High PD-L1 Expression Correlates with an Immunosuppressive Tumour Immune Microenvironment and Worse Prognosis in ALK-Rearranged Non-Small Cell Lung Cancer
Abstract
1. Introduction
2. Materials and Methods
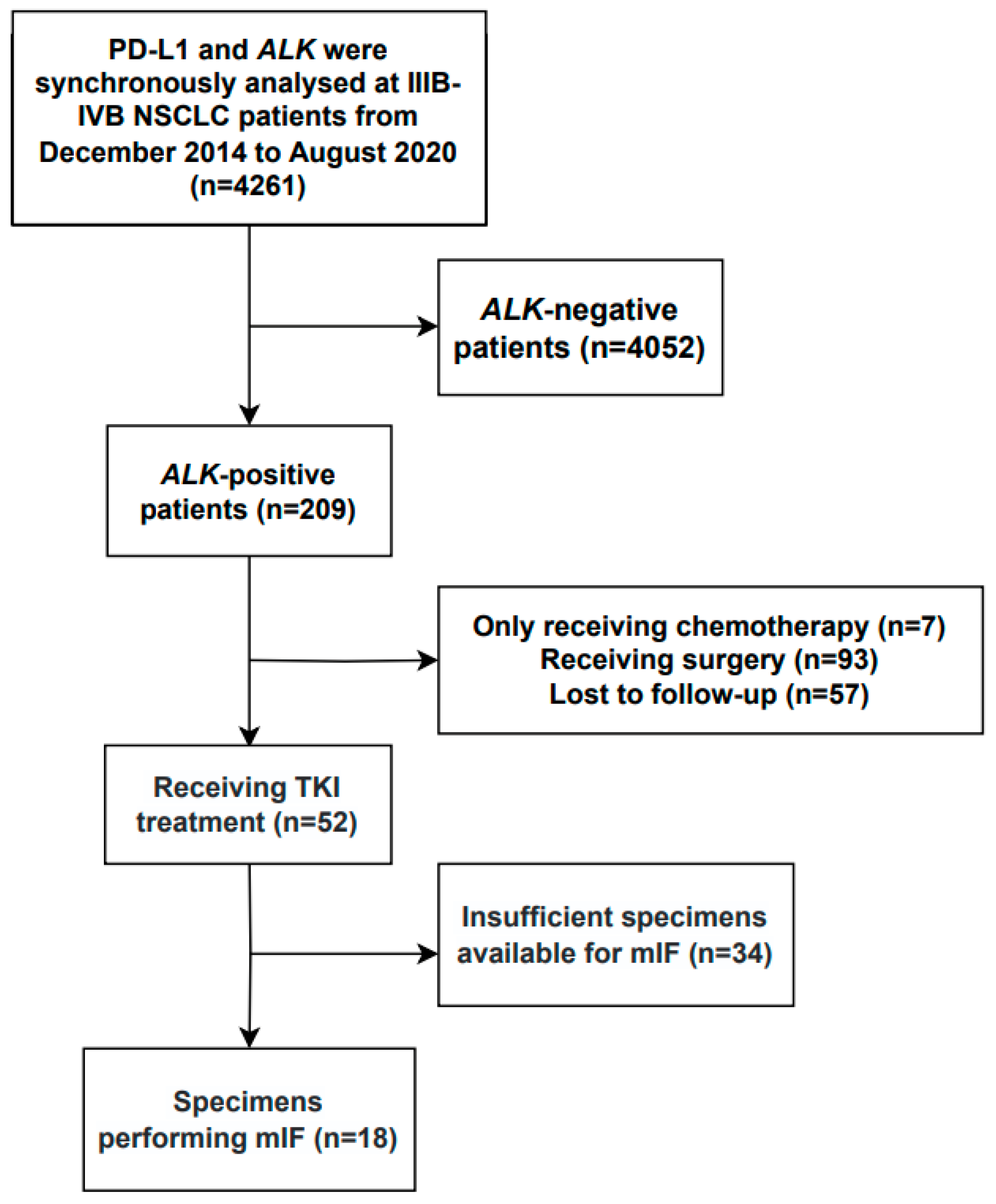
2.1. Patients
2.2. Immunohistochemical Staining of ALK
2.3. mIF
2.4. Tissue Imaging and Analysis
2.5. Evaluation of Immune Cell Spatial Distribution
2.6. Treatment Evaluation
2.7. Statistical Analysis
3. Results
3.1. Patient Characteristics
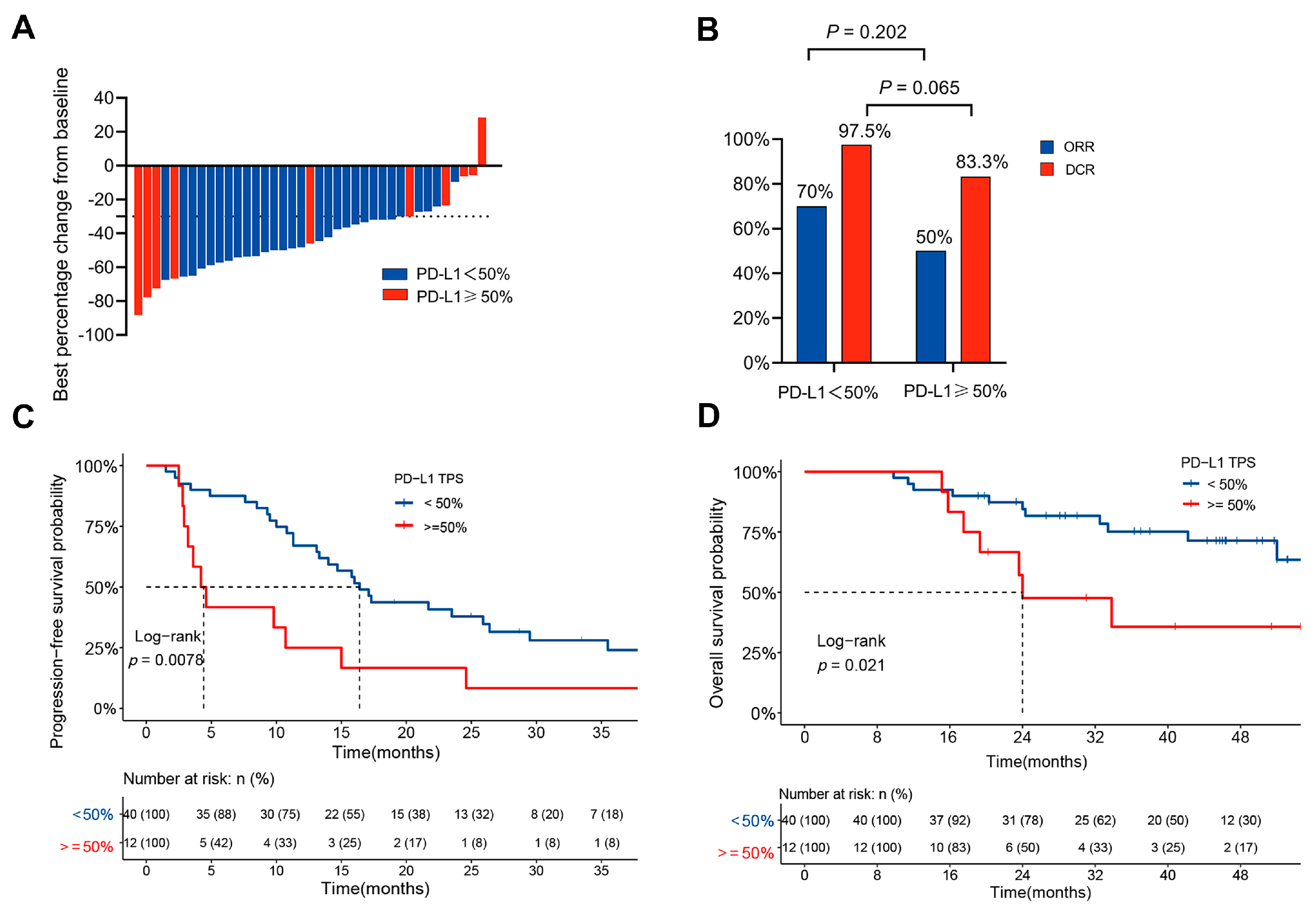
3.2. Clinical Outcomes in Patients with Different PD-L1 Expression Levels
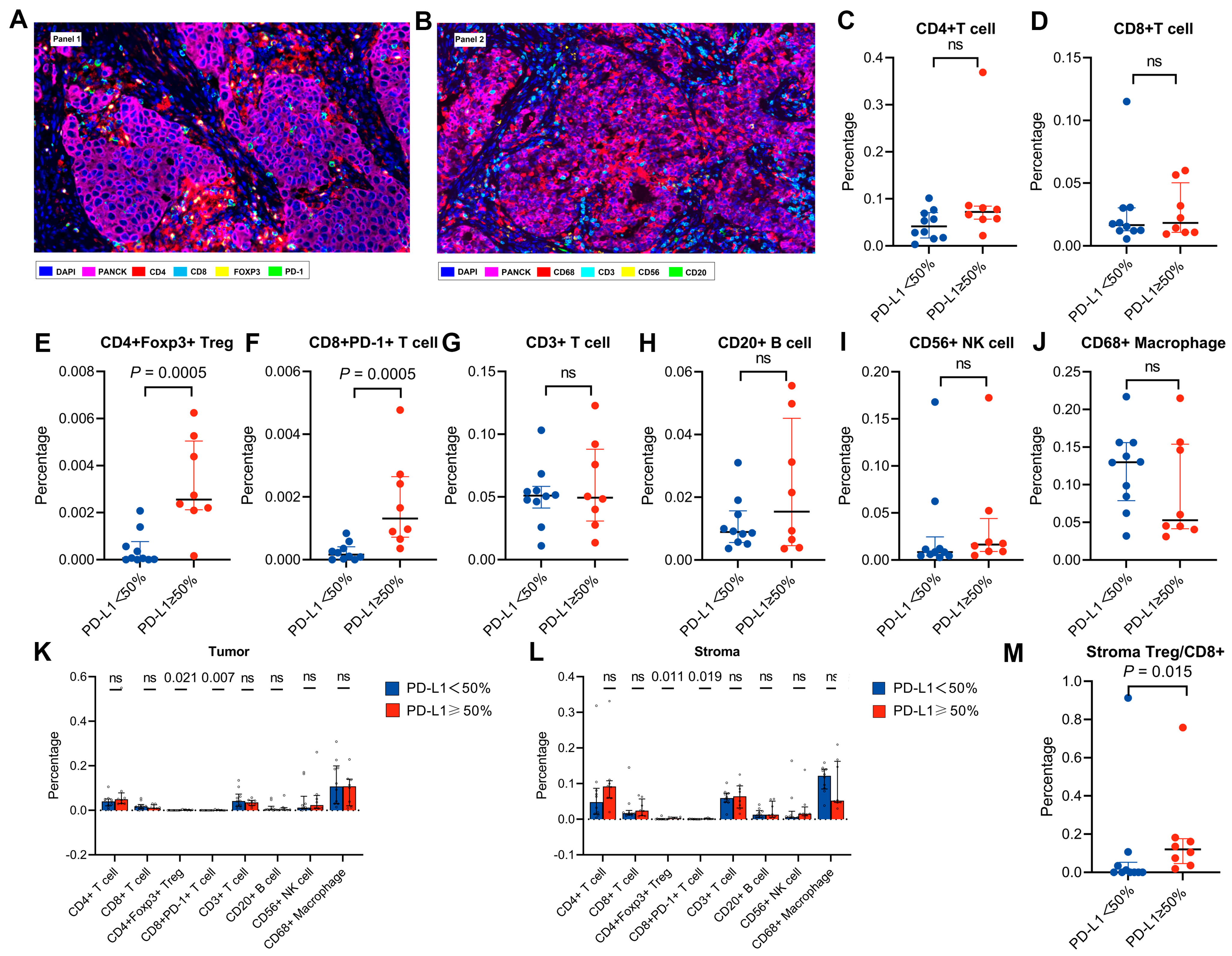
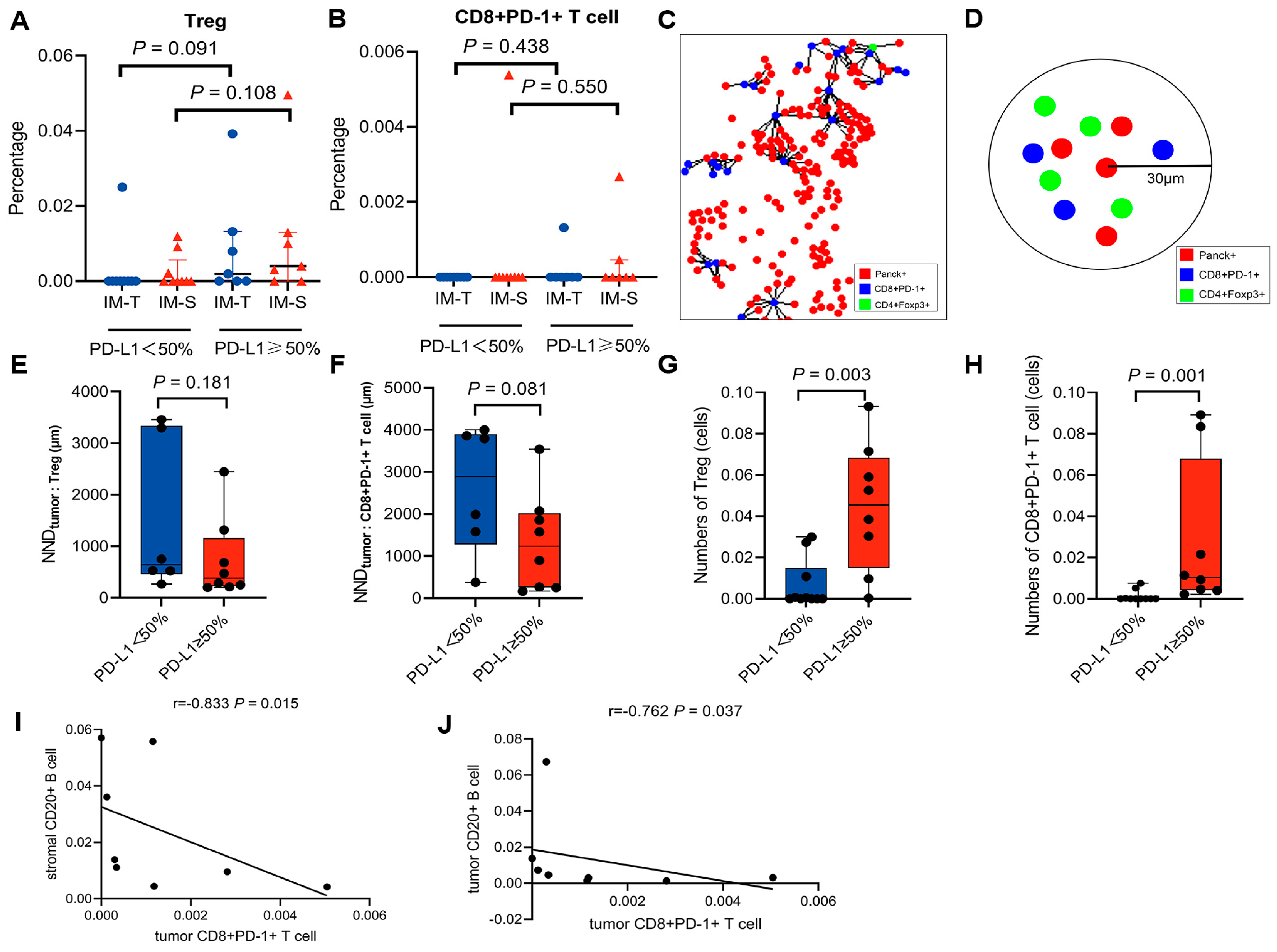
3.3. Features of the Tumour Immune Microenvironment in Patients with High PD-L1 Expression
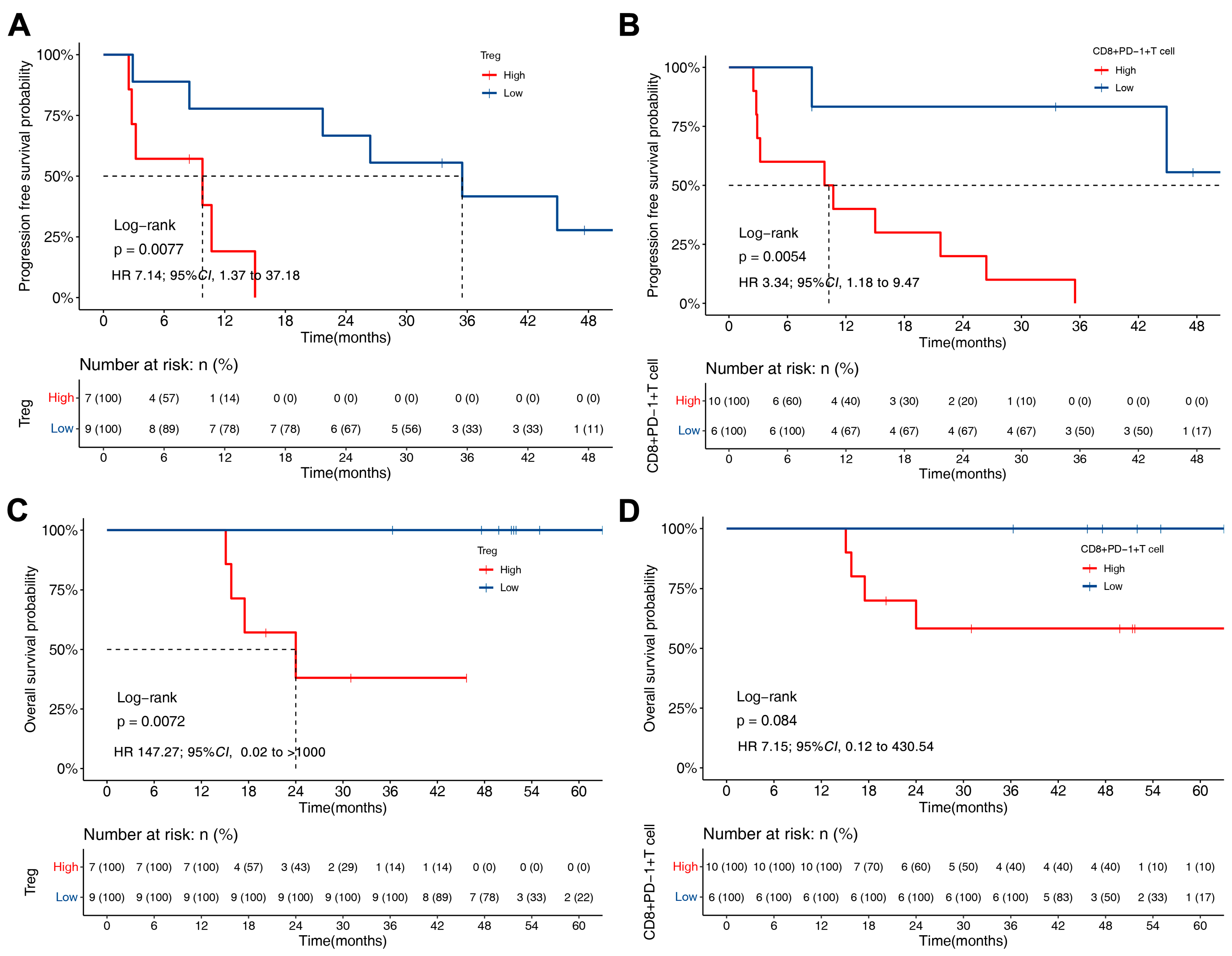
3.4. PFS and OS of ALK-Rearranged NSCLC Based on TME
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Planchard, D.; Popat, S.; Kerr, K.; Novello, S.; Smit, E.F.; Faivre-Finn, C.; Mok, T.S.; Reck, M.; Van Schil, P.E.; Hellmann, M.D.; et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, iv192–iv237. [Google Scholar] [CrossRef] [PubMed]
- Solomon, B.; Varella-Garcia, M.; Camidge, D.R. ALK Gene Rearrangements: A New Therapeutic Target in a Molecularly Defined Subset of Non-small Cell Lung Cancer. J. Thorac. Oncol. 2009, 4, 1450–1454. [Google Scholar] [CrossRef] [PubMed]
- Majeed, U.; Manochakian, R.; Zhao, Y.; Lou, Y. Targeted therapy in advanced non-small cell lung cancer: Current advances and future trends. J. Hematol. Oncol. 2021, 14, 108. [Google Scholar] [CrossRef] [PubMed]
- Doroshow, D.B.; Bhalla, S.; Beasley, M.B.; Sholl, L.M.; Kerr, K.M.; Gnjatic, S.; Wistuba, I.I.; Rimm, D.L.; Tsao, M.S.; Hirsch, F.R. PD-L1 as a biomarker of response to immune-checkpoint inhibitors. Nat. Rev. Clin. Oncol. 2021, 18, 345–362. [Google Scholar] [CrossRef] [PubMed]
- Yoneshima, Y.; Ijichi, K.; Anai, S.; Ota, K.; Otsubo, K.; Iwama, E.; Tanaka, K.; Oda, Y.; Nakanishi, Y.; Okamoto, I. PD-L1 expression in lung adenocarcinoma harboring EGFR mutations or ALK rearrangements. Lung Cancer 2018, 118, 36–40. [Google Scholar] [CrossRef]
