Isoallopregnanolone Inhibits Estrus Cycle-Dependent Aggressive Behavior
Abstract
1. Introduction
2. Materials and Methods
2.1. In Vitro Experiments
2.1.1. Transfection of HEK-293 Cells for Electrophysiological Measurements
2.1.2. Electrophysiological Recordings
2.1.3. GABA-A Receptor Pharmacology
2.1.4. Dynaflow™ System
2.2. Data Analysis
2.3. In Vivo Experimental Set-Up
2.3.1. Animals
2.3.2. Ovariectomy of the Intruder Rats
2.3.3. Determination of Estrus Cycle Phase
2.3.4. Investigation of Estrus Cycle-Dependent Aggressive Behavior
2.3.5. Video Film Analysis of Behavior
2.3.6. Treatment
2.3.7. Pharmacokinetic Study
2.3.8. Statistics
3. Results
3.1. In Vitro Experiments
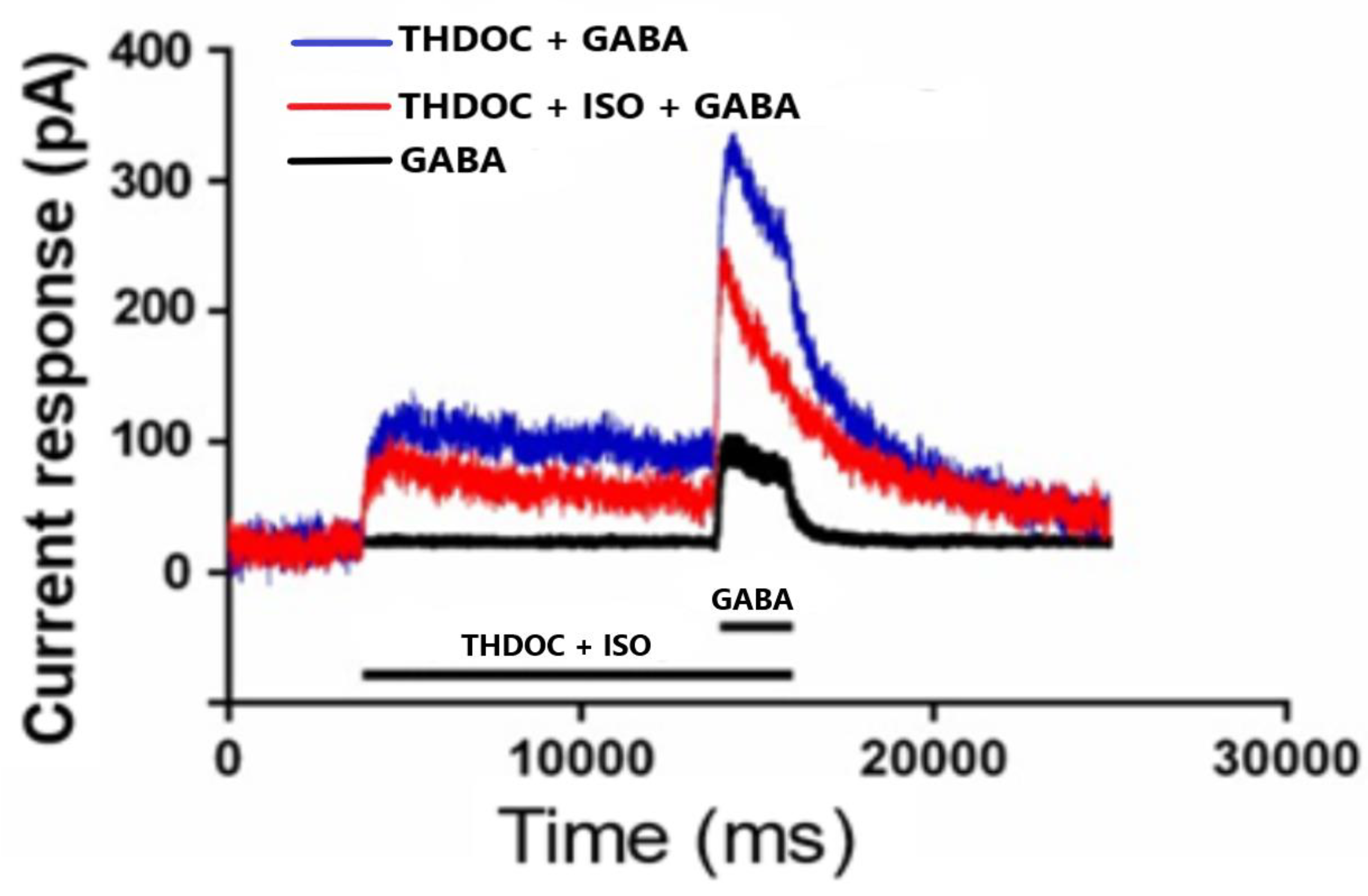
3.1.1. The Effect of THDOC + GABA and ISO on α4β3δ GABA-A Receptor Subtypes
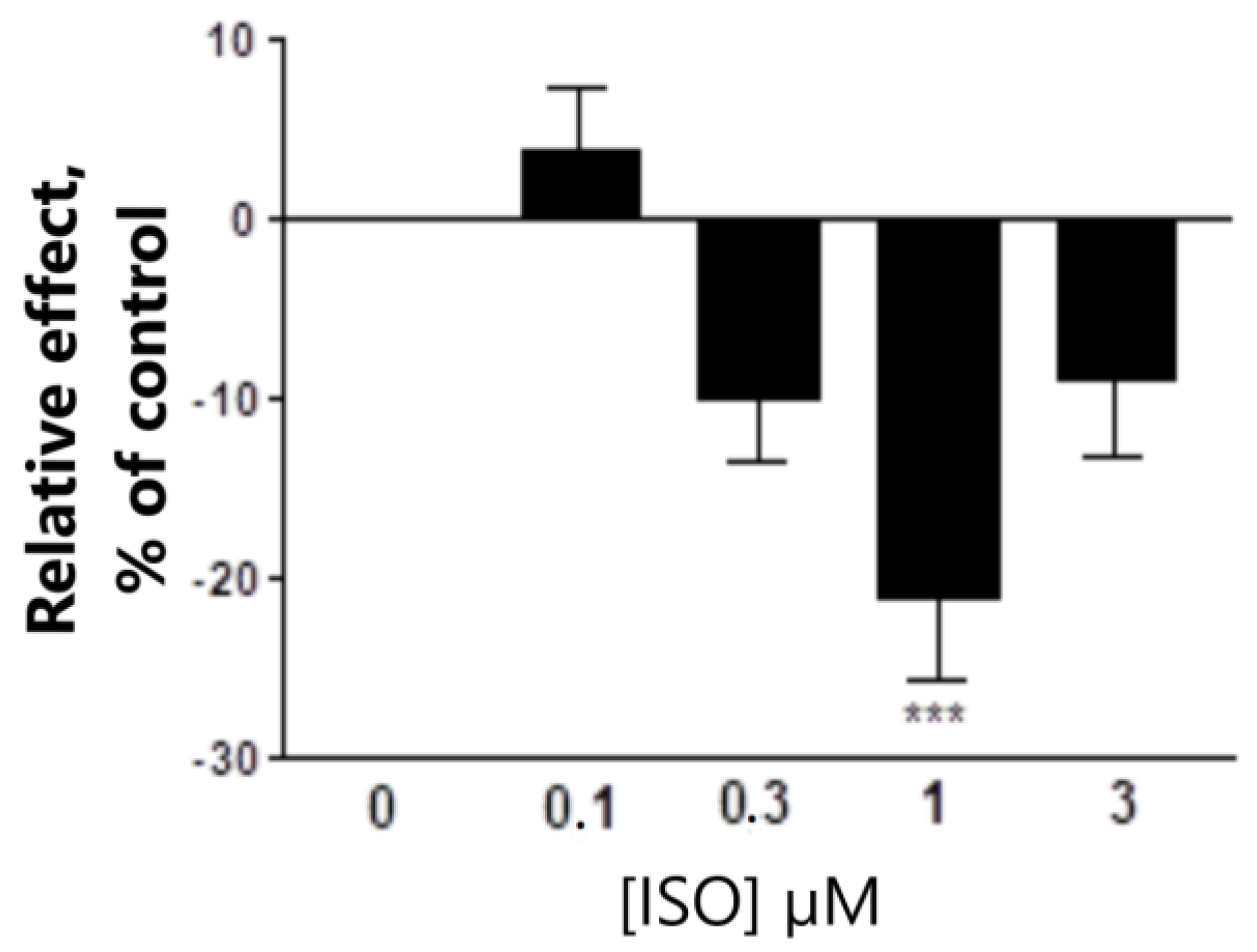
3.1.2. The Effect of THDOC Alone, with or without ISO at α4β3δ GABA-A Receptor Subtype
3.2. In Vivo Experiments
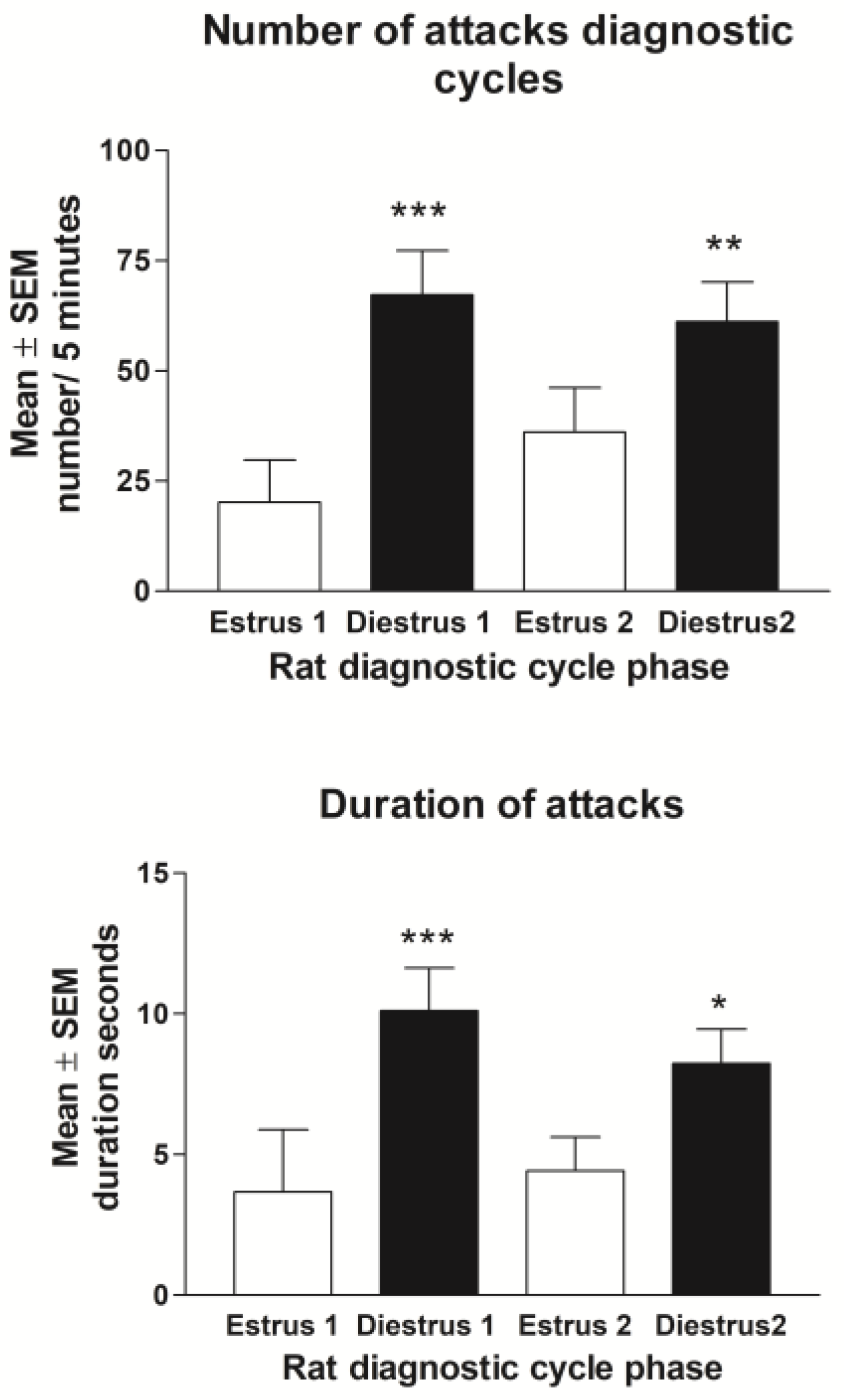
3.2.1. Baseline Behavior
3.2.2. Estrus Cycle and Behavior during ISO Treatment
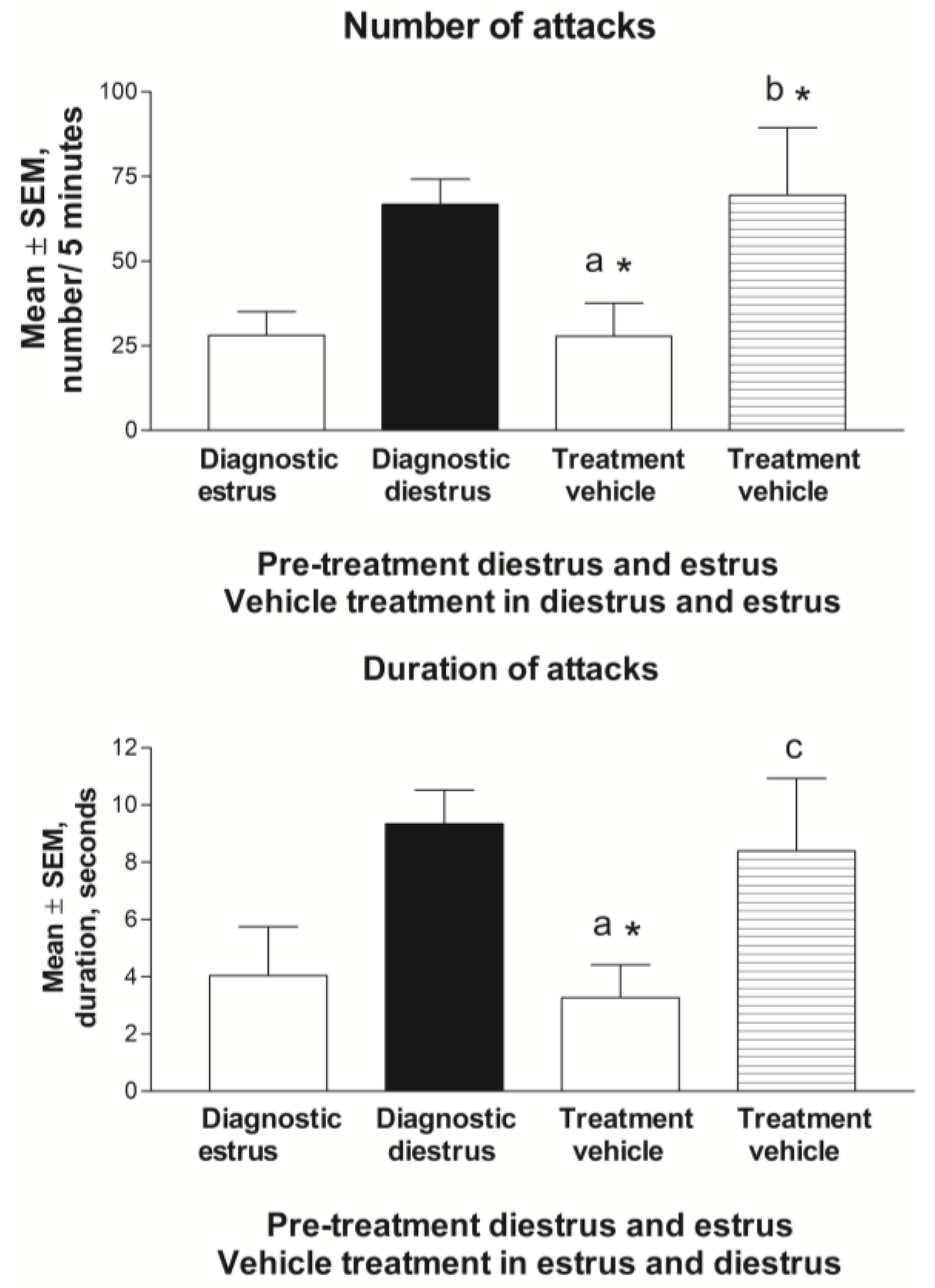
3.2.3. Estrus Cycle and Behavior during Vehicle Treatment
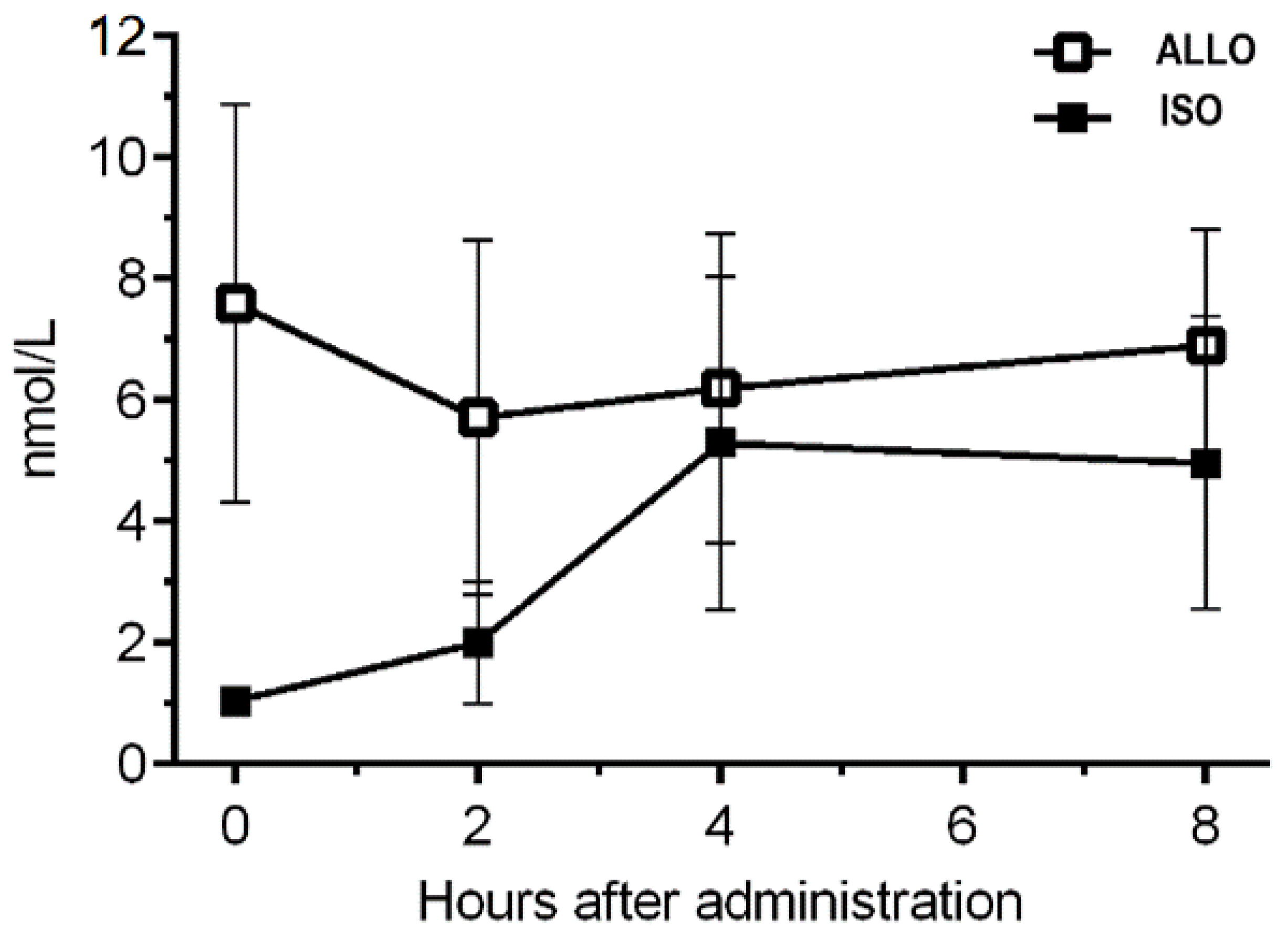
3.2.4. Plasma Concentrations of ISO and ALLO
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Declarations
References
- Schneider, T.; Popik, P. Increased depressive-like traits in an animal model of premenstrual irritability. Horm. Behav. 2007, 51, 142–148. [Google Scholar] [CrossRef]
- Ho, H.P.; Olsson, M.; Westberg, L.; Melke, J.; Eriksson, E. The serotonin reuptake inhibitor fluoxetine reduces sex steroid-related aggression in female rats: An animal model of premenstrual irritability? Neuropsychopharmacology 2001, 24, 502–510. [Google Scholar] [CrossRef]
