Chemical Composition of the Essential Oils of Three Popular Sideritis Species Cultivated in Greece Using GC-MS Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical and Reagents
2.2. Plant Material
2.3. Isolation of the Essential Oil
2.4. Gas Chromatography–Mass Spectrometry (GC-MS)
2.5. Identification of the Components of the Essential Oils
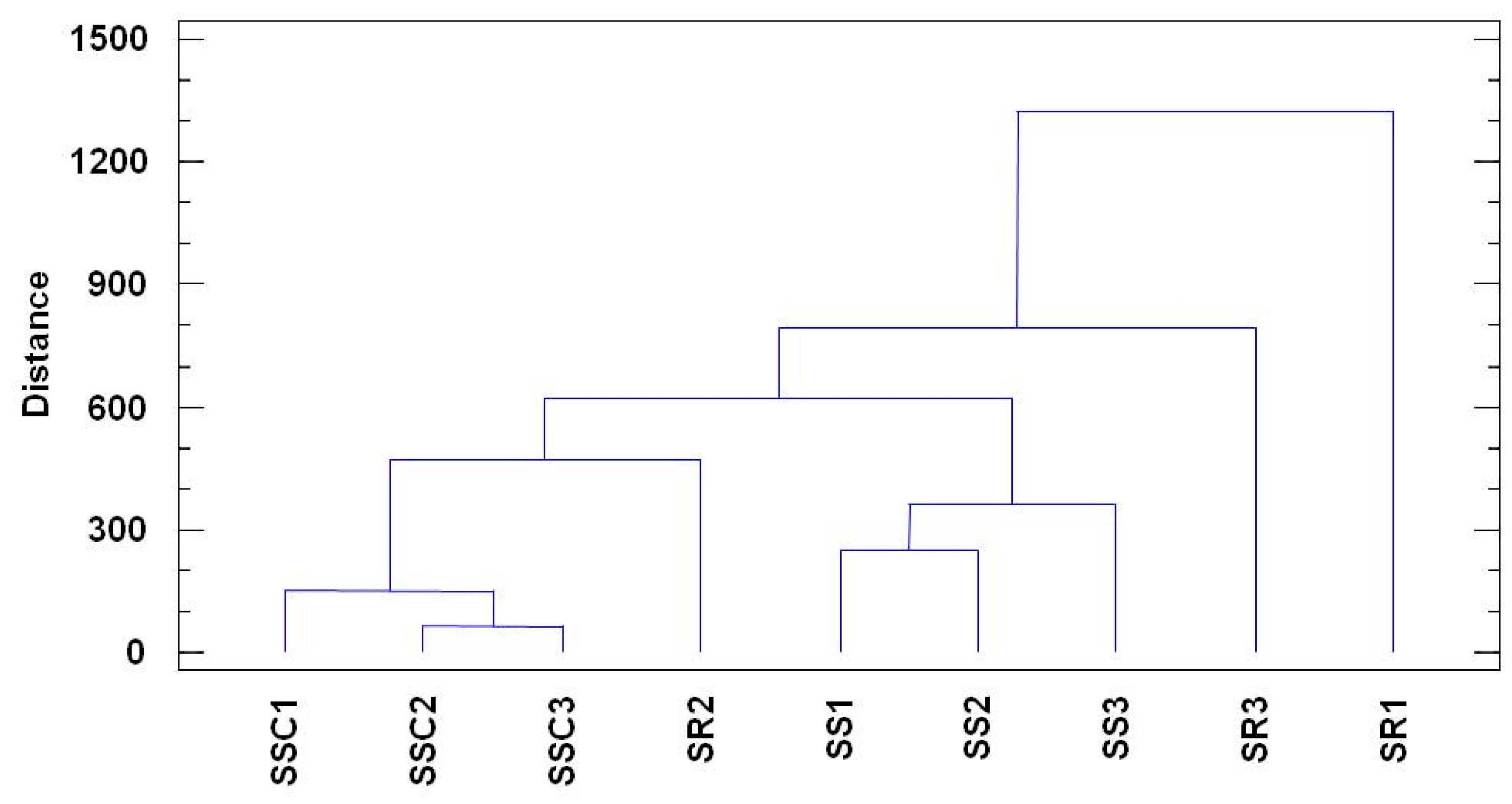
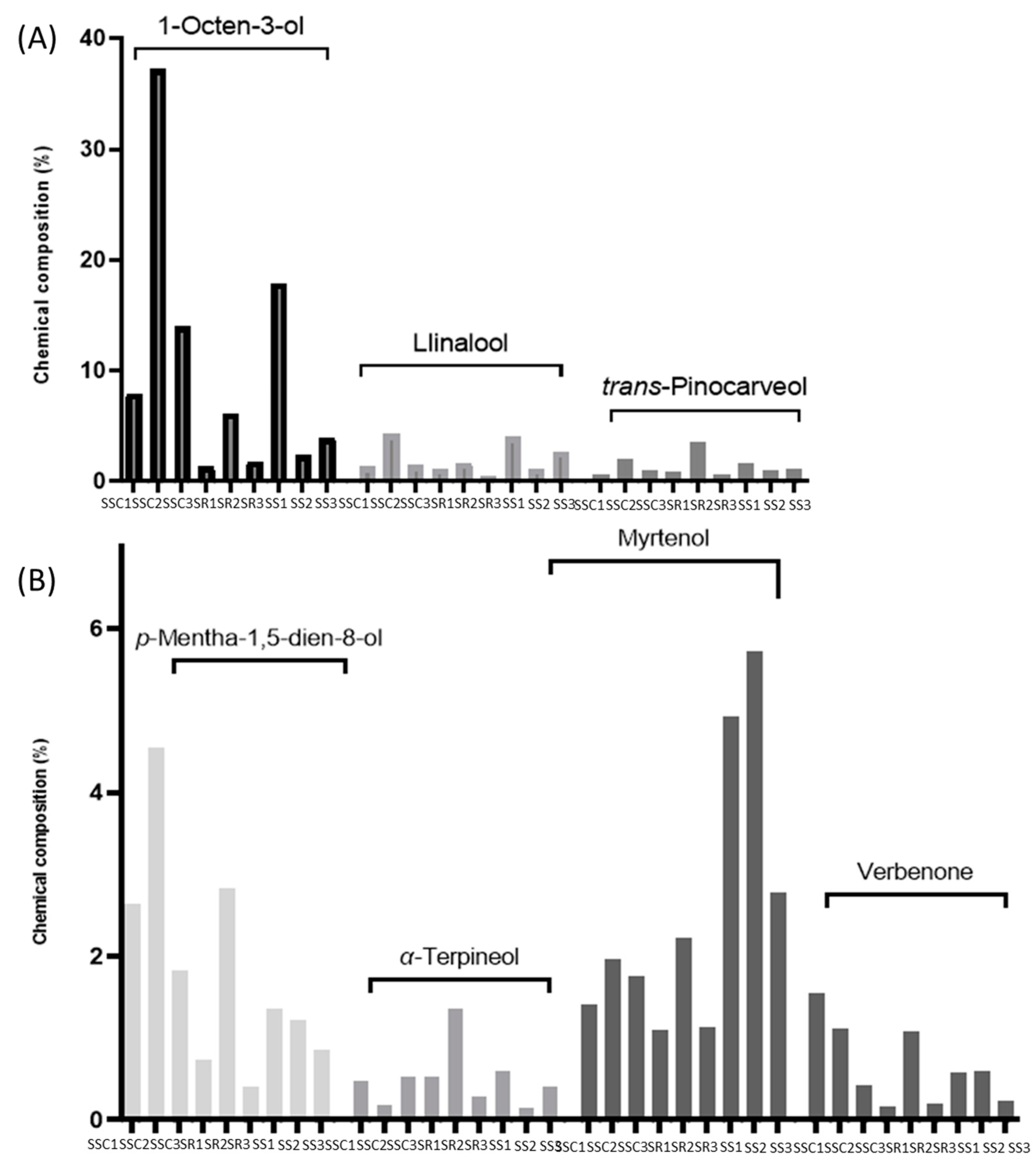
3. Results
4. Discussion
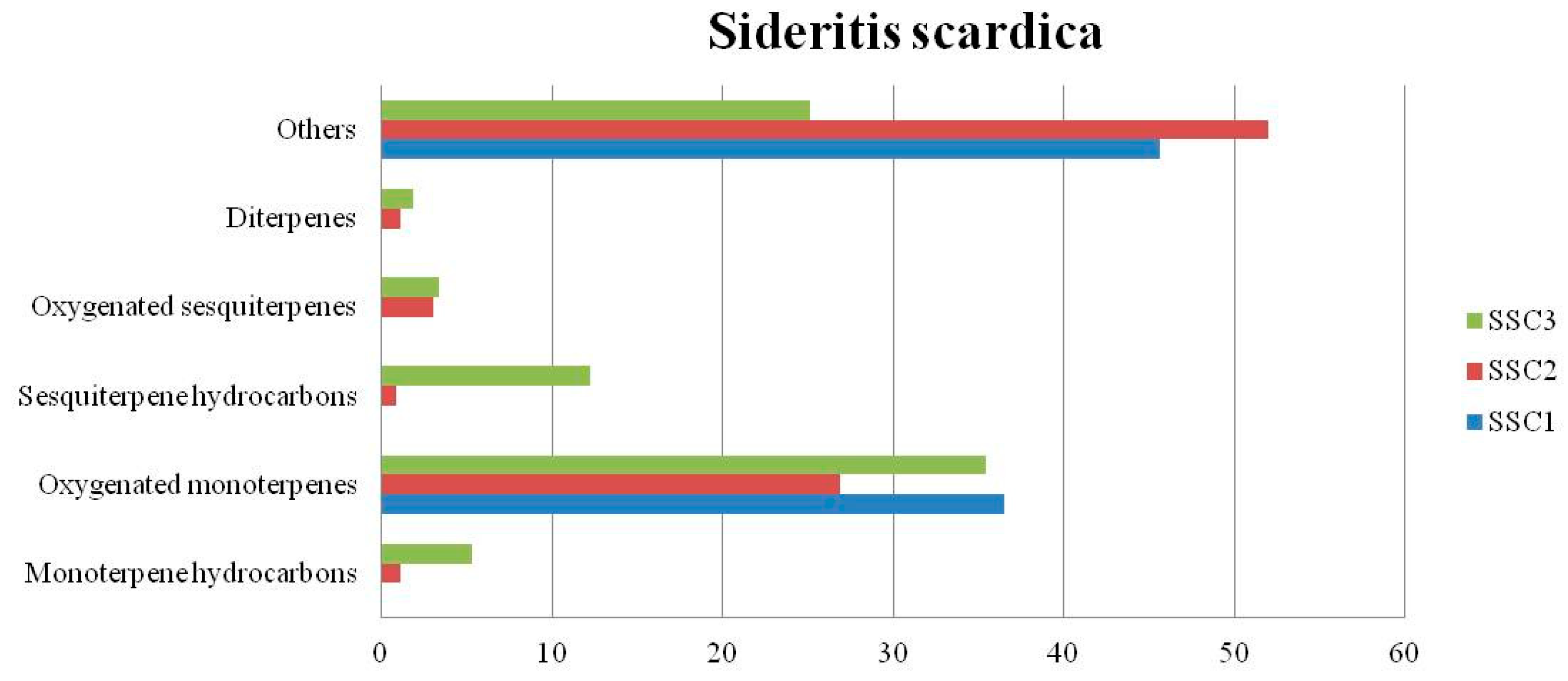
4.1. Sideritis scardica
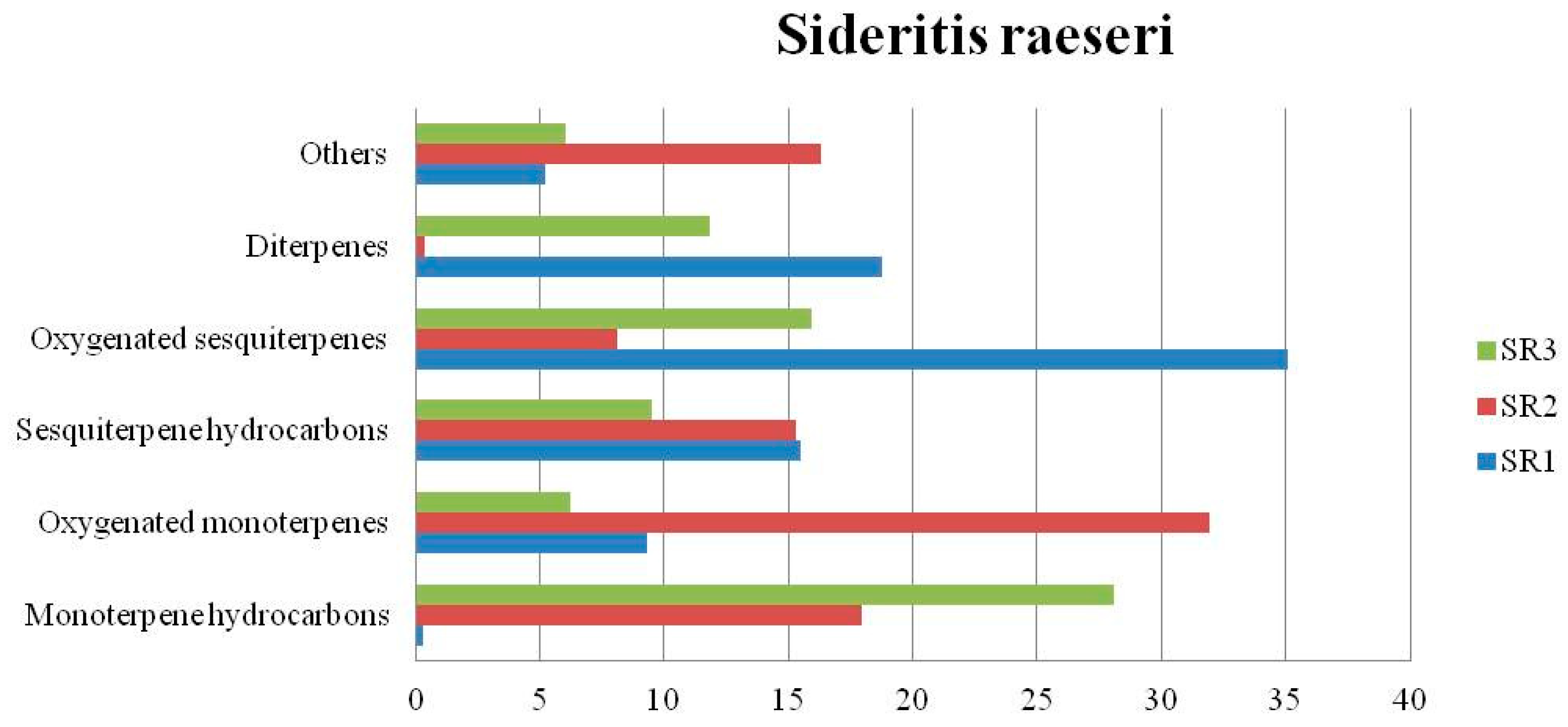
4.2. Sideritis raeseri
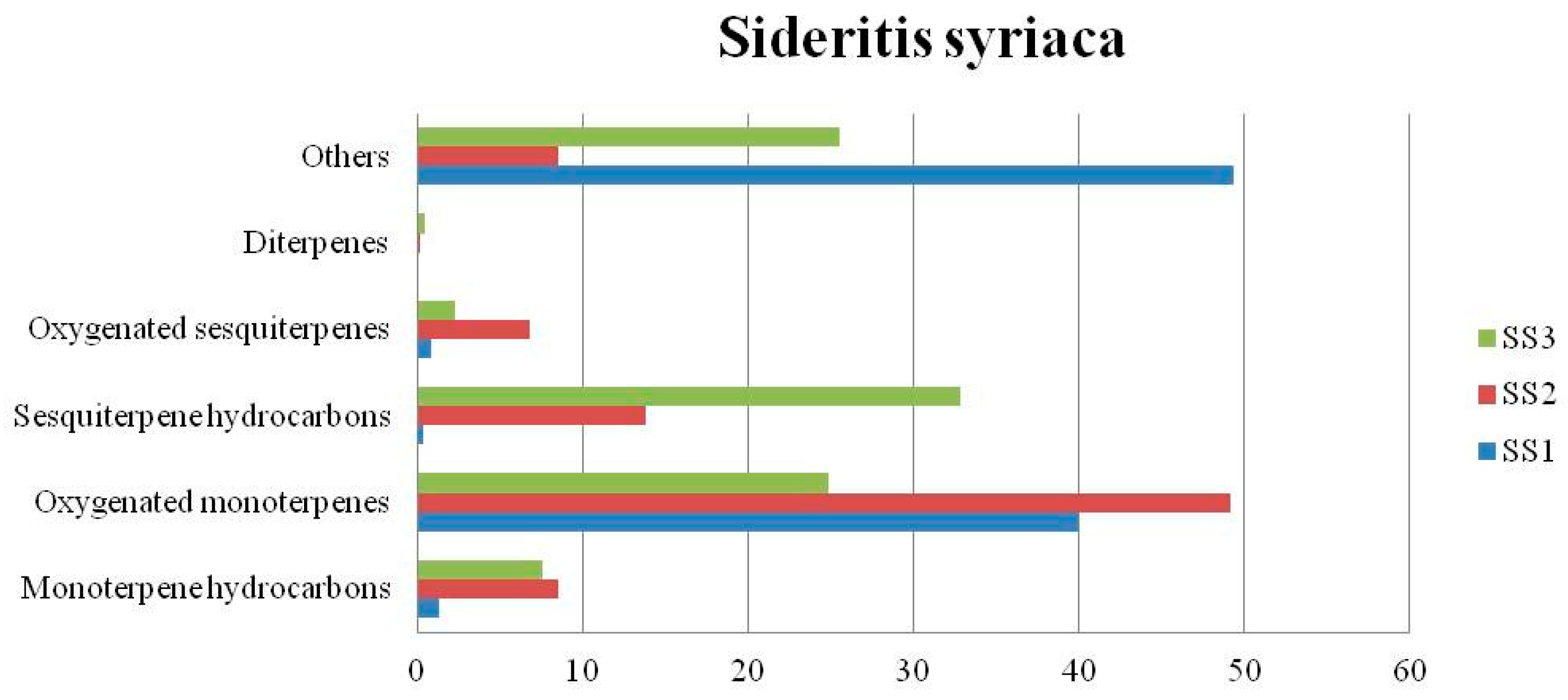
4.3. Sideritis syriaca
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- González-Burgos, E.; Carretero, M.; Gómez-Serranillos, M.P. Sideritis spp.: Uses, chemical composition and pharmacological activities—A review. J. Ethnopharmacol. 2011, 135, 209–225. [Google Scholar] [CrossRef] [PubMed]
- Stefanaki, A.; Van Andel, T. Mediterranean Aromatic Herbs and Their Culinary Use. Aromatic Herbs in Food: Bioactive Compounds, Processing, and Applications; Galanakis, C.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 3, pp. 93–121. [Google Scholar]
- Font Quer, P. El Dioscorides Renovado. In Plantas Medicinales; Ediciones Peninsula: Barcelona, Spain, 2000. [Google Scholar]
- Todorova, M.; Trendafilova, A. Sideritis scardica Griseb., an endemic species of Balkan peninsula: Traditional uses, cultivation, chemical composition, biological activity. J. Ethnopharmacol. 2014, 152, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Tadic, V.; Jeremic, I.; Dobric, S.; Isakovic, A.; Markovi´c, I.; Trajkovic, V.; Bojovic, D.; Arsic, I. Anti-inflammatory, gastroprotective, and cytotoxic Effects of Sideritis scardica extracts. Planta Med. 2012, 78, 415–427. [Google Scholar] [CrossRef] [PubMed]
- El-Molla, M.M.; El-Ghorab, A.H. Extraction of eco-friendly essential oils and their utilization in finishing polyester fabrics for fragrant and medical textiles. J. Eng. Fiber Fabr. 2022, 17, 15589250221104475. [Google Scholar] [CrossRef]
- Božovića, M.; Garzoli, S.; Navarra, A.; Garzoli, S.; Pepi, F.; Ragno, R. Essential oils extraction: A 24-hour steam distillation systematic methodology. Nat. Prod. Res. 2017, 31, 2387–2396. [Google Scholar] [CrossRef] [PubMed]
- Aligiannis, N.; Kalpoutzakis, E.; Chinou, I.B.; Mitakou, S.; Gikas, E.; Tsarbopoulos, A. Composition and antimicrobial activity of the essential oils of five taxa of Sideritis from Greece. J. Agric. Food Chem. 2001, 49, 811–815. [Google Scholar] [CrossRef] [PubMed]
- Mitropoulou, M.; Sidira, M.; Skitsa, M.; Tsochantaridis, I.; Pappa, A.; Dimtsoulidis, C.; Proestos, C.; Kourkoutas, Y. Assessment of the antimicrobial, antioxidant, and antiproliferative potential of Sideritis raeseri subps. raeseri essential oil. Foods 2020, 9, 860. [Google Scholar] [CrossRef] [PubMed]