- Kim, S.-J.; Kim, S.; Kim, D.-W.; Kim, M.; Keam, B.; Kim, T.M.; Lee, Y.; Koh, J.; Jeon, Y.K.; Heo, D.S. Alterations in PD-L1 Expression Associated with Acquisition of Resistance to ALK Inhibitors in ALK-Rearranged Lung Cancer. Cancer Res. Treat. 2019, 51, 1231–1240. [Google Scholar] [CrossRef]
- Yang, C.-Y.; Liao, W.-Y.; Ho, C.-C.; Chen, K.-Y.; Tsai, T.-H.; Liu, Y.-N.; Su, K.-Y.; Chang, Y.-L.; Wu, C.-T.; Liao, B.-C.; et al. Association of Programmed Death-Ligand 1 Expression with Fusion Variants and Clinical Outcomes in Patients with Anaplastic Lymphoma Kinase-Positive Lung Adenocarcinoma Receiving Crizotinib. Oncologist 2020, 25, 702–711. [Google Scholar] [CrossRef]
- Li, M.; Hou, X.; Chen, J.; Zhang, B.; Wang, N.; Han, H.; Chen, L. ALK fusion variant 3a/b, concomitant mutations, and high PD-L1 expression were associated with unfavorable clinical response to second-generation ALK TKIs in patients with advanced ALK-rearranged non-small cell lung cancer (GASTO 1061). Lung Cancer 2022, 165, 54–62. [Google Scholar] [CrossRef]
- Zhou, Y.; Song, L.; Xu, Q.; Zeng, L.; Jiang, W.; Yang, N.; Zhang, Y. Investigation on the survival implications of PD-L1 expression status in ALK-rearranged advanced non-small cell lung cancer treated with first-line crizotinib. Lung Cancer 2022, 167, 58–64. [Google Scholar] [CrossRef]
- Chang, G.-C.; Yang, T.-Y.; Chen, K.-C.; Hsu, K.-H.; Huang, Y.-H.; Su, K.-Y.; Yu, S.-L.; Tseng, J.-S. ALK variants, PD-L1 expression, and their association with outcomes in ALK-positive NSCLC patients. Sci. Rep. 2020, 10, 21063. [Google Scholar] [CrossRef]
- Teng, M.W.; Ngiow, S.F.; Ribas, A.; Smyth, M.J. Classifying Cancers Based on T-cell Infiltration and PD-L1. Cancer Res. 2015, 75, 2139–2145. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, L. Classification of Advanced Human Cancers Based on Tumor Immunity in the MicroEnvironment (TIME) for Cancer Immunotherapy. JAMA Oncol. 2016, 2, 1403–1404. [Google Scholar] [CrossRef]
- Mazzaschi, G.; Madeddu, D.; Falco, A.; Bocchialini, G.; Goldoni, M.; Sogni, F.; Armani, G.; Lagrasta, C.A.; Lorusso, B.; Mangiaracina, C.; et al. Low PD-1 Expression in Cytotoxic CD8+ Tumor-Infiltrating Lymphocytes Confers an Immune-Privileged Tissue Microenvironment in NSCLC with a Prognostic and Predictive Value. Clin. Cancer Res. 2018, 24, 407–419. [Google Scholar] [CrossRef]
