Effects of Antiplatelet Drugs on Platelet-Dependent Coagulation Reactions
Abstract
1. Introduction
2. Materials and Methods
2.1. Donors and Patients
2.2. Blood Collection and Platelet Preparation
2.3. Platelet Aggregation
2.4. Plasma Recalcification Assay
2.5. Thrombin Generation Test
2.6. Phosphatidylserine Exposure—Washed Platelets
2.7. Phosphatidylserine Exposure—Whole Blood
2.8. Statistics
3. Results
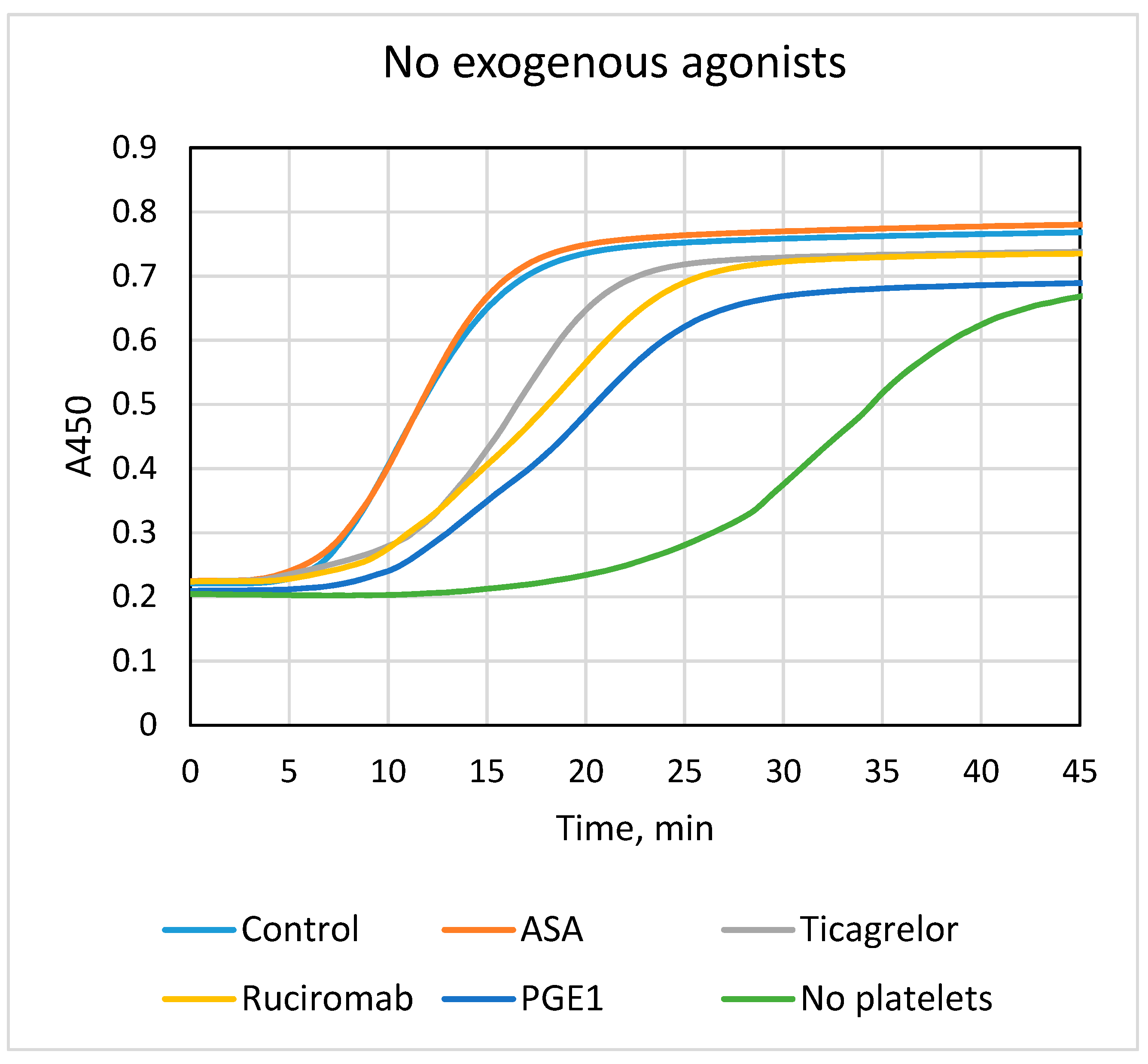
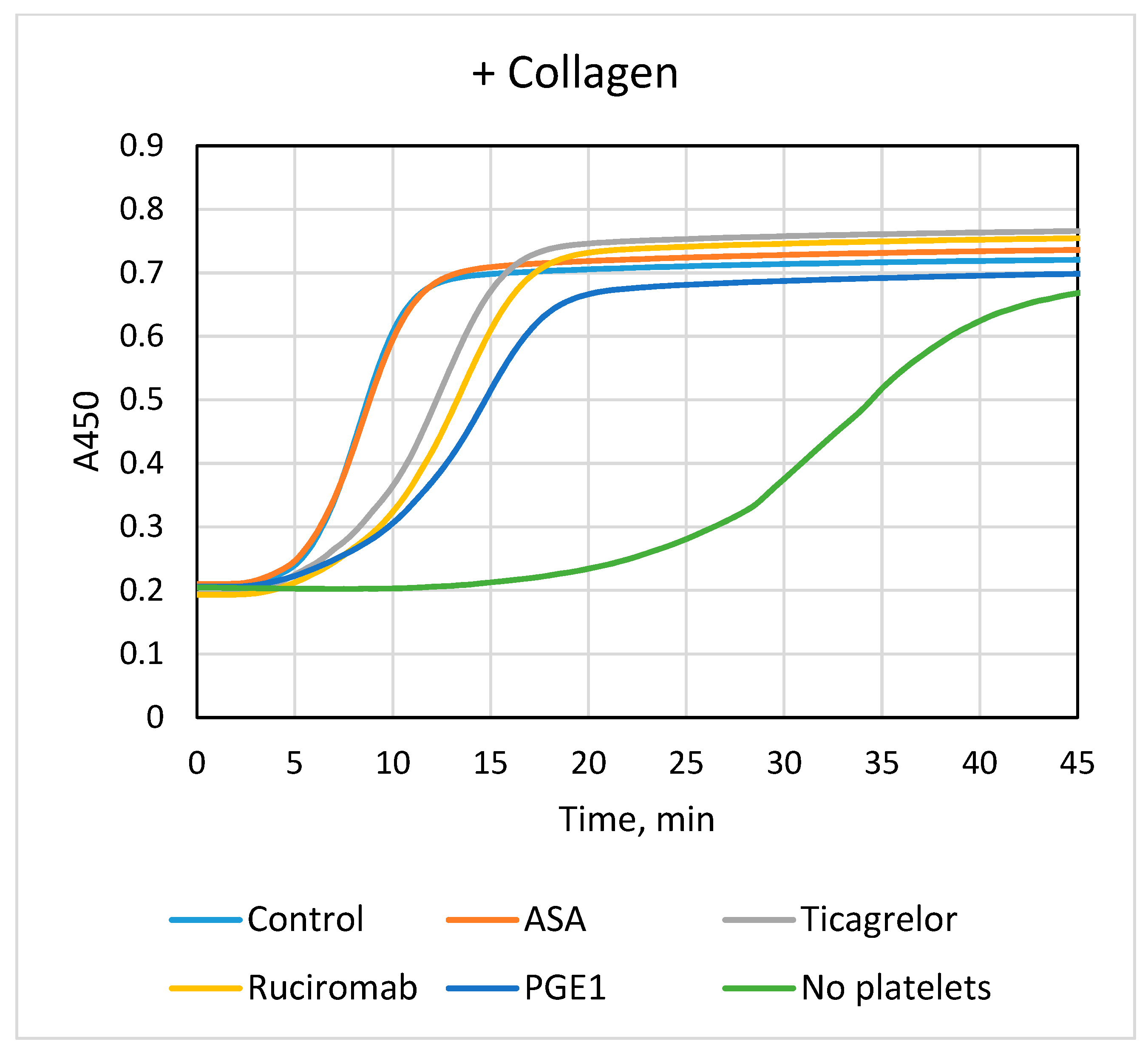
3.1. Effects of Antiplatelet Drugs on Platelet-Dependent Fibrin Formation
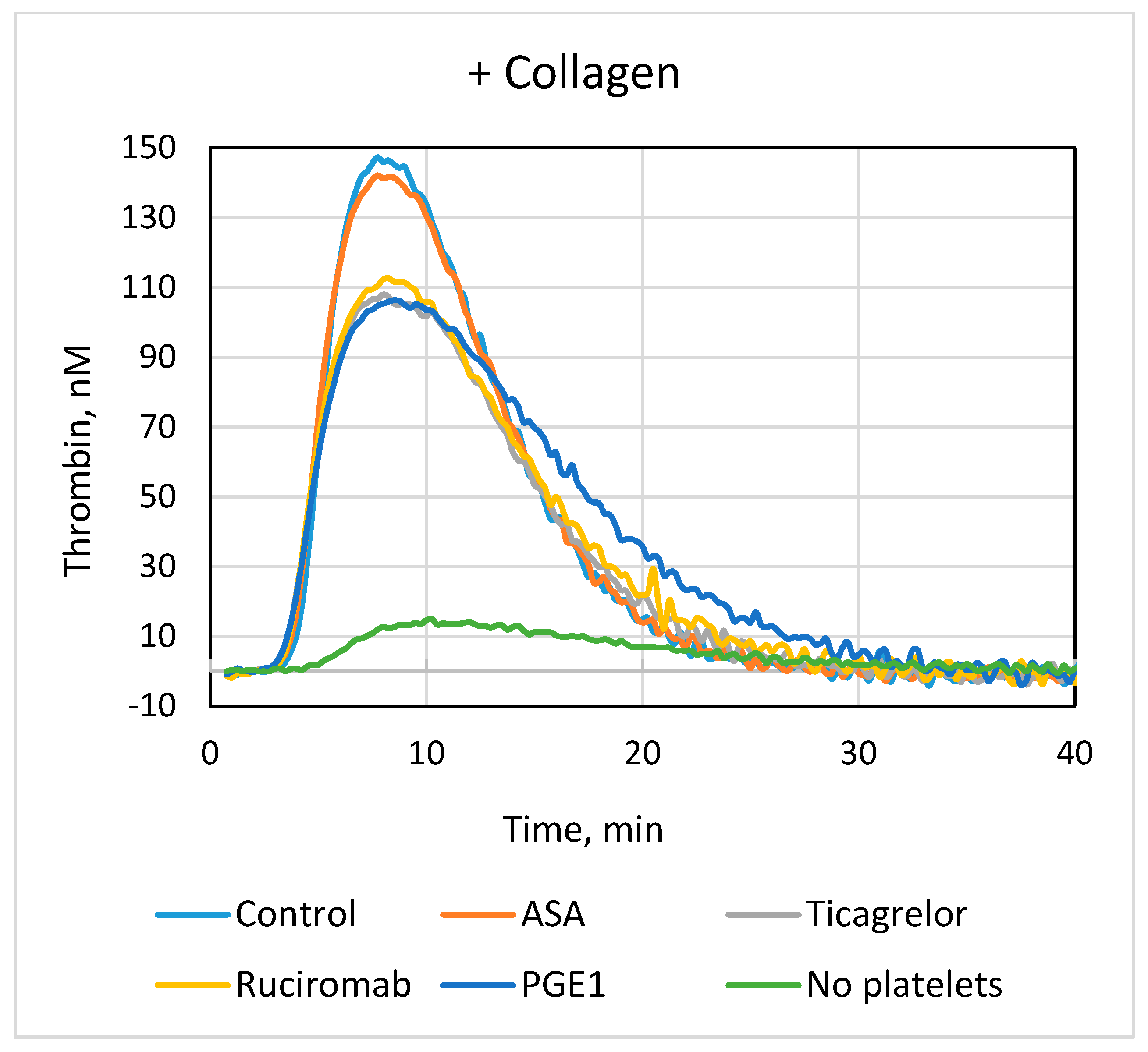
3.2. Effects of Antiplatelet Drugs on Platelet-Dependent Thrombin Generation
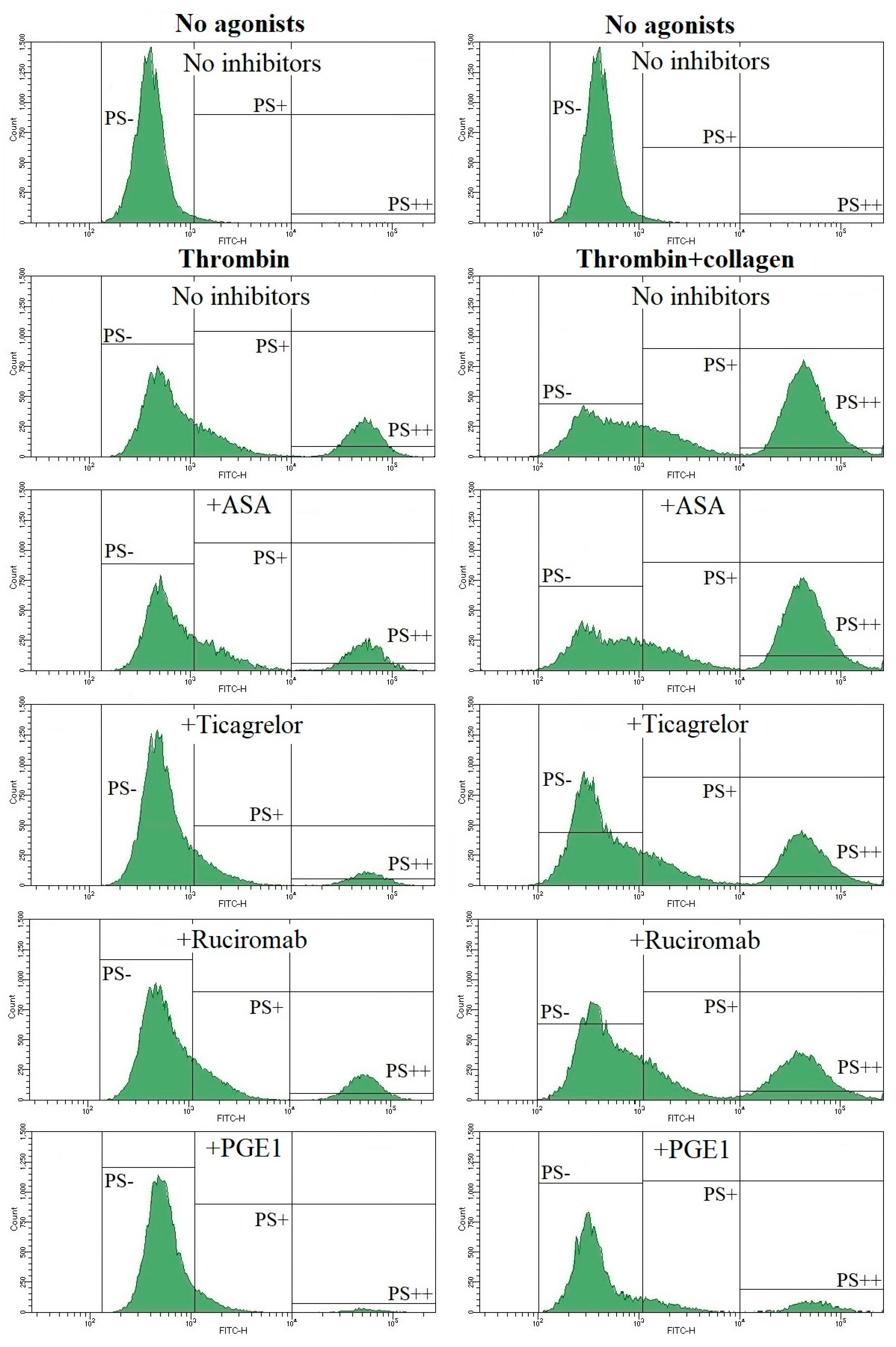
3.3. Effects of Antiplatelet Drugs on PS Exposure
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Heemskerk, J.W.M.; Cosemans, J.M.E.M.; van der Meijden, P.E.J. Platelets and coagulation. In Platelets in Thrombotic and Non-Thrombotic Disorders; Gresele, P., Kleimanat, N.S., Lopez, J.A., Page, C.P., Eds.; Springer: Cham, Switzerland, 2017; pp. 447–462. [Google Scholar] [CrossRef]
- Swieringa, F.; Spronk, H.M.H.; Heemskerk, J.W.M.; van der Meijden, P.E.J. Integrating platelet and coagulation activation in fibrin clot formation. Res. Pract. Thromb. Haemost. 2018, 2, 450–460. [Google Scholar] [CrossRef]
- Wielders, S.J.H.; Ungethüm, L.; Reutelingsperger, C.P.M.; Bevers, E.M.; Lindhout, T. Factor Xa-driven thrombin generation in plasma: Dependency on the aminophospholipid density of membranes and inhibition by phospholipid-binding proteins. Thromb. Haemost. 2007, 98, 1056–1062. [Google Scholar] [CrossRef]
