ER Stress, the Unfolded Protein Response and Osteoclastogenesis: A Review
Abstract
:1. Introduction
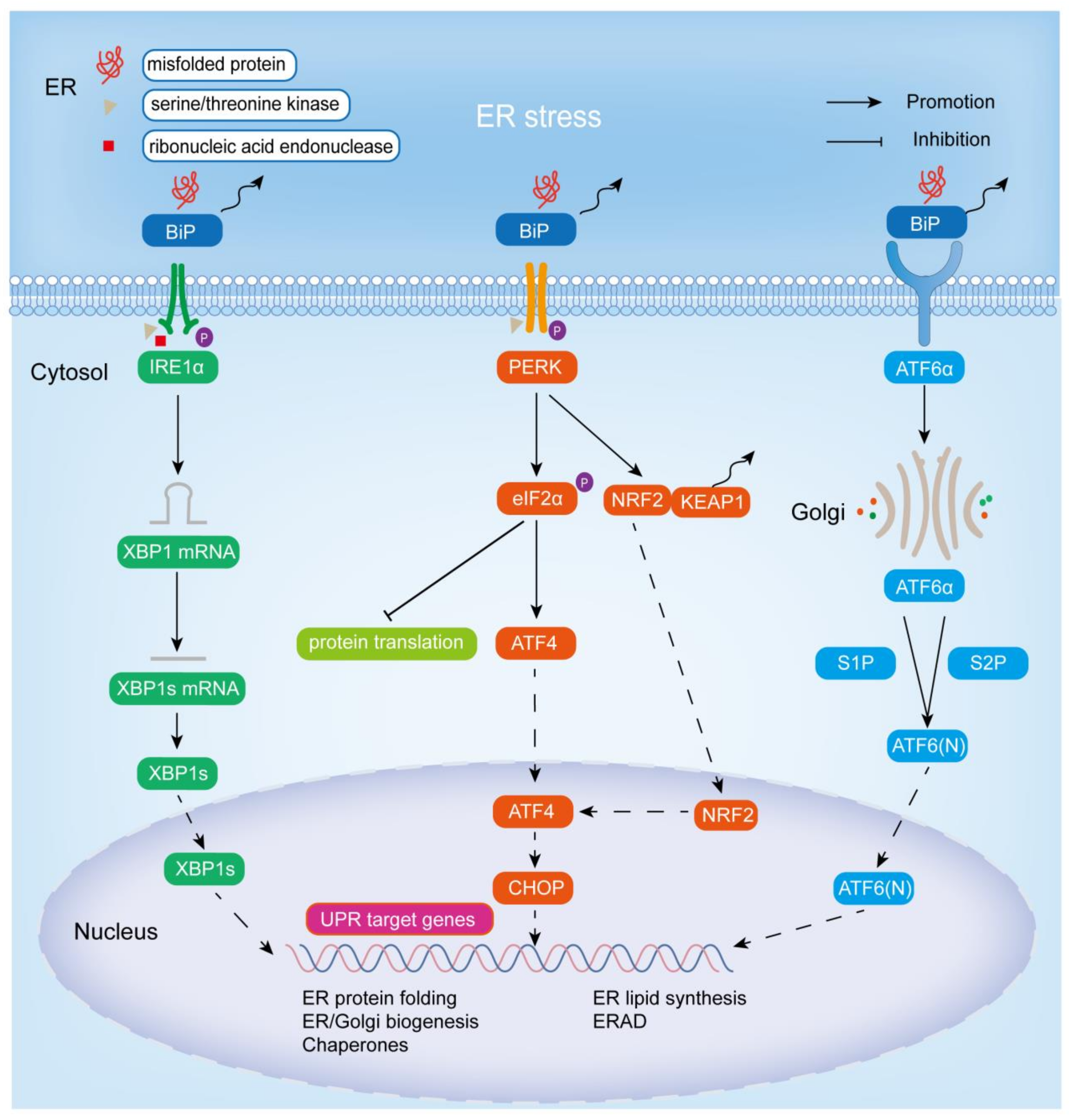
2. ER Stress and UPR
2.1. IRE1α Signaling Cascade Pathway
2.2. PERK Signaling Cascade Pathway
2.3. ATF6 Signaling Cascade Pathway
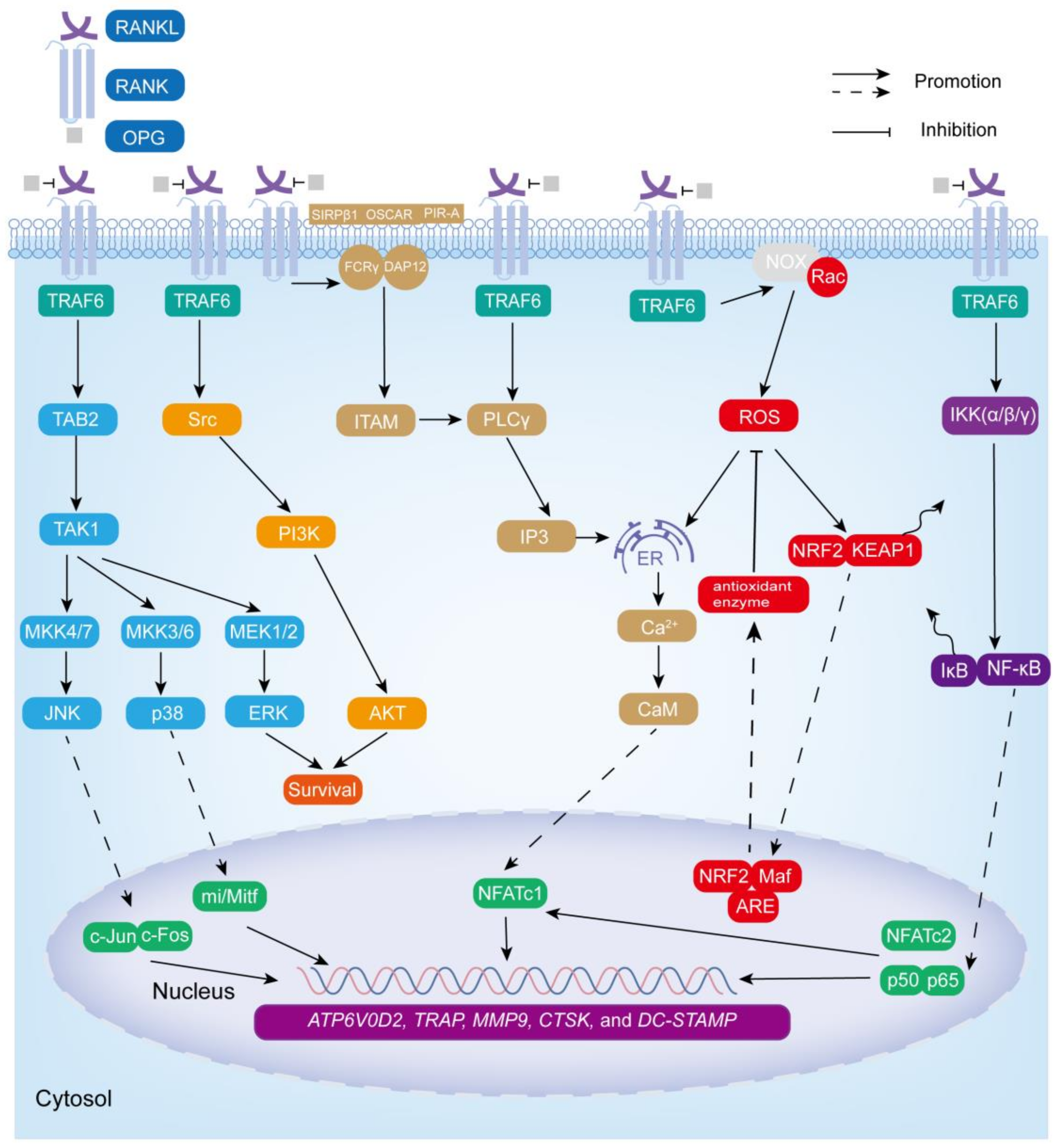
3. The Bone Remodeling and Osteoclastogenesis
3.1. NF-κB Signaling Cascade Pathway
3.2. MAPK Signaling Cascade Pathway
3.3. ROS Signaling Cascade Pathway
3.4. Ca2+-NFATc1 Signaling Cascade Pathway
3.5. Src Signaling Cascade Pathway
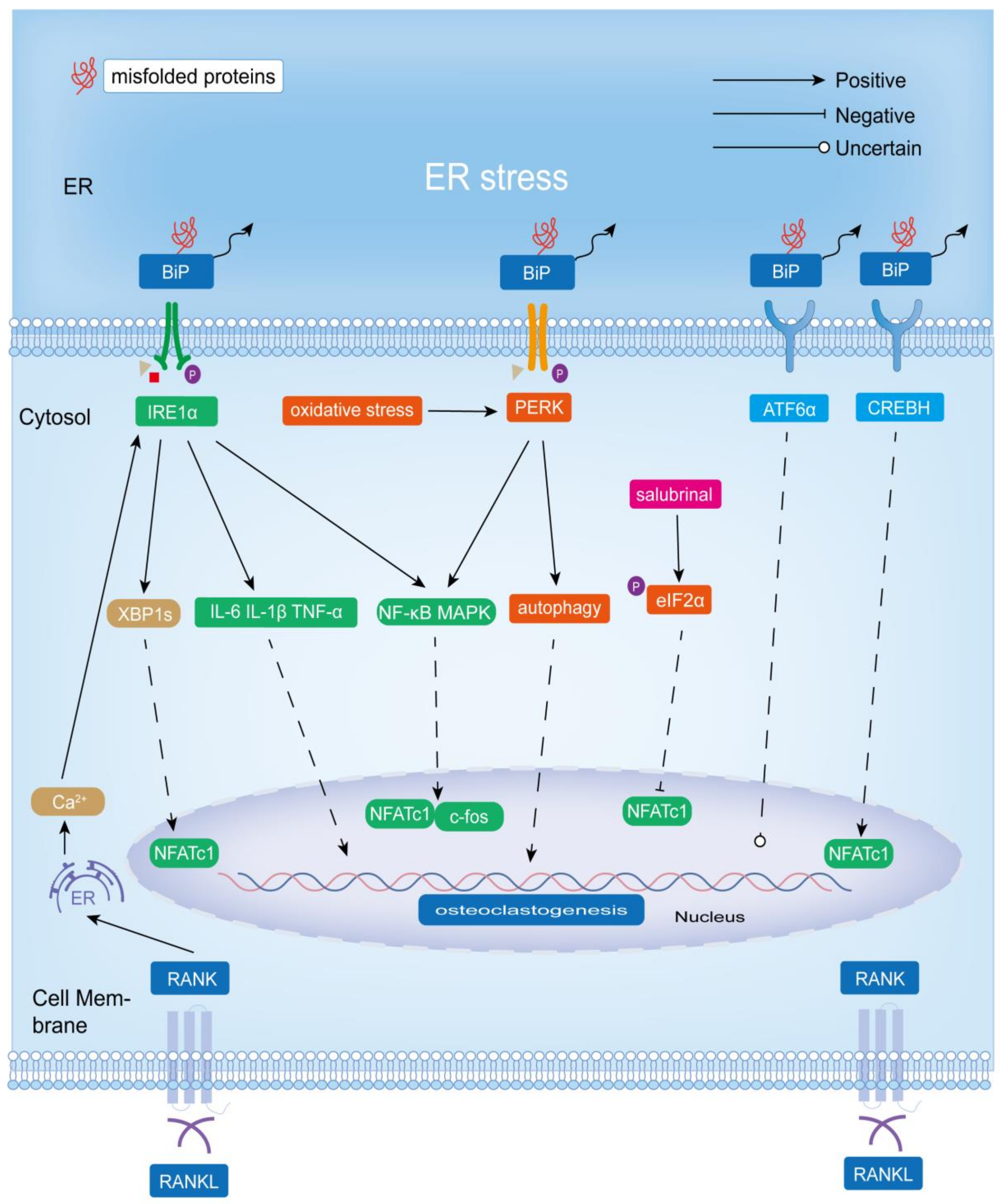
4. The Role of UPR in Osteoclastogenesis and Bone Resorption
4.1. IRE1α-XBP1
4.2. PERK-eIF2α/PERK-NRF2
4.3. ATF6/CREBH
4.4. BIP
4.5. UPR and the Expression of RANKL in Osteoblasts
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, X.; Cubillos-Ruiz, J.R. Endoplasmic reticulum stress signals in the tumour and its microenvironment. Nat. Rev. Cancer 2021, 21, 71–88. [Google Scholar] [CrossRef] [PubMed]
- Ajoolabady, A.; Lebeaupin, C.; Wu, N.N.; Kaufman, R.J.; Ren, J. ER stress and inflammation crosstalk in obesity. Med. Res. Rev. 2023, 43, 5–30. [Google Scholar] [CrossRef]
- Oakes, S.A.; Papa, F.R. The role of endoplasmic reticulum stress in human pathology. Annu. Rev. Pathol. 2015, 10, 173–194. [Google Scholar] [CrossRef] [Green Version]
- Ren, J.; Bi, Y.; Sowers, J.R.; Hetz, C.; Zhang, Y. Endoplasmic reticulum stress and unfolded protein response in cardiovascular diseases. Nat. Rev. Cardiol. 2021, 18, 499–521. [Google Scholar] [CrossRef] [PubMed]
- Hetz, C.; Zhang, K.; Kaufman, R.J. Mechanisms, regulation and functions of the unfolded protein response. Nat. Rev. Mol. Cell Biol. 2020, 21, 421–438. [Google Scholar] [CrossRef] [PubMed]
- Kenkre, J.S.; Bassett, J. The bone remodelling cycle. Ann. Clin. Biochem. 2018, 55, 308–327. [Google Scholar] [CrossRef]
- Schwarz, D.S.; Blower, M.D. The endoplasmic reticulum: Structure, function and response to cellular signaling. Cell. Mol. Life Sci. 2016, 73, 79–94. [Google Scholar] [CrossRef] [Green Version]
- Hotamisligil, G.S.; Davis, R.J. Cell Signaling and Stress Responses. Cold Spring Harb. Perspect Biol. 2016, 8, a006072. [Google Scholar] [CrossRef] [Green Version]
- Jin, J.K.; Blackwood, E.A.; Azizi, K.; Thuerauf, D.J.; Fahem, A.G.; Hofmann, C.; Kaufman, R.J.; Doroudgar, S.; Glembotski, C.C. ATF6 Decreases Myocardial Ischemia/Reperfusion Damage and Links ER Stress and Oxidative Stress Signaling Pathways in the Heart. Circ. Res. 2017, 120, 862–875. [Google Scholar] [CrossRef] [Green Version]
- Mehrtash, A.B.; Hochstrasser, M. Ubiquitin-dependent protein degradation at the endoplasmic reticulum and nuclear envelope. Semin. Cell Dev. Biol. 2019, 93, 111–124. [Google Scholar] [CrossRef]
- Moncan, M.; Mnich, K.; Blomme, A.; Almanza, A.; Samali, A.; Gorman, A.M. Regulation of lipid metabolism by the unfolded protein response. J. Cell. Mol. Med. 2021, 25, 1359–1370. [Google Scholar] [CrossRef]
- McGuckin, M.A.; Eri, R.D.; Das, I.; Lourie, R.; Florin, T.H. ER stress and the unfolded protein response in intestinal inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 298, G820–G832. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Ren, R.; Sun, K.; He, J.; Shao, J. PERK signaling pathway in bone metabolism: Friend or foe? Cell Prolif. 2021, 54, e13011. [Google Scholar] [CrossRef]