- Yang, C.-Y.; Lin, M.-W.; Chang, Y.-L.; Wu, C.-T.; Yang, P.-C. Programmed cell death-ligand 1 expression is associated with a favourable immune microenvironment and better overall survival in stage I pulmonary squamous cell carcinoma. Eur. J. Cancer 2016, 57, 91–103. [Google Scholar] [CrossRef]
- Gainor, J.F.; Shaw, A.T.; Sequist, L.V.; Fu, X.; Azzoli, C.G.; Piotrowska, Z.; Huynh, T.G.; Zhao, L.; Fulton, L.; Schultz, K.R.; et al. EGFR Mutations and ALK Rearrangements Are Associated with Low Response Rates to PD-1 Pathway Blockade in Non–Small Cell Lung Cancer: A Retrospective Analysis. Clin. Cancer Res. 2016, 22, 4585–4593. [Google Scholar] [CrossRef]
- Detterbeck, F.C.; Boffa, D.J.; Kim, A.W.; Tanoue, L.T. The Eighth Edition Lung Cancer Stage Classification. Chest 2017, 151, 193–203. [Google Scholar] [CrossRef] [PubMed]
- McLane, L.M.; Abdel-Hakeem, M.S.; Wherry, E.J. CD8 T Cell Exhaustion During Chronic Viral Infection and Cancer. Annu. Rev. Immunol. 2019, 37, 457–495. [Google Scholar] [CrossRef]
- Väyrynen, J.P.; Lau, M.C.; Haruki, K.; Väyrynen, S.A.; Costa, A.D.; Borowsky, J.; Zhao, M.; Fujiyoshi, K.; Arima, K.; Twombly, T.S.; et al. Prognostic Significance of Immune Cell Populations Identified by Machine Learning in Colorectal Cancer Using Routine Hematoxylin and Eosin–Stained Sections. Clin. Cancer Res. 2020, 26, 4326–4338. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, S.; Gong, D.; Qin, Y.; Shen, Q. Programmed death-1 upregulation is correlated with dysfunction of tumor-infiltrating CD8+ T lymphocytes in human non-small cell lung cancer. Cell. Mol. Immunol. 2010, 7, 389–395. [Google Scholar] [CrossRef]
- Que, Y.; Xiao, W.; Guan, Y.-X.; Liang, Y.; Yan, S.-M.; Chen, H.-Y.; Li, Q.-Q.; Xu, B.-S.; Zhou, Z.-W.; Zhang, X. PD-L1 Expression Is Associated with FOXP3+ Regulatory T-Cell Infiltration of Soft Tissue Sarcoma and Poor Patient Prognosis. J. Cancer 2017, 8, 2018–2025. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Wei, X.; Jiang, H.; Lan, C.; Yang, S.; Wang, H.; Yang, Y.; Tian, C.; Xu, Z.; et al. PD-L1 is a direct target of cancer-FOXP3 in pancreatic ductal adenocarcinoma (PDAC), and combined immunotherapy with antibodies against PD-L1 and CCL5 is effective in the treatment of PDAC. Signal Transduct. Target. Ther. 2020, 5, 38. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.-W.; Li, C.; Zhang, R.-L.; Xue, H.-G.; Zhang, F.-X.; Zhang, F.; Gai, X.-D. B7-H1 and B7-H4 expression in colorectal carcinoma: Correlation with tumor FOXP3+ regulatory T-cell infiltration. Acta Histochem. 2014, 116, 1163–1168. [Google Scholar] [CrossRef]
- Amarnath, S.; Mangus, C.W.; Wang, J.C.M.; Wei, F.; He, A.; Kapoor, V.; Foley, J.E.; Massey, P.R.; Felizardo, T.C.; Riley, J.L.; et al. The PDL1-PD1 Axis Converts Human TH1 Cells into Regulatory T Cells. Sci. Transl. Med. 2011, 3, 111ra120. [Google Scholar] [CrossRef]
- Fanelli, G.; Romano, M.; Nova-Lamperti, E.; Sunderland, M.W.; Nerviani, A.; Scottà, C.; Bombardieri, M.; Quezada, S.A.; Sacks, S.H.; Noelle, R.J.; et al. PD-L1 signaling on human memory CD4+ T cells induces a regulatory phenotype. PLoS Biol. 2021, 19, e3001199. [Google Scholar] [CrossRef]
- Zhang, B.; Zeng, J.; Zhang, H.; Zhu, S.; Wang, H.; He, J.; Yang, L.; Zhou, N.; Zu, L.; Xu, X.; et al. Characteristics of the immune microenvironment and their clinical significance in non-small cell lung cancer patients with ALK-rearranged mutation. Front. Immunol. 2022, 13, 974581. [Google Scholar] [CrossRef]
- Budczies, J.; Kirchner, M.; Kluck, K.; Kazdal, D.; Glade, J.; Allgäuer, M.; Kriegsmann, M.; Heußel, C.-P.; Herth, F.J.; Winter, H.; et al. Deciphering the immunosuppressive tumor microenvironment in ALK- and EGFR-positive lung adenocarcinoma. Cancer Immunol. Immunother. 2022, 71, 251–265. [Google Scholar] [CrossRef]
- Li, C.; Jiang, P.; Wei, S.; Xu, X.; Wang, J. Regulatory T cells in tumor microenvironment: New mechanisms, potential therapeutic strategies and future prospects. Mol. Cancer 2020, 19, 116. [Google Scholar] [CrossRef]
