Dysregulation of SWI/SNF Chromatin Remodelers in NSCLC: Its Influence on Cancer Therapies including Immunotherapy
Abstract
1. Introduction
2. Cancer Epigenetics and SWI/SNF Chromatin Remodelers
3. The Composition and Modular Structure of Human SWI/SNF Chromatin Remodelers
4. The Most Common Dysregulation of SWI/SNF in NSCLC
5. Mechanisms of Dysregulation of SWI/SNF Complexes Contributing to the Tumorigenesis of NSCLC
5.1. Decreased Function of Tumor Suppressor Genes via the Dysfunction of SWI/SNF Complexes
5.2. Increased Expression of some Oncogenes Caused by the Dysfunction of SWI/SNF Complexes
5.3. Impairment of DNA Damage Repair Pathways and Genomic Instability by the Dysfunction of SWI/SNF Complexes
6. Vulnerability of NSCLC with Dysregulation of SWI/SNF Complexes
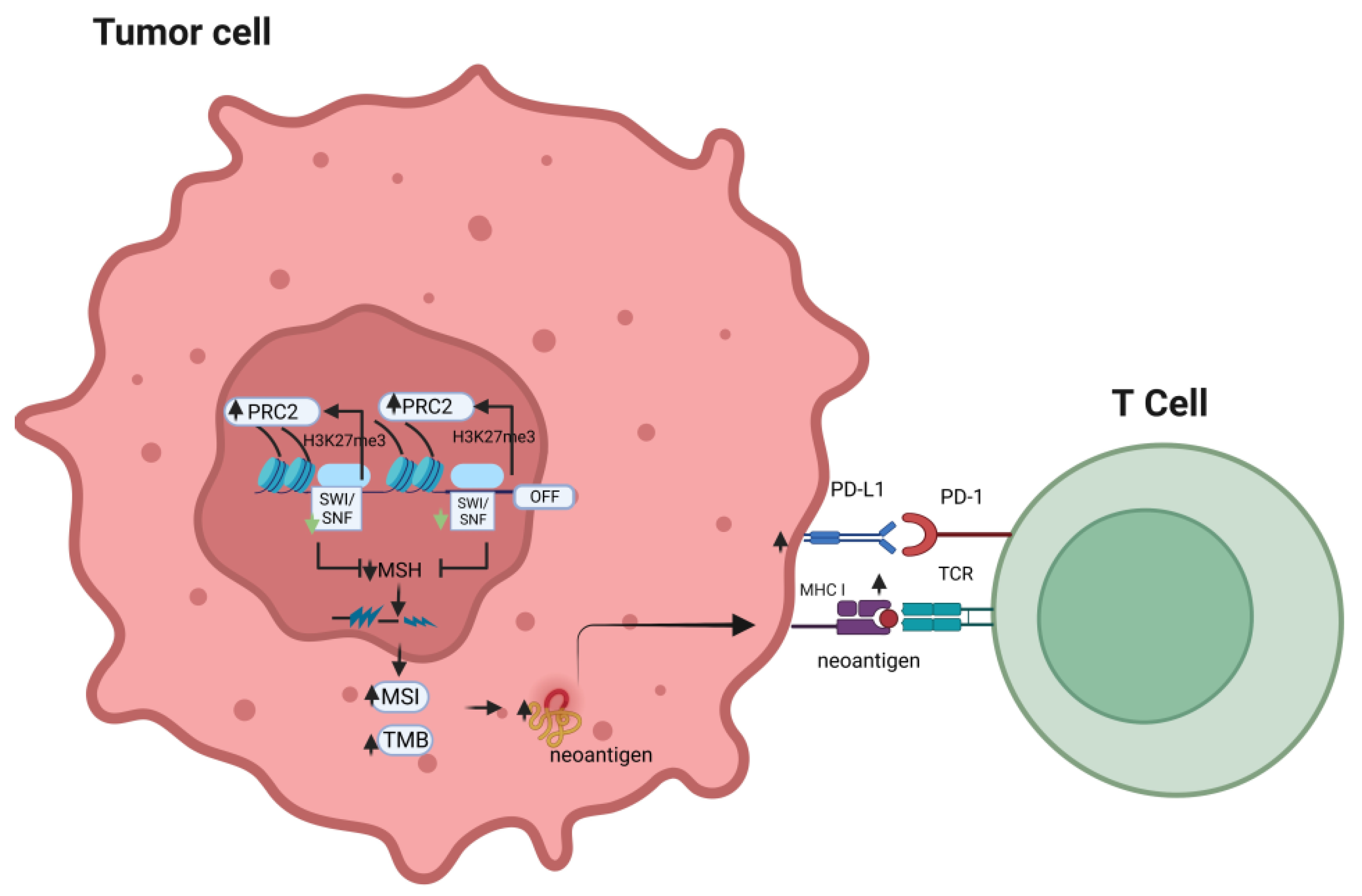
7. SWI/SNF Deficiency Influences the Immunogenicity of Malignancies
8. Outcome of ICI Treatment for NSCLC with Deficiency of SWI/SNF
9. Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Ganti, A.K.; Klein, A.B.; Cotarla, I.; Seal, B.; Chou, E. Update of Incidence, Prevalence, Survival, and Initial Treatment in Patients With Non-Small Cell Lung Cancer in the US. JAMA Oncol. 2021, 7, 1824–1832. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, I.B. Cancer. Addiction to Oncogenes--the Achilles Heal of Cancer. Science 2002, 297, 63–64. [Google Scholar] [CrossRef] [PubMed]
- Chevallier, M.; Borgeaud, M.; Addeo, A.; Friedlaender, A. Oncogenic Driver Mutations in Non-Small Cell Lung Cancer: Past, Present and Future. World J. Clin. Oncol. 2021, 12, 217–237. [Google Scholar] [CrossRef]
- Andrews Wright, N.M.; Goss, G.D. Third-Generation Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors for the Treatment of Non-Small Cell Lung Cancer. Transl. Lung Cancer Res. 2019, 8, S247–S264. [Google Scholar] [CrossRef]
- Kazandjian, D.; Blumenthal, G.M.; Chen, H.-Y.; He, K.; Patel, M.; Justice, R.; Keegan, P.; Pazdur, R. FDA Approval Summary: Crizotinib for the Treatment of Metastatic Non-Small Cell Lung Cancer with Anaplastic Lymphoma Kinase Rearrangements. Oncologist 2014, 19, e5-11. [Google Scholar] [CrossRef]
- Nakajima, E.C.; Drezner, N.; Li, X.; Mishra-Kalyani, P.S.; Liu, Y.; Zhao, H.; Bi, Y.; Liu, J.; Rahman, A.; Wearne, E.; et al. FDA Approval Summary: Sotorasib for KRAS G12C-Mutated Metastatic NSCLC. Clin. Cancer Res. 2022, 28, 1482–1486. [Google Scholar] [CrossRef]
- Ettinger, D.S.; Wood, D.E.; Aisner, D.L.; Akerley, W.; Bauman, J.R.; Bharat, A.; Bruno, D.S.; Chang, J.Y.; Chirieac, L.R.; D’Amico, T.A.; et al. Non-Small Cell Lung Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 497–530. [Google Scholar] [CrossRef]
- Lin, J.J.; Shaw, A.T. Resisting Resistance: Targeted Therapies in Lung Cancer. Trends Cancer 2016, 2, 350–364. [Google Scholar] [CrossRef]
- De Silva, P.; Aiello, M.; Gu-Trantien, C.; Migliori, E.; Willard-Gallo, K.; Solinas, C. Targeting CTLA-4 in Cancer: Is It the Ideal Companion for PD-1 Blockade Immunotherapy Combinations? Int. J. Cancer 2021, 149, 31–41. [Google Scholar] [CrossRef]
- Comin-Anduix, B.; Escuin-Ordinas, H.; Ibarrondo, F.J. Tremelimumab: Research and Clinical Development. Onco Targets Ther. 2016, 9, 1767–1776. [Google Scholar] [CrossRef]
- Shields, M.D.; Marin-Acevedo, J.A.; Pellini, B. Immunotherapy for Advanced Non-Small Cell Lung Cancer: A Decade of Progress. Am. Soc. Clin. Oncol. Educ. Book 2021, 41, e105–e127. [Google Scholar] [CrossRef]
- Frisone, D.; Friedlaender, A.; Addeo, A.; Tsantoulis, P. The Landscape of Immunotherapy Resistance in NSCLC. Front. Oncol. 2022, 12, 817548. [Google Scholar] [CrossRef]
- Jenkins, R.W.; Barbie, D.A.; Flaherty, K.T. Mechanisms of Resistance to Immune Checkpoint Inhibitors. Br. J. Cancer 2018, 118, 9–16. [Google Scholar] [CrossRef]
- Bai, R.; Lv, Z.; Xu, D.; Cui, J. Predictive Biomarkers for Cancer Immunotherapy with Immune Checkpoint Inhibitors. Biomark. Res. 2020, 8, 34. [Google Scholar] [CrossRef] [PubMed]
- Mino-Kenudson, M.; Schalper, K.; Cooper, W.; Dacic, S.; Hirsch, F.R.; Jain, D.; Lopez-Rios, F.; Tsao, M.S.; Yatabe, Y.; Beasley, M.B.; et al. Predictive Biomarkers for Immunotherapy in Lung Cancer: Perspective From the International Association for the Study of Lung Cancer Pathology Committee. J. Thorac. Oncol. 2022, 17, 1335–1354. [Google Scholar] [CrossRef]
- Dai, E.; Zhu, Z.; Wahed, S.; Qu, Z.; Storkus, W.J.; Guo, Z.S. Epigenetic Modulation of Antitumor Immunity for Improved Cancer Immunotherapy. Mol. Cancer 2021, 20, 171. [Google Scholar] [CrossRef] [PubMed]
- Sager, R. Expression Genetics in Cancer: Shifting the Focus from DNA to RNA. Proc. Natl. Acad. Sci. USA 1997, 94, 952–955. [Google Scholar] [CrossRef] [PubMed]
- Richmond, T.J.; Davey, C.A. The Structure of DNA in the Nucleosome Core. Nature 2003, 423, 145–150. [Google Scholar] [CrossRef]
- Sahu, R.K.; Singh, S.; Tomar, R.S. The Mechanisms of Action of Chromatin Remodelers and Implications in Development and Disease. Biochem. Pharmacol. 2020, 180, 114200. [Google Scholar] [CrossRef]
