split compound, (+)-acrozine A (20) (IC50 = 2.3 μM) displayed better inhibition than that of (–)-acrozine A (21) (IC50 = 13.8 μM) and (±)-acrozines A []. Compound 26, which has the same planar structure and different configurations as that of 24 (IC50 = 160.6 μM) and 25 (IC50 = 121.7 μM), displayed much better AChE inhibitory activity with IC50 value of 18.9 μM [,]. These bioactivity data showed that compounds with identical planar structure may display different bioactivity and that the selectivity of biological activity is associated with the absolute configuration. Compound 28 showed anti-AChE activity with an IC50 value of 8.4 μM []. Compound 28 differs from (+)-acrozine B (22) (IC50 = 78.8 μM) [] solely in the location of SCH3 substitution, indicating the SCH3 group at C-3 in 28 is more active than the SCH3 group at C-6 in 22.
The biosynthetic pathway for compounds 7–33 is speculated as shown in Figure 4. Diketopiperazines 7–33 are biosynthesized through non-ribosomal the peptide synthetase (NRPS) pathway [], and their biosynthetic precursors might be L-Trp and L-Ala (7, 13, 14, 17, 24–26 and 29–31), or L-Trp and L-Ser (7–12, 15–23, 27, 28, 32 and 33) [,]. The sulfurs are proposed to be incorporated into the cyclopeptide frameworks (7–19, 21–23 and 28–33) by CYP450 monooxygenase and a specialized glutathione S-transferase which is similar to that in gliotoxin (GT) [,,,], and the intramolecular disulfides are generated by FAD-dependent oxidoreductase, GliT, with dithiol precursors [].
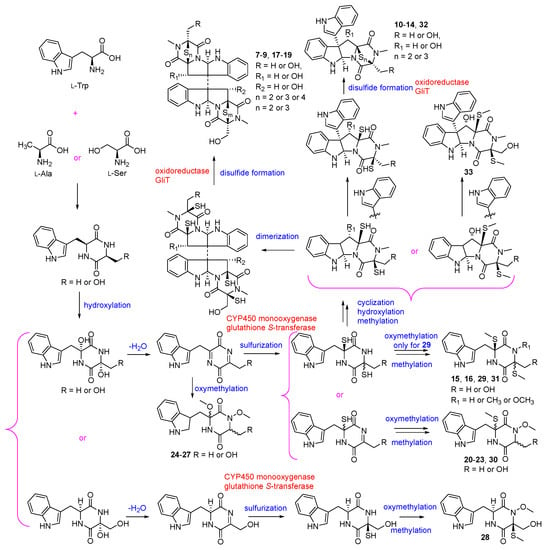
Figure 4.
Proposed biosynthesis of compounds 7–33 [,,,,,,].
Using the one strain many compounds (OSMAC) strategy to study the chemical diversity of A. luteoalbus SCSIO F457 led to one indole alkaloid, 3-(hydroxy-acetyl)-1H-indole (34, Figure 3); five cyclic dipeptides, cyclo(L-Phe-L-Pro) (38), cyclo(L-Tyr-L-Pro) (39), cyclo(L-Val-L-Pro) (40), cyclo(D-Ile-L-Pro) (41), and cyclo(D-Leu-L-Pro) (42); one pyranone derivative, 3-methoxy-2-methyl-4H-pyran-4-one (43); one benzo-tetrahydrofuran-lignin, paulownin (47); and three benzene derivatives, 1-methyoxy-4-(2-hydroxy)ethylbenzene (48), 2-(4-hydroxyphenyl)-ethanol (49), 1-phenylbutane-2,3-diol (50) [].
4. Others
4.1. Cyclic Dipeptides
Three known cyclo-dipeptides; cyclo(L-Trp-L-Ser) (35), cyclo(L-Trp-L-Ala) (36), and cyclo(L-Trp-N-methyl-L-Ala) (37) (Figure 5), were isolated from the culture extract of the deep-sea sediment-derived fungus A. luteoalbus SCSIO F457 []. Although 35–37 showed no cytotoxic activities against cancer cell lines MCF-7, SF-268, HepG-2, and NCI-H460 with the SRB method [], compound 35 displayed antimicrobial activity against Escherichia coli, Chromobacterium violaceum CV026, Pseudomonas aeruginosa PA01, S. aureus and C. albicans 00147 with the MIC values of 6.4, 3.2, 6.4, 3.2 and 6.4 mg/mL, respectively. Furthermore, 35 showed anti-quorum sensing (anti-QS) activity by inhibiting the production of violacein in C. violaceum CV026 with an inhibition of 67% in 0.2 mg/mL (the production inhibition of positive control azithromycin (AZM) was 80% in 0.05 mg/mL). The anti-QS activity of 35 was further confirmed by its reduction in elastase activity and biofilm formation. The reduced elastase activity in 35 was 40%, comparable with the positive control AZM, which induced a 49% inhibition. Interestingly, 35 resulted in a 59.9% reduction in biofilm formation in P. aeruginosa PA01 at a concentration of 0.2 mg/mL, which was better than the positive control AZM (53.9% reduction). Compound 35 or its derivatives can serve as leading compounds in the development of new antimicrobial drugs for clinical or agricultural research, playing a vital role in human health and agricultural development [,]. Compound 35 exhibited enzyme inhibition against α-glucosidase (AGS) with an IC50 value of 164.5 ± 15.5 µM, stronger than that of the positive control acarbose (IC50 = 422.3 ± 8.44 µM). In addition, 35 showed no cytotoxicity to the human normal hepatocyte (LO2) cells, suggesting its safety to be developed into hypoglycemic agent []. Compound 36 showed antibacterial activity against Bacillus cereus and Proteus vulgaris with MIC values of 1.56 and 3.13 µM (the MIC of positive control ciprofloxacin was 0.78 and 0.20 µM) []. The brine shrimp lethality of 36 was modest with an LD50 value of 25.5 µM (the LD50 of the positive control colchicine was 19.4 µM) []. Compound 36 exhibited 54.6 ± 0.6% cation radical (ABTS+•) scavenging capacity at 2 mg/mL (the positive control vitamin C displayed 79.1 ± 4.3% cation radical scavenging capacity at 0.16 mg/mL) []. Furthermore, 36 also showed potent anti-diatom attachment activity at the concentration of 50 µg/mL with an inhibition of 85% [].
