The Effects of a High-Fat Diet on Inflammatory Bowel Disease
Abstract
1. Introduction
2. High-Fat Diet and the Intestinal Barrier in IBD
2.1. TJs
2.2. Mucin 2
2.3. AMPs
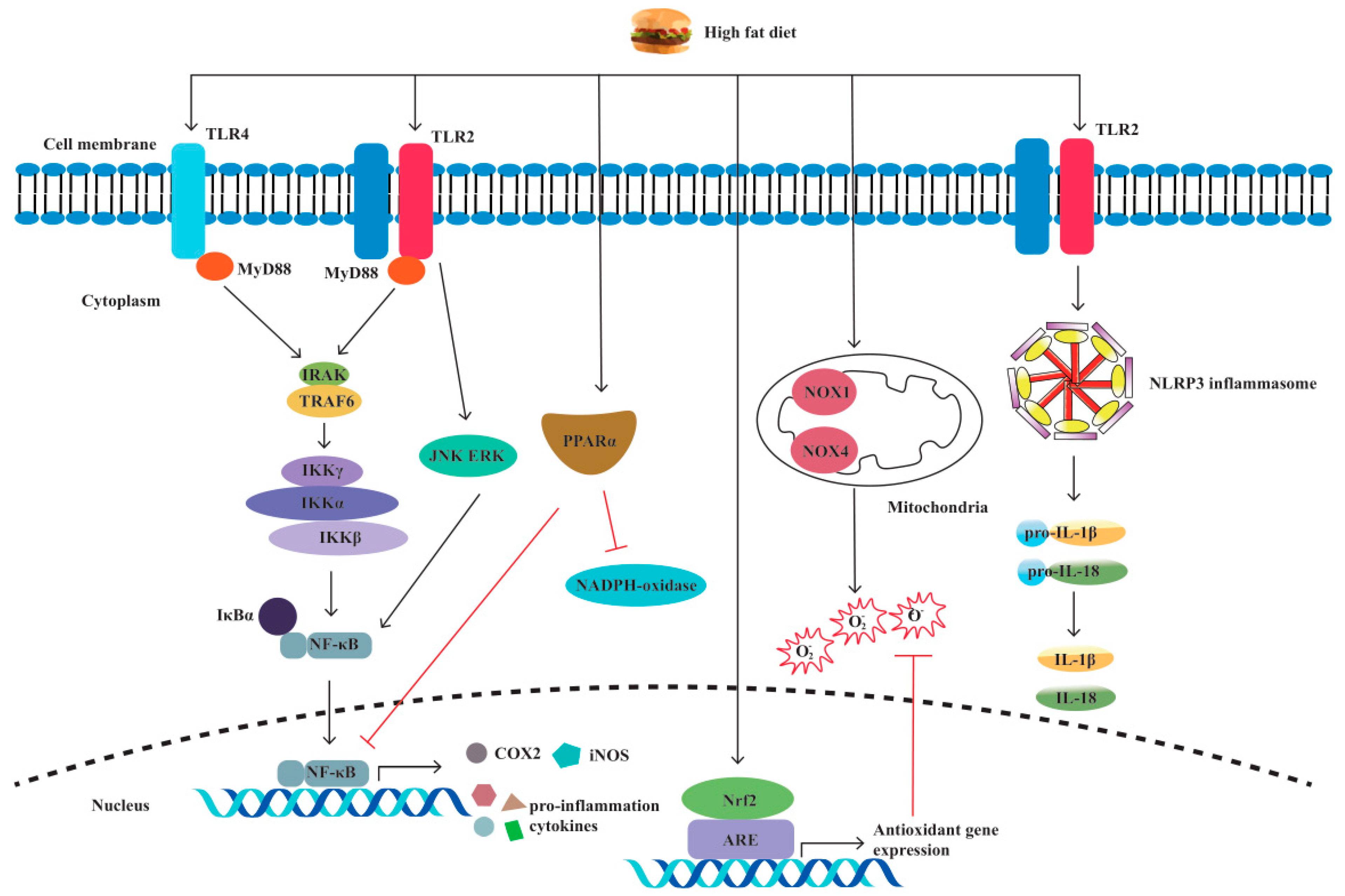
3. High-Fat Diet and Pattern Recognition Receptors in IBD
3.1. TLRs
3.2. NLRs
4. High-Fat Diet and Immune Cells in IBD
5. Polyunsaturated Fatty Acids (PUFAs) in IBD
6. Short Chain Fatty Acids (SCFAs) in IBD
7. High-Fat Diet and Intestinal Dysbacteriosis in IBD
7.1. AIEC
7.2. A. muciniphila
7.3. Clostridioides difficile
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fiocchi, C. Inflammatory bowel disease: Etiology and pathogenesis. Gastroenterology 1998, 115, 182–205. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Li, Y.; Zhou, G.; Wang, Y.; Li, L.; Han, J.; Chen, M.; He, Y.; Zhang, S. Multi-Omics Analysis of Western-style Diet Increased Susceptibility to Experimental Colitis in Mice. J. Inflamm. Res. 2022, 15, 2523–2537. [Google Scholar] [CrossRef]
- Levine, A.; Boneh, R.S.; Wine, E. Evolving role of diet in the pathogenesis and treatment of inflammatory bowel diseases. Gut 2018, 67, 1726–1738. [Google Scholar] [CrossRef] [PubMed]
- Rizzello, F.; Spisni, E.; Giovanardi, E.; Imbesi, V.; Salice, M.; Alvisi, P.; Valerii, M.C.; Gionchetti, P. Implications of the Westernized Diet in the Onset and Progression of IBD. Nutrients 2019, 11, 1033. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Gao, H.; Qi, Q.; Liu, X.; Li, J.; Gao, J.; Li, P.; Wang, Y.; Du, L.; Wang, C. High Fat Diet, Gut Microbiome and Gastro-intestinal Cancer. Theranostics 2021, 11, 5889–5910. [Google Scholar] [CrossRef]
- Molodecky, N.A.; Soon, S.; Rabi, D.M.; Ghali, W.A.; Ferris, M.; Chernoff, G.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Barkema, H.W.; et al. Increasing Incidence and Prevalence of the Inflammatory Bowel Diseases with Time, Based on Systematic Review. Gastroenterology 2012, 142, 46–54.e42, quiz e30. [Google Scholar] [CrossRef]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Bilotta, A.J.; Cong, Y. Gut Microbiota Metabolite Regulation of Host Defenses at Mucosal Surfaces: Implication in Precision Medicine. Precis. Clin. Med. 2019, 2, 110–119. [Google Scholar] [CrossRef]
- El-Salhy, M.; Solomon, T.; Hausken, T.; Gilja, O.H.; Hatlebakk, J.G. Gastrointestinal neuroendocrine peptides/amines in inflammatory bowel disease. World J. Gastroenterol. 2017, 23, 5068–5085. [Google Scholar] [CrossRef]
- Pradhan, G.; Samson, S.L.; Sun, Y. Ghrelin: Much More Than a Hunger Hormone. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 619–624. [Google Scholar] [CrossRef]
- Waseem, T.; Duxbury, M.; Ito, H.; Ashley, S.W.; Robinson, M.K. Exogenous ghrelin modulates release of pro-inflammatory and anti-inflammatory cytokines in LPS-stimulated macrophages through distinct signaling pathways. Surgery 2008, 143, 334–342. [Google Scholar] [CrossRef]
- Gonzalez–Rey, E.; Chorny, A.; Delgado, M. Therapeutic Action of Ghrelin in a Mouse Model of Colitis. Gastroenterology 2006, 130, 1707–1720. [Google Scholar] [CrossRef] [PubMed]
- Maduzia, D.; Matuszyk, A.; Ceranowicz, D.; Warzecha, Z.; Ceranowicz, P.; Fyderek, K.; Galazka, K.; Dembinski, A. The influence of pretreatment with ghrelin on the development of acetic-acid-induced colitis in rats. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2015, 66, 875–885. [Google Scholar]
- Matuszyk, A.; Ceranowicz, P.; Warzecha, Z.; Cieszkowski, J.; Ceranowicz, D.; Gałązka, K.; Bonior, J.; Jaworek, J.; Bartuś, K.; Gil, K.; et al. Exogenous Ghrelin Accelerates the Healing of Acetic Acid-Induced Colitis in Rats. Int. J. Mol. Sci. 2016, 17, 1455. [Google Scholar] [CrossRef] [PubMed]
- Matuszyk, A.; Ceranowicz, D.; Warzecha, Z.; Ceranowicz, P.; Fyderek, K.; Gałązka, K.; Cieszkowski, J.; Bonior, J.; Jaworek, J.; Pihut, M.; et al. The Influence of Ghrelin on the Development of Dextran Sodium Sulfate-Induced Colitis in Rats. Biomed. Res. Int. 2015, 2015, 718314. [Google Scholar] [CrossRef]
- Warzecha, Z.; Ceranowicz, D.; Dembiński, A.; Ceranowicz, P.; Cieszkowski, J.; Kuwahara, A.; Kato, I.; Konturek, P.C. Ghrelin accelerates the healing of cysteamine-induced duodenal ulcers in rats. Experiment 2012, 18, BR181–BR187. [Google Scholar] [CrossRef]
- Warzecha, Z.; Kownacki, P.; Ceranowicz, P.; Dembinski, M.; Cieszkowski, J.; Dembinski, A. Ghrelin accelerates the healing of oral ulcers in non-sialoadenectomized and sialoadenectomized rats. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2013, 64, 657–668. [Google Scholar]
- Stępniowska, A.; Tutaj, K.; Juśkiewicz, J.; Ognik, K. Effect of a high-fat diet and chromium on hormones level and Cr retention in rats. J. Endocrinol. Investig. 2022, 45, 527–535. [Google Scholar] [CrossRef]
- Kellard, J.A.; Rorsman, N.J.; Hill, T.G.; Armour, S.L.; van de Bunt, M.; Rorsman, P.; Knudsen, J.G.; Briant, L.J. Reduced somatostatin signalling leads to hypersecretion of glucagon in mice fed a high-fat diet. Mol. Metab. 2020, 40, 101021. [Google Scholar] [CrossRef]
- Liu, R.; Wei, N.; Guo, W.; Qiang, O.; Li, X.; Ou, Y.; Huang, W.; Tang, C.W. Octreotide alleviates obesity by reducing intestinal glucose absorption and inhibiting low-grade inflammation. Eur. J. Nutr. 2013, 52, 1067–1075. [Google Scholar] [CrossRef]
- Ameri, P.; Ferone, D. Diffuse Endocrine System, Neuroendocrine Tumors and Immunity: What’s New? Neuroendocrinology 2012, 95, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Mu, J.; Zhu, M.; Mukherjee, A.; Zhang, H. Transient Receptor Potential Channels and Inflammatory Bowel Disease. Front. Immunol. 2020, 11, 180. [Google Scholar] [CrossRef] [PubMed]