- Chrysargyris, A.; Tomou, E.-M.; Goula, K.; Dimakopoulou, K.; Tzortzakis, N.; Skaltsa, H. Sideritis L. essential oils: A systematic review. Phytochemistry 2023, 209, 113607. [Google Scholar] [CrossRef] [PubMed]
- Kloukina, C.; Tomou, E.-M.; Krigas, N.; Sarropoulou, V.; Madesis, P.; Maloupa, E.; Sklatsa, H. Non-polar secondary metabolites and essential oil of ex situ propagated and cultivated Sideritis syriaca L. subsp. syriaca (Lamiaceae) with consolidated identity (DNA Barcoding): Towards a potential new industrial crop. Ind. Crops Prod. 2020, 158, 112957. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography, Mass Spectrometry, 4.1th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2017; 804p. [Google Scholar]
- Kloukina, C.; Tomou, E.M.; Skaltsa, H. Essential oil composition of two Greek cultivated Sideritis spp. Nat. Volatiles Essent. Oils 2019, 6, 16–23. [Google Scholar]
- Trendafilova, A.B.; Todorova, M.N.; Evstatieva, L.N.; Antonova, D.V. Variability in the essential-oil composition of Sideritis scardica Griseb. from native Bulgarian populations. Chem. Biodivers. 2013, 10, 484–492. [Google Scholar] [CrossRef]
- Qazimi, B.; Stefkov, G.; Karapandzova, M.; Cvetkovikj, I.; Kulevanova, S. Aroma compounds of mountain tea (Sideritis scardica and S. raeseri) from western balkan. Nat. Prod. Commun. 2014, 9, 1369–1372. [Google Scholar] [CrossRef] [PubMed]
- Pljevljakušić, D.; Šavikin, K.; Janković, T.; Zdunić, G.; Ristić, M.; Godjevac, D.; Konić-Ristić, A. Chemical properties of the cultivated Sideritis raeseri Boiss. & Heldr. subsp. raeseri. Food Chem. 2011, 124, 226–233. [Google Scholar]
- Todorova, M.; Christov, R.; Evstatieva, L. Essential oil composition of three Sideritis species from Bulgaria. J. Essent. Oil Res. 2000, 12, 418–420. [Google Scholar] [CrossRef]
- Tirillini, B.; Pellegrino, R.; Luigi Menghini, L.; Pagiotti, R.; Menghini, A. Composition of the oil of Siderifis syriaca L. from Italy. J. Essent. Oil Res. 2001, 13, 444–445. [Google Scholar] [CrossRef]
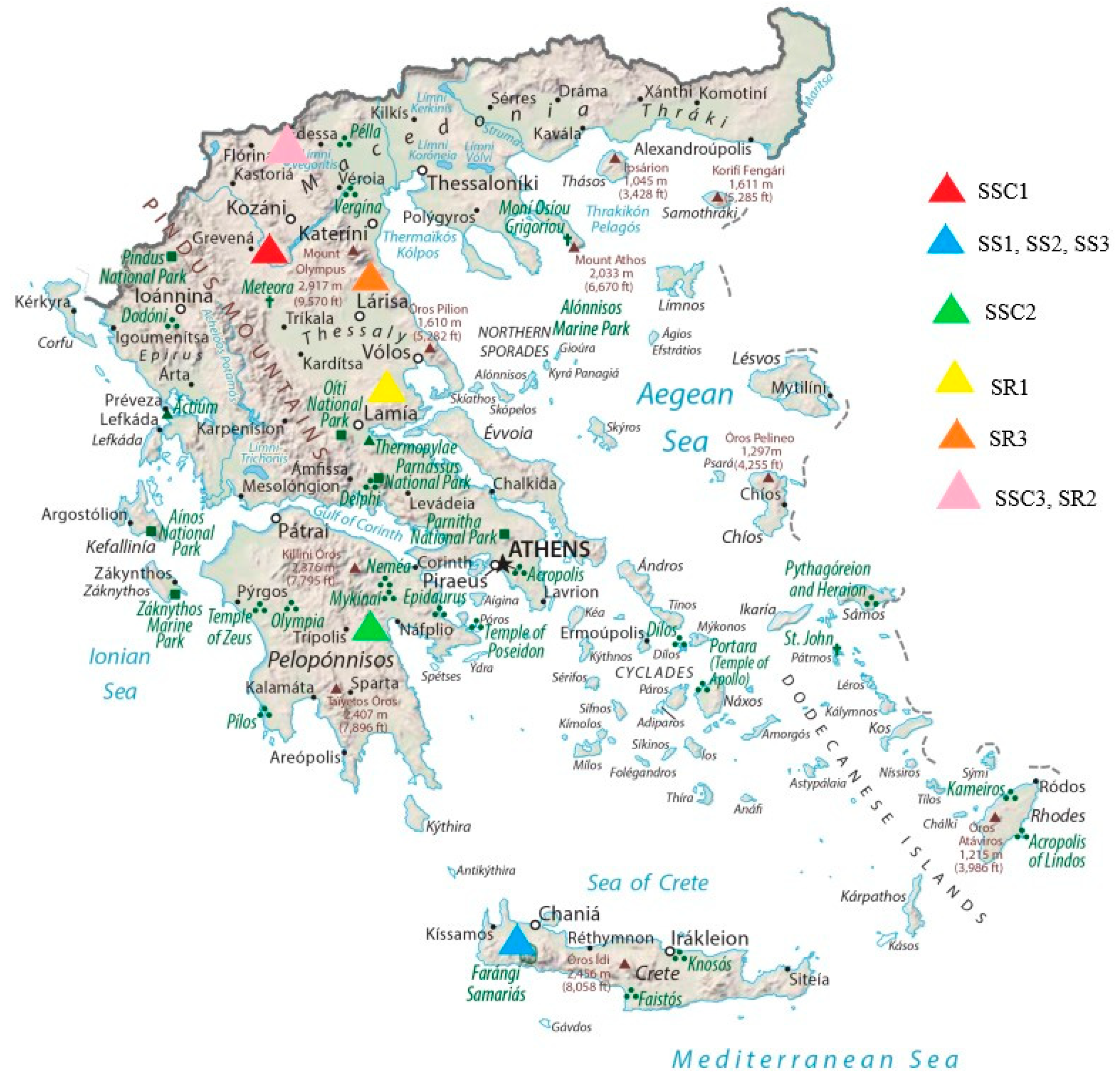
| Sample | Species | Geographical Origin | Latitude | Longitude | Elevation (m) | Voucher Number |
|---|---|---|---|---|---|---|
| SSC1 | Scardica | Mount Olympus, Central Greece | 39°59′ | 22°23′ | 900 | 012276 |
| SSC2 | Scardica | Mount Mainalo, Peloponnesos, Southern Greece | 37°57′ | 22°25′ | 900 | 012279 |
| SSC3 | Scardica | Kastoria, Northern Greece | 40°54′ | 21°34′ | 800 | 012293 |
| SR1 | Raeseri | Mount Othrys, Central Greece | 39°06′ | 22°21′ | 780 | 012277 |
| SR2 | Raeseri | Kastoria, Northern Greece | 40°54′ | 21°34′ | 800 | 012294 |
