Space Environment Impacts Homeostasis: Exposure to Spaceflight Alters Mammary Gland Transportome Genes
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Treatment Conditions
2.2. Isolation of Total RNA
2.3. RNA Preparation for Microarrays
2.4. Microarray Gene Expression Analysis
2.5. In-Vitro Glucose Metabolic Assays
2.6. Quantitative Polymerase Chain Reaction (q-PCR)
3. Results
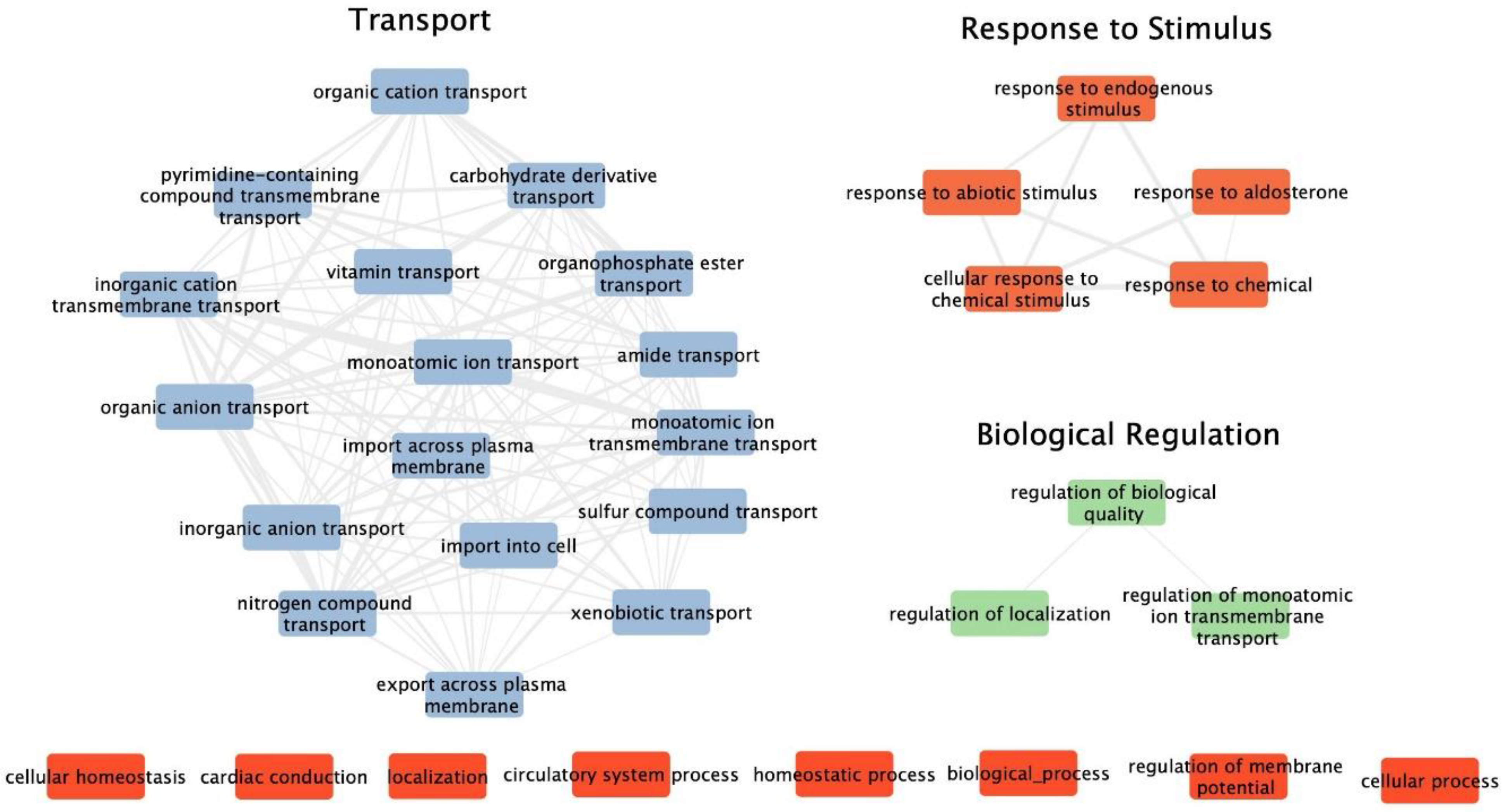
3.1. Functional Enrichment Analysis of Differentially Expressed Genes
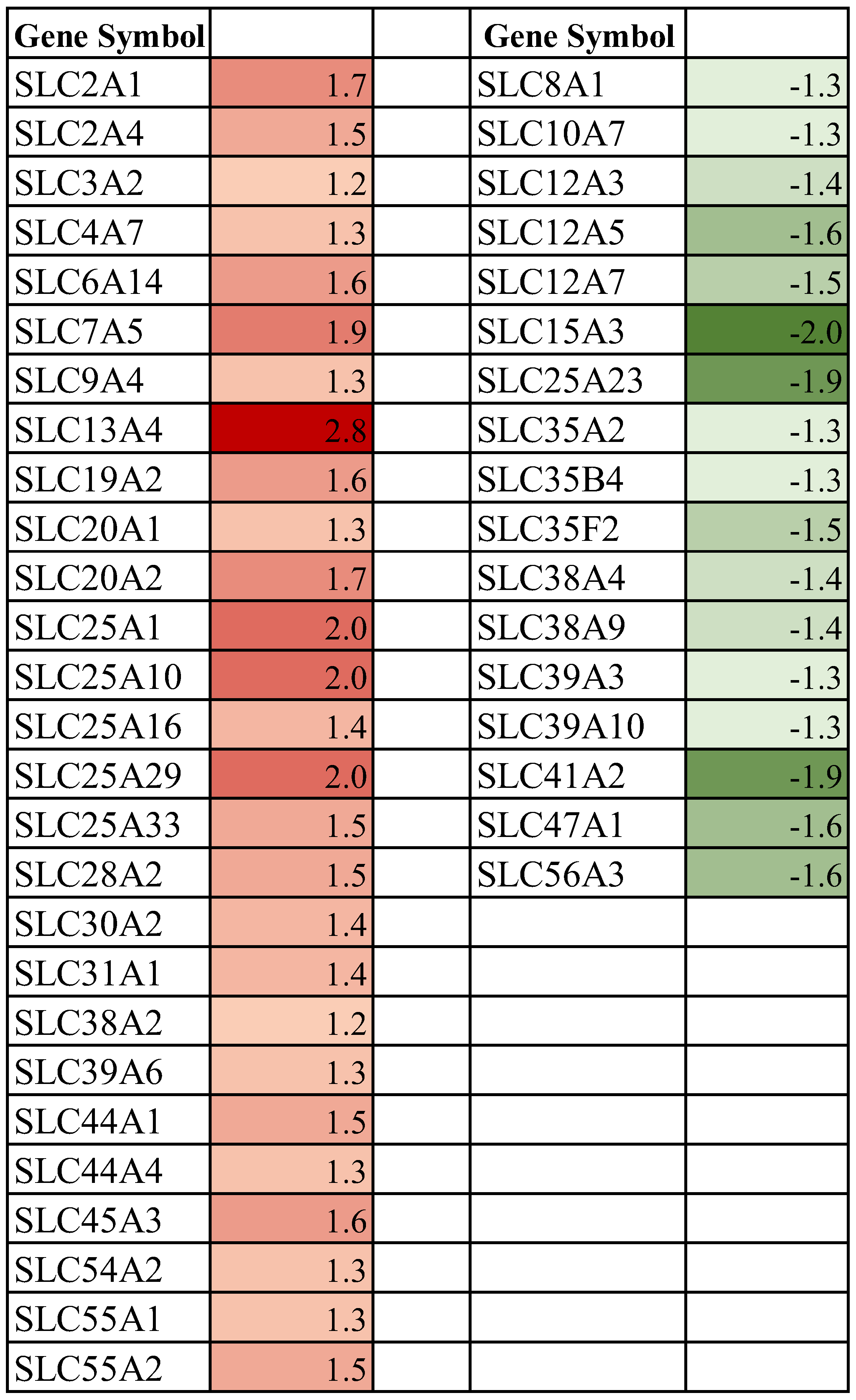
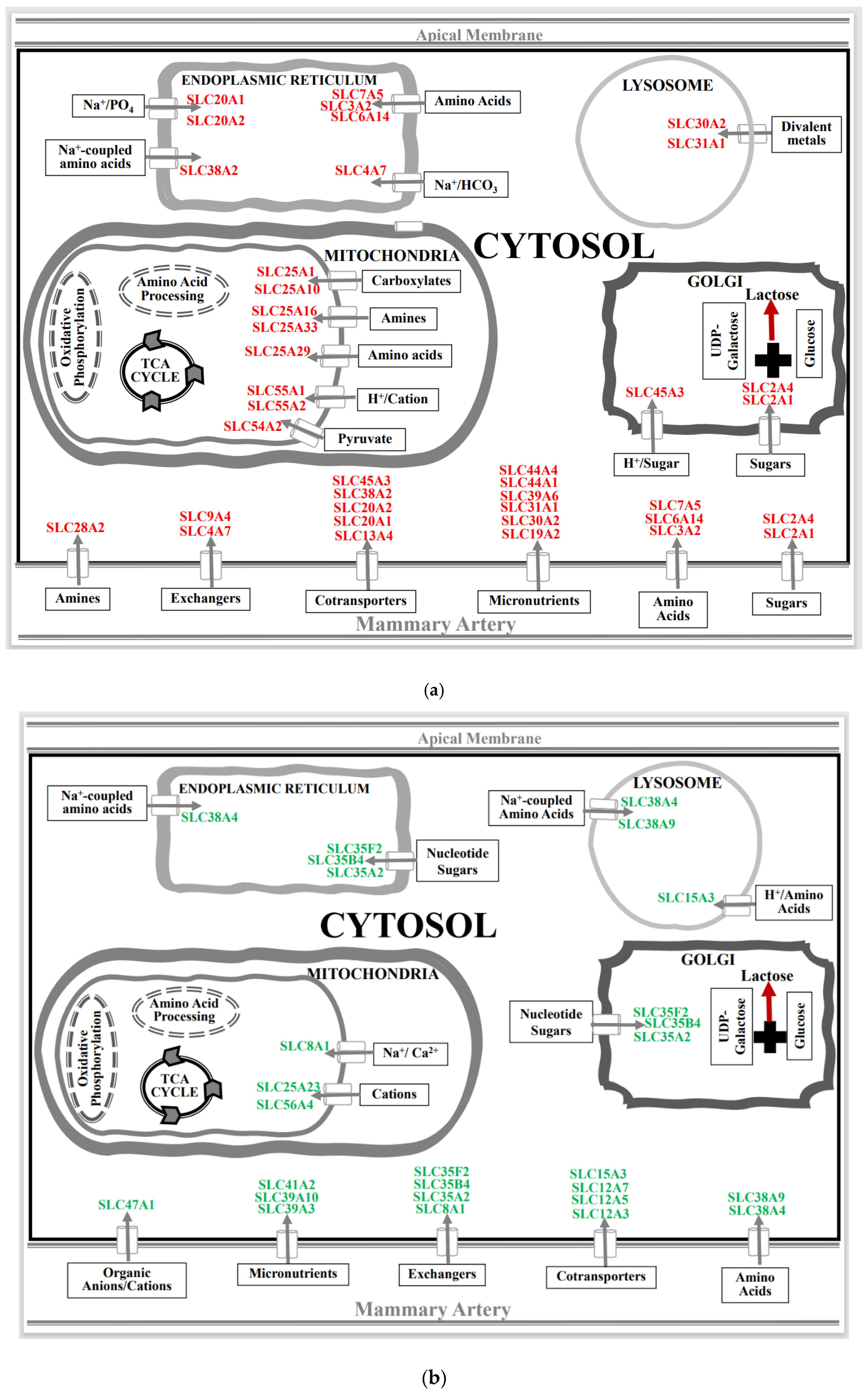
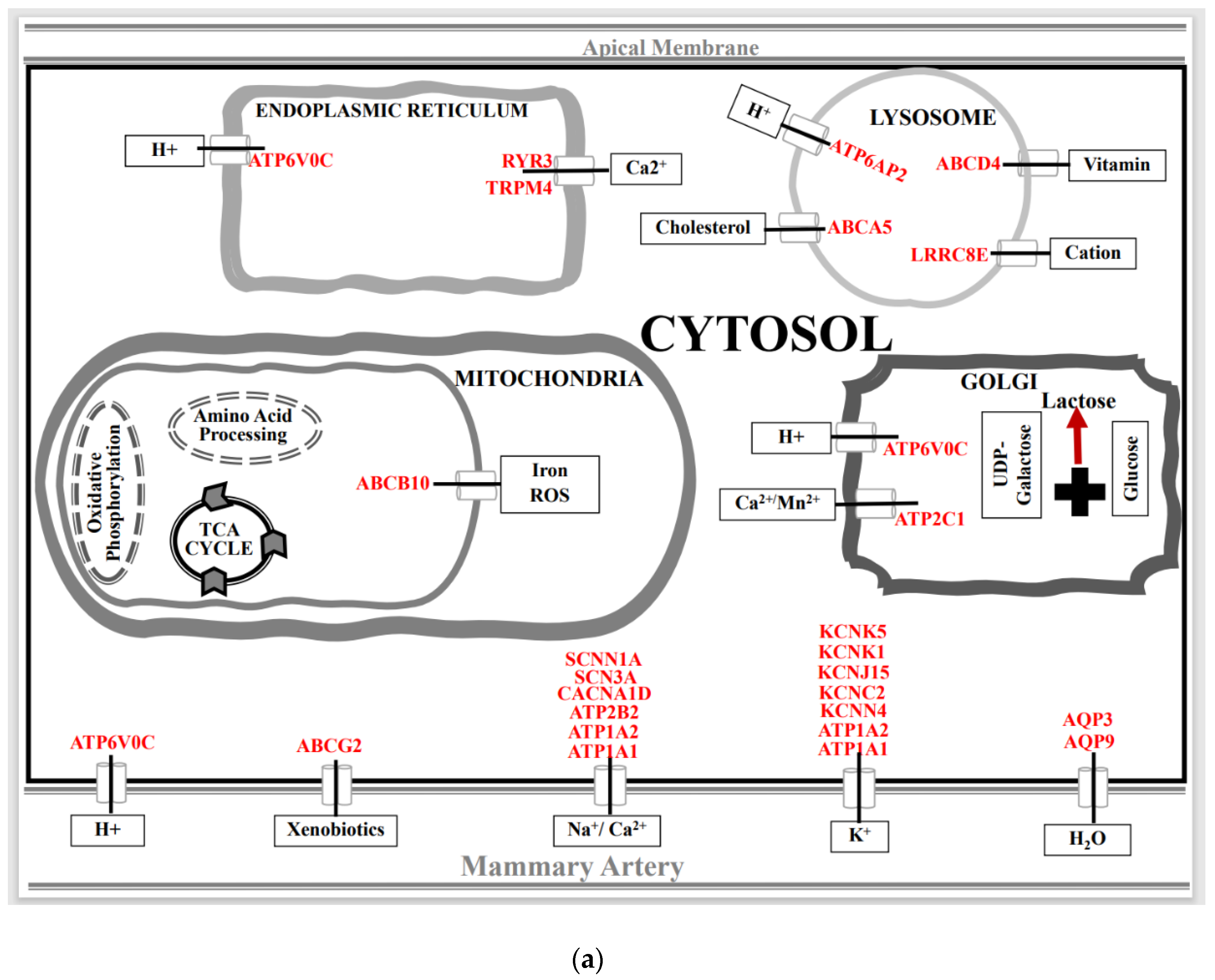
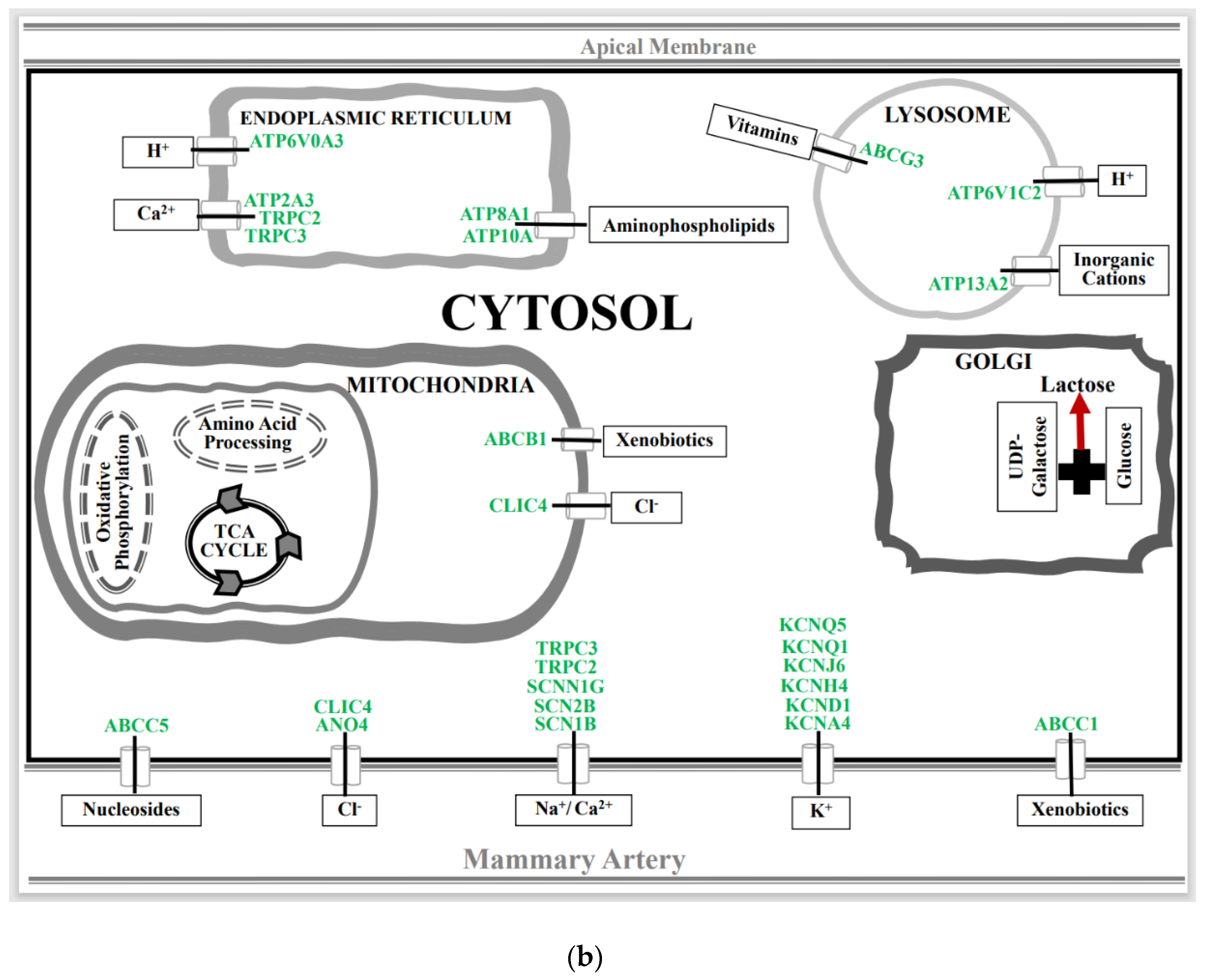
