Abstract
Type 2 diabetes mellitus (T2DM) is a common endocrine disorder which remains a large challenge for clinicians. Previous studies have suggested that mitochondrial dysfunction plays an active role in T2DM progression, but a detailed mechanism is still elusive. In the current study, two Han Chinese families with maternally inherited T2DM were evaluated using clinical, genetic, molecular, and biochemical analyses. The mitochondrial genomes were PCR amplified and sequenced. Phylogenetic and bioinformatic analyses were used to assess the potential pathogenicity of mitochondrial DNA (mtDNA) mutations. Interestingly, the matrilineal relatives of these pedigrees exhibited variable severity of T2DM, in particular, the age at onset of T2DM varied from 26 to 65 years, with an average of 49 years. Sequence analysis revealed the presence of ND4 G11696A mutation, which resulted in the substitution of an isoleucine for valine at amino acid (AA) position 312. Indeed, this mutation was present in homoplasmy only in the maternal lineage, not in other members of these families, as well as 200 controls. Furthermore, the m.C5601T in the tRNAAla and novel m.T5813C in the tRNACys, showing high evolutional conservation, may contribute to the phenotypic expression of ND4 G11696A mutation. In addition, biochemical analysis revealed that cells with ND4 G11696A mutation exhibited higher levels of reactive oxygen species (ROS) productions than the controls. In contrast, the levels of mitochondrial membrane potential (MMP), ATP, mtDNA copy number (mtDNA-CN), Complex I activity, and NAD+/NADH ratio significantly decreased in cell lines carrying the m.G11696A and tRNA mutations, suggesting that these mutations affected the respiratory chain function and led to mitochondrial dysfunction that was involved in T2DM. Thus, our study broadened the clinical phenotypes of m.G11696A mutation.
1. Introduction
T2DM is a serious public health problem that is widespread in China, affecting approximately 10% of the adult population [1]. It is a complex metabolic disorder that is characterized by abnormal levels of carbohydrates, fats, and proteins [2]. This disease can be caused by single gene mutations or multi-factorial conditions resulting from interactions between environmental and genetic risk factors [3]. Of inherited factors, a maternal excess in the transmission of T2DM was implicated in many case–control studies [4], suggesting that mutation in mtDNA is the molecular basis for this disorder [5]. mtDNA is maternally inherited and not entirely influenced by recombination events. Moreover, owing to the lack of a DNA repair system, mtDNA has a higher mutation rate than nuclear DNA [6]. In fact, since the landmark discovery of mitochondrial diabetes was the identification of a 10.4 kb large deletion in mtDNA [7] and m.A3243G mutation in tRNALeu(UUR) [8], a growing number of T2DM-associated mtDNA mutations has been reported, such as tRNAIle T4291C, tRNAGlu A14692G, and T14709C mutations [9,10,11]. However, to date, the molecular pathogenesis of these mutations in T2DM progression remains unclear.
In this study, we reported two Han Chinese families with T2DM. Through the application of PCR-Sanger sequencing, we identified the existence of ND4 G11696A mutation in both families. To see the contributions of m.G11696A mutation to T2DM progression, we generated cybrid cells which were derived from six patients with this mutation and four controls without this mutation. In addition, the cellular ATP, MMP, ROS, NAD+/NADH ratio, mtDNA-CN, and Complex I activity were determined.
2. Materials and Methods
2.1. Subjects and Clinical Assessments
We ascertained two families (Pedigree 1 and Pedigree 2) with T2DM via Hangzhou First People’s Hospital (Figure 1). Members of these pedigrees were evaluated to identify both personal and medical histories of diabetes, deafness, and other clinical abnormalities. Moreover, 200 healthy subjects (100 males and 100 females), aged from 31 to 42, with an average of 38 years, were recruited as controls. This study was approved by the Ethics Committee of the Hangzhou First People’s Hospital (Approval Number: 2021-171-01). Written informed consent, as well as consent to publish these details, was obtained from all participants.
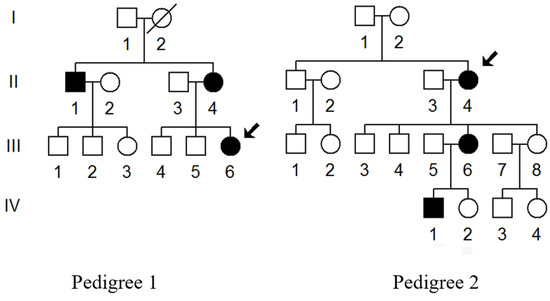
Figure 1.
Two Chinese families with T2DM; arrows indicate the probands (III-6 in Pedigree 1 and II-4 in Pedigree 2).
The diagnosis of T2DM was based on the criteria proposed by the American Diabetes Association [12], which are as follows: (1) a fasting plasma glucose (FPG) level ≥7.0 mmol/L; (2) a 2 h plasma glucose level after 75 g oral glucose tolerance test (OGTT) ≥11.1 mmol/L; and (3) the level of Hemoglobin A1c (HbA1c) ≥6.5%. For biochemical assessment, serum FPG was determined using the regular laboratory methods (Beckman Coulter AU5800). In addition, the OGTT was carried out by a measurement of plasma glucose concentrations at 0 and 2 h after the administration of 75 g of glucose.
2.2. Screening for the Entire MtDNA Variants
Genomic DNA was extracted from the blood of each participant using Paxgene Blood DNA Isolation kits (QIAGEN, Hilden, Germany). For screening the mtDNA mutations/variants, the affected individuals’ (Pedigree 1: II-1, II-4 and III-6; Pedigree 2: II-4, III-6 and IV-1) DNA fragments spanning the 16,569 bp mitochondrial genome were PCR amplified using 24 primers as described previously [13]. The PCR products were subsequently analyzed using an ABI 3700 automated DNA instrument; the data were compared to the revised Cambridge reference sequences (rCRS) to screen the mutations/variants (GenBank accession number: NC_012920.1) [14].
2.3. Haplogroup Classification
Using the nomenclature of mitochondrial haplogroups, we assigned the full mitochondrial sequences of the probands (III-6 in Pedigree 1; II-4 in Pedigree 2) to define mitochondrial haplogroups [15].
2.4. Phylogenetic Conservation Analysis
To further assess the pathogenic roles of mtDNA mutations, phylogenetic analysis was performed. In brief, 17 species were selected for this analysis, as described previously [16]. Moreover, the sequence alignment of the mtDNA was performed using the ClustalW program (https://www.ebi.ac.uk/Tools/msa/clustalw2/ (accessed on 10 May 2023) [17].
