Galectin-3’s Complex Interactions in Pancreatic Ductal Adenocarcinoma: From Cellular Signaling to Therapeutic Potential
Abstract
:1. Introduction
2. Galectin-3: Structure and Function in Cellular Biology
Galectin-3 in Various Carcinomas: Expression, Metastasis, and Prognostic Implications
3. Galectin-3 Expression in Pancreatic Ductal Adenocarcinoma: A Comprehensive Overview
Galectin-3 in Pancreatic Ductal Adenocarcinoma: Diagnostic and Prognostic Implications
4. Galectin-3 in Pancreatic Carcinoma: Apoptosis Resistance and Drug Sensitivity
5. Galectin-3 in Intracellular Signaling Pathways: Interactions and Implications in Pancreatic Cancer
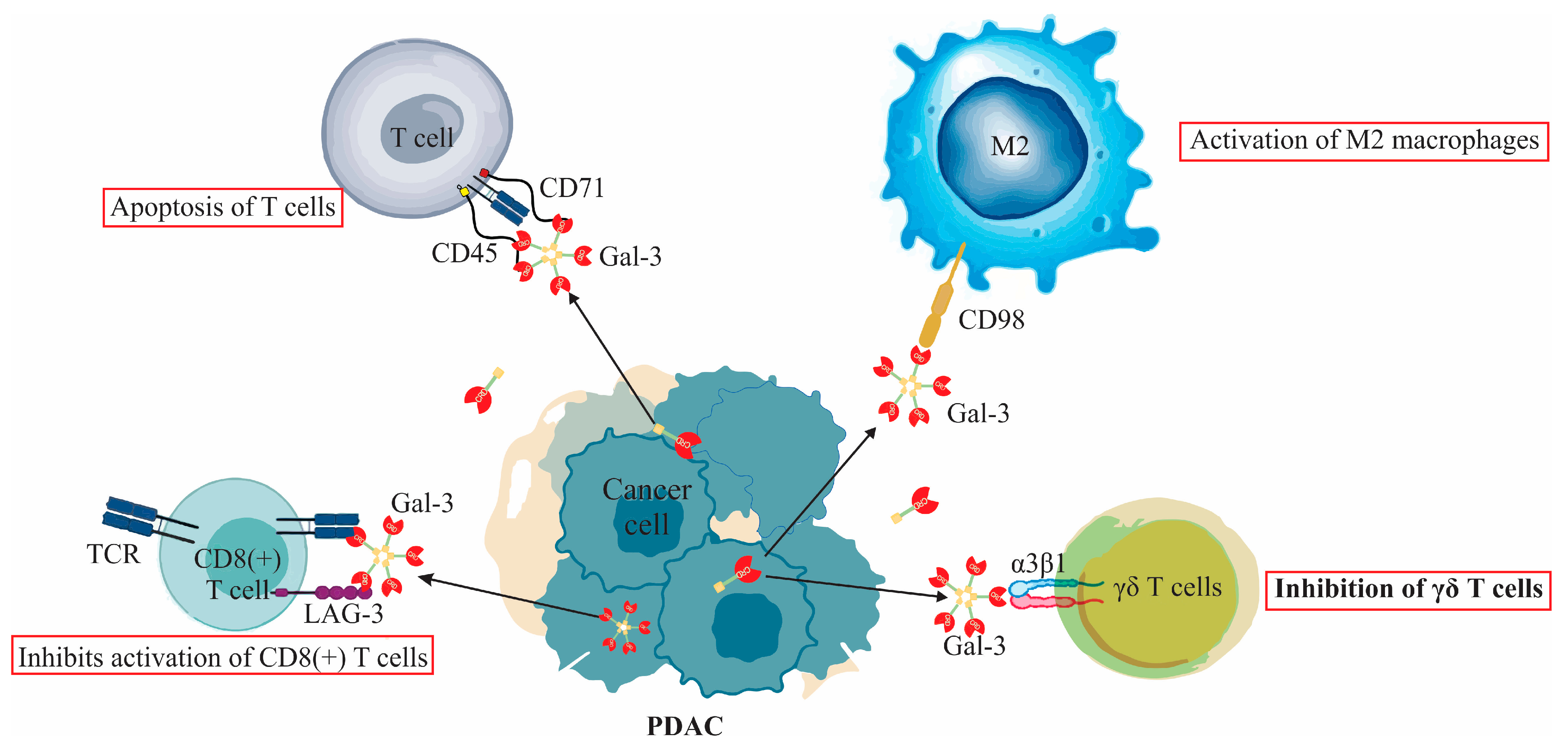
6. Galectin-3’s Interaction with the Immune Response in Pancreatic Cancer: A Dual Role
6.1. Galectin-3’s Interaction with T Lymphocytes and Its Role in Pancreatic Ductal Adenocarcinoma
6.2. Galectin-3’s Interaction with Macrophages and Its Role in Pancreatic Ductal Adenocarcinoma
7. Galectin-3 in the Tumor Microenvironment of Pancreatic Ductal Adenocarcinoma
7.1. Galectin-3 Interaction with Pancreatic Stellate Cells (PSCs) and Cancer-Associated Fibroblasts (CAFs) in PDAC
7.2. Galectin-3 Interaction with Ischemia and Nutrient Deprivation in PDAC
8. Galectin-3 Binding Protein in Pancreatic Ductal Adenocarcinoma: Function, Regulation, and Therapeutic Potential
9. Galectin-3 in PDAC: Therapeutic Implications and Future Endeavors
10. Future Directions in Researching Gal-3 in PDAC
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adamska, A.; Domenichini, A.; Falasca, M. Pancreatic Ductal Adenocarcinoma: Current and Evolving Therapies. Int. J. Mol. Sci. 2017, 18, 1338. [Google Scholar] [CrossRef] [PubMed]
- Orth, M.; Metzger, P.; Gerum, S.; Mayerle, J.; Schneider, G.; Belka, C.; Schnurr, M.; Lauber, K. Pancreatic ductal adenocarcinoma: Biological hallmarks, current status, and future perspectives of combined modality treatment approaches. Radiat. Oncol. 2019, 14, 141. [Google Scholar] [CrossRef] [PubMed]
- Sarantis, P.; Koustas, E.; Papadimitropoulou, A.; Papavassiliou, A.G.; Karamouzis, M.V. Pancreatic ductal adenocarcinoma: Treatment hurdles, tumor microenvironment and immunotherapy. World J. Gastrointest. Oncol. 2020, 12, 173–181. [Google Scholar] [CrossRef]
- Michalak, N.; Małecka-Wojciesko, E. Modifiable Pancreatic Ductal Adenocarcinoma (PDAC) Risk Factors. J. Clin. Med. 2023, 12, 4318. [Google Scholar] [CrossRef]
- Xie, D.; Xie, K. Pancreatic cancer stromal biology and therapy. Genes. Dis. 2015, 2, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Pandol, S.; Edderkaoui, M.; Gukovsky, I.; Lugea, A.; Gukovskaya, A. Desmoplasia of pancreatic ductal adenocarcinoma. Clin. Gastroenterol. Hepatol. 2009, 7, S44–S47. [Google Scholar] [CrossRef]
- Maneshi, P.; Mason, J.; Dongre, M.; Öhlund, D. Targeting Tumor-Stromal Interactions in Pancreatic Cancer: Impact of Collagens and Mechanical Traits. Front. Cell Dev. Biol. 2021, 9, 787485. [Google Scholar] [CrossRef]
- De Simoni, O.; Dal Santo, L.; Scarpa, M.; Munari, G.; Spolverato, Y.C.; Scapinello, A.; Lonardi, S.; Soldà, C.; Bergamo, F.; Fantin, A.; et al. Role of Immune Microenvironment in Pancreatic Ductal Adenocarcinoma: Could It Be Considered a Predictor of Prognosis? Curr. Oncol. 2023, 30, 5515–5528. [Google Scholar] [CrossRef]
- Martinez-Bosch, N.; Vinaixa, J.; Navarro, P. Immune Evasion in Pancreatic Cancer: From Mechanisms to Therapy. Cancers 2018, 10, 6. [Google Scholar] [CrossRef]