- Xavier, R.J.; Podolsky, D.K. Unravelling the pathogenesis of inflammatory bowel disease. Nature 2007, 448, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Libertucci, J.; Dutta, U.; Kaur, S.; Jury, J.; Rossi, L.; Fontes, M.E.; Shajib, M.S.; Khan, W.I.; Surette, M.G.; Verdu, E.F.; et al. Inflammation-related differences in mucosa-associated microbiota and intestinal barrier function in colonic Crohn’s disease. Am. J. Physiol. Liver Physiol. 2018, 315, G420–G431. [Google Scholar] [CrossRef]
- Arnott, I.D.; Kingstone, K.; Ghosh, S. Abnormal Intestinal Permeability Predicts Relapse in Inactive Crohn Disease. Scand. J. Gastroenterol. 2000, 35, 1163–1169. [Google Scholar] [PubMed]
- Choy, M.C.; Visvanathan, K.; De Cruz, P. An Overview of the Innate and Adaptive Immune System in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2017, 23, 2–13. [Google Scholar] [CrossRef]
- Munkholm, P.; Langholz, E.; Hollander, D.; Thornberg, K.; Orholm, M.; Katz, K.D.; Binder, V. Intestinal Permeability in Patients with Crohn’s Disease and Ulcerative Colitis and Their First Degree Relatives. Gut 1994, 35, 68–72. [Google Scholar] [CrossRef]
- Sartor, R.B.; Wu, G.D. Roles for Intestinal Bacteria, Viruses, and Fungi in Pathogenesis of Inflammatory Bowel Diseases and Therapeutic Approaches. Gastroenterology 2017, 152, 327–339.e4. [Google Scholar] [CrossRef]
- Matsuoka, K.; Kanai, T. The gut microbiota and inflammatory bowel disease. Semin. Immunopathol. 2015, 37, 47–55. [Google Scholar] [CrossRef]
- Sun, M.; Wu, W.; Liu, Z.; Cong, Y. Microbiota Metabolite Short Chain Fatty Acids, Gpcr, and Inflammatory Bowel Diseases. J. Gastroenterol. 2017, 52, 1–8. [Google Scholar] [CrossRef]
- Mazzarella, G.; Perna, A.; Marano, A.; Lucariello, A.; Rotondi Aufiero, V.; Sorrentino, A.; Melina, R.; Guerra, G.; Taccone, F.S.; Iaquinto, G.; et al. Pathogenic Role of Associated Adherent-Invasive Escherichia coli in Crohn’s Disease. J. Cell Physiol. 2017, 232, 2860–2868. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Vasu, R.; Zhang, H. The Role of Long-Chain Fatty Acids in Inflammatory Bowel Disease. Mediat. Inflamm. 2019, 2019, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Vancamelbeke, M.; Vermeire, S. The intestinal barrier: A fundamental role in health and disease. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 821–834. [Google Scholar] [CrossRef] [PubMed]
- Mankertz, J.; Schulzke, J.D. Altered Permeability in Inflammatory Bowel Disease: Pathophysiology and Clinical Implications. Curr. Opin. Gastroenterol. 2007, 23, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cui, W.; Li, X.; Yang, H. Interaction Between Commensal Bacteria, Immune Response and the Intestinal Barrier in Inflammatory Bowel Disease. Front. Immunol. 2021, 12, 761981. [Google Scholar] [CrossRef]
- Söderholm, J.D.; Peterson, K.H.; Olaison, G.; Franzén, L.E.; Weström, B.; Magnusson, K.E.; Sjödahl, R. Epithelial Perme-ability to Proteins in the Noninflamed Ileum of Crohn’s Disease? Gastroenterology 1999, 117, 65–72. [Google Scholar] [CrossRef]
- Rohr, M.W.; Narasimhulu, C.A.; Rudeski-Rohr, T.A.; Parthasarathy, S. Negative Effects of a High-Fat Diet on Intestinal Permeability: A Review. Adv. Nutr. 2020, 11, 77–91. [Google Scholar] [CrossRef]
- Buchert, M.; Turksen, K.; Hollande, F. Methods to Examine Tight Junction Physiology in Cancer Stem Cells: Teer, Para-cellular Permeability, and Dilution Potential Measurements. Stem Cell Rev. Rep. 2012, 8, 1030–1034. [Google Scholar] [CrossRef]
- Guo, Y.; Huang, C.; Liu, L.; Fu, X.; Lu, Y.; Zheng, J.; Mei, Q.; Huang, Z.; Fan, J.; Lu, L.; et al. Paneth Cell Ablation Aggravates Pancreatic and Intestinal Injuries in a Rat Model of Acute Necrotizing Pancreatitis after Normal and High-Fat Diet. Mediat. Inflamm. 2019, 2019, 8474523. [Google Scholar] [CrossRef]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Waget, A.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R. Changes in Gut Microbiota Control Metabolic Endotoxemia-Induced Inflammation in High-Fat Diet-Induced Obesity and Diabetes in Mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef]
- Oliveira, R.; Canuto, L.; Collares-Buzato, C. Intestinal luminal content from high-fat-fed prediabetic mice changes epithelial barrier function in vitro. Life Sci. 2019, 216, 10–21. [Google Scholar] [CrossRef]
- Aspenström-Fagerlund, B.; Sundström, B.; Tallkvist, J.; Ilbäck, N.G.; Glynn, A.W. Fatty Acids Increase Paracellular Ab-sorption of Aluminium across Caco-2 Cell Monolayers. Chem. Biol. Interact. 2009, 181, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Collares-Buzato, C.B.; McEwan, G.T.; Jepson, M.A.; Simmons, N.L.; Hirst, B.H. Paracellular barrier and junctional protein distribution depend on basolateral extracellular Ca2+ in cultured epithelia. Biochim. et Biophys. Acta (BBA)-Mol. Cell Res. 1994, 1222, 147–158. [Google Scholar] [CrossRef]
- Araki, Y.; Katoh, T.; Ogawa, A.; Bamba, S.; Andoh, A.; Koyama, S.; Fujiyama, Y.; Bamba, T. Bile acid modulates transepithelial permeability via the generation of reactive oxygen species in the Caco-2 cell line. Free. Radic. Biol. Med. 2005, 39, 769–780. [Google Scholar] [CrossRef]
- Ahmad, R.; Rah, B.; Bastola, D.; Dhawan, P.; Singh, A.B. Obesity-Induces Organ and Tissue Specific Tight Junction Re-structuring and Barrier Deregulation by Claudin Switching. Sci. Rep. 2017, 7, 5125. [Google Scholar] [CrossRef]
- Raimondi, F.; Santoro, P.; Barone, M.V.; Pappacoda, S.; Barretta, M.L.; Nanayakkara, M.; Apicella, C.; Capasso, L.; Paludetto, R. Bile acids modulate tight junction structure and barrier function of Caco-2 monolayers via EGFR activation. Am. J. Physiol. Liver Physiol. 2008, 294, G906–G913. [Google Scholar] [CrossRef]
- Joo, E.; Muraoka, A.; Hamasaki, A.; Harada, N.; Yamane, S.; Kondo, Y.; Suzuki, K.; Nasteska, D.; Shibue, K.; Harada, T.; et al. Enteral Supplementation with Glutamine, Fiber, and Oligosaccharide Modulates Incretin and Glucagon-Like Peptide-2 Secretion. J. Diabetes Investig. 2015, 6, 302–308. [Google Scholar] [CrossRef]
