Thyroid, Gonadal and Adrenal Dysfunction in Kidney Transplant Recipients: A Review for the Clinician
Abstract
1. Introduction
2. Methods
3. Thyroid Hormones and KTR
- Low T3 syndrome may persist after kidney transplantation due to corticosteroid administration, inflammation or prolongation of the uremic milieu.
- Pre-transplantation FT3 levels may help identify patients at risk for graft failure.
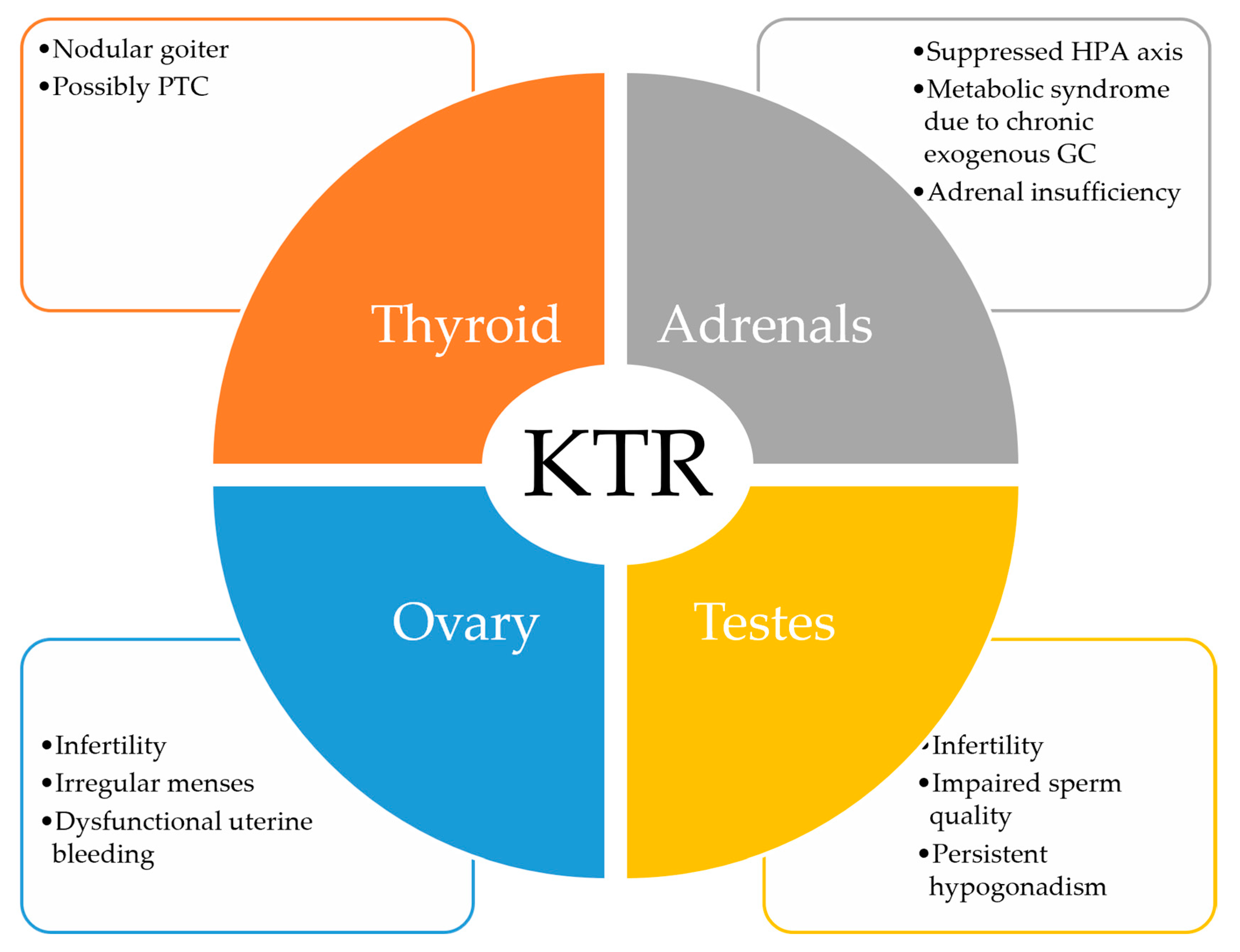
- Periodical evaluation of thyroid function in KTR is recommended, together with ultrasonographic surveillance, due to chronic immunosuppression and associated risk of malignancy.
- Primary hypothyroidism should be pronounced only when facing elevated TSH levels accompanied by clearly low free T4 concentrations.
4. Sex Hormones and KTR
- Restoration of gonadal function takes place soon after successful kidney transplantation.
- Despite testosterone and estradiol concentrations returning toward the normal range, fertility is not completely restored.
- Male KTR still exhibit low sperm quality and low inhibin B levels.
- Female KTR still exhibit abnormal uterine bleeding and anovulatory cycles.
- Steroid immunosuppressive regimens may partially contribute to infertility on the long-term and should be balanced in female KTR planning pregnancy.
- Hormonal contraception, preferably offered by the transdermal route, is beneficial for correcting bleeding abnormalities and improving quality of life in female KTR.
- Testosterone therapy may help improve anemia and hypogonadal symptoms in KTR with low serum testosterone.
- Clinicians should discuss hypogonadism-related health problems with their KTR patients, as immunosuppressive regimens have a detrimental impact upon gonadal health.
- Sirolimus has a worse impact upon gonadal function compared to calcineurin inhibitors.
