Tanshinone IIA and Cryptotanshinone Counteract Inflammation by Regulating Gene and miRNA Expression in Human SGBS Adipocytes
Abstract
1. Introduction
2. Materials and Methods
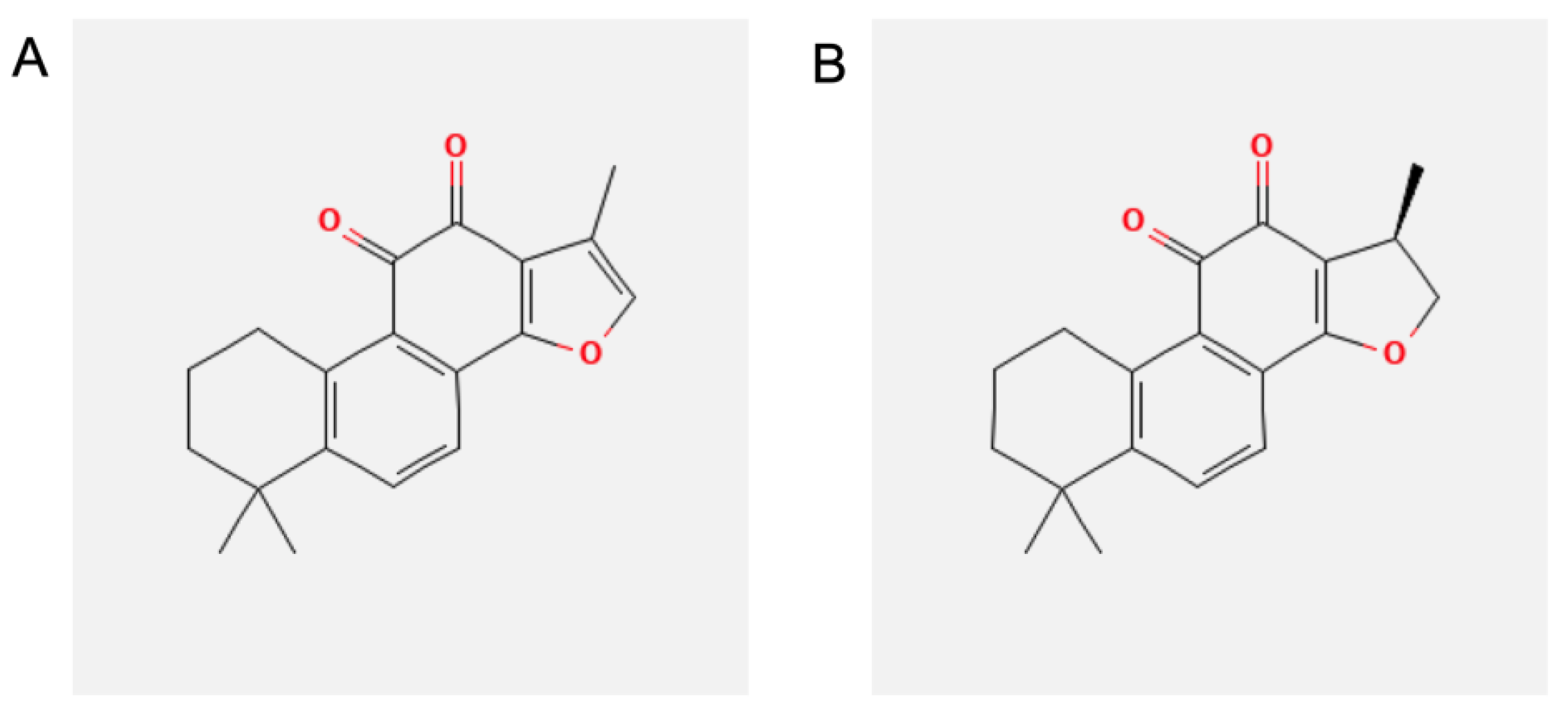
2.1. Materials
2.2. Cell Cultures and Treatments
2.3. Cell Viability
2.4. RNA Isolation and Real-Time Quantitative Polymerase Chain Reaction
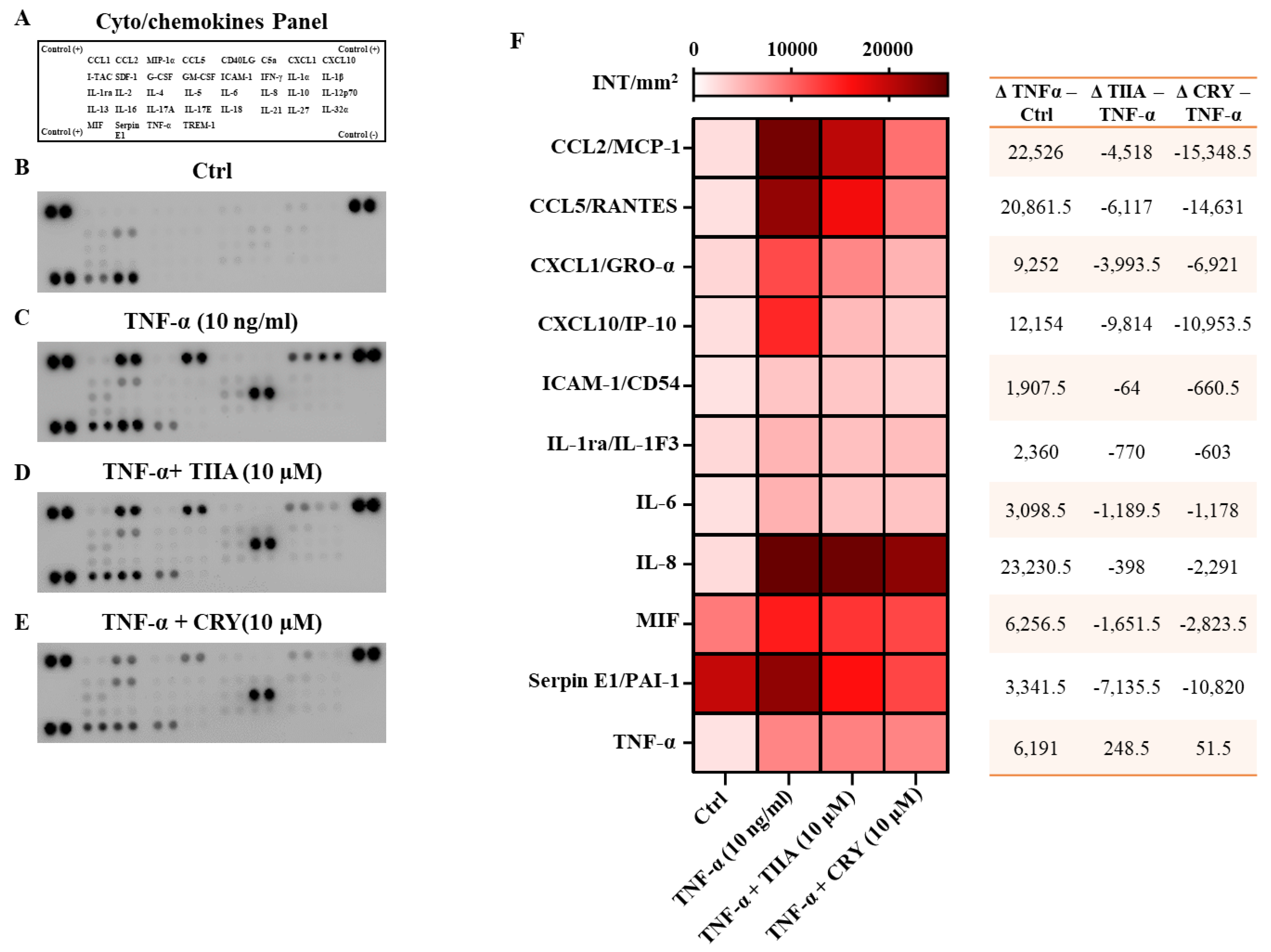
2.5. Cytokines and Chemokines Protein Array
2.6. Evaluation of miRNAs Expression in Adipocytes
2.7. miRNA Target Prediction and Pathway Analysis
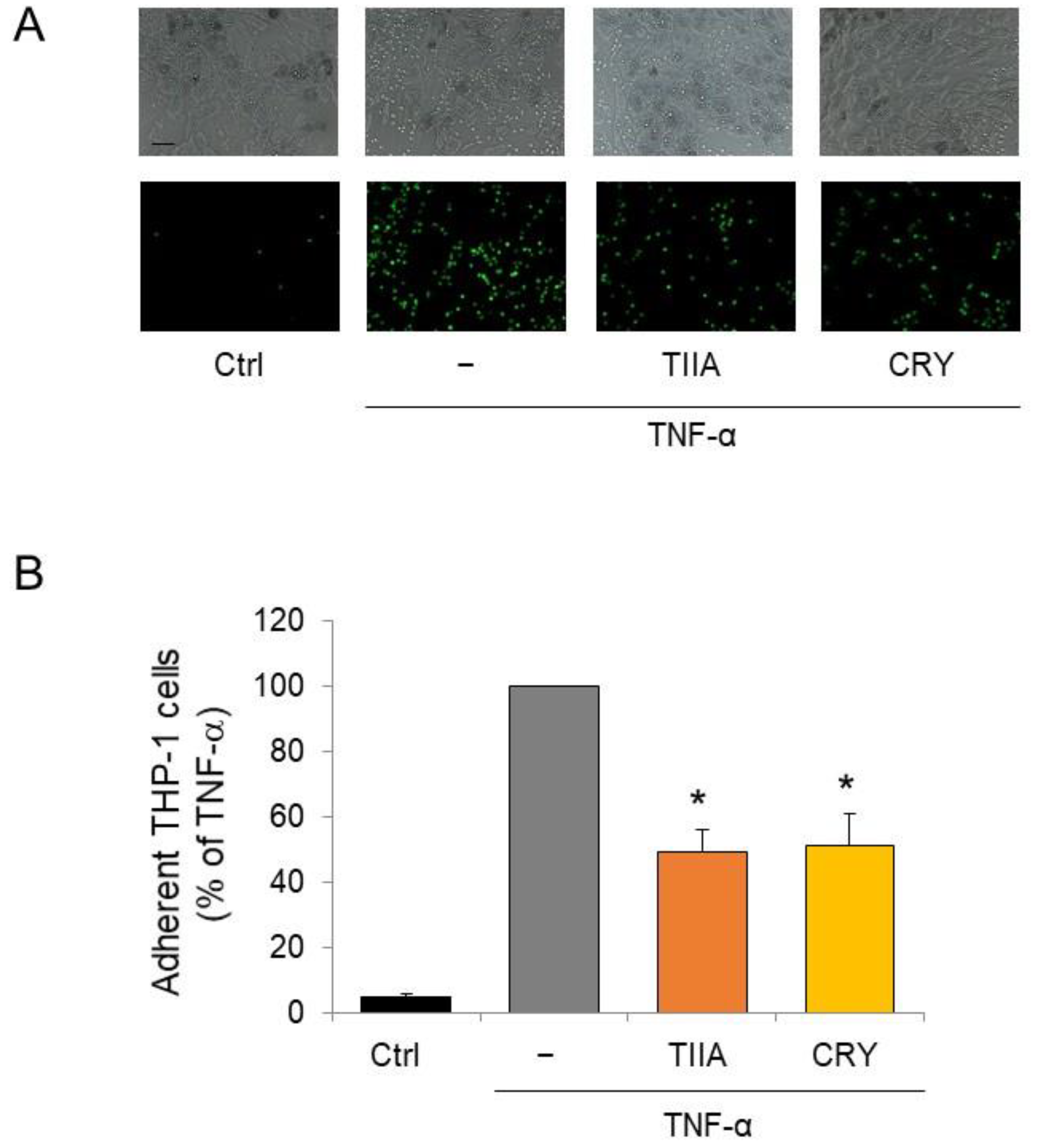
2.8. Leukocyte-Adipocyte Adhesion Assay
2.9. Statistical Analysis
3. Results
3.1. Effect of TIIA and CRY on SGBS Adipocytes Viability
3.2. TIIA and CRY Decrease the Release of Cyto/Chemokines after TNF−α Stimulation
3.3. TIIA and CRY Attenuate Monocyte Adhesion to Inflamed Adipocytes
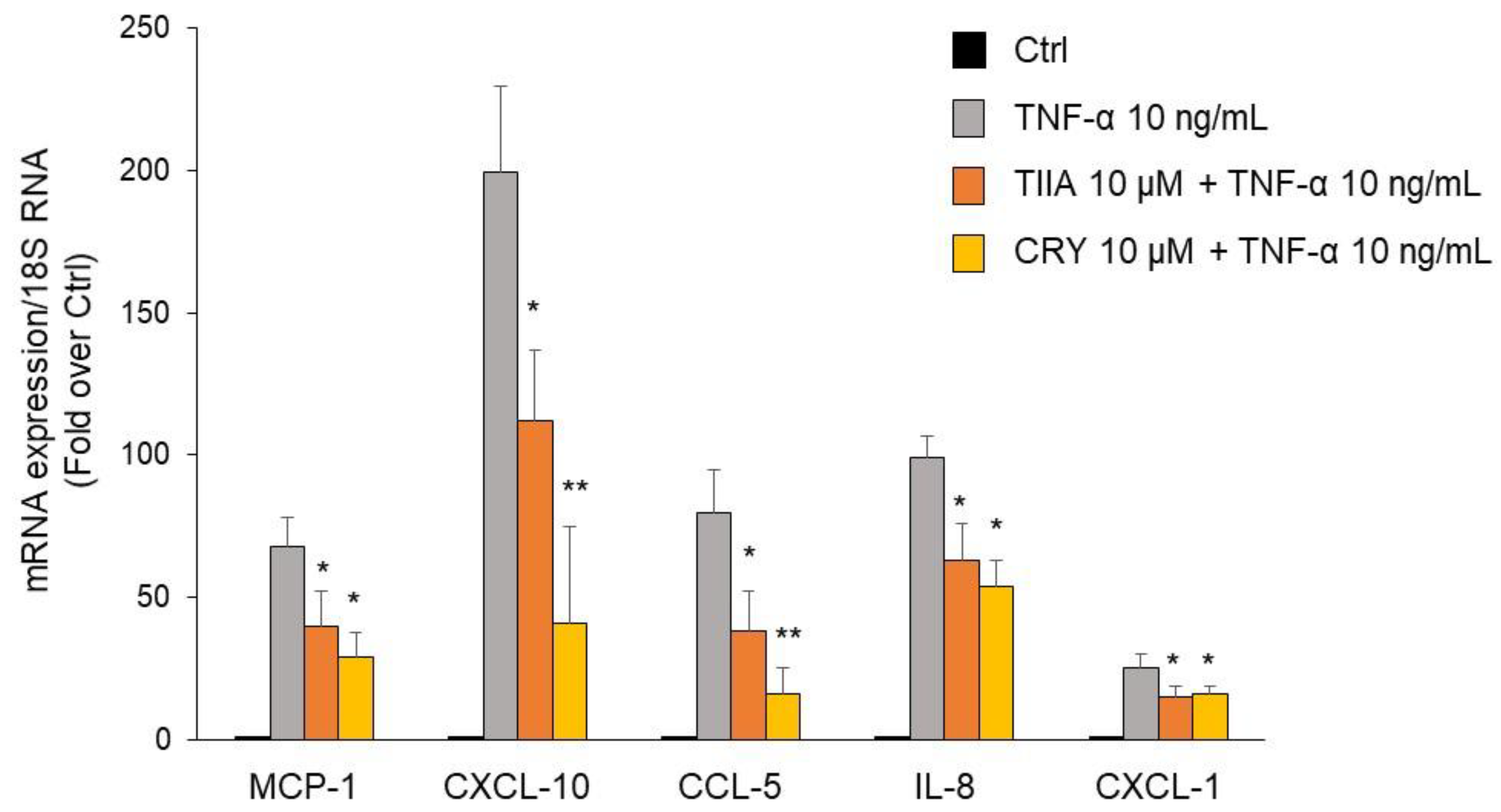
3.4. TIIA and CRY attenuate TNF-α–Mediated Inflammatory Gene Expression in Human Adipocytes
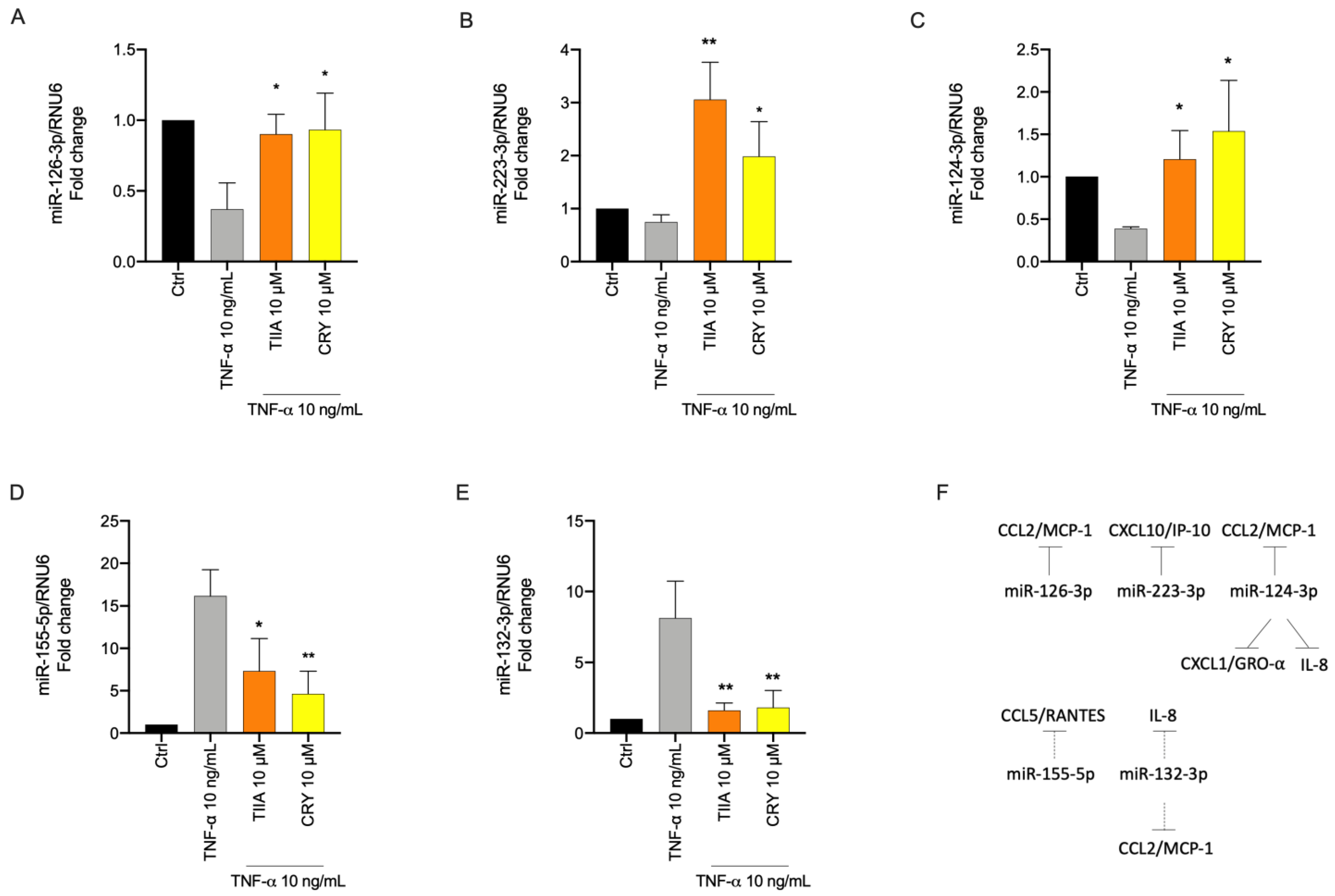
3.5. TIIA and CRY Modulate TNF-α-Induced Inflammation-Linked miRNAs Expression in Adipocytes
3.6. Biological Processes Associated to miRNAs Modulated by TIIA and CRY in Inflamed Adipocytes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tchernof, A.; Després, J.-P. Pathophysiology of Human Visceral Obesity: An Update. Physiol. Rev. 2013, 93, 359–404. [Google Scholar] [CrossRef]
- Berrington de Gonzalez, A.; Hartge, P.; Cerhan, J.R.; Flint, A.J.; Hannan, L.; MacInnis, R.J.; Moore, S.C.; Tobias, G.S.; Anton-Culver, H.; Freeman, L.B.; et al. Body-Mass Index and Mortality among 1.46 Million White Adults. N. Engl. J. Med. 2010, 363, 2211–2219. [Google Scholar] [CrossRef]
- LeRoith, D.; Novosyadlyy, R.; Gallagher, E.; Lann, D.; Vijayakumar, A.; Yakar, S. Obesity and Type 2 Diabetes Are Associated with an Increased Risk of Developing Cancer and a Worse Prognosis; Epidemiological and Mechanistic Evidence. Exp. Clin. Endocrinol. Diabetes 2008, 116, S4–S6. [Google Scholar] [CrossRef] [PubMed]
