A Systematic Review on the Role of Matrix Metalloproteinases in the Pathogenesis of Inguinal Hernias
Abstract
1. Introduction
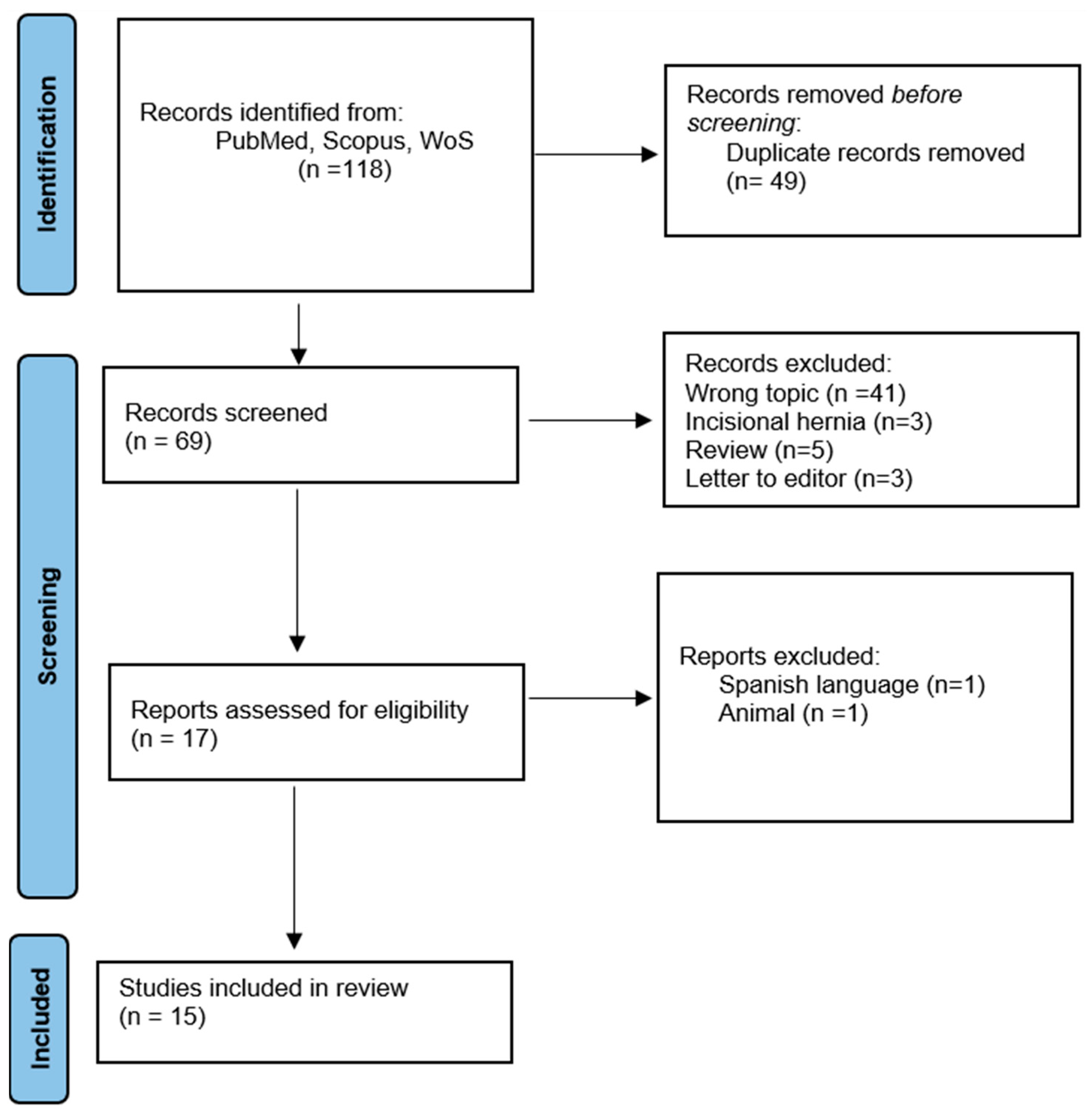
2. Materials and Methods
3. Results
3.1. MMPs
3.2. TIMPs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stabilini, C.; Cavallaro, G.; Dolce, P.; Capoccia Giovannini, S.; Corcione, F.; Frascio, M.; Sodo, M.; Merola, G.; Bracale, U. Pooled data analysis of primary ventral (PVH) and incisional hernia (IH) repair is no more acceptable: Results of a systematic review and metanalysis of current literature. Hernia 2019, 23, 831–845. [Google Scholar] [CrossRef] [PubMed]
- Stabilini, C.; Cavallaro, G.; Bocchi, P.; Campanelli, G.; Carlucci, M.; Ceci, F.; Crovella, F.; Cuccurullo, D.; Fei, L.; Gianetta, E.; et al. Defining the characteristics of certified hernia centers in Italy: The Italian society of hernia and abdominal wall surgery workgroup consensus on systematic reviews of the best available evidences. Int. J. Surg. 2018, 54, 222–235. [Google Scholar] [CrossRef] [PubMed]
- Flum, D.R.; Horvath, K.; Koepsell, T. Have outcomes of incisional hernia repair improved with time? A population-based analysis. Ann. Surg. 2003, 237, 129–135. [Google Scholar] [CrossRef]
- Liem, M.S.; Vander Graaf, Y.; Beemer, F.A.; Van Vroonhoven, T.J. Increased risk for inguinal hernia in patients with Ehlers-Danlos syndrome. Surgery 1997, 122, 114–115. [Google Scholar] [CrossRef] [PubMed]
- Lehnert, B.; Wadouh, F. High coincidence of inguinal hernias and abdominal aortic aneurysms. Ann. Vasc. Surg. 1992, 6, 578–582. [Google Scholar] [CrossRef]
- Casanova, A.B.; Trindade, E.N.; Trindade, M.R. Collagen in the transversalis fascia of patients with indirect inguinal hernia: A case-control study. Am. J. Surg. 2009, 198, 1–5. [Google Scholar] [CrossRef]
- White, B.; Osier, C.; Gletsu, N.; Jeansonne, L.; Baghai, M.; Sherman, M.; Smith, C.D.; Ramshaw, B.; Lin, E. Abnormal primary tissue collagen composition in the skin of recurrent incisional hernia patients. Am. Surg. 2007, 73, 1254–1258. [Google Scholar] [CrossRef]
- Nagase, H.; Visse, R.; Murphy, G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc. Res. 2006, 69, 562–573. [Google Scholar] [CrossRef]
- Bellon, J.M.; Bujan, J.; Honduvilla, N.G.; Jurado, F.; Gimeno, M.J.; Turnay, J.; Olmo, N.; Lizarbe, M.A. Study of biochemical substrate and role of metalloproteinases in fascia transversalis from hernial processes. Eur. J. Clin. Investig. 1997, 27, 510–516. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 29, 372. [Google Scholar]