5. The Hypothalamic-Pituitary–Adrenal Axis and KTR
- HPA axis suppression usually occurs in KTR due to chronic glucocorticoid treatment.
- The HPA axis may be suppressed even with low-dose glucocorticoid regimens.
- Patients should be informed about the signs and symptoms of hypoadrenalism that may occur after steroid withdrawal.
- Measuring morning serum cortisol either 24 h after the last glucocorticoid dose, or if specific symptoms occur, may be an option for KTR. Performing the SST should be taken into consideration if indeterminate 8 am serum cortisol values (generally accepted cutoffs for SST are between 3 and 15 µg/dL) are found, according to existing data.
- Symptomatic adrenal insufficiency may exceptionally occur in KTR due to various conditions despite low-dose glucocorticoid treatment.
- Chronic glucocorticoid administration increases the risk for metabolic syndrome, while an increased mortality from infection is seen in those with increased cortisol/cortisone ratio.
- A slight reduction in the eGFR may occur after glucocorticoid withdrawal.
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bikbov, B.; Purcell, C.A.; Levey, A.S.; Smith, M.; Abdoli, A.; Abebe, M.; Adebayo, O.M.; Afarideh, M.; Agarwal, S.K.; Agudelo-Botero, M.; et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709. [Google Scholar] [CrossRef]
- Kaka, N.; Sethi, Y.; Patel, N.; Kaiwan, O.; Al-Inaya, Y.; Manchanda, K.; Uniyal, N. Endocrine manifestations of chronic kidney disease and their evolving management: A systematic review. Dis. Mon. 2022, 68, 101466. [Google Scholar] [CrossRef] [PubMed]
- Kuczera, P.; Adamczak, M.; Wiecek, A. Endocrine Abnormalities in Patients with Chronic Kidney Disease. Pril. Makedonska Akad. Na Nauk. I Umet. Oddelenie Za Med. Nauk. 2015, 36, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Sagmeister, M.S.; Harper, L.; Hardy, R.S. Cortisol excess in chronic kidney disease—A review of changes and impact on mortality. Front. Endocrinol. 2023, 13, 1075809. [Google Scholar] [CrossRef] [PubMed]
- Mudiayi, D.; Shojai, S.; Okpechi, I.; Christie, E.A.; Wen, K.; Kamaleldin, M.; Elsadig Osman, M.; Lunney, M.; Prasad, B.; Osman, M.A.; et al. Global Estimates of Capacity for Kidney Transplantation in World Countries and Regions. Transplantation 2022, 106, 1113–1122. [Google Scholar] [CrossRef]
- Luxardo, R.; Kramer, A.; González-Bedat, M.C.; Massy, Z.A.; Jager, K.J.; Rosa-Diez, G.; Noordzij, M.; Alvarez Estevez, G.A.; Ambühl, P.M.; Andrusev, A.M.; et al. The epidemiology of renal replacement therapy in two different parts of the world: The Latin American Dialysis and Transplant Registry versus the European Renal Association-European Dialysis and Transplant Association Registry. Rev. Panam. Salud Pública 2018, 42, e87. [Google Scholar] [CrossRef]
- Szypulska-koziarska, D.; Misiakiewicz-has, K.; Wiszniewska, B. Hormonal (Im)Balance and Reproductive System’s Disorders in Transplant Recipients—A Review. Biology 2021, 10, 271. [Google Scholar] [CrossRef]
- Mariani, L.H.; Berns, J.S. The renal manifestations of thyroid disease. J. Am. Soc. Nephrol. 2012, 23, 22–26. [Google Scholar] [CrossRef]
- Slotkin, T.A.; Seidler, F.J.; Kavlock, R.J.; Bartolome, J.V. Thyroid hormone differentially regulates cellular development in neonatal rat heart and kidney. Teratology 1992, 45, 303–312. [Google Scholar] [CrossRef]
- Canavan, J.P.; Holt, J.; Easton, J.; Smith, K.; Goldspink, D.F. Thyroid-induced changes in the growth of the liver, kidney, and diaphragm of neonatal rats. J. Cell. Physiol. 1994, 161, 49–54. [Google Scholar] [CrossRef]
- Kumar, J.; Gordillo, R.; Kaskel, F.J.; Druschel, C.M.; Woroniecki, R.P. Increased prevalence of renal and urinary tract anomalies in children with congenital hypothyroidism. J. Pediatr. 2009, 154, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, R.S.; Ryan, J.; Edwards, B.S.; Klee, G.; Zimmerman, D.; Scott, N.; Burnett, J.C. Cardiorenal endocrine dynamics during volume expansion in hypothyroid dogs. Am. J. Physiol. 1988, 255, R61–R66. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, P.; Bajo, M.A.; Selgas, R.; Díez, J.J. Thyroid dysfunction and kidney disease: An update. Rev. Endocr. Metab. Disord. 2017, 18, 131–144. [Google Scholar] [CrossRef]
- Naguib, R.; Elkemary, E.; Naguib, R.; Elkemary, E. Thyroid Dysfunction and Renal Function: A Crucial Relationship to Recognize. Cureus 2023, 15, 35242. [Google Scholar] [CrossRef]
