Immunolocalization of the AT-1R Ang II Receptor in Human Kidney Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Primary Culture of Kidney Epithelial Cells
2.3. Proliferation Assay
2.4. RNA Isolation from Tissues and Real-Time PCR Assays
2.5. SDS Electrophoresis of Ang II-R
2.6. Immunoblotting
2.7. Immunocytochemistry
2.8. Image Analysis and Tumor Cell Counting
2.9. Statistical Analysis
3. Results
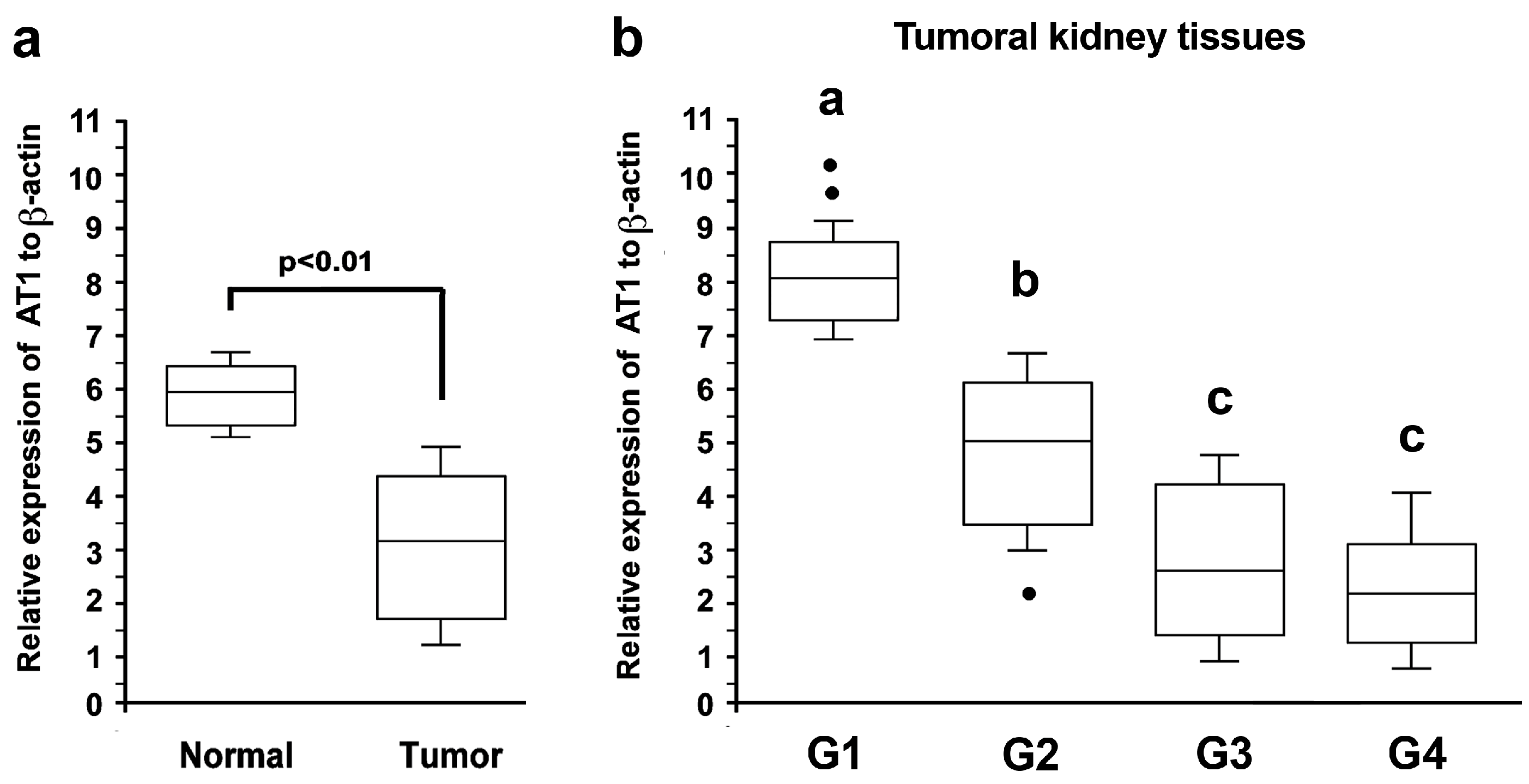
3.1. AT1-R RNA Expression in Normal and Cancerous Kidney Tissues
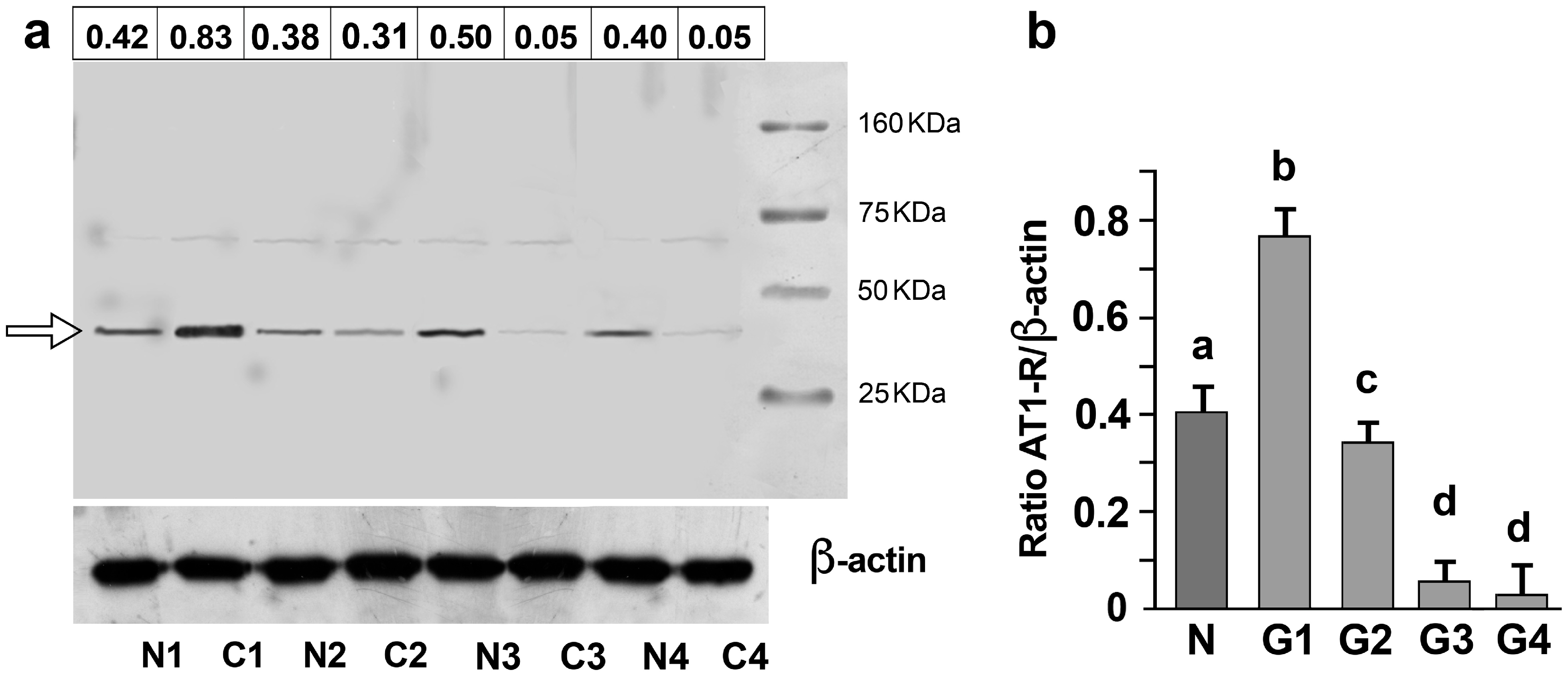
3.2. AT1-R Expression in Normal and Cancerous Kidney Tissues
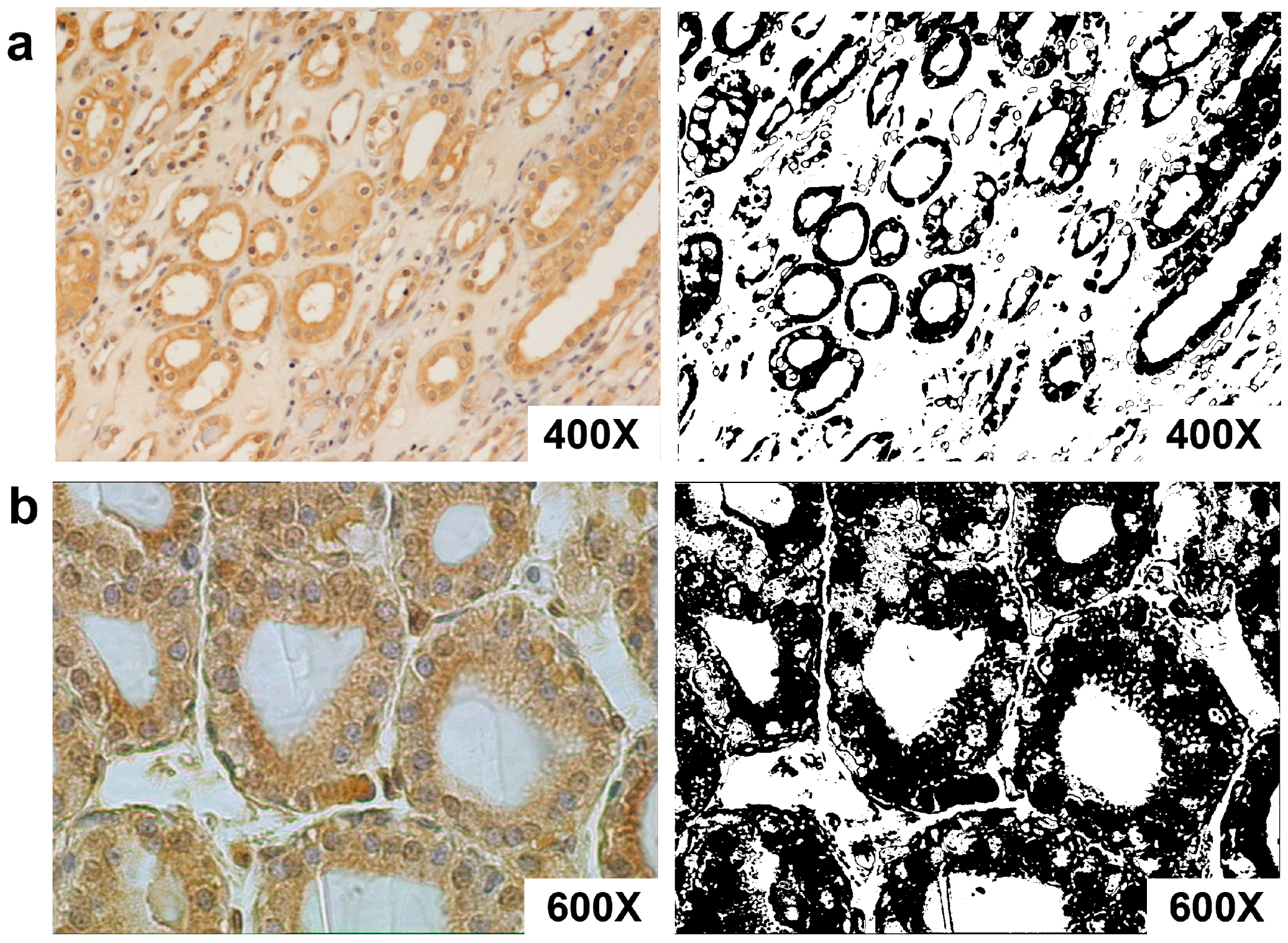
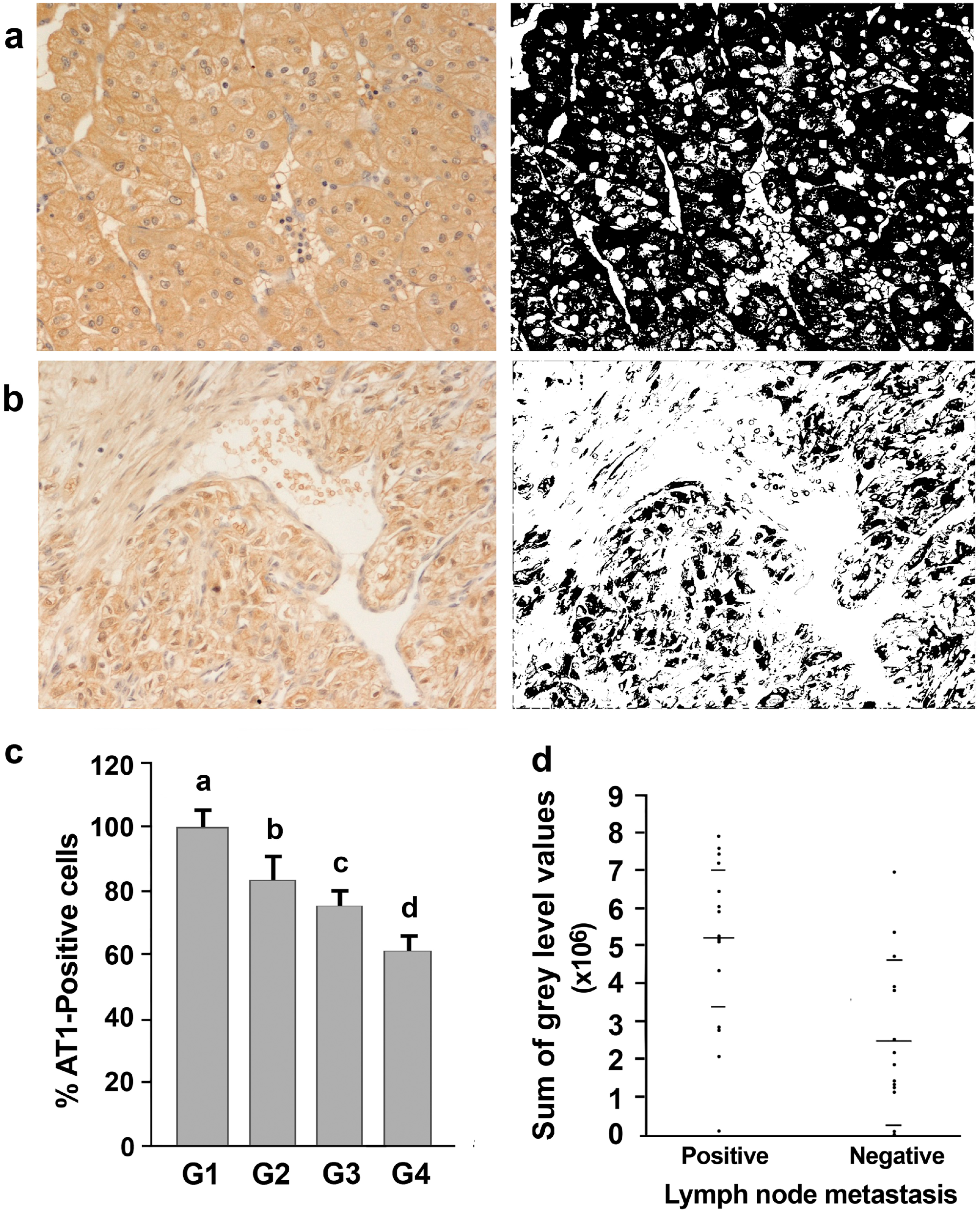
3.3. AT1-R Immunolocalization in Normal and Cancerous Kidney Tissues
3.4. Association of AT-1R Expression with Clinicopathological Characters in Kidney Cancer
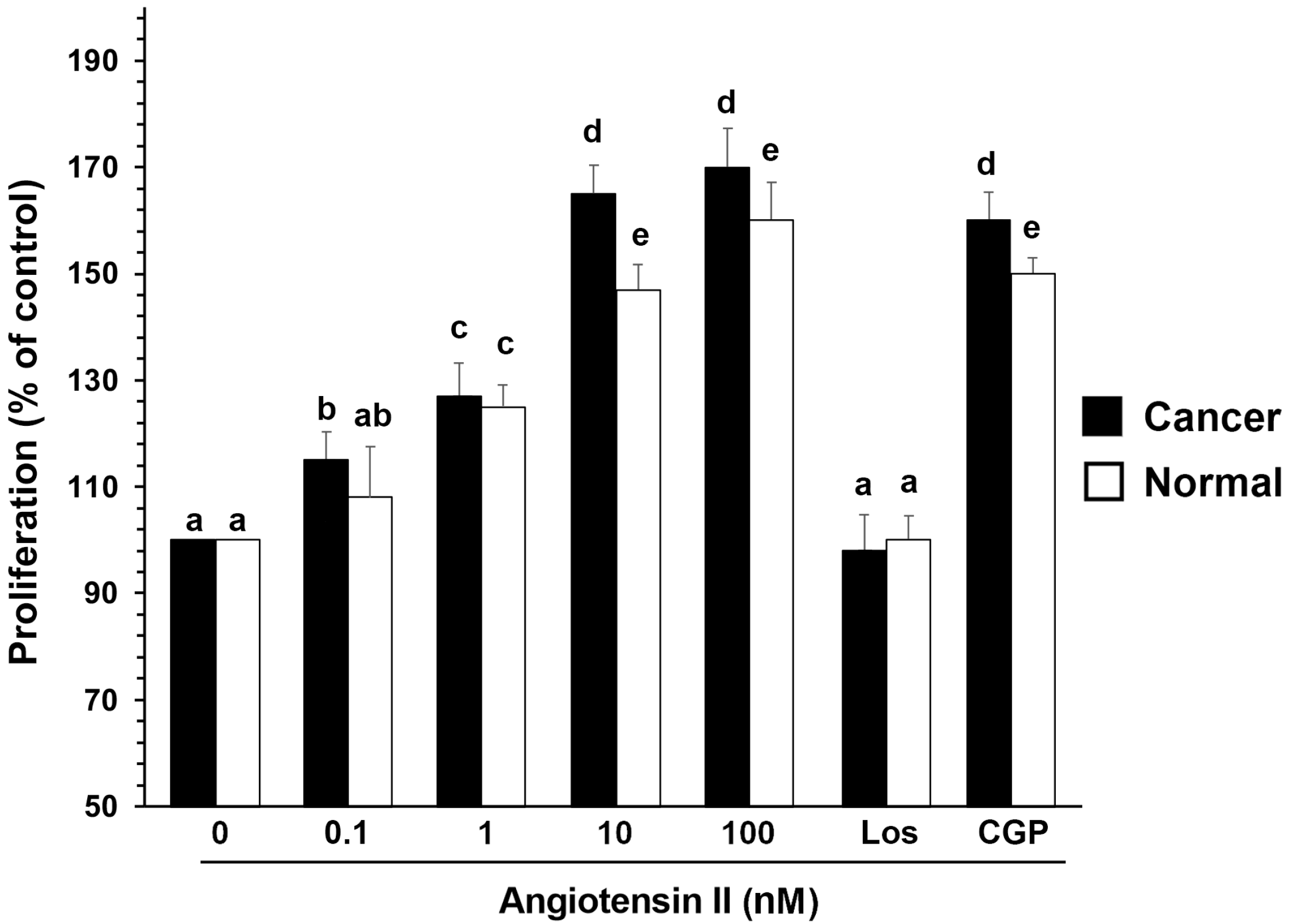
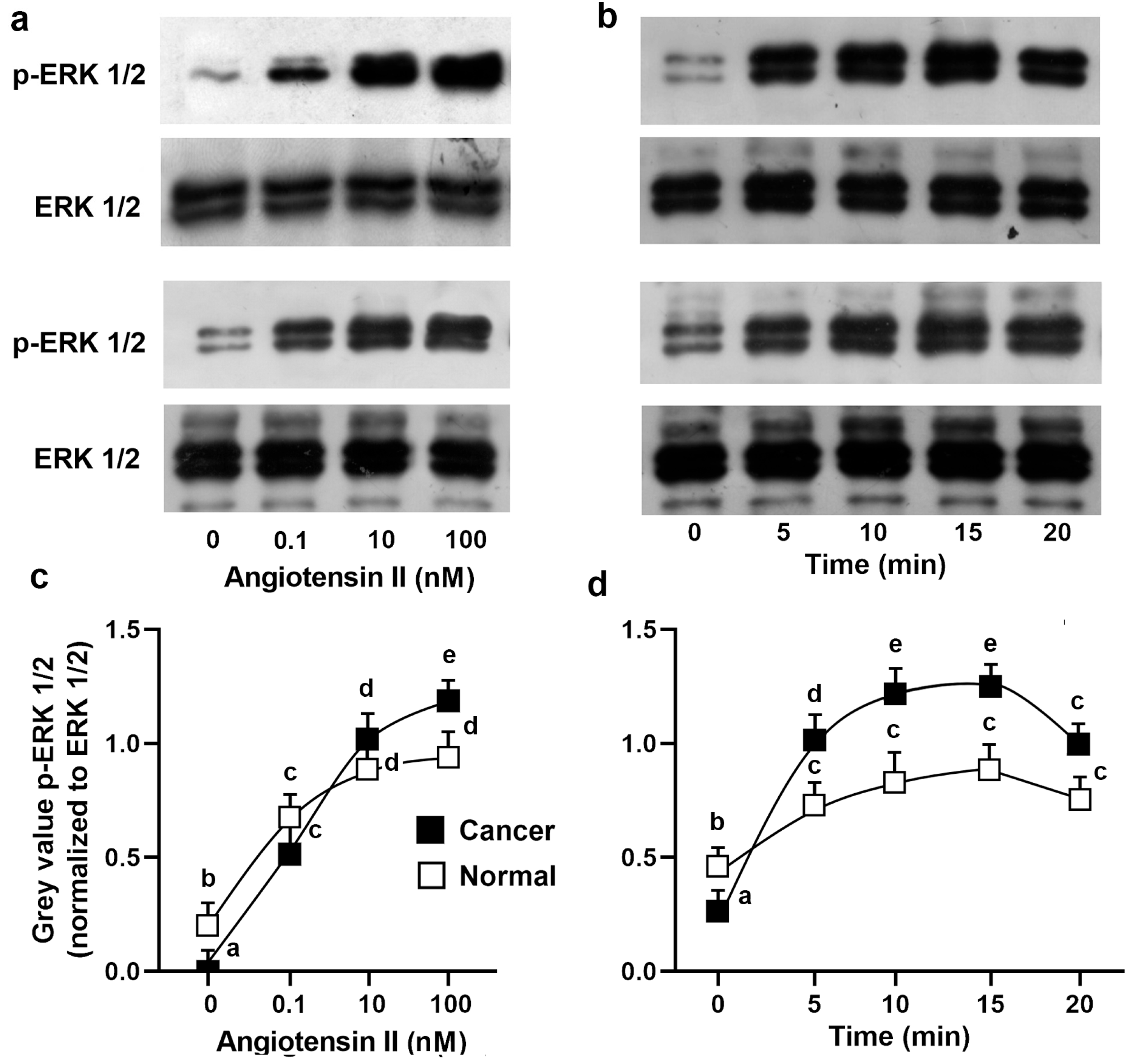
3.5. Mitogenic Effects of Ang II and ERK1/2 Activation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferrão, F.M.; Lara, L.S.; Lowe, J. Renin-angiotensin system in the kidney: What is new? World J. Nephrol. 2014, 3, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Ingelfinger, J.R.; Pratt, R.E.; Ellison, K.; Dzau, V.J. Sodium regulation of angiotensinogen mRNA expression in rat kidney cortex and medulla. J. Clin. Investig. 1986, 78, 1311–1315. [Google Scholar] [CrossRef] [PubMed]
- Seikaly, M.G.; Arant, B.S., Jr.; Seney, F.D., Jr. Endogenous angiotensin concentrations in specific intrarenal fluid compartments of the rat. J. Clin. Investig. 1990, 86, 1352–1357. [Google Scholar] [CrossRef] [PubMed]