- Yang, S.; Huang, Y.; Zhao, Q. Epigenetic Alterations and Inflammation as Emerging Use for the Advancement of Treatment in Non-Small Cell Lung Cancer. Front. Immunol. 2022, 13, 878740. [Google Scholar] [CrossRef]
- Clapier, C.R.; Iwasa, J.; Cairns, B.R.; Peterson, C.L. Mechanisms of Action and Regulation of ATP-Dependent Chromatin-Remodelling Complexes. Nat. Rev. Mol. Cell Biol. 2017, 18, 407–422. [Google Scholar] [CrossRef]
- Aeddula, N.R.; Pathireddy, S.; Ansari, A.; Juran, P.J. Hydralazine-Associated Antineutrophil Cytoplasmic Antibody Vasculitis with Pulmonary-Renal Syndrome. BMJ Case Rep. 2018, 11, e227161. [Google Scholar] [CrossRef]
- Cornacchia, E.; Golbus, J.; Maybaum, J.; Strahler, J.; Hanash, S.; Richardson, B. Hydralazine and Procainamide Inhibit T Cell DNA Methylation and Induce Autoreactivity. J. Immunol. 1988, 140, 2197–2200. [Google Scholar] [CrossRef]
- Lopes, N.; Pacheco, M.B.; Soares-Fernandes, D.; Correia, M.P.; Camilo, V.; Henrique, R.; Jerónimo, C. Hydralazine and Enzalutamide: Synergistic Partners against Prostate Cancer. Biomedicines 2021, 9, 976. [Google Scholar] [CrossRef]
- Ansari, J.; Shackelford, R.E.; El-Osta, H. Epigenetics in Non-Small Cell Lung Cancer: From Basics to Therapeutics. Transl. Lung Cancer Res. 2016, 5, 155–171. [Google Scholar] [CrossRef]
- Kadoch, C.; Hargreaves, D.C.; Hodges, C.; Elias, L.; Ho, L.; Ranish, J.; Crabtree, G.R. Proteomic and Bioinformatic Analysis of Mammalian SWI/SNF Complexes Identifies Extensive Roles in Human Malignancy. Nat. Genet. 2013, 45, 592–601. [Google Scholar] [CrossRef] [PubMed]
- Harrod, A.; Lane, K.A.; Downs, J.A. The Role of the SWI/SNF Chromatin Remodelling Complex in the Response to DNA Double Strand Breaks. DNA Repair 2020, 93, 102919. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.I. Diverse Functions of ATP-Dependent Chromatin Remodeling Complexes in Development and Cancer. Acta Biochim. Biophys. Sin. 2012, 44, 54–69. [Google Scholar] [CrossRef] [PubMed]
- Shang, X.-Y.; Shi, Y.; He, D.-D.; Wang, L.; Luo, Q.; Deng, C.-H.; Qu, Y.-L.; Wang, N.; Han, Z.-G. ARID1A Deficiency Weakens BRG1-RAD21 Interaction That Jeopardizes Chromatin Compactness and Drives Liver Cancer Cell Metastasis. Cell Death Dis. 2021, 12, 990. [Google Scholar] [CrossRef]
- Sandhya, S.; Maulik, A.; Giri, M.; Singh, M. Domain Architecture of BAF250a Reveals the ARID and ARM-Repeat Domains with Implication in Function and Assembly of the BAF Remodeling Complex. PLoS ONE 2018, 13, e0205267. [Google Scholar] [CrossRef] [PubMed]
- Mashtalir, N.; D’Avino, A.R.; Michel, B.C.; Luo, J.; Pan, J.; Otto, J.E.; Zullow, H.J.; McKenzie, Z.M.; Kubiak, R.L.; St Pierre, R.; et al. Modular Organization and Assembly of SWI/SNF Family Chromatin Remodeling Complexes. Cell 2018, 175, 1272–1288.e20. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Wu, Z.; Tian, Y.; Yu, Z.; Yu, J.; Wang, X.; Li, J.; Liu, B.; Xu, Y. Structure of Nucleosome-Bound Human BAF Complex. Science 2020, 367, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Chen, K.; Zhang, W.; Chen, Z. Structure of Human Chromatin-Remodelling PBAF Complex Bound to a Nucleosome. Nature 2022, 605, 166–171. [Google Scholar] [CrossRef]
- Jiang, T.; Chen, X.; Su, C.; Ren, S.; Zhou, C. Pan-Cancer Analysis of ARID1A Alterations as Biomarkers for Immunotherapy Outcomes. J. Cancer 2020, 11, 776–780. [Google Scholar] [CrossRef]
- Wang, N.; Qin, Y.; Du, F.; Wang, X.; Song, C. Prevalence of SWI/SNF Genomic Alterations in Cancer and Association with the Response to Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. Gene 2022, 834, 146638. [Google Scholar] [CrossRef]
- Wang, W.; Côté, J.; Xue, Y.; Zhou, S.; Khavari, P.A.; Biggar, S.R.; Muchardt, C.; Kalpana, G.V.; Goff, S.P.; Yaniv, M.; et al. Purification and Biochemical Heterogeneity of the Mammalian SWI-SNF Complex. EMBO J. 1996, 15, 5370–5382. [Google Scholar] [CrossRef]
- Shain, A.H.; Pollack, J.R. The Spectrum of SWI/SNF Mutations, Ubiquitous in Human Cancers. PLoS ONE 2013, 8, e55119. [Google Scholar] [CrossRef]
- Roberts, C.W.; Galusha, S.A.; McMenamin, M.E.; Fletcher, C.D.; Orkin, S.H. Haploinsufficiency of Snf5 (Integrase Interactor 1) Predisposes to Malignant Rhabdoid Tumors in Mice. Proc. Natl. Acad. Sci. USA 2000, 97, 13796–13800. [Google Scholar] [CrossRef]
- Versteege, I.; Sévenet, N.; Lange, J.; Rousseau-Merck, M.F.; Ambros, P.; Handgretinger, R.; Aurias, A.; Delattre, O. Truncating Mutations of HSNF5/INI1 in Aggressive Paediatric Cancer. Nature 1998, 394, 203–206. [Google Scholar] [CrossRef]
- Monterde, B.; Varela, I. Role of SWI/SNF Chromatin Remodeling Genes in Lung Cancer Development. Biochem. Soc. Trans. 2022, 50, 1143–1150. [Google Scholar] [CrossRef]
- Peinado, P.; Andrades, A.; Cuadros, M.; Rodriguez, M.I.; Coira, I.F.; Garcia, D.J.; Benitez-Cantos, M.S.; Cano, C.; Zarzuela, E.; Muñoz, J.; et al. Multi-Omic Alterations of the SWI/SNF Complex Define a Clinical Subgroup in Lung Adenocarcinoma. Clin. Epigenetics 2022, 14, 42. [Google Scholar] [CrossRef]