2.5. Population Screening
The frequencies of m.G11696A, m.C5601T, and m.T5813C mutations were further screened in 200 unrelated healthy subjects and all of the members of these two pedigrees. The primers for the amplification of ND4 gene were forward: 5′-TCA CTC TCA CTG CCC AAG AA-3′, revised: 5′-GGA GAA TGG GGG ATA GGT GT-3′, while the primers for the amplification of tRNACys and tRNAAla genes were forward: 5′-CTA ACC GGC TTT TTG CCC-3′, revised: 5′-ACC TAG AAG GTT GCC TGG CT-3′. After PCR and electrophoresis, the target products were purified and sequenced. The data were then compared with the rCRS (GenBank accession number: NC_012920.1) to detect the occurrence of m.G11696A, m.C5601T, or m.T5813C mutation [14].
2.6. Bioinformatics Analyses
To see whether m.C5601T and m.T5813C mutations affected the thermodynamic changes in tRNAAla and tRNACys, the online RNA Fold webserver program (http://rna.tbi.univie.ac.at/cgi-bin/RNAfold.cgi (accessed on 10 May 2023) was used to predict the minimum free energy (MFE) of tRNAs with and without these mutations [18]. The wild-type sequence of tRNAAla was AAG GGC TTA GCT TAA TTA AAG TGG CTG ATT TGC GTT CAG TTG ATG CAG AGG GGG TTT TGC AGT CCT TA; the sequence of tRNAAla with m.C5601T mutation was AAG GGC TTA GCT TAA TTA AAG TGG CTG ATT TGC GTT CAG TTG ATG CAG AGG GGT TTT TGC AGT CCT TA; the wild-type sequence for tRNACys was AGC TCC GAG GTG ATT TTC ATA TTG AAT TGC AAA TTC GAA GAA GCA GCT TCA AAC CTG CCG GGG CTT; and the sequence of tRNACys with m.T5813C mutation was AGC TCC GAG GTG ACT TTC ATA TTG AAT TGC AAA TTC GAA GAA GCA GCT TCA AAC CTG CCG GGG CTT.
2.7. Qualification of MtDNA-CN
The relative mtDNA-CN was determined by real-time fluorescent quantitative PCR (RT-qPCR), according to our previous study using the 2−ΔΔCt method [19]. The primer sequence for the amplification of the mt-ND1 was forward: 5′-AAC ATA CCC ATG GCC AAC CT-3′; reverse: 5′-AGC GAA GGG TTG TAG TAG CCC-3′. The primer for the amplification of the β-globin gene was forward: 5′- GAA GAG CCA AGG ACA GGT AC-3′; reverse: 5′-CAA CTT CAT CCA CGT TCA CC-3′. Each experiment was performed in triple.
2.8. Cell Lines and Cultured Conditions
Lymphoblastoid cell lines were immortalized by transformation with Epstein–Barr virus (EBV), as described elsewhere [20,21]. Cell lines were derived from six affected matrilineal relatives (Pedigree 1: II-1, II-4 and III-6; Pedigree 2: II-4, III-6 and IV-1) and four controls (Pedigree 1: III-1 and III-2; Pedigree 2: III-1 and III-2). These cell lines were grown in RPMI 1640 medium (Invitrogen Carlsbad, CA, USA) supplemented with 10% FBS.
2.9. ATP Measurements
The Cell Titer-Glo® Luminescent Cell Viability Assay kit (Promega, Madison, WI, USA) was used for the measurement of cellular ATP levels, according to the manufacturer’s instructions with some modifications [22]. Briefly, the cells were harvested and resuspended in ultrapure water. The working solution was diluted accordingly and added to the 96-well white plate. Then, ATP releasing buffer was added for ATP extraction, and cells were added to the plate. Finally, the autofluorescence signal was detected by a microplate reader. The results were corrected with cell numbers.
2.10. Analysis of MMP
MMP from cybrid cells was examined with JC-10 Assay Kit–Flow Cytometry (Abcam, Cambridge, UK) following the general manufacturer’s recommendations with some modifications [23]. The ratios of fluorescence intensity Ex/Em = 490/590 nm and 490/529 nm (FL590/FL529) were recorded to delineate the MMP level of each sample. The relative ratios of the FL590/FL529 geometric mean between mutant and control cell lines were calculated to represent the level of MMP.
2.11. Determining the ROS Production
To analyze the ROS level, a total of 2 × 106 cells were first incubated with the fluorescent probe 2,7-dichlorodihydrofluorescein for 30 min, after which the cells were analyzed using a fluorescence plate reader, as described previously [24].
2.12. Analysis of NAD+/NADH Ratio
The NAD+/NADH ratio was measured using the WST-8 NAD+/NADH Assay Kit (Beyotime, Shanghai, China), as recommended by the manufacturer’s instructions. Absorbance was measured at 450 nm on a microplate reader (ELX800, BioTek, Winooski, VT, USA) [25,26]. The NAD+/NADH ratio was calculated as follows: [NADtotal − NADH]/NADH.
2.13. Determining the Complex I Activity
For analyzing Complex I specific activity, the mitochondrion was first isolated from cybrid cell lines on ice using different centrifugations, as suggested previously [27]. Complex I activity was determined with 10 μg/mL antimycin A and 2 mm KCN by following a decrease in the absorbance due to the NADH oxidation at 340 nm in assay buffer, as detailed elsewhere [28].
2.14. Statistical Analysis
Student’s t-test was used to assess the statistical significance between unpaired samples. All analyses were performed using SPSS software version 20.0. We regarded the p < 0.05 as statistically significant.
3. Results
3.1. Clinical Features of Two Chinese Families with T2DM
We enrolled two families with maternally inherited T2DM via Hangzhou First People’s Hospital (Figure 1). In Pedigree 1, the proband (III-6) was a 40-year-old woman who came to the Hangzhou First People’s Hospital for the regular treatment of T2DM; she was administrated with metformin and insulin therapy (0.4 μ/kg). Her comprehensive personal and medical history revealed that she developed T2DM when she was 38. Her family history suggested that her matrilineal relatives (II-1 and II-4) were also diabetic carriers. Moreover, the proband’s grandmother (I-2) died from diabetes several years ago.