| SR3 | Raeseri | Elassona, Larissa, Central Greece | 40°02′ | 22°05′ | 625 | 012295 |
| SS1 | Syriaca | White Mountains (Lefka Ori), Crete island, Southern Greece | 35°23′ | 24°08′ | 700 | 012278 |
| SS2 | Syriaca | Anopoli Sfakion, Crete island, Southern Greece | 35°22′ | 24°08′ | 650 | 012291 |
| SS3 | Syriaca | Omalos Chanion, Crete island, Southern Greece | 35°232′ | 23°91′ | 1100 | 012292 |
| Sample | Oil Yield % (v/w) |
|---|---|
| SSC1 | 0.02 |
| SSC2 | 0.03 |
| SSC3 | 0.05 |
| SR1 | 0.02 |
| SR2 | 0.05 |
| SR3 | 0.10 |
| SS1 | 0.02 |
| SS2 | 0.05 |
| SS3 | 0.01 |
| No | Compound | RI Exp a | RI Lit a | SSC1 b | SSC2 b | SSC3 b | SR1 b | SR2 b | SR3 b | SS1 b | SS2 b | SS3 b |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | (E)-2-Hexenal | 846 | 846 | - c | 8.75 | - | - | - | - | - | - | - |
| 2 | Hexanol | 860 | 863 | - | 3.59 | 1.89 | - | 1.50 | 0.15 | 16.23 | 0.87 | 9.84 |
| 3 | Heptanal | 897 | 901 | - | - | - | - | - | - | 2.89 | 0.82 | 1.47 |
| 4 | 2,4-(E,E)-Hexadienal | 903 | 907 | 0.57 | 0.30 | - | - | 0.22 | - | - | - | 0.80 |
| 5 | α-Thujene | 926 | 924 | - | - | - | - | 0.20 | 0.75 | 0.89 | - | - |
| 6 | α-Pinene | 933 | 932 | - | 1.12 | 3.86 | 0.14 | 5.33 | 4.46 | 0.40 | 0.53 | 1.62 |
| 7 | Benzaldehyde | 952 | 952 | 1.96 | 0.97 | 2.04 | - | 1.44 | 0.32 | 5.83 | 2.07 | 6.65 |
| 8 | Hexanoic acid | 967 | 967 | - | 0.06 | - | - | - | - | 0.70 | - | - |
| 9 | 1-Octen-3-ol | 974 | 974 | 7.91 | 37.28 | 13.99 | 1.32 | 6.14 | 1.83 | 17.90 | 2.41 | 3.94 |
| 10 | β-Pinene | 976 | 974 | - | - | 1.43 | - | 7.50 | 3.00 | - | - | 1.26 |
| 11 | 5-Hepten-2-one, 6-methyl- | 980 | 981 | - | - | - | - | - | - | - | - | 0.38 |
| 12 | Myrcene | 988 | 988 | - | - | - | - | 0.52 | 1.45 | - | - | - |
| 13 | 3-Octanol | 991 | 988 | - | - | 0.37 | - | - | - | - | - | - |
| 14 | α-Phellandrene | 1004 | 1002 | - | - | - | - | - | 2.90 | - | - | - |
| 15 | (2E,4E)-Heptadienal | 1007 | 1005 | - | 1.07 | 0.21 | - | 0.38 | - | 2.80 | 1.03 | 1.57 |
| 16 | 3-δ-Carene | 1010 | 1008 | - | - | - | - | - | 3.73 | - | 0.38 | 0.30 |
| 17 | α-Terpinene | 1015 | 1014 | - | - | - | - | 0.09 | 1.06 | - | 0.17 | - |
| 18 | p-Cymene | 1022 | 1020 | - | - | - | 0.14 | 0.09 | 2.79 | - | 0.32 | 0.12 |
| 19 | Benzyl alcohol | 1026 | 1026 | - | - | 0.76 | - | - | - | 1.39 | - | - |
| 20 | D-Limonene | 1027 | 1024 | - | - | - | - | 3.32 | 4.44 | - | - | - |
| 21 | β-Phellandrene | 1028 | 1025 | - | - | - | - | - | - | - | 6.64 | 4.29 |
| 22 | Eucalyptol | 1029 | 1026 | - | - | - | - | - | - | 0.84 | - | - |
| 23 | β-Ocimene | 1034 | 1032 | - | - | - | - | - | 1.92 | - | 0.10 | - |
| 24 | Benzene acetaldehyde | 1035 | 1036 | 16.42 | 2.05 | 1.93 | 0.13 | 1.24 | - | 3.58 | 3.16 | 5.15 |
| 25 | (E)-2-Octen-1-al | 1053 | 1049 | - | 0.10 | - | - | - | - | - | 0.18 | - |