- Kandl, T.J.; Sagiv, O.; Curry, J.L.; Ning, J.; Ma, J.; Hudgens, C.W.; Van Arnam, J.; Wargo, J.A.; Esmaeli, B.; Tetzlaff, M.T. High expression of PD-1 and PD-L1 in ocular adnexal sebaceous carcinoma. Oncoimmunology 2018, 7, e1475874. [Google Scholar] [CrossRef]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Gao, Y.; Xiong, J.; Lu, J.; Yang, J.; Wang, X.; Cai, Y.; Li, L.; Fu, X. Tumor-infiltrating CD8+ T cells in ALK-positive lung cancer are functionally impaired despite the absence of PD-L1 on tumor cells. Lung Cancer 2020, 150, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Jin, E.A.R.; Liu, C.; Zheng, S.; Wang, X.; Feng, X.; Li, H.; Sun, N.; He, J. Molecular heterogeneity of anti-PD-1/PD-L1 immunotherapy efficacy is correlated with tumor immune microenvironment in East Asian patients with non-small cell lung cancer. Cancer Biol. Med. 2020, 17, 768–781. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Weigert, A.; Reu, S.; Guenther, S.; Mansouri, S.; Bassaly, B.; Gattenlöhner, S.; Grimminger, F.; Pullamsetti, S.S.; Seeger, W.; et al. Spatial Density and Distribution of Tumor-Associated Macrophages Predict Survival in Non–Small Cell Lung Carcinoma. Cancer Res. 2020, 80, 4414–4425. [Google Scholar] [CrossRef]
- Väyrynen, J.P.; Haruki, K.; Väyrynen, S.; Lau, M.C.; Costa, A.D.; Borowsky, J.; Zhao, M.; Ugai, T.; Kishikawa, J.; Akimoto, N.; et al. Prognostic significance of myeloid immune cells and their spatial distribution in the colorectal cancer microenvironment. J. Immunother. Cancer 2021, 9, e002297. [Google Scholar] [CrossRef]
- Barua, S.; Fang, P.; Sharma, A.; Fujimoto, J.; Wistuba, I.; Rao, A.U.; Lin, S.H. Spatial interaction of tumor cells and regulatory T cells correlates with survival in non-small cell lung cancer. Lung Cancer 2018, 117, 73–79. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, W.; Sun, J.; Yang, G.; Cai, S.; Sun, F.; Xing, L.; Sun, X. Functional status and spatial interaction of T cell subsets driven by specific tumor microenvironment correlate with recurrence of non-small cell lung cancer. Front. Immunol. 2022, 13, 1022638. [Google Scholar] [CrossRef]
- Maynard, A.; McCoach, C.E.; Rotow, J.K.; Harris, L.; Haderk, F.; Kerr, D.L.; Yu, E.A.; Schenk, E.L.; Tan, W.; Zee, A.; et al. Therapy-Induced Evolution of Human Lung Cancer Revealed by Single-Cell RNA Sequencing. Cell 2020, 182, 1232–1251.e22. [Google Scholar] [CrossRef]