- Kaneko, M.; Imaizumi, K.; Saito, A.; Kanemoto, S.; Asada, R.; Matsuhisa, K.; Ohtake, Y. ER Stress and Disease: Toward Prevention and Treatment. Biol. Pharm. Bull. 2017, 40, 1337–1343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tohmonda, T.; Yoda, M.; Mizuochi, H.; Morioka, H.; Matsumoto, M.; Urano, F.; Toyama, Y.; Horiuchi, K. The IRE1alpha-XBP1 pathway positively regulates parathyroid hormone (PTH)/PTH-related peptide receptor expression and is involved in pth-induced osteoclastogenesis. J. Biol. Chem. 2013, 288, 1691–1695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, K.; Kaufman, R.J. From endoplasmic-reticulum stress to the inflammatory response. Nature 2008, 454, 455–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Penaranda Fajardo, N.M.; Meijer, C.; Kruyt, F.A. The endoplasmic reticulum stress/unfolded protein response in gliomagenesis, tumor progression and as a therapeutic target in glioblastoma. Biochem. Pharmacol. 2016, 118, 1–8. [Google Scholar] [CrossRef]
- Bettigole, S.E.; Glimcher, L.H. Endoplasmic reticulum stress in immunity. Annu. Rev. Immunol. 2015, 33, 107–138. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Hui, Z.; Wei, S.; Li, D.; Li, W.; Daping, W.; Alahdal, M. IRE1 signaling regulates chondrocyte apoptosis and death fate in the osteoarthritis. J. Cell. Physiol. 2022, 237, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Raymundo, D.P.; Doultsinos, D.; Guillory, X.; Carlesso, A.; Eriksson, L.A.; Chevet, E. Pharmacological Targeting of IRE1 in Cancer. Trends Cancer 2020, 6, 1018–1030. [Google Scholar] [CrossRef]
- Fedeles, S.V.; So, J.S.; Shrikhande, A.; Lee, S.H.; Gallagher, A.R.; Barkauskas, C.E.; Somlo, S.; Lee, A.H. Sec63 and Xbp1 regulate IRE1alpha activity and polycystic disease severity. J. Clin. Investig. 2015, 125, 1955–1967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frakes, A.E.; Dillin, A. The UPR(ER): Sensor and Coordinator of Organismal Homeostasis. Mol. Cell 2017, 66, 761–771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, A.H.; Iwakoshi, N.N.; Glimcher, L.H. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol. Cell. Biol. 2003, 23, 7448–7459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acosta-Alvear, D.; Zhou, Y.; Blais, A.; Tsikitis, M.; Lents, N.H.; Arias, C.; Lennon, C.J.; Kluger, Y.; Dynlacht, B.D. XBP1 controls diverse cell type- and condition-specific transcriptional regulatory networks. Mol. Cell 2007, 27, 53–66. [Google Scholar] [CrossRef]
- Donnelly, N.; Gorman, A.M.; Gupta, S.; Samali, A. The eIF2alpha kinases: Their structures and functions. Cell. Mol. Life Sci. 2013, 70, 3493–3511. [Google Scholar] [CrossRef]
- Fusakio, M.E.; Willy, J.A.; Wang, Y.; Mirek, E.T.; Al Baghdadi, R.J.; Adams, C.M.; Anthony, T.G.; Wek, R.C. Transcription factor ATF4 directs basal and stress-induced gene expression in the unfolded protein response and cholesterol metabolism in the liver. Mol. Biol. Cell 2016, 27, 1536–1551. [Google Scholar] [CrossRef]
- Horiuchi, K.; Tohmonda, T.; Morioka, H. The unfolded protein response in skeletal development and homeostasis. Cell. Mol. Life Sci. 2016, 73, 2851–2869. [Google Scholar] [CrossRef]
- Ghemrawi, R.; Khair, M. Endoplasmic Reticulum Stress and Unfolded Protein Response in Neurodegenerative Diseases. Int. J. Mol. Sci. 2020, 21, 6127. [Google Scholar] [CrossRef]
- Harding, H.P.; Zhang, Y.; Ron, D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 1999, 397, 271–274. [Google Scholar] [CrossRef]
- Walter, P.; Ron, D. The unfolded protein response: From stress pathway to homeostatic regulation. Science 2011, 334, 1081–1086. [Google Scholar] [CrossRef] [Green Version]
- Ohno, M. Roles of eIF2alpha kinases in the pathogenesis of Alzheimer’s disease. Front. Mol. Neurosci. 2014, 7, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, F.; Ru, X.; Wen, T. NRF2, a Transcription Factor for Stress Response and Beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef] [PubMed]
- Zielke, S.; Kardo, S.; Zein, L.; Mari, M.; Covarrubias-Pinto, A.; Kinzler, M.N.; Meyer, N.; Stolz, A.; Fulda, S.; Reggiori, F.; et al. ATF4 links ER stress with reticulophagy in glioblastoma cells. Autophagy 2021, 17, 2432–2448. [Google Scholar] [CrossRef]
