3D Organoids for Regenerative Endodontics
Abstract
1. Introduction
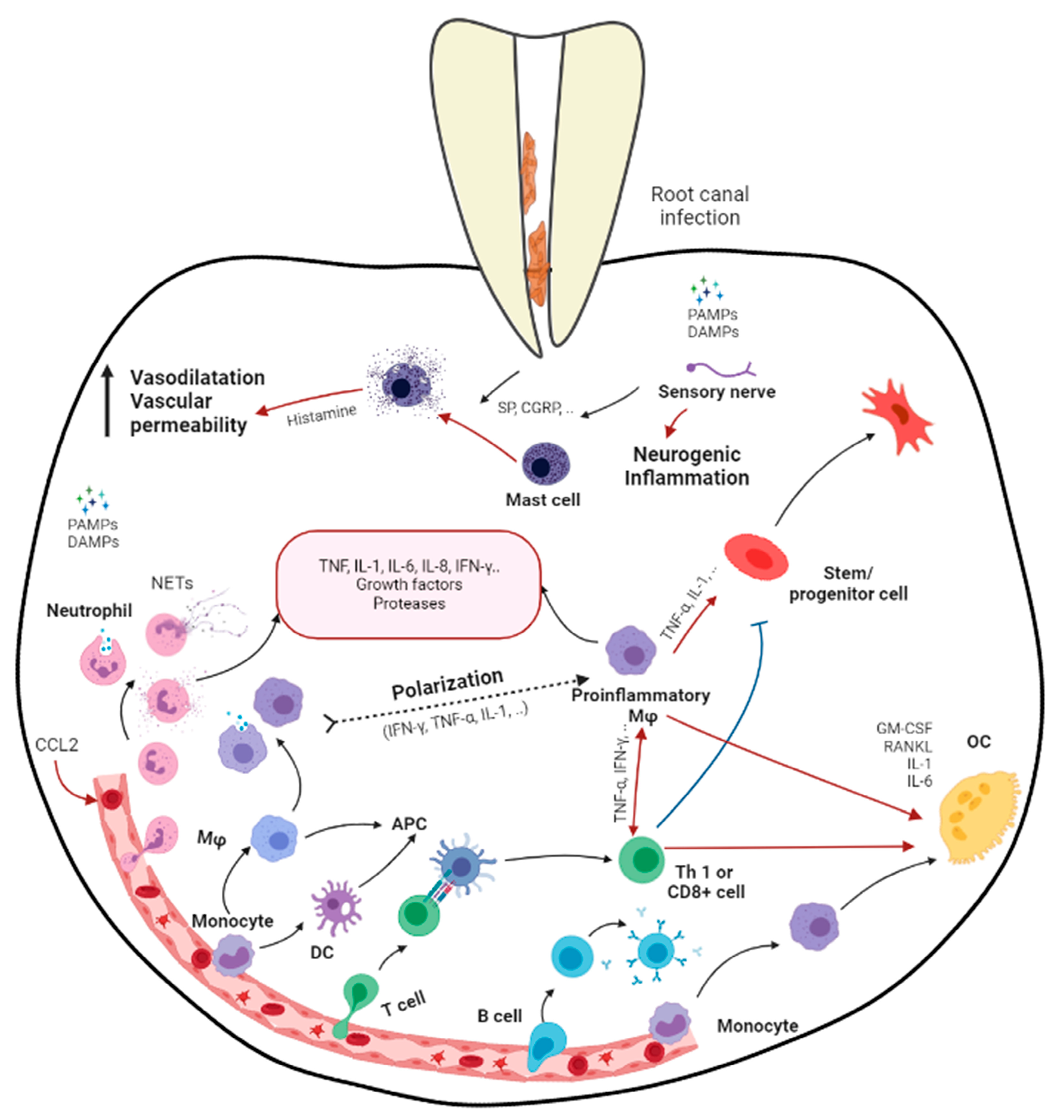
2. Apical Periodontitis
3. Effects of Apical Periodontitis in Immature Roots
4. Challenges in Regenerative Endodontic Procedures
5. Mesenchymal Stem Cells (MSCs) and Stem Cells from Apical Papilla (SCAP)
6. Macrophages
7. Mesenchymal Stem Cell–Macrophage Crosstalk
8. Three-Dimensional Cell Culture Models
| Cellular Characteristics | 2D | 3D |
|---|---|---|
| Exposure to medium/drug | Cells were equally exposed to nutrients/GF that were distributed in medium. | Nutrients/GF/drugs may not be able to fully penetrate the spheroid and reach cells near the core. |
| Morphology | Sheet-like and flat (stretched in monolayer) | Natural shape |
| Proliferation | Faster than in vivo | May be faster/slower compared to 2D-cultured cells depending on cell type/3D model system |
| Gene expression | Often display different gene/protein expression levels compared to in vivo | Often exhibit gene/protein expression profiles that are more similar to in vivo |
| Cell interaction | Paracrine/juxtacrine | Paracrine/juxtacrine/cell–matrix |
| Migration | Cells were attached on only 1 side, and less signaling was identified. | Cells were attached on all sides. More obstacles for migration but may be faster. Alteration of mechanism (more signaling). |
| Stage of cell cycle | Likely same stage (equally exposed to medium) | Spheroids contain proliferating, quiescent, hypoxic, and necrotic cells. |
| Drug sensitivity | Cells often succumbed to treatment/drug appeared to be very effective. | Cells often are more resistant to treatment and are better predictors of in vivo drug response |
| Article | Cell | Aim | 3D (Compared to 2D) |
|---|---|---|---|
| Riccio et al. [107] | Dental pulp stem cells (DPSC) | To characterize the in vitro osteogenic differentiation of DPSCs in 2D cultures and 3D biomaterials |
|
| Yamamoto et al. [108] | Mouse dental papilla cell (MDP) | To evaluate the effects of 3D spheroid culture on the phenotype of MDPs |
|
| Kawashima et al. [112] | Dental pulp mesenchymal stem cell (DPMSC) | To investigate the properties of DPMSCs cultured with different methods |
|
| Zhang et al. [116] | Dental pulp cell (DPC) | To compare the multilineage potential and extracellular matrix production of hDPC between conventional monolayer cultures and cellular spheroid cultures |
|
| Kim et al. [109] | Dental-follicle-derived mesenchymal stem cells (DFSC) | To analyze the stemness and in vitro osteogenic differentiation potential of 3D spheroid dental hDFSCs compared with conventional monolayer cultured MSCs |
|
| Xu et al. [110] | Dental pulp stem cell (DPSC) | To compare the preparation methods and preliminary mechanisms of differentiation of hDPSCs into insulin-producing cells (IPCs) under 2D or 3D culture conditions |
|
| Bu et al. [111] | Dental pulp stem cell (DPSC) | To evaluate cell morphology, cell viability, and the osteo-, adipo-, and chondrogenic differentiation potential of DPSCs cultured in 3D culture plates and 2D monolayer plate |
|
| Jeong et al. [113] | Periodontal ligament stem cells (PDLSC) | To compare the characteristics of PDLSCs cultured using 3D versus conventional 2D methods |
|
| Banavar et al. [114] | Periodontal-ligament-derived mesenchymal stem cells (PDLSCs) | To compare the effects of LPSs on PDLSCs in monolayer and 3D culture |
|
9. Current Investigations on Cellular Interactions in Regenerative Endodontics
| Article | Cells Interaction | Cell Culture | Highlight |
|---|---|---|---|
| Ding et al. [143] | SCAP–PBMC | 2D Direct contact co-culture and contact-free co-culture in transwell chamber |
|
| Tang and Ding [144] | DPSC–PBMC | 2D Direct contact co-culture and contact-free co-culture in transwell chamber |
|
| Dissanayaka et al. [145] | DPSC–endothelial cell | 2D Direct contact co-culture Direct contact co-culture on Matrigel-coated well |
|
| Yuan et al. [146] | SCAP–endothelial cell | 2D Direct contact co-culture on Matrigel-coated well |
|
| Yuan et al. [147] | SCAP–endothelial cell | 2D Direct contact co-culture on Matrigel-coated well Contact-free co-culture in transwell chamber |
|
| Lee et al. [148] | DPSC–THP-1 MQ | 2D Direct contact co-culture DPSCs directly on MQs |
|
| Omi et al. [149] | DPSC–RAW MQ | 2D DPSC-conditioned media on MQ |
|
| Jin and Kim [150] | DPSC–endothelial cell | 3D DPSC cultured in porous microcarrier co-cultured with EC in hydrogel |
|
| De Berdt et al. [151] | SCAP–BV-2 microglial cell SCAP–SCOS SCAP–SCOS–OPC | 2D Direct contact co-culture Direct contact co-culture Direct contact tri-culture |
|
| Whiting et al. [152] | DPSC–PBMC DPSC–NKC SCAP–PBMC SCAP–NKC | 2D Direct contact co-culture Direct contact co-culture |
|
| Tatic et al. [153] | SCAP–BV-2 microglial cell | 2D Contact-free co-culture in transwell chamber |
|
| Liu et al. [154] | SCAP–T cell | 2D Direct contact co-culture and contact-free co-culture in transwell chamber |
|
| Kukreti et al. [142] | SCAP–RAW MQ | 2D MQ-conditioned media on SCAP |
|
| Kanji et al. [155] | DPSC–RAW MQ | 2D Contact-free co-culture in transwell plate |
|
| Croci et al. [156] | DPSC–PBMC | 2D Direct contact co-culture PBMC directly on DPSC |
|
| Li et al. [128,129] | SCAP–THP-1 MQ | 3D Collagen type 1 Cell/matrix on each side with close contact |
|
| Anderson et al. [157] | DPSC–RAW MQ DPSC–LoVo gut epithelial cell | 2D Contact-free co-culture in transwell plate |
|