- Khatiwada, S.; Rajendra, K.C.; Gautam, S.; Lamsal, M.; Baral, N. Thyroid dysfunction and dyslipidemia in chronic kidney disease patients. BMC Endocr. Disord. 2015, 15, 65. [Google Scholar] [CrossRef] [PubMed]
- Mohamedali, M.; Reddy Maddika, S.; Vyas, A.; Iyer, V.; Cheriyath, P. Thyroid Disorders and Chronic Kidney Disease. Int. J. Nephrol. 2014, 2014, 520281. [Google Scholar] [CrossRef]
- Fan, J.; Yan, P.; Wang, Y.; Shen, B.; Ding, F.; Liu, Y. Prevalence and Clinical Significance of Low T3 Syndrome in Non-Dialysis Patients with Chronic Kidney Disease. Med. Sci. Monit. 2016, 22, 1171. [Google Scholar] [CrossRef]
- Lo, J.C.; Chertow, G.M.; Go, A.S.; Hsu, C.Y. Increased prevalence of subclinical and clinical hypothyroidism in persons with chronic kidney disease. Kidney Int. 2005, 67, 1047–1052. [Google Scholar] [CrossRef]
- Rhee, C.M.; Kalantar-Zadeh, K.; Streja, E.; Carrero, J.J.; Ma, J.Z.; Lu, J.L.; Kovesdy, C.P. The relationship between thyroid function and estimated glomerular filtration rate in patients with chronic kidney disease. Nephrol. Dial. Transplant. 2015, 30, 282–287. [Google Scholar] [CrossRef]
- Rhee, C.M. The Interaction Between Thyroid and Kidney Disease: An Overview of the Evidence. Curr. Opin. Endocrinol. Diabetes Obes. 2016, 23, 407–415. [Google Scholar] [CrossRef]
- Meuwese, C.L.; Van Diepen, M.; Cappola, A.R.; Sarnak, M.J.; Shlipak, M.G.; Bauer, D.C.; Fried, L.P.; Iacoviello, M.; Vaes, B.; Degryse, J.; et al. Low thyroid function is not associated with an accelerated deterioration in renal function. Nephrol. Dial. Transplant. 2019, 34, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Acker, C.G.; Singh, A.R.; Flick, R.P.; Bernardini, J.; Greenberg, A.; Johnson, J.P. A trial of thyroxine in acute renal failure. Kidney Int. 2000, 57, 293–298. [Google Scholar] [CrossRef]
- Rhee, C.M. Thyroid Disease in End-Stage Renal Disease. Curr. Opin. Nephrol. Hypertens. 2019, 28, 621. [Google Scholar] [CrossRef]
- Da Costa, A.B.B.A.; Pellizzari, C.; Carvalho, G.A.; Sant’Anna, B.C.; Montenegro, R.L.; Zammar Filho, R.G.; Mesa Junior, C.O.; Hauck Prante, P.R.; Olandoski, M.; Carvalho, M. High prevalence of subclinical hypothyroidism and nodular thyroid disease in patients on hemodialysis. Hemodial. Int. 2016, 20, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Fragidis, S.; Sombolos, K.; Thodis, E.; Panagoutsos, S.; Mourvati, E.; Pikilidou, M.; Papagianni, A.; Pasadakis, P.; Vargemezis, V. Low T3 syndrome and long-term mortality in chronic hemodialysis patients. World J. Nephrol. 2015, 4, 415. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Reyes, M.J.; Diez, J.J.; Collado, A.; Iglesias, P.; Bajo, M.A.; Estrada, P.; Del Peso, G.; Heras, M.; Molina, A.; Selgas, R. Are low concentrations of serum triiodothyronine a good marker for long-term mortality in hemodialysis patients? Clin. Nephrol. 2010, 73, 238–240. [Google Scholar] [CrossRef]
- Koo, H.M.; Kim, C.H.; Doh, F.M.; Lee, M.J.; Kim, E.J.; Han, J.H.; Han, J.S.; Oh, H.J.; Han, S.H.; Yoo, T.H.; et al. The impact of low triiodothyronine levels on mortality is mediated by malnutrition and cardiac dysfunction in incident hemodialysis patients. Eur. J. Endocrinol. 2013, 169, 409–419. [Google Scholar] [CrossRef]
- Rhee, C.M.; Kim, S.; Gillen, D.L.; Oztan, T.; Wang, J.; Mehrotra, R.; Kuttykrishnan, S.; Nguyen, D.V.; Brunelli, S.M.; Kovesdy, C.P.; et al. Association of thyroid functional disease with mortality in a national cohort of incident hemodialysis patients. J. Clin. Endocrinol. Metab. 2015, 100, 1386–1395. [Google Scholar] [CrossRef]
- Halilcevic, A.; Hodzic, E.; Mesic, E.; Trnacevic, S. Incidence of subclinical hypothyroidism in renal transplant patients. Mater. Sociomed. 2015, 27, 108. [Google Scholar] [CrossRef]
- Papalia, T.; Greco, R.; Lofaro, D.; Mollica, A.; Bonofiglio, R. Thyroid status and kidney transplantation outcomes. Transplant. Proc. 2011, 43, 1042–1044. [Google Scholar] [CrossRef] [PubMed]
- Schairer, B.; Jungreithmayr, V.; Schuster, M.; Reiter, T.; Herkner, H.; Gessl, A.; Sengölge, G.; Winnicki, W. Effect of Thyroid Hormones on Kidney Function in Patients after Kidney Transplantation. Sci. Rep. 2020, 10, 2156. [Google Scholar] [CrossRef] [PubMed]