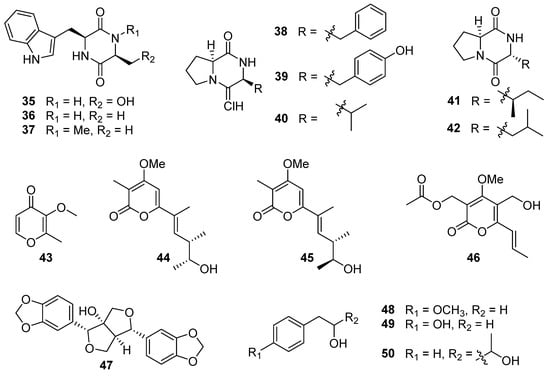
Figure 5.
Chemical structures of compounds 35–50 [,,].
Further investigation of the chemical structure diversity of the fungus A. luteoalbus SCSIO F457, using the strategy of OSMAC, led to another five cyclic dipeptides: cyclo(L-Phe-L-Pro) (38), cyclo(L-Tyr-L-Pro) (39), cyclo(L-Val-L-Pro) (40), cyclo(D-Ile-L-Pro) (41), and cyclo(D-Leu-L-Pro) (42) (Figure 5) []. Compounds 38–40 could be produced by Pseudomonas aeruginosa to promote the growth of plant with auxin-like activity through the LasI QS system. The QS-regulated bacterial production of DKPs 38–40 adjusts auxin signaling and plant growth promotion, which establishes a significant function for DKPs mediating trans-kingdom signaling between prokaryote and eukaryote []. Compounds 38 and cyclo(L-Leu-L-Pro) showed synergistic antimicrobial activity against vancomycin-resistant enterococci (VRE) and pathogenic yeasts. The combination of 38 and cyclo(L-Leu-L-Pro) exhibited significant anti-VRE activity against Enterococcus faecium (K-99-38), E. faecalis (K-99-258), E. faecalis (K-99-17), E. faecalis (K-01-511), and E. faecium (K-01-312) with MIC values of 0.25–1 μg/mL. It was also effective against E. coli, Micrococcus luteus, S. aureus, Cryptococcus neoformans, and C. albicans with MIC values of 0.25–0.5 μg/mL. And the combination of 38 and cyclo(L-Leu-L-Pro) could reduce the mutation of strains Salmonella typhimurium TA98 and TA100 [,]. Compounds 38–40 displayed antifungal activities against Ganoderma plantarum at the concentrations of 6.8, 8.2, and 8.2 mM, respectively, and 38 also showed anti-Candida activity at a concentration of 7.0 mM []. Compounds 38 and 39 also demonstrated prominent activities against agriculturally important fungi, Pencillium expansum, Rhizoctonia solani, and Fusarium oxysporum with MIC values between 2 and 8 µg/mL, much higher than the commercial fungicide bavistin (MIC values was 50, 25 and 25 µg/mL, respectively) []. Compound 40 showed antibacterial activity against MRSA 43300 with a zone of inhibition of 15 mm at a concentration of 20 µg/disc (the inhibition zone of the positive control gentamicin was 22 mm). And 40 had low toxicity against human hepatoma HepaRG cells, meaning it could be developed into a safe antibiotic []. Compound 38 displayed weak cytotoxicity against HeLa, HT-29, and MCF-7 cell lines with IC50 values of 2.92 ± 1.55, 4.04 ± 1.15, and 6.53 ± 1.26 mM, and could induce apoptosis in HT-29 colon cancer cells []. The cytotoxicity of 38 in HT-29 cells could be mediated by a caspase cascade []. Furthermore, 38 also showed enzyme inhibition to topoisomerase I with an IC50 value of 13 µM, stronger than the positive control cryptotanshinone with an IC50 value of 17 µM []. Compounds 38 and 40 exhibited anti-larval activities toward barnacle Balanus amphitrite, with effective concentrations inhibiting 50% larval attachment (EC50) after 24 h of 0.28 and 0.10 mM, respectively [,]. And 38 and 40 also showed antioxidant activities toward OH• with an inhibition of 64.9% and 54.1% at 2.5 µM, respectively []. Compound 42 exhibited weak cytotoxicity against ECA-109, Hela-S3, and PANC-1 cancer cells with the inhibition rates of 44%, 52%, and 55%, respectively, at 20 µM, and 42 could mildly increase the transcriptional activation of RXRα []. Compound 42 exhibited anti-fouling activity against cyprid larvae of the barnacle with an LC50 value of 3.5 μg/mL []. Compound 42 could obviously increase the calcium ion concentration ([Ca2+]i) in myocytes, which is heavily dependent on the extracellular Ca2+ influx []. The LPS-induced migration, adhesion, and hyperpermeability of leukocytes to a human endothelial cell monolayer and in mice could be inhibited by 42 in a dose-dependent manner, suggesting that 42 may possess the potential to be developed into therapeutic agents to treat vascular inflammatory disorders []. In addition, 42 was proved to suppress TGFBIp-mediated and CLP-induced septic responses, indicating that 42 could be a key candidate for therapy of the different vascular inflammatory diseases by repressing the TGFBIp signaling pathway [].