- Harrison-Bernard, L.M. The renal renin-angiotensin system. Adv. Physiol. Educ. 2009, 33, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Norman, J.; Badie-Dezfooly, B.; Nord, E.P.; Kurtz, I.; Schlosser, J.; Chaudhari, A.; Fine, L.G. EGF-induced mitogenesis in proximal tubular cells: Potentiation by angiotensin II. Am. J. Physiol. Physiol. 1987, 253, F299–F309. [Google Scholar] [CrossRef]
- Wolf, G.; Jablonski, K.; Schroeder, R.; Reinking, R.; Shankland, S.J.; Stahl, R.A. Angiotensin II–induced hypertrophy of proximal tubular cells requires p27Kip1. Kidney Int. 2003, 64, 71–81. [Google Scholar] [CrossRef]
- Gröne, H.J.; Helmchen, U. Impairment and recovery of the clipped kidney in two kidney, one clip hypertensive rats during and after antihypertensive therapy. Lab. Investig. 1986, 54, 645–655. [Google Scholar]
- Gröne, H.J.; Simon, M.; Fuchs, E. Autoradiographic characterization of angiotensin receptor subtypes in fetal and adult human kidney. Am. J. Physiol. Content 1992, 262, F326–F331. [Google Scholar] [CrossRef]
- Siragy, H.M. Angiotensin AT1 and AT2 receptors the battle for health and disease. Nephrol. Dial. Transpl. 2007, 22, 3128–3130. [Google Scholar] [CrossRef]
- Goldfarb, D.A.; Diz, D.I.; Tubbs, R.R.; Ferrario, C.M.; Novick, A.C. Angiotensin II Receptor Subtypes in the Human Renal Cortex and Renal Cell Carcinoma. J. Urol. 1994, 151, 208–213. [Google Scholar] [CrossRef]