- Yu, C.; Jia, G.; Jiang, Y.; Deng, Q.; Chen, Z.; Xu, Z.; Chen, X.; Wang, K. Effect of Glucagon-like Peptide 2 on Tight Junction in Jejunal Epithelium of Weaned Pigs though MAPK Signaling Pathway. Asian-Australas. J. Anim. Sci. 2014, 27, 733–742. [Google Scholar] [CrossRef]
- Richter, J.F.; Pieper, R.; Zakrzewski, S.S.; Günzel, D.; Schulzke, J.D.; Van Kessel, A.G. Diets high in fermentable protein and fibre alter tight junction protein composition with minor effects on barrier function in piglet colon. Br. J. Nutr. 2014, 111, 1040–1049. [Google Scholar] [CrossRef]
- Carlson, T.L.; Lock, J.Y.; Carrier, R.L. Engineering the Mucus Barrier. Annu. Rev. Biomed. Eng. 2018, 20, 197–220. [Google Scholar] [CrossRef]
- Hussain, M.; Ijaz, M.U.; Ahmad, M.I.; Khan, I.A.; Brohi, S.A.; Shah, A.U.; Shinwari, K.I.; Zhao, D.; Xu, X.; Zhou, G.; et al. Meat proteins in a high-fat diet have a substantial impact on intestinal barriers through mucus layer and tight junction protein suppression in C57BL/6J mice. Food Funct. 2019, 10, 6903–6914. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, K.; Ogawa, A.; Mizoguchi, E.; Shimomura, Y.; Andoh, A.; Bhan, A.K.; Blumberg, R.S.; Xavier, R.J.; Mizoguchi, A. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J. Clin. Investig. 2008, 118, 534–544. [Google Scholar] [CrossRef] [PubMed]
- Gulhane, M.; Murray, L.; Lourie, R.; Tong, H.; Sheng, Y.H.; Wang, R.; Kang, A.; Schreiber, V.; Wong, K.Y.; Magor, G.; et al. High Fat Diets Induce Colonic Epithelial Cell Stress and Inflammation that is Reversed by IL-22. Sci. Rep. 2016, 6, 28990. [Google Scholar] [CrossRef] [PubMed]
- Nagalakshmi, M.L.; Rascle, A.; Zurawski, S.; Menon, S.; Malefyt, R.D.W. Interleukin-22 activates STAT3 and induces IL-10 by colon epithelial cells. Int. Immunopharmacol. 2004, 4, 679–691. [Google Scholar] [CrossRef]
- Hruz, P.; Dann, S.; Eckmann, L. STAT3 and its activators in intestinal defense and mucosal homeostasis. Curr. Opin. Gastroenterol. 2010, 26, 109–115. [Google Scholar] [CrossRef]
- Zhao, J.; Zhao, R.; Cheng, L.; Yang, J.; Zhu, L. Peroxisome proliferator-activated receptor gamma activation promotes intestinal barrier function by improving mucus and tight junctions in a mouse colitis model. Dig. Liver Dis. 2018, 50, 1195–1204. [Google Scholar] [CrossRef]
- Tomas, J.; Mulet, C.; Saffarian, A.; Cavin, J.-B.; Ducroc, R.; Regnault, B.; Tan, C.K.; Duszka, K.; Burcelin, R.; Wahli, W.; et al. High-fat diet modifies the PPAR-γ pathway leading to disruption of microbial and physiological ecosystem in murine small intestine. Proc. Natl. Acad. Sci. USA 2016, 113, E5934–E5943. [Google Scholar] [CrossRef]
- Sanos, S.L.; Bui, V.L.; Mortha, A.; Oberle, K.; Heners, C.; Johner, C.; Diefenbach, A. Rorgammat and Commensal Microflora Are Required for the Differentiation of Mucosal Interleukin 22-Producing Nkp46+ Cells. Nat. Immunol. 2009, 10, 83–91. [Google Scholar] [CrossRef]
- Cella, M.; Fuchs, A.; Vermi, W.; Facchetti, F.; Otero, K.; Lennerz, J.K.M.; Doherty, J.M.; Mills, J.C.; Colonna, M. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature 2009, 457, 722–725. [Google Scholar] [CrossRef]
- Brand, S.; Beigel, F.; Olszak, T.; Zitzmann, K.; Eichhorst, S.T.; Otte, J.-M.; Diepolder, H.; Marquardt, A.; Jagla, W.; Popp, A.; et al. IL-22 is increased in active Crohn’s disease and promotes proinflammatory gene expression and intestinal epithelial cell migration. Am. J. Physiol. Liver Physiol. 2006, 290, G827–G838. [Google Scholar] [CrossRef]
- Mizoguchi, A.; Yano, A.; Himuro, H.; Ezaki, Y.; Sadanaga, T.; Mizoguchi, E. Clinical importance of IL-22 cascade in IBD. J. Gastroenterol. 2018, 53, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Larabi, A.; Barnich, N.; Nguyen, H.T. Nguyen. New Insights into the Interplay between Autophagy, Gut Microbiota and In-flammatory Responses in IBD. Autophagy 2020, 16, 38–51. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Li, J.; Tang, R.; Zhang, G.; Zeng, H.; Wood, R.J.; Liu, Z. High Fat Diet Alters Gut Microbiota and the Expression of Paneth Cell-Antimicrobial Peptides Preceding Changes of Circulating Inflammatory Cytokines. Mediat. Inflamm. 2017, 2017, 9474896. [Google Scholar] [CrossRef]
- Volynets, V.; Louis, S.; Pretz, D.; Lang, L.; Ostaff, M.J.; Wehkamp, J.; Bischoff, S.C. Intestinal Barrier Function and the Gut Microbiome Are Differentially Affected in Mice Fed a Western-Style Diet or Drinking Water Supplemented with Fructose. J. Nutr. 2017, 147, 770–780. [Google Scholar] [CrossRef]
- Lu, Y.; Li, X.; Liu, S.; Zhang, Y.; Zhang, D. Toll-like Receptors and Inflammatory Bowel Disease. Front. Immunol. 2018, 9, 72. [Google Scholar] [CrossRef]
- Shao, B.Z.; Xu, Z.Q.; Han, B.Z.; Su, D.F.; Liu, C. Nlrp3 Inflammasome and Its Inhibitors: A Review. Front. Pharmacol. 2015, 6, 262. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.R.; Hong, Y.P.; Mei, F.C.; Wang, C.Y.; Li, M.; Zhou, Y.; Zhao, K.L.; Yu, J.; Wang, W.X. High-Fat Diet Aggravates the Intestinal Barrier Injury via TLR4-RIP3 Pathway in a Rat Model of Severe Acute Pancreatitis. Mediat. Inflamm. 2019, 2019, 2512687. [Google Scholar] [CrossRef]
- Cremonini, E.; Wang, Z.; Bettaieb, A.; Adamo, A.M.; Daveri, E.; Mills, D.A.; Kalanetra, K.M.; Haj, F.G.; Karakas, S.; Oteiza, P.I. (-)-Epicatechin protects the intestinal barrier from high fat diet-induced permeabilization: Implications for steatosis and insulin resistance. Redox Biol. 2018, 14, 588–599. [Google Scholar] [CrossRef]
- Kim, K.-A.; Gu, W.; Lee, I.-A.; Joh, E.-H.; Kim, D.-H. High Fat Diet-Induced Gut Microbiota Exacerbates Inflammation and Obesity in Mice via the TLR4 Signaling Pathway. PLoS ONE 2012, 7, e47713. [Google Scholar] [CrossRef]
- Wang, L.; Gong, Z.; Zhang, X.; Zhu, F.; Liu, Y.; Jin, C.; Du, X.; Xu, C.; Chen, Y.; Cai, W.; et al. Gut microbial bile acid metabolite skews macrophage polarization and contributes to high-fat diet-induced colonic inflammation. Gut Microbes 2020, 12, 1819155. [Google Scholar] [CrossRef]