4.2. Pyranone Derivatives
One pyranone derivative, 3-methoxy-2-methyl-4H-pyran-4-one (43) (Figure 5), was isolated from the culture extract of the fungus A. luteoalbus SCSIO F457 []. Compound 43 displayed no DPPH free radical scavenging or antibacterial activities []. In addition, 43 exhibited antibacterial activity against S. aureus ATCC 25923, Enterococcus faecalis ATCC 29212 and E. faecium K59–68 with MIC values of 25, 12.5, and 12.5 µg/mL, respectively []. The study used bioactivity tracking and molecular networking to examine the secondary metabolites of the Antarctic soil-derived fungus A. luteoalbus CH-6, resulting in the discovery of two new α-pyrones, acrostalapyrones A (44) and B (45), along with one previously identified analog, multiforisin G (46) (Figure 5) []. Compound 46 displayed significant immunosuppressive activity against LPS or Con A-(T-cells)-induced proliferations of mouse splenic lymphocytes (B-cells), with IC50 values of 1.2 and 0.9 µg/mL, respectively, which was stronger than that of positive control azathioprine (IC50 = 2.7 µg/mL) [].
4.3. Paulownin and Benzene Derivatives
One benzo-tetrahydrofuran-lignin, paulownin (47), and three benzene derivatives, 1-methyoxy-4-(2-hydroxy)ethylbenzene (48), 2-(4-hydroxyphenyl)-ethanol (49), and 1-phenylbutane-2,3-diol (50) (Figure 5), were isolated from the deep-sea sediment-derived fungus A. luteoalbus SCSIO F457, using the OSMAC strategy []. The absolute configuration of 50 was not confirmed. Compound 48 showed antioxidant activity, and the IC50 of DPPH free radical scavenging of 48 was 240.05 μg/mL [].
5. Conclusions
Between 1969 and 2022, researchers isolated 50 natural products from the genus Acrostalagmus, and 50% of these compounds are newly discovered. Between 1975 and 2011, there was a lack of research on the secondary metabolites of the genus Acrostalagmus, with only nine compounds isolated before 1974. However, the compounds from this genus started to attract the attention of researchers after 2012. Interestingly, all the compounds isolated between 2012 and 2022 are derived from the marine Acrostalagmus, and they comprise 82% of the natural products discovered from this genus (Table 1). Thess findings highlight the ocean as a vast resource treasury and suggest that the marine-derived genus Acrostalagmus possesses the ability to produce abundant secondary metabolites.

Table 1.
Compounds isolated from Acrostalagmus during 1969–2022.
The compounds isolated from the genus Acrostalagmus exhibit diverse structures, including terpenoids, alkaloids, peptides, pyranones, benzene derivatives, and paulownin. Among these compounds, alkaloids are of particular importance, comprising 56% of the natural products derived from this genus (Figure 6). Furthermore, within the alkaloid class, 61% belong to the epipolythiodioxopiperazine family. This substantial proportion highlights the significance of epipolythiodioxopiperazine as a key characteristic structure within the genus Acrostalagmus.
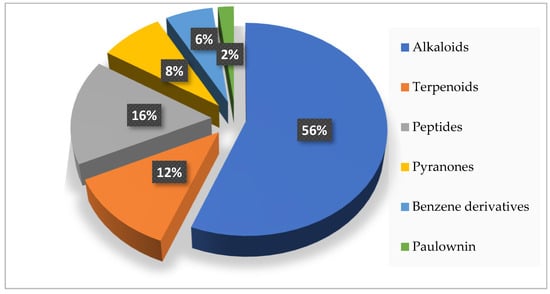
Figure 6.
Structural types of compounds isolated from Acrostalagmus during 1969–2022.
The genus Acrostalagmus has the potential to produce a variety of secondary metabolites with diverse bioactivities, including plant growth regulation, enzyme, Hsp90, and biofilm inhibitions, cytotoxic, antimicrobial, nematicidal, anti-inflammatory, immunosuppressive, antifouling, anti-QS, brine shrimp lethal, and antioxidant activities (Figure 7). Research indicates that 72% of the natural products obtained from Acrostalagmus exhibit bioactive activities, with compounds 1, 10, 12, 13, 15, 32, 35, 36, 38, 40, and 42 displaying more than three types of activity, and 50% of the bioactive compounds exhibiting prominent activities comparable or stronger than their positive controls, which further demonstrates the potential ability of this genus to produce bioactive natural products (Figure 7).
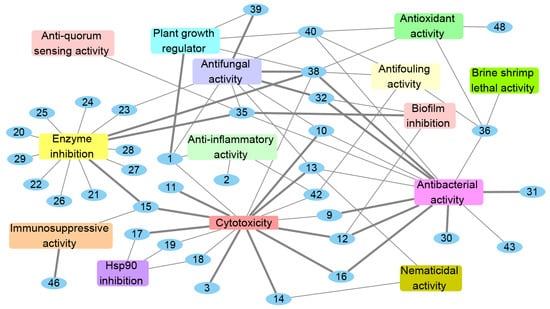
Figure 7.
Bioactivities of natural products isolated from Acrostalagmus during 1969–2022. The bold edges mean compounds with strong activities.
The bioactive compounds isolated from the genus Acrostalagmus mainly focus on cytotoxic (19%), enzyme inhibitory (17%), and antimicrobial (29%, with antibacterial (17%) and antifungal (12%) activities) activities (Figure 8), indicating considerable potential for the development of new anticancer compounds, enzyme inhibitors, and antibiotics from Acrostalagmus.
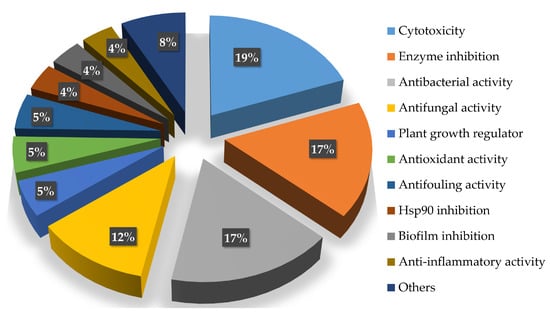
Figure 8.