- Barker, S.; Marchant, W.; Ho, M.M.; Puddefoot, J.R.; Hinson, J.P.; Clark, A.J.L.; Vinson, G.P. A monoclonal antibody to a conserved sequence in the extracellular domain recognizes the angiotensin II AT1 receptor in mammalian target tissues. J. Mol. Endocrinol. 1993, 11, 241–245. [Google Scholar] [CrossRef]
- Marsigliante, S.; Resta, L.; Muscella, A.; Vinson, G.; Marzullo, A.; Storelli, C. AT1 angiotensin II receptor subtype in the human larynx and squamous laryngeal carcinoma. Cancer Lett. 1996, 110, 19–27. [Google Scholar] [CrossRef]
- Inwang, E.R.; Puddefoot, J.R.; Brown, C.L.; Goode, A.W.; Marsigliante, S.; Ho, M.M.; Payne, J.G.; Vinson, G.P. Angiotensin II type 1 receptor expression in human breast tissues. Br. J. Cancer 1997, 75, 1279–1283. [Google Scholar] [CrossRef][Green Version]
- Piastowska-Ciesielska, A.W.; Płuciennik, E.; Wójcik-Krowiranda, K.; Bieńkiewicz, A.; Bednarek, A.; Ochędalski, T. Analysis of the expression of angiotensin II type 1 receptor and VEGF in endometrial adenocarcinoma with different clinicopathological characteristics. Tumour Biol. 2011, 33, 767–774. [Google Scholar] [CrossRef]
- Moch, H.; Humphrey, P.A.; Ulbright, T.M.; Reuter, V.E. WHO Classification of Tumours of the Urinary System and Male Genital Organs; International Agency for Research on Cancer: Lyon, France, 2016; pp. 18–21. [Google Scholar]
- Delahunt, B.; Cheville, J.C.; Martignoni, G.; Humphrey, P.A.; Magi-Galluzzi, C.; McKenney, J.; Egevad, L.; Algaba, F.; Moch, H.; Grignon, D.J.; et al. The International Society of Urological Pathology (ISUP) Grading System for Renal Cell Carcinoma and Other Prognostic Parameters. Am. J. Surg. Pathol. 2013, 37, 1490–1504. [Google Scholar] [CrossRef]