- Goulart, M.R.; Stasinos, K.; Fincham, R.E.A.; Delvecchio, F.R.; Kocher, H.M. T cells in pancreatic cancer stroma. World J. Gastroenterol. 2021, 27, 7956–7968. [Google Scholar] [CrossRef]
- Bowers, J.S.; Bailey, S.R.; Rubinstein, M.P.; Paulos, C.M.; Camp, E.R. Genomics meets immunity in pancreatic cancer: Current research and future directions for pancreatic adenocarcinoma immunotherapy. Oncol. Rev. 2019, 13, 430. [Google Scholar] [CrossRef] [PubMed]
- Hughes, R.C. Galectins. In Encyclopedia of Biological Chemistry; Lennarz, W.J., Lane, M.D., Eds.; Elsevier: New York, NY, USA, 2004; pp. 171–174. [Google Scholar]
- Vasta, G.R. Chapter 8—Lectins as Innate Immune Recognition Factors: Structural, Functional, and Evolutionary Aspects. In The Evolution of the Immune System; Malagoli, D., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 205–224. [Google Scholar]
- Compagno, D.; Tiraboschi, C.; Garcia, J.D.; Rondón, Y.; Corapi, E.; Velazquez, C.; Laderach, D.J. Galectins as Checkpoints of the Immune System in Cancers, Their Clinical Relevance, and Implication in Clinical Trials. Biomolecules 2020, 10, 750. [Google Scholar] [CrossRef] [PubMed]
- Johannes, L.; Jacob, R.; Leffler, H. Galectins at a glance. J. Cell Sci. 2018, 131, jcs208884. [Google Scholar] [CrossRef] [PubMed]
- Sciacchitano, S.; Lavra, L.; Morgante, A.; Ulivieri, A.; Magi, F.; De Francesco, G.P.; Bellotti, C.; Salehi, L.B.; Ricci, A. Galectin-3: One Molecule for an Alphabet of Diseases, from A to Z. Int. J. Mol. Sci. 2018, 19, 379. [Google Scholar] [CrossRef]
- Stojanovic, B.S.; Stojanovic, B.; Milovanovic, J.; Arsenijević, A.; Dimitrijevic Stojanovic, M.; Arsenijevic, N.; Milovanovic, M. The Pivotal Role of Galectin-3 in Viral Infection: A Multifaceted Player in Host-Pathogen Interactions. Int. J. Mol. Sci. 2023, 24, 9617. [Google Scholar] [CrossRef]
- Radosavljevic, G.; Volarevic, V.; Jovanovic, I.; Milovanovic, M.; Pejnovic, N.; Arsenijevic, N.; Hsu, D.K.; Lukic, M.L. The roles of Galectin-3 in autoimmunity and tumor progression. Immunol. Res. 2012, 52, 100–110. [Google Scholar] [CrossRef]
- Krześlak, A.; Lipińska, A. Galectin-3 as a multifunctional protein. Cell Mol. Biol. Lett. 2004, 9, 305–328. [Google Scholar]
- Nangia-Makker, P.; Hogan, V.; Balan, V.; Raz, A. Chimeric galectin-3 and collagens: Biomarkers and potential therapeutic targets in fibroproliferative diseases. J. Biol. Chem. 2022, 298, 102622. [Google Scholar] [CrossRef]
- Akahani, S.; Nangia-Makker, P.; Inohara, H.; Kim, H.R.; Raz, A. Galectin-3: A novel antiapoptotic molecule with a functional BH1 (NWGR) domain of Bcl-2 family. Cancer Res. 1997, 57, 5272–5276. [Google Scholar]
- Funasaka, T.; Raz, A.; Nangia-Makker, P. Nuclear transport of galectin-3 and its therapeutic implications. Semin. Cancer Biol. 2014, 27, 30–38. [Google Scholar] [CrossRef]
- Yu, F.; Finley, R.L.; Raz, A.; Kim, H.-R.C. Galectin-3 Translocates to the Perinuclear Membranes and Inhibits Cytochrome c Release from the Mitochondria: A Role for Synexin in Galectin-3 Translocation. J. Biol. Chem. 2002, 277, 15819–15827. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Chun, K.H. Non-classical role of Galectin-3 in cancer progression: Translocation to nucleus by carbohydrate-recognition independent manner. BMB Rep. 2020, 53, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Fukumori, T.; Oka, N.; Takenaka, Y.; Nangia-Makker, P.; Elsamman, E.; Kasai, T.; Shono, M.; Kanayama, H.-o.; Ellerhorst, J.; Lotan, R.; et al. Galectin-3 Regulates Mitochondrial Stability and Antiapoptotic Function in Response to Anticancer Drug in Prostate Cancer. Cancer Res. 2006, 66, 3114–3119. [Google Scholar] [CrossRef] [PubMed]