- Hammarstedt, A.; Gogg, S.; Hedjazifar, S.; Nerstedt, A.; Smith, U. Impaired Adipogenesis and Dysfunctional Adipose Tissue in Human Hypertrophic Obesity. Physiol. Rev. 2018, 98, 1911–1941. [Google Scholar] [CrossRef] [PubMed]
- Van Gaal, L.F.; Mertens, I.L.; De Block, C.E. Mechanisms Linking Obesity with Cardiovascular Disease. Nature 2006, 444, 875–880. [Google Scholar] [CrossRef]
- Scully, T.; Ettela, A.; LeRoith, D.; Gallagher, E.J. Obesity, Type 2 Diabetes, and Cancer Risk. Front. Oncol. 2021, 10, 615375. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Wollam, J.; Olefsky, J.M. An Integrated View of Immunometabolism. Cell 2018, 172, 22–40. [Google Scholar] [CrossRef]
- Chatzigeorgiou, A.; Karalis, K.P.; Bornstein, S.R.; Chavakis, T. Lymphocytes in Obesity-Related Adipose Tissue Inflammation. Diabetologia 2012, 55, 2583–2592. [Google Scholar] [CrossRef]
- Lumeng, C.N.; Bodzin, J.L.; Saltiel, A.R. Obesity Induces a Phenotypic Switch in Adipose Tissue Macrophage Polarization. J. Clin. Investig. 2007, 117, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Grijalva, A.; Skowronski, A.; van Eijk, M.; Serlie, M.J.; Ferrante, A.W. Obesity Activates a Program of Lysosomal-Dependent Lipid Metabolism in Adipose Tissue Macrophages Independently of Classic Activation. Cell Metab. 2013, 18, 816–830. [Google Scholar] [CrossRef]
- Kanda, H. MCP-1 Contributes to Macrophage Infiltration into Adipose Tissue, Insulin Resistance, and Hepatic Steatosis in Obesity. J. Clin. Investig. 2006, 116, 1494–1505. [Google Scholar] [CrossRef] [PubMed]
- Kamei, N.; Tobe, K.; Suzuki, R.; Ohsugi, M.; Watanabe, T.; Kubota, N.; Ohtsuka-Kowatari, N.; Kumagai, K.; Sakamoto, K.; Kobayashi, M.; et al. Overexpression of Monocyte Chemoattractant Protein-1 in Adipose Tissues Causes Macrophage Recruitment and Insulin Resistance. J. Biol. Chem. 2006, 281, 26602–26614. [Google Scholar] [CrossRef]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W. Obesity Is Associated with Macrophage Accumulation in Adipose Tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef] [PubMed]
