Carcinogenesis and Metastasis: Focus on TRPV1-Positive Neurons and Immune Cells
Abstract
1. Introduction: TRPV1, Capsaicin, and Neurogenic Inflammation
2. Neurogenic Inflammation and Tumor Promotion in the Skin
3. Neuro-Immune Regulation of Cancer
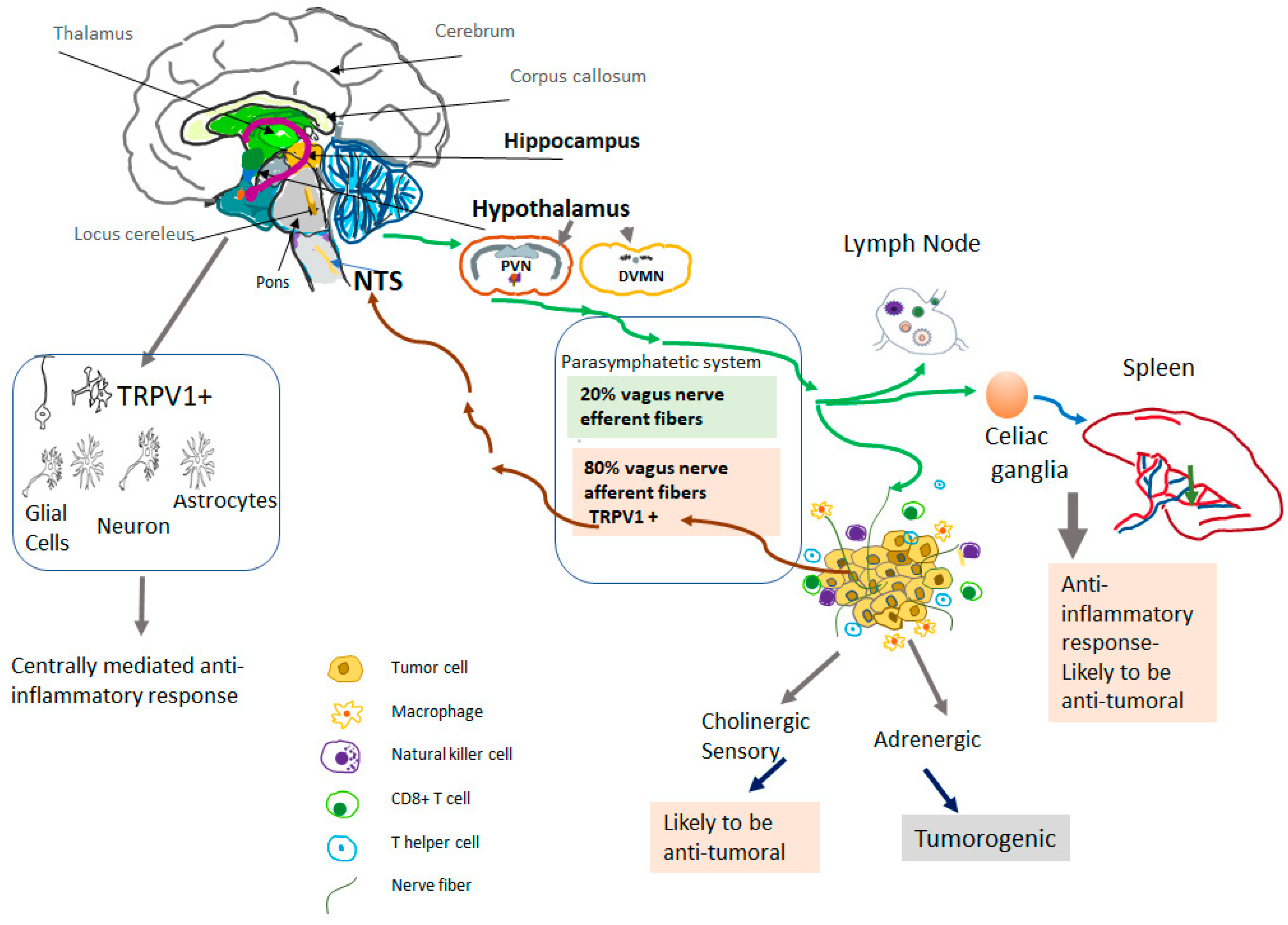
4. TRPV1-Positive Vagal Afferent Neurons and Carcinogenesis
5. Systemic Activation of Neuronal TRPV1 in Cancer
6. Role of the Central Nervous System (CNS) in the Anti-Inflammatory Effects of TRPV1 Agonists
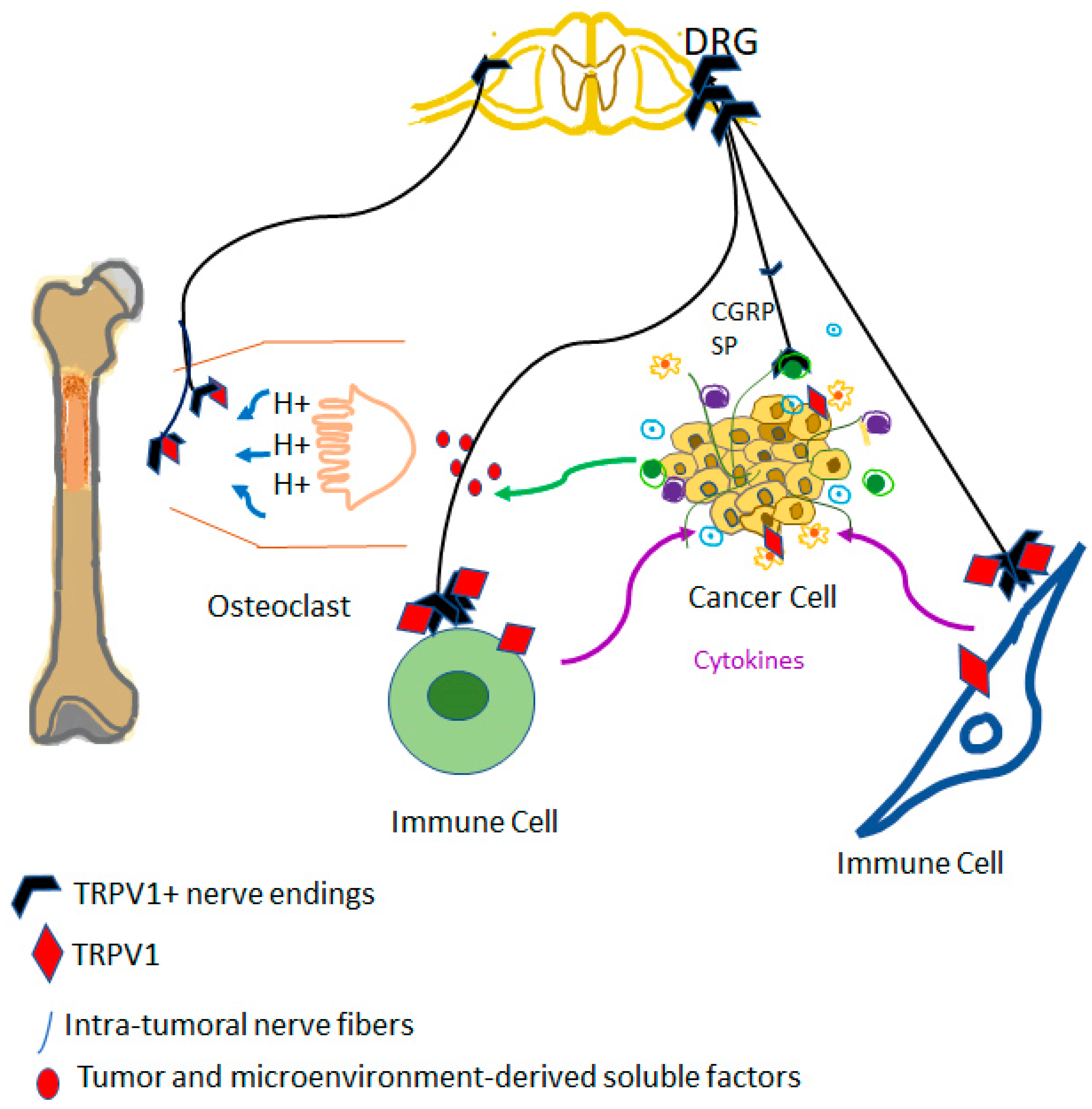
7. Innervation of the Cancer
8. TRPV1: Channel Structure, Epigenetic Regulation and Subcellular Expression
9. Capsaicin Actions in Immune Cells: On- or Off-Target?
10. Role of TRPV1 Expressing Immune Cells in Cancer
| Cell Type | TRPV1 Functions | Possible Role in Carcinogenesis |
|---|---|---|
| T cells | TRPV1 implicated in TcR-induced Ca2+ influx. Capsaicin increases Ca2+ influx and intracellular Ca2+ concentration in activated CD4+ T-cells, but not in resting T-cells [170,171]. Exposure to prolonged and high concentrations of capsaicin induces apoptosis of human peripheral T- and Jurkat cells [171,174]. | TRPV1 levels are significantly increased in T-cells isolated from B16F10 tumor-bearing mice [176]. Possible role in anti-tumoral immune response and T-cell exhaustion requires further studies. |
| NK cells | Capsaicin increases intracellular Ca2+ concentrations in human NK cells while pretreatment with specific TRPV1 antagonists inhibits this effect [159]. TRPV1 antagonist increases the population of NK cells but also downregulates activation of NK/T cells in mice infected with Plasmodium [177]. | Not known |
| Dendritic cells | TRPV1 expression significantly increases during in vitro differentiation from monocytes to immature dendritic cells [178]. Indirect effect of TRPV1 activation on dendritic cells through neuroimmune axis was suggested [118]. Possible role in antigen presentation. | Not known |
| Mixed leukocyte culture | TRPV1 activation in control mice increases IFN-γ and IL-17, does not alter IL6 release [160]. | TRPV1 activation in tumor-bearing mice (breast carcinoma) decreases IFN-γ and increases IL-6 [160]. |
11. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature 1997, 389, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Montell, C.; Birnbaumer, L.; Flockerzi, V.; Bindels, R.J.; Bruford, E.A.; Caterina, M.J.; Clapham, D.E.; Harteneck, C.; Heller, S.; Julius, D.; et al. A unified nomenclature for the superfamily of TRP cation channels. Mol. Cell 2002, 9, 229–231. [Google Scholar] [CrossRef] [PubMed]
- Patapoutian, A.; Peier, A.M.; Story, G.M.; Viswanath, V. ThermoTRP channels and beyond: Mechanisms of temperature sensation. Nat. Rev. Neurosci. 2003, 4, 529–539. [Google Scholar] [CrossRef] [PubMed]
- García-Ávila, M.; Islas, L.D. What is new about mild temperature sensing? A review of recent findings. Temperature 2019, 6, 132–141. [Google Scholar] [CrossRef]
- Moran, M.M.; Szallasi, A. Targeting nociceptive transient receptor potential channels to treat chronic pain: Current state of the field. Br. J. Pharmacol. 2018, 175, 2185–2203. [Google Scholar] [CrossRef]
- Szolcsanyi, J. A pharmacological approach to elucidation of the role of different nerve fibres and receptor endings in mediation of pain. J. Physiol. 1977, 73, 251–259. [Google Scholar]
- Karrer, T.; Bartoshuk, L. Capsaicin desensitization and recovery on the human tongue. Physiol. Behav. 1991, 49, 757–764. [Google Scholar] [CrossRef]
- Szallasi, A.; Blumberg, P.M. Vanilloid (Capsaicin) receptors and mechanisms. Pharmacol. Rev. 1999, 51, 159–212. [Google Scholar]
- Abrams, R.M.C.; Pedowitz, E.J.; Simpson, D.M. A critical review of the capsaicin 8% patch for the treatment of neuropathic pain associated with diabetic peripheral neuropathy of the feet in adults. Expert Rev. Neurother. 2021, 21, 259–266. [Google Scholar] [CrossRef]
- Campbell, J.N.; Stevens, R.; Hanson, P.; Connolly, J.; Meske, D.S.; Chung, M.K.; Lascelles, B.D.X. Injectable Capsaicin for the Management of Pain Due to Osteoarthritis. Molecules 2021, 26, 778. [Google Scholar] [CrossRef]
- Jancso, G.; Kiraly, E.; Such, G.; Joo, F.; Nagy, A. Neurotoxic effect of capsaicin in mammals. Acta Physiol. Hung. 1987, 69, 295–313. [Google Scholar] [PubMed]
- Maggi, C.A.; Meli, A. The sensory-efferent function of capsaicin-sensitive sensory neurons. Gen. Pharmacol. 1988, 19, 1–43. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.J.M.; Ciotu, C.I.; Szallasi, A. The Mysteries of Capsaicin-Sensitive Afferents. Front. Physiol. 2020, 11, 554195. [Google Scholar] [CrossRef]
- Ichikawa, H.; Sugimoto, T. The co-expression of VR1 and VRL-1 in the rat vagal sensory ganglia. Brain Res. 2003, 980, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Holzer, P. Local effector functions of capsaicin-sensitive sensory nerve endings: Involvement of tachykinins, calcitonin gene-related peptide and other neuropeptides. Neuroscience 1988, 24, 739–768. [Google Scholar] [CrossRef]
- Barnes, P.J.; Belvisi, M.G.; Rogers, D.F. Modulation of neurogenic inflammation: Novel approaches to inflammatory disease. Trends Pharmacol. Sci. 1990, 11, 185–189. [Google Scholar] [CrossRef]
- Geppetti, P.; Holzer, P. Neurogenic Inflammation; CRC Press: Boca Raton, FL, USA, 1996. [Google Scholar]
- Szallasi, A.; Blumberg, P.M. Effect of resiniferatoxin pretreatment on the inflammatory response to phorbol-12-myristate-13-acetate in mouse strains with different susceptibilities to phorbol ester tumor promotion. Carcinogenesis 1990, 11, 583–587. [Google Scholar] [CrossRef]
- Bencze, N.; Schvarcz, C.; Kriszta, G.; Danics, L.; Szoke, E.; Balogh, P.; Szallasi, A.; Hamar, P.; Helyes, Z.; Botz, B. Desensitization of Capsaicin-Sensitive Afferents Accelerates Early Tumor Growth via Increased Vascular Leakage in a Murine Model of Triple Negative Breast Cancer. Front. Oncol. 2021, 11, 685297. [Google Scholar] [CrossRef]