3.2. Effect of Spaceflight on Solute Carrier (SLC) Membrane Transporter Genes in the Mammary Gland of Pregnant Rats
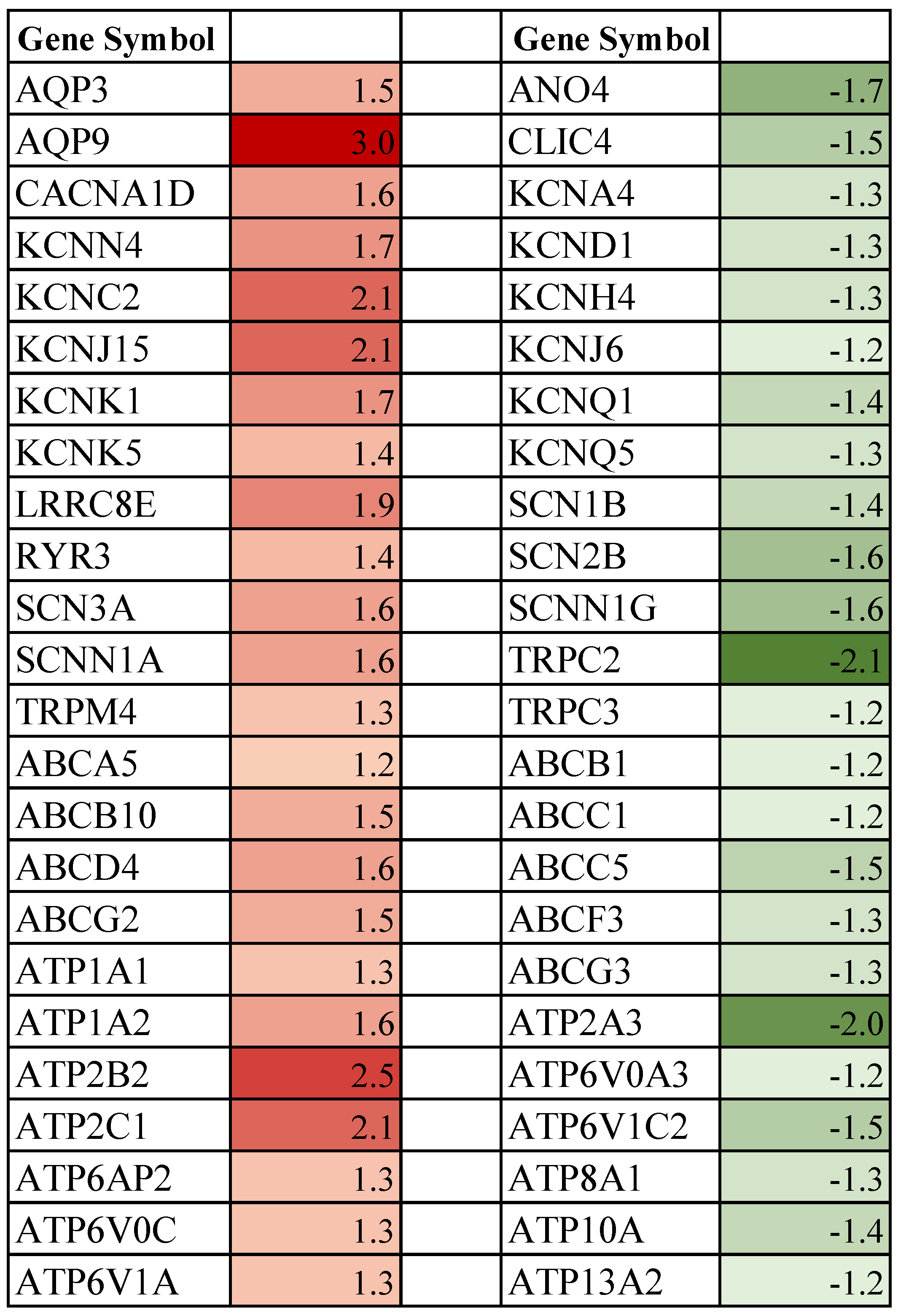
3.3. Effect of Spaceflight on Ionic Channels, ABC, and ATPase Transporter Genes in the Mammary Gland of Pregnant Rats
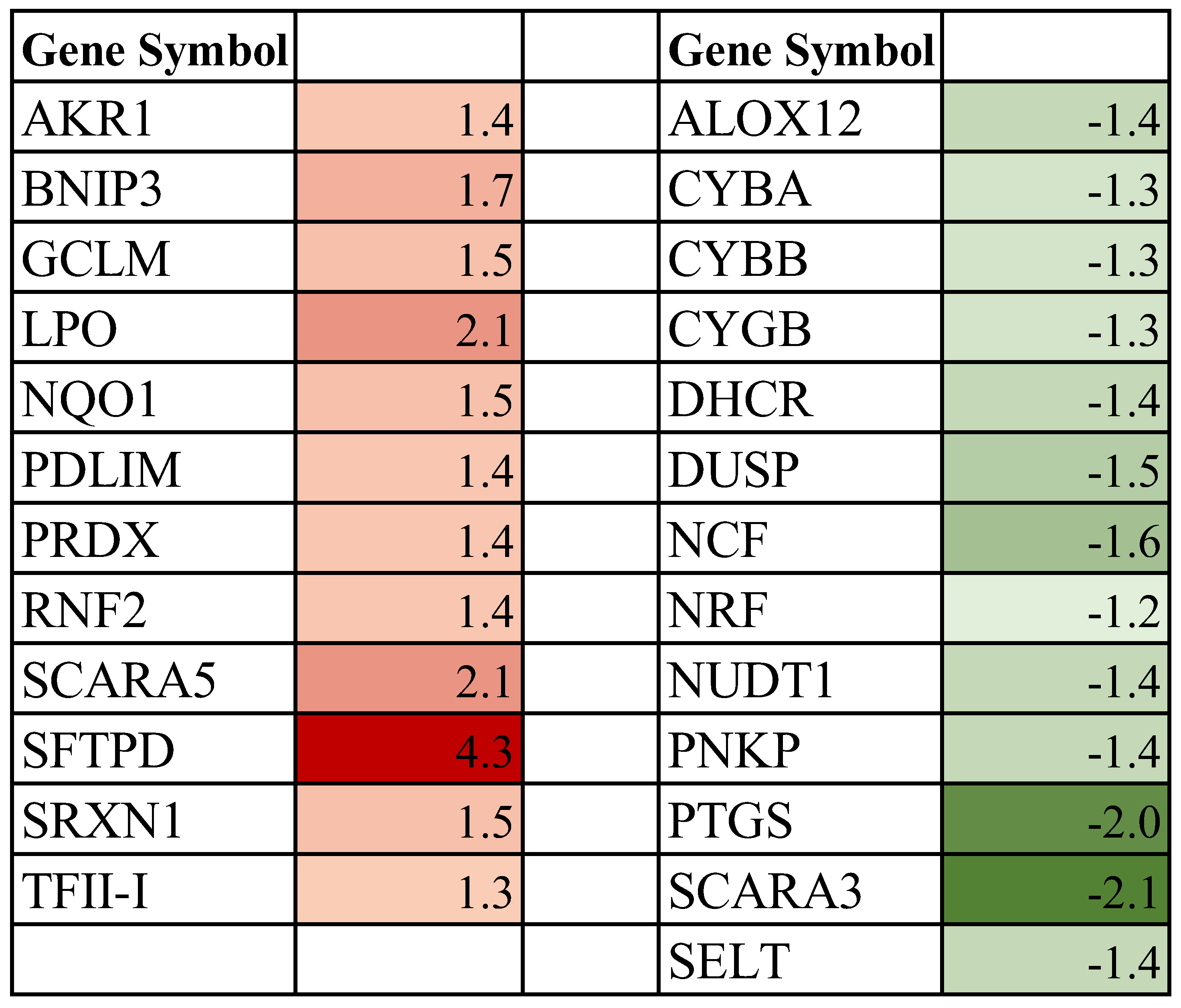
3.4. Effect of Spaceflight on Genes Associated with Cellular Redox Process in the Mammary Gland of Pregnant Rats
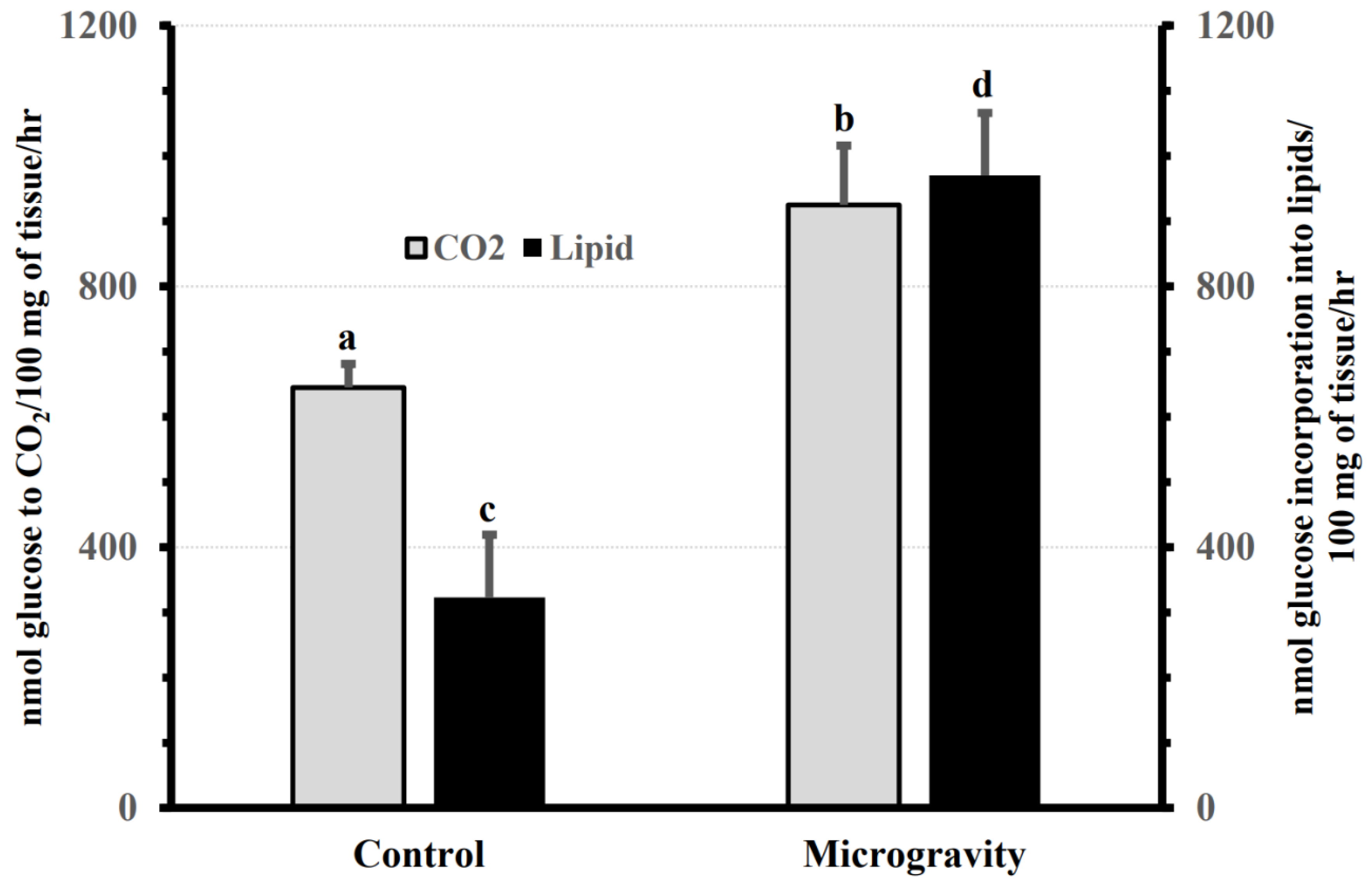
3.5. Effect of Spaceflight on the Rate of Labeled Glucose Oxidation and Incorporation in Lipids in the Mammary Gland of Pregnant Rats
4. Discussion