- Lackey, D.E.; Olefsky, J.M. Regulation of Metabolism by the Innate Immune System. Nat. Rev. Endocrinol. 2016, 12, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Han, M.S.; Jung, D.Y.; Morel, C.; Lakhani, S.A.; Kim, J.K.; Flavell, R.A.; Davis, R.J. JNK Expression by Macrophages Promotes Obesity-Induced Insulin Resistance and Inflammation. Science 2013, 339, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Chiang, S.-H.; Bazuine, M.; Lumeng, C.N.; Geletka, L.M.; Mowers, J.; White, N.M.; Ma, J.-T.; Zhou, J.; Qi, N.; Westcott, D.; et al. The Protein Kinase IKKɛ Regulates Energy Balance in Obese Mice. Cell 2009, 138, 961–975. [Google Scholar] [CrossRef] [PubMed]
- Goldfine, A.B.; Conlin, P.R.; Halperin, F.; Koska, J.; Permana, P.; Schwenke, D.; Shoelson, S.E.; Reaven, P.D. A Randomised Trial of Salsalate for Insulin Resistance and Cardiovascular Risk Factors in Persons with Abnormal Glucose Tolerance. Diabetologia 2013, 56, 714–723. [Google Scholar] [CrossRef] [PubMed]
- Di Prospero, N.A.; Artis, E.; Andrade-Gordon, P.; Johnson, D.L.; Vaccaro, N.; Xi, L.; Rothenberg, P. CCR2 Antagonism in Patients with Type 2 Diabetes Mellitus: A Randomized, Placebo-Controlled Study. Diabetes Obes. Metab. 2014, 16, 1055–1064. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Ohishi, T.; Fukutomi, R.; Shoji, Y.; Goto, S.; Isemura, M. The Beneficial Effects of Principal Polyphenols from Green Tea, Coffee, Wine, and Curry on Obesity. Molecules 2021, 26, 453. [Google Scholar] [CrossRef]
- Bhardwaj, M.; Yadav, P.; Vashishth, D.; Sharma, K.; Kumar, A.; Chahal, J.; Dalal, S.; Kataria, S.K. A Review on Obesity Management through Natural Compounds and a Green Nanomedicine-Based Approach. Molecules 2021, 26, 3278. [Google Scholar] [CrossRef] [PubMed]
- Li, X.W.; Ian, C.H. (Eds.) . Flora of China; Science Press China: Beijing, China, 1994; p. 213. [Google Scholar]
- Pharmacopoeia Committee of the People’s Republic of China; Chemical Industry Press: Beijing, China, 2005.