- Erin, N. Role of sensory neurons, neuroimmune pathways, and transient receptor potential vanilloid 1 (TRPV1) channels in a murine model of breast cancer metastasis. Cancer Immunol. Immunother. 2020, 69, 307–314. [Google Scholar] [CrossRef]
- Omari, S.A.; Adams, M.J.; Geraghty, D.P. TRPV1 Channels in Immune Cells and Hematological Malignancies. Adv. Pharmacol. 2017, 79, 173–198. [Google Scholar]
- Palma, C. Tachykinins and their receptors in human malignancies. Curr. Drug Targets 2006, 7, 1043–1052. [Google Scholar] [CrossRef] [PubMed]
- Perner, C.; Flayer, C.H.; Zhu, X.; Aderhold, P.A.; Dewan, Z.N.A.; Voisin, T.; Camire, R.B.; Chow, O.A.; Chiu, I.M.; Sokol, C.L. Substance P Release by Sensory Neurons Triggers Dendritic Cell Migration and Initiates the Type-2 Immune Response to Allergens. Immunity 2020, 53, 1063–1077.e7. [Google Scholar] [CrossRef] [PubMed]
- Payan, D.G.; Brewster, D.R.; Goetzl, E.J. Specific stimulation of human T lymphocytes by substance P. J. Immunol. 1983, 131, 1613–1615. [Google Scholar] [CrossRef] [PubMed]
- Munoz, M.; Covenas, R.; Esteban, F.; Redondo, M. The substance P/NK-1 receptor system: NK-1 receptor antagonists as anti-cancer drugs. J. Biosci. 2015, 40, 441–463. [Google Scholar] [CrossRef]
- Amantini, C.; Mosca, M.; Nabissi, M.; Lucciarini, R.; Caprodossi, S.; Arcella, A.; Giangaspero, F.; Santoni, G. Capsaicin-induced apoptosis of glioma cells is mediated by TRPV1 vanilloid receptor and requires p38 MAPK activation. J. Neurochem. 2007, 102, 977–990. [Google Scholar] [CrossRef]
- Schulz, S.; Stumm, R.; Rocken, C.; Mawrin, C.; Schulz, S. Immunolocalization of full-length NK1 tachykinin receptors in human tumors. J. Histochem. Cytochem. 2006, 54, 1015–1020. [Google Scholar] [CrossRef]
- Munoz, M.; Covenas, R. Glioma and Neurokinin-1 Receptor Antagonists: A New Therapeutic Approach. Anti-Cancer Agents Med. Chem. 2019, 19, 92–100. [Google Scholar] [CrossRef]
- Virchow, R. Reizung und Reizbarkeit. Arch. Pathol. Anat. Physiol. Klin. Med. 1858, 14, 1–63. [Google Scholar] [CrossRef]
- Yamagiwa, K.I.K. Åber die atypische Epithelwucherung. Gann 1914, 8, 11–15. [Google Scholar]
- Berenblum, I.; Shubik, P. The persistence of latent tumour cells induced in the mouse’s skin by a single application of 9:10-dimethyl-1:2-benzanthracene. Br. J. Cancer 1949, 3, 384–386. [Google Scholar] [CrossRef]
- Berenblum, I. The carcinogenic action of 9,10-dimethyl-1,2-benzanthracene on the skin and subcutaneous tissues of the mouse, rabbit, rat and guinea pig. J. Natl. Cancer Inst. 1949, 10, 167–174. [Google Scholar] [PubMed]
- Klein, M. Induction of skin tumors in the mouse with minute doses of 9, 10-dimethyl-1, 2-benzanthracene alone or with croton oil. Cancer Res. 1956, 16, 123–127. [Google Scholar] [PubMed]
- Brune, K.; Kalin, H.; Schmidt, R.; Hecker, E. Inflammatory, tumor initiating and promoting activities of polycyclic aromatic hydrocarbons and diterpene esters in mouse skin as compared with their prostaglandin releasing potency in vitro. Cancer Lett. 1978, 4, 333–342. [Google Scholar] [CrossRef]
- Ohuchi, K.; Watanabe, M.; Takahashi, C.; Hayashi, Y.; Hirasawa, N.; Tsurufuji, S.; Fujiki, H.; Sugimura, T. Analysis of tumor-promoter-induced inflammation in rats: Participation of histamine and prostaglandin E2. Biochim. Biophys. Acta 1987, 925, 156–163. [Google Scholar]
- Hergenhahn, M.; Kusumoto, S.; Hecker, E. On the active principles of the spurge family (Euphorbiaceae). V. Extremely skin-irritant and moderately tumor-promoting diterpene esters from Euphorbia resinifera Berg. J. Cancer Res. Clin. Oncol. 1984, 108, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Hausen, H.Z.; Bornkamm, G.W.; Schmidt, R.; Hecker, E. Tumor initiators and promoters in the induction of Epstein-Barr virus. Proc. Natl. Acad. Sci. USA 1979, 76, 782–785. [Google Scholar] [CrossRef]
- Driedger, P.E.; Blumberg, P.M. Different biological targets for resiniferatoxin and phorbol 12-myristate 13-acetate. Cancer Res. 1980, 40, 1400–1404. [Google Scholar]
- Blumberg, P.M.; Pettit, G.R.; Warren, B.S.; Szallasi, A.; Schuman, L.D.; Sharkey, N.A.; Nakakuma, H.; Dell’Aquila, M.L.; de Vries, D.J. The protein kinase C pathway in tumor promotion. Prog. Clin. Biol. Res. 1989, 298, 201–212. [Google Scholar]
- Szallasi, A.; Blumberg, P.M. Specific binding of resiniferatoxin, an ultrapotent capsaicin analog, by dorsal root ganglion membranes. Brain Res. 1990, 524, 106–111. [Google Scholar] [CrossRef]
- Szallasi, A.; Blumberg, P.M. Neurogenic component of phorbol ester-induced mouse skin inflammation. Cancer Res. 1989, 49, 6052–6057. [Google Scholar]
- Inoue, K.; Koizumi, S.; Fuziwara, S.; Denda, S.; Inoue, K.; Denda, M. Functional vanilloid receptors in cultured normal human epidermal keratinocytes. Biochem. Biophys. Res. Commun. 2002, 291, 124–129. [Google Scholar] [CrossRef]
- Southall, M.D.; Li, T.; Gharibova, L.S.; Pei, Y.; Nicol, G.D.; Travers, J.B. Activation of epidermal vanilloid receptor-1 induces release of proinflammatory mediators in human keratinocytes. J. Pharmacol. Exp. Ther. 2003, 304, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Surh, Y.J.; Han, S.S.; Keum, Y.S.; Seo, H.J.; Lee, S.S. Inhibitory effects of curcumin and capsaicin on phorbol ester-induced activation of eukaryotic transcription factors, NF-kappaB and AP-1. Biofactors 2000, 12, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Hwang, M.K.; Bode, A.M.; Byun, S.; Song, N.R.; Lee, H.J.; Lee, K.W.; Dong, Z. Cocarcinogenic effect of capsaicin involves activation of EGFR signaling but not TRPV1. Cancer Res. 2010, 70, 6859–6869. [Google Scholar] [CrossRef] [PubMed]
- Mundt, S.; Greter, M.; Flugel, A.; Becher, B. The CNS Immune Landscape from the Viewpoint of a T Cell. Trends Neurosci. 2019, 42, 667–679. [Google Scholar] [CrossRef]
- Antoni, M.H.; Dhabhar, F.S. The impact of psychosocial stress and stress management on immune responses in patients with cancer. Cancer 2019, 125, 1417–1431. [Google Scholar] [CrossRef]