In Pedigree 2, the proband (II-4) was a 70-year-old woman who also lived in Hangzhou city of Zhejiang Province. She started to suffer from T2DM when she was 65. The HbA1c, FGP, as well as OGTT results strongly indicated that she was a diabetic carrier. Furthermore, among 11 matrilineal relatives, 3 of them developed DM at different ages (Table 1). Moreover, these members showed no other clinical abnormalities, including cancer, hearing loss, or neurological or infection disorders.

Table 1.
Clinical and biochemical features of several members of two Chinese families with T2DM.
3.2. Screening for Mitochondrial Mutations
To explore the molecular basis of T2DM, we screened the mtDNA mutations of affected matrilineal relatives (Pedigree 1: II-1, II-4 and III-6; Pedigree 2: II-4, III-6 and IV-1) using PCR and direct sequencing analysis. As shown in Table 2, a comparison of the resultant sequences with rCRS led us to identify 60 variants in the mitochondrial genome which belonged to Eastern Asian mitochondrial haplogroup D4 and D4a, respectively [15]. There were 11 variants in D-loop, 3 variants in 12S rRNA, 3 variants in 16S rRNA, and 2 mutations in tRNAs (m.C5601T and m.T5813C), while the rest of the variants were located as OXPHOS-related genes. In addition, 13 missense mutations were identified, including ND1 C3497T (Ala to Val), ND2 A4833G (Thr to Ala) and C5178A (Leu to Met), A8 C8414T (Leu to Phe), A6 A8701G (Thr to Ala) and A8860G (Thr to Ala), ND3 A10398G (Thr to Ala), ND4 G11696A (Val to Ile) and A12026G (Ile to Val), ND5 G13928C (Ser to Thr), Cyt b C14766T (Thr to Ile), A15326G (Thr to Ala), and A15851G (Ile to Val). These variants in RNAs and polypeptides were further evaluated using phylogenetic analysis and sequences from other 16 vertebrates, including mouse [29], bovine [30], and Xenopus laevis [31]. We noticed that except for the ND4 G11696A, tRNAAla C5601T, and tRNACys T5813C mutations (Figure 2 and Figure 3), others were not well conserved, suggesting that they may be involved in the pathogenesis of T2DM (Table 3).

Table 2.
mtDNA sequence variants in two families with T2DM.
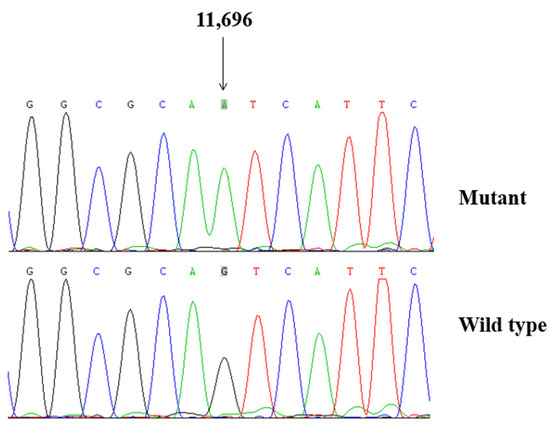
Figure 2.
Identification of ND4 G11696A mutation by Sanger sequencing.
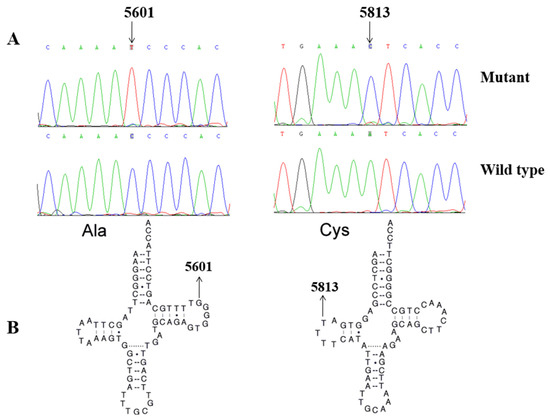
Figure 3.
(A). Identification of tRNAAla C5601T and tRNACys T5813C mutations by Sanger sequencing. (B) The locations of m.C5601T in tRNAAla and m.T5813C in tRNACys.

Table 3.
Molecular characterization of m.C5601T and m.T5813C mutations identified in this study.
3.3. Population Screening
To see the potential pathogenicity of ND4 G11696A, tRNAAla C5601T, and tRNACys T5813C mutations, we screened the frequencies of these mutations in all members of 2 pedigrees, as well as 200 healthy controls using PCR-Sanger sequencing. We noticed that the m.G11696A, m.C5601T, and m.T5813C mutations were only presented in the matrilineal relatives of two pedigrees, but they were absent in the control subjects.
3.4. M.C5601T and m.T5813C Mutations Altered tRNAs Secondary Structures
To see whether m.C5601T and m.T5813C mutations influenced the tRNA structure, the online RNA Fold webserver (http://rna.tbi.univie.ac.at/cgi-bin/RNAfold.cgi (accessed on 10 May 2023) was used to assess the thermodynamic changes in tRNAs with and without these mutations. As shown in Figure 4 and Figure 5, we found that these mutations affected the secondary structures of corresponding tRNAs, strongly indicating that they may be pathogenic.
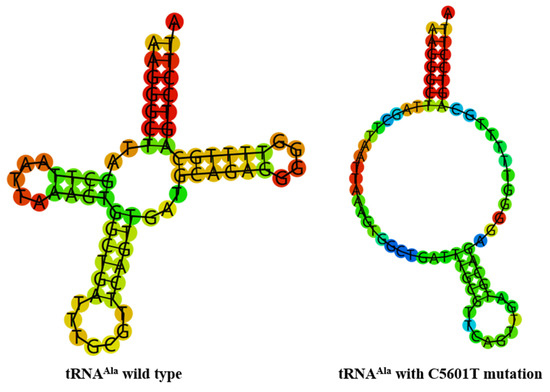
Figure 4.
Predict the secondary structure of tRNAAla with and without m.C5601T mutation.
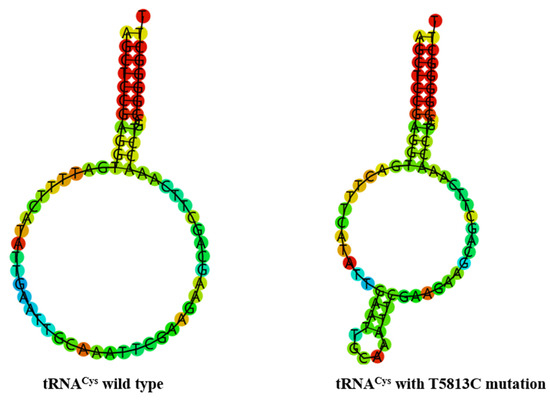
Figure 5.