| 26 | γ-Terpinene | 1056 | 1054 | - | - | - | - | 0.29 | 1.05 | - | 0.37 | - |
| 27 | Acetophenone | 1061 | 1059 | 0.15 | - | - | - | - | - | 0.48 | - | 0.32 |
| 28 | 2-Methyl-,benzaldehyde | 1061 | - | - | - | - | - | - | - | 0.34 | 0.56 | - |
| 29 | Octanol | 1063 | 1063 | - | - | - | - | 0.29 | - | 1.36 | - | 1.29 |
| 30 | cis-Linalool oxide | 1067 | 1067 | - | - | - | 0.14 | 0.24 | - | 0.37 | 0.29 | 0.28 |
| 31 | Isopinocampheol | 1071 | - | - | - | - | 0.08 | - | - | - | - | - |
| 32 | Tetramethyl-pyrazine | 1081 | 1081 | - | - | - | - | - | - | 0.30 | - | - |
| 33 | trans-Linalool oxide | 1084 | 1084 | 0.21 | - | - | - | - | - | - | - | - |
| 34 | Terpinolene | 1086 | 1086 | - | - | - | - | 0.59 | 0.57 | - | - | - |
| 35 | Linalool | 1096 | 1095 | 1.33 | 4.32 | 1.47 | 1.14 | 1.65 | 0.53 | 4.04 | 1.14 | 2.70 |
| 36 | Nonanal | 1102 | 1100 | - | 0.03 | - | 0.21 | 0.19 | - | - | 1.00 | |
| 37 | Phenylethyl Alcohol | 1110 | 1106 | 5.71 | 0.33 | - | - | - | - | - | - | 0.39 |
| 38 | endo-Fenchol | 1111 | 1114 | - | - | - | - | - | - | 0.12 | - | - |
| 39 | cis-p-Menth-2-en-1-ol | 1119 | 1118 | - | - | - | - | - | - | 0.41 | 1.00 | 0.40 |
| 40 | α-Campholenal | 1123 | 1122 | - | 0.31 | - | 0.24 | 0.11 | 0.14 | 0.17 | 0.13 | 0.13 |
| 41 | Nopinone | 1132 | 1135 | 0.53 | 0.38 | - | 0.10 | 1.92 | 0.15 | 0.48 | - | 0.27 |
| 42 | cis-p-Mentha-1(7),8-dien-2-ol | 1133 | - | - | - | - | - | - | - | - | 0.25 | - |
| 43 | trans-Pinocarveol | 1136 | 1135 | 0.66 | 2.07 | 1.02 | 0.82 | 3.60 | 0.56 | 1.66 | 1.01 | 1.11 |
| 44 | cis-Verbenol | 1142 | 1137 | - | - | 1.29 | 0.12 | 1.52 | 0.28 | 1.00 | 1.42 | 0.20 |
| 45 | trans-Verbenol | 1144 | 1140 | - | 1.36 | - | - | 1.15 | - | 0.97 | - | 0.73 |
| 46 | Sabina ketone | 1151 | 1154 | - | - | - | - | 0.56 | - | - | - | - |
| 47 | Pinocarvone | 1158 | 1160 | - | 0.85 | - | 0.47 | 1.15 | 0.22 | 0.58 | - | 0.46 |
| 48 | p-Mentha-1,5-dien-8-ol | 1167 | 1166 | 2.64 | 4.55 | 1.82 | 0.74 | 2.84 | 0.41 | 1.36 | 1.22 | 0.85 |
| 49 | Terpinen-4-ol | 1174 | 1174 | - | 0.44 | 0.84 | 0.31 | 2.51 | 0.92 | 2.59 | 4.44 | 1.56 |
| 50 | p-Methyl-acetophenone | 1177 | - | - | - | - | - | - | - | 0.28 | - | - |
| 51 | p-Cymen-8-ol | 1183 | 1179 | 0.56 | 0.27 | - | 0.13 | - | - | - | - | - |
| 52 | Cryptone | 1184 | 1183 | - | - | - | - | - | 0.24 | 2.67 | 6.21 | 2.55 |
| 53 | α-Terpineol | 1187 | 1186 | 1.41 | 1.96 | 1.76 | 1.10 | 2.23 | 1.14 | 4.92 | 5.73 | 2.78 |
| 54 | Methyl salicylate | 1188 | 1190 | 0.37 | 0.55 | - | - | - | - | - | - | - |
| 55 | Myrtenol | 1194 | 1194 | 0.48 | 0.19 | 0.52 | 0.52 | 1.37 | 0.29 | 0.59 | 0.15 | 0.41 |
| 56 | Myrtenal | 1195 | 1195 | - | - | 0.39 | - | 2.00 | 0.29 | 0.89 | 0.28 | 0.48 |