- Reynolds, C.M.; McGillicuddy, F.C.; Harford, K.A.; Finucane, O.M.; Mills, K.H.G.; Roche, H.M. Dietary saturated fatty acids prime the NLRP3 inflammasome via TLR4 in dendritic cells-implications for diet-induced insulin resistance. Mol. Nutr. Food Res. 2012, 56, 1212–1222. [Google Scholar] [CrossRef] [PubMed]
- Snodgrass, R.G.; Huang, S.; Choi, I.-W.; Rutledge, J.C.; Hwang, D.H. Inflammasome-Mediated Secretion of IL-1β in Human Monocytes through TLR2 Activation; Modulation by Dietary Fatty Acids. J. Immunol. 2013, 191, 4337–4347. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Zhang, H. The Role of Proinflammatory Pathways in the Pathogenesis of Colitis-Associated Colorectal Cancer. Mediat. Inflamm. 2017, 2017, 5126048. [Google Scholar] [CrossRef]
- Li, S.; Sun, Y.; Hu, X.; Qin, W.; Li, C.; Liu, Y.; Liu, A.; Zhao, Y.; Wu, D.; Lin, D.; et al. Effect of arabinoxylan on colonic bacterial metabolites and mucosal barrier in high-fat diet-induced rats. Food Sci. Nutr. 2019, 7, 3052–3061. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Cheng, D.; Huang, C.; Li, Y.; Lao, C.; Xia, Y.; Liu, W.; Gong, X.; Hu, D.; Li, B.; et al. Improvement of Colonic Immune Function with Soy Isoflavones in High-Fat Diet-Induced Obese Rats. Molecules 2019, 24, 1139. [Google Scholar] [CrossRef]
- Kordjazy, N.; Haj-Mirzaian, A.; Haj-Mirzaian, A.; Rohani, M.M.; Gelfand, E.W.; Rezaei, N.; Abdolghaffari, A.H. Role of toll-like receptors in inflammatory bowel disease. Pharmacol. Res. 2018, 129, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Varady, J.; Eder, K.; Ringseis, R. Dietary Oxidized Fat Activates the Oxidative Stress-Responsive Transcription Factors Nf-Κb and Nrf2 in Intestinal Mucosa of Mice. Eur. J. Nutr. 2011, 50, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska, M.; Swierczynski, M.; Fichna, J.; Piechota-Polanczyk, A. The Nrf2 in the pathophysiology of the intestine: Molecular mechanisms and therapeutic implications for inflammatory bowel diseases. Pharmacol. Res. 2021, 163, 105243. [Google Scholar] [CrossRef]
- Khor, T.O.; Huang, M.T.; Kwon, K.H.; Chan, J.Y.; Reddy, B.S.; Kong, A.N. Nrf2-Deficient Mice Have an Increased Sus-ceptibility to Dextran Sulfate Sodium-Induced Colitis. Cancer Res. 2006, 66, 11580–11584. [Google Scholar] [CrossRef]
- Osburn, W.O.; Karim, B.; Dolan, P.M.; Liu, G.; Yamamoto, M.; Huso, D.L.; Kensler, T.W. Increased colonic inflammatory injury and formation of aberrant crypt foci in Nrf2-deficient mice upon dextran sulfate treatment. Int. J. Cancer 2007, 121, 1883–1891. [Google Scholar] [CrossRef]
- Wan, Z.; Durrer, C.; Mah, D.; Simtchouk, S.; Little, J.P. One-week high-fat diet leads to reduced toll-like receptor 2 expression and function in young healthy men. Nutr. Res. 2014, 34, 1045–1051. [Google Scholar] [CrossRef] [PubMed]
- Davis, B.K.; Wen, H.; Ting, J.P. The Inflammasome Nlrs in Immunity, Inflammation, and Associated Diseases. Annu. Rev. Immunol. 2011, 29, 707–735. [Google Scholar] [CrossRef]
- Bauer, C.; Duewell, P.; Mayer, C.; Lehr, H.A.; Fitzgerald, K.A.; Dauer, M.; Tschopp, J.; Endres, S.; Latz, E.; Schnurr, M. Colitis induced in mice with dextran sulfate sodium (DSS) is mediated by the NLRP3 inflammasome. Gut 2010, 59, 1192–1199. [Google Scholar] [CrossRef] [PubMed]
- Zhen, Y.; Zhang, H. NLRP3 Inflammasome and Inflammatory Bowel Disease. Front. Immunol. 2019, 10, 276. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zan, Y.; Sui, K.; Zhu, S. The latest breakthrough on NLRP6 inflammasome. Precis. Clin. Med. 2022, 5, pbac022. [Google Scholar] [CrossRef] [PubMed]
- Kummer, J.A.; Broekhuizen, R.; Everett, H.; Agostini, L.; Kuijk, L.; Martinon, F.; van Bruggen, R.; Tschopp, J. Inflammasome Components NALP 1 and 3 Show Distinct but Separate Expression Profiles in Human Tissues Suggesting a Site-specific Role in the Inflammatory Response. J. Histochem. Cytochem. 2007, 55, 443–452. [Google Scholar] [CrossRef]
- Progatzky, F.; Sangha, N.J.; Yoshida, N.; McBrien, M.; Cheung, J.; Shia, A.; Scott, J.; Marchesi, J.R.; Lamb, J.R.; Bugeon, L.; et al. Dietary cholesterol directly induces acute inflammasome-dependent intestinal inflammation. Nat. Commun. 2014, 5, 5864. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Zhao, G.; Liu, L.; Liu, F.; Gong, W.; Liu, X.; Yang, L.; Wang, J.; Hou, Y. Pre-treatment with IL-1β enhances the efficacy of MSC transplantation in DSS-induced colitis. Cell. Mol. Immunol. 2012, 9, 473–481. [Google Scholar] [CrossRef]
- Martin, M.; Neumann, D.; Hoff, T.; Resch, K.; DeWitt, D.L.; Goppelt-Struebe, M. Interleukin-1-induced cyclooxygenase 2 expression is suppressed by cyclosporin A in rat mesangial cells. Kidney Int. 1994, 45, 150–158. [Google Scholar] [CrossRef]
- Kabashima, K.; Saji, T.; Murata, T.; Nagamachi, M.; Matsuoka, T.; Segi, E.; Tsuboi, K.; Sugimoto, Y.; Kobayashi, T.; Miyachi, Y.; et al. The Prostaglandin Receptor Ep4 Suppresses Colitis, Mucosal Damage and Cd4 Cell Activation in the Gut. J. Clin. Investig. 2002, 109, 883–893. [Google Scholar] [CrossRef]
- Maynard, C.L.; Weaver, C.T. Intestinal Effector T Cells in Health and Disease. Immunity 2009, 31, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Elson, C.O.; Cong, Y.; McCracken, V.J.; Dimmitt, R.A.; Lorenz, R.G.; Weaver, C.T. Experimental Models of Inflammatory Bowel Disease Reveal Innate, Adaptive, and Regulatory Mechanisms of Host Dialogue with the Microbiota. Immunol. Rev. 2005, 206, 260–276. [Google Scholar] [CrossRef] [PubMed]