- Bieg, D.; Sypniewski, D.; Nowak, E.; Bednarek, I. MiR-424-3p suppresses galectin-3 expression and sensitizes ovarian cancer cells to cisplatin. Arch. Gynecol. Obstet. 2019, 299, 1077–1087. [Google Scholar] [CrossRef] [PubMed]
- Stojanovic, B.; Milovanovic, J.; Arsenijevic, A.; Stojanovic, B.; Strazic Geljic, I.; Arsenijevic, N.; Jonjic, S.; Lukic, M.L.; Milovanovic, M. Galectin-3 Deficiency Facilitates TNF-α-Dependent Hepatocyte Death and Liver Inflammation in MCMV Infection. Front. Microbiol. 2019, 10, 185. [Google Scholar] [CrossRef]
- Hsu, D.K.; Yang, R.Y.; Pan, Z.; Yu, L.; Salomon, D.R.; Fung-Leung, W.P.; Liu, F.T. Targeted disruption of the galectin-3 gene results in attenuated peritoneal inflammatory responses. Am. J. Pathol. 2000, 156, 1073–1083. [Google Scholar] [CrossRef]
- Manero-Rupérez, N.; Martínez-Bosch, N.; Barranco, L.E.; Visa, L.; Navarro, P. The Galectin Family as Molecular Targets: Hopes for Defeating Pancreatic Cancer. Cells 2020, 9, 689. [Google Scholar] [CrossRef]
- Ahmed, H.; AlSadek, D.M. Galectin-3 as a Potential Target to Prevent Cancer Metastasis. Clin. Med. Insights Oncol. 2015, 9, 113–121. [Google Scholar] [CrossRef]
- Nakahara, S.; Oka, N.; Raz, A. On the role of galectin-3 in cancer apoptosis. Apoptosis 2005, 10, 267–275. [Google Scholar] [CrossRef]
- Sedlář, A.; Trávníčková, M.; Bojarová, P.; Vlachová, M.; Slámová, K.; Křen, V.; Bačáková, L. Interaction between Galectin-3 and Integrins Mediates Cell-Matrix Adhesion in Endothelial Cells and Mesenchymal Stem Cells. Int. J. Mol. Sci. 2021, 22, 5144. [Google Scholar] [CrossRef]
- Farhad, M.; Rolig, A.S.; Redmond, W.L. The role of Galectin-3 in modulating tumor growth and immunosuppression within the tumor microenvironment. Oncoimmunology 2018, 7, e1434467. [Google Scholar] [CrossRef]
- Petrovic, S.; Radosavljevic, G.D.; Pantic, J.; Jovanovic, I.; Jankovic, N.; Arsenijevic, N. Circulating and tissue galectin-1 and galectin-3 in colorectal carcinoma: Association with clinicopathological parameters, serum CEA, IL-17 and IL23. J. Buon 2016, 21, 941–949. [Google Scholar] [PubMed]
- Jovanovic, M.; Gajovic, N.; Zdravkovic, N.; Jovanovic, M.; Jurisevic, M.; Vojvodic, D.; Maric, V.; Arsenijevic, A.; Jovanovic, I. Fecal Galectin-3: A New Promising Biomarker for Severity and Progression of Colorectal Carcinoma. Mediat. Inflamm. 2018, 2018, 8031328. [Google Scholar] [CrossRef] [PubMed]
- Blanda, V.; Bracale, U.M.; Di Taranto, M.D.; Fortunato, G. Galectin-3 in Cardiovascular Diseases. Int. J. Mol. Sci. 2020, 21, 9232. [Google Scholar] [CrossRef] [PubMed]
- Sumana, B.S.; Shashidhar, S.; Shivarudrappa, A.S. Galectin-3 Immunohistochemical Expression in Thyroid Neoplasms. J. Clin. Diagn. Res. 2015, 9, EC07–EC11. [Google Scholar] [CrossRef]
- Al-Maghrabi, J.A.; Khabaz, M.N. Clinical significance of galectin-3 expression in urinary bladder carcinoma. J. Int. Med. Res. 2023, 51, 3000605231153323. [Google Scholar] [CrossRef]
- Chang, W.A.; Tsai, M.J.; Kuo, P.L.; Hung, J.Y. Role of galectins in lung cancer (Review). Oncol. Lett. 2017, 14, 5077–5084. [Google Scholar] [CrossRef]