- Smith, M.S.; Freeman, M.E.; Neill, J.D. The control of progesterone secretion during the estrous cycle and early pseudopregnancy in the rat: Prolactin, gonadotropin and steroid levels associated with rescue of the corpus luteum of pseudopregnancy. Endocrinology 1975, 96, 219–226. [Google Scholar] [CrossRef]
- Majewska, M.D.; Harrison, N.L.; Schwartz, R.D.; Barker, J.L.; Paul, S.M. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science 1986, 232, 1004–1007. [Google Scholar] [CrossRef]
- Belelli, D.; Lambert, J.J. Neurosteroids: Endogenous regulators of the GABA-A receptor. Nat. Rev. Neurosci. 2005, 6, 565–575. [Google Scholar] [CrossRef]
- Carver, C.M.; Reddy, D.S. Neurosteroid interactions with synaptic and extrasynaptic GABA-A receptors: Regulation of subunit plasticity, phasic and tonic inhibition, and neuronal network excitability. Psychopharmacology 2013, 230, 151–188. [Google Scholar] [CrossRef]
- Andreen, L.; Nyberg, S.; Turkmen, S.; van Wingen, G.; Fernandez, G.; Bäckström, T. Sex steroid induced negative mood may be explained by the paradoxical effect mediated by GABA-A modulators. Psychoneuroendocrinology 2009, 34, 1121–1132. [Google Scholar] [CrossRef]
- Bäckström, T.; Haage, D.; Lofgren, M.; Johansson, I.M.; Stromberg, J.; Nyberg, S.; Andreen, L.; Ossewaarde, L.; van Wingen, G.A.; Turkmen, S.; et al. Paradoxical Effects of GABA-A Modulators May Explain Sex Steroid Induced Negative Mood Symptoms in Some Persons. Neuroscience 2011, 191, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Miczek, K.A.; Fish, E.W.; De Bold, J.F. Neurosteroids, GABA-A receptors, and escalated aggressive behavior. Horm. Behav. 2003, 44, 242–257. [Google Scholar] [CrossRef] [PubMed]