- Dhabhar, F.S. Enhancing versus suppressive effects of stress on immune function: Implications for immunoprotection and immunopathology. Neuroimmunomodulation 2009, 16, 300–317. [Google Scholar] [CrossRef]
- Oh, P.J.; Shin, S.R.; Ahn, H.S.; Kim, H.J. Meta-analysis of psychosocial interventions on survival time in patients with cancer. Psychol. Health 2016, 31, 396–419. [Google Scholar] [CrossRef]
- Shurin, M.R.; Shurin, G.V.; Zlotnikov, S.B.; Bunimovich, Y.L. The Neuroimmune Axis in the Tumor Microenvironment. J. Immunol. 2020, 204, 280–285. [Google Scholar] [CrossRef]
- Candido, J.; Hagemann, T. Cancer-related inflammation. J. Clin. Immunol. 2013, 33 (Suppl. 1), S79–S84. [Google Scholar] [CrossRef]
- Mueller, S.N. Neural control of immune cell trafficking. J. Exp. Med. 2022, 219, e20211604. [Google Scholar] [CrossRef] [PubMed]
- Yun, H.; Yin, X.T.; Stuart, P.M.; St Leger, A.J. Sensory Nerve Retraction and Sympathetic Nerve Innervation Contribute to Immunopathology of Murine Recurrent Herpes Stromal Keratitis. Investig. Ophthalmol. Vis. Sci. 2022, 63, 4. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Chen, W.; Shen, L.; Chen, Z.; Huang, J. Crosstalk between the peripheral nervous system and breast cancer influences tumor progression. Biochim. Biophys. Acta Rev. Cancer 2022, 1877, 188828. [Google Scholar] [CrossRef] [PubMed]
- Nance, D.M.; Sanders, V.M. Autonomic innervation and regulation of the immune system (1987–2007). Brain Behav. Immun. 2007, 21, 736–745. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, A.; Hayama, Y.; Kato, S.; Shimomura, A.; Shimomura, T.; Irie, K.; Kaneko, R.; Yanagawa, Y.; Kobayashi, K.; Ochiya, T. Genetic manipulation of autonomic nerve fiber innervation and activity and its effect on breast cancer progression. Nat. Neurosci. 2019, 22, 1289–1305. [Google Scholar] [CrossRef]
- Andersson, U.; Tracey, K.J. Neural reflexes in inflammation and immunity. J. Exp. Med. 2012, 209, 1057–1068. [Google Scholar] [CrossRef]
- Pavlov, V.A.; Tracey, K.J. Neural circuitry and immunity. Immunol. Res. 2015, 63, 38–57. [Google Scholar] [CrossRef] [PubMed]
- Berthoud, H.R.; Neuhuber, W.L. Functional and chemical anatomy of the afferent vagal system. Auton. Neurosci. 2000, 85, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Bonaz, B.; Sinniger, V.; Pellissier, S. Vagus Nerve Stimulation at the Interface of Brain-Gut Interactions. Cold Spring Harb. Perspect. Med. 2019, 9, a034199. [Google Scholar] [CrossRef] [PubMed]
- Reardon, C.; Murray, K.; Lomax, A.E. Neuroimmune Communication in Health and Disease. Physiol. Rev. 2018, 98, 2287–2316. [Google Scholar] [CrossRef] [PubMed]
- Erin, N.; Shurin, G.V.; Baraldi, J.H.; Shurin, M.R. Regulation of Carcinogenesis by Sensory Neurons and Neuromediators. Cancers 2022, 14, 2333. [Google Scholar] [CrossRef]
- Sternini, C. Organization of the peripheral nervous system: Autonomic and sensory ganglia. J. Investig. Dermatol. Symp. Proc. 1997, 2, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, P.S.; Hadaya, J.; Khalsa, S.S.; Yu, C.; Chang, R.; Shivkumar, K. The Vagus Nerve in Cardiovascular Physiology and Pathophysiology: From Evolutionary Insights to Clinical Medicine. In Seminars in Cell and Developmental Biology; Academic Press: Berlin/Heidelberg, Germany, 2023. [Google Scholar]
- Cirillo, G.; Negrete-Diaz, F.; Yucuma, D.; Virtuoso, A.; Korai, S.A.; De Luca, C.; Kaniusas, E.; Papa, M.; Panetsos, F. Vagus Nerve Stimulation: A Personalized Therapeutic Approach for Crohn’s and Other Inflammatory Bowel Diseases. Cells 2022, 11, 4103. [Google Scholar] [CrossRef]
- Ruffoli, R.; Giorgi, F.S.; Pizzanelli, C.; Murri, L.; Paparelli, A.; Fornai, F. The chemical neuroanatomy of vagus nerve stimulation. J. Chem. Neuroanat. 2011, 42, 288–296. [Google Scholar] [CrossRef]
- Fernández-Ballester, G.; Fernández-Carvajal, A.; Ferrer-Montiel, A. Progress in the structural basis of thermoTRP channel polymodal gating. Int. J. Mol. Med. 2023, 24, 743. [Google Scholar] [CrossRef]
- Luu, D.D.; Owens, A.M.; Mebrat, M.D.; Van Horn, W.D. A molecular perspective on identifying TRPV1 thermosensitive regions and disentangling polymodal activation. Temperature 2021, 10, 67–101. [Google Scholar] [CrossRef]
- Ulloa, L. The vagus nerve and the nicotinic anti-inflammatory pathway. Nature Rev. Drug Discov. 2005, 4, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Gallowitsch-Puerta, M.; Pavlov, V.A. Neuro-immune interactions via the cholinergic anti-inflammatory pathway. Life Sci. 2007, 80, 2325–2329. [Google Scholar] [CrossRef]
- Jo, B.G.; Kim, S.H.; Namgung, U. Vagal afferent fibers contribute to the anti-inflammatory reactions by vagus nerve stimulation in concanavalin A model of hepatitis in rats. Mol. Med. 2020, 26, 119. [Google Scholar] [CrossRef] [PubMed]
- Erin, N.; Akdas Barkan, G.; Harms, J.F.; Clawson, G.A. Vagotomy enhances experimental metastases of 4THMpc breast cancer cells and alters substance P level. Regul. Pept. 2008, 151, 35–42. [Google Scholar] [CrossRef]
- Erin, N.; Boyer, P.J.; Bonneau, R.H.; Clawson, G.A.; Welch, D.R. Capsaicin-mediated denervation of sensory neurons promotes mammary tumor metastasis to lung and heart. Anticancer Res. 2004, 24, 1003–1009. [Google Scholar] [PubMed]
- Erin, N.; Barkan, G.A.; Clawson, G.A. Vagus nerve regulates breast cancer metastasis to the adrenal gland. Anticancer Res. 2013, 33, 3675–3682. [Google Scholar]
- Erin, N.; Duymus, O.; Ozturk, S.; Demir, N. Activation of vagus nerve by semapimod alters substance P levels and decreases breast cancer metastasis. Regul. Pept. 2012, 179, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yu, Q.; Zhang, X.; Wang, X.; Su, Y.; He, W.; Li, J.; Wan, H.; Jing, X. Electroacupuncture regulates inflammatory cytokines by activating the vagus nerve to enhance antitumor immunity in mice with breast tumors. Life Sci. 2021, 272, 119259. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yi, M.; Niu, M.; Mei, Q.; Wu, K. Myeloid-derived suppressor cells: An emerging target for anticancer immunotherapy. Mol. Cancer 2022, 21, 184. [Google Scholar] [CrossRef] [PubMed]
