Chimera and Tandem-Repeat Type Galectins: The New Targets for Cancer Immunotherapy
Abstract
1. Introduction
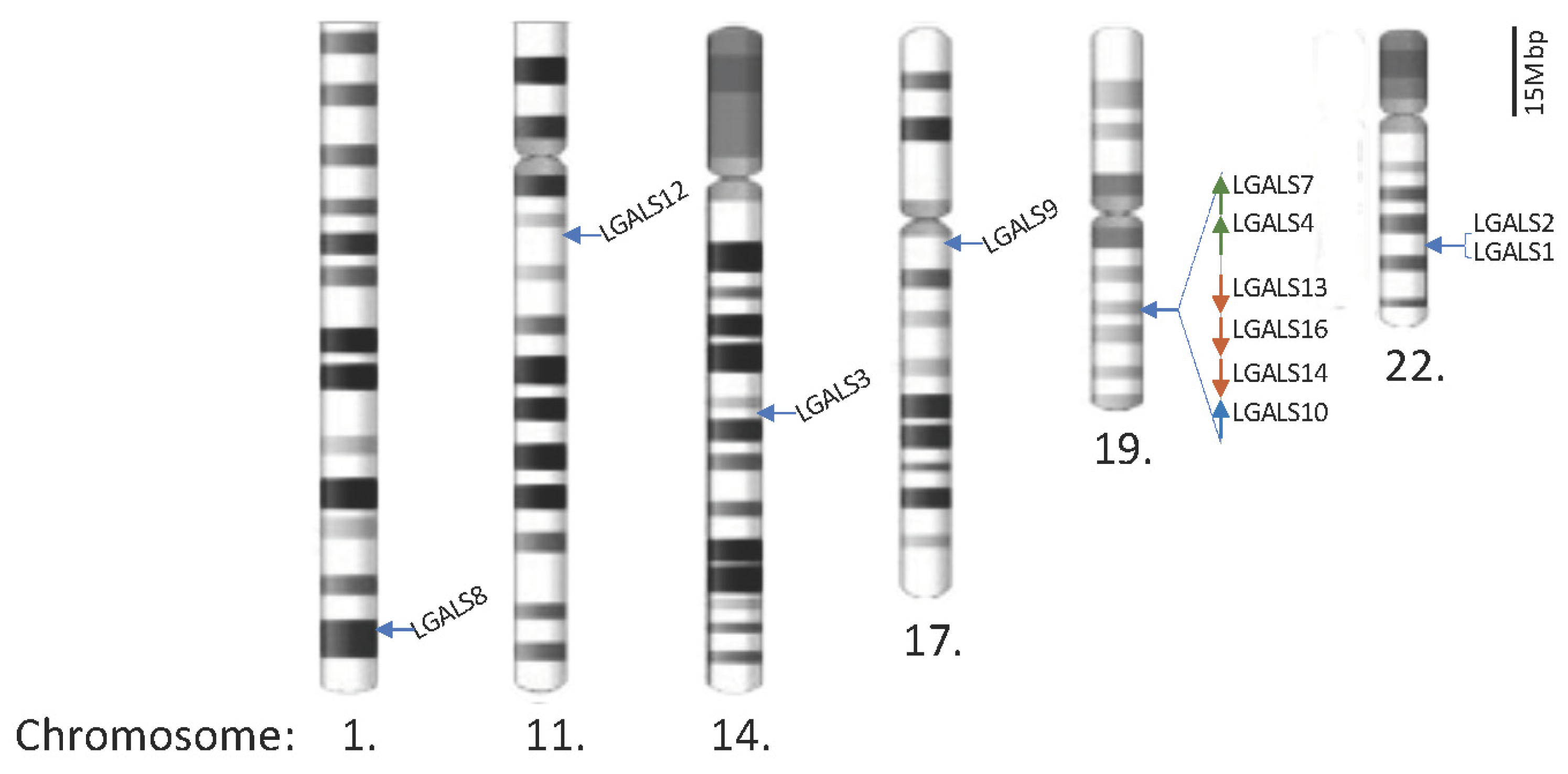
2. Human Galectins
2.1. Galectin-3: The Only Chimera-Type Galectin with Oncogenic Functioning
| Gene/Protein Name (Chromosome Position [48]) | Intracellular (Cytoplasmic/Nucleus) | Extracellular |
|---|---|---|
| LGALS1/Galectin-1 (Chr. 22q13.1) | Cytoplasmic: GRP78 [49] Gemin4 [37] H-Ras [50] PCDH24 [38] | CC and CXC chemokines [42] CD43 [51,52] CD45 [51,53] NRP1 [54] VEGFR2 [55] |
| LGALS2/Galectin-2 (Chr. 22q13.1) | Binds to surface of CD14(interm.–high) monocyte and promote M1 macrophage differentiation [56] | |
| LGALS3/Galectin-3 (Chr. 14q22.3) | Cytoplasmic: Alix (EGFR trafficking) [34,35,57] Gemin4 [37] K-Ras [32,40] PCDH24 [38] Nucleus: hnRNPA2B1 [58] Sp1 [39] | CC and CXC chemokines [42] CD29 [43] CD43 [43] CD45 [43] CD71 [43] EGFR [59] Interferon-γ [60] Integrin αvβ3 [40] LAG3 [61] MUC1 [62] |
| LGALS4/Galectin-4 (Chr. 19q13.2) | CD3 [63] | |
| LGALS7/Galectin-7 (Chr. 19q13.2) | Cytoplasmic: Bcl-2 [64] | |
| LGALS8/Galectin-8 (Chr. 1q43) | αM (CD11b, neutrophils) [65] CD166 [66] Podoplanin [67,68] | |
| LGALS9/Galectin-9 (Chr. 17q11.2) | Cytoplasmic: Binding to intracellular TIM-3 to modulate mTOR phosphorylation [69] Cytoplasmic–Lysosomes: Interact with Lamp2 to regulate lysosomal functions and autophagy [70] | 4-1BB [71] CD40 [72] CD44 [73] CD206 [74] Dectin-1 (macrophages) [75] DR3 [76] PD-1 [77] PDI [78,79] TCR [80,81] TIM-3 [77,82,83] VISTA [84] |
| LGALS10/Galectin-10/Charcot-Leyden crystal protein CLC (Chr. 19q13.2) | Cytoplasmic–Granules: Eosinophil-derived neurotoxin EDN (RNS2) and eosinophil cationic protein ECP (RNS3) co-localised with CD63. It is required for the maturation of eosinophil during granulogenesis [85] | |
| LGALS12/Galectin-12/GRIP1 (Chr. 11q12.3) | Cytoplasmic–Endosome/Lysosomes: VPS13C in lipid droplets and promotes the polarisation to M1 macrophage via TLR4 pathway [86,87] | |
| LGALS13/Galectin-13/ placental protein 13 (Chr. 19q13.2) | Nucleus: HOXA1 [88] | Binds to T lymphocytes and induces apoptosis [89]; Binds to neutrophils and shifts to immunoregulatory phenotype and promotes high PD-L1 expression [90] |
| LGALS14/Galectin-14 (Chr. 19q13.2) | Binds to T lymphocytes and induces apoptosis [89] c-Rel [91] | |
| LGALS16/Galectin-16 (Chr. 19q13.2) | c-Rel [92] |
2.2. Tandem-Repeat Type Galectins
2.2.1. Galectin-9 Acts on the Immunosuppression of Cancers
2.2.2. Other Tandem-Repeat Type Galectins: Galectin-4, -8, and -12
2.3. Prototype Galectins
2.3.1. Galectin-1 Is a Prototype Galectin with Diverse Roles in Intra- and Extracellular Processes
2.3.2. Another Prototype, Galectin-2, Has Controversial Roles in Different Cancers
2.3.3. Other Prototype Galectins (-7, -10, -13, -14, and -16) Are Located on Chromosome 19
3. From Bench to Bedside
3.1. Availability of Galectin-Specific Inhibitors
3.2. Applications, Safety/Pitfalls/Limitations, and Ongoing Clinical Trials
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CRD | Carbohydrate-recognition domain |
| CTLA-4 | Cytotoxic T lymphocyte-associated antigen-4 |
| EMT | Epithelial–Mesenchymal Transition |
| ERK | Extracellular Signal-regulated kinases |
| HCC | Hepatocellular carcinoma |
| ICIs | Immune checkpoint inhibitors |
| I-O | Immuno-oncology |
| irAEs | Immune-related adverse events |
| ITAM | Immunoreceptor tyrosine-based activation motif |
| ITIM | Immunoreceptor tyrosine-based inhibitory motif |
| MEK | Mitogen-activated protein kinase/ ERK kinases |
| NK cells | Natural Killer Cells |
| NSCLC | Non-small cell lung cancer |
| PD-1/-L1 | Programmed cell death-1/-Ligand 1 |
| PI3K | Phosphoinositide 3-kinases |
| PLC/PKC | Phospholipase C/Protein Kinase C |
| Rac | Ras-related C3 botulinum toxin substrates |
| Raf | Rapidly accelerated fibrosarcoma kinases |
| RALGDS | Ras-like (Ral) guanine nucleotide dissociation stimulator |
| SCLC | Small cell lung cancer |
| TIAM1 | T-lymphoma Invasion and Metastasis 1 |
| TIGIT | T-cell immunoreceptor with immunoglobulin and ITIM domains |
| TILs | Tumour-infiltrating lymphocytes |
| TKIs | Tyrosine kinase inhibitors |
| TME | Tumour microenvironment |
| Tregs | Regulatory T cells |
| VEGF/Rs | Vascular Endothelial Growth Factor/Receptors |
References
- Balkwill, F.; Mantovani, A. Inflammation and cancer: Back to Virchow? Lancet 2001, 357, 539–545. [Google Scholar] [CrossRef]
- Coley, W.B. II. Contribution to the Knowledge of Sarcoma. Ann. Surg. 1891, 14, 199–220. [Google Scholar] [CrossRef]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef]
- Hargadon, K.M.; Johnson, C.E.; Williams, C.J. Immune checkpoint blockade therapy for cancer: An overview of FDA-approved immune checkpoint inhibitors. Int. Immunopharmacol. 2018, 62, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.; Khan, F.; Qari, H.A.; Upadhyay, T.K.; Alkhateeb, A.F.; Oves, M. Revolutionization in Cancer Therapeutics via Targeting Major Immune Checkpoints PD-1, PD-L1 and CTLA-4. Pharmaceuticals 2022, 15, 335. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.; Pelosof, L.; Lemery, S.; Gong, Y.; Goldberg, K.B.; Farrell, A.T.; Keegan, P.; Veeraraghavan, J.; Wei, G.; Blumenthal, G.M.; et al. Systematic Review of PD-1/PD-L1 Inhibitors in Oncology: From Personalized Medicine to Public Health. Oncologist 2021, 26, e1786–e1799. [Google Scholar] [CrossRef] [PubMed]