- Zhang, S.; Huang, G.; Yuan, K.; Zhu, Q.; Sheng, H.; Yu, R.; Luo, G.; Xu, A. Tanshinone IIA Ameliorates Chronic Arthritis in Mice by Modulating Neutrophil Activities. Clin. Exp. Immunol. 2017, 190, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Zheng, W.; Li, X.; Lin, J.; Xie, C.; Li, H.; Cheng, L.; Wu, A.; Ni, W. Cryptotanshinone Protects against IL-1β-Induced Inflammation in Human Osteoarthritis Chondrocytes and Ameliorates the Progression of Osteoarthritis in Mice. Int. Immunopharmacol 2017, 50, 161–167. [Google Scholar] [CrossRef]
- Bonito, M.C.; Cicala, C.; Marcotullio, M.C.; Maione, F.; Mascolo, N. Biological Activity of Bicyclic and Tricyclic Diterpenoids from Salvia Species of Immediate Pharmacological and Pharmaceutical Interest. Nat. Prod. Commun. 2011, 6, 1205–1215. [Google Scholar] [CrossRef]
- Maione, F.; De Feo, V.; Caiazzo, E.; De Martino, L.; Cicala, C.; Mascolo, N. Tanshinone IIA, a Major Component of Salvia Milthorriza Bunge, Inhibits Platelet Activation via Erk-2 Signaling Pathway. J. Ethnopharmacol. 2014, 155, 1236–1242. [Google Scholar] [CrossRef] [PubMed]
- Maione, F.; Piccolo, M.; de Vita, S.; Chini, M.G.; Cristiano, C.; de Caro, C.; Lippiello, P.; Miniaci, M.C.; Santamaria, R.; Irace, C.; et al. Down Regulation of Pro-Inflammatory Pathways by Tanshinone IIA and Cryptotanshinone in a Non-Genetic Mouse Model of Alzheimer’s Disease. Pharm. Res. 2018, 129, 482–490. [Google Scholar] [CrossRef]
- Zhang, X.-Z.; Qian, S.-S.; Zhang, Y.-J.; Wang, R.-Q. Salvia Miltiorrhiza: A Source for Anti-Alzheimer’s Disease Drugs. Pharm. Biol. 2016, 54, 18–24. [Google Scholar] [CrossRef]
- Chen, X.; Guo, J.; Bao, J.; Lu, J.; Wang, Y. The Anticancer Properties of Salvia Miltiorrhiza Bunge (Danshen): A Systematic Review. Med. Res. Rev. 2014, 34, 768–794. [Google Scholar] [CrossRef]
- Maione, F.; Mascolo, N. Danshen and the Cardiovascular System: New Advances for an Old Remedy. Semin. Thromb. Hemost. 2016, 42, 321–322. [Google Scholar] [CrossRef]
- Maione, F.; Cantone, V.; Chini, M.G.; de Feo, V.; Mascolo, N.; Bifulco, G. Molecular Mechanism of Tanshinone IIA and Cryptotanshinone in Platelet Anti-Aggregating Effects: An Integrated Study of Pharmacology and Computational Analysis. Fitoterapia 2015, 100, 174–178. [Google Scholar] [CrossRef]
- Available online: https://Clinicaltrials.Gov/Ct2/Results?Cond=&term=Danshen+Dripping+Pills&cntry=&state=&city=&dist= (accessed on 11 May 2023).
- Li, Z.; Xu, S.; Liu, P. Salvia MiltiorrhizaBurge (Danshen): A Golden Herbal Medicine in Cardiovascular Therapeutics. Acta Pharm. Sin. 2018, 39, 802–824. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Wang, Y.; Zeng, Y.; Huang, X.; Liu, D.; Ye, L.; Li, Y.; Chen, X.; Liu, T.; Li, H.; et al. Tanshinone IIA Suppresses Proliferation and Inflammatory Cytokine Production of Synovial Fibroblasts from Rheumatoid Arthritis Patients Induced by TNF-α and Attenuates the Inflammatory Response in AIA Mice. Front. Pharm. 2020, 11, 568. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Wang, Y.; Li, D.; Guo, Y.; Zhu, M.; Xu, S.; Mao, J.; Fan, G. TanshinoneIIA Alleviates Inflammatory Response and Directs Macrophage Polarization in Lipopolysaccharide-Stimulated RAW264.7 Cells. Inflammation 2019, 42, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Fan, G.; Jiang, X.; Wu, X.; Fordjour, P.A.; Miao, L.; Zhang, H.; Zhu, Y.; Gao, X. Anti-Inflammatory Activity of Tanshinone IIA in LPS-Stimulated RAW264.7 Macrophages via MiRNAs and TLR4–NF-ΚB Pathway. Inflammation 2016, 39, 375–384. [Google Scholar] [CrossRef]
- Chen, Z.; Gao, X.; Jiao, Y.; Qiu, Y.; Wang, A.; Yu, M.; Che, F.; Li, S.; Liu, J.; Li, J.; et al. Tanshinone IIA Exerts Anti-Inflammatory and Immune-Regulating Effects on Vulnerable Atherosclerotic Plaque Partially via the TLR4/MyD88/NF-ΚB Signal Pathway. Front. Pharm. 2019, 10, 850. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.-C.; Wu, Y.-H.; Chen, W.-Y.; Hung, Y.-C. Targeting Oxidative Stress and Endothelial Dysfunction Using Tanshinone IIA for the Treatment of Tissue Inflammation and Fibrosis. Oxid. Med. Cell Longev. 2022, 2022, 2811789. [Google Scholar] [CrossRef]
- Wu, Y.H.; Wu, Y.R.; Li, B.; Yan, Z.Y. Cryptotanshinone: A Review of Its Pharmacology Activities and Molecular Mechanisms. Fitoterapia 2020, 145, 104633. [Google Scholar] [CrossRef]
- Ma, S.; Zhang, D.; Lou, H.; Sun, L.; Ji, J. Evaluation of the Anti-Inflammatory Activities of Tanshinones Isolated from Salvia Miltiorrhiza Var. Alba Roots in THP-1 Macrophages. J. Ethnopharmacol. 2016, 188, 193–199. [Google Scholar] [CrossRef]
- Tang, S.; Shen, X.-Y.; Huang, H.-Q.; Xu, S.-W.; Yu, Y.; Zhou, C.-H.; Chen, S.-R.; Le, K.; Wang, Y.-H.; Liu, P.-Q. Cryptotanshinone Suppressed Inflammatory Cytokines Secretion in RAW264.7 Macrophages through Inhibition of the NF-ΚB and MAPK Signaling Pathways. Inflammation 2011, 34, 111–118. [Google Scholar] [CrossRef]
- Wabitsch, M.; Brenner, R.; Melzner, I.; Braun, M.; Möller, P.; Heinze, E.; Debatin, K.-M.; Hauner, H. Characterization of a Human Preadipocyte Cell Strain with High Capacity for Adipose Differentiation. Int. J. Obes. 2001, 25, 8–15. [Google Scholar] [CrossRef]
- Scoditti, E.; Massaro, M.; Carluccio, M.A.; Pellegrino, M.; Wabitsch, M.; Calabriso, N.; Storelli, C.; De Caterina, R. Additive Regulation of Adiponectin Expression by the Mediterranean Diet Olive Oil Components Oleic Acid and Hydroxytyrosol in Human Adipocytes. PLoS ONE 2015, 10, e0128218. [Google Scholar] [CrossRef]
- Quarta, S.; Massaro, M.; Carluccio, M.A.; Calabriso, N.; Bravo, L.; Sarria, B.; García-Conesa, M.-T. An Exploratory Critical Review on TNF-α as a Potential Inflammatory Biomarker Responsive to Dietary Intervention with Bioactive Foods and Derived Products. Foods 2022, 11, 2524. [Google Scholar] [CrossRef]