- Delahunt, B.; McKenney, J.K.; Lohse, C.M.; Leibovich, B.C.; Thompson, R.H.; Boorjian, S.A.; Cheville, J.C. A Novel Grading System for Clear Cell Renal Cell Carcinoma Incorporating Tumor Necrosis. Am. J. Surg. Pathol. 2013, 37, 311–322. [Google Scholar] [CrossRef]
- Valente, M.J.; Henrique, R.; Costa, V.L.; Jerónimo, C.; Carvalho, F.; Bastos, M.L.; de Pinho, P.G.; Carvalho, M. A Rapid and Simple Procedure for the Establishment of Human Normal and Cancer Renal Primary Cell Cultures from Surgical Specimens. PLoS ONE 2011, 6, e19337. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Barca, A.; Gatti, F.; Spagnolo, D.; Ippati, S.; Vetrugno, C.; Verri, T. Responsiveness of Carnosine Homeostasis Genes in the Pancreas and Brain of Streptozotocin-Treated Mice Exposed to Dietary Carnosine. Int. J. Mol. Sci. 2018, 19, 1713. [Google Scholar] [CrossRef]
- Muscella, A.; Marsigliante, S.; Verri, T.; Urso, L.; Dimitri, C.; Bottà, G.; Paulmichl, M.; Beck-Peccoz, P.; Fugazzola, L.; Storelli, C. PKC-ε-dependent cytosol-to-membrane translocation of pendrin in rat thyroid PC Cl3 cells. J. Cell. Physiol. 2008, 217, 103–112. [Google Scholar] [CrossRef]
- Vinson, G.P.; Ho, M.M.; Puddefoot, J.; Teja, R.; Barker, S. Internalisation of the type I angiotensin II receptor (AT1) and angiotensin II function in the rat adrenal zona glomerulosa cell. J. Endocrinol. 1994, 141, R5–R9. [Google Scholar] [CrossRef] [PubMed]