Predict the secondary structure of tRNACys with and without m.T5813C mutation.
3.5. MtDNA-CN Decreased
MtDNA-CN was considered as a proxy for mitochondrial function [35]. To see whether m.G11696A mutation affected mtDNA function, RT-qPCR was performed to determine the mtDNA-CN. As shown in Figure 6A, a ~37% average reduction was identified in individuals who carried the m.G11696A mutation when compared with the controls (p < 0.0001).
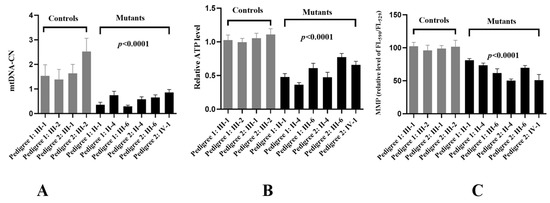
Figure 6.
Analysis of mitochondrial functions in cybrids. (A) Relative levels of mtDNA-CN. (B) ATP determination. (C) MMP analysis.
3.6. Reduced in ATP Production
To see whether m.G11696A mutation affected mitochondrial functions, we generated cybrid cell lines derived from six patients and four controls. As shown in Figure 6B, an approximately 33.6% average drop in ATP was observed in mutant cell lines with the m.G11696A mutation when compared with the controls (p < 0.0001).
3.7. MMP Analysis
MMP generated by proton pumps (Complexes I, III, and IV) was an essential component in the process of energy storage during oxidative phosphorylation (OXPHOS) [36]. The MMP levels in cell lines were measured using a fluorescence probe JC-10 assay via flow cytometry. As shown in Figure 6C, subjects with m.G11696A mutation exhibited about a 23.5% decrease in MMP levels compared with the controls (p < 0.0001).
3.8. The Enhanced in ROS Levels
Mitochondrial ROS have been gradually recognized as important signaling mediators in a wide range of cellular processes, such as metabolic adaptation, adaptive responses to hypoxia, cellular differentiation, and autophagy [37]. The ROS levels of cybrid cells were measured using a fluorescence plate reader, as shown in Figure 7A. The levels of ROS generation in the mutant cell lines harboring the G11696A mutation were much higher than the controls without this mutation (p < 0.0001).
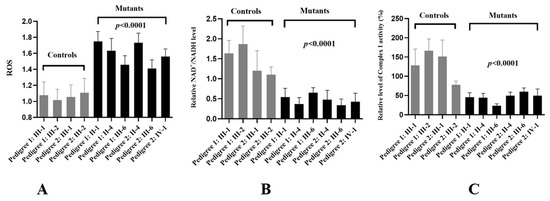
Figure 7.
Mitochondrial dysfunction in mutant cells. (A) Analysis of ROS levels in cybrids; (B) determining the NAD+/NADH ratio between two groups; (C) complex I activity analysis.
3.9. NAD+/NADH Ratio Decreased
The NAD+/NADH ratio was a key regulator of the cells’ metabolism and believed to influence oxidative stress (OS) [38]. We found that, compared with the controls, patients with m.G11696A mutation exhibited much lower levels of the NAD+/NADH ratio (Figure 7B).
3.10. Reduced Activity of Complex I
To see the effects of m.G11696A mutation on OXPHOS function, we analyzed the activity of respiratory Complex I in ten cybrid cells. As shown in Figure 7C, we noticed that Complex I was markedly decreased in cells with m.G11696A mutation, as compared with the controls (p < 0.0001).
4. Discussion
In this study, we performed clinical, genetic, molecular, and biochemical characterizations of two genetic unrelated Chinese pedigrees with maternally inherited T2DM. Notably, T2DM was only presented in the matrilineal lineage of these pedigrees, strongly indicating that mutations in mtDNA were the important contributors to this disease. In fact, the most important role of mitochondria was generating ATP for the maintenance of cellular process [39]. Mitochondria also played an essential role in ROS-mediated signaling transduction, apoptosis, and hormone biogenesis [40]. Herein, we determined that ND4 G11696A mutation was associated with T2DM in two pedigrees. Indeed, the A-to-g transition at 11,696 resulted in the substitution of an isoleucine for valine at amino acid position 312 [41]. Notably, the valine at position 312 in ND4 protein was located in a predicted transmembrane region, 28 AAs aminoterminal to the R340H Leber’s hereditary optic neuropathy (LHON) mutation [42]. A previous study revealed a severe Complex I deficiency in muscle biopsy carrying this mutation, highlighting the importance of m.G11696A to mitochondrial dysfunction [43]. In addition, the m.G11696A mutation was regarded to be a risk factor for mitochondrial disorders [41,44,45,46,47,48] (Table 4).

Table 4.
Overview of ND4 G11696A mutation.
Interestingly, the m.C5601T mutation in tRNAAla and m.T5813C mutation in tRNACys was also detected in our study (Figure 2 and Figure 3). At the molecular level, the m.C5601T mutation resided at the extremely conserved nucleotide of tRNAAla (position 59); the mutation at that position was involved in the biochemical and molecular interactions between the TψC loop and D-arm [49]. Importantly, the m.C5601T mutation created a novel Watson–Crick base-pairing (55T-59C) [32,33,34]. Furthermore, the novel m.T5813C mutation occurred at position 14 in the D-arm of tRNACys; intriguingly, the m.T7501C mutation, which was located at the same position of tRNASer(UCN), was found to be associated with cardiovascular diseases [50,51]. Thus, we speculated that the m.T5813C mutation, which was similar to the m.T7501C mutation, may also have a pathological consequence for T2DM. Moreover, bioinformatics analysis revealed that the m.C5601T and m.T5813C mutations affected the secondary structures of tRNAAla and tRNACys, respectively (Figure 4 and Figure 5). Therefore, m.C5601T and m.T5813C mutations may cause failure in tRNAs metabolism and lead to mitochondrial dysfunction which was responsible for T2DM.