- Pearlstein, T. Prevalence, impact on morbidity, and disease burden. In The Premestrual Syndromes: PMS and PMDD; O’Brien, P.M.S., Rapkin, A.J., Schmidt, P.J., Eds.; Informa UK Ltd.: London, UK, 2007; pp. 27–47. [Google Scholar]
- Sveinsdottir, H.; Bäckström, T. Menstrual cycle symptom variation in a community sample of women using and not using oral contraceptives. Acta Obstet. Gynecol. Scand. 2000, 79, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Belelli, D.; Casula, A.; Ling, A.; Lambert, J.J. The influence of subunit composition on the interaction of neurosteroids with GABA-A receptors. Neuropharmacology 2002, 43, 651–661. [Google Scholar] [CrossRef]
- Lundgren, P.; Stromberg, J.; Bäckström, T.; Wang, M.D. Allopregnanolone-stimulated GABA-mediated chloride ion flux is inhibited by 3 beta-hydroxy-5 alpha-pregnan-20-one (isoallopregnanolone). Brain Res. 2003, 982, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; He, Y.; Eisenman, L.N.; Fields, C.; Zeng, C.-M.; Mathews, J.; Benz, A.; Fu, T.; Zorumski, E.; Steinbach, J.H.; et al. 3β-Hydroxypregnane Steroids Are Pregnenolone Sulfate-Like GABA-A Receptor Antagonists. J. Neurosci. 2002, 22, 3366–3375. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.D.; Bäckström, T.; Landgren, S. The inhibitory effects of allopregnanolone and pregnanolone on the population spike, evoked in the rat hippocampal CA1 stratum pyramidale in vitro, can be blocked selectively by epiallopregnanolone. Acta Physiol. Scand. 2000, 169, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Bäckström, T.; Wahlstrom, G.; Wahlstrom, K.; Zhu, D.; Wang, M.D. Isoallopregnanolone; an antagonist to the anaesthetic effect of allopregnanolone in male rats. Eur. J. Pharmacol. 