Percentage of Acrostalagmus isolated compounds with different bioactivities during 1969–2022.
According to research, 72% of natural products derived from Acrostalagmus display bioactivities, with 50% of the bioactive compounds exhibiting more significant or comparable activities than their positive controls (Table 2, Table 3 and Table 4). Most of the compounds with remarkable activities (67%) belong to the family of epipolythiodioxopiperazine, confirming the potential of this structure as a precursor for the development of novel drugs. Eighty-nine percent of potent active compounds are isolated from marine derived fungi, further demonstrating the development potential of marine fungi.

Table 2.
Cytotoxicity of compounds isolated from Acrostalagmus during 1969–2022.

Table 3.
Antimicrobial activities of compounds isolated from Acrostalagmus during 1969–2022.

Table 4.
Other bioactivities of compounds isolated from Acrostalagmus during 1969–2022.
The stronger cytotoxic activities of compounds 3 and 10–17 compared to their positive control (Figure 7, Table 2) support their potential as new anticancer drugs. Compounds 9, 12, 31, and 32 exhibit more significant antibacterial activities than their positive controls, and 16 and 30 show comparable antibacterial activities compared to their positive control (Figure 7, Table 3), meaning they could be valuable starting points for the development of new antibiotics. Notably, compounds 9, 12, and 32 demonstrate stronger antibacterial activities against MRSA than their positive controls (Figure 7, Table 3), addressing the challenge of bacterial drug resistance. The combination of 38 and cyclo(L-Leu-L-Pro) exhibited obvious synergistic effect, with significant antimicrobial activity against VRE and pathogenic yeasts, which supports their potential use as synergistic antibiotics. Compounds 38 and 39 demonstrate prominent activities against agriculturally important fungi, much higher than the commercial fungicide bavistin, declaring the potential of 38 and 39 to be applied in agricultural fungicide (Figure 7, Table 3).
Compound 1 exhibits greater inhibition of germination and growth development at a concentration of 10−4 M compared to the commercial herbicide LOGRAN®. This indicates the potential for developing compound 1 as a new herbicide (Figure 7, Table 4). Compound 15 displays more potent inhibition of mushroom tyrosinase compared to the positive control kojic acid (Figure 7, Table 4), which demonstrates that 1 could be employed in various fields such as whitening and health care, treatment of pigmented skin diseases, pest control, and food preservation. Compound 35 exhibits stronger inhibition of the biofilm formation in P. aeruginosa PA01 than the positive control AZM, indicating 35 can serve as leading compound in developing new antimicrobial drugs for clinical or agricultural research. Compound 35 also shows more significant enzyme inhibition against α-glucosidase (AGS) than the positive control acarbose. In addition, 35 shows no cytotoxicity to the human normal hepatocyte (LO2) cells, suggesting the safety of 35 to be developed into hypoglycemic agent (Figure 7, Table 4). Compound 38 displays potent enzyme inhibition to topoisomerase I, stronger than the positive control cryptotanshinone, suggesting it can be developed into new antitumor drugs (Figure 7, Table 4). Compound 46 displays significant immunosuppressive activity, stronger than that of positive control azathioprine, which has the potential to be developed into immunomodulatory drugs (Figure 7, Table 4). These results further suggest that the genus Acrostalagmus holds promise as a source of bioactive compounds.
In this review, we comprehensively summarized the chemical structure types, biosynthesis, bioactivity, sources, and distribution of the secondary metabolites isolated from Acrostalagmus in the period between 1969 and 2022. The literature survey indicates that Acrostalagmus, especially marine derived Acrostalagmus, has great potential to produce abundant and diverse new bioactive natural products, and the family of epipolythiodioxopiperazine, with its significant bioactivities, could be one of the characteristic compound groups of the genus Acrostalagmus.
Author Contributions
T.S. collected the literature regarding natural products isolated from Acrostalagmus, and wrote the paper; H.W., Y.-J.L., Y.-F.W., and Q.P. revised the manuscript; B.W. and E.-L.S. organized and guided the writing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (No. 82104029 and 21868011) and the Talent Support Program of Shandong University of Science and Technology in 2019–2023.
Conflicts of Interest
The authors declare no conflict of interest.