- Croitoru, K.; Ernst, P.B.; Bienenstock, J.; Padol, I.; Stanisz, A.M. Selective modulation of the natural killer activity of murine intestinal intraepithelial leucocytes by the neuropeptide substance P. Immunology 1990, 71, 196–201. [Google Scholar]
- Janelsins, B.M.; Sumpter, T.L.; Tkacheva, O.A.; Rojas-Canales, D.M.; Erdos, G.; Mathers, A.R.; Shufesky, W.J.; Storkus, W.J.; Falo, L.D.; Jr Morelli, A.E.; et al. Neurokinin-1 receptor agonists bias therapeutic dendritic cells to induce type 1 immunity by licensing host dendritic cells to produce IL-12. Blood 2013, 121, 2923–2933. [Google Scholar] [CrossRef]
- Erin, N.; Korcum, A.F.; Tanriover, G.; Kale, S.; Demir, N.; Koksoy, S. Activation of neuroimmune pathways increases therapeutic effects of radiotherapy on poorly differentiated breast carcinoma. Brain Behav. Immun. 2015, 48, 174–185. [Google Scholar] [CrossRef]
- Chu, C.J.; Huang, S.M.; De Petrocellis, L.; Bisogno, T.; Ewing, S.A.; Miller, J.D.; Zipkin, R.E.; Daddario, N.; Appendino, G.; Di Marzo, V.; et al. N-oleoyldopamine, a novel endogenous capsaicin-like lipid that produces hyperalgesia. J. Biol. Chem. 2003, 278, 13633–13639. [Google Scholar] [CrossRef]
- Liu, L.; Lo, Y.; Chen, I.; Simon, S.A. The responses of rat trigeminal ganglion neurons to capsaicin and two nonpungent vanilloid receptor agonists, olvanil and glyceryl nonamide. J. Neurosci. 1997, 17, 4101–4111. [Google Scholar] [CrossRef]
- Erin, N.; Akman, M.; Aliyev, E.; Tanriover, G.; Korcum, A.F. Olvanil activates sensory nerve fibers, increases T cell response and decreases metastasis of breast carcinoma. Life Sci. 2022, 291, 120305. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.P.; Franco-Cereceda, A.; Lundberg, J.M. Different ioan channel mechanisms between low concentrations of capsaicin and high concentrations of capsaicin and nicotine regarding peptide release from pulmonary afferents. Acta Physiol. Scand. 1992, 146, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Assas, B.M.; Abdulaal, W.H.; Wakid, M.H.; Zakai, H.A.; Miyan, J.; Pennock, J.L. The use of flow cytometry to examine calcium signalling by TRPV1 in mixed cell populations. Anal. Biochem. 2017, 527, 13–19. [Google Scholar] [CrossRef]
- Liu, L.; SA, S. A rapid capsaicin-activated current in rat trigeminal ganglion neurons. Proc. Natl. Acad. Sci. USA 1994, 91, 738–741. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.; Lamotte, R.H.; Klusch, A.; Kniffki, K.D. Multiple capsaicin-evoked currents in isolated rat sensory neurons. Neuroscience 1996, 75, 495–505. [Google Scholar] [CrossRef]
- Grüter, T.; Blusch, A.; Motte, J.; Sgodzai, M.; Bachir, H.; Klimas, R.; Ambrosius, B.; Gold, R.; Ellrichmann, G.; Pitarokoili, K. Immunomodulatory and anti-oxidative effect of the direct TRPV1 receptor agonist capsaicin on Schwann cells. J. Neuroinflamm. 2020, 17, 145. [Google Scholar] [CrossRef]
- Motte, J.; Ambrosius, B.; Gruter, T.; Bachir, H.; Sgodzai, M.; Pedreiturria, X.; Pitarokoili, K.; Gold, R. Capsaicin-enriched diet ameliorates autoimmune neuritis in rats. J. Neuroinflamm. 2018, 15, 122. [Google Scholar] [CrossRef]
- Nevius, E.; Srivastava, P.K.; Basu, S. Oral ingestion of Capsaicin, the pungent component of chili pepper, enhances a discreet population of macrophages and confers protection from autoimmune diabetes. Mucosal. Immunol. 2012, 5, 76–86. [Google Scholar] [CrossRef]
- Lv, J.; Qi, L.; Yu, C.; Yang, L.; Guo, Y.; Chen, Y.; Bian, Z.; Sun, D.; Du, J.; Ge, P.; et al. Consumption of spicy foods and total and cause specific mortality: Population based cohort study. BMJ 2015, 351, h3942. [Google Scholar] [CrossRef]
- Feng, J.; Yang, P.; Mack, M.R.; Dryn, D.; Luo, J.; Gong, X.; Liu, S.; Oetjen, L.K.; Zholos, A.V.; Mei, Z.; et al. Sensory TRP channels contribute differentially to skin inflammation and persistent itch. Nat. Commun. 2017, 8, 980. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, D.H. TRPV1 ablation aggravates inflammatory responses and organ damage during endotoxic shock. Clin. Vaccine Immunol. 2013, 20, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, E.S.; Liang, L.; Smillie, S.J.; Kaiser, F.; Purcell, R.; Rivett, D.W.; Alam, S.; Howat, S.; Collins, H.; Thompson, S.J.; et al. TRPV1 deletion enhances local inflammation and accelerates the onset of systemic inflammatory response syndrome. J. Immunol. 2012, 188, 5741–5751. [Google Scholar] [CrossRef] [PubMed]
- Vinuesa, A.G.; Sancho, R.; Garcia-Limones, C.; Behrens, A.; ten Dijke, P.; Calzado, M.A.; Munoz, E. Vanilloid receptor-1 regulates neurogenic inflammation in colon and protects mice from colon cancer. Cancer Res. 2012, 72, 1705–1716. [Google Scholar] [CrossRef] [PubMed]
- Bryant, P.; Shumate, M.; Yumet, G.; Lang, C.H.; Vary, T.C.; Cooney, R.N. Capsaicin-sensitive nerves regulate the metabolic response to abdominal sepsis. J. Surg. Res. 2003, 112, 152–161. [Google Scholar] [CrossRef]
- Kobayashi, M.; Watanabe, K.; Yokoyama, S.; Matsumoto, C.; Hirata, M.; Tominari, T.; Inada, M.; Miyaura, C. Capsaicin, a TRPV1 Ligand, Suppresses Bone Resorption by Inhibiting the Prostaglandin E Production of Osteoblasts, and Attenuates the Inflammatory Bone Loss Induced by Lipopolysaccharide. ISRN Pharmacol. 2012, 2012, 439860. [Google Scholar] [CrossRef]
- Clark, N.; Keeble, J.; Fernandes, E.S.; Starr, A.; Liang, L.; Sugden, D.; de Winter, P.; Brain, S.D. The transient receptor potential vanilloid 1 (TRPV1) receptor protects against the onset of sepsis after endotoxin. FASEB J. 2007, 21, 3747–3755. [Google Scholar] [CrossRef]
- Szallasi, A.; Di Marzo, V. New perspectives on enigmatic vanilloid receptors. Trends Neurosci. 2000, 23, 491–507. [Google Scholar] [CrossRef]
- Zsombok, A.; Gao, H.; Miyata, K.; Issa, A.; Derbenev, A.V. Immunohistochemical localization of transient receptor potential vanilloid type 1 and insulin receptor substrate 2 and their co-localization with liver-related neurons in the hypothalamus and brainstem. Brain Res. 2011, 1398, 30–39. [Google Scholar] [CrossRef]