- Hotamisligil, G.S.; Shargill, N.S.; Spiegelman, B.M. Adipose Expression of Tumor Necrosis Factor-α: Direct Role in Obesity-Linked Insulin Resistance. Science 1993, 259, 87–91. [Google Scholar] [CrossRef]
- Kern, P.A.; Ranganathan, S.; Li, C.; Wood, L.; Ranganathan, G. Adipose Tissue Tumor Necrosis Factor and Interleukin-6 Expression in Human Obesity and Insulin Resistance. Am. J. Physiol. Endocrinol. Metab. 2001, 280, E745–E751. [Google Scholar] [CrossRef] [PubMed]
- Kern, P.A.; Saghizadeh, M.; Ong, J.M.; Bosch, R.J.; Deem, R.; Simsolo, R.B. The Expression of Tumor Necrosis Factor in Human Adipose Tissue. Regulation by Obesity, Weight Loss, and Relationship to Lipoprotein Lipase. J. Clin. Investig. 1995, 95, 2111–2119. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S.; Arner, P.; Caro, J.F.; Atkinson, R.L.; Spiegelman, B.M. Increased Adipose Tissue Expression of Tumor Necrosis Factor-Alpha in Human Obesity and Insulin Resistance. J. Clin. Investig. 1995, 95, 2409–2415. [Google Scholar] [CrossRef]
- Souza, S.C.; Palmer, H.J.; Kang, Y.-H.; Yamamoto, M.T.; Muliro, K.V.; Eric Paulson, K.; Greenberg, A.S. TNF-? Induction of Lipolysis Is Mediated through Activation of the Extracellular Signal Related Kinase Pathway in 3T3-L1 Adipocytes. J. Cell. Biochem. 2003, 89, 1077–1086. [Google Scholar] [CrossRef]
- Jack, B.U.; Mamushi, M.; Viraragavan, A.; Dias, S.; Pheiffer, C. Comparing the Effects of Tumor Necrosis Factor Alpha, Lipopolysaccharide and Palmitic Acid on Lipid Metabolism and Inflammation in Murine 3T3-L1 Adipocytes. Life Sci. 2022, 297, 120422. [Google Scholar] [CrossRef] [PubMed]
- Hauner, H.; Petruschke, T.; Russ, M.; Röhrig, K.; Eckel, J. Effects of Tumour Necrosis Factor Alpha (TNFα) on Glucose Transport and Lipid Metabolism of Newly-Differentiated Human Fat Cells in Cell Culture. Diabetologia 1995, 38, 764–771. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.H.; Halbleib, M.; Ahmad, F.; Manganiello, V.C.; Greenberg, A.S. Tumor Necrosis Factor-α Stimulates Lipolysis in Differentiated Human Adipocytes Through Activation of Extracellular Signal-Related Kinase and Elevation of Intracellular CAMP. Diabetes 2002, 51, 2929–2935. [Google Scholar] [CrossRef]
- Caso, F.; Saviano, A.; Tasso, M.; Raucci, F.; Marigliano, N.; Passavanti, S.; Frallonardo, P.; Ramonda, R.; Brancaleone, V.; Bucci, M.; et al. Analysis of Rheumatoid- vs Psoriatic Arthritis Synovial Fluid Reveals Differential Macrophage (CCR2) and T Helper Subsets (STAT3/4 and FOXP3) Activation. Autoimmun. Rev. 2022, 21, 103207. [Google Scholar] [CrossRef]
- Vellecco, V.; Saviano, A.; Raucci, F.; Casillo, G.M.; Mansour, A.A.; Panza, E.; Mitidieri, E.; Femminella, G.D.; Ferrara, N.; Cirino, G.; et al. Interleukin-17 (IL-17) Triggers Systemic Inflammation, Peripheral Vascular Dysfunction, and Related Prothrombotic State in a Mouse Model of Alzheimer’s Disease. Pharm. Res. 2023, 187, 106595. [Google Scholar] [CrossRef]
- Carpi, S.; Polini, B.; Poli, G.; Alcantara Barata, G.; Fogli, S.; Romanini, A.; Tuccinardi, T.; Guella, G.; Frontini, F.; Nieri, P.; et al. Anticancer Activity of Euplotin C, Isolated from the Marine Ciliate Euplotes Crassus, Against Human Melanoma Cells. Mar. Drugs 2018, 16, 166. [Google Scholar] [CrossRef] [PubMed]
- Carpi, S.; Polini, B.; Manera, C.; Digiacomo, M.; Salsano, J.E.; Macchia, M.; Scoditti, E.; Nieri, P. MiRNA Modulation and Antitumor Activity by the Extra-Virgin Olive Oil Polyphenol Oleacein in Human Melanoma Cells. Front. Pharm. 2020, 11, 574317. [Google Scholar] [CrossRef] [PubMed]
- Krämer, A.; Green, J.; Pollard, J.; Tugendreich, S. Causal Analysis Approaches in Ingenuity Pathway Analysis. Bioinformatics 2014, 30, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Curtis, M.J.; Alexander, S.; Cirino, G.; Docherty, J.R.; George, C.H.; Giembycz, M.A.; Hoyer, D.; Insel, P.A.; Izzo, A.A.; Ji, Y.; et al. Experimental Design and Analysis and Their Reporting II: Updated and Simplified Guidance for Authors and Peer Reviewers. Br. J. Pharm. 2018, 175, 987–993. [Google Scholar] [CrossRef]
- Alexander, S.P.H.; Roberts, R.E.; Broughton, B.R.S.; Sobey, C.G.; George, C.H.; Stanford, S.C.; Cirino, G.; Docherty, J.R.; Giembycz, M.A.; Hoyer, D.; et al. Goals and Practicalities of Immunoblotting and Immunohistochemistry: A Guide for Submission to the British Journal of Pharmacology. Br. J. Pharm. 2018, 175, 407–411. [Google Scholar] [CrossRef] [PubMed]
- George, C.H.; Stanford, S.C.; Alexander, S.; Cirino, G.; Docherty, J.R.; Giembycz, M.A.; Hoyer, D.; Insel, P.A.; Izzo, A.A.; Ji, Y.; et al. Updating the Guidelines for Data Transparency in the British Journal of Pharmacology—Data Sharing and the Use of Scatter Plots Instead of Bar Charts. Br. J. Pharm. 2017, 174, 2801–2804. [Google Scholar] [CrossRef]
- Ansari, M.A.; Khan, F.B.; Safdari, H.A.; Almatroudi, A.; Alzohairy, M.A.; Safdari, M.; Amirizadeh, M.; Rehman, S.; Equbal, M.J.; Hoque, M. Prospective Therapeutic Potential of Tanshinone IIA: An Updated Overview. Pharm. Res. 2021, 164, 105364. [Google Scholar] [CrossRef]
- Zorena, K.; Jachimowicz-Duda, O.; Ślęzak, D.; Robakowska, M.; Mrugacz, M. Adipokines and Obesity. Potential Link to Metabolic Disorders and Chronic Complications. Int. J. Mol. Sci. 2020, 21, 3570. [Google Scholar] [CrossRef]
- Blüher, M. Adipokines—Removing Road Blocks to Obesity and Diabetes Therapy. Mol. Metab. 2014, 3, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, B.; Gogg, S.; Hedjazifar, S.; Jenndahl, L.; Hammarstedt, A.; Smith, U. Inflammation and Impaired Adipogenesis in Hypertrophic Obesity in Man. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E999–E1003. [Google Scholar] [CrossRef] [PubMed]
- Laurencikiene, J.; Van Harmelen, V.; Nordström, E.A.; Dicker, A.; Blomqvist, L.; Näslund, E.; Langin, D.; Arner, P.; Rydén, M. NF-ΚB Is Important for TNF-α-Induced Lipolysis in Human Adipocytes. J. Lipid Res. 2007, 48, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.D.; Nedjai, B.; Hurst, T.; Pennington, D.J. Cytokines and Chemokines: At the Crossroads of Cell Signalling and Inflammatory Disease. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2014, 1843, 2563–2582. [Google Scholar] [CrossRef]