- Balsa, E.; Soustek, M.S.; Thomas, A.; Cogliati, S.; Garcia-Poyatos, C.; Martin-Garcia, E.; Jedrychowski, M.; Gygi, S.P.; Enriquez, J.A.; Puigserver, P. ER and Nutrient Stress Promote Assembly of Respiratory Chain Supercomplexes through the PERK-eIF2alpha Axis. Mol. Cell 2019, 74, 877–890.e6. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Fan, Z.; Rauh, M.; Buchfelder, M.; Eyupoglu, I.Y.; Savaskan, N. ATF4 promotes angiogenesis and neuronal cell death and confers ferroptosis in a xCT-dependent manner. Oncogene 2017, 36, 5593–5608. [Google Scholar] [CrossRef] [Green Version]
- Sharma, R.B.; Darko, C.; Alonso, L.C. Intersection of the ATF6 and XBP1 ER stress pathways in mouse islet cells. J. Biol. Chem. 2020, 295, 14164–14177. [Google Scholar] [CrossRef]
- Hillary, R.F.; FitzGerald, U. A lifetime of stress: ATF6 in development and homeostasis. J. Biomed. Sci. 2018, 25, 48. [Google Scholar] [CrossRef] [Green Version]
- Glembotski, C.C.; Arrieta, A.; Blackwood, E.A.; Stauffer, W.T. ATF6 as a Nodal Regulator of Proteostasis in the Heart. Front. Physiol. 2020, 11, 267. [Google Scholar] [CrossRef] [Green Version]
- Schroder, M.; Kaufman, R.J. ER stress and the unfolded protein response. Mutat. Res. 2005, 569, 29–63. [Google Scholar] [CrossRef]
- Ye, J.; Rawson, R.B.; Komuro, R.; Chen, X.; Dave, U.P.; Prywes, R.; Brown, M.S.; Goldstein, J.L. ER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPs. Mol. Cell 2000, 6, 1355–1364. [Google Scholar] [CrossRef]
- Yoshida, H.; Matsui, T.; Yamamoto, A.; Okada, T.; Mori, K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell 2001, 107, 881–891. [Google Scholar] [CrossRef] [Green Version]
- Adachi, Y.; Yamamoto, K.; Okada, T.; Yoshida, H.; Harada, A.; Mori, K. ATF6 is a transcription factor specializing in the regulation of quality control proteins in the endoplasmic reticulum. Cell Struct. Funct. 2008, 33, 75–89. [Google Scholar] [CrossRef] [Green Version]
- Hetz, C. The unfolded protein response: Controlling cell fate decisions under ER stress and beyond. Nat. Rev. Mol. Cell Biol. 2012, 13, 89–102. [Google Scholar] [CrossRef]
- Clarke, B. Normal bone anatomy and physiology. Clin. J. Am. Soc. Nephrol. 2008, 3 (Suppl. S3), S131–S139. [Google Scholar] [CrossRef] [Green Version]
- Eriksen, E.F. Cellular mechanisms of bone remodeling. Rev. Endocr. Metab. Disord. 2010, 11, 219–227. [Google Scholar] [CrossRef] [Green Version]
- Florencio-Silva, R.; Sasso, G.R.; Sasso-Cerri, E.; Simoes, M.J.; Cerri, P.S. Biology of Bone Tissue: Structure, Function, and Factors That Influence Bone Cells. Biomed. Res. Int. 2015, 2015, 421746. [Google Scholar] [CrossRef] [Green Version]
- Rodan, G.A.; Martin, T.J. Therapeutic approaches to bone diseases. Science 2000, 289, 1508–1514. [Google Scholar] [CrossRef] [PubMed]
- Ponzetti, M.; Rucci, N. Osteoblast Differentiation and Signaling: Established Concepts and Emerging Topics. Int. J. Mol. Sci. 2021, 22, 6651. [Google Scholar] [CrossRef]
- Dirckx, N.; Moorer, M.C.; Clemens, T.L.; Riddle, R.C. The role of osteoblasts in energy homeostasis. Nat. Rev. Endocrinol. 2019, 15, 651–665. [Google Scholar] [CrossRef] [PubMed]
- Dallas, S.L.; Prideaux, M.; Bonewald, L.F. The osteocyte: An endocrine cell … and more. Endocr. Rev. 2013, 34, 658–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delgado-Calle, J.; Bellido, T. The osteocyte as a signaling cell. Physiol. Rev. 2022, 102, 379–410. [Google Scholar] [CrossRef] [PubMed]