- Mezey, E.; Toth, Z.E.; Cortright, D.N.; Arzubi, M.K.; Krause, J.E.; Elde, R.; Guo, A.; Blumberg, P.M.; Szallasi, A. Distribution of mRNA for vanilloid receptor subtype 1 (VR1), and VR1-like immunoreactivity, in the central nervous system of the rat and human. Proc. Natl. Acad. Sci. USA 2000, 97, 3655–3660. [Google Scholar] [CrossRef]
- Tóth, A.; Boczán, J.; Kedei, N.; Lizanecz, E.; Bagi, Z.; Papp, Z.; Édes, I.; Csiba, L.; Blumberg, P.M. Expression and distribution of vanilloid receptor 1 (TRPV1) in the adult rat brain. Brain Res. Mol. Brain Res. 2005, 135, 162–168. [Google Scholar] [CrossRef]
- Steenland, H.W.; Ko, S.W.; Wu, L.J.; Zhuo, M. Hot receptors in the brain. Mol. Pain 2006, 2, 34. [Google Scholar] [CrossRef] [PubMed]
- Storozhuk, M.V.; Moroz, O.F.; Zholos, A.V. Multifunctional TRPV1 Ion Channels in Physiology and Pathology with Focus on the Brain, Vasculature, and Some Visceral Systems. BioMed Res. Int. 2019, 2019, 5806321. [Google Scholar] [CrossRef] [PubMed]
- Marinelli, S.; Vaughan, C.W.; Christie, M.J.; Connor, M. Capsaicin activation of glutamatergic synaptic transmission in the rat locus coeruleus in vitro. J. Physiol. 2002, 543, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Chavez, A.E.; Chiu, C.Q.; Castillo, P.E. TRPV1 activation by endogenous anandamide triggers postsynaptic long-term depression in dentate gyrus. Nat. Neurosci. 2010, 13, 1511–1518. [Google Scholar] [CrossRef]
- Cui, Y.; Perez, S.; Venance, L. Endocannabinoid-LTP Mediated by CB1 and TRPV1 Receptors Encodes for Limited Occurrences of Coincident Activity in Neocortex. Front. Cell. Neurosci. 2018, 12, 182. [Google Scholar] [CrossRef]
- Anstotz, M.; Lee, S.K.; Maccaferri, G. Expression of TRPV1 channels by Cajal-Retzius cells and layer-specific modulation of synaptic transmission by capsaicin in the mouse hippocampus. J. Physiol. 2018, 596, 3739–3758. [Google Scholar] [CrossRef]
- Kim, S.R.; Kim, S.U.; Oh, U.; Jin, B.K. Transient receptor potential vanilloid subtype 1 mediates microglial cell death in vivo and in vitro via Ca2+-mediated mitochondrial damage and cytochrome c release. J. Immunol. 2006, 177, 4322–4329. [Google Scholar] [CrossRef]
- Wang, X.; Yang, X.L.; Kong, W.L.; Zeng, M.L.; Shao, L.; Jiang, G.T.; Cheng, J.J.; Kong, S.; He, X.H.; Liu, W.H.; et al. TRPV1 translocated to astrocytic membrane to promote migration and inflammatory infiltration thus promotes epilepsy after hypoxic ischemia in immature brain. J. Neuroinflamm. 2019, 16, 214. [Google Scholar] [CrossRef]
- Marrone, M.C.; Morabito, A.; Giustizieri, M.; Chiurchiu, V.; Leuti, A.; Mattioli, M.; Marinelli, S.; Riganti, L.; Lombardi, M.; Murana, E.; et al. TRPV1 channels are critical brain inflammation detectors and neuropathic pain biomarkers in mice. Nat. Commun. 2017, 8, 15292. [Google Scholar] [CrossRef]
- Nam, J.H.; Park, E.S.; Won, S.Y.; Lee, Y.A.; Kim, K.I.; Jeong, J.Y.; Baek, J.Y.; Cho, E.J.; Jin, M.; Chung, Y.C.; et al. TRPV1 on astrocytes rescues nigral dopamine neurons in Parkinson’s disease via CNTF. Brain 2015, 138, 3610–3622. [Google Scholar] [CrossRef]
- Di Marzo, V.; Blumberg, P.M.; Szallasi, A. Endovanilloid signaling in pain. Curr. Opin. Neurobiol. 2002, 12, 372–379. [Google Scholar] [CrossRef]
- Joffre, J.; Wong, E.; Lawton, S.; Lloyd, E.; Nguyen, N.; Xu, F.; Sempio, C.; Kobzik, L.; Zlatanova, I.; Schumacher, M.; et al. N-Oleoyl dopamine induces IL-10 via central nervous system TRPV1 and improves endotoxemia and sepsis outcomes. J. Neuroinflamm. 2022, 19, 118. [Google Scholar] [CrossRef]
- Zou, S.; Kumar, U. Cannabinoid Receptors and the Endocannabinoid System: Signaling and Function in the Central Nervous System. Int. J. Mol. Sci. 2018, 19, 833. [Google Scholar] [CrossRef] [PubMed]
- Lawton, S.K.; Xu, F.; Tran, A.; Wong, E.; Prakash, A.; Schumacher, M.; Hellman, J.; Wilhelmsen, K. N-Arachidonoyl Dopamine Modulates Acute Systemic Inflammation via Nonhematopoietic TRPV1. J. Immunol. 2017, 199, 1465–1475. [Google Scholar] [CrossRef]
- Rollyson, W.D.; Stover, C.A.; Brown, K.C.; Perry, H.E.; Stevenson, C.D.; McNees, C.A.; Ball, J.G.; Valentovic, M.A.; Dasgupta, P. Bioavailability of capsaicin and its implications for drug delivery. J. Control. Release 2014, 196, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Bujak, J.K.; Kosmala, D.; Szopa, I.M.; Majchrzak, K.; Bednarczyk, P. Inflammation, Cancer and Immunity-Implication of TRPV1 Channel. Front. Oncol. 2019, 9, 1087. [Google Scholar] [CrossRef] [PubMed]
- Mravec, B. Neurobiology of cancer: Definition, historical overview, and clinical implications. Cancer Med. 2022, 11, 903–921. [Google Scholar] [CrossRef]
- Palm, D.; Entschladen, F. Neoneurogenesis and the neuro-neoplastic synapse. Neuronal Act. Tumor Tissue 2007, 39, 91–98. [Google Scholar]
- Magnon, C.; Hall, S.J.; Lin, J.; Xue, X.; Gerber, L.; Freedland, S.J.; Frenette, P.S. Autonomic nerve development contributes to prostate cancer progression. Science 2013, 341, 1236361. [Google Scholar] [CrossRef]
- Ferdoushi, A.; Griffin, N.; Marsland, M.; Xu, X.; Faulkner, S.; Gao, F.; Liu, H.; King, S.J.; Denham, J.W.; van Helden, D.F.; et al. Tumor innervation and clinical outcome in pancreatic cancer. Sci. Rep. 2021, 11, 7390. [Google Scholar] [CrossRef]
- Huang, D.; Su, S.; Cui, X.; Shen, X.; Zeng, Y.; Wu, W.; Chen, J.; Chen, F.; He, C.; Liu, J.; et al. Nerve fibers in breast cancer tissues indicate aggressive tumor progression. Medicine 2014, 93, e172. [Google Scholar] [CrossRef]
- McCallum, G.A.; Shiralkar, J.; Suciu, D.; Covarrubias, G.; Yu, J.S.; Karathanasis, E.; Durand, D.M. Chronic neural activity recorded within breast tumors. Sci. Rep. 2020, 10, 14824. [Google Scholar] [CrossRef]
- Allen, J.K.; Armaiz-Pena, G.N.; Nagaraja, A.S.; Sadaoui, N.C.; Ortiz, T.; Dood, R.; Ozcan, M.; Herder, D.M.; Haemmerle, M.; Gharpure, K.M.; et al. Sustained Adrenergic Signaling Promotes Intratumoral Innervation through BDNF Induction. Cancer Res. 2018, 78, 3233–3242. [Google Scholar] [CrossRef]