| Luo et al. [158] | SCAP–SCAP-derived neuronal cell | 3D Collagen type I Cell/matrix on each channel Without direct contact (contact-free) |
|
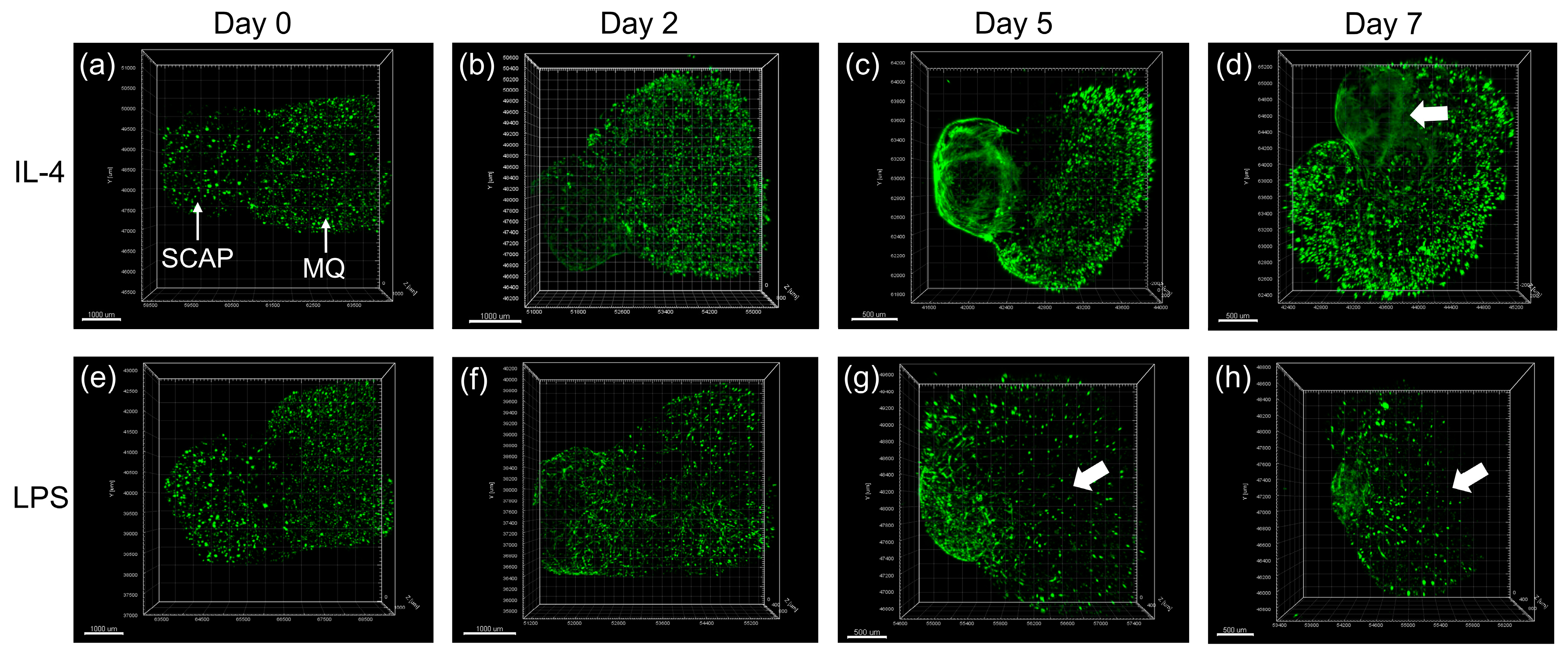
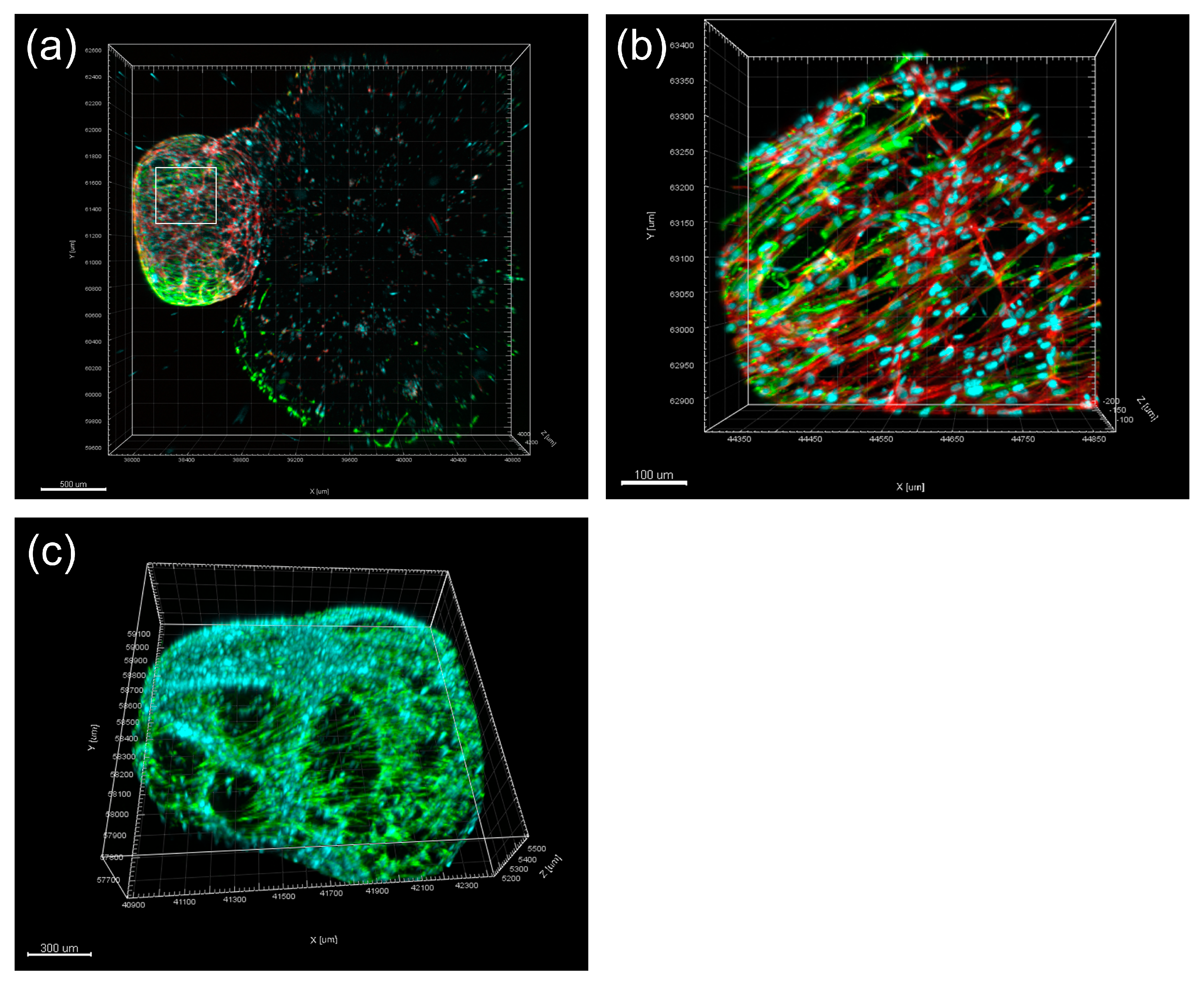
10. Dentin–Pulp Organoid Developed for Regenerative Endodontics
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kakehashi, S.; Stanley, H.R.; Fitzgerald, R.J. The effects of surgical exposures of dental pulps in germ-free and conventional laboratory rats. Oral. Surg. Oral. Med. Oral. Pathol. 1965, 20, 340–349. [Google Scholar] [CrossRef]
- Nair, P. Pathogenesis of Apical Periodontitis and the Causes of Endodontic Failures. Crit. Rev. Oral Biol. Med. 2004, 15, 348–381. [Google Scholar] [CrossRef] [PubMed]
- Márton, I.J.; Kiss, C. Protective and destructive immune reactions in apical periodontitis. Oral Microbiol. Immunol. 2000, 15, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Trope, M. Treatment of the Immature Tooth with a Non–Vital Pulp and Apical Periodontitis. Dent. Clin. N. Am. 2010, 54, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Danwittayakorn, S.; Banomyong, D.; Ongchavalit, L.; Ngoenwiwatkul, Y.; Porkaew, P. Comparison of the Effects of Intraradicular Materials on the Incidence of Fatal Root Fracture in Immature Teeth Treated with Mineral Trioxide Aggregate Apexification: A Retrospective Study. J. Endod. 2019, 45, 977–984.e1. [Google Scholar] [CrossRef]
- Diogenes, A.; Hargreaves, K.M. Microbial Modulation of Stem Cells and Future Directions in Regenerative Endodontics. J. Endod. 2017, 43, S95–S101. [Google Scholar] [CrossRef] [PubMed]
- Diogenes, A.; Ruparel, N.B.; Shiloah, Y.; Hargreaves, K.M. Regenerative endodontics: A way forward. J. Am. Dent. Assoc. 2016, 147, 372–380. [Google Scholar] [CrossRef]
- Stern, M.H.; Dreizen, S.; Mackler, B.F.; Selbst, A.G.; Levy, B.M. Quantitative analysis of cellular composition of human periapical granuloma. J. Endod. 1981, 7, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Piattelli, A.; Artese, L.; Rosini, S.; Quaranta, M.; Musiani, P. Immune cells in periapical granuloma: Morphological and immunohistochemical characterization. J. Endod. 1991, 17, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Liapatas, S.; Nakou, M.; Rontogianni, D. Inflammatory infiltrate of chronic periradicular lesions: An immunohistochemical study. Int. Endod. J. 2003, 36, 464–471. [Google Scholar] [CrossRef]
- Metzger, Z. Macrophages in periapical lesions. Endod. Dent. Traumatol. 2000, 16, 1–8. [Google Scholar] [CrossRef]
- Yumoto, H.; Hirao, K.; Hosokawa, Y.; Kuramoto, H.; Takegawa, D.; Nakanishi, T.; Matsuo, T. The roles of odontoblasts in dental pulp innate immunity. Jpn. Dent. Sci. Rev. 2018, 54, 105–117. [Google Scholar] [CrossRef]
- Henderson, B.; Poole, S.; Wilson, M. Bacterial modulins: A novel class of virulence factors which cause host tissue pathology by inducing cytokine synthesis. Microbiol Rev. 1996, 60, 316–341. [Google Scholar] [CrossRef] [PubMed]
- Marinho, A.C.; Martinho, F.C.; Leite, F.R.; Nascimento, G.G.; Gomes, B.P. Proinflammatory Activity of Primarily Infected Endodontic Content against Macrophages after Different Phases of the Root Canal Therapy. J. Endod. 2015, 41, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Stashenko, P.; Teles, R.; D’Souza, R. Periapical Inflammatory Responses and Their Modulation. Crit. Rev. Oral Biol. Med. 1998, 9, 498–521. [Google Scholar] [CrossRef] [PubMed]
- Akamine, A.; Hashiguchi, I.; Toriya, Y.; Maeda, K. Immunohistochemical examination on the localization of macrophages and plasma cells in induced rat periapical lesions. Endod. Dent. Traumatol. 1994, 10, 121–128. [Google Scholar] [CrossRef]
- Kawashima, N.; Okiji, T.; Kosaka, T.; Suda, H. Kinetics of macrophages and lymphoid cells during the development of experimentally induced periapical lesions in rat molars: A quantitative immunohistochemical study. J. Endod. 1996, 22, 311–316. [Google Scholar] [CrossRef]
- Rahimi, P.; Wang, C.Y.; Stashenko, P.; Lee, S.K.; Lorenzo, J.A.; Graves, D.T. Monocyte chemoattractant protein-1 expression and monocyte recruitment in osseous inflammation in the mouse. Endocrinology 1995, 136, 2752–2759. [Google Scholar] [CrossRef]
- Tani-Ishii, N.; Wang, C.-Y.; Stashenko, P. Immunolocalization of bone-resorptive cytokines in rat pulp and periapical lesions following surgical pulp exposure. Oral Microbiol. Immunol. 1995, 10, 213–219. [Google Scholar] [CrossRef]
- Stashenko, P.; Dewhirst, F.E.; Peros, W.J.; Kent, R.L.; Ago, J.M. Synergistic interactions between interleukin 1, tumor necrosis factor, and lymphotoxin in bone resorption. J. Immunol. 1987, 138, 1464–1468. [Google Scholar] [CrossRef]
- Saito, S.; Ngan, P.; Saito, M.; Kim, K.; Lanese, R.; Shanfeld, J.; Davidovitch, Z. Effects of cytokines on prostaglandin E and cAMP levels in human periodontal ligament fibroblasts in vitro. Arch. Oral Biol. 1990, 35, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Meikle, M.C.; Atkinson, S.J.; Ward, R.V.; Murphy, G.; Reynolds, J.J. Gingival fibroblasts degrade type I collagen films when stimulated with tumor necrosis factor and interleukin 1: Evidence that breakdown is mediated by metalloproteinases. J. Periodontal Res. 1898, 24, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T.R. Regulation of Immune Responses by T Cells with Different Cytokine Secretion Phenotypes: Role of a New Cytokine, Cytokine Synthesis Inhibitory Factor (IL10). Int. Arch. Allergy Appl. Immunol. 1991, 94, 110–115. [Google Scholar] [CrossRef]