2005, 512, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Stromberg, J.; Haage, D.; Taube, M.; Bäckström, T.; Lundgren, P. Neurosteroid modulation of allopregnanolone and GABA effect on the GABA-A receptor. Neuroscience 2006, 143, 73–81. [Google Scholar] [CrossRef]
- Stiernman, L.; Dubol, M.; Comasco, E.; Sundström-Poromaa, I.; Boraxbekk, C.J.; Johansson, M.; Bixo, M. Emotion-induced brain activation across the menstrual cycle in individuals with premenstrual dysphoric disorder and associations to serum levels of progesterone-derived neurosteroids. Transl. Psychiatry 2023, 13, 124. [Google Scholar] [CrossRef]
- Parato, J.; Shen, H.; Smith, S.S. α4βδ GABA-A Receptors Trigger Synaptic Pruning and Reduce Dendritic Length of Female Mouse CA3 Hippocampal Pyramidal Cells at Puberty. Neuroscience 2019, 398, 23–36. [Google Scholar] [CrossRef]
- Smith, S.S.; Gong, Q.H.; Li, X.; Moran, M.H.; Bitran, D.; Frye, C.A.; Hsu, F.-C. Withdrawal from 3α-OH-5α-pregnan-20-one withdrawal using a pseudopregnancy model alters the kinetics of hippocampal GABA-A-gated current and increases the GABA-A receptor α4 subunit in association with increased anxiety. J. Neurosci. 1998, 18, 5275–5284. [Google Scholar] [CrossRef]
- Smith, S.S.; Gong, Q.H.; Hsu, F.C.; Markowitz, R.S.; ffrench-Mullen, J.M.H.; Li, X. GABA-A receptor α4 subunit suppression prevents withdrawal properties of an endogenous steroid. Nature 1998, 392, 926–929. [Google Scholar] [CrossRef]
- Gulinello, M.; Smith, S.S. Anxiogenic effects of neurosteroid exposure—Sex differences and altered GABA-A receptor pharmacology in adult rats. J. Pharm. Exp. Ther. 2003, 305, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Gulinello, M.; Smith, S.S. Progesterone withdrawal increases the α4 subunit of the GABA-A receptor in male rats in association with anxiety and altered pharmacology—A comparison with female rats. Neuropharmacology 2002, 434, 702–715. [Google Scholar]