- Renz, B.W.; Takahashi, R.; Tanaka, T.; Macchini, M.; Hayakawa, Y.; Dantes, Z.; Maurer, H.C.; Chen, X.; Jiang, Z.; Westphalen, C.B.; et al. Beta2 Adrenergic-Neurotrophin Feedforward Loop Promotes Pancreatic Cancer. Cancer Cell 2018, 33, 75–90e77. [Google Scholar] [CrossRef] [PubMed]
- Kappos, E.A.; Engels, P.E.; Tremp, M.; Sieber, P.K.; von Felten, S.; Madduri, S.; Meyer Zu Schwabedissen, M.; Fischmann, A.; Schaefer, D.J.; Kalbermatten, D.F. Denervation leads to volume regression in breast cancer. J. Plast. Reconstr. Aesthet. Surg. 2018, 71, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Cole, S.W.; Nagaraja, A.S.; Lutgendorf, S.K.; Green, P.A.; Sood, A.K. Sympathetic nervous system regulation of the tumour microenvironment. Nat. Rev. Cancer 2015, 15, 563–572. [Google Scholar] [CrossRef]
- Tibensky, M.; Mravec, B. Role of the parasympathetic nervous system in cancer initiation and progression. Clin. Transl. Oncol. 2021, 23, 669–681. [Google Scholar] [CrossRef] [PubMed]
- Lackovicova, L.; Gaykema, R.P.; Banovska, L.; Kiss, A.; Goehler, L.E.; Mravec, B. The time-course of hindbrain neuronal activity varies according to location during either intraperitoneal or subcutaneous tumor growth in rats: Single Fos and dual Fos/dopamine beta-hydroxylase immunohistochemistry. J. Neuroimmunol. 2013, 260, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Horvathova, L.; Tillinger, A.; Padova, A.; Bizik, J.; Mravec, B. Changes in gene expression in brain structures related to visceral sensation, autonomic functions, food intake, and cognition in melanoma-bearing mice. Eur. J. Neurosci. 2020, 51, 2376–2393. [Google Scholar] [CrossRef] [PubMed]
- Szpunar, M.J.; Belcher, E.K.; Dawes, R.P.; Madden, K.S. Sympathetic innervation, norepinephrine content, and norepinephrine turnover in orthotopic and spontaneous models of breast cancer. Brain Behav. Immun. 2016, 53, 223–233. [Google Scholar] [CrossRef]
- Zahalka, A.H.; Frenette, P.S. Nerves in cancer. Nat. Rev. Cancer 2020, 20, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Mauffrey, P.; Tchitchek, N.; Barroca, V.; Bemelmans, A.P.; Firlej, V.; Allory, Y.; Romeo, P.H.; Magnon, C. Progenitors from the central nervous system drive neurogenesis in cancer. Nature 2019, 569, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Amit, M.; Takahashi, H.; Dragomir, M.P.; Lindemann, A.; Gleber-Netto, F.O.; Pickering, C.R.; Anfossi, S.; Osman, A.A.; Cai, Y.; Wang, R.; et al. Loss of p53 drives neuron reprogramming in head and neck cancer. Nature 2020, 578, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Rutlin, M.; Huang, S.; Barrick, C.A.; Wang, F.; Jones, K.R.; Tessarollo, L.; Ginty, D.D. Sexually dimorphic BDNF signaling directs sensory innervation of the mammary gland. Science 2012, 338, 1357–1360. [Google Scholar] [CrossRef]
- Wang, Z.; Dong, J.; Tian, W.; Qiao, S.; Wang, H. Role of TRPV1 ion channel in cervical squamous cell carcinoma genesis. Front. Mol. Biosci. 2022, 9, 980262. [Google Scholar] [CrossRef]
- Zheng, L.; Dou, X.; Song, H.; Gao, R.; Tang, X. TRPV1 acts as a Tumor Suppressor and is associated with Immune Cell Infiltration in Clear Cell Renal Cell Carcinoma: Evidence from integrated analysis. J. Cancer 2020, 11, 5678–5688. [Google Scholar] [CrossRef]
- Nie, R.; Liu, Q.; Wang, X. TRPV1 Is a Potential Tumor Suppressor for Its Negative Association with Tumor Proliferation and Positive Association with Antitumor Immune Responses in Pan-Cancer. J. Oncol. 2022, 2022, 6964550. [Google Scholar] [CrossRef]
- Balood, M.; Ahmadi, M.; Eichwald, T.; Ahmadi, A.; Majdoubi, A.; Roversi, K.; Roversi, K.; Lucido, C.T.; Restaino, A.C.; Huang, S.; et al. Nociceptor neurons affect cancer immunosurveillance. Nature 2022, 611, 405–412. [Google Scholar] [CrossRef]
- Kwon, D.H.; Zhang, F.R.; Fedor, J.G.; Suo, Y.; Lee, S.-Y. Vanilloid-dependent TRPV1 opening trajectory from cryoEM ensemble analysis. Nat. Commun. 2022, 13, 2874. [Google Scholar] [CrossRef]
- Fujimura, S.; Kazuhiro, K.; Kuramochi, M.; Sekiguchi, H.; Ikezaki, K.; Mio, M.; Hengphasatporn, K.; Shigeta, Y.; Kubo, T.; Sasaki, Y.C. Agonist and antagonist-diverted twisting motions of a single TRPV1 channel. J. Phys. Chem. B 2020, 124, 11617–11624. [Google Scholar] [CrossRef]
- Zhang, K.; Julius, D.; Cheng, Y. Structural snapshots of TRPV1 revel mechanism of polymodal functionality. Cell 2021, 184, 5138–5150. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Nguyen, P.T.; Vu, S.; Yarov-Yarovoy, V.; Zheng, J. Opening of capsaicin receptor TRPV1 is stabilized equally by its four subunits. J. Biol. Chem. 2023, 15, 104828. [Google Scholar] [CrossRef]
- Wang, C.; Hu, H.-Z.; Colton, C.K.; Wood, J.D.; Zhu, M.X. An alternative splicing product of the murine trpv1 gene dominant negatively modulates the activity of the TRPV1 channel. J. Biol. Chem. 2004, 279, 37423–37430. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Sanz, N.; Fernández-Carjaval, A.; Moranelle-Palao, C.; Planells-Cases, R.; Fajardo-Sánchez, E.; Fernández-Ballester, G.; Ferrer-Montiel, A. Identification of a tetramerization domain in the C terminus of the vanilloid receptor. J. Neurosci. 2004, 24, 5307–5314. [Google Scholar] [CrossRef]
- Sadovsky, L.R.; Sreekhrisna, K.T.; Schinaman, R.; Gorka, K.; Mantri, Y.; Haught, J.C.; Huggins, T.G.; Isfort, R.J. Unique responses are observed in transient receptor potential ankyrin 1 and vanilloid 1 (TRPA1 and TRPV1) co-expressing cells. Cells 2014, 3, 616–626. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Zheng, G.; Wiley, J.W. Epigenetic regulation of genes that modulate chronic stress-induced visceral pain in the peripheral nervous system. Gastroenterology 2015, 148, 148–157. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, Y.; Tian, Q.; Deng, Q.; Wang, Y.; Zhou, T.; Liu, Q.; Mei, K.; Wang, Y.; Liu, H.; et al. TRPV1 SUMOylation regulates nociceptive signaling in models of inflammatory pain. Nat. Commun. 2018, 9, 1529. [Google Scholar] [CrossRef]