- Boyce, B.F. Advances in the regulation of osteoclasts and osteoclast functions. J. Dent. Res. 2013, 92, 860–867. [Google Scholar] [CrossRef] [Green Version]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Udagawa, N.; Koide, M.; Nakamura, M.; Nakamichi, Y.; Yamashita, T.; Uehara, S.; Kobayashi, Y.; Furuya, Y.; Yasuda, H.; Fukuda, C.; et al. Osteoclast differentiation by RANKL and OPG signaling pathways. J. Bone Miner. Metab. 2021, 39, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Rigoni, T.S.; Vellozo, N.S.; Cabral-Piccin, M.; Fabiano-Coelho, L.; Lopes, U.G.; Filardy, A.A.; DosReis, G.A.; Lopes, M.F. RANK Ligand Helps Immunity to Leishmania major by Skewing M2-Like Into M1 Macrophages. Front. Immunol. 2020, 11, 886. [Google Scholar] [CrossRef] [PubMed]
- Sobacchi, C.; Abinun, M. Osteoclast-poor osteopetrosis. Bone 2022, 164, 116541. [Google Scholar] [CrossRef] [PubMed]
- Ono, T.; Hayashi, M.; Sasaki, F.; Nakashima, T. RANKL biology: Bone metabolism, the immune system, and beyond. Inflamm. Regen. 2020, 40, 2. [Google Scholar] [CrossRef] [Green Version]
- Nagy, V.; Penninger, J.M. The RANKL-RANK Story. Gerontology 2015, 61, 534–542. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, S.; Wang, Y.; Yang, Z.; Chen, Z.; Wen, N.; Yang, M.; Huang, Z.; Xie, Y.; Cai, L. ULK1 Suppresses Osteoclast Differentiation and Bone Resorption via Inhibiting Syk-JNK through DOK3. Oxid. Med. Cell. Longev. 2021, 2021, 2896674. [Google Scholar] [CrossRef]
- Mun, S.H.; Park, P.S.U.; Park-Min, K.H. The M-CSF receptor in osteoclasts and beyond. Exp. Mol. Med. 2020, 52, 1239–1254. [Google Scholar] [CrossRef]
- Ikebuchi, Y.; Aoki, S.; Honma, M.; Hayashi, M.; Sugamori, Y.; Khan, M.; Kariya, Y.; Kato, G.; Tabata, Y.; Penninger, J.M.; et al. Coupling of bone resorption and formation by RANKL reverse signalling. Nature 2018, 561, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Lee, N.K.; Lee, S.Y. Current Understanding of RANK Signaling in Osteoclast Differentiation and Maturation. Mol. Cells 2017, 40, 706–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asagiri, M.; Takayanagi, H. The molecular understanding of osteoclast differentiation. Bone 2007, 40, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, A.S., Jr. The NF-kappa B and I kappa B proteins: New discoveries and insights. Annu. Rev. Immunol. 1996, 14, 649–683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soysa, N.S.; Alles, N. NF-kappaB functions in osteoclasts. Biochem. Biophys. Res. Commun. 2009, 378, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Abu-Amer, Y. NF-kappaB signaling and bone resorption. Osteoporos. Int. 2013, 24, 2377–2386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Li, Y.; Peng, X.; Li, B.; Yuan, X.; Chen, Y. Emodin attenuates titanium particle-induced osteolysis and RANKL-mediated osteoclastogenesis through the suppression of IKK phosphorylation. Mol. Immunol. 2018, 96, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, N. Signaling Pathways in Osteoclast Differentiation. Chonnam Med. J. 2016, 52, 12–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wada, T.; Nakashima, T.; Hiroshi, N.; Penninger, J.M. RANKL-RANK signaling in osteoclastogenesis and bone disease. Trends Mol. Med. 2006, 12, 17–25. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, C.; Wang, G.; Sun, Y.; Deng, Z.; Chen, L.; Chen, K.; Tickner, J.; Kenny, J.; Song, D.; et al. Loureirin B suppresses RANKL-induced osteoclastogenesis and ovariectomized osteoporosis via attenuating NFATc1 and ROS activities. Theranostics 2019, 9, 4648–4662. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, X.; Liu, Y.; He, A.; Jia, R. NFATc1: Functions in osteoclasts. Int. J. Biochem. Cell Biol. 2010, 42, 576–579. [Google Scholar] [CrossRef] [PubMed]
- Herb, M.; Schramm, M. Functions of ROS in Macrophages and Antimicrobial Immunity. Antioxidants 2021, 10, 313. [Google Scholar] [CrossRef] [PubMed]
- Fukai, T.; Ushio-Fukai, M. Cross-Talk between NADPH Oxidase and Mitochondria: Role in ROS Signaling and Angiogenesis. Cells 2020, 9, 1849. [Google Scholar] [CrossRef] [PubMed]