- Takahashi, N.; Mundy, G.R.; Roodman, G.D. Recombinant human interferon-gamma inhibits formation of human osteoclast-like cells. J. Immunol. 1986, 137, 3544–3549. [Google Scholar] [CrossRef]
- Watanabe, K.; Tanaka, Y.; Morimoto, I.; Yahata, K.; Zeki, K.; Fujihira, T.; Yamashita, U.; Eto, S. Interleukin-4 as a potent inhibitor of bone resorption. Biochem. Biophys. Res. Commun. 1990, 172, 1035–1041. [Google Scholar] [CrossRef]
- Massagué, J.; Cheifetz, S.; Laiho, M.; Ralph, D.A.; Weis, F.M.; Zentella, A. Transforming growth factor-beta. Cancer Surv. 1992, 12, 81–103. [Google Scholar]
- Wahl, S.M. Transforming growth factor beta: The good, the bad, and the ugly. J. Exp. Med. 1994, 180, 1587–1590. [Google Scholar] [CrossRef]
- Espevik, T.; Waage, A.; Faxvaag, A.; Shalaby, M.R. Regulation of interleukin-2 and interleukin-6 production from T-cells: Involvement of interleukin-1 beta and transforming growth factor-beta. Cell Immunol. 1990, 126, 47–56. [Google Scholar] [CrossRef]
- Xu, X.; Zheng, L.; Yuan, Q.; Zhen, G.; Crane, J.L.; Zhou, X.; Cao, X. Transforming growth factor-β in stem cells and tissue homeostasis. Bone Res. 2018, 6, 2. [Google Scholar] [CrossRef]
- D’Souza, R.; Qin, C.D. Development of the Pulpodentin Complex. In Seltzer and Bender’s Dental Pulp; Hargreaves, K.M., Goodis, H.E., Eds.; Quintessence Publishing Co. Inc.: Chicago, IL, USA, 2002; pp. 13–40. [Google Scholar]
- Huang, G.T.-J.; Sonoyama, W.; Liu, Y.; Liu, H.; Wang, S.; Shi, S. The Hidden Treasure in Apical Papilla: The Potential Role in Pulp/Dentin Regeneration and BioRoot Engineering. J. Endod. 2008, 34, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Cvek, M. Prognosis of luxated non-vital maxillary incisors treated with calcium hydroxide and filled with gutta-percha. A retrospective clinical study. Endod. Dent. Traumatol. 1992, 8, 45–55. [Google Scholar] [CrossRef]
- Kahler, S.L.; Shetty, S.; Andreasen, F.M.; Kahler, B. The Effect of Long-term Dressing with Calcium Hydroxide on the Fracture Susceptibility of Teeth. J. Endod. 2018, 44, 464–469. [Google Scholar] [CrossRef]
- Ricucci, D.; Siqueira, J.F.; Loghin, S.; Lin, L.M. Pulp and apical tissue response to deep caries in immature teeth: A histologic and histobacteriologic study. J. Dent. 2017, 56, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Horan, M.A.; Ashcroft, G.S. Ageing, Defence Mechanisms and the Immune System. Age Ageing 1997, 26, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Yoo, Y.-J.; Oh, J.-H.; Lee, W.; Woo, K.M. Regenerative Characteristics of Apical Papilla–derived Cells from Immature Teeth with Pulpal and Periapical Pathosis. J. Endod. 2016, 42, 1626–1632. [Google Scholar] [CrossRef] [PubMed]
- Chrepa, V.; Pitcher, B.; Henry, M.A.; Diogenes, A. Survival of the Apical Papilla and Its Resident Stem Cells in a Case of Advanced Pulpal Necrosis and Apical Periodontitis. J. Endod. 2017, 43, 561–567. [Google Scholar] [CrossRef]
- Hargreaves, K.M.; Giesler, T.; Henry, M.; Wang, Y. Regeneration potential of the young permanent tooth: What does the future hold? J. Endod. 2008, 34, S51–S56. [Google Scholar] [CrossRef]
- Murray, P.E.; Garcia-Godoy, F.; Hargreaves, K.M. Regenerative Endodontics: A Review of Current Status and a Call for Action. J. Endod. 2007, 33, 377–390. [Google Scholar] [CrossRef]
- Diogenes, A.; Henry, M.A.; Teixeira, F.B.; Hargreaves, K.M. An update on clinical regenerative endodontics. Endod. Top. 2013, 28, 2–23. [Google Scholar] [CrossRef]
- Nagy, M.M.; Tawfik, H.E.; Hashem, A.A.R.; Abu-Seida, A.M. Regenerative Potential of Immature Permanent Teeth with Necrotic Pulps after Different Regenerative Protocols. J. Endod. 2014, 40, 192–198. [Google Scholar] [CrossRef]
- Kahler, B.; Mistry, S.; Moule, A.; Ringsmuth, A.K.; Case, P.; Thomson, A.; Holcombe, T. Revascularization Outcomes: A Prospective Analysis of 16 Consecutive Cases. J. Endod. 2014, 40, 333–338. [Google Scholar] [CrossRef]
- Lin, J.; Zeng, Q.; Wei, X.; Zhao, W.; Cui, M.; Gu, J.; Lu, J.; Yang, M.; Ling, J. Regenerative Endodontics Versus Apexification in Immature Permanent Teeth with Apical Periodontitis: A Prospective Randomized Controlled Study. J. Endod. 2017, 43, 1821–1827. [Google Scholar] [CrossRef]
- Ulusoy, A.T.; Turedi, I.; Cimen, M.; Cehreli, Z.C. Evaluation of Blood Clot, Platelet-rich Plasma, Platelet-rich Fibrin, and Platelet Pellet as Scaffolds in Regenerative Endodontic Treatment: A Prospective Randomized Trial. J. Endod. 2019, 45, 560–566. [Google Scholar] [CrossRef]
- Shimizu, E.; Ricucci, D.; Albert, J.; Alobaid, A.S.; Gibbs, J.L.; Huang, G.T.-J.; Lin, L.M. Clinical, Radiographic, and Histological Observation of a Human Immature Permanent Tooth with Chronic Apical Abscess after Revitalization Treatment. J. Endod. 2013, 39, 1078–1083. [Google Scholar] [CrossRef]
- Martin, G.; Ricucci, D.; Gibbs, J.L.; Lin, L.M. Histological Findings of Revascularized/Revitalized Immature Permanent Molar with Apical Periodontitis Using Platelet-rich Plasma. J. Endod. 2013, 39, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Liu, J.; Yu, Z.; Chen, C.-A.; Aksel, H.; Azim, A.A.; Huang, G.T.-J. A Miniature Swine Model for Stem Cell-Based De Novo Regeneration of Dental Pulp and Dentin-Like Tissue. Tissue Eng. Part C Methods 2018, 24, 108–120. [Google Scholar] [CrossRef]