References
- He, S.; Jin, X.; Wang, S. Antagonistic activity of Acrostalagmus luteo-albus against plant pathogenic fungi. J. Gansu Agric. Univ. 2010, 45, 60–65. [Google Scholar]
- Bondarenko, S.A.; Georgieva, M.L.; Bilanenko, E.N. Alkalitolerant micromycetes in acidic and neutral soils of the temperate zone. Microbiology 2016, 85, 737–744. [Google Scholar] [CrossRef]
- Bondarenko, S.A.; Ianutsevich, E.A.; Sinitsyna, N.A.; Georgieva, M.L.; Bilanenko, E.N.; Tereshina, B.M. Dynamics of the cytosol soluble carbohydrates and membrane lipids in response to ambient pH in alkaliphilic and alkalitolerant fungi. Microbiology 2018, 87, 21–32. [Google Scholar] [CrossRef]
- Rojas, N.L.; Cavalitto, S.F.; Cabello, M.; Hours, R.A.; Voget, C.E. Alkaline polysaccharidases produced in solid state cultures by alkalophilic fungi isolated from Argentina. J. Pure Appl. Microbiol. 2008, 2, 1–10. [Google Scholar]
- Kavitha, P.G.; Sudha, A.; Devi, P.A.; Kumaran, K. A comparative study on forest soil microbial diversity and biomass in nilgiri biosphere of Southern India. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 3701–3715. [Google Scholar] [CrossRef]
- Monoson, H.L.; Conway, T.D.; Nelson, R.E. Four endoparasitic nematode destroying fungi isolated from sand ridge state forest soil. Mycopathologia 1975, 57, 59–62. [Google Scholar] [CrossRef]
- Nguyen, M.T.H.D.; Thomas, T. Diversity, host-specificity and stability of sponge-associated fungal communities of co-occurring sponges. PeerJ 2018, 6, e4965. [Google Scholar] [CrossRef] [PubMed]
- Youssef, F.S.; Simal-Gandara, J. Comprehensive overview on the chemistry and biological activities of selected alkaloid producing marine-derived fungi as a valuable reservoir of drug entities. Biomedicines 2021, 9, 485. [Google Scholar] [CrossRef]
- Shi, T.; Li, X.-Q.; Wang, Z.-M.; Yu, Y.-Y.; Dai, J.-J.; Shi, D.-Y.; Zheng, L. Bioactivity-guided screening of antimicrobial secondary metabolites from Antarctic cultivable fungus Acrostalagmus luteoalbus CH-6 combined with molecular networking. Mar. Drugs 2022, 20, 334. [Google Scholar] [CrossRef]
- Amatayakul, T. Synthesis of fibrinolysin by fungi. Ohio J. Sci. 1955, 55, 343–353. [Google Scholar]
- Artigues, M.; Davet, P. β-(1 → 3)-glucanase and chitinase activities in some fungi in relation to their antisclerotic activity towards Corticium rolfsii in sterile soil. Soil Biol. Biochem. 1984, 16, 527–528. [Google Scholar] [CrossRef]
- Rojas, N.L.; Voget, C.E.; Hours, R.A.; Cavalitto, S.F. Purification and characterization of a novel alkaline α-L-rhamnosidase produced by Acrostalagmus luteoalbus. J. Ind. Microbiol. Biotechnol. 2011, 38, 1515–1522. [Google Scholar] [CrossRef]
- Soprunov, F.F.; Galiulina, Z.A. Predatory hyphomycetes from Turkmenistan soils. Mikrobiologiya 1951, 20, 489–499. [Google Scholar]
- Jackson, R.M. Some aspects of soil fungistasis. J. Gen. Microbiol. 1958, 19, 390–401. [Google Scholar] [CrossRef]
- Jensen, H.L. Carbon nutrition of some microorganisms decomposing halogen-substituted aliphatic acids. Acta Agric. Scand. 1963, 13, 404–412. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, F.; Tian, X.; Li, J.; Zhang, S. Identification and activities of fungal strain 00457 isolated from the deep-sea sediment of northern south china sea. Shengwu Jishu Tongbao 2012, 10, 199–204. [Google Scholar]
- Khalmuratovalt, I.; Choilt, D.-H.; Yoon, H.-J.; Yoon, T.-W.; Kim, J.-G. Diversity and plant growth promotion of fungal endophytes in five halophytes from the buan salt marsh. J. Microbiol. Biotechnol. 2021, 31, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Andersson, A.M.A.; Salo, J.; Mikkola, R.; Kurnitski, J.; Salonen, H.; Marik, T.; Kredics, L. Melinacidin-producing Acrostalagmus luteoalbus, a major constituent of mixed mycobiota contaminating insulation material in an outdoor wall. Pathogens 2021, 10, 843. [Google Scholar] [CrossRef]
- Ellestad, G.A.; Evans, R.H., Jr.; Kunstmann, M.P. Structure of a C17 antifungal terpenoid from an unidentified Acrostalagmus species. J. Am. Chem. Soc. 1969, 91, 2134–2136. [Google Scholar] [CrossRef]
- Ellestad, G.A.; Evans, R.H., Jr.; Kunstmann, M.P.; Lancaster, J.E.; Morton, G.O. Structure and chemistry of antibiotic LL-Z1271α, an antifungal carbon-17 terpene. J. Am. Chem. Soc. 1970, 92, 5483–5489. [Google Scholar] [CrossRef] [PubMed]
- Ellestad, G.A.; Evans, R.H., Jr.; Kunstmann, M.P. LL-Z1271β [C16H24O5], an additional C16 terpenoid metabolite from an Acrostalagmus species. Tetrahedron Lett. 1971, 12, 497–500. [Google Scholar] [CrossRef]
- Sato, M.; Ruo, T.-I.; Hayashi, T.; Kakisawa, H.; Miyaki, T.; Yamamoto, H.; Fujisawa, K. Structure of C16-terpenes from Acrostalagmus. Tetrahedron Lett. 1974, 15, 2183–2186. [Google Scholar] [CrossRef]