- Naito, T.; Udagawa, H.; Umemura, S.; Sakai, T.; Zenke, Y.; Kirita, K.; Matsumoto, S.; Yoh, K.; Niho, S.; Tsuboi, M.; et al. Non-Small Cell Lung Cancer with Loss of Expression of the SWI/SNF Complex Is Associated with Aggressive Clinicopathological Features, PD-L1-Positive Status, and High Tumor Mutation Burden. Lung Cancer 2019, 138, 35–42. [Google Scholar] [CrossRef]
- Wu, J.N.; Roberts, C.W.M. ARID1A Mutations in Cancer: Another Epigenetic Tumor Suppressor? Cancer Discov. 2013, 3, 35–43. [Google Scholar] [CrossRef]
- Rodriguez-Nieto, S.; Cañada, A.; Pros, E.; Pinto, A.I.; Torres-Lanzas, J.; Lopez-Rios, F.; Sanchez-Verde, L.; Pisano, D.G.; Sanchez-Cespedes, M. Massive Parallel DNA Pyrosequencing Analysis of the Tumor Suppressor BRG1/SMARCA4 in Lung Primary Tumors. Hum. Mutat. 2011, 32, E1999–E2017. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.A.; Tansey, W.P.; Weissmiller, A.M. Emerging Themes in Mechanisms of Tumorigenesis by SWI/SNF Subunit Mutation. Epigenet Insights 2022, 15, 25168657221115656. [Google Scholar] [CrossRef]
- Alver, B.H.; Kim, K.H.; Lu, P.; Wang, X.; Manchester, H.E.; Wang, W.; Haswell, J.R.; Park, P.J.; Roberts, C.W.M. The SWI/SNF Chromatin Remodelling Complex Is Required for Maintenance of Lineage Specific Enhancers. Nat. Commun. 2017, 8, 14648. [Google Scholar] [CrossRef]
- Romero, O.A.; Sanchez-Cespedes, M. The SWI/SNF Genetic Blockade: Effects in Cell Differentiation, Cancer and Developmental Diseases. Oncogene 2014, 33, 2681–2689. [Google Scholar] [CrossRef]
- Jancewicz, I.; Siedlecki, J.A.; Sarnowski, T.J.; Sarnowska, E. BRM: The Core ATPase Subunit of SWI/SNF Chromatin-Remodelling Complex—A Tumour Suppressor or Tumour-Promoting Factor? Epigenetics Chromatin 2019, 12, 68. [Google Scholar] [CrossRef]
- Hung, Y.P.; Redig, A.; Hornick, J.L.; Sholl, L.M. ARID1A Mutations and Expression Loss in Non-Small Cell Lung Carcinomas: Clinicopathologic and Molecular Analysis. Mod. Pathol. 2020, 33, 2256–2268. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Xu, L.; Li, X.; Li, H.; Zhao, M. SMARCA4: Current Status and Future Perspectives in Non-Small-Cell Lung Cancer. Cancer Lett. 2023, 554, 216022. [Google Scholar] [CrossRef]
- Rada-Iglesias, A.; Bajpai, R.; Swigut, T.; Brugmann, S.A.; Flynn, R.A.; Wysocka, J. A Unique Chromatin Signature Uncovers Early Developmental Enhancers in Humans. Nature 2011, 470, 279–283. [Google Scholar] [CrossRef]
- Bitler, B.G.; Aird, K.M.; Garipov, A.; Li, H.; Amatangelo, M.; Kossenkov, A.V.; Schultz, D.C.; Liu, Q.; Shih, I.-M.; Conejo-Garcia, J.R.; et al. Synthetic Lethality by Targeting EZH2 Methyltransferase Activity in ARID1A-Mutated Cancers. Nat. Med. 2015, 21, 231–238. [Google Scholar] [CrossRef]
- Suryo Rahmanto, Y.; Shen, W.; Shi, X.; Chen, X.; Yu, Y.; Yu, Z.-C.; Miyamoto, T.; Lee, M.-H.; Singh, V.; Asaka, R.; et al. Inactivation of Arid1a in the Endometrium Is Associated with Endometrioid Tumorigenesis through Transcriptional Reprogramming. Nat. Commun. 2020, 11, 2717. [Google Scholar] [CrossRef]
- Guan, B.; Wang, T.-L.; Shih, I.-M. ARID1A, a Factor That Promotes Formation of SWI/SNF-Mediated Chromatin Remodeling, Is a Tumor Suppressor in Gynecologic Cancers. Cancer Res. 2011, 71, 6718–6727. [Google Scholar] [CrossRef]
- Nagl, N.G.; Wang, X.; Patsialou, A.; Van Scoy, M.; Moran, E. Distinct Mammalian SWI/SNF Chromatin Remodeling Complexes with Opposing Roles in Cell-Cycle Control. EMBO J. 2007, 26, 752–763. [Google Scholar] [CrossRef]
- Gramling, S.; Reisman, D. Discovery of BRM Targeted Therapies: Novel Reactivation of an Anti-Cancer Gene. Lett. Drug Des. Discov. 2011, 8, 93–99. [Google Scholar] [CrossRef]
- Naidu, S.R.; Love, I.M.; Imbalzano, A.N.; Grossman, S.R.; Androphy, E.J. The SWI/SNF Chromatin Remodeling Subunit BRG1 Is a Critical Regulator of P53 Necessary for Proliferation of Malignant Cells. Oncogene 2009, 28, 2492–2501. [Google Scholar] [CrossRef]
- Lee, D.; Kim, J.W.; Seo, T.; Hwang, S.G.; Choi, E.-J.; Choe, J. SWI/SNF Complex Interacts with Tumor Suppressor P53 and Is Necessary for the Activation of P53-Mediated Transcription. J. Biol. Chem. 2002, 277, 22330–22337. [Google Scholar] [CrossRef] [PubMed]
- Strobeck, M.W.; Knudsen, K.E.; Fribourg, A.F.; DeCristofaro, M.F.; Weissman, B.E.; Imbalzano, A.N.; Knudsen, E.S. BRG-1 Is Required for RB-Mediated Cell Cycle Arrest. Proc. Natl. Acad. Sci. USA 2000, 97, 7748–7753. [Google Scholar] [CrossRef]
- Strober, B.E.; Dunaief, J.L.; Guha, N.; Goff, S.P. Functional Interactions between the HBRM/HBRG1 Transcriptional Activators and the PRB Family of Proteins. Mol. Cell Biol. 1996, 16, 1576–1583. [Google Scholar] [CrossRef] [PubMed]