- Fuss, I.J.; Neurath, M.F.; Boirivant, M.; Fiocchi, C.; Strober, W. Disparate Cd4+ Lamina Propria (Lp) Lymphokine Secretion Profiles in Inflammatory Bowel Disease. Crohn’s Disease Lp Cells Manifest Increased Secretion of Ifn-Gamma, Whereas Ulcerative Colitis Lp Cells Manifest Increased Secretion of Il-5. J. Immunol. 1996, 157, 1261–1270. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Yu, T.; Cong, Y. CD4+ T cell metabolism, gut microbiota, and autoimmune diseases: Implication in precision medicine of autoimmune diseases. Precis. Clin. Med. 2022, 5, pbac018. [Google Scholar] [CrossRef]
- Ma, X.; Torbenson, M.; Hamad, A.R.A.; Soloski, M.J.; Li, Z. High-fat diet modulates non-CD1d-restricted natural killer T cells and regulatory T cells in mouse colon and exacerbates experimental colitis. Clin. Exp. Immunol. 2008, 151, 130–138. [Google Scholar] [CrossRef]
- Park, C.; Cheung, K.P.; Limon, N.; Costanzo, A.; Barba, C.; Miranda, N.; Gargas, S.; Johnson, A.M.; Olefsky, J.M.; Jameson, J.M. Obesity Modulates Intestinal Intraepithelial T Cell Persistence, Cd103 and Ccr9 Expression, and Outcome in Dextran Sulfate Sodium-Induced Colitis. J. Immunol. 2019, 203, 3427–3435. [Google Scholar] [CrossRef]
- Okada, Y.; Tsuzuki, Y.; Sato, H.; Narimatsu, K.; Hokari, R.; Kurihara, C.; Watanabe, C.; Tomita, K.; Komoto, S.; Kawaguchi, A.; et al. Trans fatty acids exacerbate dextran sodium sulphate-induced colitis by promoting the up-regulation of macrophage-derived proinflammatory cytokines involved in T helper 17 cell polarization. Clin. Exp. Immunol. 2013, 174, 459–471. [Google Scholar] [CrossRef]
- Ding, N.; Zhang, X.; Di Zhang, X.; Jing, J.; Liu, S.S.; Mu, Y.P.; Peng, L.L.; Yan, Y.J.; Xiao, G.M.; Bi, X.; et al. Impairment of spermatogenesis and sperm motility by the high-fat diet-induced dysbiosis of gut microbes. Gut 2020, 69, 1608–1619. [Google Scholar] [CrossRef]
- Rohr, M.; Narasimhulu, C.A.; Keewan, E.; Hamid, S.; Parthasarathy, S. The dietary peroxidized lipid, 13-HPODE, promotes intestinal inflammation by mediating granzyme B secretion from natural killer cells. Food Funct. 2020, 11, 9526–9534. [Google Scholar] [CrossRef]
- Saez, A.; Gomez-Bris, R.; Herrero-Fernandez, B.; Mingorance, C.; Rius, C.; Gonzalez-Granado, J.M. Innate Lymphoid Cells in Intestinal Homeostasis and Inflammatory Bowel Disease. Int. J. Mol. Sci. 2021, 22, 7618. [Google Scholar] [CrossRef]
- Babu, S.T.; Niu, X.; Raetz, M.; Savani, R.C.; Hooper, L.V.; Mirpuri, J. Maternal High-Fat Diet Results in Microbio-ta-Dependent Expansion of Ilc3s in Mice Offspring. JCI Insight 2018, 3, e99223. [Google Scholar] [CrossRef] [PubMed]
- Mxinwa, V.; Nkambule, B.B.; Nyambuya, T.M.; Dludla, P.V. Expression of Caspase-3 in Circulating Innate Lymphoid Cells Subtypes Is Altered by Treatment with Metformin and Fluvastatin in High-Fat Diet Fed C57BL/6 Mice. Cells 2022, 11, 1430. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Xiao, Y.; Shi, Y.-H.; Wang, B.; Le, G.-W. Lipoic acid attenuates high-fat-diet–induced oxidative stress and B-cell–related immune depression. Nutrition 2012, 28, 275–280. [Google Scholar] [CrossRef]
- Gurzell, E.A.; Teague, H.; Harris, M.; Clinthorne, J.; Shaikh, S.R.; Fenton, J.I. DHA-enriched fish oil targets B cell lipid microdomains and enhances ex vivo and in vivo B cell function. J. Leukoc. Biol. 2013, 93, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Iftikhar, R.; Penrose, H.M.; King, A.N.; Kim, Y.; Ruiz, E.; Kandil, E.; Machado, H.L.; Savkovic, S.D. FOXO3 Expression in Macrophages Is Lowered by a High-Fat Diet and Regulates Colonic Inflammation and Tumorigenesis. Metabolites 2022, 12, 250. [Google Scholar] [CrossRef]
- Senokuchi, T.; Liang, C.P.; Seimon, T.A.; Han, S.; Matsumoto, M.; Banks, A.S.; Paik, J.H.; DePinho, R.A.; Accili, D.; Tabas, I.; et al. Forkhead Transcription Factors (Foxos) Promote Apoptosis of Insulin-Resistant Macrophages During Cholester-ol-Induced Endoplasmic Reticulum Stress. Diabetes 2008, 57, 2967–2976. [Google Scholar] [CrossRef]
- Chen, H.; Wu, X.; Xu, C.; Lin, J.; Liu, Z. Dichotomous roles of neutrophils in modulating pathogenic and repair processes of inflammatory bowel diseases. Precis. Clin. Med. 2021, 4, 246–257. [Google Scholar] [CrossRef]
- Guo, H.; Diao, N.; Yuan, R.; Chen, K.; Geng, S.; Li, M.; Li, L. Subclinical-Dose Endotoxin Sustains Low-Grade Inflammation and Exacerbates Steatohepatitis in High-Fat Diet–Fed Mice. J. Immunol. 2016, 196, 2300–2308. [Google Scholar] [CrossRef]
- Bibi, S.; Kang, Y.; Du, M.; Zhu, M.-J. Maternal high-fat diet consumption enhances offspring susceptibility to DSS-induced colitis in mice. Obesity 2017, 25, 901–908. [Google Scholar] [CrossRef]
- Pérez, M.M.; Martins, L.M.; Dias, M.S.; Pereira, C.A.; Leite, J.A.; Gonçalves, E.C.; de Almeida, P.Z.; de Freitas, E.N.; Tostes, R.C.; Ramos, S.G.; et al. Interleukin-17/Interleukin-17 Receptor Axis Elicits Intestinal Neutrophil Migration, Restrains Gut Dysbiosis and Lipopolysaccharide Translocation in High-Fat Diet-Induced Metabolic Syndrome Model. Immunology 2019, 156, 339–355. [Google Scholar] [CrossRef]
- Yoshida, H.; Kishikawa, H.; Hirokawa, M.; Nakamizo, H.; Nakatsumi, R.C.; Suzuki, H.; Saito, H.; Miura, S.; Ishii, H. Fatty Acids Enhance GRO/CINC-1 and Interleukin-6 Production in Rat Intestinal Epithelial Cells. J. Nutr. 2001, 131, 2943–2950. [Google Scholar] [CrossRef] [PubMed]
- Gruber, L.; Kisling, S.; Lichti, P.; Martin, F.-P.; May, S.; Klingenspor, M.; Lichtenegger, M.; Rychlik, M.; Haller, D. High Fat Diet Accelerates Pathogenesis of Murine Crohn’s Disease-Like Ileitis Independently of Obesity. PLoS ONE 2013, 8, e71661. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Jin, H.; Qiang, Y.; Wu, S.; Yan, C.; Han, M.; Xiao, T.; Yan, N.; An, H.; Zhou, X.; et al. High fat diet exacerbates dextran sulfate sodium induced colitis through disturbing mucosal dendritic cell homeostasis. Int. Immunopharmacol. 2016, 40, 1–10. [Google Scholar] [CrossRef]