4.1. Spaceflight and Solute Carrier (SLC) Membrane Transporters
4.2. Spaceflight and Membrane Cotransporters and Exchangers
4.3. Spaceflight and Micronutrient Membrane Transporters
4.4. Spaceflight and Ion-Channels
4.5. Spaceflight and ABC and ATPase Transporters
4.6. Spaceflight and Cellular Redox Process
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Crane, L. SpaceX’s first crewed flight is a go. New Sci. 2020, 246, 17. [Google Scholar] [CrossRef] [PubMed]
- Witze, A. SpaceX to Launch Astronauts—And a New Era of Private Human Spaceflight. Nature 2020. Available online: https://www.nature.com/articles/d41586-020-01554-8 (accessed on 14 March 2022).
- Patel, Z.S.; Brunstetter, T.J.; Tarver, W.J.; Whitmire, A.M.; Zwart, S.R.; Smith, S.M.; Huff, J.L. Red Risks for a Journey to the Red Planet: The Highest Priority Human Health Risks for a Mission to Mars. NPJ Microgravity 2020, 6, 33. Available online: https://pubmed.ncbi.nlm.nih.gov/33298950/ (accessed on 14 March 2022). [CrossRef] [PubMed]
- Haws, T.D.; Zimmerman, J.S.; Fuller, M.E. SLS, the Gateway, and a Lunar Outpost in the Early 2030s. In Proceedings of the IEEE Aerospace Conference, Big Sky, MT, USA, 2–9 March 2019; pp. 1–15. Available online: https://ieeexplore.ieee.org/abstract/document/8741598/ (accessed on 14 March 2022).