- Rusman, Y.; Wilson, M.B.; Williams, J.M.; Held, B.W.; Blanchette, R.A.; Anderson, B.N.; Lupfer, C.R.; Salomon, C.E. Antifungal norditerpene oidiolactones from the fungus Oidiodendron truncatum, a potential biocontrol agent for White-Nose Syndrome in bats. J. Nat. Prod. 2020, 83, 344–353. [Google Scholar] [CrossRef]
- Pettit, G.R.; Tan, R.; Herald, D.L.; Hamblin, J.; Pettit, R.K. Antineoplastic agents. 488. isolation and structure of Yukonin from a yukon territory fungus. J. Nat. Prod. 2003, 66, 276–278. [Google Scholar] [CrossRef]
- Deng, C.; Huang, C.; Wu, Q.; Pang, J.; Lin, Y. A new sesquiterpene from the mangrove endophytic fungus Aspergillus terreus (No. GX7-3B). Nat. Prod. Res. 2013, 27, 1882–1887. [Google Scholar] [CrossRef]
- Kakisawa, H.; Sato, M.; Ruo, T.-i.; Hayashi, T. Biosynthesis of a C16-terpenoid lactone, a plant growth regulator. J. Chem. Soc. Chem. Commun. 1973, 20, 802–803. [Google Scholar] [CrossRef]
- Barrero, A.F.; Sánchez, J.F.; Elmerabet, J.; Jiménez-González, D.; Macías, F.A.; Simonet, A.M. Enantiospecific syntheses of the potent bioactives nagilactone F and the mould metabolite LL-Z1271α an evaluation of their allelopathic potential. Tetrahedron 1999, 55, 7289–7304. [Google Scholar] [CrossRef]
- Dinarello, C.A. Inflammatory cytokines: Interleukin-1 and tumor necrosis factor as effector molecules in autoimmune diseases. Curr. Opin. Immunol. 1991, 3, 941–948. [Google Scholar] [CrossRef]
- Ichikawa, K.; Inagaki, T.; Kachi-Tonai, H.; Kojima, Y.; Nakamura, T.-a.; Nishida, H.; Ueno, Y.; Binding, P.; Gabel, C.A.; Lucas, V. LL-Z1271α: An interleukin-1β production inhibitor. Biochem. Biophys. Res. Commun. 2001, 286, 697–700. [Google Scholar] [CrossRef]
- Ichikawa, K.; Hirai, H.; Ishiguro, M.; Kambara, T.; Kato, Y.; Kim, Y.J.; Kojima, Y.; Matsunaga, Y.; Nishida, H.; Shiomi, Y. Cytokine production inhibitors produced by a fungus, Oidiodendron griseum. J. Antibiot. 2001, 54, 697–702. [Google Scholar] [CrossRef][Green Version]
- Argoudelis, A.D.; Reusser, F. Melinacidins, a new family of antibiotics. J. Antibiot. 1971, 24, 383. [Google Scholar] [CrossRef]
- Reusser, F. Mode of action of melinacidin, an inhibitor of nicotinic acid biosynthesis. J. Bacteriol. 1968, 96, 1285. [Google Scholar] [CrossRef]
- Argoudelis, A.D. Melinacidins II, III, and IV. New 3,6-epidithiadiketopiperazine antibiotics. J. Antibiot. 1972, 25, 171. [Google Scholar] [CrossRef]
- Argoudelis, A.D.; Mizsak, S.A. Melinacidins II, III and IV structural studies. J. Antibiot. 1977, 30, 468. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Blunt, J.W.; Cole, A.L.J.; Cannon, J.F.; Robinson, W.T.; Munro, M.H.G. Two novel cytotoxic cyclodepsipeptides from a mycoparasitic Cladobotryum sp. J. Org. Chem. 2003, 68, 2002–2005. [Google Scholar] [CrossRef] [PubMed]
- Ebead, G.A.; Overy, D.P.; Berrué, F.; Kerr, R.G. Westerdykella reniformis sp. nov., producing the antibiotic metabolites melinacidin IV and chetracin B. IMA Fungus 2012, 3, 189–201. [Google Scholar] [CrossRef]
- Wang, F.-Z.; Huang, Z.; Shi, X.-F.; Chen, Y.-C.; Zhang, W.-M.; Tian, X.-P.; Li, J.; Zhang, S. Cytotoxic indole diketopiperazines from the deep sea-derived fungus Acrostalagmus luteoalbus SCSIO F457. Bioorg. Med. Chem. Lett. 2012, 22, 7265–7267. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Wang, Y.; Yu, R.; Feng, Y.; Wang, L.; Che, Q.; Gu, Q.; Li, D.; Li, J.; Zhu, T. Chetracins E and F, cytotoxic epipolythiodioxopiperazines from the marine-derived fungus Acrostalagmus luteoalbus HDN13-530. RSC Adv. 2018, 8, 53–58. [Google Scholar] [CrossRef]
- Cao, J.; Li, X.-M.; Meng, L.-H.; Konuklugil, B.; Li, X.; Li, H.-L.; Wang, B.-G. Isolation and characterization of three pairs of indolediketopiperazine enantiomers containing infrequent N-methoxy substitution from the marine algal-derived endophytic fungus Acrostalagmus luteoalbus TK-43. Bioorg. Chem. 2019, 90, 103030. [Google Scholar] [CrossRef]
- Cao, J.; Li, X.-M.; Li, X.; Li, H.-L.; Konuklugil, B.; Wang, B.-G. Uncommon N-methoxyindolediketopiperazines from Acrostalagmus luteoalbus, a marine algal isolate of endophytic fungus. Chin. J. Chem. 2021, 39, 2808–2814. [Google Scholar] [CrossRef]
- Chen, X.-Y.; Zhong, W.-M.; Zeng, Q.; Wang, F.-Z. A preliminary study on the chemical diversity of the deep-sea derived fungus Acrostalagmus luteoalbus SCSIO F457 based on OSMAC strategy. Zhongguo Haiyang Yaowu 2020, 39, 11–19. [Google Scholar]