- Zhang, H.; Luo, M.; Liang, X.; Wang, D.; Gu, X.; Duan, C.; Gu, H.; Chen, G.; Zhao, X.; Zhao, Z.; et al. Galectin-3 as a marker and potential therapeutic target in breast cancer. PLoS ONE 2014, 9, e103482. [Google Scholar] [CrossRef]
- Radosavljevic, G.; Jovanovic, I.; Majstorovic, I.; Mitrovic, M.; Lisnic, V.J.; Arsenijevic, N.; Jonjic, S.; Lukic, M.L. Deletion of galectin-3 in the host attenuates metastasis of murine melanoma by modulating tumor adhesion and NK cell activity. Clin. Exp. Metastasis 2011, 28, 451–462. [Google Scholar] [CrossRef]
- Pang, Y.; Maxwell, E.; Sindrewicz-Goral, P.; Shapanis, A.; Li, S.; Morgan, M.; Yu, L.-G. Galectin-3 Is a Natural Binding Ligand of MCAM (CD146, MUC18) in Melanoma Cells and Their Interaction Promotes Melanoma Progression. Biomolecules 2022, 12, 1451. [Google Scholar] [CrossRef]
- Wang, L.; Du, D.D.; Zheng, Z.X.; Shang, P.F.; Yang, X.X.; Sun, C.; Wang, X.Y.; Tang, Y.J.; Guo, X.L. Circulating galectin-3 promotes tumor-endothelium-adhesion by upregulating ICAM-1 in endothelium-derived extracellular vesicles. Front. Pharmacol. 2022, 13, 979474. [Google Scholar] [CrossRef] [PubMed]
- Aureli, A.; Del Cornò, M.; Marziani, B.; Gessani, S.; Conti, L. Highlights on the Role of Galectin-3 in Colorectal Cancer and the Preventive/Therapeutic Potential of Food-Derived Inhibitors. Cancers 2022, 15, 52. [Google Scholar] [CrossRef] [PubMed]
- Debray, C.; Vereecken, P.; Belot, N.; Teillard, P.; Brion, J.-P.; Pandolfo, M.; Pochet, R. Multifaceted role of galectin-3 on human glioblastoma cell motility. Biochem. Biophys. Res. Commun. 2004, 325, 1393–1398. [Google Scholar] [CrossRef]
- Setayesh, T.; Colquhoun, S.D.; Wan, Y.Y. Overexpression of Galectin-1 and Galectin-3 in hepatocellular carcinoma. Liver Res. 2020, 4, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Shimada, C.; Xu, R.; Al-Alem, L.; Stasenko, M.; Spriggs, D.R.; Rueda, B.R. Galectins and Ovarian Cancer. Cancers 2020, 12, 1421. [Google Scholar] [CrossRef]
- Castronovo, V.; Van Den Brûle, F.A.; Jackers, P.; Clausse, N.; Liu, F.T.; Gillet, C.; Sobel, M.E. Decreased expression of galectin-3 is associated with progression of human breast cancer. J. Pathol. 1996, 179, 43–48. [Google Scholar] [CrossRef]
- Caputo, S.; Grioni, M.; Brambillasca, C.S.; Monno, A.; Brevi, A.; Freschi, M.; Piras, I.S.; Elia, A.R.; Pieri, V.; Baccega, T.; et al. Galectin-3 in Prostate Cancer Stem-Like Cells Is Immunosuppressive and Drives Early Metastasis. Front. Immunol. 2020, 11, 1820. [Google Scholar] [CrossRef]
- Schaffert, C.; Pour, P.M.; Chaney, W.G. Localization of galectin-3 in normal and diseased pancreatic tissue. Int. J. Pancreatol. 1998, 23, 1–9. [Google Scholar] [CrossRef]
- Jiang, K.; Lawson, D.; Cohen, C.; Siddiqui, M.T. Galectin-3 and PTEN expression in pancreatic ductal adenocarcinoma, pancreatic neuroendocrine neoplasms and gastrointestinal tumors on fine-needle aspiration cytology. Acta Cytol. 2014, 58, 281–287. [Google Scholar] [CrossRef]
- Hann, A.; Gruner, A.; Chen, Y.; Gress, T.M.; Buchholz, M. Comprehensive analysis of cellular galectin-3 reveals no consistent oncogenic function in pancreatic cancer cells. PLoS ONE 2011, 6, e20859. [Google Scholar] [CrossRef]
- Berberat, P.O.; Friess, H.; Wang, L.; Zhu, Z.; Bley, T.; Frigeri, L.; Zimmermann, A.; Büchler, M.W. Comparative analysis of galectins in primary tumors and tumor metastasis in human pancreatic cancer. J. Histochem. Cytochem. 2001, 49, 539–549. [Google Scholar] [CrossRef] [PubMed]