- Muravlev, I.A.; Dobrovolsky, A.B.; Antonova, O.A.; Khaspekova, S.G.; Mazurov, A.V. Effects of platelets activated by different agonists on fibrin formation and thrombin generation. Platelets 2023, 34, 139365. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Konings, J.; de Laat, B.; Hackeng, T.M.; Roest, M. Added value of blood cells in thrombin generation testing. Thromb. Haemost. 2021, 121, 1574–1587. [Google Scholar] [CrossRef]
- Patrono, C. Aspirin. In Platelets, 4th ed.; Michelson, A.D., Cattaneo, M., Frelinger, A.L., III, Newman, P.J., Eds.; Elsevier Academic Press: Amsterdam, The Netherlands, 2019; pp. 921–936. [Google Scholar]
- Cattaneo, M. P2Y12 Antagonists. In Platelets, 4th ed.; Michelson, A.D., Cattaneo, M., Frelinger, A.L., III, Newman, P.J., Eds.; Elsevier Academic Press: Amsterdam, The Netherlands, 2019; pp. 937–956. [Google Scholar]
- Mahtta, D.; Bavry, A.A. αIIbβ3 (GPIIb-IIIa) Antagonists. In Platelets, 4th ed.; Michelson, A.D., Cattaneo, M., Frelinger, A.L., III, Newman, P.J., Eds.; Elsevier Academic Press: Amsterdam, The Netherlands, 2019; pp. 957–971. [Google Scholar]
- Mazurov, A.V.; Pevzner, D.V.; Antonova, O.A.; Byzova, T.V.; Khaspekova, S.G.; Semenov, A.V.; Vlasik, T.N.; Samko, A.N.; Staroverov, I.I.; Ruda, M.Y. Safety, inhibition of platelet aggregation and pharmacokinetics of F(ab’)2 fragments of the anti-glycoprotein IIb-IIIa monoclonal antibody FRaMon in high-risk coronary angioplasty. Platelets 2002, 13, 465–477. [Google Scholar] [CrossRef]