- Koh, J.; Go, H.; Keam, B.; Kim, M.-Y.; Nam, S.J.; Kim, T.M.; Lee, S.-H.; Min, H.S.; Kim, Y.T.; Kim, D.-W.; et al. Clinicopathologic analysis of programmed cell death-1 and programmed cell death-ligand 1 and 2 expressions in pulmonary adenocarcinoma: Comparison with histology and driver oncogenic alteration status. Mod. Pathol. 2015, 28, 1154–1166. [Google Scholar] [CrossRef]
- Sullivan, J.A.; Tomita, Y.; Jankowska-Gan, E.; Lema, D.A.; Arvedson, M.P.; Nair, A.; Bracamonte-Baran, W.; Zhou, Y.; Meyer, K.K.; Zhong, W.; et al. Treg-Cell-Derived IL-35-Coated Extracellular Vesicles Promote Infectious Tolerance. Cell Rep. 2020, 30, 1039–1051.e5. [Google Scholar] [CrossRef]
- Sawant, D.V.; Yano, H.; Chikina, M.; Zhang, Q.; Liao, M.; Liu, C.; Callahan, D.J.; Sun, Z.; Sun, T.; Tabib, T.; et al. Adaptive plasticity of IL-10(+) and IL-35(+) T(reg) cells cooperatively promotes tumor T cell exhaustion. Nat. Immunol. 2019, 20, 724–735. [Google Scholar] [CrossRef]
- Garnelo, M.; Tan, A.; Her, Z.; Yeong, J.; Lim, C.J.; Chen, J.; Lim, K.H.; Weber, A.; Chow, P.; Chung, A.; et al. Interaction between tumour-infiltrating B cells and T cells controls the progression of hepatocellular carcinoma. Gut 2017, 66, 342–351. [Google Scholar] [CrossRef]

| Variable | Total |
|---|---|
| Age | |
| Median (25%, 75%) | 51 (41, 61) |
| Sex, n (%) | |
| Male | 21 (40%) |
| Female | 31 (60%) |
| Smoking status, n (%) | |
| Non-smoker | 38 (73%) |
| Former/current smoker | 14 (27%) |
| Stage, n (%) | |
| IIIB–IIIC | 3 (6%) |
| IV | 49 (94%) |
| Histology, n (%) | |
| Adenocarcinoma | 52 (100%) |
| ECOG PS, n (%) | |
| 0 | 18 (35%) |
| 1 | 27 (52%) |
| 2 | 7 (13%) |
| Metastasis, n (%) | |
| Brain | 18 (35%) |
| Bone | 24 (46%) |
| Liver | 11 (21%) |
| Adrenal glands | 4 (8%) |
| Pleura | 13 (25%) |
| Contralateral lung | 18 (35%) |
| TKI, n (%) | |
| Crizotinib | 42 (81%) |
| Alectinib | 10 (19%) |
| PD-L1 TPS, n (%) | |
| 0% | 23 (44%) |
| 1–49% | 17 (33%) |
| ≥50% | 12 (23%) |
| Variable | PD-L1 < 50% (n = 40) | PD-L1 ≥ 50% (n = 12) | p Value |