- Hodges, H.C.; Stanton, B.Z.; Cermakova, K.; Chang, C.-Y.; Miller, E.L.; Kirkland, J.G.; Ku, W.L.; Veverka, V.; Zhao, K.; Crabtree, G.R. Dominant-Negative SMARCA4 Mutants Alter the Accessibility Landscape of Tissue-Unrestricted Enhancers. Nat. Struct. Mol. Biol. 2018, 25, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Romero, O.A.; Setien, F.; John, S.; Gimenez-Xavier, P.; Gómez-López, G.; Pisano, D.; Condom, E.; Villanueva, A.; Hager, G.L.; Sanchez-Cespedes, M. The Tumour Suppressor and Chromatin-Remodelling Factor BRG1 Antagonizes Myc Activity and Promotes Cell Differentiation in Human Cancer. EMBO Mol. Med. 2012, 4, 603–616. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Whyte, W.A.; Zepeda-Mendoza, C.J.; Milazzo, J.P.; Shen, C.; Roe, J.-S.; Minder, J.L.; Mercan, F.; Wang, E.; Eckersley-Maslin, M.A.; et al. Role of SWI/SNF in Acute Leukemia Maintenance and Enhancer-Mediated Myc Regulation. Genes Dev. 2013, 27, 2648–2662. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.R.; Reske, J.J.; Holladay, J.; Neupane, S.; Ngo, J.; Cuthrell, N.; Wegener, M.; Rhodes, M.; Adams, M.; Sheridan, R.; et al. ARID1A Mutations Promote P300-Dependent Endometrial Invasion through Super-Enhancer Hyperacetylation. Cell Rep. 2020, 33, 108366. [Google Scholar] [CrossRef]
- Concepcion, C.P.; Ma, S.; LaFave, L.M.; Bhutkar, A.; Liu, M.; DeAngelo, L.P.; Kim, J.Y.; Del Priore, I.; Schoenfeld, A.J.; Miller, M.; et al. Smarca4 Inactivation Promotes Lineage-Specific Transformation and Early Metastatic Features in the Lung. Cancer Discov. 2022, 12, 562–585. [Google Scholar] [CrossRef]
- Hays, E.; Nettleton, E.; Carter, C.; Morales, M.; Vo, L.; Passo, M.; Vélez-Cruz, R. The SWI/SNF ATPase BRG1 Stimulates DNA End Resection and Homologous Recombination by Reducing Nucleosome Density at DNA Double Strand Breaks and by Promoting the Recruitment of the CtIP Nuclease. Cell Cycle 2020, 19, 3096–3114. [Google Scholar] [CrossRef]
- Mimitou, E.P.; Yamada, S.; Keeney, S. A Global View of Meiotic Double-Strand Break End Resection. Science 2017, 355, 40–45. [Google Scholar] [CrossRef]
- Watanabe, R.; Ui, A.; Kanno, S.-I.; Ogiwara, H.; Nagase, T.; Kohno, T.; Yasui, A. SWI/SNF Factors Required for Cellular Resistance to DNA Damage Include ARID1A and ARID1B and Show Interdependent Protein Stability. Cancer Res. 2014, 74, 2465–2475. [Google Scholar] [CrossRef]
- Shen, J.; Peng, Y.; Wei, L.; Zhang, W.; Yang, L.; Lan, L.; Kapoor, P.; Ju, Z.; Mo, Q.; Shih, I.-M.; et al. ARID1A Deficiency Impairs the DNA Damage Checkpoint and Sensitizes Cells to PARP Inhibitors. Cancer Discov. 2015, 5, 752–767. [Google Scholar] [CrossRef]
- Park, J.-H.; Park, E.-J.; Lee, H.-S.; Kim, S.J.; Hur, S.-K.; Imbalzano, A.N.; Kwon, J. Mammalian SWI/SNF Complexes Facilitate DNA Double-Strand Break Repair by Promoting Gamma-H2AX Induction. EMBO J. 2006, 25, 3986–3997. [Google Scholar] [CrossRef]
- Shen, J.; Ju, Z.; Zhao, W.; Wang, L.; Peng, Y.; Ge, Z.; Nagel, Z.D.; Zou, J.; Wang, C.; Kapoor, P.; et al. ARID1A Deficiency Promotes Mutability and Potentiates Therapeutic Antitumor Immunity Unleashed by Immune Checkpoint Blockade. Nat. Med. 2018, 24, 556–562. [Google Scholar] [CrossRef]
- Mandal, J.; Mandal, P.; Wang, T.-L.; Shih, I.-M. Treating ARID1A Mutated Cancers by Harnessing Synthetic Lethality and DNA Damage Response. J. Biomed. Sci. 2022, 29, 71. [Google Scholar] [CrossRef]
- Mou, P.K.; Yang, E.J.; Shi, C.; Ren, G.; Tao, S.; Shim, J.S. Aurora Kinase A, a Synthetic Lethal Target for Precision Cancer Medicine. Exp. Mol. Med. 2021, 53, 835–847. [Google Scholar] [CrossRef] [PubMed]
- Tagal, V.; Wei, S.; Zhang, W.; Brekken, R.A.; Posner, B.A.; Peyton, M.; Girard, L.; Hwang, T.; Wheeler, D.A.; Minna, J.D.; et al. SMARCA4-Inactivating Mutations Increase Sensitivity to Aurora Kinase A Inhibitor VX-680 in Non-Small Cell Lung Cancers. Nat. Commun. 2017, 8, 14098. [Google Scholar] [CrossRef]
- Wanior, M.; Krämer, A.; Knapp, S.; Joerger, A.C. Exploiting Vulnerabilities of SWI/SNF Chromatin Remodelling Complexes for Cancer Therapy. Oncogene 2021, 40, 3637–3654. [Google Scholar] [CrossRef]
- Centore, R.C.; Sandoval, G.J.; Soares, L.M.M.; Kadoch, C.; Chan, H.M. Mammalian SWI/SNF Chromatin Remodeling Complexes: Emerging Mechanisms and Therapeutic Strategies. Trends Genet. 2020, 36, 936–950. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Ogiwara, H. Synthetic Lethal Therapy Based on Targeting the Vulnerability of SWI/SNF Chromatin Remodeling Complex-Deficient Cancers. Cancer Sci. 2020, 111, 774–782. [Google Scholar] [CrossRef]
- Chabanon, R.M.; Morel, D.; Postel-Vinay, S. Exploiting Epigenetic Vulnerabilities in Solid Tumors: Novel Therapeutic Opportunities in the Treatment of SWI/SNF-Defective Cancers. Semin. Cancer Biol. 2020, 61, 180–198. [Google Scholar] [CrossRef]
- Pyziak, K.; Sroka-Porada, A.; Rzymski, T.; Dulak, J.; Łoboda, A. Potential of Enhancer of Zeste Homolog 2 Inhibitors for the Treatment of SWI/SNF Mutant Cancers and Tumor Microenvironment Modulation. Drug Dev. Res. 2021, 82, 730–753. [Google Scholar] [CrossRef]
- Morel, D.; Almouzni, G.; Soria, J.-C.; Postel-Vinay, S. Targeting Chromatin Defects in Selected Solid Tumors Based on Oncogene Addiction, Synthetic Lethality and Epigenetic Antagonism. Ann. Oncol. 2017, 28, 254–269. [Google Scholar] [CrossRef]
- Research, C. FDA Granted Accelerated Approval to Tazemetostat for Follicular Lymphoma. FDA, 2020. Available online: https://www.fda.gov/drugs/fda-granted-accelerated-approval-tazemetostat-follicular-lymphoma (accessed on 18 December 2022).