- Brizuela, C.; Meza, G.; Urrejola, D.; Quezada, M.; Concha, G.; Ramirez, V.; Angelopoulos, I.; Cadiz, M.; Tapia-Limonchi, R.; Khoury, M. Cell-Based Regenerative Endodontics for Treatment of Periapical Lesions: A Randomized, Controlled Phase I/II Clinical Trial. J. Dent. Res. 2020, 99, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Xin, X.; Moioli, E.K.; Chung, J.; Lee, C.H.; Chen, M.; Fu, S.Y.; Koch, P.D.; Mao, J.J. Regeneration of Dental-Pulp-like Tissue by Chemotaxis-Induced Cell Homing. Tissue Eng. Part A 2010, 16, 3023–3031. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-W.; Zhang, Y.-F.; Wan, C.-Y.; Sun, Z.-Y.; Nie, S.; Jian, S.-J.; Zhang, L.; Song, G.-T.; Chen, Z. Autophagy in SDF-1α-mediated DPSC migration and pulp regeneration. Biomaterials 2015, 44, 11–23. [Google Scholar] [CrossRef]
- Caplan, A.I. Mesenchymal stem cells. J. Orthop. Res. 1991, 9, 641–650. [Google Scholar] [CrossRef]
- Bobis, S.; Jarocha, D.; Majka, M. Mesenchymal stem cells: Characteristics and clinical applications. Folia Histochem. et Cytobiol. 2006, 44, 215–230. [Google Scholar]
- Caplan, A.I. The Mesengenic Process. Clin. Plast. Surg. 1994, 21, 429–435. [Google Scholar] [CrossRef]
- Egusa, H.; Sonoyama, W.; Nishimura, M.; Atsuta, I.; Akiyama, K. Stem cells in dentistry--part I: Stem cell sources. J. Prosthodont. Res. 2012, 56, 151–165. [Google Scholar] [CrossRef] [PubMed]
- Sonoyama, W.; Liu, Y.; Yamaza, T.; Tuan, R.S.; Wang, S.; Shi, S.; Huang, G.T. Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: A pilot study. J. Endod. 2008, 34, 166–171. [Google Scholar] [CrossRef]
- Sonoyama, W.; Liu, Y.; Fang, D.; Yamaza, T.; Seo, B.-M.; Zhang, C.; Liu, H.; Gronthos, S.; Wang, C.-Y.; Shi, S.; et al. Mesenchymal Stem Cell-Mediated Functional Tooth Regeneration in Swine. PLoS ONE 2006, 1, e79. [Google Scholar] [CrossRef] [PubMed]
- Mullen, L.M.; Richards, D.W.; Quaranta, V. Evidence that laminin-5 is a component of the tooth surface internal basal lamina, supporting epithelial cell adhesion. J. Periodontal Res. 1999, 34, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Bègue-Kirn, C.; Smith, A.J.; Loriot, M.; Kupferle, C.; Ruch, J.V.; Lesot, H. Comparative analysis of TGF beta s, BMPs, IGF1, msxs, fibronectin, osteonectin and bone sialoprotein gene expression during normal and in vitro-induced odontoblast differentiation. Int. J. Dev. Biol. 1994, 38, 405–420. [Google Scholar]
- Bakopoulou, A.; Leyhausen, G.; Volk, J.; Koidis, P.; Geurtsen, W. Comparative characterization of STRO-1(neg)/CD146(pos) and STRO-1(pos)/CD146(pos) apical papilla stem cells enriched with flow cytometry. Arch. Oral Biol. 2013, 58, 1556–1568. [Google Scholar] [CrossRef] [PubMed]
- Patil, R.; Kumar, B.M.; Lee, W.-J.; Jeon, R.-H.; Jang, S.-J.; Lee, Y.-M.; Park, B.-W.; Byun, J.-H.; Ahn, C.-S.; Kim, J.-W.; et al. Multilineage potential and proteomic profiling of human dental stem cells derived from a single donor. Exp. Cell Res. 2014, 320, 92–107. [Google Scholar] [CrossRef]
- Kang, J.; Fan, W.; Deng, Q.; He, H.; Huang, F. Stem Cells from the Apical Papilla: A Promising Source for Stem Cell-Based Therapy. BioMed Res. Int. 2019, 2019, 6104738. [Google Scholar] [CrossRef] [PubMed]
- Vishwanat, L.; Duong, R.; Takimoto, K.; Phillips, L.; Espitia, C.O.; Diogenes, A.; Ruparel, S.B.; Kolodrubetz, D.; Ruparel, N.B. Effect of Bacterial Biofilm on the Osteogenic Differentiation of Stem Cells of Apical Papilla. J. Endod. 2017, 43, 916–922. [Google Scholar] [CrossRef]
- Lei, S.; Liu, X.M.; Liu, Y.; Bi, J.; Zhu, S.; Chen, X. Lipopolysaccharide Downregulates the Osteo-/Odontogenic Differentiation of Stem Cells from Apical Papilla by Inducing Autophagy. J. Endod. 2020, 46, 502–508. [Google Scholar] [CrossRef]
- Liu, C.; Xiong, H.; Chen, K.; Huang, Y.; Yin, X. Long-term exposure to pro-inflammatory cytokines inhibits the osteogenic/dentinogenic differentiation of stem cells from the apical papilla. Int. Endod. J. 2016, 49, 950–959. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S. The macrophage: Past, present and future. Eur. J. Immunol. 2007, 37 (Suppl. S1), S9–S17. [Google Scholar] [CrossRef]
- Rodini, C.D.O.; Lara, V.S. Study of the expression of CD68+ macrophages and CD8+ T cells in human granulomas and periapical cysts. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2001, 92, 221–227. [Google Scholar] [CrossRef]
- Mantovani, A.; Sica, A.; Sozzani, S.; Allavena, P.; Vecchi, A.; Locati, M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004, 25, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Sica, A.; Mantovani, A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef]
- Mantovani, A.; Biswas, S.K.; Galdiero, M.R.; Sica, A.; Locati, M. Macrophage plasticity and polarization in tissue repair and remodelling. J. Pathol. 2013, 229, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Liang, H.; Zen, K. Molecular mechanisms that influence the macrophage m1-m2 polarization balance. Front. Immunol. 2014, 5, 614. [Google Scholar] [CrossRef]
- Yao, Y.; Xu, X.-H.; Jin, L. Macrophage Polarization in Physiological and Pathological Pregnancy. Front. Immunol. 2019, 10, 792. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Darzi, S.; Paul, K.; Werkmeister, J.A.; Gargett, C.E. Mesenchymal stem cell-based bioengineered constructs: Foreign body response, cross-talk with macrophages and impact of biomaterial design strategies for pelvic floor disorders. Interface Focus 2019, 9, 20180089. [Google Scholar] [CrossRef]
- Rees, A.J. Monocyte and Macrophage Biology: An Overview. Semin. Nephrol. 2010, 30, 216–233. [Google Scholar] [CrossRef]
- Davies, L.C.; Boberg, E.; Le Blanc, K. Commentary: Role of Mesenchymal Stromal Cell–Mediated Crosstalk with Macrophages in Graft-versus-Host Disease and Tissue Repair. Biol. Blood Marrow Transplant. 2017, 23, 861–862. [Google Scholar] [CrossRef]