- da Silva Filho, A.F.; Tavares, L.B.; Pitta, M.G.R.; Beltrão, E.I.C.; Rêgo, M. Galectin-3 is modulated in pancreatic cancer cells under hypoxia and nutrient deprivation. Biol. Chem. 2020, 401, 1153–1165. [Google Scholar] [CrossRef] [PubMed]
- Yi, N.; Zhao, X.; Ji, J.; Xu, M.; Jiao, Y.; Qian, T.; Zhu, S.; Jiang, F.; Chen, J.; Xiao, M. Serum galectin-3 as a biomarker for screening, early diagnosis, prognosis and therapeutic effect evaluation of pancreatic cancer. J. Cell Mol. Med. 2020, 24, 11583–11591. [Google Scholar] [CrossRef] [PubMed]
- Shimura, T.; Shibata, M.; Gonda, K.; Kofunato, Y.; Okada, R.; Ishigame, T.; Kimura, T.; Kenjo, A.; Kono, K.; Marubashi, S. Significance of Circulating Galectin-3 in Patients with Pancreatobiliary Cancer. Anticancer Res. 2017, 37, 4979–4986. [Google Scholar] [CrossRef]
- Sun, Q.; Zhang, Y.; Liu, M.; Ye, Z.; Yu, X.; Xu, X.; Qin, Y. Prognostic and diagnostic significance of galectins in pancreatic cancer: A systematic review and meta-analysis. Cancer Cell Int. 2019, 19, 309. [Google Scholar] [CrossRef] [PubMed]
- Modi, S.; Kir, D.; Banerjee, S.; Saluja, A. Control of Apoptosis in Treatment and Biology of Pancreatic Cancer. J. Cell Biochem. 2016, 117, 279–288. [Google Scholar] [CrossRef]
- Kobayashi, T.; Shimura, T.; Yajima, T.; Kubo, N.; Araki, K.; Wada, W.; Tsutsumi, S.; Suzuki, H.; Kuwano, H.; Raz, A. Transient silencing of galectin-3 expression promotes both in vitro and in vivo drug-induced apoptosis of human pancreatic carcinoma cells. Clin. Exp. Metastasis 2011, 28, 367–376. [Google Scholar] [CrossRef]
- Takenaka, Y.; Fukumori, T.; Yoshii, T.; Oka, N.; Inohara, H.; Kim, H.R.; Bresalier, R.S.; Raz, A. Nuclear export of phosphorylated galectin-3 regulates its antiapoptotic activity in response to chemotherapeutic drugs. Mol. Cell Biol. 2004, 24, 4395–4406. [Google Scholar] [CrossRef]
- da Silva Filho, A.F.; de Sousa, L.M.; Consonni, S.R.; da Rocha Pitta, M.G.; Carvalho, H.F.; de Melo Rêgo, M.J.B. Galectin-3 Expression in Pancreatic Cell Lines Under Distinct Autophagy-Inducing Stimulus. Microsc. Microanal. 2020, 26, 1187–1197. [Google Scholar] [CrossRef]
- Yao, Y.; Zhou, L.; Liao, W.; Chen, H.; Du, Z.; Shao, C.; Wang, P.; Ding, K. HH1-1, a novel Galectin-3 inhibitor, exerts anti-pancreatic cancer activity by blocking Galectin-3/EGFR/AKT/FOXO3 signaling pathway. Carbohydr. Polym. 2019, 204, 111–123. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, P.; Qin, Y.; Cong, Q.; Shao, C.; Du, Z.; Ni, X.; Li, P.; Ding, K. RN1, a novel galectin-3 inhibitor, inhibits pancreatic cancer cell growth in vitro and in vivo via blocking galectin-3 associated signaling pathways. Oncogene 2017, 36, 1297–1308. [Google Scholar] [CrossRef]
- Zhao, W.; Ajani, J.A.; Sushovan, G.; Ochi, N.; Hwang, R.; Hafley, M.; Johnson, R.L.; Bresalier, R.S.; Logsdon, C.D.; Zhang, Z.; et al. Galectin-3 Mediates Tumor Cell-Stroma Interactions by Activating Pancreatic Stellate Cells to Produce Cytokines via Integrin Signaling. Gastroenterology 2018, 154, 1524–1537. [Google Scholar] [CrossRef]
- Song, S.; Ji, B.; Ramachandran, V.; Wang, H.; Hafley, M.; Logsdon, C.; Bresalier, R.S. Overexpressed galectin-3 in pancreatic cancer induces cell proliferation and invasion by binding Ras and activating Ras signaling. PLoS ONE 2012, 7, e42699. [Google Scholar] [CrossRef]