- Antoniou, S.A.; Antoniou, G.A.; Granderath, F.A.; Simopoulos, C. The role of matrix metalloproteinases in the pathogenesis of abdominal wall hernias. Eur. J. Clin. Investig. 2009, 39, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, X.; Sun, Q.; Li, W.; Yu, A.; Fu, H.; Chen, K. Circulating matrix metalloproteinases and procollagen propeptides in inguinal hernia. Hernia 2018, 22, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Han, Y.; Xu, X.; Chen, J.; Chen, Y. Matrix Metalloproteinases (MMP-2) and Tissue Inhibitors of Metalloproteinases (TIMP-2) in Patients with Inguinal Hernias. World J. Surg. 2021, 45, 2747–2751. [Google Scholar] [CrossRef] [PubMed]
- Smigielski, J.; Brocki, M.; Kuzdak, K.; Kołomecki, K. Serum MMP 2 and TIMP 2 in patients with inguinal hernias. Eur. J. Clin. Investig. 2011, 41, 584–588. [Google Scholar] [CrossRef]
- Antoniou, G.A.; Tentes, I.K.; Antoniou, S.A.; Simopoulos, C.; Lazarides, M.K. Matrix Metalloproteinase Imbalance in Inguinal Hernia Formation. J. Investig. Surg. 2011, 24, 145–150. [Google Scholar] [CrossRef]
- Aren, A.; Gökçe, A.H.; Gökçe, F.S.; Dursun, N. Roles of matrix metalloproteinases in the etiology of inguinal hernia. Hernia 2011, 15, 667–671. [Google Scholar] [CrossRef]
- Isik, A.; Gursul, C.; Peker, K.; Aydın, M.; Fırat, D.; Yılmaz, I. Metalloproteinases and Their Inhibitors in Patients with Inguinal Hernia. World J. Surg. 2017, 41, 1259–1266. [Google Scholar] [CrossRef]
- Pascual, G.; Rodriguez, M.; Gomez-Gil, V.; Trejo, C.; Buja, J.; Bellon, J.M. Active matrix metalloproteinase-2 upregulation in the abdominal skin of patients with direct inguinal hernia. Eur. J. Clin. Investig. 2010, 40, 1113–1121. [Google Scholar] [CrossRef]
- Peng, X.; Guo, Z.; Zhang, Y.; Sun, B.; Zhang, Q. EFEMP1 in Direct Inguinal Hernia: Correlation with TIMP3 and Regulation Toward Elastin Homoeostasis as Well as Fibroblast Mobility. J. Investig. Surg. 2022, 35, 203–211. [Google Scholar] [CrossRef]
- Strohalmová, S.; Levová, K.; Kubĕna, A.A.; Krška, Z.; Hoskovec, D.; Zima, T.; Kalousovà, M. The Effect of Surgery on the Levels of Matrix Metalloproteinases in Patients with Inguinal Hernia. Physiol. Res. 2021, 70, 627–634. [Google Scholar] [CrossRef]
- Nizar, N.; Afriwardi, A.; Yanwirasti, Y.; Arlan, A. Matrix Metalloproteinase-2, COL1A1, and COL3A1 mRNA Expression in Aponeurosis Musculus obliquus Externus Abdominis of Adult Inguinal Hernias. J. Med. Sci. 2021, 16, 318–323. [Google Scholar] [CrossRef]
- Antoniou, G.A.; Tentes, I.K.; Antoniou, S.A.; Georgiadis, G.S.; Giannoukas, A.D.; Simopoulos, C.; Lazarides, M.K. Circulating matrix metalloproteinases and their inhibitors in inguinal hernia and abdominal aortic aneurysm. Int. Angiol. 2010, 30, 123–129. [Google Scholar]