- Martinez, F.O.; Gordon, S.; Locati, M.; Mantovani, A. Transcriptional Profiling of the Human Monocyte-to-Macrophage Differentiation and Polarization: New Molecules and Patterns of Gene Expression. J. Immunol. 2006, 177, 7303–7311. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.K.; Mantovani, A. Macrophage plasticity and interaction with lymphocyte subsets: Cancer as a paradigm. Nat. Immunol. 2010, 11, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.O.; Sica, A.; Mantovani, A.; Locati, M. Macrophage activation and polarization. Front. Biosci. 2008, 13, 453–461. [Google Scholar] [CrossRef]
- Veloso, P.; Fernández, A.; Terraza-Aguirre, C.; Álvarez, C.; Vernal, R.; Escobar, A.; Hernández, M. Macrophages skew towards M1 profile through reduced CD163 expression in symptomatic apical periodontitis. Clin. Oral Investig. 2020, 24, 4571–4581. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Schlittenbauer, T.; Moebius, P.; Büttner-Herold, M.; Ries, J.; Preidl, R.; Geppert, C.-I.; Neukam, F.W.; Wehrhan, F. Macrophage polarization differs between apical granulomas, radicular cysts, and dentigerous cysts. Clin. Oral Investig. 2018, 22, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Hussein, H.; Kishen, A. Local Immunomodulatory Effects of Intracanal Medications in Apical Periodontitis. J. Endod. 2022, 48, 430–456. [Google Scholar] [CrossRef]
- Dal-Fabbro, R.; Swanson, W.B.; Capalbo, L.C.; Sasaki, H.; Bottino, M.C. Next-generation biomaterials for dental pulp tissue immunomodulation. Dent. Mater. 2023, 39, 333–349. [Google Scholar] [CrossRef]
- Pajarinen, J.; Lin, T.; Gibon, E.; Kohno, Y.; Maruyama, M.; Nathan, K.; Lu, L.; Yao, Z.; Goodman, S.B. Mesenchymal stem cell-macrophage crosstalk and bone healing. Biomaterials 2019, 196, 80–89. [Google Scholar] [CrossRef]
- Lu, D.; Xu, Y.; Liu, Q.; Zhang, Q. Mesenchymal Stem Cell-Macrophage Crosstalk and Maintenance of Inflammatory Microenvironment Homeostasis. Front. Cell Dev. Biol. 2021, 9, 681171. [Google Scholar] [CrossRef]
- Yu, B.; Sondag, G.R.; Malcuit, C.; Kim, M.-H.; Safadi, F.F. Macrophage-Associated Osteoactivin/GPNMB Mediates Mesenchymal Stem Cell Survival, Proliferation, and Migration Via a CD44-Dependent Mechanism. J. Cell. Biochem. 2016, 117, 1511–1521. [Google Scholar] [CrossRef]
- Xia, Y.; He, X.-T.; Xu, X.-Y.; Tian, B.-M.; An, Y.; Chen, F.-M. Exosomes derived from M0, M1 and M2 macrophages exert distinct influences on the proliferation and differentiation of mesenchymal stem cells. PeerJ 2020, 8, e8970. [Google Scholar] [CrossRef] [PubMed]
- Belema-Bedada, F.; Uchida, S.; Martire, A.; Kostin, S.; Braun, T. Efficient Homing of Multipotent Adult Mesenchymal Stem Cells Depends on FROUNT-Mediated Clustering of CCR2. Cell Stem Cell 2008, 2, 566–575. [Google Scholar] [CrossRef] [PubMed]
- Kitaori, T.; Ito, H.; Schwarz, E.M.; Tsutsumi, R.; Yoshitomi, H.; Oishi, S.; Nakano, M.; Fujii, N.; Nagasawa, T.; Nakamura, T. Stromal cell-derived factor 1/CXCR4 signaling is critical for the recruitment of mesenchymal stem cells to the fracture site during skeletal repair in a mouse model. Arthritis. Rheum. 2009, 60, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Guihard, P.; Danger, Y.; Brounais, B.; David, E.; Brion, R.; Delecrin, J.; Richards, C.D.; Chevalier, S.; Rédini, F.; Heymann, D.; et al. Induction of osteogenesis in mesenchymal stem cells by activated monocytes/macrophages depends on oncostatin M signaling. Stem Cells 2012, 30, 762–772. [Google Scholar] [CrossRef] [PubMed]
- Nathan, K.; Lu, L.Y.; Lin, T.; Pajarinen, J.; Jämsen, E.; Huang, J.-F.; Romero-Lopez, M.; Maruyama, M.; Kohno, Y.; Yao, Z.; et al. Precise immunomodulation of the M1 to M2 macrophage transition enhances mesenchymal stem cell osteogenesis and differs by sex. Bone Jt. Res. 2019, 8, 481–488. [Google Scholar] [CrossRef]
- Tang, H.; Husch, J.F.; Zhang, Y.; Jansen, J.A.; Yang, F.; Beucken, J.J.V.D. Coculture with monocytes/macrophages modulates osteogenic differentiation of adipose-derived mesenchymal stromal cells on poly(lactic-co-glycolic) acid/polycaprolactone scaffolds. J. Tissue Eng. Regen. Med. 2019, 13, 785–798. [Google Scholar] [CrossRef]
- Németh, K.; Leelahavanichkul, A.; Yuen, P.S.; Mayer, B.; Parmelee, A.; Doi, K.; Robey, P.G.; Leelahavanichkul, K.; Koller, B.H.; Brown, J.M.; et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E2–dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat. Med. 2009, 15, 42–49. [Google Scholar] [CrossRef]
- Reading, J.L.; Vaes, B.; Hull, C.; Sabbah, S.; Hayday, T.; Wang, N.S.; DiPiero, A.; Lehman, N.A.; Taggart, J.M.; Carty, F.; et al. Suppression of IL-7-dependent Effector T-cell Expansion by Multipotent Adult Progenitor Cells and PGE2. Mol. Ther. 2015, 23, 1783–1793. [Google Scholar] [CrossRef]
- Castro-Manrreza, M.E.; Montesinos, J.J. Immunoregulation by Mesenchymal Stem Cells: Biological Aspects and Clinical Applications. J. Immunol. Res. 2015, 2015, 394917. [Google Scholar] [CrossRef]
- Ren, G.; Zhang, L.; Zhao, X.; Xu, G.; Zhang, Y.; Roberts, A.I.; Zhao, R.C.; Shi, Y. Mesenchymal Stem Cell-Mediated Immunosuppression Occurs via Concerted Action of Chemokines and Nitric Oxide. Cell Stem Cell 2008, 2, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, D.; Xu, L.; Dong, L.; Zheng, J.; Lin, Y.; Huang, J.; Zhang, Y.; Tao, Y.; Zang, X.; et al. Cell–cell contact with proinflammatory macrophages enhances the immunotherapeutic effect of mesenchymal stem cells in two abortion models. Cell. Mol. Immunol. 2019, 16, 908–920. [Google Scholar] [CrossRef] [PubMed]