- Chow, A.; Perica, K.; Klebanoff, C.A.; Wolchok, J.D. Clinical implications of T cell exhaustion for cancer immunotherapy. Nat. Rev. Clin. Oncol. 2022, 19, 775–790. [Google Scholar] [CrossRef]
- Togashi, Y.; Shitara, K.; Nishikawa, H. Regulatory T cells in cancer immunosuppression—Implications for anticancer therapy. Nat. Rev. Clin. Oncol. 2019, 16, 356–371. [Google Scholar] [CrossRef]
- Preillon, J.; Cuende, J.; Rabolli, V.; Garnero, L.; Mercier, M.; Wald, N.; Pappalardo, A.; Denies, S.; Jamart, D.; Michaux, A.C.; et al. Restoration of T-cell Effector Function, Depletion of Tregs, and Direct Killing of Tumor Cells: The Multiple Mechanisms of Action of a-TIGIT Antagonist Antibodies. Mol. Cancer Ther. 2021, 20, 121–131. [Google Scholar] [CrossRef]
- Florou, V.; Garrido-Laguna, I. Clinical Development of Anti-TIGIT Antibodies for Immunotherapy of Cancer. Curr. Oncol. Rep. 2022, 24, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.C.; Abreu, D.R.; Hussein, M.; Cobo, M.; Patel, A.J.; Secen, N.; Lee, K.H.; Massuti, B.; Hiret, S.; Yang, J.C.H.; et al. Tiragolumab plus atezolizumab versus placebo plus atezolizumab as a first-line treatment for PD-L1-selected non-small-cell lung cancer (CITYSCAPE): Primary and follow-up analyses of a randomised, double-blind, phase 2 study. Lancet Oncol. 2022, 23, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Brazel, D.; Ou, S.I.; Nagasaka, M. Tiragolumab (Anti-TIGIT) in SCLC: Skyscraper-02, a Towering Inferno. Lung Cancer Targets Ther. 2023, 14, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Marino, K.V.; Cagnoni, A.J.; Croci, D.O.; Rabinovich, G.A. Targeting galectin-driven regulatory circuits in cancer and fibrosis. Nat. Rev. Drug Discov. 2023, 22, 295–316. [Google Scholar] [CrossRef] [PubMed]
- Zetterberg, F.R.; MacKinnon, A.; Brimert, T.; Gravelle, L.; Johnsson, R.E.; Kahl-Knutson, B.; Leffler, H.; Nilsson, U.J.; Pedersen, A.; Peterson, K.; et al. Discovery and Optimization of the First Highly Effective and Orally Available Galectin-3 Inhibitors for Treatment of Fibrotic Disease. J. Med. Chem. 2022, 65, 12626–12638. [Google Scholar] [CrossRef]
- Aslanis, V.; Slack, R.J.; MacKinnon, A.C.; McClinton, C.; Tantawi, S.; Gravelle, L.; Nilsson, U.J.; Leffler, H.; Brooks, A.; Khindri, S.K.; et al. Safety and pharmacokinetics of GB1211, an oral galectin-3 inhibitor: A single- and multiple-dose first-in-human study in healthy participants. Cancer Chemother. Pharmacol. 2023, 91, 267–280. [Google Scholar] [CrossRef]
- Astorgues-Xerri, L.; Riveiro, M.E.; Tijeras-Raballand, A.; Serova, M.; Rabinovich, G.A.; Bieche, I.; Vidaud, M.; de Gramont, A.; Martinet, M.; Cvitkovic, E.; et al. OTX008, a selective small-molecule inhibitor of galectin-1, downregulates cancer cell proliferation, invasion and tumour angiogenesis. Eur. J. Cancer 2014, 50, 2463–2477. [Google Scholar] [CrossRef]
- Greer, P.F.C.; Rich, A.; Coates, D.E. Effects of galectin-1 inhibitor OTX008 on oral squamous cell carcinoma cells in vitro and the role of AP-1 and the MAPK/ERK pathway. Arch. Oral. Biol. 2022, 134, 105335. [Google Scholar] [CrossRef]
- Koonce, N.A.; Griffin, R.J.; Dings, R.P.M. Galectin-1 Inhibitor OTX008 Induces Tumor Vessel Normalization and Tumor Growth Inhibition in Human Head and Neck Squamous Cell Carcinoma Models. Int. J. Mol. Sci. 2017, 18, 2671. [Google Scholar] [CrossRef]
- Leung, Z.; Ko, F.C.F.; Tey, S.K.; Kwong, E.M.L.; Mao, X.; Liu, B.H.M.; Ma, A.P.Y.; Fung, Y.M.E.; Che, C.M.; Wong, D.K.H.; et al. Galectin-1 promotes hepatocellular carcinoma and the combined therapeutic effect of OTX008 galectin-1 inhibitor and sorafenib in tumor cells. J. Exp. Clin. Cancer Res. 2019, 38, 423. [Google Scholar] [CrossRef]
- Curti, B.D.; Koguchi, Y.; Leidner, R.S.; Rolig, A.S.; Sturgill, E.R.; Sun, Z.; Wu, Y.; Rajamanickam, V.; Bernard, B.; Hilgart-Martiszus, I.; et al. Enhancing clinical and immunological effects of anti-PD-1 with belapectin, a galectin-3 inhibitor. J. Immunother. Cancer 2021, 9, e002371. [Google Scholar] [CrossRef] [PubMed]
- Lau, E.S.; Liu, E.; Paniagua, S.M.; Sarma, A.A.; Zampierollo, G.; Lopez, B.; Diez, J.; Wang, T.J.; Ho, J.E. Galectin-3 Inhibition With Modified Citrus Pectin in Hypertension. JACC Basic Transl. Sci. 2021, 6, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Sigamani, A.; Mayo, K.H.; Miller, M.C.; Chen-Walden, H.; Reddy, S.; Platt, D. An Oral Galectin Inhibitor in COVID-19-A Phase II Randomized Controlled Trial. Vaccines 2023, 11, 731. [Google Scholar] [CrossRef]
- von Heijne, G. Membrane-protein topology. Nat. Rev. Mol. Cell Biol. 2006, 7, 909–918. [Google Scholar] [CrossRef]
- Nielsen, H.; Tsirigos, K.D.; Brunak, S.; von Heijne, G. A Brief History of Protein Sorting Prediction. Protein J. 2019, 38, 200–216. [Google Scholar] [CrossRef]
- Zanetti, G.; Pahuja, K.B.; Studer, S.; Shim, S.; Schekman, R. COPII and the regulation of protein sorting in mammals. Nat. Cell Biol. 2011, 14, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Popa, S.J.; Stewart, S.E.; Moreau, K. Unconventional secretion of annexins and galectins. Semin. Cell Dev. Biol. 2018, 83, 42–50. [Google Scholar] [CrossRef]