- Gulinello, M.; Gong, Q.H.; Li, X.; Smith, S.S. Short-term exposure to a neuroactive steroid increases α4 GABA-A receptor subunit levels in association with increased anxiety. Brain Res. 2001, 910, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.; Augusti, A.; Lansola, M.; Montoliu, C.; Strömberg, J.; Malinina, E.; Ragagnin, G.; Doverskog, M.; Bäckström, T.; Felipo, V. GR3027 Antagonizes GABA-A receptor potentiating neurosteroids and restores spatial learning and motor coordination in rats with chronic hyperammonmeia and hepatic encephalopathy. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, G400-9. [Google Scholar] [CrossRef]
- Wang, M.D.; Rahman, M.; Johansson, I.M.; Bäckström, T. Agonist function of the recombinant alpha 4 beta 3 delta GABA-A receptor is dependent on the human and rat variants of the alpha 4-subunit. Clin. Exp. Pharmacol. Physiol. 2010, 37, 662–669. [Google Scholar] [CrossRef]
- Rahman, M.; Borra, V.B.; Isaksson, M.; Johansson, I.M.; Ragagnin, G.; Bäckström, T.; Wang, M.D. A comparison of the pharmacological properties of recombinant human and rat α1β2γ2L GABAA receptors in Xenopus oocytes. Clin. Exp. Pharmacol. Physiol. 2008, 35, 1002–1011. [Google Scholar] [CrossRef]
- Hubscher, C.H.; Brooks, D.L.; Johnson, J.R. A quantitative method for assessing stages of the rat estrous cycle. Biotech. Histochem. 2005, 80, 79–87. [Google Scholar] [CrossRef]
- Marcondes, F.K.; Bianchi, F.J.; Tanno, A.P. Determination of the estrous cycle phases of rats: Some helpful considerations. Braz. J. Biol. 2002, 62, 609–614. [Google Scholar] [CrossRef]