- Italiano, A.; Soria, J.-C.; Toulmonde, M.; Michot, J.-M.; Lucchesi, C.; Varga, A.; Coindre, J.-M.; Blakemore, S.J.; Clawson, A.; Suttle, B.; et al. Tazemetostat, an EZH2 Inhibitor, in Relapsed or Refractory B-Cell Non-Hodgkin Lymphoma and Advanced Solid Tumours: A First-in-Human, Open-Label, Phase 1 Study. Lancet Oncol. 2018, 19, 649–659. [Google Scholar] [CrossRef]
- Shin, D.S.; Park, K.; Garon, E.; Dubinett, S. Targeting EZH2 to Overcome the Resistance to Immunotherapy in Lung Cancer. Semin. Oncol. 2022, 49, 306–318. [Google Scholar] [CrossRef] [PubMed]
- Januario, T.; Ye, X.; Bainer, R.; Alicke, B.; Smith, T.; Haley, B.; Modrusan, Z.; Gould, S.; Yauch, R.L. PRC2-Mediated Repression of SMARCA2 Predicts EZH2 Inhibitor Activity in SWI/SNF Mutant Tumors. Proc. Natl. Acad. Sci. USA 2017, 114, 12249–12254. [Google Scholar] [CrossRef]
- Kaelin, W.G. Synthetic Lethality: A Framework for the Development of Wiser Cancer Therapeutics. Genome Med. 2009, 1, 99. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, G.R.; Rahal, R.; Buxton, F.; Xiang, K.; McAllister, G.; Frias, E.; Bagdasarian, L.; Huber, J.; Lindeman, A.; Chen, D.; et al. Functional Epigenetics Approach Identifies BRM/SMARCA2 as a Critical Synthetic Lethal Target in BRG1-Deficient Cancers. Proc. Natl. Acad. Sci. USA 2014, 111, 3128–3133. [Google Scholar] [CrossRef]
- Hughes, S.J.; Testa, A.; Thompson, N.; Churcher, I. The Rise and Rise of Protein Degradation: Opportunities and Challenges Ahead. Drug Discov. Today 2021, 26, 2889–2897. [Google Scholar] [CrossRef]
- Hulse, M.; Agarwal, A.; Wang, M.; Carter, J.; Sivakumar, M.; Vidal, B.; Brown, J.; Moore, A.; Grego, A.; Bhagwat, N.; et al. Abstract 3263: Preclinical Characterization of PRT3789, a Potent and Selective SMARCA2 Targeted Degrader. Cancer Res. 2022, 82, 3263. [Google Scholar] [CrossRef]
- Prelude Therapeutics A Phase 1 Open-Label, Multi-Center, Safety and Efficacy Study of PRT3789 in Participants with Select Advanced or Metastatic Solid Tumors with a SMARCA4 Mutation. 2022. Available online: https://clinicaltrials.gov/ (accessed on 18 December 2022).
- Moreno, T.; Monterde, B.; González-Silva, L.; Betancor-Fernández, I.; Revilla, C.; Agraz-Doblas, A.; Freire, J.; Isidro, P.; Quevedo, L.; Blanco, R.; et al. ARID2 Deficiency Promotes Tumor Progression and Is Associated with Higher Sensitivity to Chemotherapy in Lung Cancer. Oncogene 2021, 40, 2923–2935. [Google Scholar] [CrossRef]
- Xue, Y.; Meehan, B.; Fu, Z.; Wang, X.Q.D.; Fiset, P.O.; Rieker, R.; Levins, C.; Kong, T.; Zhu, X.; Morin, G.; et al. SMARCA4 Loss Is Synthetic Lethal with CDK4/6 Inhibition in Non-Small Cell Lung Cancer. Nat. Commun. 2019, 10, 557. [Google Scholar] [CrossRef]
- Lissanu Deribe, Y.; Sun, Y.; Terranova, C.; Khan, F.; Martinez-Ledesma, J.; Gay, J.; Gao, G.; Mullinax, R.A.; Khor, T.; Feng, N.; et al. Mutations in the SWI/SNF Complex Induce a Targetable Dependence on Oxidative Phosphorylation in Lung Cancer. Nat. Med. 2018, 24, 1047–1057. [Google Scholar] [CrossRef]
- Gebuhr, T.C.; Kovalev, G.I.; Bultman, S.; Godfrey, V.; Su, L.; Magnuson, T. The Role of Brg1, a Catalytic Subunit of Mammalian Chromatin-Remodeling Complexes, in T Cell Development. J. Exp. Med. 2003, 198, 1937–1949. [Google Scholar] [CrossRef]
- Guo, A.; Huang, H.; Zhu, Z.; Chen, M.J.; Shi, H.; Yuan, S.; Sharma, P.; Connelly, J.P.; Liedmann, S.; Dhungana, Y.; et al. CBAF Complex Components and MYC Cooperate Early in CD8+ T Cell Fate. Nature 2022, 607, 135–141. [Google Scholar] [CrossRef]
- Chi, T. A BAF-Centred View of the Immune System. Nat. Rev. Immunol. 2004, 4, 965–977. [Google Scholar] [CrossRef]
- Holley, D.W.; Groh, B.S.; Wozniak, G.; Donohoe, D.R.; Sun, W.; Godfrey, V.; Bultman, S.J. The BRG1 Chromatin Remodeler Regulates Widespread Changes in Gene Expression and Cell Proliferation during B Cell Activation. J. Cell Physiol. 2014, 229, 44–52. [Google Scholar] [CrossRef]
- Wang, L.; Qu, J.; Zhou, N.; Hou, H.; Jiang, M.; Zhang, X. Effect and Biomarker of Immune Checkpoint Blockade Therapy for ARID1A Deficiency Cancers. Biomed. Pharmacother. 2020, 130, 110626. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Kobayashi, A.; Jiang, P.; Ferrari de Andrade, L.; Tay, R.E.; Luoma, A.M.; Tsoucas, D.; Qiu, X.; Lim, K.; Rao, P.; et al. A Major Chromatin Regulator Determines Resistance of Tumor Cells to T Cell-Mediated Killing. Science 2018, 359, 770–775. [Google Scholar] [CrossRef] [PubMed]
- Miao, D.; Margolis, C.A.; Gao, W.; Voss, M.H.; Li, W.; Martini, D.J.; Norton, C.; Bossé, D.; Wankowicz, S.M.; Cullen, D.; et al. Genomic Correlates of Response to Immune Checkpoint Therapies in Clear Cell Renal Cell Carcinoma. Science 2018, 359, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Abou El Hassan, M.; Huang, K.; Eswara, M.B.K.; Zhao, M.; Song, L.; Yu, T.; Liu, Y.; Liu, J.C.; McCurdy, S.; Ma, A.; et al. Cancer Cells Hijack PRC2 to Modify Multiple Cytokine Pathways. PLoS ONE 2015, 10, e0126466. [Google Scholar] [CrossRef]
- Odnokoz, O.; Wavelet-Vermuse, C.; Hophan, S.L.; Bulun, S.; Wan, Y. ARID1 Proteins: From Transcriptional and Post-Translational Regulation to Carcinogenesis and Potential Therapeutics. Epigenomics 2021, 13, 809–823. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Cui, K.; Murray, D.M.; Ling, C.; Xue, Y.; Gerstein, A.; Parsons, R.; Zhao, K.; Wang, W. PBAF Chromatin-Remodeling Complex Requires a Novel Specificity Subunit, BAF200, to Regulate Expression of Selective Interferon-Responsive Genes. Genes Dev. 2005, 19, 1662–1667. [Google Scholar] [CrossRef]