- Tai, T.W.; Chen, C.Y.; Su, F.C.; Tu, Y.K.; Tsai, T.T.; Lin, C.F.; Jou, I.M. Reactive oxygen species are required for zoledronic acid-induced apoptosis in osteoclast precursors and mature osteoclast-like cells. Sci. Rep. 2017, 7, 44245. [Google Scholar] [CrossRef]
- Agidigbi, T.S.; Kim, C. Reactive Oxygen Species in Osteoclast Differentiation and Possible Pharmaceutical Targets of ROS-Mediated Osteoclast Diseases. Int. J. Mol. Sci. 2019, 20, 3576. [Google Scholar] [CrossRef] [Green Version]
- Tao, H.; Ge, G.; Liang, X.; Zhang, W.; Sun, H.; Li, M.; Geng, D. ROS signaling cascades: Dual regulations for osteoclast and osteoblast. Acta Biochim. Biophys. Sin. 2020, 52, 1055–1062. [Google Scholar] [CrossRef]
- Badila, A.E.; Radulescu, D.M.; Ilie, A.; Niculescu, A.G.; Grumezescu, A.M.; Radulescu, A.R. Bone Regeneration and Oxidative Stress: An Updated Overview. Antioxidants 2022, 11, 318. [Google Scholar] [CrossRef]
- Ha, H.; Kwak, H.B.; Lee, S.W.; Jin, H.M.; Kim, H.M.; Kim, H.H.; Lee, Z.H. Reactive oxygen species mediate RANK signaling in osteoclasts. Exp. Cell Res. 2004, 301, 119–127. [Google Scholar] [CrossRef]
- Sun, Y.X.; Xu, A.H.; Yang, Y.; Li, J. Role of Nrf2 in bone metabolism. J. Biomed. Sci. 2015, 22, 101. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.Y.; Kang, N.; Yang, Y.M.; Hong, J.H.; Shin, D.M. The Role of Ca(2+)-NFATc1 Signaling and Its Modulation on Osteoclastogenesis. Int. J. Mol. Sci. 2020, 21, 3646. [Google Scholar] [CrossRef]
- Macian, F. NFAT proteins: Key regulators of T-cell development and function. Nat. Rev. Immunol. 2005, 5, 472–484. [Google Scholar] [CrossRef]
- Kanemoto, S.; Kobayashi, Y.; Yamashita, T.; Miyamoto, T.; Cui, M.; Asada, R.; Cui, X.; Hino, K.; Kaneko, M.; Takai, T.; et al. Luman is involved in osteoclastogenesis through the regulation of DC-STAMP expression, stability and localization. J. Cell Sci. 2015, 128, 4353–4365. [Google Scholar] [CrossRef] [Green Version]
- Tohmonda, T.; Yoda, M.; Iwawaki, T.; Matsumoto, M.; Nakamura, M.; Mikoshiba, K.; Toyama, Y.; Horiuchi, K. IRE1alpha/XBP1-mediated branch of the unfolded protein response regulates osteoclastogenesis. J. Clin. Investig. 2015, 125, 3269–3279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choe, C.U.; Ehrlich, B.E. The inositol 1,4,5-trisphosphate receptor (IP3R) and its regulators: Sometimes good and sometimes bad teamwork. Sci. STKE 2006, 2006, re15. [Google Scholar] [CrossRef] [PubMed]
- Krebs, J.; Agellon, L.B.; Michalak, M. Ca(2+) homeostasis and endoplasmic reticulum (ER) stress: An integrated view of calcium signaling. Biochem. Biophys. Res. Commun. 2015, 460, 114–121. [Google Scholar] [CrossRef]
- Jaskulska, A.; Janecka, A.E.; Gach-Janczak, K. Thapsigargin-From Traditional Medicine to Anticancer Drug. Int. J. Mol. Sci. 2020, 22, 4. [Google Scholar] [CrossRef]
- Lee, E.G.; Sung, M.S.; Yoo, H.G.; Chae, H.J.; Kim, H.R.; Yoo, W.H. Increased RANKL-mediated osteoclastogenesis by interleukin-1beta and endoplasmic reticulum stress. Jt. Bone Spine 2014, 81, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.S.; Jeong, J.H.; Lee, E.G.; Choi, Y.; Kim, J.H.; Kim, H.R.; Yoo, W.H. Tacrolimus regulates endoplasmic reticulum stress-mediated osteoclastogenesis and inflammation: In vitro and collagen-induced arthritis mouse model. Cell Biol. Int. 2018, 42, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Niu, J.; Kim, H.; Kolattukudy, P.E. Osteoclast precursor differentiation by MCPIP via oxidative stress, endoplasmic reticulum stress, and autophagy. J. Mol. Cell Biol. 2011, 3, 360–368. [Google Scholar] [CrossRef]
- Yang, F.; Wu, W.; Cao, L.; Huang, Y.; Zhu, Z.; Tang, T.; Dai, K. Pathways of macrophage apoptosis within the interface membrane in aseptic loosening of prostheses. Biomaterials 2011, 32, 9159–9167. [Google Scholar] [CrossRef]