- Maggini, J.; Mirkin, G.; Geffner, J.R.; Bognanni, I.; Holmberg, J.; Piazzón, I.M.; Nepomnaschy, I.; Costa, H.; Cañones, C.; Raiden, S.; et al. Mouse Bone Marrow-Derived Mesenchymal Stromal Cells Turn Activated Macrophages into a Regulatory-Like Profile. PLoS ONE 2010, 5, e9252. [Google Scholar] [CrossRef] [PubMed]
- Eggenhofer, E.; Hoogduijn, M.J. Mesenchymal stem cell-educated macrophages. Transplant. Res. 2012, 1, 12. [Google Scholar] [CrossRef]
- Kyurkchiev, D.; Bochev, I.; Ivanova-Todorova, E.; Mourdjeva, M.; Oreshkova, T.; Belemezova, K.; Kyurkchiev, S. Secretion of immunoregulatory cytokines by mesenchymal stem cells. World J. Stem Cells 2014, 6, 552–570. [Google Scholar] [CrossRef]
- Eggenhofer, E.; Luk, F.; Dahlke, M.; Hoogduijn, M. The Life and Fate of Mesenchymal Stem Cells. Front. Immunol. 2014, 5, 148. [Google Scholar] [CrossRef]
- Liu, F.; Qiu, H.; Xue, M.; Zhang, S.; Zhang, X.; Xu, J.; Chen, J.; Yang, Y.; Xie, J. MSC-secreted TGF-β regulates lipopolysaccharide-stimulated macrophage M2-like polarization via the Akt/FoxO1 pathway. Stem Cell Res. Ther. 2019, 10, 345. [Google Scholar] [CrossRef]
- Huh, D.; Hamilton, G.A.; Ingber, D.E. From 3D cell culture to organs-on-chips. Trends Cell Biol. 2011, 21, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Simian, M.; Bissell, M.J. Organoids: A historical perspective of thinking in three dimensions. J. Cell Biol. 2017, 216, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Bhadriraju, K.; Chen, C.S. Engineering cellular microenvironments to improve cell-based drug testing. Drug Discov. Today 2002, 7, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Edmondson, R.; Broglie, J.J.; Adcock, A.F.; Yang, L. Three-Dimensional Cell Culture Systems and Their Applications in Drug Discovery and Cell-Based Biosensors. ASSAY Drug Dev. Technol. 2014, 12, 207–218. [Google Scholar] [CrossRef]
- Lee, J.; Cuddihy, M.J.; Kotov, N.A. Three-Dimensional Cell Culture Matrices: State of the Art. Tissue Eng. Part B Rev. 2008, 14, 61–86. [Google Scholar] [CrossRef]
- Shield, K.; Ackland, M.L.; Ahmed, N.; Rice, G.E. Multicellular spheroids in ovarian cancer metastases: Biology and pathology. Gynecol. Oncol. 2009, 113, 143–148. [Google Scholar] [CrossRef]
- Riccio, M.; Resca, E.; Maraldi, T.; Pisciotta, A.; Ferrari, A.; Bruzzesi, G.; DE Pol, A. Human dental pulp stem cells produce mineralized matrix in 2D and 3D cultures. Eur. J. Histochem. 2010, 54, e46. [Google Scholar] [CrossRef]
- Yamamoto, M.; Kawashima, N.; Takashino, N.; Koizumi, Y.; Takimoto, K.; Suzuki, N.; Saito, M.; Suda, H. Three-dimensional spheroid culture promotes odonto/osteoblastic differentiation of dental pulp cells. Arch. Oral Biol. 2014, 59, 310–317. [Google Scholar] [CrossRef]
- Kim, H.-J.; Sung, I.-Y.; Cho, Y.-C.; Kang, M.-S.; Rho, G.-J.; Byun, J.-H.; Park, W.-U.; Son, M.-G.; Park, B.-W.; Lee, H.-J.; et al. Three-Dimensional Spheroid Formation of Cryopreserved Human Dental Follicle-Derived Stem Cells Enhances Pluripotency and Osteogenic Induction Properties. Tissue Eng. Regen. Med. 2019, 16, 513–523. [Google Scholar] [CrossRef]
- Xu, B.; Fan, D.; Zhao, Y.; Li, J.; Wang, Z.; Wang, J.; Wang, X.; Guan, Z.; Niu, B. Three-Dimensional Culture Promotes the Differentiation of Human Dental Pulp Mesenchymal Stem Cells into Insulin-Producing Cells for Improving the Diabetes Therapy. Front. Pharmacol. 2019, 10, 1576. [Google Scholar] [CrossRef] [PubMed]
- Bu, N.-U.; Lee, H.-S.; Lee, B.-N.; Hwang, Y.-C.; Kim, S.-Y.; Chang, S.W.; Choi, K.-K.; Kim, D.-S.; Jang, J.-H. In Vitro Characterization of Dental Pulp Stem Cells Cultured in Two Microsphere-Forming Culture Plates. J. Clin. Med. 2020, 9, 242. [Google Scholar] [CrossRef]
- Kawashima, N.; Noda, S.; Yamamoto, M.; Okiji, T. Properties of Dental Pulp–derived Mesenchymal Stem Cells and the Effects of Culture Conditions. J. Endod. 2017, 43, S31–S34. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.Y.; Kim, M.S.; Lee, K.E.; Nam, O.H.; Jang, J.-H.; Choi, S.-C.; Lee, H.-S. Comparison of 2- and 3-Dimensional Cultured Periodontal Ligament Stem Cells; a Pilot Study. Appl. Sci. 2021, 11, 1083. [Google Scholar] [CrossRef]
- Banavar, S.R.; Rawal, S.Y.; Pulikkotil, S.J.; Daood, U.; Paterson, I.C.; Davamani, F.A.; Kajiya, M.; Kurihara, H.; SKhoo, P.; Tan, E.L. 3D Clumps/Extracellular Matrix Complexes of Periodontal Ligament Stem Cells Ameliorate the Attenuating Effects of LPS on Proliferation and Osteogenic Potential. J. Pers. Med. 2021, 11, 528. [Google Scholar] [CrossRef] [PubMed]
- Itoh, Y.; Sasaki, J.I.; Hashimoto, M.; Katata, C.; Hayashi, M.; Imazato, S. Pulp Regeneration by 3-dimensional Dental Pulp Stem Cell Constructs. J. Dent. Res. 2018, 97, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Buttler-Buecher, P.; Denecke, B.; Arana-Chavez, V.E.; Apel, C. A comprehensive analysis of human dental pulp cell spheroids in a three-dimensional pellet culture system. Arch. Oral Biol. 2018, 91, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Oortgiesen, D.A.; Yu, N.; Bronckers, A.L.; Yang, F.; Walboomers, X.F.; Jansen, J.A. A Three-Dimensional Cell Culture Model to Study the Mechano-Biological Behavior in Periodontal Ligament Regeneration. Tissue Eng. Part C Methods 2012, 18, 81–89. [Google Scholar] [CrossRef]
- Shahin-Shamsabadi, A.; Selvaganapathy, P.R. A rapid biofabrication technique for self-assembled collagen-based multicellular and heterogeneous 3D tissue constructs. Acta Biomater. 2019, 92, 172–183. [Google Scholar] [CrossRef]