- Mazurov, A.V.; Pevzner, D.V.; Vlasik, T.N.; Ruda, M.I. Antiplatelet effects of glycoproteins IIb-IIIa antagonist monafram. Ross. Fiziol. Zhurnal Im. I.M. Sechenova 2004, 90, 586–599. (In Russian) [Google Scholar]
- Braune, S.; Küpper, J.-H.; Jung, F. Effect of Prostanoids on Human Platelet Function: An Overview. Int. J. Mol. Sci. 2020, 21, 9020. [Google Scholar] [CrossRef]
- Altman, R.; Scazziota, A.; Rouvier, J.; Gonzalez, C. Effect of sodium arachidonate on thrombin generation through platelet activation–inhibitory effect of aspirin. Thromb. Haemost. 2000, 84, 1109–1112. [Google Scholar] [CrossRef] [PubMed]
- Altman, R.; Scazziota, A.; de Lourdes Herrera, M.; Gonzalez, C. Recombinant factor VIIa reverses the inhibitory effect of aspirin or aspirin plus clopidogrel on in vitro thrombin generation. J. Thromb. Haemost. 2006, 4, 2022–2027. [Google Scholar] [CrossRef]
- Van der Meijden, P.E.J.; Schoenwaelder, S.M.; Feijge, M.A.H.; Cosemans, J.M.E.M.; Munnix, I.C.A.; Wetzker, R.; Heller, R.; Jackson, S.P.; Heemskerk, J.W.M. Dual P2Y12 receptor signaling in thrombin-stimulated platelets-involvement of phosphoinositide 3-kinase β but not γ isoform in Ca2+ mobilization and procoagulant activity. FEBS J. 2008, 275, 371–385. [Google Scholar] [CrossRef] [PubMed]
- Reverter, J.C.; Béguin, S.; Kessels, H.; Kumar, R.; Hemker, H.C.; Coller, B.S. Inhibition of platelet-mediated tissue factor-induced thrombin generation by the mouse/human chimeric 7E3 antibody. Potential implications for the effect of c7E3 Fab treatment on acute thrombosis and “clinical restenosis”. J. Clin. Investig. 1996, 98, 863–874. [Google Scholar] [CrossRef]