- Kim, J.; Chung, K.; Choi, C.; Beloor, J.; Ullah, I.; Kim, N.; Lee, K.Y.; Lee, S.-K.; Kumar, P. Silencing CCR2 in Macrophages Alleviates Adipose Tissue Inflammation and the Associated Metabolic Syndrome in Dietary Obese Mice. Mol. Ther. Nucleic Acids 2016, 5, e280. [Google Scholar] [CrossRef]
- Ohman, M.K.; Wright, A.P.; Wickenheiser, K.J.; Luo, W.; Russo, H.M.; Eitzman, D.T. Monocyte Chemoattractant Protein-1 Deficiency Protects Against Visceral Fat-Induced Atherosclerosis. Arter. Thromb. Vasc. Biol. 2010, 30, 1151–1158. [Google Scholar] [CrossRef]
- Tourniaire, F.; Romier-Crouzet, B.; Lee, J.H.; Marcotorchino, J.; Gouranton, E.; Salles, J.; Malezet, C.; Astier, J.; Darmon, P.; Blouin, E.; et al. Chemokine Expression in Inflamed Adipose Tissue Is Mainly Mediated by NF-ΚB. PLoS ONE 2013, 8, e66515. [Google Scholar] [CrossRef] [PubMed]
- Huber, J.; Kiefer, F.W.; Zeyda, M.; Ludvik, B.; Silberhumer, G.R.; Prager, G.; Zlabinger, G.J.; Stulnig, T.M. CC Chemokine and CC Chemokine Receptor Profiles in Visceral and Subcutaneous Adipose Tissue Are Altered in Human Obesity. J. Clin. Endocrinol. Metab. 2008, 93, 3215–3221. [Google Scholar] [CrossRef] [PubMed]
- Keophiphath, M.; Rouault, C.; Divoux, A.; Clément, K.; Lacasa, D. CCL5 Promotes Macrophage Recruitment and Survival in Human Adipose Tissue. Arter. Thromb. Vasc. Biol. 2010, 30, 39–45. [Google Scholar] [CrossRef]
- Chan, P.-C.; Lu, C.-H.; Chien, H.-C.; Tian, Y.-F.; Hsieh, P.-S. Adipose Tissue-Derived CCL5 Enhances Local Pro-Inflammatory Monocytic MDSCs Accumulation and Inflammation via CCR5 Receptor in High-Fat Diet-Fed Mice. Int. J. Mol. Sci. 2022, 23, 14226. [Google Scholar] [CrossRef]
- Matter, C.M.; Handschin, C. RANTES (Regulated on Activation, Normal T Cell Expressed and Secreted), Inflammation, Obesity, and the Metabolic Syndrome. Circulation 2007, 115, 946–948. [Google Scholar] [CrossRef]
- Zhou, H.; Liao, X.; Zeng, Q.; Zhang, H.; Song, J.; Hu, W.; Sun, X.; Ding, Y.; Wang, D.; Xiao, Y.; et al. Metabolic Effects of CCL5 Deficiency in Lean and Obese Mice. Front. Immunol. 2023, 13, 1059687. [Google Scholar] [CrossRef] [PubMed]
- Faber, D.R.; Moll, F.L.; Vink, A.; van der Waal, C.; Kalkhoven, E.; Schipper, H.S.; Hajer, G.R.; Monajemi, H.; Visseren, F.L.J. Adipose Tissue Quantity and Composition Contribute to Adipokine Concentrations in the Subclavian Vein and the Inferior Mesenteric Vein. Int. J. Obes. 2012, 36, 1078–1085. [Google Scholar] [CrossRef]
- Faber, D.R.; van der Graaf, Y.; Westerink, J.; Kanhai, D.A.; Monajemi, H.; Visseren, F.L.J. Hepatocyte Growth Factor and Interferon-γ Inducible Protein-10 Are Related to Visceral Adiposity. Eur. J. Clin. Investig. 2013, 43, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Nunemaker, C.S.; Chung, H.G.; Verrilli, G.M.; Corbin, K.L.; Upadhye, A.; Sharma, P.R. Increased Serum CXCL1 and CXCL5 Are Linked to Obesity, Hyperglycemia, and Impaired Islet Function. J. Endocrinol. 2014, 222, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Straczkowski, M.; Kowalska, I.; Nikolajuk, A.; Dzienis-Straczkowska, S.; Szelachowska, M.; Kinalska, I. Plasma Interleukin 8 Concentrations in Obese Subjects with Impaired Glucose Tolerance. Cardiovasc. Diabetol. 2003, 2, 5. [Google Scholar] [CrossRef] [PubMed]
- Viola, A.; Luster, A.D. Chemokines and Their Receptors: Drug Targets in Immunity and Inflammation. Annu. Rev. Pharm. Toxicol. 2008, 48, 171–197. [Google Scholar] [CrossRef]
- Wu, H.; Ghosh, S.; Perrard, X.D.; Feng, L.; Garcia, G.E.; Perrard, J.L.; Sweeney, J.F.; Peterson, L.E.; Chan, L.; Smith, C.W.; et al. T-Cell Accumulation and Regulated on Activation, Normal T Cell Expressed and Secreted Upregulation in Adipose Tissue in Obesity. Circulation 2007, 115, 1029–1038. [Google Scholar] [CrossRef]
- Elgazar-Carmon, V.; Rudich, A.; Hadad, N.; Levy, R. Neutrophils Transiently Infiltrate Intra-Abdominal Fat Early in the Course of High-Fat Feeding. J. Lipid Res. 2008, 49, 1894–1903. [Google Scholar] [CrossRef]
- Morrison, M.C.; Kleemann, R. Role of Macrophage Migration Inhibitory Factor in Obesity, Insulin Resistance, Type 2 Diabetes, and Associated Hepatic Co-Morbidities: A Comprehensive Review of Human and Rodent Studies. Front. Immunol. 2015, 6, 308. [Google Scholar] [CrossRef]
- Finucane, O.M.; Reynolds, C.M.; McGillicuddy, F.C.; Harford, K.A.; Morrison, M.; Baugh, J.; Roche, H.M. Macrophage Migration Inhibitory Factor Deficiency Ameliorates High-Fat Diet Induced Insulin Resistance in Mice with Reduced Adipose Inflammation and Hepatic Steatosis. PLoS ONE 2014, 9, e113369. [Google Scholar] [CrossRef]
- Skurk, T.; Hauner, H. Obesity and Impaired Fibrinolysis: Role of Adipose Production of Plasminogen Activator Inhibitor-1. Int. J. Obes. 2004, 28, 1357–1364. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.-J.; Mao, S.-L.; Taylor, K.L.; Kanjanabuch, T.; Guan, Y.; Zhang, Y.; Brown, N.J.; Swift, L.L.; McGuinness, O.P.; Wasserman, D.H.; et al. Prevention of Obesity and Insulin Resistance in Mice Lacking Plasminogen Activator Inhibitor 1. Diabetes 2004, 53, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Spranger, J.; Kroke, A.; Möhlig, M.; Hoffmann, K.; Bergmann, M.M.; Ristow, M.; Boeing, H.; Pfeiffer, A.F.H. Inflammatory Cytokines and the Risk to Develop Type 2 Diabetes. Diabetes 2003, 52, 812–817. [Google Scholar] [CrossRef] [PubMed]
- Bastard, J.-P.; Jardel, C.; Bruckert, E.; Blondy, P.; Capeau, J.; Laville, M.; Vidal, H.; Hainque, B. Elevated Levels of Interleukin 6 Are Reduced in Serum and Subcutaneous Adipose Tissue of Obese Women after Weight Loss. J. Clin. Endocrinol. Metab. 2000, 85, 3338–3342. [Google Scholar] [CrossRef]