- Zhang, L.; Tian, Z.; Li, W.; Wang, X.; Man, Z.; Sun, S. Inhibitory effect of quercetin on titanium particle-induced endoplasmic reticulum stress (ERS)-related apoptosis and in vivoosteolysis. Biosci. Rep. 2017, 37, BSR20170961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Bao, D.; Li, P.; Lu, Z.; Pang, L.; Chen, Z.; Guo, H.; Gao, Z.; Jin, Q. Particle-induced SIRT1 downregulation promotes osteoclastogenesis and osteolysis through ER stress regulation. Biomed. Pharmacother. 2018, 104, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Doan, N.T.; Paulsen, E.S.; Sehgal, P.; Moller, J.V.; Nissen, P.; Denmeade, S.R.; Isaacs, J.T.; Dionne, C.A.; Christensen, S.B. Targeting thapsigargin towards tumors. Steroids 2015, 97, 2–7. [Google Scholar] [CrossRef] [Green Version]
- Abdullahi, A.; Stanojcic, M.; Parousis, A.; Patsouris, D.; Jeschke, M.G. Modeling Acute ER Stress in Vivo and in Vitro. Shock 2017, 47, 506–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dawood, A.A.; Altobje, M.A. Inhibition of N-linked Glycosylation by Tunicamycin May Contribute to The Treatment of SARS-CoV-2. Microb. Pathog. 2020, 149, 104586. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.S.; Aryal, Y.P.; Kim, T.Y.; Pokharel, E.; Kim, J.Y.; Yamamoto, H.; An, C.H.; An, S.Y.; Jung, J.K.; Lee, Y.; et al. The effects of 4-Phenylbutyric acid on ER stress during mouse tooth development. Front. Physiol. 2022, 13, 1079355. [Google Scholar] [CrossRef]
- Chen, S.Z.; Ling, Y.; Yu, L.X.; Song, Y.T.; Chen, X.F.; Cao, Q.Q.; Yu, H.; Chen, C.; Tang, J.J.; Fan, Z.C.; et al. 4-phenylbutyric acid promotes hepatocellular carcinoma via initiating cancer stem cells through activation of PPAR-alpha. Clin. Transl. Med. 2021, 11, e379. [Google Scholar] [CrossRef]
- Yamada, H.; Nakajima, T.; Domon, H.; Honda, T.; Yamazaki, K. Endoplasmic reticulum stress response and bone loss in experimental periodontitis in mice. J. Periodontal Res. 2015, 50, 500–508. [Google Scholar] [CrossRef]
- Khalaf, K.; Tornese, P.; Cocco, A.; Albanese, A. Tauroursodeoxycholic acid: A potential therapeutic tool in neurodegenerative diseases. Transl. Neurodegener. 2022, 11, 33. [Google Scholar] [CrossRef]
- Hamamura, K.; Tanjung, N.; Yokota, H. Suppression of osteoclastogenesis through phosphorylation of eukaryotic translation initiation factor 2 alpha. J. Bone Miner. Metab. 2013, 31, 618–628. [Google Scholar] [CrossRef]
- Benmerzouga, I.; Checkley, L.A.; Ferdig, M.T.; Arrizabalaga, G.; Wek, R.C.; Sullivan, W.J., Jr. Guanabenz repurposed as an antiparasitic with activity against acute and latent toxoplasmosis. Antimicrob. Agents Chemother. 2015, 59, 6939–6945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Li, Z.; Wang, G.; Sun, Y.; Yuan, Y.; Nie, H. Salubrinal Alleviates Collagen-Induced Arthritis through Promoting P65 Degradation in Osteoclastogenesis. Int. J. Mol. Sci. 2021, 22, 3501. [Google Scholar] [CrossRef]
- Liu, G.; Liu, N.; Xu, Y.; Ti, Y.; Chen, J.; Chen, J.; Zhang, J.; Zhao, J. Endoplasmic reticulum stress-mediated inflammatory signaling pathways within the osteolytic periosteum and interface membrane in particle-induced osteolysis. Cell Tissue Res. 2016, 363, 427–447. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Wang, Z.; Ma, Y.; Liu, G.; Shi, H.; Chen, J.; Dong, L.; Zhao, J.; Zhang, J. Particle-induced osteolysis mediated by endoplasmic reticulum stress in prosthesis loosening. Biomaterials 2013, 34, 2611–2623. [Google Scholar] [CrossRef]
- Chen, S.; Chen, J.; Hua, X.; Sun, Y.; Cui, R.; Sha, J.; Zhu, X. The emerging role of XBP1 in cancer. Biomed. Pharm. 2020, 127, 110069. [Google Scholar] [CrossRef] [PubMed]
- Raimondi, L.; De Luca, A.; Fontana, S.; Amodio, N.; Costa, V.; Carina, V.; Bellavia, D.; Raimondo, S.; Siragusa, S.; Monteleone, F.; et al. Multiple Myeloma-Derived Extracellular Vesicles Induce Osteoclastogenesis through the Activation of the XBP1/IRE1alpha Axis. Cancers 2020, 12, 2167. [Google Scholar] [CrossRef]