|---|---|---|---|
| Age | |||
| <65 year | 31 (77.5%) | 10 (83.3%) | 1.000 |
| ≥65 year | 9 (22.5%) | 2 (16.7%) | |
| Sex | |||
| Male | 16 (40.0%) | 5 (41.7%) | 1.000 |
| Female | 24 (60.0%) | 7 (58.3%) | |
| Smoking status | |||
| Never | 28 (70.0%) | 10 (83.3%) | 0.475 |
| Former/current | 12 (30.0%) | 2 (16.7%) | |
| Stage | |||
| IIIB–IIIC | 3 (7.5%) | 0 | 1.000 |
| IV | 37 (92.5%) | 12 (100.0%) | |
| ECOG PS | 0.103 | ||
| 0 | 15 (37.5%) | 3 (25.0%) | |
| 1 | 22 (55.0%) | 5 (41.7%) | |
| 2 | 3 (7.5%) | 4 (33.3%) | |
| Metastasis | |||
| Brain | 12 (30.0%) | 6 (50.0%) | 0.300 |
| Bone | 17 (42.5%) | 7 (58.3%) | 0.510 |
| Liver | 8 (20.0%) | 3 (25.0%) | 0.701 |
| Adrenal glands | 3 (7.5%) | 1 (8.3%) | 1.000 |
| Pleura | 10 (25.0%) | 3 (25.0%) | 1.000 |
| Contralateral lung | 11 (27.5%) | 7 (58.3%) | 0.082 |
| TKI | |||
| Crizotinib | 32 (80.0%) | 10 (83.3%) | 1.000 |
| Alectinib | 8 (20.0%) | 2 (16.7%) |
| Variable | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Age | ||||
| <65 year | Reference [1] | |||
| ≥65 year | 1.342 (0.610, 2.952) | 0.465 | ||
| Sex | ||||
| Male | Reference [1] | |||
| Female | 0.689 (0.367, 1.292) | 0.245 | ||
| Smoking status | ||||
| Never | Reference [1] | |||
| Former/current | 1.723 (0.869, 3.418) | 0.119 | ||
| Stage | ||||
| IIIB–IIIC | Reference [1] | |||
| IV | 1.319 (0.317, 5.495) | 0.704 | ||
| ECOG PS | ||||
| 0 | Reference [1] | |||
| 1–2 | 1.087 (0.573, 2.061) | 0.798 | ||
| Metastasis | ||||
| Brain | 1.710 (0.902, 3.245) | 0.100 | ||
| Bone | 1.956 (1.045, 3.660) | 0.036 | 2.123 (1.119, 4.028) | 0.021 |
| Liver | 1.773 (0.862, 3.647) | 0.120 | ||
| Adrenal glands | 2.842 (0.993, 8.132) | 0.051 | ||
| Pleura | 1.004 (0.490, 2.057) | 0.990 | ||
| Contralateral lung | 1.733 (0.922, 3.255) | 0.088 | ||
| PD-L1 expression | ||||
| <50% | Reference [1] | |||
| ≥50% | 2.532 (1.247, 5.141) | 0.010 | 2.792 (1.348, 5.785) | 0.006 |
| TKI | ||||
| Crizotinib | Reference [1] | |||
| Alectinib | 0.397 (0.155, 1.019) | 0.055 | ||
| Variable | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Age | ||||
| <65 year | Reference [1] | |||
| ≥65 year | 1.130 (0.371, 3.444) | 0.830 | ||