- Franckhauser, S.; Elias, I.; Rotter Sopasakis, V.; Ferré, T.; Nagaev, I.; Andersson, C.X.; Agudo, J.; Ruberte, J.; Bosch, F.; Smith, U. Overexpression of Il6 Leads to Hyperinsulinaemia, Liver Inflammation and Reduced Body Weight in Mice. Diabetologia 2008, 51, 1306–1316. [Google Scholar] [CrossRef]
- Mortensen, R.F. C-Reactive Protein, Inflammation, and Innate Immunity. Immunol. Res. 2001, 24, 163–176. [Google Scholar] [CrossRef]
- Charo, I.F.; Ransohoff, R.M. The Many Roles of Chemokines and Chemokine Receptors in Inflammation. N. Engl. J. Med. 2006, 354, 610–621. [Google Scholar] [CrossRef]
- Li, H.; Gao, C.; Liu, C.; Liu, L.; Zhuang, J.; Yang, J.; Zhou, C.; Feng, F.; Sun, C.; Wu, J. A Review of the Biological Activity and Pharmacology of Cryptotanshinone, an Important Active Constituent in Danshen. Biomed. Pharmacother. 2021, 137, 111332. [Google Scholar] [CrossRef]
- Su, C.-Y.; Ming, Q.-L.; Rahman, K.; Han, T.; Qin, L.-P. Salvia Miltiorrhiza: Traditional Medicinal Uses, Chemistry, and Pharmacology. Chin. J. Nat. Med. 2015, 13, 163–182. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Liu, Z.; Li, H.; Little, P.J.; Liu, P.; Xu, S. Cardiovascular Actions and Therapeutic Potential of Tanshinone IIA. Atherosclerosis 2012, 220, 3–10. [Google Scholar] [CrossRef]
- Huang, L.; Ding, W.; Wang, M.-Q.; Wang, Z.-G.; Chen, H.-H.; Chen, W.; Yang, Q.; Lu, T.-N.; Yang, Q.; He, J.-M. Tanshinone IIA Ameliorates Non-Alcoholic Fatty Liver Disease through Targeting Peroxisome Proliferator-Activated Receptor Gamma and Toll-like Receptor 4. J. Int. Med. Res. 2019, 47, 5239–5255. [Google Scholar] [CrossRef]
- Gong, Z.; Huang, C.; Sheng, X.; Zhang, Y.; Li, Q.; Wang, M.-W.; Peng, L.; Zang, Y.Q. The Role of Tanshinone IIA in the Treatment of Obesity through Peroxisome Proliferator-Activated Receptor γ Antagonism. Endocrinology 2009, 150, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Imran, K.M.; Rahman, N.; Yoon, D.; Jeon, M.; Lee, B.-T.; Kim, Y.-S. Cryptotanshinone Promotes Commitment to the Brown Adipocyte Lineage and Mitochondrial Biogenesis in C3H10T1/2 Mesenchymal Stem Cells via AMPK and P38-MAPK Signaling. Biochim. Biophys. Acta BBA Mol. Cell Biol. Lipids 2017, 1862, 1110–1120. [Google Scholar] [CrossRef]
- Kim, E.J.; Jung, S.-N.; Son, K.H.; Kim, S.R.; Ha, T.Y.; Park, M.G.; Jo, I.G.; Park, J.G.; Choe, W.; Kim, S.-S.; et al. Antidiabetes and Antiobesity Effect of Cryptotanshinone via Activation of AMP-Activated Protein Kinase. Mol. Pharm. 2007, 72, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Rahman, N.; Jeon, M.; Song, H.-Y.; Kim, Y.-S. Cryptotanshinone, a Compound of Salvia Miltiorrhiza Inhibits Pre-Adipocytes Differentiation by Regulation of Adipogenesis-Related Genes Expression via STAT3 Signaling. Phytomedicine 2016, 23, 58–67. [Google Scholar] [CrossRef]
- Hwang, S.-L.; Yang, J.H.; Jeong, Y.-T.; Kim, Y.D.; Li, X.; Lu, Y.; Chang, Y.-C.; Son, K.H.; Chang, H.W. Tanshinone IIA Improves Endoplasmic Reticulum Stress-Induced Insulin Resistance through AMP-Activated Protein Kinase. Biochem. Biophys. Res. Commun. 2013, 430, 1246–1252. [Google Scholar] [CrossRef]
- Jung, S.H.; Seol, H.J.; Jeon, S.J.; Son, K.H.; Lee, J.R. Insulin-Sensitizing Activities of Tanshinones, Diterpene Compounds of the Root of Salvia Miltiorrhiza Bunge. Phytomedicine 2009, 16, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Zhang, M.; Xu, P.; Xu, D.; Chen, P.; Ren, M.; Sun, Q.; Chen, J.; Du, J.; Tang, X. Tanshinone IIA Improves Diabetes Mellitus via the NF-κB-induced AMPK Signal Pathway. Exp. Ther. Med. 2018, 16, 4225–4231. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, W.; Wang, C.C.; Wu, X.; Zheng, J. Cryptotanshinone Reverses Ovarian Insulin Resistance in Mice through Activation of Insulin Signaling and the Regulation of Glucose Transporters and Hormone Synthesizing Enzymes. Fertil. Steril. 2014, 102, 589–596.e4. [Google Scholar] [CrossRef]
- Gebert, L.F.R.; MacRae, I.J. Regulation of MicroRNA Function in Animals. Nat. Rev. Mol. Cell Biol. 2019, 20, 21–37. [Google Scholar] [CrossRef]
- Scoditti, E.; Naccarati, A.; Carpi, S.; Polini, B.; Ebada, S.S.; Nieri, P. Editorial: Non-Coding RNAs as Mediators of the Activity of Natural Compounds. Front. Pharm. 2021, 12, 751956. [Google Scholar] [CrossRef]
- Deiuliis, J.A. MicroRNAs as Regulators of Metabolic Disease: Pathophysiologic Significance and Emerging Role as Biomarkers and Therapeutics. Int. J. Obes. 2016, 40, 88–101. [Google Scholar] [CrossRef]
- Ferrero, G.; Carpi, S.; Polini, B.; Pardini, B.; Nieri, P.; Impeduglia, A.; Grioni, S.; Tarallo, S.; Naccarati, A. Intake of Natural Compounds and Circulating MicroRNA Expression Levels: Their Relationship Investigated in Healthy Subjects with Different Dietary Habits. Front. Pharm. 2021, 11, 619200. [Google Scholar] [CrossRef]
- Castaño, C.; Kalko, S.; Novials, A.; Párrizas, M. Obesity-Associated Exosomal MiRNAs Modulate Glucose and Lipid Metabolism in Mice. Proc. Natl. Acad. Sci. USA 2018, 115, 12158–12163. [Google Scholar] [CrossRef] [PubMed]
- van Rooij, E.; Purcell, A.L.; Levin, A.A. Developing MicroRNA Therapeutics. Circ. Res. 2012, 110, 496–507. [Google Scholar] [CrossRef] [PubMed]
- Cerutti, C.; Edwards, L.J.; de Vries, H.E.; Sharrack, B.; Male, D.K.; Romero, I.A. MiR-126 and MiR-126* Regulate Shear-Resistant Firm Leukocyte Adhesion to Human Brain Endothelium. Sci. Rep. 2017, 7, 45284. [Google Scholar] [CrossRef]
- He, Y.; Hwang, S.; Cai, Y.; Kim, S.; Xu, M.; Yang, D.; Guillot, A.; Feng, D.; Seo, W.; Hou, X.; et al. MicroRNA-223 Ameliorates Nonalcoholic Steatohepatitis and Cancer by Targeting Multiple Inflammatory and Oncogenic Genes in Hepatocytes. Hepatology 2019, 70, 1150–1167. [Google Scholar] [CrossRef]
- Available online: https://Mirtarbase.Cuhk.Edu.Cn/~miRTarBase/MiRTarBase_2022/Php/Search.Php?Org=hsa&opt=mirna_id&kw=miR-124-3p) (accessed on 11 May 2023).