- Ullian, M.E.; Linas, S.L. Role of receptor cycling in the regulation of angiotensin II surface receptor number and angiotensin II uptake in rat vascular smooth muscle cells. J. Clin. Investig. 1989, 84, 840–846. [Google Scholar] [CrossRef]
- Herblin, W.; Diamond, S.; Timmermans, P. Localization of angiotensin II receptor subtypes in the rabbit adrenal and kidney. Peptides 1991, 12, 581–584. [Google Scholar] [CrossRef]
- Liu, F.Y.; Cogan, M.G. Angiotensin II stimulation of hydrogen ion secretion in the rat early proximal tubule. Modes of action, mechanism, and kinetics. J. Clin. Investig. 1988, 82, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Quan, A.; Baum, M. Endogenous production of angiotensin II modulates rat proximal tubule transport. J. Clin. Investig. 1996, 97, 2878–2882. [Google Scholar] [CrossRef] [PubMed]
- Wong, P.C.; Hart, S.D.; Zaspel, A.M.; Chiu, A.T.; Ardecky, R.J.; Smith, R.D.; Timmermans, P.B. Functional studies of nonpeptide angiotensin II receptor subtype-specific ligands: DuP 753 (AII-1) and PD123177 (AII-2). Experiment 1990, 255, 584–592. [Google Scholar]
- Spieker, C.; Zidek, W.; Rahn, K.-H. Characterization of the renal microvascular effects of angiotensin II antagonist, DuP 753: Studies in isolated perfused hydronephrotic kidneys. Am. J. Hypertens. 1991, 4, 309S–314S. [Google Scholar] [CrossRef]
- Wong, P.C.; Hart, S.D.; Duncia, J.V.; Timmermans, P.B. Nonpeptide angiotensin II receptor antagonists. Studies with DuP 753 and EXP3174 in dogs. Eur. J. Pharmacol. 1991, 202, 323–330. [Google Scholar] [CrossRef]