- Van der Meijden, P.E.J.; Feijge, M.A.H.; Swieringa, F.; Gilio, K.; Nergiz-Unal, R.; Hamulyák, K.; Heemskerk, J.W.M. Key role of integrin αIIbβ3 signaling to Syk kinase in tissue factor-induced thrombin generation. Cell Mol. Life Sci. 2012, 69, 3481–3492. [Google Scholar] [CrossRef]
- Léon, C.; Ravanat, C.; Freund, M.; Cazenave, J.-P.; Gachet, C. Differential Involvement of the P2Y1 and P2Y12 receptors in platelet procoagulant activity. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1941–1947. [Google Scholar] [CrossRef] [PubMed]
- Kotova, Y.N.; Ataullakhanov, F.I.; Panteleev, M.A. Formation of coated platelets is regulated by the dense granule secretion of adenosine 5′diphosphate acting via the P2Y12 receptor. J. Thromb. Haemost. 2008, 6, 1603–1605. [Google Scholar] [CrossRef]
- Pedicord, D.L.; Thomas, B.E.; Mousa, S.A.; Dicker, I.B. Glycoprotein IIb/IIIa receptor antagonists inhibit the development of platelet procoagulant activity. Thromb. Res. 1998, 90, 247–258. [Google Scholar] [CrossRef]
- Goto, S.; Tamura, N.; Li, M.; Handa, M.; Ikeda, Y.; Handa, S.; Ruggeri, Z.M. Different effects of various anti-GPIIb-IIIa agents on shear-induced platelet activation and expression of procoagulant activity. J. Thromb. Haemost. 2003, 1, 2022–2030. [Google Scholar] [CrossRef]
- Ramström, S.; Rånby, M.; Lindahl, T.L. Platelet phosphatidylserine exposure and procoagulant activity in clotting whole blood–different effects of collagen, TRAP and calcium ionophore A23187. Thromb. Haemost. 2003, 89, 132–141. [Google Scholar] [PubMed]