- Kutzner, T.J.; Higuero, A.M.; Sussmair, M.; Kopitz, J.; Hingar, M.; Diez-Revuelta, N.; Caballero, G.G.; Kaltner, H.; Lindner, I.; Abad-Rodriguez, J.; et al. How presence of a signal peptide affects human galectins-1 and -4: Clues to explain common absence of a leader sequence among adhesion/growth-regulatory galectins. Biochim. Biophys. Acta Gen. Subj. 2020, 1864, 129449. [Google Scholar] [CrossRef]
- Wan, L.; Hsu, Y.A.; Wei, C.C.; Liu, F.T. Galectins in allergic inflammatory diseases. Mol. Aspects Med. 2021, 79, 100925. [Google Scholar] [CrossRef]
- Clementy, N.; Piver, E.; Bisson, A.; Andre, C.; Bernard, A.; Pierre, B.; Fauchier, L.; Babuty, D. Galectin-3 in Atrial Fibrillation: Mechanisms and Therapeutic Implications. Int. J. Mol. Sci. 2018, 19, 976. [Google Scholar] [CrossRef]
- Mazurek, N.; Sun, Y.J.; Price, J.E.; Ramdas, L.; Schober, W.; Nangia-Makker, P.; Byrd, J.C.; Raz, A.; Bresalier, R.S. Phosphorylation of galectin-3 contributes to malignant transformation of human epithelial cells via modulation of unique sets of genes. Cancer Res. 2005, 65, 10767–10775. [Google Scholar] [CrossRef] [PubMed]
- Mysore, V.P.; Zhou, Z.W.; Ambrogio, C.; Li, L.; Kapp, J.N.; Lu, C.; Wang, Q.; Tucker, M.R.; Okoro, J.J.; Nagy-Davidescu, G.; et al. A structural model of a Ras-Raf signalosome. Nat. Struct. Mol. Biol. 2021, 28, 847–857. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Guo, Z.; Wang, F.; Fu, L. KRAS mutation: From undruggable to druggable in cancer. Signal Transduct. Target Ther. 2021, 6, 386. [Google Scholar] [CrossRef]
- Liu, W.; Hsu, D.K.; Chen, H.Y.; Yang, R.Y.; Carraway, K.L., 3rd; Isseroff, R.R.; Liu, F.T. Galectin-3 regulates intracellular trafficking of EGFR through Alix and promotes keratinocyte migration. J. Investig. Dermatol. 2012, 132, 2828–2837. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.F.; Tsao, C.H.; Lin, Y.T.; Hsu, D.K.; Chiang, M.L.; Lo, C.H.; Chien, F.C.; Chen, P.; Arthur Chen, Y.M.; Chen, H.Y.; et al. Galectin-3 promotes HIV-1 budding via association with Alix and Gag p6. Glycobiology 2014, 24, 1022–1035. [Google Scholar] [CrossRef]
- Monypenny, J.; Milewicz, H.; Flores-Borja, F.; Weitsman, G.; Cheung, A.; Chowdhury, R.; Burgoyne, T.; Arulappu, A.; Lawler, K.; Barber, P.R.; et al. ALIX Regulates Tumor-Mediated Immunosuppression by Controlling EGFR Activity and PD-L1 Presentation. Cell Rep. 2018, 24, 630–641. [Google Scholar] [CrossRef]
- Park, J.W.; Voss, P.G.; Grabski, S.; Wang, J.L.; Patterson, R.J. Association of galectin-1 and galectin-3 with Gemin4 in complexes containing the SMN protein. Nucleic Acids Res. 2001, 29, 3595–3602. [Google Scholar] [CrossRef]
- Ose, R.; Oharaa, O.; Nagase, T. Galectin-1 and Galectin-3 Mediate Protocadherin-24-Dependent Membrane Localization of beta-catenin in Colon Cancer Cell Line HCT116. Curr. Chem. Genom. 2012, 6, 18–26. [Google Scholar] [CrossRef]
- Jia, W.; Kong, L.; Kidoya, H.; Naito, H.; Muramatsu, F.; Hayashi, Y.; Hsieh, H.Y.; Yamakawa, D.; Hsu, D.K.; Liu, F.T.; et al. Indispensable role of Galectin-3 in promoting quiescence of hematopoietic stem cells. Nat. Commun. 2021, 12, 2118. [Google Scholar] [CrossRef]
- Seguin, L.; Camargo, M.F.; Wettersten, H.I.; Kato, S.; Desgrosellier, J.S.; von Schalscha, T.; Elliott, K.C.; Cosset, E.; Lesperance, J.; Weis, S.M.; et al. Galectin-3, a Druggable Vulnerability for KRAS-Addicted Cancers. Cancer Discov. 2017, 7, 1464–1479. [Google Scholar] [CrossRef]
- Diaz-Alvarez, L.; Ortega, E. The Many Roles of Galectin-3, a Multifaceted Molecule, in Innate Immune Responses against Pathogens. Mediators Inflamm. 2017, 2017, 9247574. [Google Scholar] [CrossRef]
- Eckardt, V.; Miller, M.C.; Blanchet, X.; Duan, R.; Leberzammer, J.; Duchene, J.; Soehnlein, O.; Megens, R.T.; Ludwig, A.K.; Dregni, A.; et al. Chemokines and galectins form heterodimers to modulate inflammation. EMBO Rep. 2020, 21, e47852. [Google Scholar] [CrossRef] [PubMed]
- Stillman, B.N.; Hsu, D.K.; Pang, M.; Brewer, C.F.; Johnson, P.; Liu, F.T.; Baum, L.G. Galectin-3 and galectin-1 bind distinct cell surface glycoprotein receptors to induce T cell death. J. Immunol. 2006, 176, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Huang, Q.; Xiao, W.; Zhao, Y.; Pi, J.; Xu, H.; Zhao, H.; Xu, J.; Evans, C.E.; Jin, H. Advances in Anti-Tumor Treatments Targeting the CD47/SIRPalpha Axis. Front. Immunol. 2020, 11, 18. [Google Scholar] [CrossRef] [PubMed]
- Al Attar, A.; Antaramian, A.; Noureddin, M. Review of galectin-3 inhibitors in the treatment of nonalcoholic steatohepatitis. Expert Rev. Clin. Pharmacol. 2021, 14, 457–464. [Google Scholar] [CrossRef]
- Huh, Y.; Cho, Y.J.; Nam, G.E. Recent Epidemiology and Risk Factors of Nonalcoholic Fatty Liver Disease. J. Obes. Metab. Syndr. 2022, 31, 17–27. [Google Scholar] [CrossRef]
- Slack, R.J.; Mills, R.; Mackinnon, A.C. The therapeutic potential of galectin-3 inhibition in fibrotic disease. Int. J. Biochem. Cell Biol. 2021, 130, 105881. [Google Scholar] [CrossRef]
- Ensembl—Galectins. Available online: https://www.ensembl.org/Homo_sapiens/Search/Results?q=galectins;site=ensembl;facet_species=Human (accessed on 2 April 2023).