- Czekalski, S.; Chansel, D.; Vandermeersch, S.; Ronco, P.; Ardaillou, R. Evidence for angiotensin IV receptors in human collecting duct cells. Kidney Int. 1996, 50, 1125–1131. [Google Scholar] [CrossRef]
- Schlatter, E.; Haxelmans, S.; Ankorina, I.; Kleta, R. Regulation of Na+/H+ exchange by diadenosine polyphosphates, angiotensin II, and vasopressin in rat cortical collecting duct. J. Am. Soc. Nephrol. 1995, 6, 1223–1229. [Google Scholar] [CrossRef]
- Weiner, I.D.; New, A.R.; Milton, A.E.; Tisher, C.C. Regulation of luminal alkalinization and acidification in the cortical collecting duct by angiotensin II. Am. J. Physiol. Physiol. 1995, 269, F730–F738. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Giebisch, G. Effects of angiotensin II on electrolyte transport in the early and late distal tubule in rat kidney. Am. J. Physiol. 1996, 271, F143–F149. [Google Scholar] [CrossRef]
- Cook, M.D.; Phillips, M.I.; Cook, V.I.; Kimura, B.; Wilcox, C.S. Angiotensin II receptor subtypes on adrenal adenoma in primary hyperaldosteronism. J. Am. Soc. Nephrol. 1993, 4, 111–116. [Google Scholar] [CrossRef]
- Dolley-Hitze, T.; Jouan, F.; Martin, B.; Mottier, S.; Edeline, J.; Moranne, O.; Le Pogamp, P.; Belaud-Rotureau, M.-A.; Patard, J.-J.; Rioux-Leclercq, N.; et al. Angiotensin-2 receptors (AT1-R and AT2-R), new prognostic factors for renal clear-cell carcinoma? Br. J. Cancer 2010, 103, 1698–1705. [Google Scholar] [CrossRef]
- Escobar, E.; Rodríguez-Reyna, T.S.; Arrieta, O.; Sotelo, J. Angiotensin II, cell proliferation and angiogenesis regulator: Biologic and therapeutic implications in cancer. Curr. Vasc. Pharmacol. 2004, 2, 385–399. [Google Scholar] [CrossRef]