MtDNA-CN was a mitochondrial function marker that reflected its depletion, energy reserves, and OS [52]. Recent studies demonstrated that lower mtDNA content was found to be associated with insulin resistance, T2DM, and impair pancreatic β-cell functions [53,54,55], which was consistent with our results.
Trans-mitochondrial technology was frequently used to evaluate the contribution of mtDNA mutations to the OXPHOS function since noise from the nuclear genetic background was adjusted [56]. In this study, we used this technology to test mitochondrial functions in cybrids carrying m.G11696A mutation. We found that, compared with control cell lines, mutant cybrids had much lower levels of ATP, MMP, NAD+/NADH ratio, and Complex I activity. In fact, the reduction in ATP production in m.G11696A cells was likely a consequence of the decrease in the proton electrochemical potential gradient of mutant mitochondria [57]. MMP was a major component of proton motive force, which played an essential role in mitochondrial bioenergetics, metabolic, and signaling functions [58,59]. Loss of MMP was a critical event in deciding cell fate [60].
NAD+ and NADH were the most important redox pairs in the cell. In particular, NAD+, a key co-enzyme in cellular energy metabolism, could be adaptive response to OS and affect mitochondrial function [61]. The NAD+/NADH ratio set the intracellular redox environment, controlled antioxidant capacity, and regulated cell signaling [62]. Mutant cells showed a marked decreased in their ratio indicated that m.G11696A exhibited more OS and mitochondrial dysfunction than the controls.
The cell lines with m.G11696A showed significant reductions in Complex I activity, as in the case of T2DM-associated ND6 T14502C mutation [63]. Complex I was a large protein complex responsible for acquiring electrons by oxidizing NADH to NAD+ and transferring these electrons to CoQ [64]. It was also a primary site where electrons leaked into the mitochondrial matrix and bound with molecular oxygen to form superoxide [65]. The deficient activity of Complex I caused by m.G11696A mutation would increase the production of ROS [66]. Excess ROS can increase mitochondrial dysfunction, protein damage, lipid peroxidation, and impair antioxidant functions [67]. Thus promoting pancreatic β-cell death or apoptosis and contributing to T2DM progression [68].
In conclusion, our study suggested that m.G11696A mutation was associated with T2DM. Moreover, tRNAAla C5601T and tRNACys T5813C mutations may alter the structure and tRNA functions, subsequently leading to failure in tRNAs metabolism, and they play as synergistic role in the phenotypic expression of m.G11696A mutation. Thus, our study broadened the clinical presentations of m.G11696A mutation. The main limitation of the current study was the relatively small sample size. Further studies including more T2DM samples are needed to verify this conclusion.
Author Contributions
Y.D.: conceptualization; funding acquisition; investigation; writing—review and editing, S.Z.: formal analysis; software, Q.G.: investigation; software, J.L.: investigation; writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the Health Commission of Zhejiang Province (2021RC022), Hangzhou Bureau of Science and Technology (20201203B210), Hangzhou Municipal Health Commission (ZD20220010), and Quzhou Bureau of Science and Technology (2022K51).
Institutional Review Board Statement
The ethics committee of Hangzhou First People’s Hospital approved this study (No. 2021-171-01).
Informed Consent Statement
Written informed consent was obtained from all subjects enrolled in this study.
Data Availability Statement
The datasets for this study will be available from the corresponding authors upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Xu, Y.; Wang, L.; He, J.; Bi, Y.; Li, M.; Wang, T.; Wang, L.; Jiang, Y.; Dai, M.; Lu, J.; et al. 2010 China Noncommunicable Disease Surveillance Group. Prevalence and control of diabetes in Chinese adults. JAMA 2013, 310, 948–959. [Google Scholar] [CrossRef]
- Akash, M.S.H.; Rehman, K.; Chen, S. Role of inflammatory mechanisms in pathogenesis of type 2 diabetes mellitus. J. Cell. Biochem. 2013, 114, 525–531. [Google Scholar] [CrossRef]
- Wong, Y.H.; Wong, S.H.; Wong, X.T.; Yi Yap, Q.; Yip, K.Y.; Wong, L.Z.; Wong, L.Z.; Chellappan, D.K.; Bhattamisra, S.K.; Candasamy, M. Genetic associated complications of type 2 Diabetes Mellitus: A review. Panminerva Med. 2022, 64, 274–288. [Google Scholar] [CrossRef]
- Avital, G.; Buchshtav, M.; Zhidkov, I.; Feder, J.T.; Dadon, S.; Rubin, E.; Glass, D.; Spector, T.D.; Mishmar, D. Mitochondrial DNA heteroplasmy in diabetes and normal adults: Role of acquired and inherited mutational patterns in twins. Hum. Mol. Genet. 2012, 21, 4214–4224. [Google Scholar] [CrossRef]
- Alharbi, K.K.; Alsaikhan, A.S.; Alshammary, A.F.; Al-Hakeem, M.M.; Khan, I.A. Screening of mitochondrial mutations in Saudi women diagnosed with gestational diabetes mellitus: A non-replicative case-control study. Saudi J. Biol. Sci. 2022, 29, 360–365. [Google Scholar] [CrossRef]