- Zhang, Q.; Ali, M.; Wang, Y.; Sun, Q.N.; Zhu, X.D.; Tang, D.; Wang, W.; Zhang, C.Y.; Zhou, H.H.; Wang, D.R. Galectin-1 binds GRP78 to promote the proliferation and metastasis of gastric cancer. Int. J. Oncol. 2022, 61, 1–18. [Google Scholar] [CrossRef]
- Paz, A.; Haklai, R.; Elad-Sfadia, G.; Ballan, E.; Kloog, Y. Galectin-1 binds oncogenic H-Ras to mediate Ras membrane anchorage and cell transformation. Oncogene 2001, 20, 7486–7493. [Google Scholar] [CrossRef]
- Fulcher, J.A.; Chang, M.H.; Wang, S.; Almazan, T.; Hashimi, S.T.; Eriksson, A.U.; Wen, X.; Pang, M.; Baum, L.G.; Singh, R.R.; et al. Galectin-1 co-clusters CD43/CD45 on dendritic cells and induces cell activation and migration through Syk and protein kinase C signaling. J. Biol. Chem. 2009, 284, 26860–26870. [Google Scholar] [CrossRef]
- Hernandez, J.D.; Nguyen, J.T.; He, J.; Wang, W.; Ardman, B.; Green, J.M.; Fukuda, M.; Baum, L.G. Galectin-1 binds different CD43 glycoforms to cluster CD43 and regulate T cell death. J. Immunol. 2006, 177, 5328–5336. [Google Scholar] [CrossRef] [PubMed]
- Auvynet, C.; Moreno, S.; Melchy, E.; Coronado-Martinez, I.; Montiel, J.L.; Aguilar-Delfin, I.; Rosenstein, Y. Galectin-1 promotes human neutrophil migration. Glycobiology 2013, 23, 32–42. [Google Scholar] [CrossRef]
- Hsieh, S.H.; Ying, N.W.; Wu, M.H.; Chiang, W.F.; Hsu, C.L.; Wong, T.Y.; Jin, Y.T.; Hong, T.M.; Chen, Y.L. Galectin-1, a novel ligand of neuropilin-1, activates VEGFR-2 signaling and modulates the migration of vascular endothelial cells. Oncogene 2008, 27, 3746–3753. [Google Scholar] [CrossRef] [PubMed]
- Croci, D.O.; Cerliani, J.P.; Dalotto-Moreno, T.; Mendez-Huergo, S.P.; Mascanfroni, I.D.; Dergan-Dylon, S.; Toscano, M.A.; Caramelo, J.J.; Garcia-Vallejo, J.J.; Ouyang, J.; et al. Glycosylation-dependent lectin-receptor interactions preserve angiogenesis in anti-VEGF refractory tumors. Cell 2014, 156, 744–758. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, C.; Vogel, D.Y.; Hollander, M.R.; Baggen, J.M.; Fontijn, R.D.; Nieuwenhuis, S.; Haverkamp, A.; de Vries, M.R.; Quax, P.H.; Garcia-Vallejo, J.J.; et al. Galectin-2 induces a proinflammatory, anti-arteriogenic phenotype in monocytes and macrophages. PLoS ONE 2015, 10, e0124347. [Google Scholar] [CrossRef]
- Chen, H.Y.; Fermin, A.; Vardhana, S.; Weng, I.C.; Lo, K.F.; Chang, E.Y.; Maverakis, E.; Yang, R.Y.; Hsu, D.K.; Dustin, M.L.; et al. Galectin-3 negatively regulates TCR-mediated CD4+ T-cell activation at the immunological synapse. Proc. Natl. Acad. Sci. USA 2009, 106, 14496–14501. [Google Scholar] [CrossRef]
- Fritsch, K.; Mernberger, M.; Nist, A.; Stiewe, T.; Brehm, A.; Jacob, R. Galectin-3 interacts with components of the nuclear ribonucleoprotein complex. BMC Cancer 2016, 16, 502. [Google Scholar] [CrossRef]
- Kuo, H.Y.; Hsu, H.T.; Chen, Y.C.; Chang, Y.W.; Liu, F.T.; Wu, C.W. Galectin-3 modulates the EGFR signalling-mediated regulation of Sox2 expression via c-Myc in lung cancer. Glycobiology 2016, 26, 155–165. [Google Scholar] [CrossRef]
- Gordon-Alonso, M.; Hirsch, T.; Wildmann, C.; van der Bruggen, P. Galectin-3 captures interferon-gamma in the tumor matrix reducing chemokine gradient production and T-cell tumor infiltration. Nat. Commun. 2017, 8, 793. [Google Scholar] [CrossRef]
- Kouo, T.; Huang, L.; Pucsek, A.B.; Cao, M.; Solt, S.; Armstrong, T.; Jaffee, E. Galectin-3 Shapes Antitumor Immune Responses by Suppressing CD8+ T Cells via LAG-3 and Inhibiting Expansion of Plasmacytoid Dendritic Cells. Cancer Immunol. Res. 2015, 3, 412–423. [Google Scholar] [CrossRef]
- Piyush, T.; Chacko, A.R.; Sindrewicz, P.; Hilkens, J.; Rhodes, J.M.; Yu, L.G. Interaction of galectin-3 with MUC1 on cell surface promotes EGFR dimerization and activation in human epithelial cancer cells. Cell Death Differ. 2017, 24, 1937–1947. [Google Scholar] [CrossRef] [PubMed]
- Paclik, D.; Danese, S.; Berndt, U.; Wiedenmann, B.; Dignass, A.; Sturm, A. Galectin-4 controls intestinal inflammation by selective regulation of peripheral and mucosal T cell apoptosis and cell cycle. PLoS ONE 2008, 3, e2629. [Google Scholar] [CrossRef] [PubMed]
- Villeneuve, C.; Baricault, L.; Canelle, L.; Barboule, N.; Racca, C.; Monsarrat, B.; Magnaldo, T.; Larminat, F. Mitochondrial proteomic approach reveals galectin-7 as a novel BCL-2 binding protein in human cells. Mol. Biol. Cell 2011, 22, 999–1013. [Google Scholar] [CrossRef]
- Nishi, N.; Shoji, H.; Seki, M.; Itoh, A.; Miyanaka, H.; Yuube, K.; Hirashima, M.; Nakamura, T. Galectin-8 modulates neutrophil function via interaction with integrin alphaM. Glycobiology 2003, 13, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Renard, H.F.; Tyckaert, F.; Lo Giudice, C.; Hirsch, T.; Valades-Cruz, C.A.; Lemaigre, C.; Shafaq-Zadah, M.; Wunder, C.; Wattiez, R.; Johannes, L.; et al. Endophilin-A3 and Galectin-8 control the clathrin-independent endocytosis of CD166. Nat. Commun. 2020, 11, 1457. [Google Scholar] [CrossRef]
- Bieniasz-Krzywiec, P.; Martin-Perez, R.; Ehling, M.; Garcia-Caballero, M.; Pinioti, S.; Pretto, S.; Kroes, R.; Aldeni, C.; Di Matteo, M.; Prenen, H.; et al. Podoplanin-Expressing Macrophages Promote Lymphangiogenesis and Lymphoinvasion in Breast Cancer. Cell. Metab. 2019, 30, 917–936.e910. [Google Scholar] [CrossRef]
- Cueni, L.N.; Detmar, M. Galectin-8 interacts with podoplanin and modulates lymphatic endothelial cell functions. Exp. Cell Res. 2009, 315, 1715–1723. [Google Scholar] [CrossRef]
- Goncalves Silva, I.; Ruegg, L.; Gibbs, B.F.; Bardelli, M.; Fruehwirth, A.; Varani, L.; Berger, S.M.; Fasler-Kan, E.; Sumbayev, V.V. The immune receptor Tim-3 acts as a trafficker in a Tim-3/galectin-9 autocrine loop in human myeloid leukemia cells. Oncoimmunology 2016, 5, e1195535. [Google Scholar] [CrossRef]
- Sudhakar, J.N.; Lu, H.H.; Chiang, H.Y.; Suen, C.S.; Hwang, M.J.; Wu, S.Y.; Shen, C.N.; Chang, Y.M.; Li, F.A.; Liu, F.T.; et al. Lumenal Galectin-9-Lamp2 interaction regulates lysosome and autophagy to prevent pathogenesis in the intestine and pancreas. Nat. Commun. 2020, 11, 4286. [Google Scholar] [CrossRef]
- Madireddi, S.; Eun, S.Y.; Lee, S.W.; Nemcovicova, I.; Mehta, A.K.; Zajonc, D.M.; Nishi, N.; Niki, T.; Hirashima, M.; Croft, M. Galectin-9 controls the therapeutic activity of 4-1BB-targeting antibodies. J. Exp. Med. 2014, 211, 1433–1448. [Google Scholar] [CrossRef]
- Vaitaitis, G.M.; Wagner, D.H., Jr. Galectin-9 controls CD40 signaling through a Tim-3 independent mechanism and redirects the cytokine profile of pathogenic T cells in autoimmunity. PLoS ONE 2012, 7, e38708. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Thalhamer, T.; Franca, R.F.; Xiao, S.; Wang, C.; Hotta, C.; Zhu, C.; Hirashima, M.; Anderson, A.C.; Kuchroo, V.K. Galectin-9-CD44 interaction enhances stability and function of adaptive regulatory T cells. Immunity 2014, 41, 270–282. [Google Scholar] [CrossRef] [PubMed]
- Enninga, E.A.L.; Chatzopoulos, K.; Butterfield, J.T.; Sutor, S.L.; Leontovich, A.A.; Nevala, W.K.; Flotte, T.J.; Markovic, S.N. CD206-positive myeloid cells bind galectin-9 and promote a tumor-supportive microenvironment. J. Pathol. 2018, 245, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Daley, D.; Mani, V.R.; Mohan, N.; Akkad, N.; Ochi, A.; Heindel, D.W.; Lee, K.B.; Zambirinis, C.P.; Pandian, G.S.B.; Savadkar, S.; et al. Dectin 1 activation on macrophages by galectin 9 promotes pancreatic carcinoma and peritumoral immune tolerance. Nat. Med. 2017, 23, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Madireddi, S.; Eun, S.Y.; Mehta, A.K.; Birta, A.; Zajonc, D.M.; Niki, T.; Hirashima, M.; Podack, E.R.; Schreiber, T.H.; Croft, M. Regulatory T Cell-Mediated Suppression of Inflammation Induced by DR3 Signaling Is Dependent on Galectin-9. J. Immunol. 2017, 199, 2721–2728. [Google Scholar] [CrossRef]