- Sundstrom-Poromaa, I.; Smith, D.H.; Gong, Q.-H.; Li, X.; Light, A.; Wiedmann, M.; Williams, K.; Smith, S.S. Hormonally regulated α4β2δ GABA-A receptors are a target for alcohol. Nat. Neurosci. 2002, 5, 721–722. [Google Scholar] [CrossRef]
- Bengtsson, S.K.; Nyberg, S.; Hedstrom, H.; Zingmark, E.; Jonsson, B.; Bäckström, T.; Bixo, M. Isoallopregnanolone antagonize allopregnanolone-induced effects on saccadic eye velocity and self-reported sedation in humans. Psychoneuroendocrinology 2015, 52, 22–31. [Google Scholar] [CrossRef]
- Bäckström, T.; Bixo, M.; Johansson, M.; Nyberg, S.; Ossewaarde, L.; Ragagnin, G.; Savic, I.; Stromberg, J.; Timby, E.; van Broekhoven, F.; et al. Allopregnanolone and mood disorders. Prog. Neurobiol. 2014, 113, 88–94. [Google Scholar] [CrossRef]
- Ichikawa, S.; Sawada, T.; Nakamura, Y.; Morioka, H. Ovarian secretion of pregnane compounds during the estrous cycle and pregnancy in rats. Endocrinology 1974, 94, 1615–1620. [Google Scholar] [CrossRef]
- Nyberg, S.; Bäckström, T.; Zingmark, E.; Purdy, R.H.; Poromaa, I.S. Allopregnanolone decrease with symptom improvement during placebo and gonadotropin-releasing hormone agonist treatment in women with severe premenstrual syndrome. Gynecol. Endocrinol. 2007, 23, 257–266. [Google Scholar] [CrossRef]
- Hammarback, S.; Bäckström, T. Induced anovulation as treatment of premenstrual tension syndrome. A double-blind cross-over study with GnRH-agonist versus placebo. Acta Obstet. Gynecol. Scand. 1988, 67, 159–166. [Google Scholar] [CrossRef]
- Hammarback, S.; Ekholm, U.B.; Bäckström, T. Spontaneous anovulation causing disappearance of cyclical symptoms in women with the premenstrual syndrome. Acta Endocrinol. 1991, 125, 132–137. [Google Scholar] [CrossRef]