- Strum, J.C.; Johnson, J.H.; Ward, J.; Xie, H.; Feild, J.; Hester, A.; Alford, A.; Waters, K.M. MicroRNA 132 Regulates Nutritional Stress-Induced Chemokine Production through Repression of SirT1. Mol. Endocrinol. 2009, 23, 1876–1884. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Deng, P.; Sun, Y.; Ye, P.; Zhang, A.; Wu, C.; Yue, Z.; Chen, Z.; Xia, J. MicroRNA-155 Promotes Neointimal Hyperplasia through Smooth Muscle-like Cell-Derived RANTES in Arteriovenous Fistulas. J. Vasc. Surg. 2018, 67, 933–944.e3. [Google Scholar] [CrossRef]
- Ortega, F.J.; Moreno, M.; Mercader, J.M.; Moreno-Navarrete, J.M.; Fuentes-Batllevell, N.; Sabater, M.; Ricart, W.; Fernández-Real, J.M. Inflammation Triggers Specific MicroRNA Profiles in Human Adipocytes and Macrophages and in Their Supernatants. Clin. Epigenetics 2015, 7, 49. [Google Scholar] [CrossRef]
- Karkeni, E.; Astier, J.; Tourniaire, F.; el Abed, M.; Romier, B.; Gouranton, E.; Wan, L.; Borel, P.; Salles, J.; Walrand, S.; et al. Obesity-Associated Inflammation Induces MicroRNA-155 Expression in Adipocytes and Adipose Tissue: Outcome on Adipocyte Function. J. Clin. Endocrinol. Metab. 2016, 101, 1615–1626. [Google Scholar] [CrossRef]
- Mahesh, G.; Biswas, R. MicroRNA-155: A Master Regulator of Inflammation. J. Interferon Cytokine Res. 2019, 39, 321–330. [Google Scholar] [CrossRef]
- Ying, W.; Riopel, M.; Bandyopadhyay, G.; Dong, Y.; Birmingham, A.; Seo, J.B.; Ofrecio, J.M.; Wollam, J.; Hernandez-Carretero, A.; Fu, W.; et al. Adipose Tissue Macrophage-Derived Exosomal MiRNAs Can Modulate In Vivo and In Vitro Insulin Sensitivity. Cell 2017, 171, 372–384.e12. [Google Scholar] [CrossRef]
- Gaudet, A.D.; Fonken, L.K.; Gushchina, L.V.; Aubrecht, T.G.; Maurya, S.K.; Periasamy, M.; Nelson, R.J.; Popovich, P.G. MiR-155 Deletion in Female Mice Prevents Diet-Induced Obesity. Sci. Rep. 2016, 6, 22862. [Google Scholar] [CrossRef]
- Zhang, Y.; Mei, H.; Chang, X.; Chen, F.; Zhu, Y.; Han, X. Adipocyte-Derived Microvesicles from Obese Mice Induce M1 Macrophage Phenotype through Secreted MiR-155. J. Mol. Cell Biol. 2016, 8, 505–517. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, G.; Meng, C.; Guo, X.; Cheruku, P.S.; Shi, L.; Xu, H.; Li, H.; Wang, G.; Evans, A.R.; Safe, S.; et al. A Novel Regulator of Macrophage Activation. Circulation 2012, 125, 2892–2903. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Li, L.-H.; Tan, L.-M.; Luo, W.-B.; Xiong, H.; Lu, X.-L.; Liu, D.; Li, W.-Y.; Guo, Y.-X.; Tang, Z.; et al. Tanshinone IIA Ameliorates Inflammation Response in Osteoarthritis via Inhibition of MiR-155/FOXO3 Axis. Pharmacology 2021, 106, 20–28. [Google Scholar] [CrossRef]
- Xu, H.; Li, H.; Zhu, P.; Liu, Y.; Zhou, M.; Chen, A. Tanshinone IIA Ameliorates Progression of CAD Through Regulating Cardiac H9c2 Cells Proliferation and Apoptosis by MiR-133a-3p/EGFR Axis. Drug Des. Dev. Ther. 2020, 14, 2853–2863. [Google Scholar] [CrossRef]
- Du, X.; Wang, X.; Cui, K.; Chen, Y.; Zhang, C.; Yao, K.; Hao, Y.; Chen, Y. Tanshinone IIA and Astragaloside IV Inhibit MiR-223/JAK2/STAT1 Signalling Pathway to Alleviate Lipopolysaccharide-Induced Damage in Nucleus Pulposus Cells. Dis. Mrk. 2021, 2021, 6554480. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, Y.; Zhang, Y.; Liu, W.; Wang, J. Cryptotanshinone Inhibits Lung Cancer Invasion via MicroRNA-133a/Matrix Metalloproteinase 14 Regulation. Oncol. Lett. 2019, 18, 2554–2559. [Google Scholar] [CrossRef] [PubMed]
- Thomou, T.; Mori, M.A.; Dreyfuss, J.M.; Konishi, M.; Sakaguchi, M.; Wolfrum, C.; Rao, T.N.; Winnay, J.N.; Garcia-Martin, R.; Grinspoon, S.K.; et al. Adipose-Derived Circulating MiRNAs Regulate Gene Expression in Other Tissues. Nature 2017, 542, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.; Jacob, S. A Simple Practice Guide for Dose Conversion between Animals and Human. J. Basic Clin. Pharm. 2016, 7, 27. [Google Scholar] [CrossRef] [PubMed]


| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| CCL2/MCP-1 | 5′-CCCCAGTCACCTGCTGTTAT-3′ | 5′-TCCTGAACCCACTTCTGCTT-3′ |
| CXCL1/GRO-α | 5′-CCCCAAGAACATCCAAAGTG-3′ | 5′-TGGATTTGTCACTGTTCAGCA-3′ |
| CXCL10/IP-10 | 5′-CAAGGATGGACCACACAGAG-3′ | 5′-GCAGGGTCAGAACATCCACT-3′ |
| CCL5/RANTES | 5′-CGCTGTCATCCTCATTGCTA-3′ | 5′-GAGCACTTGCCACTGGTGTA-3′ |
| IL-8 | 5′-GTGCAGTTTTGCCAAGGAGT-3′ | 5′-CTCTGCACCCAGTTTTCCTT-3′ |
| 18S | 5′-AAACGGCTACCACATCCAAG-3′ | 5′-CCTCCAATGGATCCTCGTTA-3′ |
| miRNA Name | Mature miRNA Sequences | miRBase Accession |
|---|---|---|
| hsa-miR-126-3p | 5′-UCGUACCGUGAGUAAUAAUGCG-3′ | MIMAT0000445 |
| hsa-miR-223-3p | 5′-UGUCAGUUUGUCAAAUACCCCA-3′ | MIMAT0000280 |
| hsa-miR-124-3p | 5′-UAAGGCACGCGGUGAAUGCC-3′ | MIMAT0000422 |
| hsa-miR-155-5p | 5′-UUAAUGCUAAUCGUGAUAGGGGU-3′ | MIMAT0000646 |
| hsa-miR-132-3p | 5′-UAACAGUCUACAGCCAUGGUCG-3 | MIMAT0000426 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carpi, S.; Quarta, S.; Doccini, S.; Saviano, A.; Marigliano, N.; Polini, B.; Massaro, M.; Carluccio, M.A.; Calabriso, N.; Wabitsch, M.; et al. Tanshinone IIA and Cryptotanshinone Counteract Inflammation by Regulating Gene and miRNA Expression in Human SGBS Adipocytes. Biomolecules 2023, 13, 1029. https://doi.org/10.3390/biom13071029
Carpi S, Quarta S, Doccini S, Saviano A, Marigliano N, Polini B, Massaro M, Carluccio MA, Calabriso N, Wabitsch M, et al. Tanshinone IIA and Cryptotanshinone Counteract Inflammation by Regulating Gene and miRNA Expression in Human SGBS Adipocytes. Biomolecules. 2023; 13(7):1029. https://doi.org/10.3390/biom13071029
Chicago/Turabian StyleCarpi, Sara, Stefano Quarta, Stefano Doccini, Anella Saviano, Noemi Marigliano, Beatrice Polini, Marika Massaro, Maria Annunziata Carluccio, Nadia Calabriso, Martin Wabitsch, and et al. 2023. "Tanshinone IIA and Cryptotanshinone Counteract Inflammation by Regulating Gene and miRNA Expression in Human SGBS Adipocytes" Biomolecules 13, no. 7: 1029. https://doi.org/10.3390/biom13071029
APA StyleCarpi, S., Quarta, S., Doccini, S., Saviano, A., Marigliano, N., Polini, B., Massaro, M., Carluccio, M. A., Calabriso, N., Wabitsch, M., Santorelli, F. M., Cecchini, M., Maione, F., Nieri, P., & Scoditti, E. (2023). Tanshinone IIA and Cryptotanshinone Counteract Inflammation by Regulating Gene and miRNA Expression in Human SGBS Adipocytes. Biomolecules, 13(7), 1029. https://doi.org/10.3390/biom13071029

