| Sex | ||||
| Male | Reference [1] | |||
| Female | 0.859 (0.332, 2.219) | 0.753 | ||
| Smoking status | ||||
| Never | Reference [1] | |||
| Former/current | 1.193 (0.425, 3.349) | 0.738 | ||
| Stage | ||||
| IIIB–IIIC | Reference [1] | |||
| IV | 0.308 (0.068, 1.393) | 0.126 | ||
| ECOG PS | ||||
| 0 | Reference [1] | Reference [1] | ||
| 1–2 | 5.870 (1.344, 25.644) | 0.019 | 6.420 (1.449, 28.439) | 0.014 |
| Metastasis | ||||
| Brain | 1.602 (0.616, 4.166) | 0.334 | ||
| Bone | 2.349 (0.901, 6.128) | 0.081 | ||
| Liver | 2.260 (0.835, 6.117) | 0.109 | ||
| Adrenal glands | 1.306 (0.300, 5.688) | 0.722 | ||
| Pleura | 0.891 (0.293, 2.710) | 0.839 | ||
| Contralateral lung | 1.864 (0.739, 4.699) | 0.187 | ||
| PD-L1 expression | ||||
| <50% | Reference [1] | Reference [1] | ||
| ≥50% | 2.945 (1.128, 7.691) | 0.027 | 3.348 (1.231, 9.107) | 0.018 |
| TKI | ||||
| Crizotinib | Reference [1] | |||
| Alectinib | 0.275 (0.036, 2.076) | 0.211 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, X.; Li, Y.; Huang, Q.; Zeng, H.; Wei, Q.; Tian, P. High PD-L1 Expression Correlates with an Immunosuppressive Tumour Immune Microenvironment and Worse Prognosis in ALK-Rearranged Non-Small Cell Lung Cancer. Biomolecules 2023, 13, 991. https://doi.org/10.3390/biom13060991
Tian X, Li Y, Huang Q, Zeng H, Wei Q, Tian P. High PD-L1 Expression Correlates with an Immunosuppressive Tumour Immune Microenvironment and Worse Prognosis in ALK-Rearranged Non-Small Cell Lung Cancer. Biomolecules. 2023; 13(6):991. https://doi.org/10.3390/biom13060991
Chicago/Turabian StyleTian, Xia, Yalun Li, Qin Huang, Hao Zeng, Qi Wei, and Panwen Tian. 2023. "High PD-L1 Expression Correlates with an Immunosuppressive Tumour Immune Microenvironment and Worse Prognosis in ALK-Rearranged Non-Small Cell Lung Cancer" Biomolecules 13, no. 6: 991. https://doi.org/10.3390/biom13060991
APA StyleTian, X., Li, Y., Huang, Q., Zeng, H., Wei, Q., & Tian, P. (2023). High PD-L1 Expression Correlates with an Immunosuppressive Tumour Immune Microenvironment and Worse Prognosis in ALK-Rearranged Non-Small Cell Lung Cancer. Biomolecules, 13(6), 991. https://doi.org/10.3390/biom13060991