- Topalov, N.N.; Kotova, Y.N.; Vasil’ev, S.A.; Panteleev, M.A. Identification of signal transduction pathways involved in the formation of platelet subpopulations upon activation. Br. J. Haematol. 2012, 157, 105–115. [Google Scholar] [CrossRef]
- Jones, M.L.; Harper, M.T.; Aitken, E.W.; Williams, C.M.; Poole, A.W. RGD-ligand mimetic antagonists of integrin αIIbβ3 paradoxically enhance GPVI-induced human platelet activation. J. Thromb. Haemost. 2010, 8, 567–576. [Google Scholar] [CrossRef]
- Prodan, C.I.; Joseph, P.M.; Vincent, A.S.; Dale, G.L. Coated-platelet levels are influenced by smoking, aspirin, and selective serotonin reuptake inhibitors. J. Thromb. Haemost. 2007, 5, 2149–2151. [Google Scholar] [CrossRef] [PubMed]
- Mazurov, A.V.; Vinogradov, D.V.; Kabaeva, N.V.; Antonova, G.N.; Romanov, Y.A.; VIasik, T.N.; Antonov, A.S.; Smirnov, V.N. A monoclonal antibody, VM64, reacts with a 130 kDa glycoprotein common to platelets and endothelial cells: Heterogeneity in antibody binding to human aortic endothelial cells. Thromb. Haemost. 1991, 66, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Khaspekova, S.G.; Zyuryaev, I.T.; Yakushkin, V.V.; Naimushin, Y.A.; Sirotkina, O.V.; Zaytseva, N.O.; Ruda, M.Y.; Mazurov, A.V. Mean platelet volume: Interrelation with platelet aggregation activity and glycoprotein IIb-IIIa and Ib expression levels. Biochem. (Mosc.) Suppl. Ser. B Biomed. Chem. 2014, 8, 134–142. [Google Scholar] [CrossRef]