- Reisman, D.; Glaros, S.; Thompson, E.A. The SWI/SNF Complex and Cancer. Oncogene 2009, 28, 1653–1668. [Google Scholar] [CrossRef] [PubMed]
- Takada, K.; Sugita, S.; Murase, K.; Kikuchi, T.; Oomori, G.; Ito, R.; Hayasaka, N.; Miyanishi, K.; Iyama, S.; Ikeda, H.; et al. Exceptionally Rapid Response to Pembrolizumab in a SMARCA4-Deficient Thoracic Sarcoma Overexpressing PD-L1: A Case Report. Thorac. Cancer 2019, 10, 2312–2315. [Google Scholar] [CrossRef]
- Velut, Y.; Decroix, E.; Blons, H.; Alifano, M.; Leroy, K.; Petitprez, F.; Boni, A.; Garinet, S.; Biton, J.; Cremer, I.; et al. SMARCA4-Deficient Lung Carcinoma Is an Aggressive Tumor Highly Infiltrated by FOXP3+ Cells and Neutrophils. Lung Cancer 2022, 169, 13–21. [Google Scholar] [CrossRef]
- Malla, R.R.; Vasudevaraju, P.; Vempati, R.K.; Rakshmitha, M.; Merchant, N.; Nagaraju, G.P. Regulatory T Cells: Their Role in Triple-Negative Breast Cancer Progression and Metastasis. Cancer 2022, 128, 1171–1183. [Google Scholar] [CrossRef]
- McFarlane, A.J.; Fercoq, F.; Coffelt, S.B.; Carlin, L.M. Neutrophil Dynamics in the Tumor Microenvironment. J. Clin. Investig. 2021, 131, 1–10. [Google Scholar] [CrossRef]
- Agaimy, A. SWI/SNF-Deficient Malignancies: Optimal Candidates for Immune-Oncological Therapy? Adv. Anat. Pathol. 2022, 30, 211–217. [Google Scholar] [CrossRef]
- Zhou, H.; Shen, J.; Liu, J.; Fang, W.; Zhang, L. Efficacy of Immune Checkpoint Inhibitors in SMARCA4-Mutant NSCLC. J. Thorac. Oncol. 2020, 15, e133–e136. [Google Scholar] [CrossRef]
- Dagogo-Jack, I.; Schrock, A.B.; Kem, M.; Jessop, N.; Lee, J.; Ali, S.M.; Ross, J.S.; Lennerz, J.K.; Shaw, A.T.; Mino-Kenudson, M. Clinicopathologic Characteristics of BRG1-Deficient NSCLC. J. Thorac. Oncol. 2020, 15, 766–776. [Google Scholar] [CrossRef]
- Schoenfeld, A.J.; Bandlamudi, C.; Lavery, J.A.; Montecalvo, J.; Namakydoust, A.; Rizvi, H.; Egger, J.; Concepcion, C.P.; Paul, S.; Arcila, M.E.; et al. The Genomic Landscape of SMARCA4 Alterations and Associations with Outcomes in Patients with Lung Cancer. Clin. Cancer Res. 2020, 26, 5701–5708. [Google Scholar] [CrossRef]
- Naito, T.; Umemura, S.; Nakamura, H.; Zenke, Y.; Udagawa, H.; Kirita, K.; Matsumoto, S.; Yoh, K.; Niho, S.; Motoi, N.; et al. Successful Treatment with Nivolumab for SMARCA4-Deficient Non-Small Cell Lung Carcinoma with a High Tumor Mutation Burden: A Case Report. Thorac. Cancer 2019, 10, 1285–1288. [Google Scholar] [CrossRef] [PubMed]
- Chang, G.; Li, W.; Bai, H.; Duan, J.; Wang, Z.; Du, X.; Yu, R.; Wang, Y.; Wang, M.; Zhu, Y.; et al. Correlations of Switch/Sucrose Nonfermentable Complex Mutations with Clinical Outcomes in Advanced Non-Small Cell Lung Cancer. Thorac. Cancer 2022, 13, 2951–2959. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Shi, R.; Li, Y.; Zhang, Z.; Xu, S.; Chen, C.; Cao, P.; Zhang, H.; Liu, M.; Pan, Z.; et al. ARID1A, ARID1B, and ARID2 Mutations Serve as Potential Biomarkers for Immune Checkpoint Blockade in Patients With Non-Small Cell Lung Cancer. Front. Immunol. 2021, 12, 670040. [Google Scholar] [CrossRef] [PubMed]
- Alessi, J.V.; Ricciuti, B.; Spurr, L.F.; Gupta, H.; Li, Y.Y.; Glass, C.; Nishino, M.; Cherniack, A.D.; Lindsay, J.; Sharma, B.; et al. SMARCA4 and Other SWItch/Sucrose NonFermentable Family Genomic Alterations in NSCLC: Clinicopathologic Characteristics and Outcomes to Immune Checkpoint Inhibition. J. Thorac. Oncol. 2021, 16, 1176–1187. [Google Scholar] [CrossRef]
- Sun, D.; Tian, L.; Zhu, Y.; Wo, Y.; Liu, Q.; Liu, S.; Li, H.; Hou, H. Subunits of ARID1 Serve as Novel Biomarkers for the Sensitivity to Immune Checkpoint Inhibitors and Prognosis of Advanced Non-Small Cell Lung Cancer. Mol. Med. 2020, 26, 78. [Google Scholar] [CrossRef]
- Miao, X.-Y.; Wu, H.; Ye, B.-C.; Yi, Q.-W.; Lin, F.-N.; Wang, Y.-L.; Ren, C.-L.; Jiang, Y.-F.; Li, A. Non-small Cell Lung Cancer Carrying PBRM1 Mutation Suggests an Immunologically Cold Phenotype Leading to Immunotherapy Failure Even with High TMB. Sci. Rep. 2022, 12, 20734. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, J.; Zhang, Y.; Huang, Y.; Shen, J.; Yang, Y.; Fang, W.; Zhang, L. PBRM1 Mutation and Preliminary Response to Immune Checkpoint Blockade Treatment in Non-Small Cell Lung Cancer. NPJ Precis. Oncol. 2020, 4, 6. [Google Scholar] [CrossRef]
- Marinelli, D.; Mazzotta, M.; Scalera, S.; Terrenato, I.; Sperati, F.; D’Ambrosio, L.; Pallocca, M.; Corleone, G.; Krasniqi, E.; Pizzuti, L.; et al. KEAP1-Driven Co-Mutations in Lung Adenocarcinoma Unresponsive to Immunotherapy despite High Tumor Mutational Burden. Ann. Oncol. 2020, 31, 1746–1754. [Google Scholar] [CrossRef]
- Cyrta, J.; Augspach, A.; Filippo, M.R.d.; Prandi, D.; Thienger, P.; Benelli, M.; Cooley, V.; Bareja, R.; Wilkes, D.; Chae, S.-S.; et al. Role of Specialized Composition of SWI/SNF Complexes in Prostate Cancer Lineage Plasticity. Nat. Commun. 2020, 11, 5549. [Google Scholar] [CrossRef]
- Khongorzul, P.; Ling, C.J.; Khan, F.U.; Ihsan, A.U.; Zhang, J. Antibody-Drug Conjugates: A Comprehensive Review. Mol. Cancer Res. 2020, 18, 3–19. [Google Scholar] [CrossRef]
| Name | Target | Year of Approval | Approved Clinical Indication |
|---|---|---|---|
| Nivolumab | PD-1 | 2022 | Nivolumab + chemotherapy as neoadjuvant treatment |
| 2020 | Nivolumab + ipilimumab + limited chemotherapy as 1st treatment of metastatic or recurrent NSCLC | ||
| 2020 | Nivolumab + ipilimumab as 1st treatment of Metastatic NSCLC (PD-L1 ≥ 1%) | ||