| 57 | Verbenone | 1204 | 1204 | 1.55 | 1.12 | 0.42 | 0.16 | 1.08 | 0.20 | 0.58 | 0.60 | 0.24 |
| 58 | Eucarvone | 1206 | - | - | - | - | 0.07 | - | - | - | - | - |
| 59 | trans-Carveol | 1217 | 1215 | 0.37 | - | - | 0.09 | 0.66 | 0.16 | 0.42 | 0.45 | 0.23 |
| 60 | Nerol | 1224 | 1227 | - | - | - | - | - | - | 0.22 | - | - |
| 61 | cis-Carveol | 1226 | 1226 | - | - | - | - | 0.08 | - | - | - | - |
| 62 | cis-3-Hexenyl-α-methylbu-tyrate | 1228 | 1229 | - | - | - | 0.04 | - | - | - | - | - |
| 63 | Pulegone | 1233 | 1233 | - | 1.96 | 0.47 | - | 0.24 | - | 0.53 | - | - |
| 64 | Cumin aldehyde | 1233 | - | - | - | - | - | - | 0.14 | - | 0.65 | 0.38 |
| 65 | Carvone | 1240 | 1239 | - | 0.19 | - | 0.16 | 0.41 | 0.18 | 0.47 | 0.29 | 0.20 |
| 66 | Geraniol | 1251 | 1249 | 1.07 | - | - | 0.12 | 0.35 | - | 0.46 | - | - |
| 67 | trans-2-Decenal | 1258 | - | - | - | - | 0.02 | - | - | - | - | - |
| 68 | Geranial | 1264 | 1264 | - | - | - | 0.01 | - | - | - | - | - |
| 69 | Nonanoic acid | 1275 | 1267 | 3.87 | - | - | 0.66 | 0.22 | - | 0.87 | - | - |
| 70 | Bornyl acetate | 1281 | 1254 | - | - | - | 0.18 | 0.18 | - | - | - | - |
| 71 | Thymol | 1286 | 1289 | 0.56 | - | 1.16 | 0.18 | 0.29 | - | 0.11 | - | - |
| 72 | Carvacrol | 1296 | 1298 | 7.98 | - | 19.91 | 1.54 | 3.88 | 0.22 | 4.52 | 19.75 | 0.19 |
| 73 | 2-Methoxy-4-vinylphenol | 1303 | - | 2.74 | - | - | - | - | - | - | - | - |
| 74 | 2-Methylpropyl ester-Benzoic acid | 1324 | - | - | - | - | 0.03 | - | - | - | - | |
| 75 | δ-Elemene | 1338 | 1335 | - | - | - | 0.40 | - | - | - | - | 0.16 |
| 76 | 4-Methoxy-acetophenone | 1343 | - | - | - | - | - | - | 1.86 | 0.26 | 0.86 | |
| 77 | α-Cubebene | 1344 | 1345 | - | - | - | 0.10 | - | - | - | - | - |
| 78 | Eugenol | 1351 | 1356 | 21.52 | 3.83 | 1.70 | 0.31 | 0.36 | - | 1.30 | - | 0.35 |
| 79 | γ-Nonanolactone | 1354 | - | 0.27 | - | - | - | - | - | - | - | - |
| 80 | Ylangene | 1373 | 1373 | - | - | - | 0.60 | - | - | - | - | - |
| 81 | α-Copaene | 1378 | 1374 | - | - | 0.41 | - | 0.18 | 0.28 | - | 0.12 | 1.42 |
| 82 | (E)-β-Damascenone | 1380 | 1383 | - | - | - | 0.20 | - | - | 0.28 | 0.22 | 0.26 |
| 83 | β-Bourbonene | 1386 | 1387 | - | - | - | 0.12 | 0.11 | - | - | - | - |
| 84 | 4-(2,2-Dimethyl-6-methyle-ne-cyclohexyl)-2-butanone, | 1390 | - | - | - | - | 0.13 | - | - | - | - | - |
| 85 | β-Elemene | 1392 | 1389 | - | - | - | - | 0.22 | - | - | - | 0.43 |
| 86 | 4-Dimethyl-γ-benzenebuta-nal | 1396 | - | - | - | - | 0.24 | - | - | - | - | - |
| 87 | α-Gurjunene | 1404 | 1409 | - | - | - | 0.06 | - | - | - | - | 0.56 |
| 88 | 4-(2,6,6-Trimethyl-1,3-cycl-ohexadien-1-yl)-2-butanone | 1409 | - | - | - | - | 0.30 | - | - | - | - | - |
| 89 | α-Cedrene | 1415 | 1410 | - | - | - | - | - | - | - | - | 0.14 |