- Wien, M.; Rajaram, S.; Oda, K.; Sabaté, J. Decreasing the Linoleic Acid to Alpha-Linolenic Acid Diet Ratio Increases Eicosapentaenoic Acid in Erythrocytes in Adults. Lipids 2010, 45, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Belluzzi, A.; Boschi, S.; Brignola, C.; Munarini, A.; Cariani, G.; Miglio, F. Polyunsaturated fatty acids and inflammatory bowel disease. Am. J. Clin. Nutr. 2000, 71, 339S–342S. [Google Scholar] [CrossRef] [PubMed]
- John, S.; Luben, R.; Shrestha, S.S.; Welch, A.; Khaw, K.-T.; Hart, A.R. Dietary n-3 polyunsaturated fatty acids and the aetiology of ulcerative colitis: A UK prospective cohort study. Eur. J. Gastroenterol. Hepatol. 2010, 22, 602–606. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, K.; Nakamura, M.; Odahara, S.; Koido, S.; Katahira, K.; Shiraishi, H.; Ohkusa, T.; Fujise, K.; Tajiri, H. N-3 Poly-unsaturated Fatty Acid Diet Therapy for Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2010, 16, 1696–1707. [Google Scholar] [CrossRef]
- Beguin, P.; Errachid, A.; Larondelle, Y.; Schneider, Y.-J. Effect of polyunsaturated fatty acids on tight junctions in a model of the human intestinal epithelium under normal and inflammatory conditions. Food Funct. 2013, 4, 923–931. [Google Scholar] [CrossRef]
- Charpentier, C.; Chan, R.; Salameh, E.; Mbodji, K.; Ueno, A.; Coëffier, M.; Guérin, C.; Ghosh, S.; Savoye, G.; Marion-Letellier, R. Dietary n-3 PUFA May Attenuate Experimental Colitis. Mediat. Inflamm. 2018, 2018, 8430614. [Google Scholar] [CrossRef]
- Hawthorne, A.B.; Daneshmend, T.K.; Hawkey, C.J.; Belluzzi, A.; Everitt, S.J.; Holmes, G.K.; Malkinson, C.; Shaheen, M.Z.; EWillars, J. Treatment of ulcerative colitis with fish oil supplementation: A prospective 12 month randomised controlled trial. Gut 1992, 33, 922–928. [Google Scholar] [CrossRef]
- Teague, H.; Rockett, B.D.; Harris, M.; Brown, D.A.; Shaikh, S.R. Dendritic Cell Activation, Phagocytosis and Cd69 Ex-pression on Cognate T Cells Are Suppressed by N-3 Long-Chain Polyunsaturated Fatty Acids. Immunology 2013, 139, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Ly, L.H.; Smith, R.; Switzer, K.C.; Chapkin, R.S.; McMurray, D.N. Dietary Eicosapentaenoic Acid Modulates Ctla-4 Ex-pression in Murine Cd4+ T-Cells. Prostaglandins Leukot Essent Fat. Acids 2006, 74, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Pearl, D.S.; Masoodi, M.; Eiden, M.; Brümmer, J.; Gullick, D.; Mckeever, T.M.; Whittaker, M.A.; Nitch-Smith, H.; Brown, J.F.; Shute, J.K.; et al. Altered colonic mucosal availability of n-3 and n-6 polyunsaturated fatty acids in ulcerative colitis and the relationship to disease activity. J. Crohn’s Colitis 2014, 8, 70–79. [Google Scholar] [CrossRef]
- Krause, P.; Bruckner, M.; Uermösi, C.; Singer, E.; Groettrup, M.; Legler, D.F. Prostaglandin E(2) Enhances T-Cell Prolifer-ation by Inducing the Costimulatory Molecules Ox40l, Cd70, and 4-1bbl on Dendritic Cells. Blood 2009, 113, 2451–2460. [Google Scholar] [CrossRef] [PubMed]
- Arimoto-Miyamoto, K.; Kadowaki, N.; Kitawaki, T.; Iwata, S.; Morimoto, C.; Uchiyama, T. Optimal stimulation for CD70 induction on human monocyte-derived dendritic cells and the importance of CD70 in naive CD4+T-cell differentiation. Immunology 2010, 130, 137–149. [Google Scholar] [CrossRef]
- Monk, J.M.; Hou, T.Y.; Turk, H.F.; Weeks, B.; Wu, C.; McMurray, D.N.; Chapkin, R.S. Dietary n-3 Polyunsaturated Fatty Acids (PUFA) Decrease Obesity-Associated Th17 Cell-Mediated Inflammation during Colitis. PLoS ONE 2012, 7, e49739. [Google Scholar] [CrossRef]
- Monk, J.M.; Jia, Q.; Callaway, E.; Weeks, B.; Alaniz, R.C.; McMurray, D.N.; Chapkin, R.S. Th17 Cell Accumulation Is De-creased During Chronic Experimental Colitis by (N-3) Pufa in Fat-1 Mice. J. Nutr. 2012, 142, 117–124. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, S. Gut metabolites: Make orphans adopted. Precis. Clin. Med. 2019, 2, 87–89. [Google Scholar] [CrossRef]
- Zheng, L.; Kelly, C.J.; Battista, K.D.; Schaefer, R.; Lanis, J.M.; Alexeev, E.E.; Wang, R.X.; Onyiah, J.C.; Kominsky, D.J.; Colgan, S.P. Microbial-Derived Butyrate Promotes Epithelial Barrier Function through IL-10 Receptor–Dependent Repression of Claudin-2. J. Immunol. 2017, 199, 2976–2984. [Google Scholar] [CrossRef]
- Hatayama, H.; Iwashita, J.; Kuwajima, A.; Abe, T. The short chain fatty acid, butyrate, stimulates MUC2 mucin production in the human colon cancer cell line, LS174T. Biochem. Biophys. Res. Commun. 2007, 356, 599–603. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, F.; Wu, W.; Sun, M.; Bilotta, A.J.; Yao, S.; Xiao, Y.; Huang, X.; Eaves-Pyles, T.D.; Golovko, G.; et al. GPR43 mediates microbiota metabolite SCFA regulation of antimicrobial peptide expression in intestinal epithelial cells via activation of mTOR and STAT3. Mucosal Immunol. 2018, 11, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Wu, W.; Chen, L.; Yang, W.; Huang, X.; Ma, C.; Chen, F.; Xiao, Y.; Zhao, Y.; Ma, C.; et al. Microbiota-derived short-chain fatty acids promote Th1 cell IL-10 production to maintain intestinal homeostasis. Nat. Commun. 2018, 9, 3555. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, M.; Kang, S.; Jannasch, A.; Cooper, B.; Patterson, J.; Kim, C. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR–S6K pathway. Mucosal Immunol. 2015, 8, 80–93. [Google Scholar] [CrossRef]
- Haidinger, M.; Poglitsch, M.; Geyeregger, R.; Kasturi, S.; Zeyda, M.; Zlabinger, G.J.; Pulendran, B.; Hörl, W.H.; Säemann, M.D.; Weichhart, T. A Versatile Role of Mammalian Target of Rapamycin in Human Dendritic Cell Function and Differentiation. J. Immunol. 2010, 185, 3919–3931. [Google Scholar] [CrossRef] [PubMed]
- Schulthess, J.; Pandey, S.; Capitani, M.; Rue-Albrecht, K.C.; Arnold, I.; Franchini, F.; Chomka, A.; Ilott, N.E.; Johnston, D.G.W.; Pires, E.; et al. The Short Chain Fatty Acid Butyrate Imprints an Antimicrobial Program in Macrophages. Immunity 2019, 50, 432–445.e437. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.V.; Hao, L.; Offermanns, S.; Medzhitov, R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc. Natl. Acad. Sci. USA 2014, 111, 2247–2252. [Google Scholar] [CrossRef]