| 2015 | Advanced (metastatic) NSCLC progressed during or after platinum-based chemotherapy. | ||
| 2015 | Advanced (metastatic) squamous NSCLC with progression on or after platinum-based chemotherapy. | ||
| Pembrolizumab | PD-1 | 2018 | Pembrolizumab +chemotherapy for the 1st treatment of metastatic squamous NSCLC |
| 2017 | Pembrolizumab + chemotherapy for the 1st treatment of metastatic non-squamous NSCLC, ± PD-L1 | ||
| 2016 | Metastatic NSCLC (PD-L1 ≥ 50%)) without EGFR /ALK genomic tumor aberrations | ||
| 2015 | Advanced NSCLC progressed after other treatments and with tumors that express PD-L1 | ||
| Cemiplimab | PD-1 | 2022 | Cemiplimab + chemotherapy as 1st treatment for advanced NSCL |
| Atezolizumab | PD L-1 | 2021 | Adjuvant treatment following surgery and chemotherapy for stage II-IIIA NSCLC (PD-L1 ≥ 1%) |
| 2020 | 1st treatment for NSCLC with PD-L1 expression no EGFR/ALK genomic tumor aberrations | ||
| 2019 | Atezolizumab + chemotherapy for the 1st treatment of NSCLC no EGFR/ALK aberrations. | ||
| 2018 | Atezolizumab +bevacizumab+chemotherapy 1st treatment of metastatic NSCLC no EGFR/ALK aberrations | ||
| 2016 | Metastatic NSCLC who have disease progression during/following chemotherapy | ||
| Durvalumab | PD L-1 | 2022 | Durvalumab + Tremelimumab + chemotherapy for the treatment of metastatic NSCLC |
| 2018 | Unresectable stage III not progressed NSCLC after treatment with chemotherapy and radiation | ||
| Ipilimumab | CTLA-4 | 2020 | Ipilimumab+nivolumab + chemotherapy as 1st treatment of metastatic or recurrent NSCLC |
| 2020 | Ipilimumab + nivolumab as 1st treatment of NSCLC (PD-L1 ≥ 1%) | ||
| Tremelimumab | CTLA-4 | 2022 | Tremelimumab + Durvalumab+chemotherapy for the treatment of metastatic NSCLC |
| Name | Year of Approval | Target | Clinical Use |
|---|---|---|---|
| Azacitidine | 2004 | DNMT | Myelodysplastic syndrome |
| Decitabine | 2006 | DNMT | Myelodysplastic syndrome |
| Hydralazine | 1953 | DNMT | Hypertension |
| Belinostat | 2014 | HDAC | Peripheral T-cell lymphoma |
| Panobinostat | 2015 | HDAC | Multiple myeloma |
| Vorinostat | 2006 | HDAC | Cutaneous T-cell lymphoma |
| Romidepsin | 2009 | HDAC | Cutaneous T-cell lymphoma |
| Enasidenib | 2017 | IDH2 | Acute myeloid leukemia |
| Ivosidenib | 2018 | IDH1 | Acute myeloid leukemia |
| Tazemetostat | 2020 | EZH2 | Epithelioid sarcoma/follicular lymphoma |
| Function | Subunit | Alias | BAF/PBAF/ncBAF | Mutual Exclusive Paralog | Domains |
|---|---|---|---|---|---|
| ATPase | SMARCA2 | BRM | +/−/+ * | SMARCA2/A4 | Bromodomain, HSA, SnAC |
| ATPase | SMARCA4 | BRG1 | +/+/+ | Bromodomain, HAS, SnAC | |
| Core | SMARCC1 | BAF155 | +/+/+ | SMARCC1/C2 | Chromodomain, SWIRM, SANT |
| Core | SMARCC2 | BAF170 | +/+/+ | Chromodomain, SWIRM, SANT | |
| Core | SMARCD1 | BAF60A | +/+/+ | SMARCD1/D2/D3 | |
| Core | SMARCD2 | BAF60B | +/+/+ | ||
| Core | SMARCD3 | BAF60C | +/+/+ | ||
| Core | SMARCB1 | BAF47 | +/+/− | WH | |
| Core | SMARCE1 | BAF57 | +/+/− | HMG | |
| Accessory | BCL7A | BCL7A | +/+/+ | ||
| Accessory | BCL7B | BCL7B | +/+/+ | ||
| Accessory | BCL7C | BCL7C | +/+/+ | ||
| Actin | ACTL6A | BAF53A | +/+/+ | ACTL6A/6B | |
| Actin | ACTL6B | BAF53AB | +/+/+ | ||
| Actin | ACTB | -ACTIN | +/+/+ | ||
| Accessory | SS18 | SSXT | +/−/+ | ||
| Accessory | SS18L1 | CREST | +/−/+ | ||
| BAF unique | ARID1A | BAF250A | +/−/− | ARID1A/1B | ARID, ARM, ZNF |
| BAF unique | ARID1B | BAF250B | +/−/− | ARID, ARM, ZNF | |
| BAF unique | DPF1 | BAF45B | +/−/− | DPF1/F2/F3/ | PHD finger |
| BAF unique | DPF2 | BAF45C | +/−/− | PHD finger, ZNF | |
| BAF unique | DPF3 | BAF45BD | +/−/− | PHD finger, ZNF | |
| PBAF unique | PHF10 | BAF45A | −/+/− | PHD finger, ZNF | |
| PBAF unique | PBRM1 | BAF180 | −/+/− | Bromodomain, BAH, HMG, ZNF | |
| PBAF unique | ARID2 | BAF200 | −/+/− | ARID, WH, ZNF, ARM | |
| PBAF unique | BRD7 | BRD7 | −/+/− | Bromodomain | |
| ncBAF unique | BICRA | GLTSCR1 | -/-/+ | ||
| ncBAF unique | BICRAL | GLTSCR1 | −/−/+ | ||
| ncBAF unique | BRD9 | BRD9 | −/−/+ | Bromodomain |
| Subunit | Mutation Freq (%) | Misssense N (%) | Truncating N (%) | Inframe N (%) | Splice N (%) |
|---|---|---|---|---|---|
| SMARCA4. | 7 | 344 (54.5) | 206 (32.6) | 10 (1.6) | 71 (11.3) |
| ARID1A | 6 | 271 (42.7) | 322 (50.8) | 6 (0.9) | 35 (5.5) |
| ARID1B | 4 | 279 (75.0) | 70 (18.8) | 13 (3.5) | 0 (2.7) |
| ARID2 | 4 | 227 (52.8) | 168 (39.1) | 0 (0.0) | 35 (8.1) |
| SMARCA2. | 2.8 | 8 (5.8) | 0 (0.0) | 1 (0.7) | |
| PBRM1 | 2.5 | 126 (57.0) | 64 (29.0) | 1 (0.5) | 30 (13.6) |
| DPF3 | 1.9 | ||||
| SMARCC2 | 1.7 | ||||
| BICRAL | 1.7 | ||||
| SMARCC1 | 1.6 | ||||
| ACTL6B | 1.4 | ||||
| BICRA | 1.3 | ||||
| DPF2 | 1.2 | ||||
| ACTB | 1.1 | ||||
| SS18 | 1 | ||||
| ACTL6A | 0.9 | ||||
| BRD7 | 0.8 | ||||
| BRD9 | 0.8 | ||||
| SMARCD2 | 0.8 | ||||
| DPF1 | 0.8 | ||||
| SMARCD1 | 0.7 | ||||
| SS18L1 | 0.6 | ||||
| SMARCB1 | 0.5 | ||||
| SMARCD3 | 0.3 | ||||
| SMARCE1 | 0.3 | ||||
| BCL7A | 0.3 | ||||
| BCL7B | 0.2 | ||||
| BCL7C | 0.2 | ||||
| PHF10 | 0.2 |
| Identifier | Status | Drugs | Target | Subject | Phase |
|---|---|---|---|---|---|
| NCT05467748 | Not yet recruiting | * Tazemetostat + PD-1 mAb | EZH2 | Progressed with an anti-PD-1/L1 mAb | Phase 1/2 |
| NCT05639751 | Not yet recruiting | PRT3789 | BRM | Advanced solid tumor with loss of SMARCA4 | Phase 1 |
| NCT01082549 | Completed | Chmo ± * Iniparib | PARP | Untreated NSCLC stage III | Phase 3 |
| NCT04538378 | Recruiting | * Olaparib + Durvalumab | PARP | EGFR Mutated NSCLC | Phase 2 |