| 90 | (E)-Caryophyllene | 1421 | 1417 | - | - | 3.40 | 4.03 | 4.13 | 0.91 | 0.33 | 9.09 | 10.18 |
| 91 | β-Copaene | 1426 | 1430 | - | - | - | 0.07 | - | - | - | - | - |
| 92 | α-Bergamotene | 1430 | 1432 | - | - | - | 0.03 | - | - | - | - | - |
| 93 | 3-Methyl,-1-Butanol, benzoate | 1433 | - | - | - | - | 0.20 | - | - | - | - | - |
| 94 | (Z)-β-Famesene | 1437 | 1440 | - | - | - | 0.04 | - | - | - | - | - |
| 95 | cis-Muurola-3,5-diene | 1445 | 1448 | - | - | - | 0.34 | - | 0.16 | - | - | - |
| 96 | (E)-β-Farnesene | 1454 | 1454 | - | - | 1.34 | 0.56 | 1.29 | 0.43 | - | 1.20 | 1.26 |
| 97 | Alloaromadendrene | 1455 | 1458 | - | - | - | 0.09 | - | - | - | - | - |
| 98 | 2,6,10-Trimethyltridecane | 1461 | - | - | - | - | 0.08 | - | - | - | - | - |
| 99 | epi-β-Caryophyllene | 1463 | 1464 | - | - | - | - | - | 0.13 | - | - | - |
| 100 | α-Acoradiene | 1466 | 1464 | - | - | - | - | - | - | - | - | 0.60 |
| 101 | trans-Cadina-1(6),4-diene | 1468 | - | - | - | - | 0.15 | - | - | - | - | - |
| 102 | Pentyl benzoate | 1471 | 1476 | - | - | - | 0.12 | - | - | - | - | - |
| 103 | γ-Curcumene | 1479 | 1479 | - | - | - | - | - | - | - | - | 1.62 |
| 104 | Phenyl ethyl 2-methylbutanoate | 1480 | 1486 | - | - | - | 0.06 | - | - | - | - | - |
| 105 | D-Germacrene | 1482 | 1484 | - | 0.87 | 3.80 | 1.10 | 5.25 | 0.39 | - | 3.41 | 7.22 |
| 106 | γ-Amorphene | 1487 | 1495 | - | - | - | 0.47 | - | - | - | - | - |
| 107 | α-Zingiberene | 1494 | 1493 | - | - | - | - | - | - | - | - | 2.51 |
| 108 | α-Muurolene | 1494 | 1500 | - | - | - | 0.01 | - | - | - | - | - |
| 109 | Bicyclogermacrene | 1497 | 1500 | - | - | 3.33 | 3.20 | 3.17 | 5.81 | - | - | 2.66 |
| 110 | β-Bisabolene | 1505 | 1505 | - | - | - | 0.74 | 0.53 | 0.98 | - | - | - |
| 111 | β-Curcumene | 1511 | 1514 | - | - | - | - | - | - | - | - | 1.20 |
| 112 | Cubebol | 1515 | 1514 | - | - | - | - | - | 0.09 | - | - | - |
| 113 | trans-Calamenene | 1522 | 1521 | - | - | - | 0.65 | - | - | - | - | - |
| 114 | δ-Cadinene | 1523 | 1522 | - | - | - | 0.94 | 0.45 | - | - | - | 2.91 |
| 115 | trans-Cadina-1,4-diene | 1528 | - | - | - | - | 0.72 | - | 0.42 | - | - | - |
| 116 | α-Calacorene | 1536 | 1544 | - | - | - | 0.37 | - | - | - | - | - |
| 117 | β-Calacorene | 1556 | - | - | - | - | 0.08 | - | - | - | - | - |
| 118 | trans-Nerolidol | 1560 | 1561 | - | - | - | 0.09 | - | 0.14 | - | - | - |
| 119 | (Z)-3-Hexen-1-ol, benzoate | 1568 | - | - | - | - | 0.58 | - | 0.44 | - | - | - |
| 120 | Spathulenol | 1578 | 1577 | - | - | 1.02 | 6.46 | 0.81 | 5.03 | 0.33 | 1.12 | 0.91 |
| 121 | Caryophyllene oxide | 1583 | 1582 | - | - | 1.17 | 2.92 | 0.77 | 1.11 | 0.37 | 4.21 | 1.37 |
| 122 | Globulol | 1590 | 1590 | - | - | - | 0.46 | - | - | - | - | - |
| 123 | Viridiflorol | 1592 | 1592 | - | - | - | 0.97 | - | - | - | - | - |