- Vinolo, M.A.R.; Rodrigues, H.G.; Hatanaka, E.; Hebeda, C.B.; Farsky, S.H.P.; Curi, R. Short-chain fatty acids stimulate the migration of neutrophils to inflammatory sites. Clin. Sci. 2009, 117, 331–338. [Google Scholar] [CrossRef]
- Vinolo, M.A.R.; Rodrigues, H.G.; Hatanaka, E.; Sato, F.T.; Sampaio, S.C.; Curi, R. Suppressive effect of short-chain fatty acids on production of proinflammatory mediators by neutrophils. J. Nutr. Biochem. 2011, 22, 849–855. [Google Scholar] [CrossRef]
- Weingarden, A.R.; Vaughn, B.P. Intestinal Microbiota, Fecal Microbiota Transplantation, and Inflammatory Bowel Disease. Gut Microbes 2017, 8, 238–252. [Google Scholar] [CrossRef]
- Ding, X.; Li, Q.; Li, P.; Zhang, T.; Cui, B.; Ji, G.; Lu, X.; Zhang, F. Long-Term Safety and Efficacy of Fecal Microbiota Transplant in Active Ulcerative Colitis. Drug Saf. 2019, 42, 869–880. [Google Scholar] [CrossRef]
- Rapozo, D.C.; Bernardazzi, C.; de Souza, H.S. Diet and Microbiota in Inflammatory Bowel Disease: The Gut in Dis-harmony. World J. Gastroenterol. 2017, 23, 2124–2240. [Google Scholar] [CrossRef] [PubMed]
- Fritsch, J.; Garces, L.; Quintero, M.A.; Pignac-Kobinger, J.; Santander, A.M.; Fernández, I.; Ban, Y.J.; Kwon, D.; Phillips, M.C.; Knight, K.; et al. Low-Fat, High-Fiber Diet Reduces Markers of Inflammation and Dysbiosis and Improves Quality of Life in Patients with Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2021, 19, 1189–1199.e30. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Medina, M.; Denizot, J.; Dreux, N.; Robin, F.; Billard, E.; Bonnet, R.; Darfeuille-Michaud, A.; Barnich, N. Western Diet Induces Dysbiosis with Increased E Coli in Ceabac10 Mice, Alters Host Barrier Function Favouring Aiec Colonisation. Gut 2014, 63, 116–124. [Google Scholar] [CrossRef]
- Shawki, A.; McCole, D.F. Mechanisms of Intestinal Epithelial Barrier Dysfunction by Adherent-Invasive Escherichia coli. Cell. Mol. Gastroenterol. Hepatol. 2017, 3, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Viladomiu, M.; Kivolowitz, C.; Abdulhamid, A.; Dogan, B.; Victorio, D.; Castellanos, J.G.; Woo, V.; Teng, F.; Tran, N.L.; Sczesnak, A.; et al. Iga-Coated E. Coli Enriched in Crohn’s Disease Spondyloarthritis Promote T(H)17-Dependent Inflammation. Sci. Transl. Med. 2017, 9, eaaf9655. [Google Scholar] [CrossRef]
- EavEaves-Pyles, T.; Allen, C.A.; Taormina, J.; Swidsinski, A.; Tutt, C.B.; Jezek, G.E.; Islas-Islas, M.; Torres, A.G. Escherichia coli Isolated from a Crohn’s Disease Patient Adheres, Invades, and Induces Inflammatory Responses in Polarized Intestinal Epi-thelial Cells. Int. J. Med. Microbiol. 2008, 298, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Carter, G.P.; Chakravorty, A.; Pham Nguyen, T.A.; Mileto, S.; Schreiber, F.; Li, L.; Howarth, P.; Clare, S.; Cunningham, B.; Sambol, S.P.; et al. Defining the Roles of Tcda and Tcdb in Localized Gastrointestinal Disease, Systemic Organ Damage, and the Host Response During Clostridium Difficile Infections. mBio 2015, 6, e00551. [Google Scholar] [CrossRef]
- Leslie, J.L.; Huang, S.; Opp, J.S.; Nagy, M.S.; Kobayashi, M.; Young, V.B.; Spence, J.R. Persistence and Toxin Production by Clostridium difficile within Human Intestinal Organoids Result in Disruption of Epithelial Paracellular Barrier Function. Infect. Immun. 2015, 83, 138–145. [Google Scholar] [CrossRef]
- Ng, J.; Hirota, S.A.; Groß, O.; Li, Y.; Ulke–Lemee, A.; Potentier, M.S.; Schenck, L.P.; Vilaysane, A.; Seamone, M.E.; Feng, H.; et al. Clostridium difficile Toxin–Induced Inflammation and Intestinal Injury Are Mediated by the Inflammasome. Gastroenterology 2010, 139, 542–552.e3. [Google Scholar] [CrossRef]
- Ryan, A.; Lynch, M.; Smith, S.M.; Amu, S.; Nel, H.J.; McCoy, C.E.; Dowling, J.K.; Draper, E.; O’Reilly, V.; McCarthy, C.; et al. A Role for TLR4 in Clostridium difficile Infection and the Recognition of Surface Layer Proteins. PLoS Pathog. 2011, 7, e1002076. [Google Scholar] [CrossRef]
- Ashrafian, F.; Behrouzi, A.; Shahriary, A.; Badi, S.A.; Davari, M.; Khatami, S.; Jamnani, F.R.; Fateh, A.; Vaziri, F.; Siadat, S.D. Comparative study of effect of Akkermansia muciniphila and its extracellular vesicles on toll-like receptors and tight junction. Gastroenterol. Hepatol. Bed Bench 2019, 12, 163–168. [Google Scholar] [PubMed]
- Ottman, N.; Reunanen, J.; Meijerink, M.; Pietilä, T.E.; Kainulainen, V.; Klievink, J.; Huuskonen, L.; Aalvink, S.; Skurnik, M.; Boeren, S.; et al. Pili-Like Proteins of Akkermansia muciniphila Modulate Host Immune Responses and Gut Barrier Function. PLoS ONE 2017, 12, e0173004. [Google Scholar] [CrossRef] [PubMed]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef] [PubMed]
- Kosciow, K.; Deppenmeier, U. Characterization of a Phospholipid-Regulated Β-Galactosidase from Akkermansia muciniphila Involved in Mucin Degradation. Microbiologyopen 2019, 8, e00796. [Google Scholar] [CrossRef] [PubMed]
- Shin, N.R.; Lee, J.C.; Lee, H.Y.; Kim, M.S.; Whon, T.W.; Lee, M.S.; Bae, J.W. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut 2014, 63, 727–735. [Google Scholar] [CrossRef]
- Ansaldo, E.; Slayden, L.C.; Ching, K.L.; Koch, M.A.; Wolf, N.K.; Plichta, D.R.; Brown, E.M.; Graham, D.B.; Xavier, R.J.; Moon, J.J.; et al. Akkermansia muciniphila induces intestinal adaptive immune responses during homeostasis. Science 2019, 364, 1179–1184. [Google Scholar] [CrossRef]
- Tsuzuki, Y.; Miyazaki, J.; Matsuzaki, K.; Okada, Y.; Hokari, R.; Kawaguchi, A.; Nagao, S.; Itoh, K.; Miura, S. Differential modulation in the functions of intestinal dendritic cells by long- and medium-chain fatty acids. J. Gastroenterol. 2006, 41, 209–216. [Google Scholar] [CrossRef]