- Guo, J.; Ren, R.; Sun, K.; Yao, X.; Lin, J.; Wang, G.; Guo, Z.; Xu, T.; Guo, F. PERK controls bone homeostasis through the regulation of osteoclast differentiation and function. Cell Death Dis. 2020, 11, 847. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.; Zhang, Y.; Yan, M.; Mao, H.; Pan, C.; Gan, M.; Fan, J.; Wang, G. Inhibition of osteolysis after local administration of osthole in a TCP particles-induced osteolysis model. Int. Orthop. 2016, 40, 1545–1552. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Dong, F.; Yang, P.; Wang, Z.; Yan, M.; Fang, J.; Zhang, Y. Boric Acid Inhibits RANKL-Stimulated Osteoclastogenesis In Vitro and Attenuates LPS-Induced Bone Loss In Vivo. Biol. Trace Elem. Res. 2022, 201, 1388–1397. [Google Scholar] [CrossRef]
- Li, J.; Yang, S.; Li, X.; Liu, D.; Wang, Z.; Guo, J.; Tan, N.; Gao, Z.; Zhao, X.; Zhang, J.; et al. Role of endoplasmic reticulum stress in disuse osteoporosis. Bone 2017, 97, 2–14. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Li, X.; Liu, D.; Hamamura, K.; Wan, Q.; Na, S.; Yokota, H.; Zhang, P. eIF2alpha signaling regulates autophagy of osteoblasts and the development of osteoclasts in OVX mice. Cell Death Dis. 2019, 10, 921. [Google Scholar] [CrossRef] [Green Version]
- Kimura, F.; Miyazawa, K.; Hamamura, K.; Tabuchi, M.; Sato, T.; Asano, Y.; Kako, S.; Aoki, Y.; Sugita, Y.; Maeda, H.; et al. Suppression of alveolar bone resorption by salubrinal in a mouse model of periodontal disease. Life Sci. 2021, 284, 119938. [Google Scholar] [CrossRef]
- Takigawa, S.; Frondorf, B.; Liu, S.; Liu, Y.; Li, B.; Sudo, A.; Wallace, J.M.; Yokota, H.; Hamamura, K. Salubrinal improves mechanical properties of the femur in osteogenesis imperfecta mice. J. Pharmacol. Sci. 2016, 132, 154–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamamura, K.; Chen, A.; Tanjung, N.; Takigawa, S.; Sudo, A.; Yokota, H. In vitro and in silico analysis of an inhibitory mechanism of osteoclastogenesis by salubrinal and guanabenz. Cell. Signal. 2015, 27, 353–362. [Google Scholar] [CrossRef]
- Hamamura, K.; Chen, A.; Yokota, H. Enhancement of osteoblastogenesis and suppression of osteoclastogenesis by inhibition of de-phosphorylation of eukaryotic translation initiation factor 2 alpha. Recept. Clin. Investig. 2015, 2, e493. [Google Scholar] [CrossRef]
- Yokota, H.; Hamamura, K.; Chen, A.; Dodge, T.R.; Tanjung, N.; Abedinpoor, A.; Zhang, P. Effects of salubrinal on development of osteoclasts and osteoblasts from bone marrow-derived cells. BMC Musculoskelet. Disord. 2013, 14, 197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, L.; Lee, J.; Jang, J.H.; Sakchaisri, K.; Hwang, J.; Cha-Molstad, H.J.; Kim, K.A.; Ryoo, I.J.; Lee, H.G.; Kim, S.O.; et al. Osteoporosis regulation by salubrinal through eIF2alpha mediated differentiation of osteoclast and osteoblast. Cell. Signal. 2013, 25, 552–560. [Google Scholar] [CrossRef] [Green Version]
- Sun, F.; Li, X.; Yang, C.; Lv, P.; Li, G.; Xu, H. A role for PERK in the mechanism underlying fluoride-induced bone turnover. Toxicology 2014, 325, 52–66. [Google Scholar] [CrossRef]
- Lu, P.; Li, X.; Ruan, L.; Xu, H.; Liu, Q. Effect of siRNA PERK on fluoride-induced osteoblastic differentiation in OS732 cells. Biol. Trace Elem. Res. 2014, 159, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.; Baird, T.D.; Zhou, D.; Palam, L.R.; Spandau, D.F.; Wek, R.C. Both transcriptional regulation and translational control of ATF4 are central to the integrated stress response. J. Biol. Chem. 2010, 285, 33165–33174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Z.; Zhang, X.; Huang, B.; Liu, J.; Wei, X.; Shan, Z.; Wu, H.; Feng, Z.; Chen, Y.; Fan, S.; et al. Site-1 protease controls osteoclastogenesis by mediating LC3 transcription. Cell Death Differ. 