- Adams, T.C.; Payette, J.N.; Cheah, J.H.; Movassaghi, M. Concise total synthesis of (+)-luteoalbusins A and B. Org. Lett. 2015, 17, 4268–4271. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Blunt, J.W.; Cole, A.L.J.; Munro, M.H.G. Novel cytotoxic thiodiketopiperazine derivatives from a Tilachlidium sp. J. Nat. Prod. 2004, 67, 2090–2092. [Google Scholar] [CrossRef]
- Dong, J.-Y.; He, H.-P.; Shen, Y.-M.; Zhang, K.-Q. Nematicidal epipolysulfanyldioxopiperazines from Gliocladium roseum. J. Nat. Prod. 2005, 68, 1510–1513. [Google Scholar] [CrossRef]
- Fujimoto, H.; Sumino, M.; Okuyama, E.; Ishibashi, M. Immunomodulatory constituents from an ascomycete, Chaetomium seminudum. J. Nat. Prod. 2004, 67, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Marmouzi, I.; El Abbes Faouzi, M.; Saidi, N.; Cherrah, Y.; Rehberg, N.; Ebada, S.S.; Ebrahim, W.; Kalscheuer, R.; Proksch, P. Bioactive secondary metabolites from Chaetomium globosum, an endophyte from the Moroccan plant Avena sativa. Chem. Nat. Compd. 2017, 53, 1208–1211. [Google Scholar] [CrossRef]
- Zhai, Y.-J.; Huo, G.-M.; Zhang, Q.; Li, D.; Wang, D.-C.; Qi, J.-Z.; Han, W.-B.; Gao, J.-M. Phaeosphaones: Tyrosinase inhibitory thiodiketopiperazines from an endophytic Phaeosphaeria fuckelii. J. Nat. Prod. 2020, 83, 1592–1597. [Google Scholar] [CrossRef]
- Li, L.; Li, D.; Luan, Y.; Gu, Q.; Zhu, T. Cytotoxic metabolites from the Antarctic psychrophilic fungus Oidiodendron truncatum. J. Nat. Prod. 2012, 75, 920–927. [Google Scholar] [CrossRef]
- Wei, W.; Jiang, N.; Mei, Y.N.; Chu, Y.L.; Ge, H.M.; Song, Y.C.; Ng, S.W.; Tan, R.X. An antibacterial metabolite from Lasiodiplodia pseudotheobromae F2. Phytochemistry 2014, 100, 103–109. [Google Scholar] [CrossRef]
- Arora, P.; Wani, Z.A.; Nalli, Y.; Ali, A.; Riyaz-Ul-Hassan, S. Antimicrobial potential of thiodiketopiperazine derivatives produced by Phoma sp., an endophyte of Glycyrrhiza glabra Linn. Microb. Ecology 2016, 72, 802–812. [Google Scholar] [CrossRef]
- Scharf, D.H.; Remme, N.; Habel, A.; Chankhamjon, P.; Scherlach, K.; Heinekamp, T.; Hortschansky, P.; Brakhage, A.A.; Hertweck, C. A Dedicated glutathione S-transferase mediates carbon-sulfur bond formation in gliotoxin biosynthesis. J. Am. Chem. Soc. 2011, 133, 12322–12325. [Google Scholar] [CrossRef]
- Zhao, P.; Xue, Y.; Li, J.; Li, X.; Zu, X.; Zhao, Z.; Quan, C.; Gao, W.; Feng, S. Non-lipopeptide fungi-derived peptide antibiotics developed since 2000. Biotechnol. Lett. 2019, 41, 651–673. [Google Scholar] [CrossRef] [PubMed]
- Scharf, D.H.; Heinekamp, T.; Remme, N.; Hortschansky, P.; Brakhage, A.A.; Hertweck, C. Biosynthesis and function of gliotoxin in Aspergillus fumigatus. Appl. Microbiol. Biotechnol. 2012, 93, 467–472. [Google Scholar] [CrossRef]
- Kim, J.; Movassaghi, M. Biogenetically-inspired total synthesis of epidithiodiketopiperazines and related alkaloids. Acc. Chem. Res. 2015, 48, 1159–1171. [Google Scholar] [CrossRef]
- Scharf, D.H.; Remme, N.; Heinekamp, T.; Hortschansky, P.; Brakhage, A.A.; Hertweck, C. Transannular disulfide formation in gliotoxin biosynthesis and its role in self-resistance of the human pathogen Aspergillus fumigatus. J. Am. Chem. Soc. 2010, 132, 10136–10141. [Google Scholar] [CrossRef]
- Sun, S.; Dai, X.; Sun, J.; Bu, X.; Weng, C.; Li, H.; Zhu, H. A diketopiperazine factor from Rheinheimera aquimaris QSI02 exhibits anti-quorum sensing activity. Sci. Rep. 2016, 6, 39637. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Sun, S.; Li, H.; Sun, J.; Liu, A.; Zhou, W. Preparation, structural identification and application of quorum sensing inhibitor. CN105130963, 9 December 2015. [Google Scholar]
- Lu, X.; Zhang, M.; Qiu, Y.; Liu, X.; Wang, C.; Chen, J.; Zhang, H.; Wei, B.; Yu, Y.; Ying, Y.; et al. α-Glucosidase inhibitors from two mangrove-derived actinomycetes. Molecules 2023, 28, 3822. [Google Scholar] [CrossRef]
- Zhao, D.; Cao, F.; Guo, X.-J.; Zhang, Y.-R.; Kang, Z.; Zhu, H.-J. Antibacterial indole alkaloids and anthraquinones from a sewage-derived fungus Eurotium sp. Chem. Nat. Compd. 2018, 54, 399–401. [Google Scholar] [CrossRef]
- Meng, L.-H.; Du, F.-Y.; Li, X.-M.; Pedpradab, P.; Xu, G.-M.; Wang, B.-G. Rubrumazines A-C, indolediketopiperazines of the isoechinulin class from Eurotium rubrum MA-150, a fungus obtained from marine mangrove-derived rhizospheric soil. J. Nat. Prod. 2015, 78, 909–913. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.-Z.; Pu, X.; Luo, G.; Zhao, L.-X.; Xu, L.-H.; Li, W.-J.; Luo, Y. Isolation and characterization of new p-terphenyls with antifungal, antibacterial, and antioxidant activities from halophilic actinomycete Nocardiopsis gilva YIM 90087. J. Agric. Food Chem. 2013, 61, 3006–3012. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Jiang, W.; Miao, L.; Jin, C.; Bao, W. Anti-diatom compounds from marine bacterium Pseudomonas putida. Weishengwu Xuebao 2013, 53, 825–831. [Google Scholar]