- Fine, L.G. Looking Back 50 Years at the Biology of Mankind in Space: The Renal-Cardiovascular Fluid Shift Conundrum. J. Am. Soc. Nephrol. 2019, 30, 2288–2292. [Google Scholar] [CrossRef]
- Bonnefoy, J.; Ghislin, S.; Beyrend, J.; Coste, F.; Calcagno, G.; Lartaud, I.; Gauquelin-Koch, G.; Poussier, S.; Frippiat, J.-P. Gravitational Experimental Platform for Animal Models, a New Platform at ESA’s Terrestrial Facilities to Study the Effects of Micro- and Hypergravity on Aquatic and Rodent Animal Models. Int. J. Mol. Sci. 2021, 22, 2961. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.; Luderer, U. Reproductive hazards of space travel in women and men. Nat. Rev. Endocrinol. 2019, 15, 713–730. [Google Scholar] [CrossRef]
- Kim, H.; Shin, Y.; Kim, D.-H. Mechanobiological Implications of Cancer Progression in Space. Front. Cell Dev. Biol. 2021, 9, 740009. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8692837/ (accessed on 14 March 2022). [CrossRef]
- Morey-Holton, E.R.; Hill, E.L.; Souza, K.A. Animals and spaceflight: From survival to understanding. J. Musculoskelet. Neuronal Interact. 2007, 7, 9. [Google Scholar]
- Adamopoulos, K.; Koutsouris, D.; Zaravinos, A.; Lambrou, G.I. Gravitational Influence on Human Living Systems and the Evolution of Species on Earth. Molecules 2021, 26, 2784. [Google Scholar] [CrossRef]
- Ehler, E. Cardiac cytoarchitecture—Why the “hardware” is important for heart function! Biochim. Biophys. Acta BBA Mol. Cell Res. 2016, 1863, 1857–1863. [Google Scholar] [CrossRef]
- Garoffolo, G.; Pesce, M. Mechanotransduction in the Cardiovascular System: From Developmental Origins to Homeostasis and Pathology. Cells 2019, 8, 1607. [Google Scholar] [CrossRef]
- Felsenthal, N.; Zelzer, E. Mechanical regulation of musculoskeletal system development. Dev. Camb. Engl. 2017, 144, 4271. [Google Scholar] [CrossRef] [PubMed]
- Murphy, A.C.; Muldoon, S.F.; Baker, D.; Lastowka, A.; Bennett, B.; Yang, M.; Bassett, D.S. Structure, function, and control of the human musculoskeletal network. PLoS Biol. 2018, 16, e2002811. [Google Scholar] [CrossRef] [PubMed]
- Vernice, N.A.; Meydan, C.; Afshinnekoo, E.; Mason, C.E. Long-term spaceflight and the cardiovascular system. Precis. Clin. Med. 2020, 3, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Baran, R.; Marchal, S.; Campos, S.G.; Rehnberg, E.; Tabury, K.; Baselet, B.; Wehland, M.; Grimm, D.; Baatout, S. The Cardiovascular System in Space: Focus on In Vivo and In Vitro Studies. Biomedicines 2022, 10, 59. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8773383/ (accessed on 14 March 2022). [CrossRef]
- Juhl, O.J.; Buettmann, E.G.; Friedman, M.A.; DeNapoli, R.C.; Hoppock, G.A.; Donahue, H.J. Update on the effects of microgravity on the musculoskeletal system. Npj Microgravity 2021, 7, 28. [Google Scholar] [CrossRef]
- Comfort, P.; McMahon, J.J.; Jones, P.A.; Cuthbert, M.; Kendall, K.; Lake, J.P.; Haff, G.G. Effects of Spaceflight on Musculoskeletal Health: A Systematic Review and Meta-analysis, Considerations for Interplanetary Travel. Sports Med. 2021, 51, 2097–2114. [Google Scholar] [CrossRef]
- Teodori, L.; Costa, A.; Campanella, L.; Albertini, M.C. Skeletal Muscle Atrophy in Simulated Microgravity Might Be Triggered by Immune-Related microRNAs. Front. Physiol. 2019, 9, 1926. Available online: https://www.frontiersin.org/articles/ (accessed on 14 March 2022). [CrossRef]
- Afshinnekoo, E.; Scott, R.T.; MacKay, M.J.; Pariset, E.; Cekanaviciute, E.; Barker, R.; Gilroy, S.; Hassane, D.; Smith, S.M.; Zwart, S.R.; et al. Fundamental Biological Features of Spaceflight: Advancing the Field to Enable Deep-Space Exploration. Cell 2020, 183, 1162–1184. [Google Scholar] [CrossRef]
- Capuco, A.V.; Ellis, S.E. Comparative aspects of mammary gland development and homeostasis. Annu. Rev. Anim. Biosci. 2013, 1, 179–202. [Google Scholar] [CrossRef]
- Macias, H.; Hinck, L. Mammary Gland Development. Wiley Interdiscip. Rev. Dev. Biol. 2012, 1, 533–557. [Google Scholar] [CrossRef]
- Slepicka, P.F.; Somasundara, A.V.H.; Dos Santos, C.O. The molecular basis of mammary gland development and epithelial differentiation. Semin. Cell Dev. Biol. 2021, 114, 93–112. [Google Scholar] [CrossRef] [PubMed]
- Inman, J.L.; Robertson, C.; Mott, J.D.; Bissell, M.J. Mammary Gland Development: Cell Fate Specification, Stem Cells and the Microenvironment. Dev. Camb. Engl. 2015, 142, 1028–1042. Available online: https://pubmed.ncbi.nlm.nih.gov/25758218/ (accessed on 14 March 2022). [CrossRef] [PubMed]
- Fu, N.Y.; Nolan, E.; Lindeman, G.J.; Visvader, J.E. Stem Cells and the Differentiation Hierarchy in Mammary Gland Development. Physiol. Rev. 2020, 100, 489–523. [Google Scholar] [CrossRef] [PubMed]
- Pizzagalli, M.D.; Bensimon, A.; Superti-Furga, G. A guide to plasma membrane solute carrier proteins. FEBS J. 2021, 288, 2784–2835. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Moraes, T.F.; Reithmeier, R.A.F. Structural biology of solute carrier (SLC) membrane transport proteins. Mol. Membr. Biol. 2017, 34, 1–32. [Google Scholar] [CrossRef]
- Jennings, M.L. Carriers, exchangers, and cotransporters in the first 100 years of the Journal of General Physiology. J. Gen. Physiol. 2018, 150, 1063–1080. [Google Scholar] [CrossRef]
- Zeidel, M.L. Water homeostasis: Evolutionary Medicine. Trans. Am. Clin. Climatol. Assoc. 2012, 123, 93–105. Available online: https://pubmed.ncbi.nlm.nih.gov/23303973/ (accessed on 14 March 2022).
- Delpire, E.; Gagnon, K.B. Water Homeostasis and Cell Volume Maintenance and Regulation. Curr. Top. Membr. 2018, 81, 3–52. Available online: https://pubmed.ncbi.nlm.nih.gov/30243436/ (accessed on 14 March 2022).