- Yang, R.; Sun, L.; Li, C.F.; Wang, Y.H.; Yao, J.; Li, H.; Yan, M.; Chang, W.C.; Hsu, J.M.; Cha, J.H.; et al. Galectin-9 interacts with PD-1 and TIM-3 to regulate T cell death and is a target for cancer immunotherapy. Nat. Commun. 2021, 12, 832. [Google Scholar] [CrossRef]
- Schaefer, K.; Webb, N.E.; Pang, M.; Hernandez-Davies, J.E.; Lee, K.P.; Gonzalez, P.; Douglass, M.V.; Lee, B.; Baum, L.G. Galectin-9 binds to O-glycans on protein disulfide isomerase. Glycobiology 2017, 27, 878–887. [Google Scholar] [CrossRef]
- Bi, S.; Hong, P.W.; Lee, B.; Baum, L.G. Galectin-9 binding to cell surface protein disulfide isomerase regulates the redox environment to enhance T-cell migration and HIV entry. Proc. Natl. Acad. Sci. USA 2011, 108, 10650–10655. [Google Scholar] [CrossRef]
- Yang, R.; Sun, L.; Li, C.F.; Wang, Y.H.; Xia, W.; Liu, B.; Chu, Y.Y.; Bover, L.; Vien, L.; Hung, M.C. Development and characterization of anti-galectin-9 antibodies that protect T cells from galectin-9-induced cell death. J. Biol. Chem. 2022, 298, 101821. [Google Scholar] [CrossRef]
- Colomb, F.; Giron, L.B.; Premeaux, T.A.; Mitchell, B.I.; Niki, T.; Papasavvas, E.; Montaner, L.J.; Ndhlovu, L.C.; Abdel-Mohsen, M. Galectin-9 Mediates HIV Transcription by Inducing TCR-Dependent ERK Signaling. Front. Immunol. 2019, 10, 267. [Google Scholar] [CrossRef]
- Pang, N.; Alimu, X.; Chen, R.; Muhashi, M.; Ma, J.; Chen, G.; Zhao, F.; Wang, L.; Qu, J.; Ding, J. Activated Galectin-9/Tim3 promotes Treg and suppresses Th1 effector function in chronic lymphocytic leukemia. FASEB J. 2021, 35, e21556. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Anderson, A.C.; Schubart, A.; Xiong, H.; Imitola, J.; Khoury, S.J.; Zheng, X.X.; Strom, T.B.; Kuchroo, V.K. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat. Immunol. 2005, 6, 1245–1252. [Google Scholar] [CrossRef] [PubMed]
- Yasinska, I.M.; Meyer, N.H.; Schlichtner, S.; Hussain, R.; Siligardi, G.; Casely-Hayford, M.; Fiedler, W.; Wellbrock, J.; Desmet, C.; Calzolai, L.; et al. Ligand-Receptor Interactions of Galectin-9 and VISTA Suppress Human T Lymphocyte Cytotoxic Activity. Front. Immunol. 2020, 11, 580557. [Google Scholar] [CrossRef]
- Grozdanovic, M.M.; Doyle, C.B.; Liu, L.; Maybruck, B.T.; Kwatia, M.A.; Thiyagarajan, N.; Acharya, K.R.; Ackerman, S.J. Charcot-Leyden crystal protein/galectin-10 interacts with cationic ribonucleases and is required for eosinophil granulogenesis. J. Allergy Clin. Immunol. 2020, 146, 377–389.e10. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Lin, H.J.; Huang, C.C.; Chen, Y.C.; Hsu, Y.A.; Lin, C.H.; Lin, H.C.; Chang, C.Y.; Huang, S.H.; Lin, J.M.; et al. Galectin-12 enhances inflammation by promoting M1 polarization of macrophages and reduces insulin sensitivity in adipocytes. Glycobiology 2016, 26, 732–744. [Google Scholar] [CrossRef]
- Hancock-Cerutti, W.; Wu, Z.; Xu, P.; Yadavalli, N.; Leonzino, M.; Tharkeshwar, A.K.; Ferguson, S.M.; Shadel, G.S.; De Camilli, P. ER-lysosome lipid transfer protein VPS13C/PARK23 prevents aberrant mtDNA-dependent STING signaling. J. Cell Biol. 2022, 221, e202106046. [Google Scholar] [CrossRef]
- Yang, T.; Yao, Y.; Wang, X.; Li, Y.; Si, Y.; Li, X.; Ayala, G.J.; Wang, Y.; Mayo, K.H.; Tai, G.; et al. Galectin-13/placental protein 13: Redox-active disulfides as switches for regulating structure, function and cellular distribution. Glycobiology 2020, 30, 120–129. [Google Scholar] [CrossRef]
- Balogh, A.; Toth, E.; Romero, R.; Parej, K.; Csala, D.; Szenasi, N.L.; Hajdu, I.; Juhasz, K.; Kovacs, A.F.; Meiri, H.; et al. Placental Galectins Are Key Players in Regulating the Maternal Adaptive Immune Response. Front. Immunol. 2019, 10, 1240. [Google Scholar] [CrossRef]
- Vokalova, L.; Balogh, A.; Toth, E.; Van Breda, S.V.; Schafer, G.; Hoesli, I.; Lapaire, O.; Hahn, S.; Than, N.G.; Rossi, S.W. Placental Protein 13 (Galectin-13) Polarizes Neutrophils Toward an Immune Regulatory Phenotype. Front. Immunol. 2020, 11, 145. [Google Scholar] [CrossRef]
- Si, Y.; Li, Y.; Yang, T.; Li, X.; Ayala, G.J.; Mayo, K.H.; Tai, G.; Su, J.; Zhou, Y. Structure-function studies of galectin-14, an important effector molecule in embryology. FEBS J. 2021, 288, 1041–1055. [Google Scholar] [CrossRef]
- Si, Y.; Yao, Y.; Jaramillo Ayala, G.; Li, X.; Han, Q.; Zhang, W.; Xu, X.; Tai, G.; Mayo, K.H.; Zhou, Y.; et al. Human galectin-16 has a pseudo ligand binding site and plays a role in regulating c-Rel-mediated lymphocyte activity. Biochim. Biophys. Acta Gen. Subj. 2021, 1865, 129755. [Google Scholar] [CrossRef]
- Wada, J.; Kanwar, Y.S. Identification and characterization of galectin-9, a novel beta-galactoside-binding mammalian lectin. J. Biol. Chem. 1997, 272, 6078–6086. [Google Scholar] [CrossRef] [PubMed]
- Tureci, O.; Schmitt, H.; Fadle, N.; Pfreundschuh, M.; Sahin, U. Molecular definition of a novel human galectin which is immunogenic in patients with Hodgkin’s disease. J. Biol. Chem. 1997, 272, 6416–6422. [Google Scholar] [CrossRef]
- Teo Hansen Selno, A.; Schlichtner, S.; Yasinska, I.M.; Sakhnevych, S.S.; Fiedler, W.; Wellbrock, J.; Berger, S.M.; Klenova, E.; Gibbs, B.F.; Fasler-Kan, E.; et al. High Mobility Group Box 1 (HMGB1) Induces Toll-Like Receptor 4-Mediated Production of the Immunosuppressive Protein Galectin-9 in Human Cancer Cells. Front. Immunol. 2021, 12, 675731. [Google Scholar] [CrossRef]
- He, Y.; Jia, K.; Dziadziuszko, R.; Zhao, S.; Zhang, X.; Deng, J.; Wang, H.; Hirsch, F.R.; Zhou, C. Galectin-9 in non-small cell lung cancer. Lung Cancer 2019, 136, 80–85. [Google Scholar] [CrossRef]
- Li, E.; Xu, J.; Chen, Q.; Zhang, X.; Xu, X.; Liang, T. Galectin-9 and PD-L1 antibody blockade combination therapy inhibits tumour progression in pancreatic cancer. Immunotherapy 2023, 15, 135–147. [Google Scholar] [CrossRef]
- Blair, B.B.; Funkhouser, A.T.; Goodwin, J.L.; Strigenz, A.M.; Chaballout, B.H.; Martin, J.C.; Arthur, C.M.; Funk, C.R.; Edenfield, W.J.; Blenda, A.V. Increased Circulating Levels of Galectin Proteins in Patients with Breast, Colon, and Lung Cancer. Cancers 2021, 13, 4819. [Google Scholar] [CrossRef] [PubMed]
- Okoye, I.; Xu, L.; Motamedi, M.; Parashar, P.; Walker, J.W.; Elahi, S. Galectin-9 expression defines exhausted T cells and impaired cytotoxic NK cells in patients with virus-associated solid tumors. J. Immunother. Cancer 2020, 8, e001849. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, R.M.; Emam, A.; Abdelfattah, M.M.; Abdel-Mageed, A.I.; Abdelhafeez, M.A.; Helwa, R. Assessment of galectins -1, -3, -4, -8, and -9 expression in ovarian carcinoma patients with clinical implications. World J. Surg. Oncol. 2022, 20, 276. [Google Scholar] [CrossRef]
- Liu, D.; Zhu, H.; Li, C. Galectins and galectin-mediated autophagy regulation: New insights into targeted cancer therapy. Biomark Res. 2023, 11, 22. [Google Scholar] [CrossRef]
- Fuselier, C.; Dumoulin, A.; Pare, A.; Nehme, R.; Ajarrag, S.; Granger Joly de Boissel, P.; Chatenet, D.; Doucet, N.; St-Pierre, Y. Placental Galectins in Cancer: Why We Should Pay More Attention. Cells 2023, 12, 437. [Google Scholar] [CrossRef]
- Vladoiu, M.C.; Labrie, M.; St-Pierre, Y. Intracellular galectins in cancer cells: Potential new targets for therapy (Review). Int. J. Oncol. 2014, 44, 1001–1014. [Google Scholar] [CrossRef] [PubMed]
- The Human Protein Altas—Galectins. Available online: https://www.proteinatlas.org/search/Galectins (accessed on 2 April 2023).