| 124 | Rosifoliol | 1601 | 1600 | - | - | - | 0.11 | - | - | - | - | - |
| 125 | Humulene epoxide II | 1604 | 1608 | - | - | - | 0.52 | - | - | - | 0.50 | - |
| 126 | Isospathulenol | 1628 | - | - | - | - | 0.35 | - | - | - | - | - |
| 127 | Caryophylladienol II | 1634 | - | - | - | - | 0.21 | - | - | - | 0.48 | - |
| 128 | tau.-Muurolol | 1639 | 1640 | - | - | 1.20 | 2.02 | 0.09 | - | - | - | - |
| 129 | Bisabolol oxide II | 1650 | - | - | - | - | 0.51 | - | 0.41 | - | - | - |
| 130 | α-Cadinol | 1653 | 1652 | - | - | - | - | 0.10 | - | - | - | - |
| 131 | (Z,Z)-1,8,11-Heptadecatrie-ne | 1659 | - | - | - | - | 0.24 | - | - | - | - | - |
| 132 | (Z,Z,Z)-,1,8,11,14-Heptade-catetraene | 1663 | - | - | - | - | 0.28 | - | - | - | - | - |
| 133 | Valeranone | 1668 | 1674 | - | - | - | 1.82 | - | 0.65 | - | - | - |
| 134 | Aromadendrene oxide-(1) | 1671 | - | - | - | - | 0.47 | - | - | - | - | - |
| 135 | α-Bisabolol | 1683 | 1683 | - | - | - | 3.24 | 0.21 | 4.56 | - | - | - |
| 136 | Pentadecanal | 1712 | - | - | - | - | 0.16 | - | - | - | - | - |
| 137 | trans-Nuciferol | 1718 | 1713 | - | - | - | 0.11 | - | - | - | - | - |
| 138 | Benzyl benzoate | 1758 | 1759 | 1.31 | - | - | 0.45 | 0.84 | 0.26 | 0.16 | 0.46 | - |
| 139 | Tetradecanoic acid | 1761 | - | - | - | - | 0.10 | - | - | - | - | - |
| 140 | 6,10,14-Trimethyl-2-Pentadecanone | 1838 | - | - | - | - | 0.55 | 0.19 | - | - | - | - |
| 141 | Geranyl-α-terpinene d | 1845 | - | - | - | - | 0.58 | - | - | - | - | - |
| 142 | Benzyl salicylate | 1859 | 1864 | - | - | - | 0.05 | - | - | - | - | - |
| 143 | Geranyl-α-terpinene d | 1863 | - | - | - | - | 0.23 | - | - | - | - | - |
| 144 | (E,E)-7,11,15-Trimethyl-3-methylene-hexadeca-1,6,10,14-tetraene | 1902 | - | - | - | - | 0.40 | - | - | - | - | - |
| 145 | Geranyl-α-terpinene d | 1910 | - | - | - | - | 1.52 | - | - | - | - | - |
| 146 | Geranyl-α-terpinene d | 1918 | - | - | - | - | 0.30 | - | - | - | - | - |
| 147 | Cembrene | 1922 | 1937 | - | - | - | - | - | 1.39 | - | - | - |
| 148 | Isopimara-9(11),15-diene | 1926 | - | - | - | - | 0.17 | - | - | - | - | - |
| 149 | Pimaradiene | 1936 | - | - | - | - | 0.85 | - | - | - | - | - |
| 150 | Geranyl-p-cymene | 1952 | - | - | 1.15 | 1.88 | 3.60 | 0.20 | 3.13 | - | 0.21 | 0.44 |
| 151 | Sandaracopimaradiene | 1955 | 1968 | - | - | - | 0.17 | - | - | - | - | - |
| 152 | Geranyl-α-terpinene e | 1960 | - | - | - | - | 4.58 | - | - | - | - | - |
| 153 | Hexadecanoic acid | 1962 | - | - | - | 4.54 | 3.10 | 4.16 | 1.96 | - | 1.03 | - |
| 154 | Geranyl-α-terpinene e | 1968 | - | - | - | - | - | - | 5.84 | - | - | - |
| 155 | (Z,Z)-Geranyl linalool | 1977 | - | - | - | - | 1.75 | - | - | - | - | - |