- Shimomoto, T.; Ohmori, H.; Luo, Y.; Chihara, Y.; Denda, A.; Sasahira, T.; Tatsumoto, N.; Fujii, K.; Kuniyasu, H. Diabetes-associated angiotensin activation enhances liver metastasis of colon cancer. Clin. Exp. Metastasis 2012, 29, 915–925. [Google Scholar] [CrossRef]
- Batra, V.K.; Gopalakrishnan, V.; McNeill, J.R.; Hickie, R.A. Angiotensin II elevates cytosolic free calcium in human lung adenocarcinoma cells via activation of AT1 receptors. Cancer Lett. 1994, 76, 19–24. [Google Scholar] [CrossRef]
- Ishikane, S.; Takahashi-Yanaga, F. The role of angiotensin II in cancer metastasis: Potential of renin-angiotensin system blockade as a treatment for cancer metastasis. Biochem. Pharmacol. 2018, 151, 96–103. [Google Scholar] [CrossRef]
- Miyajima, A.; Kosaka, T.; Asano, T.; Asano, T.; Seta, K.; Kawai, T.; Hayakawa, M. Angiotensin II type I antagonist prevents pulmonary metastasis of murine renal cancer by inhibiting tumor angiogenesis. Cancer Res. 2002, 62, 4176–4179. [Google Scholar]
- Sun, H.; Li, T.; Zhuang, R.; Cai, W.; Zheng, Y. Do renin–angiotensin system inhibitors influence the recurrence, metastasis, and survival in cancer patients? Evidence from a meta-analysis including 55 studies. Medicine 2017, 96, e6394. [Google Scholar] [CrossRef]
| CLINICOPATHOLOGICAL CHARACTERS | N |
|---|---|
| Age (years) | |
| <59 | 22 |
| ≥59 | 20 |
| Gender | |
| Male | 25 |
| Female | 17 |
| Smoke history | |
| Positive | 19 |
| Negative | 23 |
| Tumor location | |
| Left | 20 |
| Right | 22 |
| Tumor size (cm) | |