- Kapetanakis, N.I.; Busson, P. Galectins as pivotal components in oncogenesis and immune exclusion in human malignancies. Front. Immunol. 2023, 14, 1145268. [Google Scholar] [CrossRef] [PubMed]
- Wykes, M.N.; Lewin, S.R. Immune checkpoint blockade in infectious diseases. Nat. Rev. Immunol. 2018, 18, 91–104. [Google Scholar] [CrossRef]
- Pauls, S.D.; Marshall, A.J. Regulation of immune cell signaling by SHIP1: A phosphatase, scaffold protein, and potential therapeutic target. Eur. J. Immunol. 2017, 47, 932–945. [Google Scholar] [CrossRef]
- Elahi, S.; Dinges, W.L.; Lejarcegui, N.; Laing, K.J.; Collier, A.C.; Koelle, D.M.; McElrath, M.J.; Horton, H. Protective HIV-specific CD8+ T cells evade Treg cell suppression. Nat. Med. 2011, 17, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.C.; Joller, N.; Kuchroo, V.K. Lag-3, Tim-3, and TIGIT: Co-inhibitory Receptors with Specialized Functions in Immune Regulation. Immunity 2016, 44, 989–1004. [Google Scholar] [CrossRef]
- Liu, T.; Soong, L.; Liu, G.; Konig, R.; Chopra, A.K. CD44 expression positively correlates with Foxp3 expression and suppressive function of CD4+ Treg cells. Biol. Direct. 2009, 4, 40. [Google Scholar] [CrossRef]
- Nishikii, H.; Kim, B.S.; Yokoyama, Y.; Chen, Y.; Baker, J.; Pierini, A.; Alvarez, M.; Mavers, M.; Maas-Bauer, K.; Pan, Y.; et al. DR3 signaling modulates the function of Foxp3+ regulatory T cells and the severity of acute graft-versus-host disease. Blood 2016, 128, 2846–2858. [Google Scholar] [CrossRef]
- Rahmati, A.; Bigam, S.; Elahi, S. Galectin-9 promotes natural killer cells activity via interaction with CD44. Front. Immunol. 2023, 14, 1131379. [Google Scholar] [CrossRef]
- Sideras, K.; de Man, R.A.; Harrington, S.M.; Polak, W.G.; Zhou, G.; Schutz, H.M.; Pedroza-Gonzalez, A.; Biermann, K.; Mancham, S.; Hansen, B.E.; et al. Circulating levels of PD-L1 and Galectin-9 are associated with patient survival in surgically treated Hepatocellular Carcinoma independent of their intra-tumoral expression levels. Sci. Rep. 2019, 9, 10677. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, T.; Sperandio, M.; Mocsai, A. Neutrophils as emerging therapeutic targets. Nat. Rev. Drug Discov. 2020, 19, 253–275. [Google Scholar] [CrossRef] [PubMed]
- Robinson, B.S.; Arthur, C.M.; Evavold, B.; Roback, E.; Kamili, N.A.; Stowell, C.S.; Vallecillo-Zuniga, M.L.; Van Ry, P.M.; Dias-Baruffi, M.; Cummings, R.D.; et al. The Sweet-Side of Leukocytes: Galectins as Master Regulators of Neutrophil Function. Front. Immunol. 2019, 10, 1762. [Google Scholar] [CrossRef] [PubMed]
- Ustyanovska Avtenyuk, N.; Choukrani, G.; Ammatuna, E.; Niki, T.; Cendrowicz, E.; Lourens, H.J.; Huls, G.; Wiersma, V.R.; Bremer, E. Galectin-9 Triggers Neutrophil-Mediated Anticancer Immunity. Biomedicines 2021, 10, 66. [Google Scholar] [CrossRef]
- Dunsmore, G.; Rosero, E.P.; Shahbaz, S.; Santer, D.M.; Jovel, J.; Lacy, P.; Houston, S.; Elahi, S. Neutrophils promote T-cell activation through the regulated release of CD44-bound Galectin-9 from the cell surface during HIV infection. PLoS Biol. 2021, 19, e3001387. [Google Scholar] [CrossRef]
- Uribe-Querol, E.; Rosales, C. Neutrophils in Cancer: Two Sides of the Same Coin. J. Immunol. Res. 2015, 2015, 983698. [Google Scholar] [CrossRef]
- Mauracher, L.M.; Hell, L.; Moik, F.; Krall, M.; Englisch, C.; Roiss, J.; Grilz, E.; Hofbauer, T.M.; Brostjan, C.; Knapp, S.; et al. Neutrophils in lung cancer patients: Activation potential and neutrophil extracellular trap formation. Res. Pract. Thromb. Haemost. 2023, 7, 100126. [Google Scholar] [CrossRef]
- Yan, M.; Zheng, M.; Niu, R.; Yang, X.; Tian, S.; Fan, L.; Li, Y.; Zhang, S. Roles of tumor-associated neutrophils in tumor metastasis and its clinical applications. Front. Cell Dev. Biol. 2022, 10, 938289. [Google Scholar] [CrossRef]
- Li, Y.R.; Zhou, K.; Wilson, M.; Kramer, A.; Zhu, Y.; Dawson, N.; Yang, L. Mucosal-associated invariant T cells for cancer immunotherapy. Mol. Ther. 2023, 31, 631–646. [Google Scholar] [CrossRef]
- Bozorgmehr, N.; Hnatiuk, M.; Peters, A.C.; Elahi, S. Depletion of polyfunctional CD26(high)CD8(+) T cells repertoire in chronic lymphocytic leukemia. Exp. Hematol. Oncol. 2023, 12, 13. [Google Scholar] [CrossRef]
- Stowell, S.R.; Arthur, C.M.; Dias-Baruffi, M.; Rodrigues, L.C.; Gourdine, J.P.; Heimburg-Molinaro, J.; Ju, T.; Molinaro, R.J.; Rivera-Marrero, C.; Xia, B.; et al. Innate immune lectins kill bacteria expressing blood group antigen. Nat. Med. 2010, 16, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.H.; Tzeng, S.F.; Chao, T.K.; Tsai, C.Y.; Yang, Y.C.; Lee, M.T.; Hwang, J.J.; Chou, Y.C.; Tsai, M.H.; Cha, T.L.; et al. Metastatic Progression of Prostate Cancer Is Mediated by Autonomous Binding of Galectin-4-O-Glycan to Cancer Cells. Cancer Res. 2016, 76, 5756–5767. [Google Scholar] [CrossRef] [PubMed]
- Huflejt, M.E.; Leffler, H. Galectin-4 in normal tissues and cancer. Glycoconj J. 2004, 20, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Duckworth, C.A.; Fu, B.; Pritchard, D.M.; Rhodes, J.M.; Yu, L.G. Circulating galectins -2, -4 and -8 in cancer patients make important contributions to the increased circulation of several cytokines and chemokines that promote angiogenesis and metastasis. Br. J. Cancer 2014, 110, 741–752. [Google Scholar] [CrossRef]
- Danguy, A.; Rorive, S.; Decaestecker, C.; Bronckart, Y.; Kaltner, H.; Hadari, Y.R.; Goren, R.; Zich, Y.; Petein, M.; Salmon, I.; et al. Immunohistochemical profile of galectin-8 expression in benign and malignant tumors of epithelial, mesenchymatous and adipous origins, and of the nervous system. Histol. Histopathol. 2001, 16, 861–868. [Google Scholar]
- Li, Z.L.; Prakash, P.; Buck, M. A “Tug of War” Maintains a Dynamic Protein-Membrane Complex: Molecular Dynamics Simulations of C-Raf RBD-CRD Bound to K-Ras4B at an Anionic Membrane. ACS Cent. Sci. 2018, 4, 298–305. [Google Scholar] [CrossRef]
- Carthew, R.W.; Sontheimer, E.J. Origins and Mechanisms of miRNAs and siRNAs. Cell 2009, 136, 642–655. [Google Scholar] [CrossRef]