- Lin, L.; Yee, S.W.; Kim, R.B.; Giacomini, K.M. SLC Transporters as Therapeutic Targets: Emerging Opportunities. Nat. Rev. Drug Discov. 2015, 14, 543–560. [Google Scholar] [CrossRef]
- Schumann, T.; König, J.; Henke, C.; Willmes, D.M.; Bornstein, S.R.; Jordan, J.; Fromm, M.F.; Birkenfeld, A.L. Solute Carrier Transporters as Potential Targets for the Treatment of Metabolic Disease. Pharmacol. Rev. 2020, 72, 343–379. Available online: https://pubmed.ncbi.nlm.nih.gov/31882442/ (accessed on 14 March 2022). [CrossRef]
- Demirbilek, H.; Galcheva, S.; Vuralli, D.; Al-Khawaga, S.; Hussain, K. Ion Transporters, Channelopathies, and Glucose Disorders. Int. J. Mol. Sci. 2019, 20, 2590. Available online: https://pubmed.ncbi.nlm.nih.gov/31137773/ (accessed on 14 March 2022). [CrossRef] [PubMed]
- Ayka, A.; Şehirli, A.Ö. The Role of the SLC Transporters Protein in the Neurodegenerative Disorders. Clin. Psychopharmacol. Neurosci. 2020, 18, 174–187. Available online: https://pubmed.ncbi.nlm.nih.gov/32329299/ (accessed on 14 March 2022). [CrossRef] [PubMed]
- Sutherland, R.; Meeson, A.; Lowes, S. Solute Transporters and Malignancy: Establishing the Role of Uptake Transporters in Breast Cancer and Breast Cancer Metastasis. Cancer Metastasis Rev. 2020, 39, 919–932. Available online: https://pubmed.ncbi.nlm.nih.gov/32388639/ (accessed on 14 March 2022). [CrossRef] [PubMed]
- Masini, M.A.; Albi, E.; Barmo, C.; Bonfiglio, T.; Bruni, L.; Canesi, L.; Cataldi, S.; Curcio, F.; D’Amora, M.; Ferri, I.; et al. The Impact of Long-Term Exposure to Space Environment on Adult Mammalian Organisms: A Study on Mouse Thyroid and Testis. PLoS ONE 2012, 7, e35418. [Google Scholar] [CrossRef]
- Costa, F.; Ambesi-Impiombato, F.S.; Beccari, T.; Conte, C.; Cataldi, S.; Curcio, F.; Albi, E. Spaceflight Induced Disorders: Potential Nutritional Countermeasures. Front. Bioeng. Biotechnol. 2021, 9, 666683. Available online: https://www.frontiersin.org/articles/10.3389/fbioe.2021.666683 (accessed on 14 March 2022). [CrossRef] [PubMed]
- Crucian, B.E.; Choukèr, A.; Simpson, R.J.; Mehta, S.; Marshall, G.; Smith, S.M.; Zwart, S.R.; Heer, M.; Ponomarev, S.; Whitmire, A.; et al. Immune System Dysregulation During Spaceflight: Potential Countermeasures for Deep Space Exploration Missions. Front. Immunol. 2018, 9, 1437. Available online: https://www.frontiersin.org/articles/10.3389/fimmu.2018.01437/full (accessed on 14 March 2022). [CrossRef]
- Akiyama, T.; Horie, K.; Hinoi, E.; Hiraiwa, M.; Kato, A.; Maekawa, Y.; Takahashi, A.; Furukawa, S. How does spaceflight affect the acquired immune system? Npj Microgravity 2020, 6, 14. [Google Scholar] [CrossRef]
- Neutelings, T.; Nusgens, B.V.; Liu, Y.; Tavella, S.; Ruggiu, A.; Cancedda, R.; Gabriel, M.; Colige, A.; Lambert, C. Skin physiology in microgravity: A 3-month stay aboard ISS induces dermal atrophy and affects cutaneous muscle and hair follicles cycling in mice. Npj Microgravity 2015, 1, 15002. [Google Scholar] [CrossRef]
- Terada, M.; Seki, M.; Takahashi, R.; Yamada, S.; Higashibata, A.; Majima, H.J.; Sudoh, M.; Mukai, C.; Ishioka, N. Effects of a Closed Space Environment on Gene Expression in Hair Follicles of Astronauts in the International Space Station. PLoS ONE 2016, 11, e0150801. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4814050/ (accessed on 14 March 2022).
- Yang, J.-Q.; Jiang, N.; Li, Z.-P.; Guo, S.; Chen, Z.-Y.; Li, B.-B.; Chai, S.-B.; Lu, S.-Y.; Yan, H.-F.; Sun, P.-M.; et al. The effects of microgravity on the digestive system and the new insights it brings to the life sciences. Life Sci. Space Res. 2020, 27, 74–82. [Google Scholar] [CrossRef]
- Turroni, S.; Magnani, M.; Kc, P.; Lesnik, P.; Vidal, H.; Heer, M. Gut Microbiome and Space Travelers’ Health: State of the Art and Possible Pro/Prebiotic Strategies for Long-Term Space Missions. Front. Physiol. 2020, 11, 553929. Available online: https://www.frontiersin.org/articles/10.3389/fphys.2020.553929/full (accessed on 14 March 2022). [CrossRef] [PubMed]
- Jandial, R.; Hoshide, R.; Waters, J.D.; Limoli, C.L. Space–Brain: The Negative Effects of Space Exposure on the Central Nervous System. Surg. Neurol. Int. 2018, 9, 9. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5791508/ (accessed on 14 March 2022). [PubMed]
- Roy-O’Reilly, M.; Mulavara, A.; Williams, T. A review of alterations to the brain during spaceflight and the potential relevance to crew in long-duration space exploration. Npj Microgravity 2021, 7, 5. [Google Scholar] [CrossRef]
- Lee, A.G.; Mader, T.H.; Gibson, C.R.; Tarver, W.; Rabiei, P.; Riascos, R.F.; Galdamez, L.A.; Brunstetter, T. Spaceflight associated neuro-ocular syndrome (SANS) and the neuro-ophthalmologic effects of microgravity: A review and an update. Npj Microgravity 2020, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.S.; Stenger, M.B.; Macias, B.R. Gravitational Influence on Intraocular Pressure: Implications for Spaceflight and Disease. J. Glaucoma 2019, 28, 756–764. Available online: https://pubmed.ncbi.nlm.nih.gov/31162175/ (accessed on 14 March 2022). [CrossRef] [PubMed]
- Ronca, A.E.; Baker, E.S.; Bavendam, T.G.; Beck, K.D.; Miller, V.M.; Tash, J.S.; Jenkins, M. Effects of Sex and Gender on Adaptations to Space: Reproductive Health. J. Womens Health 2014, 23, 967. [Google Scholar] [CrossRef] [PubMed]
- Patel, O.V.; Casey, T.; Dover, H.; Plaut, K. Homeorhetic adaptation to lactation: Comparative transcriptome analysis of mammary, liver, and adipose tissue during the transition from pregnancy to lactation in rats. Funct. Integr. Genom. 2011, 11, 193–202. [Google Scholar] [CrossRef]
- Patel, O.V.; Casey, T.; Plaut, K. Profiling Solute-Carrier Transporters in Key Metabolic Tissues during the Postpartum Evolution of Mammary Epithelial Cells from Nonsecretory to Secretory. Physiol. Genom. 2019, 51, 539–552. [Google Scholar] [CrossRef]
- Casey, T.; Patel, O.V.; Plaut, K. Transcriptomes reveal alterations in gravity impact circadian clocks and activate mechanotransduction pathways with adaptation through epigenetic change. Physiol. Genom. 2015, 47, 113–128. [Google Scholar] [CrossRef]
- Plaut, K.; Maple, R.; Vyas, C.; Munaim, S.; Darling, A.; Casey, T.; Alberts, J.R. The effects of spaceflight on mammary metabolism in pregnant rats. Proc. Soc. Exp. Biol. Med. 1999, 222, 85–89. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef] [PubMed]
- Pawitan, Y.; Michiels, S.; Koscielny, S.; Gusnanto, A.; Ploner, A. False discovery rate, sensitivity and sample size for microarray studies. Bioinformatics 2005, 21, 3017–3024. [Google Scholar] [CrossRef] [PubMed]
- van Iterson, M.; Boer, J.M.; Menezes, R.X. Filtering, FDR and power. BMC Bioinform. 2010, 11, 450. [Google Scholar] [CrossRef] [PubMed]
- Tauber, S.; Christoffel, S.; Thiel, C.S.; Ullrich, O. Transcriptional Homeostasis of Oxidative Stress-Related Pathways in Altered Gravity. Int. J. Mol. Sci. 2018, 19, 2814. [Google Scholar] [CrossRef]
- Ushio-Fukai, M.; Ash, D.; Nagarkoti, S.; Belin de Chantemèle, E.J.; Fulton, D.J.R.; Fukai, T. Interplay Between Reactive Oxygen/Reactive Nitrogen Species and Metabolism in Vascular Biology and Disease. Antioxid. Redox Signal. 2021, 34, 1319–1354. [Google Scholar] [CrossRef]
- Patel, O.V.; Suchyta, S.; Sipkovsky, S.; Yao, J.; Ireland, J.; Coussens, P.; Smith, G.W. Validation and application of a high fidelity mRNA linear amplification procedure for profiling gene expression. Vet. Immunol. Immunopathol. 2005, 105, 331–342. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Rm, A. A 100-Year Review: Mammary Development and Lactation. J. Dairy Sci. 2017, 100, 10332–10352. Available online: https://pubmed.ncbi.nlm.nih.gov/29153168/ (accessed on 14 March 2022).