2021, 28, 2001–2018. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, K.; Kim, I.; Seong, S.; Nam, K.I.; Kim, K.K.; Kim, N. Endoplasmic Reticulum-Bound Transcription Factor CREBH Stimulates RANKL-Induced Osteoclastogenesis. J. Immunol. 2018, 200, 1661–1670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Copley, S.D. Moonlighting is mainstream: Paradigm adjustment required. Bioessays 2012, 34, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Zaiss, M.M.; Hall, C.; McGowan, N.W.A.; Babb, R.; Devlia, V.; Lucas, S.; Meghji, S.; Henderson, B.; Bozec, A.; Schett, G.; et al. Binding Immunoglobulin Protein (BIP) Inhibits TNF-alpha-Induced Osteoclast Differentiation and Systemic Bone Loss in an Erosive Arthritis Model. ACR Open Rheumatol. 2019, 1, 382–393. [Google Scholar] [CrossRef] [Green Version]
- Iyer, S.; Melendez-Suchi, C.; Han, L.; Baldini, G.; Almeida, M.; Jilka, R.L. Elevation of the unfolded protein response increases RANKL expression. FASEB Bioadv. 2020, 2, 207–218. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Yang, M.; Fan, J.; Peng, Y.; Deng, L.; Ding, Y.; Yang, R.; Zhou, J.; Miao, D.; Fu, Q. Deficiency of osteoblastic Arl6ip5 impaired osteoblast differentiation and enhanced osteoclastogenesis via disturbance of ER calcium homeostasis and induction of ER stress-mediated apoptosis. Cell Death Dis. 2014, 5, e1464. [Google Scholar] [CrossRef] [Green Version]
| Compound | Concentration | Role in ER Stress | Mechanism of Action | Other Activities | References |
|---|---|---|---|---|---|
| thapsigargin | 0.5 nM | ER stress inducer | an inhibitor of the sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) pump | initiation of ER stress and stress-induced apoptosis by inhibition of the SERCA pump; anti-tumor activity | [86,93] |
| tunicamycin | 100 ng/mL | ER stress inducer | an inhibitor of protein glycosylation | induced apoptosis in colon or prostate cancer cells; inhibited the glycoprotein of coronavirus | [94,95] |
| 4-Phenylbutyric acid | 20 mM (in vivo) 1 and 2 mM (in vitro) | ER stress inhibitor | chemical chaperone (alleviating ER stress and preventing UPR dysfunction by enhancing protein folding capacity) | ammonia clearance; a histone deacetylase inhibitor | [96,97,98] |
| tauroursodeoxycholic acid | / | ER stress inhibitor | chemical chaperone | reduced oxidative stress; regulated and inhibited the apoptotic cascade response; protected mitochondria; reduced the inflammatory response | [99] |
| guanabenz | 20 μM | inhibitor of dephosphorylation of eIF2α | interacting with protein phosphatase 1 (PP1) | treatment of hypertension (an agonist of α2 adrenergic receptors); antiparasitic (interfering with translation control) | [100,101] |
| salubrinal | 1 and 2 mg/kg (in vivo) 1, 2, 5, 10, 20, and 30 μM (in vitro) | inhibitor of dephosphorylation of eIF2α | interacting with protein phosphatase 1 (PP1) | suppressed inflammation (by inhibiting dual-specificity phosphatase 2) | [100,102] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, W.; Gong, Y.; Yan, L. ER Stress, the Unfolded Protein Response and Osteoclastogenesis: A Review. Biomolecules 2023, 13, 1050. https://doi.org/10.3390/biom13071050
Huang W, Gong Y, Yan L. ER Stress, the Unfolded Protein Response and Osteoclastogenesis: A Review. Biomolecules. 2023; 13(7):1050. https://doi.org/10.3390/biom13071050
Chicago/Turabian StyleHuang, Wangli, Yining Gong, and Liang Yan. 2023. "ER Stress, the Unfolded Protein Response and Osteoclastogenesis: A Review" Biomolecules 13, no. 7: 1050. https://doi.org/10.3390/biom13071050
APA StyleHuang, W., Gong, Y., & Yan, L. (2023). ER Stress, the Unfolded Protein Response and Osteoclastogenesis: A Review. Biomolecules, 13(7), 1050. https://doi.org/10.3390/biom13071050