- Ortiz-Castro, R.; Diaz-Perez, C.; Martinez-Trujillo, M.; del Rio, R.E.; Campos-Garcia, J.; Lopez-Bucio, J. Transkingdom signaling based on bacterial cyclodipeptides with auxin activity in plants. Proc. Natl. Acad. Sci. USA 2011, 108, 7253. [Google Scholar] [CrossRef]
- Wang, G.; Dai, S.; Chen, M.; Wu, H.; Xie, L.; Luo, X.; Li, X. Two diketopiperazine cyclo(Pro-Phe) isomers from marine bacterium Bacillus subtilis sp. 13-2. Chem. Nat. Compd. 2010, 46, 583–585. [Google Scholar] [CrossRef]
- Rhee, K.-H. Cyclic dipeptides exhibit synergistic, broad spectrum antimicrobial effects and have anti-mutagenic properties. Int. J. Antimicrob. Agents 2004, 24, 423–427. [Google Scholar] [CrossRef]
- Kwak, M.-K.; Liu, R.; Kim, M.-K.; Moon, D.; Kim, A.H.; Song, S.-H.; Kang, S.-O. Cyclic dipeptides from lactic acid bacteria inhibit the proliferation of pathogenic fungi. J. Microbiol. 2014, 52, 64–70. [Google Scholar] [CrossRef]
- Kumar, N.; Mohandas, C.; Nambisan, B.; Kumar, D.R.S.; Lankalapalli, R.S. Isolation of proline-based cyclic dipeptides from Bacillus sp. N strain associated with rhabitid entomopathogenic nematode and its antimicrobial properties. World J. Microbiol. Biotechnol. 2013, 29, 355–364. [Google Scholar] [CrossRef]
- Alshaibani, M.M.; MohamadZin, N.; Jalil, J.; Sidik, N.M.; Ahmad, S.J.; Karna, N.; Edrada-Ebel, R. Isolation, purification, and characterization of five active diketopiperazine derivatives from endophytic Streptornyces SUK 25 with antimicrobial and cytotoxic activities. J. Microbiol. Biotechnol. 2017, 27, 1249–1256. [Google Scholar] [CrossRef]
- Brauns, S.C.; Milne, P.; Naude, R.; van de Venter, M. Selected cyclic dipeptides inhibit cancer cell growth and induce apoptosis in HT-29 colon cancer cells. Anticancer Res. 2004, 24, 1713–1719. [Google Scholar]
- Brauns, S.C.; Dealtry, G.; Milne, P.; Naude, R.; Van De Venter, M. Caspase-3 activation and induction of PARP cleavage by cyclic dipeptide cyclo(Phe-Pro) in HT-29 cells. Anticancer Res. 2005, 25, 4197–4202. [Google Scholar]
- Rhee, K.-H. Inhibition of DNA topoisomerase I by cyclo(L-prolyl-L-phenylalanyl) isolated from Streptomyces sp. AMLK-335. J. Microbiol. Biotechnol. 2002, 12, 1013–1016. [Google Scholar]
- Li, X.; Dobretsov, S.; Xu, Y.; Xiao, X.; Hungi, O.S.; Qian, P.-Y. Antifouling diketopiperazines produced by a deep-sea bacterium, Streptomyces fungicidicus. Biofouling 2006, 22, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.-H.; Xu, Y.; Gao, J.; Qian, P.-Y.; Zhang, S. Antibacterial and antilarval compounds from marine bacterium Pseudomonas rhizosphaerae. Ann. Microbiol. 2009, 59, 229–233. [Google Scholar] [CrossRef]
- Takaya, Y.; Furukawa, T.; Miura, S.; Akutagawa, T.; Hotta, Y.; Ishikawa, N.; Niwa, M. Antioxidant constituents in distillation residue of awamori spirits. J. Agric. Food Chem. 2007, 55, 75–79. [Google Scholar] [CrossRef]
- Lin, W.-X.; Xie, C.-L.; Zhou, M.; Xia, M.-L.; Zhou, T.-T.; Chen, H.-F.; Yang, X.-W.; Yang, Q. Chemical constituents from the deep sea-derived Streptomyces xiamenensis MCCC 1A01570 and their effects on RXRα transcriptional regulation. Nat. Prod. Res. 2020, 34, 1461–1464. [Google Scholar] [CrossRef]
- Yang, B.; Huang, J.; Lin, X.; Zhang, Y.; Tao, H.; Liu, Y. A new diketopiperazine from the marine sponge Callyspongia species. Rec. Nat. Prod. 2016, 10, 117–121. [Google Scholar]
- Hou, Y.; Sun, S.; Wu, L.; Wang, X.; Li, T.; Zhang, M.; Wang, J.; Wang, L. Calcium sensitizers isolated from the edible pine mushroom, Tricholoma matsutake (S. Ito & Imai) Sing. Z. Naturforsch. C J. Biosci. 2013, 68, 113–117. [Google Scholar]
- Kang, H.; Ku, S.-K.; Choi, H.; Bae, J.-S. Three diketopiperazines from marine-derived bacteria inhibit LPS-induced endothelial inflammatory responses. Bioorg. Med. Chem. Lett. 2016, 26, 1873–1876. [Google Scholar] [CrossRef]
- Jung, B.; Ku, S.-K.; Gao, M.; Kim, K.-M.; Han, M.-S.; Choi, H.; Bae, J.-S. Suppressive effects of three diketopiperazines from marine-derived bacteria on TGFBIp-mediated septic responses in human endothelial cells and mice. Arch. Pharmacal Res. 2016, 39, 843–854. [Google Scholar] [CrossRef]
- Maglangit, F.; Kyeremeh, K.; Deng, H. Deletion of the accramycin pathway-specific regulatory gene accJ activates the production of unrelated polyketide metabolites. Nat. Prod. Res. 2022, 37, 2753–2758. [Google Scholar] [CrossRef]
- Fujimoto, H.; Sumino, M.; Nagano, J.; Natori, H.; Okuyama, E.; Yamazaki, M. Immunomodulatory constituents from three Ascomycetes, Gelasinospora heterospora, G. multiforis, and G. longispora. Chem. Pharm. Bull. 1999, 47, 71–76. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).