- Marshall, B.A.; Hansen, P.A.; Ensor, N.J.; Ogden, M.A.; Mueckler, M. GLUT-1 or GLUT-4 transgenes in obese mice improve glucose tolerance but do not prevent insulin resistance. Am. J. Physiol. 1999, 276, E390–E400. [Google Scholar] [CrossRef]
- Vitavska, O.; Edemir, B.; Wieczorek, H. Putative role of the H(+)/sucrose symporter SLC45A3 as an osmolyte transporter in the kidney. Pflug. Arch. 2016, 468, 1353–1362. [Google Scholar] [CrossRef]
- Hadsell, D.L.; Olea, W.; Wei, J.; Fiorotto, M.L.; Matsunami, R.K.; Engler, D.A.; Collier, R.J. Developmental regulation of mitochondrial biogenesis and function in the mouse mammary gland during a prolonged lactation cycle. Physiol. Genom. 2011, 43, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Mowry, A.V.; Donoviel, Z.S.; Kavazis, A.N.; Hood, W.R. Mitochondrial function and bioenergetic trade-offs during lactation in the house mouse (Mus musculus). Ecol. Evol. 2017, 7, 2994–3005. [Google Scholar] [CrossRef] [PubMed]
- Vacanti, N.M.; Divakaruni, A.S.; Green, C.R.; Parker, S.J.; Henry, R.R.; Ciaraldi, T.P.; Murphy, A.N.; Metallo, C.M. Regulation of substrate utilization by the mitochondrial pyruvate carrier. Mol. Cell 2014, 56, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Catalina-Rodriguez, O.; Kolukula, V.K.; Tomita, Y.; Preet, A.; Palmieri, F.; Wellstein, A.; Byers, S.; Giaccia, A.J.; Glasgow, E.; Albanese, C.; et al. The mitochondrial citrate transporter, CIC, is essential for mitochondrial homeostasis. Oncotarget 2012, 3, 1220–1235. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, R.; Tabata, S.; Shindo, Y.; Hotta, K.; Suzuki, K.; Soga, T.; Oka, K. Mitochondrial Mg(2+) homeostasis decides cellular energy metabolism and vulnerability to stress. Sci. Rep. 2016, 6, 30027. [Google Scholar] [CrossRef] [PubMed]
- Bhutia, Y.D.; Babu, E.; Prasad, P.D.; Ganapathy, V. The amino acid transporter SLC6A14 in cancer and its potential use in chemotherapy. Asian J. Pharm. Sci. 2014, 9, 293–303. [Google Scholar] [CrossRef]
- Karunakaran, S.; Ramachandran, S.; Coothankandaswamy, V.; Elangovan, S.; Babu, E.; Periyasamy-Thandavan, S.; Gurav, A.; Gnanaprakasam, J.P.; Singh, N.; Schoenlein, P.V.; et al. SLC6A14 (ATB0,+) protein, a highly concentrative and broad specific amino acid transporter, is a novel and effective drug target for treatment of estrogen receptor-positive breast cancer. J. Biol. Chem. 2011, 286, 31830–31838. [Google Scholar] [CrossRef] [PubMed]
- Menchini, R.J.; Chaudhry, F.A. Multifaceted regulation of the system A transporter Slc38a2 suggests nanoscale regulation of amino acid metabolism and cellular signaling. Neuropharmacology 2019, 161, 107789. [Google Scholar] [CrossRef]
- Birukov, A.; Rakova, N.; Lerchl, K.; Olde Engberink, R.H.; Johannes, B.; Wabel, P.; Moissl, U.; Rauh, M.; Luft, F.C.; Titze, J. Ultra-long-term human salt balance studies reveal interrelations between sodium, potassium, and chloride intake and excretion. Am. J. Clin. Nutr. 2016, 104, 49–57. [Google Scholar] [CrossRef]
- Crawford, B.E.; Garner, O.B.; Bishop, J.R.; Zhang, D.Y.; Bush, K.T.; Nigam, S.K.; Esko, J.D. Loss of the heparan sulfate sulfotransferase, Ndst1, in mammary epithelial cells selectively blocks lobuloalveolar development in mice. PLoS ONE 2010, 5, e10691. [Google Scholar] [CrossRef]
- Biber, J.; Hernando, N.; Forster, I. Phosphate transporters and their function. Annu. Rev. Physiol. 2013, 75, 535–550. [Google Scholar] [CrossRef] [PubMed]
- Onaga, C.; Tamori, S.; Motomura, H.; Ozaki, A.; Matsuda, C.; Matsuoka, I.; Fujita, T.; Nozaki, Y.; Hara, Y.; Kawano, Y.; et al. High SLC20A1 Expression Is Associated With Poor Prognoses in Claudin-low and Basal-like Breast Cancers. Anticancer Res. 2021, 41, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Hadley, B.; Litfin, T.; Day, C.J.; Haselhorst, T.; Zhou, Y.; Tiralongo, J. Nucleotide Sugar Transporter SLC35 Family Structure and Function. Comput. Struct. Biotechnol. J. 2019, 17, 1123–1134. [Google Scholar] [CrossRef] [PubMed]
- Hara, T.; Takeda, T.-A.; Takagishi, T.; Fukue, K.; Kambe, T.; Fukada, T. Physiological roles of zinc transporters: Molecular and genetic importance in zinc homeostasis. J. Physiol. Sci. JPS 2017, 67, 283–301. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Hennigar, S.R.; Alam, S.; Nishida, K.; Kelleher, S.L. Essential Role for Zinc Transporter 2 (ZnT2)-mediated Zinc Transport in Mammary Gland Development and Function during Lactation. J. Biol. Chem. 2015, 290, 13064–13078. [Google Scholar] [CrossRef]
- Puchkova, L.V.; Babich, P.S.; Zatulovskaia, Y.A.; Ilyechova, E.Y.; Di Sole, F. Copper Metabolism of Newborns Is Adapted to Milk Ceruloplasmin as a Nutritive Source of Copper: Overview of the Current Data. Nutrients 2018, 10, 1591. [Google Scholar] [CrossRef]
- Chao, C.K.; Pomfret, E.A.; Zeisel, S.H. Uptake of choline by rat mammary-gland epithelial cells. Biochem. J. 1988, 254, 33–38. [Google Scholar] [CrossRef]
- Kim, J.-B. Channelopathies. Korean J. Pediatr. 2014, 57, 1–18. [Google Scholar] [CrossRef]
- Calamita, G.; Delporte, C. Involvement of aquaglyceroporins in energy metabolism in health and disease. Biochimie 2021, 188, 20–34. [Google Scholar] [CrossRef]
- Medina, Y.; Acosta, L.; Reppetti, J.; Corominas, A.; Bustamante, J.; Szpilbarg, N.; Damiano, A.E. Lactic Acid Transport Mediated by Aquaporin-9: Implications on the Pathophysiology of Preeclampsia. Front. Physiol. 2021, 12, 774095. [Google Scholar] [CrossRef]
- Tradtrantip, L.; Jin, B.-J.; Yao, X.; Anderson, M.O.; Verkman, A.S. Aquaporin-Targeted Therapeutics: State-of-the-Field. Adv. Exp. Med. Biol. 2017, 969, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Abir-Awan, M.; Kitchen, P.; Salman, M.M.; Conner, M.T.; Conner, A.C.; Bill, R.M. Inhibitors of Mammalian Aquaporin Water Channels. Int. J. Mol. Sci. 2019, 20, 1589. [Google Scholar] [CrossRef] [PubMed]
- Lang, F.; Föller, M.; Lang, K.S.; Lang, P.A.; Ritter, M.; Gulbins, E.; Vereninov, A.; Huber, S.M. Ion channels in cell proliferation and apoptotic cell death. J. Membr. Biol. 2005, 205, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Azimi, I.; Roberts-Thomson, S.J.; Monteith, G.R. Calcium influx pathways in breast cancer: Opportunities for pharmacological intervention. Br. J. Pharmacol. 2014, 171, 945–960. [Google Scholar] [CrossRef]
- Cohen, B.J.; Lechene, C. (Na,K)-pump: Cellular role and regulation in nonexcitable cells. Biol. Cell 1989, 66, 191–195. [Google Scholar] [CrossRef]
- Therien, A.G.; Blostein, R. Mechanisms of sodium pump regulation. Am. J. Physiol. Cell Physiol. 2000, 279, C541–C566. [Google Scholar] [CrossRef]