- Yarham, J.W.; Elson, J.L.; Blakely, E.L.; McFarland, R.; Taylor, R.W. Mitochondrial tRNA mutations and disease. Wiley Interdiscip. Rev. RNA 2010, 1, 304–324. [Google Scholar] [CrossRef]
- Ballinger, S.W.; Shoffner, J.M.; Hedaya, E.V.; Trounce, I.; Polak, M.A.; Koontz, D.A.; Wallace, D.C. Maternally transmitted diabetes and deafness associated with a 10.4 kb mitochondrial DNA deletion. Nat. Genet. 1992, 1, 11–15. [Google Scholar] [CrossRef]
- van den Ouweland, J.M.; Lemkes, H.H.; Ruitenbeek, W.; Sandkuijl, L.A.; de Vijlder, M.F.; Struyvenberg, P.A.; Van de Kamp, J.J.P.; Maassen, J.A. Mutation in mitochondrial tRNA(Leu)(UUR) gene in a large pedigree with maternally transmitted type II diabetes mellitus and deafness. Nat Genet. 1992, 1, 368–371. [Google Scholar] [CrossRef]
- Wilson, F.H.; Hariri, A.; Farhi, A.; Zhao, H.; Petersen, K.F.; Toka, H.R.; Nelson-Williams, C.; Raja, K.M.; Kashgarian, M.; Shulman, G.I.; et al. A cluster of metabolic defects caused by mutation in a mitochondrial tRNA. Science 2004, 306, 1190–1194. [Google Scholar] [CrossRef]
- Wang, M.; Liu, H.; Zheng, J.; Chen, B.; Zhou, M.; Fan, W.; Wang, H.; Liang, X.; Zhou, X.; Eriani, G.; et al. A Deafness- and Diabetes-associated tRNA Mutation Causes Deficient Pseudouridinylation at Position 55 in tRNAGlu and Mitochondrial Dysfunction. J. Biol. Chem. 2016, 291, 21029–21041. [Google Scholar] [CrossRef]
- Damore, M.; Speiser, P.; Slonim, A.; New, M.; Shanske, S.; Xia, W.; Santorelli, F.; DiMauro, S. Early Onset of Diabetes Mellitus Associated with the Mitochondrial DNA T14709C Point Mutation: Patient Report and Literature Review. J. Pediatr. Endocrinol. Metab. 1999, 12, 207–214. [Google Scholar] [CrossRef]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2010, 33, S62–S69. [Google Scholar] [CrossRef]
- You, X.; Huang, X.; Bi, L.; Li, R.; Zheng, L.; Xin, C. Clinical and molecular features of two diabetes families carrying mitochondrial ND1 T3394C mutation. Ir. J. Med. Sci. (1971-) 2022, 191, 749–758. [Google Scholar] [CrossRef]
- Andrews, R.M.; Kubacka, I.; Chinnery, P.F.; Lightowlers, R.N.; Turnbull, D.M.; Howell, N. Reanalysis and revision of the Cambridge reference sequence for human mitochondrial DNA. Nat. Genet. 1999, 23, 147. [Google Scholar] [CrossRef]
- Kong, Q.-P.; Bandelt, H.-J.; Sun, C.; Yao, Y.-G.; Salas, A.; Achilli, A.; Wang, C.-Y.; Zhong, L.; Zhu, C.-L.; Wu, S.-F.; et al. Updating the East Asian mtDNA phylogeny: A prerequisite for the identification of pathogenic mutations. Hum. Mol. Genet. 2006, 15, 2076–2086. [Google Scholar] [CrossRef]
- Ding, Y.; Teng, Y.-S.; Zhuo, G.-C.; Xia, B.-H.; Leng, J.-H. The Mitochondrial tRNAHis G12192A Mutation May Modulate the Clinical Expression of Deafness-Associated tRNAThr G15927A Mutation in a Chinese Pedigree. Curr. Mol. Med. 2019, 19, 136–146. [Google Scholar] [CrossRef]
- Hall, B.G. Building Phylogenetic Trees from Molecular Data with MEGA. Mol. Biol. Evol. 2013, 30, 1229–1235. [Google Scholar] [CrossRef]
- LLorenz, R.; Bernhart, S.H.; Höner zu Siederdissen, C.; Tafer, H.; Flamm, C.; Stadler, P.F.; Hofacker, I.L. ViennaRNA Package 2.0. Algorithms Mol. Biol. 2011, 6, 26. [Google Scholar] [CrossRef]
- Ding, Y.; Zhuo, G.; Zhang, C. The mitochondrial tRNALeu(UUR) A3302G mutation may be associated with insulin resistance in woman with polycystic ovary syndrome. Reprod Sci. 2016, 23, 228–233. [Google Scholar] [CrossRef]
- Miller, G.; Lipman, M. Release of Infectious Epstein-Barr Virus by Transformed Marmoset Leukocytes. Proc. Natl. Acad. Sci. USA 1973, 70, 190–194. [Google Scholar] [CrossRef]
- Soler-Agesta, R.; Marco-Brualla, J.; Fernández-Silva, P.; Mozas, P.; Anel, A.; Loshuertos, R.M. Transmitochondrial Cybrid Generation Using Cancer Cell Lines. J. Vis. Exp. 2023, 193, e65186. [Google Scholar] [CrossRef]
- Jiang, P.; Wang, M.; Xue, L.; Xiao, Y.; Yu, J.; Wang, H.; Yao, J.; Liu, H.; Peng, Y.; Liu, H.; et al. A Hypertension-Associated tRNAAla Mutation Alters tRNA Metabolism and Mitochondrial Function. Mol. Cell. Biol. 2016, 36, 1920–1930. [Google Scholar] [CrossRef] [PubMed]
- Acton, B.; Jurisicova, A.; Jurisica, I.; Casper, R. Alterations in mitochondrial membrane potential during preimplantation stages of mouse and human embryo development. Mol. Hum. Reprod. 2004, 10, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Xia, B.-H.; Zhang, C.-J.; Zhuo, G.-C. Mitochondrial tRNALeu(UUR) C3275T, tRNAGln T4363C and tRNALys A8343G mutations may be associated with PCOS and metabolic syndrome. Gene 2018, 642, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-G.; Zeng, Q.-Z.; Chen, M.-Y.; Xu, L.-H.; Zhang, C.-C.; Mai, F.-Y.; Zeng, C.-Y.; He, X.-H.; Ouyang, D.-Y. Evodiamine Augments NLRP3 Inflammasome Activation and Anti-bacterial Responses Through Inducing α-Tubulin Acetylation. Front. Pharmacol. 2019, 10, 290. [Google Scholar] [CrossRef]
- Xue, C.; Chen, W.; Yuan, A.; Chen, C.; Li, S.; Chen, K.; Zhao, Y.; Xiao, T.; Shao, G.; Zou, Y.; et al. Dezocine, An Opioid Analgesic, Exerts Antitumor Effects in Triple-Negative Breast Cancer by Targeting Nicotinamide Phosphoribosyltransferase. Front. Pharmacol. 2021, 12, 600296. [Google Scholar] [CrossRef] [PubMed]