- Łebkowska, U.; Małyszko, J.; Brzósko, S.; Łebkowski, W.; Małyszko, J.S.; Janica, J.; Kowalewski, R.; Gacko, M.; Myśliwiec, M.; Walecki, J. Renal artery resistance index, thyroid hormones, and thyroid volume in the early kidney transplants recipients. Transplant. Proc. 2006, 38, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Łebkowska, U.; Małyszko, J.; Lebkowski, W.J.; Walecki, J.; Myśliwiec, M. Is there any relation between thyroid gland function and kidney transplant function? Transplant. Proc. 2003, 35, 2222–2223. [Google Scholar] [CrossRef]
- Tauchmanovà, L.; Carrano, R.; Musella, T.; Orio, F.; Sabbatini, M.; Lombardi, G.; Fenzi, G.; Federico, S.; Colao, A. Thyroid function and morphology after a successful kidney transplantation. J. Endocrinol. Investig. 2006, 29, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Malyszko, J.; Malyszko, J.S.; Pawlak, K.; Mysliwiec, M. Possible relations between thyroid function, endothelium, and kidney and liver function in kidney allograft recipients. Transplant. Proc. 2006, 38, 3509–3513. [Google Scholar] [CrossRef]
- Acker, C.G.; Flick, R.; Shapiro, R.; Scantlebury, V.P.; Jordan, M.L.; Vivas, C.; Greenberg, A.; Johnson, J.P. Thyroid hormone in the treatment of post-transplant acute tubular necrosis (ATN). Am. J. Transplant. 2002, 2, 57–61. [Google Scholar] [CrossRef]
- Hekmat, R.; Javadi, Z.; Javan, M.L.; Sanadgol, H.; Gholami, F.; Mohebbi, M.; Zeraati, A.A.; Ahmadnia, H.; Tabarraiei, H.; Baradaran, M.; et al. Thyroid hormone changes in early kidney transplantation and its correlation with delayed graft function. Urol. J. 2010, 7, 30–34. [Google Scholar]
- Junik, R.; Wlodarczyk, Z.; Masztalerz, M.; Odrowaz-Sypniewska, G.; Jendryczka, E.; Manitius, J. Function, structure, and volume of thyroid gland following allogenic kidney transplantation. Transplant. Proc. 2003, 35, 2224–2226. [Google Scholar] [CrossRef]
- Rotondi, M.; Netti, G.S.; Rosati, A.; Mazzinghi, B.; Magri, F.; Ronconi, E.; Becherucci, F.; Pradella, F.; Salvadori, M.; Serio, M.; et al. Pretransplant serum FT3 levels in kidney graft recipients are useful for identifying patients with higher risk for graft failure. Clin. Endocrinol. 2008, 68, 220–225. [Google Scholar] [CrossRef]
- Karakan, S.; Sezer, S.; Ozdemir, F.N.; Haberal, M. Pretransplantation serum FT3 concentration in kidney recipients is useful to identify higher risk of graft failure. Transplant. Proc. 2011, 43, 448–450. [Google Scholar] [CrossRef]
- Lebkowska, U.; Malyszko, J.S.; Malyszko, J.; Dzieciol, J.; Walecki, J.; Mysliwiec, M. Thyroid function and incidentalomas in kidney transplant recipients. Med. Sci. Monit. 2003, 9, MT8–MT11. [Google Scholar] [PubMed]
- Lee, J.; Jeong, J.J.; Lee, Y.S.; Nam, K.H.; Chang, H.S.; Chung, W.Y.; Soh, E.Y.; Kim, Y.S.; Park, C.S. Incidence and clinical behavior of papillary thyroid carcinoma in renal allograft recipients: A single center experience. Transplant. Proc. 2008, 40, 3751–3754. [Google Scholar] [CrossRef] [PubMed]
- Sabia, R.; Wagner, M.; Susa, K.; Lemke, J.; Rothermund, L.; Henne-Bruns, D.; Hillenbrand, A. Calcitonin concentrations in patients with chronic kidney disease on hemodialysis in reference to parathyroidectomy. BMC Res. Notes 2019, 12, 439. [Google Scholar] [CrossRef] [PubMed]
- Sobki, S.H.; Sobki, S.H.; Henry, J.G.; Mujeebuddin, S.; Khan, H.A.; Fedail, H.M.; Khader, A.A. Serum calcitonin in renal transplant patients. Ren. Fail. 2001, 23, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Saller, B.; Gorges, R.; Reinhardt, W.; Haupt, K.; Janssen, O.E.; Mann, K. Sensitive calcitonin measurement by two-site immunometric assays: Implications for calcitonin screening in nodular thyroid disease. Clin. Lab. 2002, 48, 191–200. [Google Scholar]
- Zdunek, M.; Silbiger, S.; Lei, J.; Neugarten, J. Protein kinase CK2 mediates TGF-beta1-stimulated type IV collagen gene transcription and its reversal by estradiol. Kidney Int. 2001, 60, 2097–2108. [Google Scholar] [CrossRef]
- Negulescu, O.; Bognar, I.; Lei, J.; Devarajan, P.; Silbiger, S.; Neugarten, J. Estradiol reverses TGF-beta1-induced mesangial cell apoptosis by a casein kinase 2-dependent mechanism. Kidney Int. 2002, 62, 1989–1998. [Google Scholar] [CrossRef]
- Neugarten, J.; Medve, I.; Lei, J.; Silbiger, S.R. Estradiol suppresses mesangial cell type I collagen synthesis via activation of the MAP kinase cascade. Am. J. Physiol. 1999, 277, F875–F881. [Google Scholar] [CrossRef]