- Denizot, J.; Sivignon, A.; Barreau, F.; Darcha, C.; Chan, C.H.; Stanners, C.P.; Hofman, P.; Darfeuille-Michaud, A.; Barnich, N. Adherent-Invasive Escherichia Coli Induce Claudin-2 Expression and Barrier Defect in Ceabac10 Mice and Crohn’s Disease Patients. Inflamm. Bowel Dis. 2012, 18, 294–304. [Google Scholar] [CrossRef]
- Hemmasi, S.; Czulkies, B.A.; Schorch, B.; Veit, A.; Aktories, K.; Papatheodorou, P. Interaction of the Clostridium difficile Binary Toxin Cdt and Its Host Cell Receptor, Lipolysis-Stimulated Lipoprotein Receptor (LSR). J. Biol. Chem. 2015, 290, 14031–14044. [Google Scholar] [CrossRef]
- Higashi, T.; Tokuda, S.; Kitajiri, S.I.; Masuda, S.; Nakamura, H.; Oda, Y.; Furuse, M. Furuse. Analysis of the ‘Angulin’ Proteins Lsr, Ildr1 and Ildr2--Tricellulin Recruitment, Epithelial Barrier Function and Implication in Deafness Pathogenesis. J. Cell Sci. 2013, 126 Pt 4, 966–977. [Google Scholar] [CrossRef]
- Farrow, M.A.; Chumbler, N.M.; Lapierre, L.A.; Franklin, J.L.; Rutherford, S.A.; Goldenring, J.R.; Lacy, D.B. Clostridium difficile toxin B-induced necrosis is mediated by the host epithelial cell NADPH oxidase complex. Proc. Natl. Acad. Sci. USA 2013, 110, 18674–18679. [Google Scholar] [CrossRef] [PubMed]
- Jefferson, K.K.; Smith, M.F., Jr.; Bobak, D.A. Roles of Intracellular Calcium and Nf-Kappa B in the Clostridium Difficile Toxin a-Induced up-Regulation and Secretion of Il-8 from Human Monocytes. J. Immunol. 1999, 163, 5183–5191. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Sougioultzis, S.; Hagen, S.; Liu, J.; Keates, S.; Keates, A.C.; Pothoulakis, C.; LaMont, J. Clostridium difficile toxin A triggers human colonocyte IL-8 release via mitochondrial oxygen radical generation. Gastroenterology 2002, 122, 1048–1057. [Google Scholar] [CrossRef] [PubMed]
- Cowardin, C.A.; Buonomo, E.L.; Saleh, M.M.; Wilson, M.G.; Burgess, S.L.; Kuehne, S.A.; Schwan, C.; Eichhoff, A.M.; Koch-Nolte, F.; Lyras, D.; et al. The binary toxin CDT enhances Clostridium difficile virulence by suppressing protective colonic eosinophilia. Nat. Microbiol. 2016, 1, 16108. [Google Scholar] [CrossRef] [PubMed]
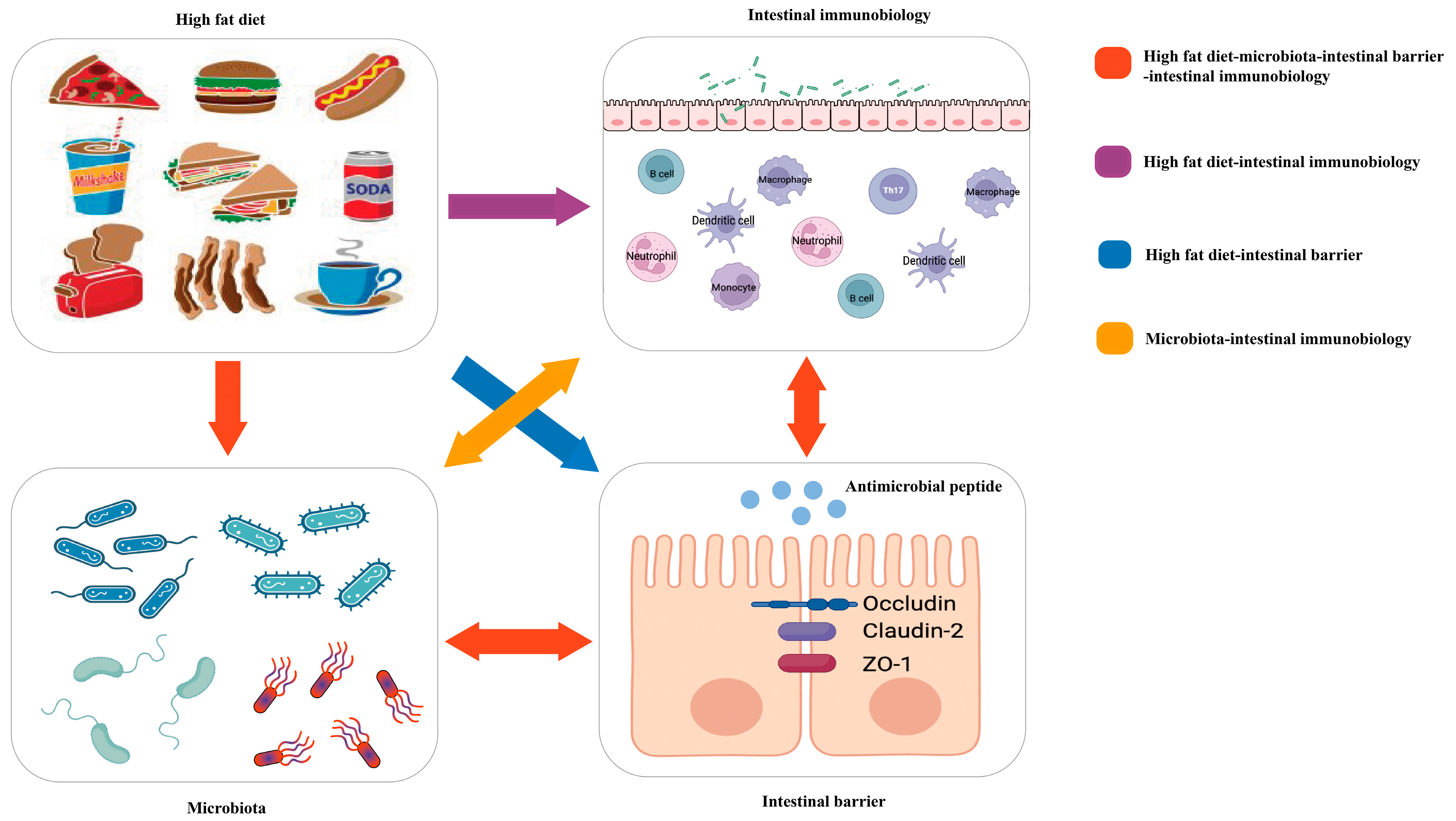

| Effects of HFD on Intestinal Products or Microbiota | Mechanisms of Intestinal Products or Microbiota on the Intestine | References | Data Sources |
|---|---|---|---|
| AIEC | Targeting claudin-2 exacerbates the intestinal physical barrier and decreases the expression of the Muc-2 gene to disrupt the mucus barrier. | [143] | Mice |
| Increasing TNF-α to active TNF-α-NF-κB regulatory pathways. | [144] | Mice | |
| Inducing Th17 polarization in the intestine. | [145] | Mice | |
| Eliciting the secretions of IL-8 and CCL20 to impact the recruitments of macrophages and dendritic cells in the intestine. | [146] | In vitro | |
| Clostridioides difficile | Inactivating GTPases to lead cytoskeleton disruption and barrier breakdown. | [147] | Mice |
| Inducing epithelial cell death and disrupting TJ structure. | [148] | In vitro | |
| Promoting NF-κB, IL-1 and IL-8 secretion. | [149] | Mice | |
| Decreasing neutrophil recruitment and modulating DCs and Th cell responses. | [150] | Mice | |
| n-6 families | Reducing occludin secretion. | [118] | In vitro |
| Promoting the antigen-presenting ability of DCs. | [124,125] | In vitro | |
| Decreasing the percentage of Th17 cells. | [125,127] | In vitro, Mice | |
| A. muciniphila | Increasing the gene expressions of TLR2, TLR4, occludin, and claudin3 in the intestine promotes thickness of the mucus layer. | [151,152,153,154] | In vitro, In vitro, Mice, In vitro |
| Enhancing the production of antimicrobial peptide Reg3γ. | [153] | Mice | |
| Inhibiting the expression of immunoglobulins encoding gene and receptor, chemokines CXCL13 and CCL12, and complement factors C1ra and C5ar1 in the colon. | [154] | In vitro | |
| Decreasing the presence of B cells in the colon and promoting Treg cell proliferation and antigen-specific Th cell response. | [155] | Mice | |
| Upregulating IL-8, IL-6, IL-1β, IL-10, and TNF-α secretion. | [156] | Mice | |
| n-3 families | Protecting tight junctions while reducing MUC2 secretion. | [51] | Mice |
| Upregulating the TLR-2 gene. | [119] | Mice | |
| Inhibiting neutrophil infiltration and averting the concomitant hurt caused by neutrophil production. | [120] | In vitro | |
| Reducing the antigen-presenting ability of DCs. | [121,122,157] | Mice, Mice, Rats | |
| SCFAs | Stimulating MUC2 production. | [130] | In vitro |
| Stimulating AMPs production. | [131] | Mice | |
| Promoting Th1 cells to produce IL-10 and DCs differentiation though mTOR and SATA3 pathways. | [132,134] | In vitro | |
| Increasing the antimicrobial functions of intestinal macrophages and downregulating macrophages secreting proinflammatory mediators. | [135,136] | Mice | |
| Inhibiting pro-inflammatory cytokines produced by neutrophils. | [138] | Rats |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dang, Y.; Ma, C.; Chen, K.; Chen, Y.; Jiang, M.; Hu, K.; Li, L.; Zeng, Z.; Zhang, H. The Effects of a High-Fat Diet on Inflammatory Bowel Disease. Biomolecules 2023, 13, 905. https://doi.org/10.3390/biom13060905
Dang Y, Ma C, Chen K, Chen Y, Jiang M, Hu K, Li L, Zeng Z, Zhang H. The Effects of a High-Fat Diet on Inflammatory Bowel Disease. Biomolecules. 2023; 13(6):905. https://doi.org/10.3390/biom13060905
Chicago/Turabian StyleDang, Yuan, Chunxiang Ma, Kexin Chen, Yiding Chen, Mingshan Jiang, Kehan Hu, Lili Li, Zhen Zeng, and Hu Zhang. 2023. "The Effects of a High-Fat Diet on Inflammatory Bowel Disease" Biomolecules 13, no. 6: 905. https://doi.org/10.3390/biom13060905
APA StyleDang, Y., Ma, C., Chen, K., Chen, Y., Jiang, M., Hu, K., Li, L., Zeng, Z., & Zhang, H. (2023). The Effects of a High-Fat Diet on Inflammatory Bowel Disease. Biomolecules, 13(6), 905. https://doi.org/10.3390/biom13060905