- Lin, R.Z.; Chou, L.F.; Chien, C.C.; Chang, H.Y. Dynamic analysis of hepatoma spheroid formation: Roles of E-cadherin and beta1-integrin. Cell Tissue Res. 2006, 324, 411–422. [Google Scholar] [CrossRef]
- Ruedinger, F.; Lavrentieva, A.; Blume, C.; Pepelanova, I.; Scheper, T. Hydrogels for 3D mammalian cell culture: A starting guide for laboratory practice. Appl. Microbiol. Biotechnol. 2015, 99, 623–636. [Google Scholar] [CrossRef]
- McGuigan, A.P.; Bruzewicz, D.A.; Glavan, A.; Butte, D.A.; Butte, M.; Whitesides, G.M. Cell encapsulation in sub-mm sized gel modules using replica molding. PLoS ONE 2008, 3, e2258. [Google Scholar] [CrossRef]
- Duval, K.; Grover, H.; Han, L.-H.; Mou, Y.; Pegoraro, A.F.; Fredberg, J.; Chen, Z. Modeling Physiological Events in 2D vs. 3D Cell Culture. Physiology 2017, 32, 266–277. [Google Scholar] [CrossRef]
- Jensen, C.; Teng, Y. Is It Time to Start Transitioning from 2D to 3D Cell Culture? Front. Mol. Biosci. 2020, 7, 33. [Google Scholar] [CrossRef]
- Langhans, S.A. Three-Dimensional. Front. Pharmacol. 2018, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- Gurski, L.A.; Petrelli, N.J.; Jia, X.; Farach-Carson, M.C. Three-dimensional matrices for anti-cancer drug testing and development. Oncol. Issues 2010, 25, 20–25. [Google Scholar] [CrossRef]
- Antoni, D.; Burckel, H.; Josset, E.; Noel, G. Three-Dimensional Cell Culture: A Breakthrough in Vivo. Int. J. Mol. Sci. 2015, 16, 5517–5527. [Google Scholar] [CrossRef] [PubMed]
- Booij, T.H.; Price, L.S.; Danen, E.H.J. 3D Cell-Based Assays for Drug Screens: Challenges in Imaging, Image Analysis, and High-Content Analysis. SLAS Discov. 2019, 24, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Li, F.-C.; Shahin-Shamsabadi, A.; Selvaganapathy, P.R.; Kishen, A. Engineering a Novel Stem Cells from Apical Papilla-Macrophages Organoid for Regenerative Endodontics. J. Endod. 2022, 48, 741–748. [Google Scholar] [CrossRef]
- Li, F.-C.; Hussein, H.; Magalhaes, M.; Selvaganapathy, P.R.; Kishen, A. Deciphering Stem Cell from Apical Papilla–Macrophage Choreography Using a Novel 3-dimensional Organoid System. J. Endod. 2022, 48, 1063–1072.e7. [Google Scholar] [CrossRef]
- Estrela, C.; De Alencar, A.H.G.; Kitten, G.T.; Vencio, E.F.; Gava, E. Mesenchymal stem cells in the dental tissues: Perspectives for tissue regeneration. Braz. Dent. J. 2011, 22, 91–98. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Lv, H.; Yu, Q.; Zhou, Z.; Zhu, Q.; Wang, Z.; Cooper, P.R.; Smith, A.J.; Niu, Z.; et al. Human Stem Cells from the Apical Papilla Response to Bacterial Lipopolysaccharide Exposure and Anti-inflammatory Effects of Nuclear Factor I C. J. Endod. 2013, 39, 1416–1422. [Google Scholar] [CrossRef]
- Lertchirakarn, V.; Aguilar, P. Effects of Lipopolysaccharide on the Proliferation and Osteogenic Differentiation of Stem Cells from the Apical Papilla. J. Endod. 2017, 43, 1835–1840. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-J.; Xie, J.; Lin, X.; Ou, M.-H.; Zhou, J.; Wei, X.-L. The Role of Small Extracellular Vesicles Derived from Lipopolysaccharide-preconditioned Human Dental Pulp Stem Cells in Dental Pulp Regeneration. J. Endod. 2021, 47, 961–969. [Google Scholar] [CrossRef]
- Goers, L.; Freemont, P.; Polizzi, K.M. Co-culture systems and technologies: Taking synthetic biology to the next level. J. R. Soc. Interface 2014, 11, 20140065. [Google Scholar] [CrossRef] [PubMed]
- Vis, M.A.M.; Ito, K.; Hofmann, S. Impact of Culture Medium on Cellular Interactions in in vitro Co-culture Systems. Front. Bioeng. Biotechnol. 2020, 8, 911. [Google Scholar] [CrossRef]
- Tibbitt, M.W.; Anseth, K.S. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol. Bioeng. 2009, 103, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Luca, A.C.; Mersch, S.; Deenen, R.; Schmidt, S.; Messner, I.; Schäfer, K.-L.; Baldus, S.E.; Huckenbeck, W.; Piekorz, R.P.; Knoefel, W.T.; et al. Impact of the 3D Microenvironment on Phenotype, Gene Expression, and EGFR Inhibition of Colorectal Cancer Cell Lines. PLoS ONE 2013, 8, e59689. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Ding, Y.; Sun, X.S.; Nguyen, T.A. Peptide Hydrogelation and Cell Encapsulation for 3D Culture of MCF-7 Breast Cancer Cells. PLoS ONE 2013, 8, e59482. [Google Scholar] [CrossRef]
- Clause, K.C.; Barker, T.H. Extracellular matrix signaling in morphogenesis and repair. Curr. Opin. Biotechnol. 2013, 24, 830–833. [Google Scholar] [CrossRef]
- Järveläinen, H.; Sainio, A.; Koulu, M.; Wight, T.N.; Penttinen, R. Extracellular Matrix Molecules: Potential Targets in Pharmacotherapy. Pharmacol. Rev. 2009, 61, 198–223. [Google Scholar] [CrossRef]
- Sainio, A.; Järveläinen, H. Extracellular matrix-cell interactions: Focus on therapeutic applications. Cell Signal. 2020, 66, 109487. [Google Scholar] [CrossRef] [PubMed]
- Kukreti, H.; Li, F.-C.; Singh, K.; Sodhi, R.; Kishen, A. Efficacy of bioactive nanoparticles on tissue-endotoxin induced suppression of stem cell viability, migration and differentiation. Int. Endod. J. 2020, 53, 859–870. [Google Scholar] [CrossRef]
- Ding, G.; Liu, Y.; An, Y.; Zhang, C.; Shi, S.; Wang, W.; Wang, S. Suppression of T Cell Proliferation by Root Apical Papilla Stem Cells in vitro. Cells Tissues Organs 2010, 191, 357–364. [Google Scholar] [CrossRef]
- Tang, R.; Ding, G. Swine Dental Pulp Stem Cells Inhibit T-Cell Proliferation. Transplant. Proc. 2011, 43, 3955–3959. [Google Scholar] [CrossRef]