- Neugarten, J.; Golestaneh, L. Influence of Sex on the Progression of Chronic Kidney Disease. Mayo Clin. Proc. 2019, 94, 1339–1356. [Google Scholar] [CrossRef]
- Nickenig, G.; Bäumer, A.T.; Grohè, C.; Kahlert, S.; Strehlow, K.; Rosenkranz, S.; Stäblein, A.; Beckers, F.; Smits, J.F.M.; Daemen, M.J.A.P.; et al. Estrogen Modulates AT1 Receptor Gene Expression In Vitro and In Vivo. Circulation 1998, 97, 2197–2201. [Google Scholar] [CrossRef]
- Baiardi, G.; Macova, M.; Armando, I.; Ando, H.; Tyurmin, D.; Saavedra, J.M. Estrogen upregulates renal angiotensin II AT 1 and AT 2 receptors in the rat. Regul. Pept. 2005, 124, 7–17. [Google Scholar] [CrossRef]
- Reckelhoff, J.F.; Yanes, L.L.; Iliescu, R.; Fortepiani, L.A.; Granger, J.P. Testosterone supplementation in aging men and women: Possible impact on cardiovascular-renal disease. Am. J. Physiol. Renal Physiol. 2005, 289, F941–F948. [Google Scholar] [CrossRef] [PubMed]
- Reckelhoff, J.F. Sex Steroids, Cardiovascular Disease, and Hypertension. Hypertension 2005, 45, 170–174. [Google Scholar] [CrossRef]
- Doublier, S.; Lupia, E.; Catanuto, P.; Periera-Simon, S.; Xia, X.; Korach, K.; Berho, M.; Elliot, S.J.; Karl, M. Testosterone and 17β-estradiol have opposite effects on podocyte apoptosis that precedes glomerulosclerosis in female estrogen receptor knockout mice. Kidney Int. 2011, 79, 404–413. [Google Scholar] [CrossRef]
- Verzola, D.; Villaggio, B.; Procopio, V.; Gandolfo, M.T.; Gianiorio, F.; Famà, A.; Tosetti, F.; Traverso, P.; Deferrari, G.; Garibotto, G. Androgen-mediated apoptosis of kidney tubule cells: Role of c-Jun amino terminal kinase. Biochem. Biophys. Res. Commun. 2009, 387, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Gohar, E.Y.; Giachini, F.R.; Pollock, D.M.; Tostes, R.C. Role of the Endothelin System in Sexual Dimorphism in Cardiovascular and Renal Diseases. Life Sci. 2016, 159, 20. [Google Scholar] [CrossRef]
- Dousdampanis, P.; Trigka, K.; Fourtounas, C.; Bargman, J.M. Role of testosterone in the pathogenesis, progression, prognosis and comorbidity of men with chronic kidney disease. Ther. Apher. Dial. 2014, 18, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Cigarrán, S.; Coronel, F.; Florit, E.; Calviño, J.; Villa, J.; Gonzalez Tabares, L.; Herrero, J.A.; Carrero, J.J. Testosterone deficiency in dialysis patients: Differences according to the dialysis techniques. Nefrología 2017, 37, 526–530. [Google Scholar] [CrossRef]
- Leśniak, K.; Rymarz, A.; Sobol, M.; Dymus, J.; Woźniak-Kosek, A.; Niemczyk, S. Testosterone Deficiency and Nutritional Parameters as Predictors of All-Cause Mortality among Male Dialysis Patients. Nutrients 2022, 14, 4461. [Google Scholar] [CrossRef]
- Bello, A.K.; Stenvinkel, P.; Lin, M.; Hemmelgarn, B.; Thadhani, R.; Klarenbach, S.; Chan, C.; Zimmerman, D.; Cembrowski, G.; Strippoli, G.; et al. Serum testosterone levels and clinical outcomes in male hemodialysis patients. Am. J. Kidney Dis. 2014, 63, 268–275. [Google Scholar] [CrossRef]
- Gungor, O.; Kircelli, F.; Carrero, J.J.; Asci, G.; Toz, H.; Tatar, E.; Hur, E.; Sever, M.S.; Arinsoy, T.; Ok, E. Endogenous Testosterone and Mortality in Male Hemodialysis Patients: Is It the Result of Aging? Clin. J. Am. Soc. Nephrol. 2010, 5, 2018. [Google Scholar] [CrossRef]
- Carrero, J.J.; Qureshi, A.R.; Nakashima, A.; Arver, S.; Parini, P.; Lindholm, B.; Bárány, P.; Heimbürger, O.; Stenvinkel, P. Prevalence and clinical implications of testosterone deficiency in men with end-stage renal disease. Nephrol. Dial. Transplant. 2011, 26, 184–190. [Google Scholar] [CrossRef]
- Chang, D.H.; Dumanski, S.M.; Ahmed, S.B. Female Reproductive and Gynecologic Considerations in Chronic Kidney Disease: Adolescence and Young Adulthood. Kidney Int. Rep. 2022, 7, 152–164. [Google Scholar] [CrossRef]
- Oh, E.S.; Steele, C.N.; You, Z.; Nowak, K.L.; Jovanovich, A.J. Sex hormones and the risk of cardiovascular disease and mortality in male and female patients with chronic kidney disease: A systematic review and meta-analysis. Physiol. Rep. 2022, 10, e15490. [Google Scholar] [CrossRef] [PubMed]