- Premkumar, L.S.; Ahern, G.P. Induction of vanilloid receptor channel activity by protein kinase C. Nature 2000, 408, 985–990. [Google Scholar] [CrossRef]
- Hellwig, N.; Albrech, N.; Harteneck, C.; Schultz, G.; Schaefer, M. Homo- and heteromeric assembly of TRPV channel subunits. J. Cell. Sci. 2005, 118, 917–928. [Google Scholar] [CrossRef]
- Planells-Cases, R.; Valente, P.; Ferrer-Montiel, A.; Feng, Q.; Szallasi, A. Complex regulation of TRPV1 and related thermoTRPs: Implications for therapeutic intervention. Adv. Exp. Med. Biol. 2011, 704, 491–515. [Google Scholar]
- Maggi, F.; Morelli, M.B.; Aguzzi, C.; Zeppa, L.; Nabissi, M.; Polidori, C.; Santoni, G.; Amantini, C. Calcium influx, oxidative stress, and apoptosis induced by TRPV1 in chronic myeloid leukemia cells: Synergic effects with imatinib. Front. Mol. Biosci. 2023, 10, 1129202. [Google Scholar] [CrossRef] [PubMed]
- Patapoutian, A.; Tate, S.; Wood, C.J. Transient receptor potential chanels: Targeting pain at the source. Nat. Rev. Drug Discov. 2009, 8, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Lozano, C.; Córdova, C.; Marchant, I.; Zúniga, R.; Ochova, P.; Ramirez-Barrantes, R.; González-Arriagada, W.A.; Rodriguez, B.; Olivero, P. Intracellular aggregated TRPV1 is associated with lowe survival in breast cancer patients. Breast Cancer 2018, 10, 161–168. [Google Scholar]
- Surh, Y.J.; Lee, S.S. Capsaicin, a double-edged sword: Toxicity, metabolism, and chemopreventive potential. Life Sci. 1995, 56, 1845–1855. [Google Scholar] [CrossRef]
- Torrecillas, A.; Schneider, A.; Fernández-Martínez, A.M.; Ausili, A.; de Godos, A.M.; Corbalán-Garcia, S.; Gómez-Fernández, J.C. Capsaicin fluidifies membrane and localizes itself near the lipid-water interface. ACS Chem. Neurosci. 2015, 6, 1741–1750. [Google Scholar] [CrossRef]
- Bleakman, D.; Brorson, J.R.; Miller, R.J. The effect of capsaicin on voltage-gated calcium currents and calcium sigbnals in cultures dorsal root ganglion cells. Br. J. Pharmacol. 1990, 101, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Kwon, H.J.; Kim, G.E.; Cho, M.H.; Yoon, S.Y.; Davies, A.J.; Oh, S.B.; Lee, H.; Cho, Y.K.; Joo, C.H.; et al. Attenuation of natural killer cell functions by capsaicin through a direct and TRPV1-independent mechanism. Carcinogenesis 2014, 35, 1652–1660. [Google Scholar] [CrossRef] [PubMed]
- Erin, N.; Akman, M. Effects of in-vitro modulation of TRPV1 activity on immune response of mice bearing metastatic breast carcinoma: Enhanced inflammatory response may hinder therapeutic potentials of TRPV1 agonists. Life Sci. 2021, 287, 120115. [Google Scholar] [CrossRef]
- Marek-Jozefowicz, L.; Nedoszytko, B.; Grochocka, M.; Zmijewski, M.A.; Czajkowski, R.; Cubala, W.L.; Slominski, A.T. Molecular mechanisms of neurogenic inflammation of the skin. Int. J. Mol. Sci. 2023, 24, 5001. [Google Scholar] [CrossRef]
- Majhi, R.K.; Sahoo, S.S.; Yadav, M.; Pratheek, B.M.; Chattopadhyay, S.; Goswami, C. Functional expression of TRPV channels in T cells and their implications in immune regulation. FEBS J. 2015, 282, 2661–2681. [Google Scholar] [CrossRef]
- Kunde, D.A.; Yingchoncharoen, J.; Jurkovic, S.; Geraghty, D.P. TRPV1 mediates capsaicin-stimulated metabolic activity but not cell death or inhibition of interleukin-1beta release in human THP-1 monocytes. Toxicol. Appl. Pharmacol. 2018, 360, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Sanjai Kumar, P.; Nayak, T.K.; Mahish, C.; Sahoo, S.S.; Radhakrishnan, A.; De, S.; Datey, A.; Sahu, R.P.; Goswami, C.; Chattopadhyay, S.; et al. Inhibition of transient receptor potential vanilloid 1 (TRPV1) channel regulates chikungunya virus infection in macrophages. Arch. Virol. 2021, 166, 139–155. [Google Scholar] [CrossRef] [PubMed]
- Saunders, C.I.; Kunde, D.A.; Crawford, A.; Geraghty, D.P. Expression of transient receptor potential vanilloid 1 (TRPV1) and 2 (TRPV2) in human peripheral blood. Mol. Immunol. 2007, 44, 1429–1435. [Google Scholar] [CrossRef]
- Basu, S.; Srivastava, P. Immunological role of neuronal vanilloid receptor 1 expressed on dendritic cells. Proc. Natl. Acad. Sci. USA 2005, 102, 5120–5125. [Google Scholar] [CrossRef] [PubMed]
- Amantini, C.; Farfariello, V.; Cardinali, C.; Morelli, M.B.; Marinelli, O.; Nabissi, M.; Santoni, M.; Bonfili, L.; Cecarini, V.; Eleuteri, A.M.; et al. The TRPV1 ion channel regulates thymocyte differentiation by modulating autophagy and proteasome activity. Oncotarget 2017, 8, 90766–90780. [Google Scholar] [CrossRef]
- Hogan, P.G.; Lewis, R.S.; Rao, A. Molecular basis of calcium signaling in lymphocytes: STIM and ORAI. Annu. Rev. Immunol. 2010, 28, 491–533. [Google Scholar] [CrossRef]
- Inada, H.; Iida, T.; Tominaga, M. Different expression patterns of TRP genes in murine B and T lymphocytes. Biochem. Biophys. Res. Commun. 2006, 350, 762–767. [Google Scholar] [CrossRef]
- Zhang, F.; Challapalli, S.C.; Smith, P.J. Cannabinoid CB(1) receptor activation stimulates neurite outgrowth and inhibits capsaicin-induced Ca2+ influx in an in vitro model of diabetic neuropathy. Neuropharmacology 2009, 57, 88–96. [Google Scholar] [CrossRef]
- Macho, A.; Calzado, M.A.; Munoz-Blanco, J.; Gomez-Diaz, C.; Gajate, C.; Mollinedo, F.; Navas, P.; Munoz, E. Selective induction of apoptosis by capsaicin in transformed cells: The role of reactive oxygen species and calcium. Cell Death Differ. 1999, 6, 155–165. [Google Scholar] [CrossRef]
- Bertin, S.; Aoki-Nonaka, Y.; Lee, J.; de Jong, P.R.; Kim, P.; Han, T.; Yu, T.; To, K.; Takahashi, N.; Boland, B.S.; et al. The TRPA1 ion channel is expressed in CD4+ T cells and restrains T-cell-mediated colitis through inhibition of TRPV1. Gut 2017, 66, 1584–1596. [Google Scholar] [CrossRef]
- Baker, K.; Raemdonck, K.; Dekkak, B.; Snelgrove, R.J.; Ford, J.; Shala, F.; Belvisi, M.G.; Birrell, M.A. Role of the ion channel, transient receptor potential cation channel subfamily V member 1 (TRPV1), in allergic asthma. Respir. Res. 2016, 17, 67. [Google Scholar] [CrossRef]