- Dissanayaka, W.L.; Zhan, X.; Zhang, C.; Hargreaves, K.M.; Jin, L.; Tong, E.H. Coculture of dental pulp stem cells with endothelial cells enhances osteo-/odontogenic and angiogenic potential in vitro. J. Endod. 2012, 38, 454–463. [Google Scholar] [CrossRef]
- Yuan, C.; Wang, P.; Zhu, L.; Dissanayaka, W.L.; Green, D.W.; Tong, E.H.; Jin, L.; Zhang, C. Coculture of stem cells from apical papilla and human umbilical vein endothelial cell under hypoxia increases the formation of three-dimensional vessel-like structures in vitro. Tissue Eng. Part A 2015, 21, 1163–1172. [Google Scholar] [CrossRef]
- Yuan, C.; Wang, P.; Zhu, S.; Zou, T.; Wang, S.; Xu, J.; Heng, B.C.; Diogenes, A.; Zhang, C. EphrinB2 Stabilizes Vascularlike Structures Generated by Endothelial Cells and Stem Cells from Apical Papilla. J. Endod. 2016, 42, 1362–1370. [Google Scholar] [CrossRef]
- Lee, S.; Zhang, Q.; Karabucak, B.; Le, A. DPSCs from Inflamed Pulp Modulate Macrophage Function via the TNF-α/IDO Axis. J. Dent. Res. 2016, 95, 1274–1281. [Google Scholar] [CrossRef] [PubMed]
- Omi, M.; Hata, M.; Nakamura, N.; Miyabe, M.; Kobayashi, Y.; Kamiya, H.; Nakamura, J.; Ozawa, S.; Tanaka, Y.; Takebe, J.; et al. Transplantation of dental pulp stem cells suppressed inflammation in sciatic nerves by promoting macrophage polarization towards anti-inflammation phenotypes and ameliorated diabetic polyneuropathy. J. Diabetes Investig. 2016, 7, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.-Z.; Kim, H.-W. Co-culture of Human Dental Pulp Stem Cells and Endothelial Cells Using Porous Biopolymer Microcarriers: A Feasibility Study for Bone Tissue Engineering. Tissue Eng. Regen. Med. 2017, 14, 393–401. [Google Scholar] [CrossRef] [PubMed]
- De Berdt, P.; Bottemanne, P.; Bianco, J.; Alhouayek, M.; Diogenes, A.; Llyod, A.; Gerardo-Nava, J.; Brook, G.A.; Miron, V.; Muccioli, G.G.; et al. Stem cells from human apical papilla decrease neuro-inflammation and stimulate oligodendrocyte progenitor differentiation via activin-A secretion. Cell. Mol. Life Sci. 2018, 75, 2843–2856. [Google Scholar] [CrossRef]
- Whiting, D.; Chung, W.O.; Johnson, J.D.; Paranjpe, A. Characterization of the Cellular Responses of Dental Mesenchymal Stem Cells to the Immune System. J. Endod. 2018, 44, 1126–1131. [Google Scholar] [CrossRef]
- Tatic, N.; Rose, F.R.A.J.; Rieux, A.D.; White, L.J. Stem cells from the dental apical papilla in extracellular matrix hydrogels mitigate inflammation of microglial cells. Sci. Rep. 2019, 9, 14015. [Google Scholar] [CrossRef]
- Liu, X.M.; Liu, Y.; Yu, S.; Jiang, L.M.; Song, B.; Chen, X. Potential immunomodulatory effects of stem cells from the apical papilla on Treg conversion in tissue regeneration for regenerative endodontic treatment. Int. Endod. J. 2019, 52, 1758–1767. [Google Scholar] [CrossRef] [PubMed]
- Kanji, S.; Sarkar, R.; Pramanik, A.; Kshirsagar, S.; Greene, C.J.; Das, H. Dental pulp–derived stem cells inhibit osteoclast differentiation by secreting osteoprotegerin and deactivating AKT signalling in myeloid cells. J. Cell. Mol. Med. 2021, 25, 2390–2403. [Google Scholar] [CrossRef]
- Croci, S.; Bonacini, M.; Dolci, G.; Massari, M.; Facciolongo, N.; Pignatti, E.; Pisciotta, A.; Carnevale, G.; Negro, A.; Cassone, G.; et al. Human Dental Pulp Stem Cells Modulate Cytokine Production in vitro by Peripheral Blood Mononuclear Cells from Coronavirus Disease 2019 Patients. Front. Cell Dev. Biol. 2020, 8, 609204. [Google Scholar] [CrossRef]
- Anderson, S.; Prateeksha, P.; Das, H. Dental Pulp-Derived Stem Cells Reduce Inflammation, Accelerate Wound Healing and Mediate M2 Polarization of Myeloid Cells. Biomedicines 2022, 10, 1999. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Basabrain, M.S.; Zhong, J.; Liu, J.; Zhang, Y.; Qi, Y.; Zou, T.; Zhang, C. Neuroregenerative Potential of Stem-Cells-from-Apical-Papilla–Derived Neuronal Cell Spheroids Regulated by Stem Cells from Apical Papillae Under Various Microenvironments in a Pulp-On-Chip System. J. Endod. 2022, 48, 1367–1377.e2. [Google Scholar] [CrossRef]
- Li, F.-C. Deciphering Stem Cell from Apical Papilla—Macrophage Choreography in Inflammatory Environment Using a Novel 3D Organoid. Ph.D. Thesis, University of Toronto, Toronto, ON, Canada, 2021. [Google Scholar]
- Lancaster, M.A.; Knoblich, J.A. Organogenesis in a dish: Modeling development and disease using organoid technologies. Science 2014, 345, 1247125. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.Y.; Lee, S.; Choi, W.H.; Jee, J.H.; Kim, H.-R.; Yoo, J. Fabrication of Dentin-Pulp-Like Organoids Using Dental-Pulp Stem Cells. Cells 2020, 9, 642. [Google Scholar] [CrossRef]
- Xu, X.; Li, Z.; Ai, X.; Tang, Y.; Yang, D.; Dou, L. Human three-dimensional dental pulp organoid model for toxicity screening of dental materials on dental pulp cells and tissue. Int. Endod. J. 2022, 55, 79–88. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, F.-C.; Kishen, A. 3D Organoids for Regenerative Endodontics. Biomolecules 2023, 13, 900. https://doi.org/10.3390/biom13060900
Li F-C, Kishen A. 3D Organoids for Regenerative Endodontics. Biomolecules. 2023; 13(6):900. https://doi.org/10.3390/biom13060900
Chicago/Turabian StyleLi, Fang-Chi, and Anil Kishen. 2023. "3D Organoids for Regenerative Endodontics" Biomolecules 13, no. 6: 900. https://doi.org/10.3390/biom13060900
APA StyleLi, F.-C., & Kishen, A. (2023). 3D Organoids for Regenerative Endodontics. Biomolecules, 13(6), 900. https://doi.org/10.3390/biom13060900