- Wyatt, K.M.; Dimmock, P.W.; Ismail, K.M.; Jones, P.W.; O’Brien, P.M. The effectiveness of GnRHa with and without ‘add-back’ therapy in treating premenstrual syndrome: A meta analysis. BJOG Int. J. Obstet. Gynaecol. 2004, 111, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, P.J.; Nieman, L.K.; Danaceau, M.A.; Adams, L.F.; Rubinow, D.R. Differential behavioral effects of gonadal steroids in women with and in those without premenstrual syndrome. N. Engl. J. Med. 1998, 338, 209–216. [Google Scholar] [CrossRef]
- Sundstrom-Poromaa, I.; Bixo, M.; Bjorn, I.; Nordh, O. Compliance to antidepressant drug therapy for treatment of premenstrual syndrome. J. Psychosom. Obstet. Gynaecol. 2000, 21, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Schneider, T.; Popik, P. Attenuation of estrous cycle-dependent marble burying in female rats by acute treatment with progesterone and antidepressants. Psychoneuroendocrinology 2007, 32, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Miczek, K.A.; DeBold, J.F.; van Erp, A.M.; Tornatzky, W. Alcohol, GABA-A-benzodiazepine receptor complex, and aggression. Recent Dev. Alcohol. 1997, 13, 139–171. [Google Scholar]
- de Jong, T.R.; Beiderbeck, D.I.; Neumann, I.D. Measuring Virgin Female Aggression in the Female Intruder Test (FIT): Effects of Oxytocin, Estrous Cycle, and Anxiety. PLoS ONE 2014, 9, e91701. [Google Scholar] [CrossRef] [PubMed]



| Tested Dosages Combinations: | Effect Mean ± SEM% (N; P) |
|---|---|
| 0.3–1 μM THDOC + 30 μM GABA (%) at α1β2γ2L | +115 Emax% (N = 35, P = 0.001) |
| 1 μM ISO + 30 μM GABA (%) at α1β2γ2L | +10.3 ± 7.8% (N = 9, NS) |