- Pu, X.; Roth, J.A.; Hildebrandt, M.A.; Ye, Y.; Wei, H.; Minna, J.D.; Lippman, S.M.; Wu, X. MicroRNA-related genetic variants associated with clinical outcomes in early-stage non-small cell lung cancer patients. Cancer Res. 2013, 73, 1867–1875. [Google Scholar] [CrossRef]
- Xia, S.; Duan, W.; Liu, W.; Zhang, X.; Wang, Q. GRP78 in lung cancer. J. Transl. Med. 2021, 19, 118. [Google Scholar] [CrossRef]
- Ose, R.; Yanagawa, T.; Ikeda, S.; Ohara, O.; Koga, H. PCDH24-induced contact inhibition involves downregulation of beta-catenin signaling. Mol. Oncol. 2009, 3, 54–66. [Google Scholar] [CrossRef]
- Earl, L.A.; Bi, S.; Baum, L.G. N- and O-glycans modulate galectin-1 binding, CD45 signaling, and T cell death. J. Biol. Chem. 2010, 285, 2232–2244. [Google Scholar] [CrossRef]
- Iberg, C.A.; Hawiger, D. Natural and Induced Tolerogenic Dendritic Cells. J. Immunol. 2020, 204, 733–744. [Google Scholar] [CrossRef]
- Segura, E.; Touzot, M.; Bohineust, A.; Cappuccio, A.; Chiocchia, G.; Hosmalin, A.; Dalod, M.; Soumelis, V.; Amigorena, S. Human inflammatory dendritic cells induce Th17 cell differentiation. Immunity 2013, 38, 336–348. [Google Scholar] [CrossRef]
- Hedrick, C.C.; Malanchi, I. Neutrophils in cancer: Heterogeneous and multifaceted. Nat. Rev. Immunol. 2022, 22, 173–187. [Google Scholar] [CrossRef] [PubMed]
- Salazar, Y.; Zheng, X.; Brunn, D.; Raifer, H.; Picard, F.; Zhang, Y.; Winter, H.; Guenther, S.; Weigert, A.; Weigmann, B.; et al. Microenvironmental Th9 and Th17 lymphocytes induce metastatic spreading in lung cancer. J. Clin. Investig. 2020, 130, 3560–3575. [Google Scholar] [CrossRef]
- Benwell, C.J.; Johnson, R.T.; Taylor, J.; Price, C.A.; Robinson, S.D. Endothelial VEGFR Coreceptors Neuropilin-1 and Neuropilin-2 Are Essential for Tumor Angiogenesis. Cancer Res. Commun. 2022, 2, 1626–1640. [Google Scholar] [CrossRef] [PubMed]
- Bannoud, N.; Stupirski, J.C.; Cagnoni, A.J.; Hockl, P.F.; Perez Saez, J.M.; Garcia, P.A.; Mahmoud, Y.D.; Gambarte Tudela, J.; Scheidegger, M.A.; Marshall, A.; et al. Circulating galectin-1 delineates response to bevacizumab in melanoma patients and reprograms endothelial cell biology. Proc. Natl. Acad. Sci. USA 2023, 120, e2214350120. [Google Scholar] [CrossRef] [PubMed]
- Corral, J.M.; Puerto-Nevado, L.D.; Cedeno, M.; Rio-Vilarino, A.; Mahillo-Fernandez, I.; Galeano, C.; Banos, N.; Garcia-Foncillas, J.; Domine, M.; Cebrian, A. Galectin-1, a novel promising target for outcome prediction and treatment in SCLC. Biomed. Pharmacother. 2022, 156, 113987. [Google Scholar] [CrossRef]
- Cagnoni, A.J.; Giribaldi, M.L.; Blidner, A.G.; Cutine, A.M.; Gatto, S.G.; Morales, R.M.; Salatino, M.; Abba, M.C.; Croci, D.O.; Marino, K.V.; et al. Galectin-1 fosters an immunosuppressive microenvironment in colorectal cancer by reprogramming CD8(+) regulatory T cells. Proc. Natl. Acad. Sci. USA 2021, 118, e2102950118. [Google Scholar] [CrossRef]
- Zucchetti, M.; Bonezzi, K.; Frapolli, R.; Sala, F.; Borsotti, P.; Zangarini, M.; Cvitkovic, E.; Noel, K.; Ubezio, P.; Giavazzi, R.; et al. Pharmacokinetics and antineoplastic activity of galectin-1-targeting OTX008 in combination with sunitinib. Cancer Chemother. Pharmacol. 2013, 72, 879–887. [Google Scholar] [CrossRef]
- Gheysen, L.; Soumoy, L.; Trelcat, A.; Verset, L.; Journe, F.; Saussez, S. New Treatment Strategy Targeting Galectin-1 against Thyroid Cancer. Cells 2021, 10, 1112. [Google Scholar] [CrossRef] [PubMed]
- Stowell, S.R.; Arthur, C.M.; Mehta, P.; Slanina, K.A.; Blixt, O.; Leffler, H.; Smith, D.F.; Cummings, R.D. Galectin-1, -2, and -3 exhibit differential recognition of sialylated glycans and blood group antigens. J. Biol. Chem. 2008, 283, 10109–10123. [Google Scholar] [CrossRef]
- Ko, F.C.; Lee, K.W.; Tai, W.C.; Leung, Z.; Yam, J.W. Functional characterization of novel tumour suppressor galectin-2 in hepatocellular carcinoma. Eur. J. Cancer 2016, 61, S62. [Google Scholar]
- Calvisi, D.F.; Ladu, S.; Conner, E.A.; Seo, D.; Hsieh, J.T.; Factor, V.M.; Thorgeirsson, S.S. Inactivation of Ras GTPase-activating proteins promotes unrestrained activity of wild-type Ras in human liver cancer. J. Hepatol. 2011, 54, 311–319. [Google Scholar] [CrossRef]
- Calvisi, D.F.; Ladu, S.; Gorden, A.; Farina, M.; Conner, E.A.; Lee, J.S.; Factor, V.M.; Thorgeirsson, S.S. Ubiquitous activation of Ras and Jak/Stat pathways in human HCC. Gastroenterology 2006, 130, 1117–1128. [Google Scholar] [CrossRef]
- Ko, F.C.; Chan, L.K.; Tung, E.K.; Lowe, S.W.; Ng, I.O.; Yam, J.W. Akt phosphorylation of deleted in liver cancer 1 abrogates its suppression of liver cancer tumorigenesis and metastasis. Gastroenterology 2010, 139, 1397–1407. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Krasnitz, A.; Lucito, R.; Sordella, R.; Vanaelst, L.; Cordon-Cardo, C.; Singer, S.; Kuehnel, F.; Wigler, M.; Powers, S.; et al. DLC1 is a chromosome 8p tumor suppressor whose loss promotes hepatocellular carcinoma. Genes Dev. 2008, 22, 1439–1444. [Google Scholar] [CrossRef] [PubMed]
- Ji, P.; Gong, Y.; Jin, M.L.; Wu, H.L.; Guo, L.W.; Pei, Y.C.; Chai, W.J.; Jiang, Y.Z.; Liu, Y.; Ma, X.Y.; et al. In vivo multidimensional CRISPR screens identify Lgals2 as an immunotherapy target in triple-negative breast cancer. Sci. Adv. 2022, 8, eabl8247. [Google Scholar] [CrossRef]
- Than, N.G.; Romero, R.; Xu, Y.; Erez, O.; Xu, Z.; Bhatti, G.; Leavitt, R.; Chung, T.H.; El-Azzamy, H.; LaJeunesse, C.; et al. Evolutionary origins of the placental expression of chromosome 19 cluster galectins and their complex dysregulation in preeclampsia. Placenta 2014, 35, 855–865. [Google Scholar] [CrossRef]
- Liu, J.; Liu, J.; Lu, X. HOXA1 upregulation is associated with poor prognosis and tumor progression in breast cancer. Exp. Ther. Med. 2019, 17, 1896–1902. [Google Scholar] [CrossRef]
- Zha, T.Z.; Hu, B.S.; Yu, H.F.; Tan, Y.F.; Zhang, Y.; Zhang, K. Overexpression of HOXA1 correlates with poor prognosis in patients with hepatocellular carcinoma. Tumour Biol. 2012, 33, 2125–2134. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Xu, Y.; Wang, P.; Xu, Y.; Jin, P.; Wu, Z.; Qian, Y.; Bai, L.; Dong, M. Galectin-14 Promotes Trophoblast Migration and Invasion by Upregulating the Expression of MMP-9 and N-Cadherin. Front Cell Dev. Biol. 2021, 9, 645658. [Google Scholar] [CrossRef] [PubMed]