- Hamdi, S.M.; Walschaerts, M.; Bujan, L.; Rostaing, L.; Kamar, N. A prospective study in male recipients of kidney transplantation reveals divergent patterns for inhibin B and testosterone secretions. Basic Clin. Androl. 2014, 24, 11. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zheng, J.; Xu, L.; Min, Z.; Zhu, Y.; Qi, J.; Duan, Q. Measurements of Serum Pituitary-Gonadal Hormones and Investigation of Sexual and Reproductive Functions in Kidney Transplant Recipients. Int. J. Nephrol. 2010, 2010, 612126. [Google Scholar] [CrossRef] [PubMed]
- Fritsche, L.; Budde, K.; Dragun, D.; Einecke, G.; Diekmann, F.; Neumayer, H.H. Testosterone Concentrations and Sirolimus in Male Renal Transplant Patients. Am. J. Transplant. 2004, 4, 130–131. [Google Scholar] [CrossRef]
- Lee, S.; Coco, M.; Greenstein, S.M.; Schechner, R.S.; Tellis, V.A.; Glicklich, D.G. The effect of sirolimus on sex hormone levels of male renal transplant recipients. Clin. Transplant. 2005, 19, 162–167. [Google Scholar] [CrossRef]
- Tondolo, V.; Citterio, F.; Panocchia, N.; Nanni, G.; Favi, E.; Brescia, A.; Castagneto, M. Gonadal function and immunosuppressive therapy after renal transplantation. Transplant. Proc. 2005, 37, 1915–1917. [Google Scholar] [CrossRef]
- Eckersten, D.; Giwercman, A.; Pihlsgård, M.; Bruun, L.; Christensson, A. Impact of Kidney Transplantation on Reproductive Hormone Levels in Males: A Longitudinal Study. Nephron 2018, 138, 192–201. [Google Scholar] [CrossRef]
- Tauchmanovà, L.; Carrano, R.; Sabbatini, M.; De Rosa, M.; Orio, F.; Palomba, S.; Cascella, T.; Lombardi, G.; Federico, S.; Colao, A. Hypothalamic-pituitary-gonadal axis function after successful kidney transplantation in men and women. Hum. Reprod. 2004, 19, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Majzoub, A.; Shoskes, D.A. A case series of the safety and efficacy of testosterone replacement therapy in renal failure and kidney transplant patients. Transl. Androl. Urol. 2016, 5, 81418–81818. [Google Scholar] [CrossRef] [PubMed]
- Sikora-Grabka, E.; Adamczak, M.; Kuczera, P.; Wiecek, A. Serum sex hormones concentrations in young women in the early period after successful kidney transplantation. Endokrynol. Pol. 2018, 69, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Aulakh, B.S.; Singh, S.K.; Khanna, S.; Kaura, R.; Goyal, S. Impact of renal transplantation on gonadal function in male uremic patients—Our experience. Transplant. Proc. 2003, 35, 316. [Google Scholar] [CrossRef] [PubMed]
- Eckersten, D.; Giwercman, A.; Christensson, A. Male patients with terminal renal failure exhibit low serum levels of antimüllerian hormone. Asian J. Androl. 2015, 17, 149. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, W.; Kübber, H.; Dolff, S.; Benson, S.; Führer, D.; Tan, S. Rapid recovery of hypogonadism in male patients with end stage renal disease after renal transplantation. Endocrine 2018, 60, 159–166. [Google Scholar] [CrossRef]
- Kim, J.M.; Song, R.K.; Kim, M.J.; Lee, D.Y.; Jang, H.R.; Kwon, C.H.D.; Huh, W.S.; Kim, G.S.; Kim, S.J.; Choi, D.S.; et al. Hormonal differences between female kidney transplant recipients and healthy women with the same gynecologic conditions. Transplant. Proc. 2012, 44, 740–743. [Google Scholar] [CrossRef]
- Castinetti, F.; Brue, T. Impact of Cushing’s syndrome on fertility and pregnancy. Ann. Endocrinol. 2022, 83, 188–190. [Google Scholar] [CrossRef]
- Grbac, E.; So, T.; Varshney, S.; Williamson, N.; Dimitriadis, E.; Menkhorst, E. Prednisolone Alters Endometrial Decidual Cells and Affects Decidual-Trophoblast Interactions. Front. Cell Dev. Biol. 2021, 9, 838. [Google Scholar] [CrossRef]
- Li, Q.N.; Li, L.; Hou, G.; Wang, Z.B.; Hou, Y.; Liu, Z.H.; Schatten, H.; Sun, Q.Y. Glucocorticoid exposure affects female fertility by exerting its effect on the uterus but not on the oocyte: Lessons from a hypercortisolism mouse model. Hum. Reprod. 2018, 33, 2285–2294. [Google Scholar] [CrossRef]
- Ong, S.C.; Kumar, V. Pregnancy in a kidney transplant patient. Clin. J. Am. Soc. Nephrol. 2020, 15, 120–122. [Google Scholar] [CrossRef]
- Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am. J. Transplant. 2009, 9 (Suppl. S3), S1–S155. [Google Scholar] [CrossRef] [PubMed]
- Pietrzak, B.; Bobrowska, K.; Jabiry-Zieniewicz, Z.; Kaminski, P.; Wielgos, M.; Pazik, J.; Durlik, M. Oral and transdermal hormonal contraception in women after kidney transplantation. Transplant. Proc. 2007, 39, 2759–2762. [Google Scholar] [CrossRef] [PubMed]
- Vedder, H. Physiology of the Hypothalamic–Pituitary–Adrenocortical Axis. NeuroImmune Biol. 2007, 7, 17–31. [Google Scholar] [CrossRef]