- Serra, R.; Buffone, G.; Costanzo, G.; Montemurro, R.; Scarcello, E.; Stillitano, D.M.; Damiano, R.; de Franciscis, S. Altereted Metalloproteinase-9 Expression as Least Common Denominator between Varicocele, Inguinal Hernia, and Chronic Venous Disorders. Ann. Vasc. Surg. 2014, 28, 705–709. [Google Scholar] [CrossRef] [PubMed]
- Jain, V.; Srivastava, R.; Rawat, N.S.; Misra, S.; Jha, S.; Amla, D.V. Matrix Metalloproteinase-2 and its Relation with Incisional & Inguinal Hernia. JIMSA 2011, 24, 4. [Google Scholar]
- Skiles, J.W.; Gonnella, N.C.; Jeng, A.Y. The Design, Structure, and Therapeutic Application of Matrix Metalloproteinase Inhibitors. Curr. Med. Chem. 2001, 8, 425–475. [Google Scholar] [CrossRef]
- Dolmatov, I.Y.; Nizhnichenko, V.A.; Dolmatova, L.S. Matrix Metalloproteinases and Tissue Inhibitors of Metalloproteinases in Echinoderms: Structure and Possible Functions. Cells 2021, 10, 2331. [Google Scholar] [CrossRef]
- Laronha, H.; Caldeira, J. Structure and Function of Human Matrix Metalloproteinases. Cells 2020, 9, 1076. [Google Scholar] [CrossRef]
- Van Doren, S.R. Matrix metalloproteinase interactions with collagen and elastin. Matrix Biol. 2015, 44, 224–231. [Google Scholar] [CrossRef]
- Ugur Durukan, U.; Agcaoglu, O.; Ozoran, E.; Karahan, S.N.; Ozata, I.; Duzkoylu, Y.; Pasaoglu, E.; Aren, A. The role of tissue inhibitor of metalloproteinases in the aetiology of inguinal and incisional hernias. Int. Wound J. 2022, 19, 1502–1508. [Google Scholar] [CrossRef]
- Klinge, U.; Zheng, H.; Si, Z.; Schumpelick, V.; Bhardwaj, R.S.; Muys, L. Expression of the extracellular matrix proteins collagen I, collagen III and fibronectin and matrix metalloproteinase-1 and -13 in the skin of patients with inguinal hernia. Eur. Surg. Res. 1999, 31, 480–490. [Google Scholar] [CrossRef]
- Zheng, H.; Si, Z.; Kasperk, R.; Bhardwaj, R.S.; Schumpelick, V.; Klinge, U.; Klosterhalfen, B. Recurrent inguinal hernia: Disease of the collagen matrix? World J. Surg. 2002, 26, 401–408. [Google Scholar] [PubMed]
- Chowdhury, A.; Brinson, R.; Wei, B.; Stetler-Stevenson, W.G. Tissue Inhibitor of Metalloprotease-2 (TIMP-2): Bioprocess Development, Physicochemical, Biochemical, and Biological Characterization of Highly Expressed Recombinant Protein. Biochemistry 2017, 56, 6423–6433. [Google Scholar] [CrossRef] [PubMed]
| Ref./Year | Type of Hernia | Type of Sample | Pat./Ctrl. (n) | Groups Studied (n Patients) | Substances Measured | Method Used | Results |
|---|---|---|---|---|---|---|---|
| [11], 2011 | DINGH, IINGH | Blood plasma, abdominal fascia tissue | 126/35 | INGH (91), controls (35) | MMP2, MMP9, TIMP1, TIMP2 | ELISA | MMP-9 AND MMP-2 in serum are ↓ in INGH then controls; TIMP-1 in serum is ↓ in INGH then controls; TIMP-2 in serum is ↑ in INGH then controls; MMP-9 and MMP-2 in tissue are ↑ in INGH then controls; TIMP-1 and TIMP-2 in tissue are ↓ in INGH then controls. |