- Mendes, D.; Silva, A.M.; Oliveira, M.M.; Andrade, P.B.; Videira, R.A. An Experimental Approach to Address the Functional Relationship between Antioxidant Enzymes and Mitochondrial Respiratory Complexes. Methods Protoc. 2023, 6, 32. [Google Scholar] [CrossRef]
- Li, Y.; D’Aurelio, M.; Deng, J.-H.; Park, J.-S.; Manfredi, G.; Hu, P.; Lu, J.; Bai, Y. An Assembled Complex IV Maintains the Stability and Activity of Complex I in Mammalian Mitochondria. J. Biol. Chem. 2007, 282, 17557–17562. [Google Scholar] [CrossRef]
- Bibb, M.J.; Van Etten, R.A.; Wright, C.T.; Walberg, M.W.; Clayton, D.A. Sequence and gene organization of mouse mitochondrial DNA. Cell 1981, 26, 167–180. [Google Scholar] [CrossRef]
- Gadaleta, G.; Pepe, G.; De Candia, G.; Quagliariello, C.; Sbisa, E.; Saccone, C. The complete nucleotide sequence of theRattus norvegicus mitochondrial genome: Cryptic signals revealed by comparative analysis between vertebrates. J. Mol. Evol. 1989, 28, 497–516. [Google Scholar] [CrossRef]
- Roe, B.A.; Ma, D.P.; Wilson, R.K.; Wong, J.F. The complete nucleotide sequence of the Xenopus laevis mitochondrial genome. J. Biol. Chem. 1985, 260, 9759–9774. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; Li, S.; Liu, C.; Zha, Z.; Wei, X.; Yuan, Y. Mitochondrial tRNAAla C5601T mutation may modulate the clinical expression of tRNAMet A4435G mutation in a Han Chinese family with hypertension. Clin. Exp. Hypertens. 2017, 40, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Li, S.; Ding, Y. Maternally transmitted nonsyndromic hearing impairment may be associated with mitochondrial tRNAAla 5601C>T and tRNALeu(CUN) 12311T>C mutations. J. Clin. Lab. Anal. 2022, 36, e24298. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Ye, Y.; Li, M.; Xia, B.; Leng, J. Mitochondrial tRNAAla 5601C>T variant may affect the clinical expression of the LHON-related ND4 11778G>A mutation in a family. Mol. Med. Rep. 2020, 21, 201–208. [Google Scholar] [CrossRef]
- Castellani, C.A.; Longchamps, R.J.; Sun, J.; Guallar, E.; Arking, D.E. Thinking outside the nucleus: Mitochondrial DNA copy number in health and disease. Mitochondrion 2020, 53, 214–223. [Google Scholar] [CrossRef]
- Reers, M.; Smiley, S.T.; Mottola-Hartshorn, C.; Chen, A.; Lin, M.; Chen, L.B. Mitochondrial membrane potential monitored by JC-1 dye. Methods Enzymol. 1995, 260, 406–417. [Google Scholar] [CrossRef] [PubMed]
- Sena, L.A.; Chandel, N.S. Physiological roles of mitochondrial reactive oxygen species. Mol. Cell 2012, 48, 158–167. [Google Scholar] [CrossRef]
- Teodoro, J.S.; Rolo, A.P.; Palmeira, C.M. The NAD ratio redox paradox: Why does too much reductive power cause oxidative stress? Toxicol. Mech. Methods 2013, 23, 297–302. [Google Scholar] [CrossRef]
- Wallace, D.C. Mitochondrial genetic medicine. Nat. Genet. 2018, 50, 1642–1649. [Google Scholar] [CrossRef]
- Martin, L.A.; Kennedy, B.E.; Karten, B. Mitochondrial cholesterol: Mechanisms of import and effects on mitochondrial function. J. Bioenerg. Biomembr. 2016, 48, 137–151. [Google Scholar] [CrossRef]
- Zhou, X.; Wei, Q.; Yang, L.; Tong, Y.; Zhao, F.; Lu, C.; Qian, Y.; Sun, Y.; Lu, F.; Qu, J.; et al. Leber’s hereditary optic neuropathy is associated with the mitochondrial ND4 G11696A mutation in five Chinese families. Biochem. Biophys. Res. Commun. 2006, 340, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Fearnley, I.M.; Walker, J. Conservation of sequences of subunits of mitochondrial complex I and their relationships with other proteins. Biochim. Biophys. Acta (BBA)-Bioenerg. 1992, 1140, 105–134. [Google Scholar] [CrossRef]
- De Vries, D.D.; Went, L.N.; Bruyn, G.W.; Scholte, H.R.; Hofstra, R.M.; A Bolhuis, P.; A Van Oost, B. Genetic and biochemical impairment of mitochondrial complex I activity in a family with Leber hereditary optic neuropathy and hereditary spastic dystonia. Am. J. Hum. Genet. 1996, 58, 703–711. [Google Scholar]
- Qu, J.; Li, R.; Zhou, X.; Tong, Y.; Yang, L.; Chen, J.; Zhao, F.; Lu, C.; Qian, Y.; Lu, F.; et al. Cosegregation of the ND4 G11696A mutation with the LHON-associated ND4 G11778A mutation in a four generation Chinese family. Mitochondrion 2007, 7, 140–146. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Y.; Yang, L.; Wang, S.; Guan, M.X. Maternally inherited hypertension is associated with the mitochondrial tRNA(Ile) A4295G mutation in a Chinese family. Biochem. Biophys. Res. Commun. 2008, 367, 906–911. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Xu, M.; Huang, Z.; Li, L.; Zheng, H.; Yang, S.; Li, S.; Jin, L.; Ling, X.; Xia, Y.; et al. Mitochondrial DNA sequencing and large-scale genotyping identifies MT-ND4 gene mutation m.11696G>A associated with idiopathic oligoasthenospermia. Oncotarget 2017, 8, 52975–52982. [Google Scholar] [CrossRef]
- Xie, S.; Zhang, J.; Sun, J.; Zhang, M.; Zhao, F.; Wei, Q.P.; Tong, Y.; Liu, X.; Zhou, X.; Jiang, P.; et al. Mitochondrial haplogroup D4j specific variant m.11696G > a(MT-ND4) may increase the penetrance and expressivity of the LHON-associated m.11778G > a mutation in Chinese pedigrees. Mitochondrial DNA A DNA Mapp. Seq. Anal. 2017, 28, 434–441. [Google Scholar] [CrossRef]
- Liao, Z.; Zhao, J.; Zhu, Y.; Yang, L.; Yang, A.; Sun, D.; Zhao, Z.; Wang, X.; Tao, Z.; Tang, X.; et al. The ND4 G11696A mutation may influence the phenotypic manifestation of the deafness-associated 12S rRNA A1555G mutation in a four-generation Chinese family. Biochem. Biophys. Res. Commun. 2007, 362, 670–676. [Google Scholar] [CrossRef]