- Li, X.; Xiang, X.; Hu, J.; Goswami, R.; Yang, S.; Zhang, A.; Wang, Y.; Li, Q.; Bi, X. Association Between Serum Cortisol and Chronic Kidney Disease in Patients with Essential Hypertension. Kidney Blood Press. Res. 2016, 41, 384–391. [Google Scholar] [CrossRef]
- Afsar, B. The relationship of serum cortisol levels with depression, cognitive function and sleep disorders in chronic kidney disease and hemodialysis patients. Psychiatr. Q. 2014, 85, 479–486. [Google Scholar] [CrossRef]
- Shirazian, S.; Grant, C.D.; Aina, O.; Mattana, J.; Khorassani, F. Depression in Chronic Kidney Disease and End-Stage Renal Disease: Similarities and Differences in Diagnosis, Epidemiology, and Management. Kidney Int. Rep. 2017, 2, 94. [Google Scholar] [CrossRef]
- Rapa, S.F.; Di Iorio, B.R.; Campiglia, P.; Heidland, A.; Marzocco, S. Inflammation and Oxidative Stress in Chronic Kidney Disease—Potential Therapeutic Role of Minerals, Vitamins and Plant-Derived Metabolites. Int. J. Mol. Sci. 2020, 21, 263. [Google Scholar] [CrossRef]
- Raff, H.; Trivedi, H. Circadian rhythm of salivary cortisol, plasma cortisol, and plasma ACTH in end-stage renal disease. Endocr. Connect. 2013, 2, 23–31. [Google Scholar] [CrossRef]
- Cardoso, E.M.d.L.; Arregger, A.L.; Budd, D.; Zucchini, A.E.; Contreras, L.N. Dynamics of salivary cortisol in chronic kidney disease patients at stages 1 through 4. Clin. Endocrinol. 2016, 85, 313–319. [Google Scholar] [CrossRef]
- Chiodini, I.; Ramos-Rivera, A.; Marcus, A.O.; Yau, H. Adrenal Hypercortisolism: A Closer Look at Screening, Diagnosis, and Important Considerations of Different Testing Modalities. J. Endocr. Soc. 2019, 3, 1097. [Google Scholar] [CrossRef]
- Chan, K.C.A.; Lit, L.C.W.; Law, E.L.K.; Tai, M.H.L.; Yung, C.U.; Chan, M.H.M.; Lam, C.W.K. Diminished urinary free cortisol excretion in patients with moderate and severe renal impairment. Clin. Chem. 2004, 50, 757–759. [Google Scholar] [CrossRef] [PubMed]
- Raff, H.; Cohen, E.P.; Findling, J.W. A commentary on Diagnosing Cushing’s disease in the context of renal failure. Eur. J. Endocrinol. 2019, 181, C9–C11. [Google Scholar] [CrossRef] [PubMed]
- Stroud, A.; Zhang, J.; McCormack, A. Diagnosing Cushing’s disease in the context of chronic kidney disease: A case report and literature review. Eur. J. Endocrinol. 2019, 181, K29–K35. [Google Scholar] [CrossRef]
- Szychlińska, M.; Baranowska-Jurkun, A.; Matuszewski, W.; Wołos-Kłosowicz, K.; Bandurska-Stankiewicz, E. Markers of Subclinical Cardiovascular Disease in Patients with Adrenal Incidentaloma. Medicina 2020, 56, 69. [Google Scholar] [CrossRef]
- Kim, J.; Yun, K.S.; Cho, A.; Kim, D.H.; Lee, Y.K.; Choi, M.J.; Kim, S.H.; Kim, H.; Yoon, J.W.; Park, H.C. High cortisol levels are associated with oxidative stress and mortality in maintenance hemodialysis patients. BMC Nephrol. 2022, 23, 98. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, N.; Sakao, Y.; Sato, T.; Ishigaki, S.; Isobe, S.; Fujikura, T.; Kato, A.; Yasuda, H. Characteristics of adrenal insufficiency in hemodialysis patients. Ren. Replace. Ther. 2021, 7, 17. [Google Scholar] [CrossRef]
- Sakao, Y.; Sugiura, T.; Tsuji, T.; Ohashi, N.; Yasuda, H.; Fujigaki, Y.; Kato, A. Clinical manifestation of hypercalcemia caused by adrenal insufficiency in hemodialysis patients: A case-series study. Intern. Med. 2014, 53, 1485–1490. [Google Scholar] [CrossRef]
- de Vries, L.V.; de Jong, W.H.A.; Touw, D.J.; Berger, S.P.; Navis, G.; Kema, I.P.; Bakker, S.J.L. Twenty-four hour urinary cortisol excretion and the metabolic syndrome in prednisolone-treated renal transplant recipients. Steroids 2017, 127, 31–39. [Google Scholar] [CrossRef]
- Miozzari, M.; Ambühl, P.M. Steroid withdrawal after long-term medication for immunosuppressive therapy in renal transplant patients: Adrenal response and clinical implications. Nephrol. Dial. Transplant. 2004, 19, 2615–2621. [Google Scholar] [CrossRef]
- Vulto, A.; Minović, I.; de Vries, L.V.; Timmermans, A.C.; van Faassen, M.; Gomes Neto, A.W.; Touw, D.J.; de Jong, M.F.C.; van Beek, A.P.; Dullaart, R.P.F.; et al. Endogenous urinary glucocorticoid metabolites and mortality in prednisolone-treated renal transplant recipients. Clin. Transplant. 2020, 34, e13824. [Google Scholar] [CrossRef]