- Didelot, M.; Docq, C.; Wahl, D.; Lacolley, P.; Regnault, V.; Lagrange, J. Platelet aggregation impacts thrombin generation assessed by calibrated automated thrombography. Platelets 2018, 29, 156–161. [Google Scholar] [CrossRef]
- Arima, Y.; Kaikita, K.; Ishii, M.; Ito, M.; Sueta, D.; Oimatsu, Y.; Sakamoto, K.; Tsujita, K.; Kojima, S.; Nakagawa, K.; et al. Assessment of platelet-derived thrombogenicity with the total thrombus-formation analysis system in coronary artery disease patients receiving antiplatelet therapy. J. Thromb. Haemost. 2016, 14, 850–859. [Google Scholar] [CrossRef]
- Berezovskaya, G.; Smirnova, O.; Malev, E.; Khromov-Borisov, N.; Klokova, E.; Karpenko, M.; Papayan, L.; Petrishchev, N. Thrombin generation test for evaluation of antiplatelet treatment in patients with coronary artery disease after percutaneous coronary intervention. Platelets 2018, 29, 185–191. [Google Scholar] [CrossRef] [PubMed]


| Healthy Volunteers | Patients with ACS | |
|---|---|---|
| n | 30 | 26 |
| Age, years, mean ± SD | 56 ± 16 | 63 ± 10 |
| Men/women | 18/12 | 17/9 |
| Diagnosis | - | |
| Myocardial infarction | - | 23 |
| Unstable angina | - | 3 |
| Percutaneous coronary intervention | - | 26 |
| Antiplatelet drugs | - | ASA 100 mg/daily + ticagrelor 90 mg × 2/daily |
| Time of blood collection (to the day of ACS onset) | - | 4–6 days |
| No Exogenous Agonists | ||
|---|---|---|
| Lag phase, min | Vmax, %A450/min | |
| Control (n = 15) | 6.4 ± 1.7 | 11.8 ± 2.5 |
| ASA (n = 11) | 6.1 ± 1.8 | 12.1 ± 4.3 |
| Ticagrelor (n = 15) | 8.0 ± 1.7 *** | 7.9 ± 2.3 *** |
| ASA + Ticagrelor (n = 10) | 7.2 ± 1.2 *** | 8.6 ± 2.4 ** |
| Ruciromab (n = 7) | 9.8 ± 1.5 ** | 5.7 ± 0.8 *** |