| 1 μM ISO + 30 μM GABA + 0.2 μM THDOC at α1β2γ2L | −21 ± 4.6% (N = 10, P = 0.001) |
| The Effect of 1 μM ISO on | Mean ± SEM% (P; N) |
|---|---|
| 1 μM ISO + 3 μM GABA response (%); at α4β3δ | −6.7 ± 3.3% (N = 11, NS) |
| 1 μM ISO + 0.1 μM THDOC + 3 μM GABA (%); at α4β3δ | −26 ± 2.4% (N = 13, P = 0.001) |
| 1 μM ISO + 0.1 μM THDOC, baseline (%); at α4β3δ | −43 ± 3.5% (N = 11, P = 0.003) |
| THDOC (μM) Baselineshift | Mean ± SEM pA (N; P) |
|---|---|
| 0.03 | +5 ± 3.3 pA (N = 9; P = 0.008) |
| 0.1 | +15 ± 9.5 pA (N = 9; P = 0.003) |
| 0.3 | +12 ± 7.9 pA (N = 9; P = 0.019) |
| 1 | +69 ± 35 pA (N = 3; P = 0.018) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bäckström, T.; Bengtsson, S.K.S.; Sjöstedt, J.; Malinina, E.; Johansson, M.; Ragagnin, G.; Ekberg, K.; Lundgren, P. Isoallopregnanolone Inhibits Estrus Cycle-Dependent Aggressive Behavior. Biomolecules 2023, 13, 1017. https://doi.org/10.3390/biom13061017
Bäckström T, Bengtsson SKS, Sjöstedt J, Malinina E, Johansson M, Ragagnin G, Ekberg K, Lundgren P. Isoallopregnanolone Inhibits Estrus Cycle-Dependent Aggressive Behavior. Biomolecules. 2023; 13(6):1017. https://doi.org/10.3390/biom13061017
Chicago/Turabian StyleBäckström, Torbjörn, Sara K. S. Bengtsson, Jessica Sjöstedt, Evgenya Malinina, Maja Johansson, Gianna Ragagnin, Karin Ekberg, and Per Lundgren. 2023. "Isoallopregnanolone Inhibits Estrus Cycle-Dependent Aggressive Behavior" Biomolecules 13, no. 6: 1017. https://doi.org/10.3390/biom13061017
APA StyleBäckström, T., Bengtsson, S. K. S., Sjöstedt, J., Malinina, E., Johansson, M., Ragagnin, G., Ekberg, K., & Lundgren, P. (2023). Isoallopregnanolone Inhibits Estrus Cycle-Dependent Aggressive Behavior. Biomolecules, 13(6), 1017. https://doi.org/10.3390/biom13061017