- Kaminker, J.D.; Timoshenko, A.V. Expression, Regulation, and Functions of the Galectin-16 Gene in Human Cells and Tissues. Biomolecules 2021, 11, 1909. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.C.; Nesmelova, I.V.; Daragan, V.A.; Ippel, H.; Michalak, M.; Dregni, A.; Kaltner, H.; Kopitz, J.; Gabius, H.J.; Mayo, K.H. Pro4 prolyl peptide bond isomerization in human galectin-7 modulates the monomer-dimer equilibrum to affect function. Biochem. J. 2020, 477, 3147–3165. [Google Scholar] [CrossRef] [PubMed]
- Laderach, D.J.; Compagno, D. Inhibition of galectins in cancer: Biological challenges for their clinical application. Front. Immunol. 2022, 13, 1104625. [Google Scholar] [CrossRef] [PubMed]
- Bertuzzi, S.; Gimeno, A.; Martinez-Castillo, A.; Lete, M.G.; Delgado, S.; Airoldi, C.; Rodrigues Tavares, M.; Blahova, M.; Chytil, P.; Kren, V.; et al. Cross-Linking Effects Dictate the Preference of Galectins to Bind LacNAc-Decorated HPMA Copolymers. Int. J. Mol. Sci. 2021, 22, 6000. [Google Scholar] [CrossRef]
- Wang, T.; Cai, S.; Cheng, Y.; Zhang, W.; Wang, M.; Sun, H.; Guo, B.; Li, Z.; Xiao, Y.; Jiang, S. Discovery of Small-Molecule Inhibitors of the PD-1/PD-L1 Axis That Promote PD-L1 Internalization and Degradation. J. Med. Chem. 2022, 65, 3879–3893. [Google Scholar] [CrossRef]
- Croese, T.; Castellani, G.; Schwartz, M. Immune cell compartmentalization for brain surveillance and protection. Nat. Immunol. 2021, 22, 1083–1092. [Google Scholar] [CrossRef]
- Vafaei, S.; Zekiy, A.O.; Khanamir, R.A.; Zaman, B.A.; Ghayourvahdat, A.; Azimizonuzi, H.; Zamani, M. Combination therapy with immune checkpoint inhibitors (ICIs); a new frontier. Cancer Cell Int. 2022, 22, 2. [Google Scholar] [CrossRef]
- Eruslanov, E.B.; Singhal, S.; Albelda, S.M. Mouse versus Human Neutrophils in Cancer: A Major Knowledge Gap. Trends Cancer 2017, 3, 149–160. [Google Scholar] [CrossRef]
- Douam, F.; Ploss, A. A humanized “new-trophil” mouse to study early inflammatory processes. Proc. Natl. Acad. Sci. USA 2022, 119, e2216699119. [Google Scholar] [CrossRef] [PubMed]
- Socinski, M.A.; Jotte, R.M.; Cappuzzo, F.; Nishio, M.; Mok, T.S.K.; Reck, M.; Finley, G.G.; Kaul, M.D.; Yu, W.; Paranthaman, N.; et al. Association of Immune-Related Adverse Events With Efficacy of Atezolizumab in Patients With Non-Small Cell Lung Cancer: Pooled Analyses of the Phase 3 IMpower130, IMpower132, and IMpower150 Randomized Clinical Trials. JAMA Oncol. 2023, 9, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Engelhardt, B.; Vajkoczy, P.; Weller, R.O. The movers and shapers in immune privilege of the CNS. Nat. Immunol. 2017, 18, 123–131. [Google Scholar] [CrossRef] [PubMed]


| Target | Drug | Phase | Cancer Type | Intervention |
|---|---|---|---|---|
| Galectin-1 | OTX008 | I | Solid tumours | NCT01724320 Status unknown (updated: 2012) |
| Galectin-3 | Belapectin (GR-MD-02) | I | Metastatic melanoma | NCT02117362 Completed (updated: 2019) |
| I | Metastatic melanoma, NSCLC, HNSCC | NCT02575404 [21] Active (updated: 2022) | ||
| GB1211 | I | Healthy subjects | NCT03809052 [16] Completed (updated: 2021) | |
| I/II | NSCLC | NCT05240131 Recruiting (updated: 2023) | ||
| GCS-100 | I/II | Relapsed/Refractory diffuse large-B-cell lymphoma | NCT00776802 Withdrawn as funding issue (updated: 2013) | |
| GM-CT-01 | I | Breast, colorectal, head and neck, lung, prostate | NCT00054977 Completed (updated: 2012) | |
| PectaSol-C, modified citrus pectin (MCP) [22] | N/A | Non-cancer-related: study for high blood pressure control | NCT01960946 Completed (updated: 2021) | |
| Galactomannan/ ProLectin-M [23] | III | Non-cancer-related: antagonist for COVID-19 | NCT05096052 Recruiting (updated: 2022) | |
| Galectin-9 | LYT-200 (monoclonal antibody against galectin-9) | I | Acute myeloid leukaemia | NCT05829226 Recruiting (updated: 2023) |
| I/II | Metastatic cancer in head and neck, colorectal, pancreatic, or urothelial origins | NCT04666688 Recruiting (updated: 2023) |
| Cancer Type | Galectin-1 | Galectin-2 | Galectin-3 | Galectin-4 | Galectin-7 | Galectin-8 | Galectin-9 | Galectin-12 | Galectin-13 | Galectin-14 | Galectin-16 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Melanoma | + | + | + | + | |||||||
| Lungs | + | + | + | + | + | + | + | + | |||
| Glioma/ Neuroblastoma | + | + | + | + | + | + | |||||
| Leukaemia | + | + | |||||||||
| Myeloma | + | ||||||||||
| Lymphoma | + | + | + | ||||||||
| Thyroid | + | + | + | - | - | - | |||||
| Oral | + | + | + | + | |||||||
| Gastric | + | + | + | ||||||||
| Liver | + | ! | + | + | + | + | |||||
| Pancreas | + | + | + | + | |||||||
| Colorectal | + | + | + | + | + | + | + | + | |||
| Renal | + | + | + | + | |||||||
| Bladder | + | + | + | + | |||||||
| Breast | + | + | + | + | + | + | + | + | + | + | |
| Cervical | + | + | + | + | + | + | |||||
| Endometrial | + | ||||||||||
| Ovarian | + | + | + | + | + | + | + | ||||
| Prostate | + | + | + | + | |||||||
| Testis | + | + | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, F.C.F.; Yan, S.; Lee, K.W.; Lam, S.K.; Ho, J.C.M. Chimera and Tandem-Repeat Type Galectins: The New Targets for Cancer Immunotherapy. Biomolecules 2023, 13, 902. https://doi.org/10.3390/biom13060902
Ko FCF, Yan S, Lee KW, Lam SK, Ho JCM. Chimera and Tandem-Repeat Type Galectins: The New Targets for Cancer Immunotherapy. Biomolecules. 2023; 13(6):902. https://doi.org/10.3390/biom13060902
Chicago/Turabian StyleKo, Frankie Chi Fat, Sheng Yan, Ka Wai Lee, Sze Kwan Lam, and James Chung Man Ho. 2023. "Chimera and Tandem-Repeat Type Galectins: The New Targets for Cancer Immunotherapy" Biomolecules 13, no. 6: 902. https://doi.org/10.3390/biom13060902
APA StyleKo, F. C. F., Yan, S., Lee, K. W., Lam, S. K., & Ho, J. C. M. (2023). Chimera and Tandem-Repeat Type Galectins: The New Targets for Cancer Immunotherapy. Biomolecules, 13(6), 902. https://doi.org/10.3390/biom13060902