| PGE1 (n = 7) | 10.1 ± 1.0 *** | 4.8 ± 0.7 *** |
| +Collagen | ||
| Lag phase, min | Vmax, % A450/min | |
| Control (n = 14) | 5.1 ± 1.2 | 18.7 ± 3.8 |
| ASA (n = 9) | 5.1 ± 1.1 | 17.8 ± 4.5 |
| Ticagrelor (n = 15) | 6.3 ± 1.4 *** | 12.6 ± 3.8 *** |
| ASA + Ticagrelor (n = 8) | 6.0 ± 1.1 ** | 11.8 ± 3.0 ** |
| Ruciromab (n = 6) | 7.1 ± 1.7 ** | 11.4 ± 2.2 ** |
| PGE1 (n = 6) | 7.5 ± 1.9 ** | 10.2 ± 2.3 ** |
| No Exogenous Agonists | ||||
|---|---|---|---|---|
| Lag phase, min | ETP 1, nM × min | Peak, nM | Vmax, nM/min | |
| Control (n = 9) | 3.9 ± 0.3 | 1137 ± 146 | 84 ± 23 | 17 ± 7 |
| ASA (n = 5) | 3.7 ± 0.1 | 1177 ± 204 | 93 ± 28 | 20 ± 8 |
| Ticagrelor (n = 7) | 3.8 ± 0.2 | 1058 ± 164 | 60 ± 17 ** | 10 ± 4 ** |
| ASA + Ticagrelor (n = 5) | 3.6 ± 0.3 | 1103 ± 236 | 67 ± 26 * | 12 ± 6 * |
| Ruciromab (n = 6) | 3.7 ± 0.3 | 1115 ± 190 | 62 ± 27 * | 12 ± 6 * |
| PGE1 (n = 5) | 3.5 ± 0.3 * | 1007 ± 186 * | 62 ± 18 * | 10 ± 3 * |
| +Collagen | ||||
| Lag phase, min | ETP 1, nM × min | Peak, nM | Vmax, nM/min | |
| Control (n = 8) | 3.8 ± 0.3 | 1234 ± 140 | 137 ± 24 | 36 ± 10 |
| ASA (n = 7) | 3.8 ± 0.2 | 1257 ± 125 | 131 ± 15 | 33 ± 5 |
| Ticagrelor (n = 7) | 3.8 ± 0.3 | 1277 ± 193 | 109 ± 30 ** | 25 ± 12 ** |
| ASA + Ticagrelor (n = 7) | 3.8 ± 0.3 | 1284 ± 145 | 111 ± 21 * | 25 ± 6 ** |
| Ruciromab (n = 7) | 3.7 ± 0.3 | 1327 ± 193 | 115 ± 17 * | 26 ± 5 * |
| PGE1 (n = 7) | 3.9 ± 0.2 | 1222 ± 183 | 105 ± 24 ** | 24 ± 8 ** |
| Healthy Volunteers (No Medications) | Patients with ACS (ASA + Ticagrelor) | |||
|---|---|---|---|---|