| NCT05127590 | Recruiting | RBN-2397 + Pembrolizumab | PARP | Advanced Squamous NSCLC | Phase 2 |
| NCT03330405 | Not yet recruiting | * Talazoparib + Avelumab | PARP | Primary or Recurrent or Metastatic Solid Tumors | Phase 2 |
| NCT04380636 | Recruiting | Pembrolizumab+chmo | PARP | NSCLC stage III | Phase 3 |
| Pembrolizumab ± * Olaparib | |||||
| NCT01386385 | Not yet recruiting | Veliparib ± Radiation Therapy | PARP | Stage III unremovable NSCLC | Phase 1/2 |
| Carboplatin/Paclitaxel | |||||
| NCT02944396 | Completed | Veliparib + Nivolumab | PARP | Metastatic or Advanced NSCLC | Phase 1 |
| +Platinum | |||||
| NCT03308942 | Completed | * Niraparib ± PD-1 mAb | PARP | Advanced NSCLC no chemo or PD-L/1/ mAb with high PD-L1 | Phase 2 |
| NCT02412371 | Terminated | Veliparib + chemo | PARP | NSCLC stage III | Phase 1/2 |
| NCT02264990 | Completed | Veliparib + chemo | PARP | Non-squamous NSCLC | Phase 3 |
| NCT02292550 | Completed | * Ribociclib and Ceritinib | CDK4/6 | ALK-positive NSCLC | Phase 1 |
| NCT04863248 | Terminated | * Trilaciclib + Docetaxel | CDK4/6 | Metastatic NSCLC | Phase 2 |
| NCT03455829 | Completed | G1T38, + Osimertinib | CDK4/6 | EGFR-Mutant NSCLC | Phase 1/2 |
| NCT02022982 | Not yet recruiting | * Palboclclb + PD-0325901 | CDK4/6 | KRAS Mutant NSCLC | Phase 1/2 |
| NCT03170206 | Recruiting | Palbociclib + MEK162 | CDK4/6 | Advanced KRAS Mutant NSCLC | Phase 1/2 |
| NCT03965845 | Completed | Palbociclib + Telaglenastat | CDK4/6 | Solid tumor (including NSCLC) | Phase 1/2 |
| NCT04545710 | Recruiting | Osimertinib + * Abemaciclib | CDK4/6 | EGFR Mutant NSCLC after Osimertinib | Phase 1 |
| Spartalizumab/* Ribociclib | |||||
| NCT03386929 | Not yet recruiting | Avelumab/Axitinib/ | CDK4/6 | Advanced or metastatic NSCLC | Phase ½ |
| * Palbociclib | |||||
| NCT02664935 | Not yet recruiting | Multi drug trial including | CDK4/6 | NSCLC | Phase 2 |
| * Palbociclib | |||||
| NCT05538572 | Recruiting | PRT3645 | CDK4/6 | Solid tumor (including NSCLC) | Phase 1 |
| NCT04591431 | Recruiting | Multi drug trial including | CDK4/6 | Solid tumor (including NSCLC) | Phase 2 |
| * Palbociclib | |||||
| NCT04606446 | Recruiting | Multi drug trial including | CDK4/6 | Solid tumor (including NSCLC) | Phase 1 |
| * Palbociclib | |||||
| NCT05358249 | Recruiting | JDQ443 + trametinib | CDK4/6 | K-Ras G12C Solid tumor (including NSCLC) | Phase 1/2 |
| +* Ribociclib | |||||
| NCT04491942 | Recruiting | BAY 1895344 + chemo | ATR | Advanced Solid tumor (including NSCLC) | Phase 1 |
| NCT02264678 | Recruiting | * Ceralasertib + chemo | ATR | Advanced Solid tumor (including NSCLC) | Phase 1/2 |
| NCT01471964 | Terminated | MLN8237 + Erlotinib | Aurora A | NSCLC | Phase 1/2 |
| NCT05017025 | Recruiting | LY3295668 + Osimertinib | Aurora A | Advanced or Metastatic EGFRMutant NSCLC | Phase 1/2 |
| NCT05374538 | Recruiting | VIC-1911 + Sotorasib | Aurora A | KRAS G12CMutant NSCLC | Phase 1 |
| NCT01045421 | Completed | Alisertib | Nonhematological Malignancies including NSCLC | Phase 1/2 | |
| NCT02635061 | Not recruiting | ACY 241 + Nivolumab | HDAC6 | Unresectable NSCLC | Phase 1 |
| Type of Study | Number of Subjects | Targeting Subunits | Year of Publication | Clinical OUTCOMES | References |
|---|---|---|---|---|---|
| Retrospective | 441 | BRG1 | 2020 | Longer PFS in NSCLC with ‘pure ‘alterations of BRG1 without STK11/KEAP mutations | [110] |
| Retrospective | 11 | BRG1 | 2020 | 4 NSCLC with BRG1 mutation ICI monotherapy, ¼ lost follow-up, ¾ primary progression | [111] |
| Retrospective | 445 | BRG1 | 2020 | Higher ORR in NSCLC with BRG1 mutation, higher TMB, lower PD-L1 expression | [112] |
| Case report | BRG1 | 2019 | Obvious reduction of metastasis for longer than 14 months | [113] | |
| Retrospective | 63 | BRG1 | 2022 | Shorter OS, increased FOXP3+ and neutrophil but no CD8 + cells | [104] |
| Retrospective | 146 | BRG1/ARID1A | 2022 | Longer PFS in NSCLC with alterations of BRG1/ARID1A | [114] |
| Meta study | 3416 | ARID1A/1B/ARID2, BRG1 | 2021 | Longer mOS NSCLC with mutations of ARID1A/1B/ARID2, higher TMB | [115] |
| Retrospective | 136 | ARID1A/1B/ARID2, BRG1, PBRM1 | 2021 | Shorter OS in NSCLC with BAG1 and K-RAS co-mutation, higher TMB | [116] |
| Retrospective | 240 | ARID1A/1B | 2020 | Longer OS and PFS in NSCLC with ARID1 mutation, higher TMB, higher ratio of PD-L1 | [117] |
| Retrospective | 350 | PBRM1 | 2022 | Shorter OS and PFS in NSCLC with PBRM1 mutation | [118] |
| Retrospective | 441 | PBRM1 | 2020 | Shorter OS | [119] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Y.; Shin, D.S. Dysregulation of SWI/SNF Chromatin Remodelers in NSCLC: Its Influence on Cancer Therapies including Immunotherapy. Biomolecules 2023, 13, 984. https://doi.org/10.3390/biom13060984
Shi Y, Shin DS. Dysregulation of SWI/SNF Chromatin Remodelers in NSCLC: Its Influence on Cancer Therapies including Immunotherapy. Biomolecules. 2023; 13(6):984. https://doi.org/10.3390/biom13060984
Chicago/Turabian StyleShi, Yijiang, and Daniel Sanghoon Shin. 2023. "Dysregulation of SWI/SNF Chromatin Remodelers in NSCLC: Its Influence on Cancer Therapies including Immunotherapy" Biomolecules 13, no. 6: 984. https://doi.org/10.3390/biom13060984
APA StyleShi, Y., & Shin, D. S. (2023). Dysregulation of SWI/SNF Chromatin Remodelers in NSCLC: Its Influence on Cancer Therapies including Immunotherapy. Biomolecules, 13(6), 984. https://doi.org/10.3390/biom13060984