- Takaori, K.; Hruban, R.H.; Maitra, A.; Tanigawa, N. Pancreatic intraepithelial neoplasia. Pancreas 2004, 28, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Shimura, T.; Yajima, T.; Kubo, N.; Araki, K.; Tsutsumi, S.; Suzuki, H.; Kuwano, H.; Raz, A. Transient gene silencing of galectin-3 suppresses pancreatic cancer cell migration and invasion through degradation of β-catenin. Int. J. Cancer 2011, 129, 2775–2786. [Google Scholar] [CrossRef] [PubMed]
- Merlin, J.; Stechly, L.; de Beaucé, S.; Monté, D.; Leteurtre, E.; van Seuningen, I.; Huet, G.; Pigny, P. Galectin-3 regulates MUC1 and EGFR cellular distribution and EGFR downstream pathways in pancreatic cancer cells. Oncogene 2011, 30, 2514–2525. [Google Scholar] [CrossRef] [PubMed]
- Coppin, L.; Vincent, A.; Frénois, F.; Duchêne, B.; Lahdaoui, F.; Stechly, L.; Renaud, F.; Villenet, C.; Van Seuningen, I.; Leteurtre, E.; et al. Galectin-3 is a non-classic RNA binding protein that stabilizes the mucin MUC4 mRNA in the cytoplasm of cancer cells. Sci. Rep. 2017, 7, 43927. [Google Scholar] [CrossRef]
- Stojanovic, B.; Jovanovic, I.; Stojanovic, B.S.; Stojanovic, M.D.; Gajovic, N.; Radosavljevic, G.; Pantic, J.; Arsenijevic, N.; Lukic, M.L. Deletion of Galectin-3 attenuates acute pancreatitis in mice by affecting activation of innate inflammatory cells. Eur. J. Immunol. 2019, 49, 940–946. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Zhang, W.; Sha, G.; Wang, D.; Tang, D. Galectins Are Central Mediators of Immune Escape in Pancreatic Ductal Adenocarcinoma. Cancers 2022, 14, 5475. [Google Scholar] [CrossRef]
- Tang, D.; Yuan, Z.; Xue, X.; Lu, Z.; Zhang, Y.; Wang, H.; Chen, M.; An, Y.; Wei, J.; Zhu, Y.; et al. High expression of Galectin-1 in pancreatic stellate cells plays a role in the development and maintenance of an immunosuppressive microenvironment in pancreatic cancer. Int. J. Cancer 2012, 130, 2337–2348. [Google Scholar] [CrossRef]
- Gonnermann, D.; Oberg, H.H.; Lettau, M.; Peipp, M.; Bauerschlag, D.; Sebens, S.; Kabelitz, D.; Wesch, D. Galectin-3 Released by Pancreatic Ductal Adenocarcinoma Suppresses γδ T Cell Proliferation but Not Their Cytotoxicity. Front. Immunol. 2020, 11, 1328. [Google Scholar] [CrossRef] [PubMed]
- Stojanovic, B.; Jovanovic, I.P.; Stojanovic, M.D.; Jovanovic, M.; Vekic, B.; Milosevic, B.; Cvetkovic, A.; Spasic, M.; Stojanovic, B.S. The Emerging Roles of the Adaptive Immune Response in Acute Pancreatitis. Cells 2023, 12, 1495. [Google Scholar] [CrossRef] [PubMed]
- MacKinnon, A.C.; Farnworth, S.L.; Hodkinson, P.S.; Henderson, N.C.; Atkinson, K.M.; Leffler, H.; Nilsson, U.J.; Haslett, C.; Forbes, S.J.; Sethi, T. Regulation of alternative macrophage activation by galectin-3. J. Immunol. 2008, 180, 2650–2658. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, A.C.; Andrade, L.N.; Bustos, S.O.; Chammas, R. Galectin-3 Determines Tumor Cell Adaptive Strategies in Stressed Tumor Microenvironments. Front. Oncol. 2016, 6, 127. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yu, C.; Liu, X.; Liu, B.; Wu, X.; Wu, J.; Yan, D.; Han, L.; Tang, Z.; Yuan, X.; et al. Intracellular galectin-3 is a lipopolysaccharide sensor that promotes glycolysis through mTORC1 activation. Nat. Commun. 2022, 13, 7578. [Google Scholar] [CrossRef]
- Choi, Y.S.; Kim, M.J.; Choi, E.A.; Kim, S.; Lee, E.J.; Park, M.J.; Kim, M.J.; Kim, Y.W.; Ahn, H.S.; Jung, J.Y.; et al. Antibody-mediated blockade for galectin-3 binding protein in tumor secretome abrogates PDAC metastasis. Proc. Natl. Acad. Sci. USA 2022, 119, e2119048119. [Google Scholar] [CrossRef]