- Amantini, C.; Mosca, M.; Lucciarini, R.; Perfumi, M.; Morrone, S.; Piccoli, M.; Santoni, G. Distinct thymocyte subsets express the vanilloid receptor VR1 that mediates capsaicin-induced apoptotic cell death. Cell Death Differ. 2004, 11, 1342–1356. [Google Scholar]
- Takano, F.; Yamaguchi, M.; Takada, S.; Shoda, S.; Yahagi, N.; Takahashi, T.; Ohta, T. Capsicum ethanol extracts and capsaicin enhance interleukin-2 and interferon-gamma production in cultured murine Peyer’s patch cells ex vivo. Life Sci. 2007, 80, 1553–1563. [Google Scholar] [CrossRef]
- Kumar, P.S.; Mukherjee, T.; Khamaru, S.; Radhakrishnan, A.; Nanda-Kishore, D.J.; Chawla, S.; Sahoo, S.S.; Chattopadhyay, S. Elevation of TRPV1 expression on T-cells during experimental immunosuppression. J. Biosci. 2022, 47, 42. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, E.S.; Brito, C.X.; Teixeira, S.A.; Barboza, R.; dos Reis, A.S.; Azevedo-Santos, A.P.; Muscara, M.; Costa, S.K.; Marinho, C.R.; Brain, S.D.; et al. TRPV1 antagonism by capsazepine modulates innate immune response in mice infected with Plasmodium berghei ANKA. Mediat. Inflamm. 2014, 2014, 506450. [Google Scholar] [CrossRef] [PubMed]
- Tóth, B.I.; Benkó, S.; Szőllősi, A.G.; Kovács, L.; Rajnavölgyi, E.; Bíró, T. Transient receptor potential vanilloid-1 signaling inhibits differentiation and activation of human dendritic cells. FEBS Lett. 2009, 583, 1619–1624. [Google Scholar] [CrossRef] [PubMed]
- Koebel, C.M.; Vermi, W.; Swann, J.B.; Zerafa, N.; Rodig, S.J.; Old, L.J.; Smyth, M.J.; Schreiber, R.D. Adaptive immunity maintains occult cancer in an equilibrium state. Nature 2007, 450, 903–907. [Google Scholar] [CrossRef]
- Zou, W.; Restifo, N.P. T(H)17 cells in tumour immunity and immunotherapy. Nat. Rev. Immunol. 2010, 10, 248–256. [Google Scholar] [CrossRef]
- Chopan, M.; Littenberg, B. The Association of Hot Red Chili Pepper Consumption and Mortality: A Large Population-Based Cohort Study. PLoS ONE 2017, 12, e0169876. [Google Scholar] [CrossRef]
- Bonaccio, M.; Di Castelnuovo, A.; Costanzo, S.; Ruggiero, E.; De Curtis, A.; Persichillo, M.; Tabolacci, C.; Facchiano, F.; Cerletti, C.; Donati, M.B.; et al. Chili Pepper Consumption and Mortality in Italian Adults. J. Am. Coll. Cardiol. 2019, 74, 3139–3149. [Google Scholar] [CrossRef]
- Ryan, D.P.; Hong, T.S.; Bardeesy, N. Pancreatic adenocarcinoma. N. Engl. J. Med. 2014, 371, 1039–1049. [Google Scholar] [CrossRef] [PubMed]
- Stopczynski, R.E.; Normolle, D.P.; Hartman, D.J.; Ying, H.; DeBerry, J.J.; Bielefeldt, K.; Rhim, A.D.; DePinho, R.A.; Albers, K.M.; Davis, B.M. Neuroplastic changes occur early in the development of pancreatic ductal adenocarcinoma. Cancer Res. 2014, 74, 1718–1727. [Google Scholar] [CrossRef] [PubMed]
- Swidnicka-Siergiejko, A.K.; Gomez-Chou, S.B.; Cruz-Monserrate, Z.; Deng, D.; Liu, Y.; Huang, H.; Ji, B.; Azizian, N.; Daniluk, J.; Lu, W.; et al. Chronic inflammation initiates multiple forms of K-Ras-independent mouse pancreatic cancer in the absence of TP53. Oncogene 2017, 36, 3149–3158. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, T.; Hiasa, M.; Okui, T.; Hata, K. Sensory nerves: A driver of the vicious cycle in bone metastasis? J. Bone Oncol. 2021, 30, 100387. [Google Scholar] [CrossRef]
- Brown, D.C.; Iadarola, M.J.; Perkowski, S.Z.; Erin, H.; Shofer, F.; Laszlo, K.J.; Olah, Z.; Mannes, A.J. Physiologic and antinociceptive effects of intrathecal resiniferatoxin in a canine bone cancer model. Anesthesiology 2005, 103, 1052–1059. [Google Scholar] [CrossRef]
- Brown, D.C.; Agnello, K.; Iadarola, M.J. Intrathecal resiniferatoxin in a dog model: Efficacy in bone cancer pain. Pain 2015, 156, 1018–1024. [Google Scholar] [CrossRef]
- Erin, N.; Zhao, W.; Bylander, J.; Chase, G.; Clawson, G. Capsaicin-induced inactivation of sensory neurons promotes a more aggressive gene expression phenotype in breast cancer cells. Breast Cancer Res. Treat. 2006, 99, 351–364. [Google Scholar] [CrossRef]
- Kissin, I. Vanilloid-induced conduction analgesia: Selective, dose-dependent, long-lasting, with a low level of potential neurotoxicity. Anesth. Analg. 2008, 107, 271–281. [Google Scholar] [CrossRef]
- Szallasi, A.; Cortright, D.N.; Blum, C.A.; Eid, S.R. The vanilloid receptor TRPV1: 10 years from channel cloning to antagonist proof-of-concept. Nat. Rev. Drug Discov 2007, 6, 357–372. [Google Scholar] [CrossRef]
- Gavva, N.R.; Treanor, J.J.; Garami, A.; Fang, L.; Surapaneni, S.; Akrami, A.; Alvarez, F.; Bak, A.; Darling, M.; Gore, A.; et al. Pharmacological blockade of the vanilloid receptor TRPV1 elicits marked hyperthermia in humans. Pain 2008, 136, 202–210. [Google Scholar] [CrossRef]
- Garami, A.; Shimansky, Y.P.; Rumbus, Z.; Vizin, R.C.L.; Farkas, N.; Hegyi, J.; Szakacs, Z.; Solymar, M.; Csenkey, A.; Chiche, D.A.; et al. Hyperthermia induced by transient receptor potential vanilloid-1 (TRPV1) antagonists in human clinical trials: Insights from mathematical modeling and meta-analysis. Pharmacol. Ther. 2020, 208, 107474. [Google Scholar] [CrossRef] [PubMed]
- Toth, B.; Rogan, E.; Walker, B. Tumorigenicity and mutagenicity studies with capsaicin of hot peppers. Anticancer Res. 1984, 4, 117–119. [Google Scholar] [PubMed]
- Bode, A.M.; Dong, Z. The two faces of capsaicin. Cancer Res. 2011, 71, 2809–2814. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erin, N.; Szallasi, A. Carcinogenesis and Metastasis: Focus on TRPV1-Positive Neurons and Immune Cells. Biomolecules 2023, 13, 983. https://doi.org/10.3390/biom13060983
Erin N, Szallasi A. Carcinogenesis and Metastasis: Focus on TRPV1-Positive Neurons and Immune Cells. Biomolecules. 2023; 13(6):983. https://doi.org/10.3390/biom13060983
Chicago/Turabian StyleErin, Nuray, and Arpad Szallasi. 2023. "Carcinogenesis and Metastasis: Focus on TRPV1-Positive Neurons and Immune Cells" Biomolecules 13, no. 6: 983. https://doi.org/10.3390/biom13060983
APA StyleErin, N., & Szallasi, A. (2023). Carcinogenesis and Metastasis: Focus on TRPV1-Positive Neurons and Immune Cells. Biomolecules, 13(6), 983. https://doi.org/10.3390/biom13060983