| [12], 2018 | DINGH, IINGH, RDINGH, RIINGH | Blood serum | 155/45 | DINGH (40); RDINGH (10); IINGH (45); RIINGH (15); controls (45) | MMP2; MMP9 | ELISA; spectrometer | MMP2 ↑ in RDINGH, RIINGH and DINGH; MMP9 no difference. |
| [13], 2020 | DINGH, IINGH | Anterior rectus sheath fascia | 58/10 | DIINGH (16); IINGH (32); controls (10) | MMP2 mRNA; TIMP2 mRNA | RT-PCR; Immunohistochemistry | MMP2 ↑ in DINGH/IINGH; TIMP2 ↓ in DINGH; Ratio of MMP2 mRNA/TIMP2 mRNA ↑ in DINGH/IINGH |
| [14], 2011 | DINGH, IINGH, RINGH | Blood serum | 150/30 | DINGH; IINGH; RINGH; Control; subgroup A: age 26–49 B: age 50–70 | MMP2; TIMP2; | ELISA | MMP2 ↑ in DINGH-A, IINGH-A, RINGH A; TIMP2 ↑ in RINGH |
| [16], 2011 | DIINGH, IINGH | Fascia trasversalis | 90/30 | DIINGH (30); IINGH (30); Controls (30) | MMP-1, MMP-2, MMP-9 | Immunohistochemical | ↑ MMP-1, -2, -9 in DINGH and IINGH |
| [17], 2017 | IINGH, DINGH, BINGH | Serum and FT | 44/11 | DINGH (11), IINGH (11), BINGH (11), controls (11) | MMP-1, MMP-2, MMP-9, MMP-13, TIMP-1, TIMP-2, TIMP3. | ELISA | ↑ MMP-1, -2, -9, -13 in DINGH, IINGH; ↑↑ in BINGH; TIMPs ↓ in every group then controls; TIMPs ↓↓ in BINGH |
| [18], 2021 | Primary inguinal hernia | Anterior rectus sheath fascia | 48/8 | Primary: Group A (50–59 aa) (10); Group B (60–69 aa) (10); Group C (70–79 aa) (10); Group D (80–89 aa) (10) Controls (8) | MMP-2, TIMP-2 | RT-PCR | MMP-2 ↑ in Group C and D then controls; TIMP-2 is ↓ in Group C and D then controls; positive correlation between age and MMP-s levels; negative correlation between age and TIMP-2 levels |
| [19], 2010 | DINGH, IINGH, CONTROLS | Skin biopsy | 46/10 | Group I (median age 43) DINGH (18), Group II (median age 39,7) IINGH (18), Controls (median age 39,5) (10) | MMP-2, MT-1 MMP, TIMP-2 | Immunocytochemistry, Immunoblotting, Zymography | MMP-2 expression in skin fibroblasts is ↑ in DINGH; positive correlation between MT1-MMP expression and MMP-2 expression in DINGH AND IINGH; TIMP-2 ↑ IN CONTROL GROUP |
| [20], 2020 | DINGH, CONTROLS | FT | 40/20 | DINGH (20), CONTROLS (20) | TIMP-3, MMP-9 | Immunohistochemical, western blot, RT-PCR | TIMP-3 ↓ in DINGH then Controls; MMP-9 level ↑ in DINGH then controls. Negative correlation between TIMP3 and MMP9 |
| [21], 2021 | DINGH, IINGH, RINGH, controls | Blood serum | 46/21 | DINGH (8), INGH (10), RINGH (4), CONTROLS (21) pre and post operative (24 h after the surgery) | MMP-1, MMP-2, MMP-7, MMP-9, MMP-10, TIMP-1, TIMP-2 | ELISA | preoperative: MMP-2 MMP-9 ↓ in INGH, MMP-1,-7,-10 no difference; TIMP-2 ↓ in INGH; TIMP-1 no difference; MMP-9/TIMP-1 ↓ in INGH; postoperative: MMPs significant ↓ except MMP-9 that ↑ in IH; MMP-9/TIMP-1 ↑ in IH; not observe differences in MMPs/TIMPs in subgroups of IH |