- Nigjeh, E.N.; Chen, R.; Allen-Tamura, Y.; Brand, R.E.; Brentnall, T.A.; Pan, S. Spectral library-based glycopeptide analysis-detection of circulating galectin-3 binding protein in pancreatic cancer. Proteom. Clin. Appl. 2017, 11, 1700064. [Google Scholar] [CrossRef]
- Demotte, N.; Wieërs, G.; Van Der Smissen, P.; Moser, M.; Schmidt, C.; Thielemans, K.; Squifflet, J.-L.; Weynand, B.; Carrasco, J.; Lurquin, C.; et al. A Galectin-3 Ligand Corrects the Impaired Function of Human CD4 and CD8 Tumor-Infiltrating Lymphocytes and Favors Tumor Rejection in Mice. Cancer Res. 2010, 70, 7476–7488. [Google Scholar] [CrossRef]
- Mercanti, L.; Sindaco, M.; Mazzone, M.; Di Marcantonio, M.C.; Piscione, M.; Muraro, R.; Mincione, G. PDAC, the Influencer Cancer: Cross-Talk with Tumor Microenvironment and Connected Potential Therapy Strategies. Cancers 2023, 15, 2923. [Google Scholar] [CrossRef]
| Location/Function | Activity or Role |
|---|---|
| Cell–cell Adhesion | Involved in various cellular functions |
| Cytoplasm | Functions as an apoptosis inhibitor, regulates cytoplasm–nucleus trafficking |
| Nucleus | Acts as an mRNA-splicing promoter |
| Surface of Tumor Cells | Functions as an adhesion molecule, facilitates cell interactions |
| Extracellular Function | Aids immune cell migration and metastasis |
| Condition | Gal-3 Expression | Notes |
|---|---|---|
| Normal Pancreatic Tissue | Localized to ductal cells | Weaker expression in acinar cells |
| Chronic Pancreatitis | Over 95% expression | More uniform |
| Pancreatic Cancer | Over 95% expression | Mainly cytoplasmic, with weak nuclear staining |
| Pancreatic Neuroendocrine Tumors | Undetectable | Differentiation aspect in diagnosis |
| Gastrointestinal Stromal Tumors | Undetectable | Differentiation aspect in diagnosis |
| Category | Interaction with | Mechanisms and Actions | Impact on PDAC |
|---|---|---|---|
| Pancreatic Stellate Cells (PSCs) |
|
| Encourages tumor-stroma interactions; promotes PDAC progression |
|
| Increases potential for metastasis and invasion of surrounding tissues | |
|
| Facilitates PSC activation; contributes to fibrotic environment | |
|
| Modulates inflammation, contributing to a favorable tumor microenvironment | |
| Cancer-Associated Fibroblasts (CAFs) |
|
| Enhances connectivity between cellular elements within the tumor environment |
|
| Strengthens the supportive stromal network aiding in tumor growth and development | |
|
| Reinforces the structural integrity of the tumor, facilitating progression and survival |
| Aspect | Description |
|---|---|
| Role in PDAC | Involved in tumorigenesis, enhances Gal-3-mediated EGFR signaling, and stimulates the EMT process. |
| Effect on cMyc and EMT | Stimulates the increase in cMyc; facilitates EMT, affecting cancer metastasis. |
| Influence on Cell Growth | Essential for PDAC cell growth both in vitro and in vivo. |
| Manipulation of Expression |
|
| |
| Interaction with EGFR | Noteworthy interaction with EGFR; upregulation of cMyc via EGFR activation. |
| Therapeutic Potential | Targeting with Gal-3BP antibodies inhibits EGFR-Myc signaling and metastasis in preclinical models. |
| Expression in Tumor Tissue | Overexpression in PDAC tumor tissue; abnormal N-glycosylation levels in circulating Gal-3BP. |