- Ueda, T.; Yotsumoto, Y.; Ikeda, K.; Watanabe, K. The T-loop region of animal mitochondrial tRNA(Ser)(AGY) is a main recognition site for homologous seryl-tRNA synthetase. Nucleic Acids Res. 1992, 20, 2217–2222. [Google Scholar] [CrossRef]
- Tansel, T.; Paçal, F.; Ustek, D. A novel ATP8 gene mutation in an infant with tetralogy of Fallot. Cardiol. Young 2014, 24, 531–533. [Google Scholar] [CrossRef]
- Zaragoza, M.V.; Fass, J.; Diegoli, M.; Lin, D.; Arbustini, E. Mitochondrial DNA Variant Discovery and Evaluation in Human Cardiomyopathies through Next-Generation Sequencing. PLoS ONE 2010, 5, e12295. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M.; Toshihide, Y.; Fujino, T.; Arai, S.; Saku, K.; Kakino, T.; Tyynismaa, H.; Yamasaki, T.; Yamada, K.-I.; Kang, D.; et al. Overexpression of TFAM or Twinkle Increases mtDNA Copy Number and Facilitates Cardioprotection Associated with Limited Mitochondrial Oxidative Stress. PLoS ONE 2015, 10, e0119687. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Song, J.; Shin, C.; Park, D.; Park, K.; Lee, K.; Koh, C.-S. Decreased mitochondrial DNA content in peripheral blood precedes the development of non-insulin-dependent diabetes mellitus. Diabetes Res. Clin. Pr. 1998, 42, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Fazzini, F.; Lamina, C.; Raftopoulou, A.; Koller, A.; Fuchsberger, C.; Pattaro, C.; Del Greco, F.M.; Döttelmayer, P.; Fendt, L.; GCKD Investigators; et al. Association of mitochondrial DNA copy number with metabolic syndrome and type 2 diabetes in 14 176 individuals. J. Intern. Med. 2021, 290, 190–202. [Google Scholar] [CrossRef]
- Nile, D.L.; Brown, A.E.; Kumaheri, M.A.; Blair, H.R.; Heggie, A.; Miwa, S.; Cree, L.M.; Payne, B.; Chinnery, P.F.; Brown, L.; et al. Age-Related Mitochondrial DNA Depletion and the Impact on Pancreatic Beta Cell Function. PLoS ONE 2014, 9, e115433. [Google Scholar] [CrossRef]
- Wilkins, H.M.; Carl, S.M.; Swerdlow, R.H. Cytoplasmic hybrid (cybrid) cell lines as a practical model for mitochondriopathies. Redox Biol. 2014, 2, 619–631. [Google Scholar] [CrossRef]
- James, A.M.; Sheard, P.W.; Wei, Y.-H.; Murphy, M.P. Decreased ATP synthesis is phenotypically expressed during increased energy demand in fibroblasts containing mitochondrial tRNA mutations. JBIC J. Biol. Inorg. Chem. 1999, 259, 462–469. [Google Scholar] [CrossRef]
- Duchen, M.R.; Surin, A.; Jacobson, J. Imaging mitochondrial function in intact cells. Methods Enzymol. 2003, 361, 353–389. [Google Scholar] [CrossRef]
- Gan, Z.; Audi, S.H.; Bongard, R.D.; Gauthier, K.M.; Merker, M.P. Quantifying mitochondrial and plasma membrane potentials in intact pulmonary arterial endothelial cells based on extracellular disposition of rhodamine dyes. Am. J. Physiol. Cell. Mol. Physiol. 2011, 300, L762–L772. [Google Scholar] [CrossRef]
- Chang, X.; Niu, S.; Shang, M.; Li, J.; Guo, M.; Zhang, W.; Sun, Z.; Li, Y.; Zhang, R.; Shen, X.; et al. ROS-Drp1-mediated mitochondria fission contributes to hippocampal HT22 cell apoptosis induced by silver nanoparticles. Redox Biol. 2023, 63, 102739. [Google Scholar] [CrossRef]
- Lee, C.F.; Chavez, J.D.; Garcia-Menendez, L.; Choi, Y.; Roe, N.D.; Chiao, Y.A.; Edgar, J.S.; Goo, Y.A.; Goodlett, D.R.; Bruce, J.E.; et al. Normalization of NAD + Redox Balance as a Therapy for Heart Failure. Circulation 2016, 134, 883–894. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Lee, C.F.; Wang, W.; Karamanlidis, G.; Kuroda, J.; Matsushima, S.; Sadoshima, J.; Tian, R. Elimination of NADPH Oxidase Activity Promotes Reductive Stress and Sensitizes the Heart to Ischemic Injury. J. Am. Heart Assoc. 2014, 3, e000555. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Zhang, S.; Guo, Q.; Zheng, H. Mitochondrial diabetes is associated with tRNALeu(UUR) A3243G and ND6 T14502C mutations. Diabetes Metab. Syndr. Obes. 2022, 15, 1687–1701. [Google Scholar] [CrossRef]
- Hirst, J. Mitochondrial complex I. Annu. Rev. Biochem. 2013, 82, 551–575. [Google Scholar] [CrossRef] [PubMed]
- Fearnley, I.M.; Carroll, J.; Shannon, R.J.; Runswick, M.J.; Walker, J.E.; Hirst, J. GRIM-19, a Cell Death Regulatory Gene Product, Is a Subunit of Bovine Mitochondrial NADH:Ubiquinone Oxidoreductase (Complex I). J. Biol. Chem. 2001, 276, 38345–38348. [Google Scholar] [CrossRef] [PubMed]
- Szczepanowska, J.; Malinska, D.; Wieckowski, M.R.; Duszynski, J. Effect of mtDNA point mutations on cellular bioenergetics. Biochim. Biophys. Acta. 2012, 1817, 1740–1746. [Google Scholar] [CrossRef] [PubMed]
- Pant, T.; Uche, N.; Juric, M.; Bosnjak, Z.J. Clinical Relevance of lncRNA and Mitochondrial Targeted Antioxidants as Therapeutic Options in Regulating Oxidative Stress and Mitochondrial Function in Vascular Complications of Diabetes. Antioxidants 2023, 12, 898. [Google Scholar] [CrossRef]
- Du, S.-C.; Ge, Q.-M.; Lin, N.; Dong, Y.; Su, Q. ROS-mediated lipopolysaccharide-induced apoptosis in INS-1 cells by modulation of Bcl-2 and Bax. Cell. Mol. Biol. 2012, 58, 1654–1659. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).