| [22], 2021 | INGH, CONTROLS | Aponeurosis Musculus obliquus externus | INGH (50.92 ± 13.09 years), CONTROLS (36.16 ± 12.53) | MMP-2 mRNA | RT-PCR | MMP2 expression higher in INGH | |
| [23], 2010 | AAA, INGH, CONTROLS | Blood plasma | 159/35 | AAA (33), INGH (91), CONTROLS (35) | MMP-2, MMP-9, TIMP1, TIMP2 | ELISA | MMP-9 and MMP-2 ↓ in INGH; TIMP2 ↑ in INGH; TIMP1 ↓ in INGH |
| [24], 2014 | INGH | FT (Transverse fascia); spermatic veins; GSV (great saphenous vein); plasma fluid | 37/15 | VAR + INGH (9); VAR + CVD (6); INGH + CVD (2); VAR + CVD + INGH (5); Control: VAR (7); INGH (3); CVD (5) = 15 | MMP1-2-9-12-13 | ELISA; Western blot; immunoblotting | MMP1, 2, 9, 12, 13 ↑ in groups with INGH (plasma and tissue); MMP9 ↑ in group with two or three clinical conditions; MMP9 ↑ in VAR/CVD respect INGH |
| [25], 2011 | DIINGH, IINGH, IH, CONTROLS | Blood serum, FT | 100/30 | DIINGH (30), IINGH (30), IH (10), CONTROLS (30) | MMP 2 | ELISA | MMP-2 ↑ in DINGH |
| [26], 2021 | DINGH | TF (Transverse fascia) | 90/30 | DINGH (30); IH (30); controls (30) | TIMP1; TIMP2 | Immunohistochemical | TIMP1 ↑ in DINGH and in IH; TIMP2 ↓ in DINGH |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bracale, U.; Peltrini, R.; Iacone, B.; Martirani, M.; Sannino, D.; Gargiulo, A.; Corcione, F.; Serra, R.; Bracale, U.M. A Systematic Review on the Role of Matrix Metalloproteinases in the Pathogenesis of Inguinal Hernias. Biomolecules 2023, 13, 1123. https://doi.org/10.3390/biom13071123
Bracale U, Peltrini R, Iacone B, Martirani M, Sannino D, Gargiulo A, Corcione F, Serra R, Bracale UM. A Systematic Review on the Role of Matrix Metalloproteinases in the Pathogenesis of Inguinal Hernias. Biomolecules. 2023; 13(7):1123. https://doi.org/10.3390/biom13071123
Chicago/Turabian StyleBracale, Umberto, Roberto Peltrini, Biancamaria Iacone, Mirko Martirani, Daniele Sannino, Antonio Gargiulo, Francesco Corcione, Raffaele Serra, and Umberto Marcello Bracale. 2023. "A Systematic Review on the Role of Matrix Metalloproteinases in the Pathogenesis of Inguinal Hernias" Biomolecules 13, no. 7: 1123. https://doi.org/10.3390/biom13071123
APA StyleBracale, U., Peltrini, R., Iacone, B., Martirani, M., Sannino, D., Gargiulo, A., Corcione, F., Serra, R., & Bracale, U. M. (2023). A Systematic Review on the Role of Matrix Metalloproteinases in the Pathogenesis of Inguinal Hernias. Biomolecules, 13(7), 1123. https://doi.org/10.3390/biom13071123