| N-Glycosylation in Stage II | Elevation correlates with cancer neoplasm size; insights into glycan biosynthesis and immune response. |
| Therapeutic Strategy | Description | Ongoing Research and Clinical Efforts | Major Conclusions | References |
|---|---|---|---|---|
| Polysaccharide Inhibitors | Focuses on molecules like HH1-1 that hinder Gal-3 interactions and related signaling cascades. | Identification and optimization of molecules targeting Gal-3. | HH1-1 obstructs Galectin-3/EGFR/AKT/FOXO3 signaling, affecting various cellular activities. | [62] |
| Modified Citrus Pectin (MCP) | MCP challenges Gal-3’s dominance in the tumor microenvironment. | Preclinical testing for efficacy in mitigating tumor growth. | MCP holds potential in hampering tumor proliferation and metastasis. | [64] |
| Polysaccharides (e.g., RN1) | RN1, from Panax notoginseng, inhibits Gal-3 expression and disrupts its interactions. | Delve deeper into RN1’s interaction mechanisms and clinical efficacy. | RN1 disrupts various oncogenic pathways, highlighting its therapeutic potential. | [63] |
| Antibodies and RNA Interference | Precision-targeted strategies leveraging the specificity of antibodies and RNA interference mechanisms. | Refining molecular targets and delivery mechanisms. | Precision-based approach offers specificity against Gal-3. | [63] |
| Combination Therapies | Combining Gal-3 inhibitors with other treatments like immune checkpoint inhibitors or chemotherapy. | Investigating synergies and formulating clinical trial strategies. | Combination therapies have potential for amplified therapeutic outcomes. | [63] |
| Therapeutic Vaccines | Creating vaccines, like GCS-100, to stimulate immune response against Gal-3-presenting cells. | Development and optimization of Gal-3 targeted vaccines. | Vaccines can boost immune response leading to increased cytotoxic effects. | [80,81] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dimitrijevic Stojanovic, M.; Stojanovic, B.; Radosavljevic, I.; Kovacevic, V.; Jovanovic, I.; Stojanovic, B.S.; Prodanovic, N.; Stankovic, V.; Jocic, M.; Jovanovic, M. Galectin-3’s Complex Interactions in Pancreatic Ductal Adenocarcinoma: From Cellular Signaling to Therapeutic Potential. Biomolecules 2023, 13, 1500. https://doi.org/10.3390/biom13101500
Dimitrijevic Stojanovic M, Stojanovic B, Radosavljevic I, Kovacevic V, Jovanovic I, Stojanovic BS, Prodanovic N, Stankovic V, Jocic M, Jovanovic M. Galectin-3’s Complex Interactions in Pancreatic Ductal Adenocarcinoma: From Cellular Signaling to Therapeutic Potential. Biomolecules. 2023; 13(10):1500. https://doi.org/10.3390/biom13101500
Chicago/Turabian StyleDimitrijevic Stojanovic, Milica, Bojan Stojanovic, Ivan Radosavljevic, Vojin Kovacevic, Ivan Jovanovic, Bojana S. Stojanovic, Nikola Prodanovic, Vesna Stankovic, Miodrag Jocic, and Marina Jovanovic. 2023. "Galectin-3’s Complex Interactions in Pancreatic Ductal Adenocarcinoma: From Cellular Signaling to Therapeutic Potential" Biomolecules 13, no. 10: 1500. https://doi.org/10.3390/biom13101500
APA StyleDimitrijevic Stojanovic, M., Stojanovic, B., Radosavljevic, I., Kovacevic, V., Jovanovic, I., Stojanovic, B. S., Prodanovic, N., Stankovic, V., Jocic, M., & Jovanovic, M. (2023). Galectin-3’s Complex Interactions in Pancreatic Ductal Adenocarcinoma: From Cellular Signaling to Therapeutic Potential. Biomolecules, 13(10), 1500. https://doi.org/10.3390/biom13101500