- Schroth, M.; Plank, C.; Rauh, M.; Dörr, H.G.; Rascher, W.; Dötsch, J. Pediatric renal allograft transplantation does not normalize the increased cortisol/cortisone ratios of chronic renal failure. Eur. J. Endocrinol. 2006, 154, 555–561. [Google Scholar] [CrossRef]
- Valentin, A.; Borresen, S.W.; Rix, M.; Elung-Jensen, T.; Sørensen, S.S.; Feldt-Rasmussen, U. Adrenal insufficiency in kidney transplant patients during low-dose prednisolone therapy: A cross-sectional case-control study. Nephrol. Dial. Transplant. 2020, 35, 2191–2197. [Google Scholar] [CrossRef]
- Baz-Hecht, M.; Osher, E.; Yachnin, T.; Nakache, R.; Nakache, G.; Tordjman, K.; Stern, N. The low-dose (1 microg) adrenocorticotropin stimulation test in kidney and kidney-pancreas transplant patients: A potential guideline for steroid withdrawal. Clin. Transplant. 2006, 20, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Smets, P.; Meyer, E.; Maddens, B.; Daminet, S. Cushing’s syndrome, glucocorticoids and the kidney. Gen. Comp. Endocrinol. 2010, 169, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Broersen, L.H.A.; Pereira, A.M.; Jørgensen, J.O.L.; Dekkers, O.M. Adrenal Insufficiency in Corticosteroids Use: Systematic Review and Meta-Analysis. J. Clin. Endocrinol. Metab. 2015, 100, 2171–2180. [Google Scholar] [CrossRef]
- Joseph, R.M.; Hunter, A.L.; Ray, D.W.; Dixon, W.G. Systemic glucocorticoid therapy and adrenal insufficiency in adults: A systematic review. Semin. Arthritis Rheum. 2016, 46, 133. [Google Scholar] [CrossRef]
- Pelewicz, K.; Miśkiewicz, P. Glucocorticoid Withdrawal—An Overview on When and How to Diagnose Adrenal Insufficiency in Clinical Practice. Diagnostics 2021, 11, 728. [Google Scholar] [CrossRef] [PubMed]
- Fleseriu, M.; Hashim, I.A.; Karavitaki, N.; Melmed, S.; Murad, M.H.; Salvatori, R.; Samuels, M.H. Hormonal replacement in hypopituitarism in adults: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2016, 101, 3888–3921. [Google Scholar] [CrossRef] [PubMed]
- Prete, A.; Bancos, I. Glucocorticoid induced adrenal insufficiency. BMJ 2021, 374, n1380. [Google Scholar] [CrossRef]
- Ardalan, M.; Shoja, M.M. Cytomegalovirus-Induced Adrenal Insufficiency in a Renal Transplant Recipient. Transplant. Proc. 2009, 41, 2915. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Advanced CKD/Dialysis | KTR |
|---|---|---|
| Thyroid Hormones | ||
| TSH | ↑ [15,18,23] | N/↓ [21,38] |
| FT4 | N/↓ [24] | N [37,38] |
| FT3 | ↓ [13,16,17] | ↓ [29,30] |
| Calcitonin | N/↑ [43] | N [44] |
| Parameter | Advanced CKD/Dialysis | KTR |
|---|---|---|
| Sex Hormones | ||
| Men | ||
| Testosterone | ↓ [2,57,58,59,61,62] | N [67] |
| LH | ↑ [2,65] | N |
| FSH | ↑ [2,65] | ↑ [65,70,71] |
| Inhibin B | N [65] | ↓ [65,70] |
| Women | ||
| Estradiol | ↓ [2] | N/↑ [72] |
| Progesterone | ↓ [2] | ↓ [72] |
| LH | ↑ [2] | N/↓ [66,73] |
| FSH | ↑ [2] | N/↑ [66,72,73] |
| Parameter | Advanced CKD/Dialysis | KTR |
|---|---|---|
| Hypothalamic-Pituitary–Adrenal Axis | ||
| 8 am cortisol | N/↑ [4,85] Cut-off: 8.45 µg/dL for predicting AI [97] | ↓ * [100] |
| Late-night salivary cortisol | ↑ [90,91] | - |
| UFC/24 h | ↓ [92] | ↓ * [101] |
| Cortisol/cortisone ratio | ↑ [102] | ↑ [101] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bilha, S.C.; Hogas, S.; Hogas, M.; Marcu, S.; Leustean, L.; Ungureanu, M.-C.; Branisteanu, D.D.; Preda, C. Thyroid, Gonadal and Adrenal Dysfunction in Kidney Transplant Recipients: A Review for the Clinician. Biomolecules 2023, 13, 920. https://doi.org/10.3390/biom13060920
Bilha SC, Hogas S, Hogas M, Marcu S, Leustean L, Ungureanu M-C, Branisteanu DD, Preda C. Thyroid, Gonadal and Adrenal Dysfunction in Kidney Transplant Recipients: A Review for the Clinician. Biomolecules. 2023; 13(6):920. https://doi.org/10.3390/biom13060920
Chicago/Turabian StyleBilha, Stefana Catalina, Simona Hogas, Mihai Hogas, Stefan Marcu, Letitia Leustean, Maria-Christina Ungureanu, Dumitru D. Branisteanu, and Cristina Preda. 2023. "Thyroid, Gonadal and Adrenal Dysfunction in Kidney Transplant Recipients: A Review for the Clinician" Biomolecules 13, no. 6: 920. https://doi.org/10.3390/biom13060920
APA StyleBilha, S. C., Hogas, S., Hogas, M., Marcu, S., Leustean, L., Ungureanu, M.-C., Branisteanu, D. D., & Preda, C. (2023). Thyroid, Gonadal and Adrenal Dysfunction in Kidney Transplant Recipients: A Review for the Clinician. Biomolecules, 13(6), 920. https://doi.org/10.3390/biom13060920