- Shennan, D.B.; Peaker, M. Transport of milk constituents by the mammary gland. Physiol. Rev. 2000, 80, 925–951. [Google Scholar] [CrossRef]
- Breuer, E.-K.; Fukushiro-Lopes, D.; Dalheim, A.; Burnette, M.; Zartman, J.; Kaja, S.; Wells, C.; Campo, L.; Curtis, K.J.; Romero-Moreno, R.; et al. Potassium channel activity controls breast cancer metastasis by affecting β-catenin signaling. Cell Death Dis. 2019, 10, 180. [Google Scholar] [CrossRef]
- Luo, Q.; Wu, T.; Wu, W.; Chen, G.; Luo, X.; Jiang, L.; Tao, H.; Rong, M.; Kang, S.; Deng, M. The Functional Role of Voltage-Gated Sodium Channel Nav1.5 in Metastatic Breast Cancer. Front. Pharmacol. 2020, 11, 1111. [Google Scholar] [CrossRef]
- Bagal, S.K.; Brown, A.D.; Cox, P.J.; Omoto, K.; Owen, R.M.; Pryde, D.C.; Sidders, B.; Skerratt, S.E.; Stevens, E.B.; Storer, R.I.; et al. Ion Channels as Therapeutic Targets: A Drug Discovery Perspective. J. Med. Chem. 2013, 56, 593–624. [Google Scholar] [CrossRef]
- Coleman, J.A.; Quazi, F.; Molday, R.S. Mammalian P4-ATPases and ABC transporters and their role in phospholipid transport. Biochim. Biophys. Acta 2013, 1831, 555–574. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.M.; Bell, E.L.; Hughes, R.O.; Garfield, A.S. ABC transporters: Human disease and pharmacotherapeutic potential. Trends Mol. Med. 2023, 29, 152–172. [Google Scholar] [CrossRef]
- Chloupková, M.; LeBard, L.S.; Koeller, D.M. MDL1 is a high copy suppressor of ATM1: Evidence for a role in resistance to oxidative stress. J. Mol. Biol. 2003, 331, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Kispal, G.; Csere, P.; Guiard, B.; Lill, R. The ABC transporter Atm1p is required for mitochondrial iron homeostasis. FEBS Lett. 1997, 418, 346–350. [Google Scholar] [CrossRef] [PubMed]
- Kitai, K.; Kawaguchi, K.; Tomohiro, T.; Morita, M.; So, T.; Imanaka, T. The lysosomal protein ABCD4 can transport vitamin B12 across liposomal membranes in vitro. J. Biol. Chem. 2021, 296, 100654. [Google Scholar] [CrossRef]
- Andersen, J.P.; Vestergaard, A.L.; Mikkelsen, S.A.; Mogensen, L.S.; Chalat, M.; Molday, R.S. P4-ATPases as Phospholipid Flippases—Structure, Function, and Enigmas. Front. Physiol. 2016, 7, 275. [Google Scholar] [CrossRef]
- Pamarthy, S.; Mao, L.; Katara, G.K.; Fleetwood, S.; Kulshreshta, A.; Gilman-Sachs, A.; Beaman, K.D. The V-ATPase a2 isoform controls mammary gland development through Notch and TGF-β signaling. Cell Death Dis. 2016, 7, e2443. [Google Scholar] [CrossRef]
- Hernández-García, D.; Wood, C.D.; Castro-Obregón, S.; Covarrubias, L. Reactive oxygen species: A radical role in development? Free Radic. Biol. Med. 2010, 49, 130–143. [Google Scholar] [CrossRef]
- Sena, L.A.; Chandel, N.S. Physiological roles of mitochondrial reactive oxygen species. Mol. Cell 2012, 48, 158–167. [Google Scholar] [CrossRef]
- Smith, S.M.; Zwart, S.R.; Block, G.; Rice, B.L.; Davis-Street, J.E. The nutritional status of astronauts is altered after long-term space flight aboard the International Space Station. J. Nutr. 2005, 135, 437–443. [Google Scholar] [CrossRef]
- Hayashi, G.; Cortopassi, G. Lymphoblast Oxidative Stress Genes as Potential Biomarkers of Disease Severity and Drug Effect in Friedreich’s Ataxia. PLoS ONE 2016, 11, e0153574. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; You, H.J.; Youn, C. SCARA3 inhibits cell proliferation and EMT through AKT signaling pathway in lung cancer. BMC Cancer 2022, 22, 552. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Guo, Q.; Su, T.; Xiao, Y.; Li, C.-J.; Huang, Y.; Luo, X.-H. Identification of SCARA3 with potential roles in metabolic disorders. Aging 2020, 13, 2149–2167. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, G.L. Surfactant Protein D in Respiratory and Non-Respiratory Diseases. Front. Med. 2018, 5, 18. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Okumura, H.; Guo, R.; Naruse, K. Effect of Oxidative Stress on Cardiovascular System in Response to Gravity. Int. J. Mol. Sci. 2017, 18, 1426. [Google Scholar] [CrossRef]
- da Silveira, W.A.; Fazelinia, H.; Rosenthal, S.B.; Laiakis, E.C.; Kim, M.S.; Meydan, C.; Kidane, Y.; Rathi, K.S.; Smith, S.M.; Stear, B.; et al. Comprehensive Multi-omics Analysis Reveals Mitochondrial Stress as a Central Biological Hub for Spaceflight Impact. Cell 2020, 183, 1185–1201.e20. [Google Scholar] [CrossRef]
- Kiselyov, K.; Muallem, S. ROS and intracellular ion channels. Cell Calcium 2016, 60, 108–114. [Google Scholar] [CrossRef]
- Li, B.; Li, N.; Wang, N.; Li, C.; Liu, X.; Cao, Z.; Xing, C.; Wang, S. Targeting ROS-sensitive TRP ion channels for relieving oxidative stress-related diseases based on nanomaterials. Mater. Today Adv. 2023, 17, 100335. [Google Scholar] [CrossRef]
- Matsuda, T.; Takuma, K.; Baba, A. Na+-Ca2+ Exchanger: Physiology and Pharmacology. Jpn. J. Pharmacol. 1997, 74, 1–20. [Google Scholar] [CrossRef]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell. Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef]
- Petersen, R.C. Free-radicals and advanced chemistries involved in cell membrane organization influence oxygen diffusion and pathology treatment. AIMS Biophys. 2017, 4, 240–283. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.P. Redefining oxidative stress. Antioxid. Redox Signal. 2006, 8, 1865–1879. [Google Scholar] [CrossRef] [PubMed]
- Kannan, N.; Nguyen, L.V.; Makarem, M.; Dong, Y.; Shih, K.; Eirew, P.; Raouf, A.; Emerman, J.T.; Eaves, C.J. Glutathione-dependent and -independent oxidative stress-control mechanisms distinguish normal human mammary epithelial cell subsets. Proc. Natl. Acad. Sci. USA 2014, 111, 7789–7794. [Google Scholar] [CrossRef] [PubMed]
- Verkman, A.S.; Hara-Chikuma, M.; Papadopoulos, M.C. Aquaporins--new players in cancer biology. J. Mol. Med. Berl. Ger. 2008, 86, 523–529. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, O.V.; Partridge, C.; Plaut, K. Space Environment Impacts Homeostasis: Exposure to Spaceflight Alters Mammary Gland Transportome Genes. Biomolecules 2023, 13, 872. https://doi.org/10.3390/biom13050872
Patel OV, Partridge C, Plaut K. Space Environment Impacts Homeostasis: Exposure to Spaceflight Alters Mammary Gland Transportome Genes. Biomolecules. 2023; 13(5):872. https://doi.org/10.3390/biom13050872
Chicago/Turabian StylePatel, Osman V., Charlyn Partridge, and Karen Plaut. 2023. "Space Environment Impacts Homeostasis: Exposure to Spaceflight Alters Mammary Gland Transportome Genes" Biomolecules 13, no. 5: 872. https://doi.org/10.3390/biom13050872
APA StylePatel, O. V., Partridge, C., & Plaut, K. (2023). Space Environment Impacts Homeostasis: Exposure to Spaceflight Alters Mammary Gland Transportome Genes. Biomolecules, 13(5), 872. https://doi.org/10.3390/biom13050872