| 156 | Kaur-15-ene | 1987 | 1997 | - | - | - | 1.13 | 0.20 | 0.20 | - | - | - |
| 157 | (E,Z)-Geranyl linalool | 1990 | 1987 | - | - | - | 0.77 | - | - | - | - | - |
| 158 | 13-epi-Manoyl oxide | 2004 | - | - | - | - | 1.20 | - | - | - | - | - |
| 159 | (E,E)-Geranyl linalool | 2018 | 2026 | - | - | - | 0.14 | - | - | - | - | |
| 160 | (Z)-9-Octadecen-1-ol | 2059 | - | - | - | - | 12.00 | 5.13 | 3.23 | - | - | - |
| 161 | (E)-9-Octadecen-1-ol | 2069 | - | - | 3.04 | - | - | - | - | - | - | - |
| 162 | 1-Heneicosene | 2088 | 2100 | - | - | - | 0.36 | - | - | - | - | - |
| 163 | 5-(7a-Isopropenyl-4,5-dimethyl-octahydroinden-4-yl)-3-methyl-pent-2-en-1-ol | 2094 | - | - | - | - | 1.01 | - | - | - | - | - |
| 164 | Linoleic acid | 2131 | 2132 | - | - | 2.11 | - | 2.23 | 1.80 | - | - | - |
| 165 | Cembrenol | 2165 | - | - | - | - | - | - | 1.29 | - | - | - |
| Monoterpene hydrocarbons (%) | - | 1.12 | 5.29 | 0.28 | 17.93 | 28.12 | 1.29 | 8.51 | 7.59 | |||
| Oxygenated monoterpenes (%) | 36.5 | 26.86 | 35.46 | 9.33 | 31.91 | 6.26 | 39.98 | 49.13 | 24.91 | |||
| Sesquiterpene hydrocarbons (%) | - | 0.87 | 12.28 | 15.47 | 15.33 | 9.51 | 0.33 | 13.82 | 32.87 | |||
| Oxygenated sesquiterpenes (%) | - | 3.04 | 3.39 | 35.1 | 8.14 | 15.92 | 0.86 | 6.77 | 2.28 | |||
| Diterpenes (%) | - | 1.15 | 1.88 | 18.76 | 0.40 | 11.85 | - | 0.21 | 0.44 | |||
| Others (%) | 45.65 | 52.02 | 25.15 | 5.21 | 16.29 | 6.06 | 49.38 | 8.49 | 25.51 | |||
| Total intedified (%) | 82.15 | 85.06 | 83.45 | 84.15 | 90 | 77.72 | 91.84 | 86.93 | 93.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaparakou, E.H.; Daferera, D.; Kanakis, C.D.; Skotti, E.; Kokotou, M.G.; Tarantilis, P.A. Chemical Composition of the Essential Oils of Three Popular Sideritis Species Cultivated in Greece Using GC-MS Analysis. Biomolecules 2023, 13, 1157. https://doi.org/10.3390/biom13071157
Kaparakou EH, Daferera D, Kanakis CD, Skotti E, Kokotou MG, Tarantilis PA. Chemical Composition of the Essential Oils of Three Popular Sideritis Species Cultivated in Greece Using GC-MS Analysis. Biomolecules. 2023; 13(7):1157. https://doi.org/10.3390/biom13071157
Chicago/Turabian StyleKaparakou, Eleftheria H., Dimitra Daferera, Charalabos D. Kanakis, Efstathia Skotti, Maroula G. Kokotou, and Petros A. Tarantilis. 2023. "Chemical Composition of the Essential Oils of Three Popular Sideritis Species Cultivated in Greece Using GC-MS Analysis" Biomolecules 13, no. 7: 1157. https://doi.org/10.3390/biom13071157
APA StyleKaparakou, E. H., Daferera, D., Kanakis, C. D., Skotti, E., Kokotou, M. G., & Tarantilis, P. A. (2023). Chemical Composition of the Essential Oils of Three Popular Sideritis Species Cultivated in Greece Using GC-MS Analysis. Biomolecules, 13(7), 1157. https://doi.org/10.3390/biom13071157