| ≤5 | 27 |
| >5 | 15 |
| Lymph node metastasis | |
| Positive | 18 |
| Negative | 24 |
| Nuclear grading | |
| G1 | 6 |
| G2 | 9 |
| G3 | 13 |
| G4 | 14 |
| Gene Name | GenBank ID | Function | T/N |
|---|---|---|---|
| actin | NM 001101 | Housekeeping | 1.0 |
| CASP9 | AB020979 | Apoptosis | 1.4 |
| CCND1 | M64349 | Cell cycle | 3.3 |
| CCNE2 | AF106690 | Cell cycle | 3.4 |
| CCNA1 | NM 003914 | Cell cycle | 3.1 |
| p53 | NM 000546 | Cell cycle | 1.7 |
| MYC | M14206 | Cell cycle | 4.0 |
| CTSD | M11233 | Proteolysis | 4.7 |
| c-KIT | NM000222 | Tumorigenesis | 3.2 |
| JNKK2 | AF022805 | Tumorigenesis | 3.9 |
| Survivin | AF077350 | Tumorigenesis | 9.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muscella, A.; Resta, L.; Cossa, L.G.; Marsigliante, S. Immunolocalization of the AT-1R Ang II Receptor in Human Kidney Cancer. Biomolecules 2023, 13, 1181. https://doi.org/10.3390/biom13081181
Muscella A, Resta L, Cossa LG, Marsigliante S. Immunolocalization of the AT-1R Ang II Receptor in Human Kidney Cancer. Biomolecules. 2023; 13(8):1181. https://doi.org/10.3390/biom13081181
Chicago/Turabian StyleMuscella, Antonella, Leonardo Resta, Luca Giulio Cossa, and Santo Marsigliante. 2023. "Immunolocalization of the AT-1R Ang II Receptor in Human Kidney Cancer" Biomolecules 13, no. 8: 1181. https://doi.org/10.3390/biom13081181
APA StyleMuscella, A., Resta, L., Cossa, L. G., & Marsigliante, S. (2023). Immunolocalization of the AT-1R Ang II Receptor in Human Kidney Cancer. Biomolecules, 13(8), 1181. https://doi.org/10.3390/biom13081181