| PS+ Platelets, % | PS++ Platelets, % | PS+ Platelets, % | PS++ Platelets, % | |
| No agonists | 1.9 ± 1.3 (n = 30) | 0.4 ± 0.4 (n = 30) | 1.2 ± 1.0 (n = 25) p = 0.022 | 0.2 ± 0.2 (n = 25) p = 0.014 |
| TRAP | 10.9 ± 5.1 (n = 20) | 3.1 ± 2.8 (n = 20) | 5.1 ± 2.8 (n = 19) p < 0.001 | 1.2 ± 1.3 (n = 19) p = 0.011 |
| TRAP + collagen | 52.0 ± 7.6 (n = 28) | 36.4 ± 10.2 (n = 28) | 41.7 ± 10.2 (n = 26) p < 0.001 | 26.6 ± 11.9 (n = 26) p = 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muravlev, I.A.; Dobrovolsky, A.B.; Antonova, O.A.; Khaspekova, S.G.; Alieva, A.K.; Pevzner, D.V.; Mazurov, A.V. Effects of Antiplatelet Drugs on Platelet-Dependent Coagulation Reactions. Biomolecules 2023, 13, 1124. https://doi.org/10.3390/biom13071124
Muravlev IA, Dobrovolsky AB, Antonova OA, Khaspekova SG, Alieva AK, Pevzner DV, Mazurov AV. Effects of Antiplatelet Drugs on Platelet-Dependent Coagulation Reactions. Biomolecules. 2023; 13(7):1124. https://doi.org/10.3390/biom13071124
Chicago/Turabian StyleMuravlev, Ivan A., Anatoly B. Dobrovolsky, Olga A. Antonova, Svetlana G. Khaspekova, Amina K. Alieva, Dmitry V. Pevzner, and Alexey V. Mazurov. 2023. "Effects of Antiplatelet Drugs on Platelet-Dependent Coagulation Reactions" Biomolecules 13, no. 7: 1124. https://doi.org/10.3390/biom13071124
APA StyleMuravlev, I. A., Dobrovolsky, A. B., Antonova, O. A., Khaspekova, S. G., Alieva, A. K., Pevzner, D. V., & Mazurov, A. V. (2023). Effects of Antiplatelet Drugs on Platelet-Dependent Coagulation Reactions. Biomolecules, 13(7), 1124. https://doi.org/10.3390/biom13071124





