Socio-Demographic, Lifestyle, and Cardiometabolic Characteristics Associated with Low-Grade Systemic Inflammation in Russian Adult Population
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Collection
2.3. Low-Grade Systemic Inflammation
2.4. Socio-Demographic Characteristics
2.5. Housing Characteristics
2.6. Lifestyle Characteristics
2.7. Cardiometabolic Characteristics
2.8. Self-Reported Diseases
2.9. Mental Health
2.10. Exclusions from the Study
2.11. Statistical Analysis
3. Results
3.1. Comparisons of the LGSI-Positive and LGSI-Negative Participants
3.2. Socio-Demographic Characteristics
3.3. Lifestyle Characteristics
3.4. Cardiometabolic Conditions
3.5. Self-Reported Diseases
3.6. Associations between the hs-CRP Level and Cardiometabolic Biomarkers
4. Discussion
4.1. Socio-Demographic Correlates
4.2. Lifestyle Correlates
4.3. Cardiometabolic Conditions
4.4. Self-Reported Diseases
4.5. Cardiometabolic Biomarkers
4.6. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, H.; Sun, K.; Zhao, R.; Hu, J.; Hao, Z.; Wang, F.; Lu, Y.; Liu, F.; Zhang, Y. Inflammatory biomarkers of coronary heart disease. Front. Biosci. 2018, 10, 185–196. [Google Scholar] [CrossRef]
- Markozannes, G.; Koutsioumpa, C.; Cividini, S.; Monori, G.; Tsilidis, K.K.; Kretsavos, N.; Theodoratou, E.; Gill, D.; Ioannidis, J.P.; Tzoulaki, I. Global assessment of C-reactive protein and health-related outcomes: An umbrella review of evidence from observational studies and Mendelian randomization studies. Eur. J. Epidemiol. 2021, 36, 11–36. [Google Scholar] [CrossRef]
- Boncler, M.; Wu, Y.; Watala, C. The Multiple Faces of C-Reactive Protein-Physiological and Pathophysiological Implications in Cardiovascular Disease. Molecules 2019, 24, 2062. [Google Scholar] [CrossRef]
- Minihane, A.M.; Vinoy, S.; Russell, W.R.; Baka, A.; Roche, H.M.; Tuohy, K.M.; Teeling, J.L.; Blaak, E.E.; Fenech, M.; Vauzour, D.; et al. Low-grade inflammation, diet composition and health: Current research evidence and its translation. Br. J. Nutr. 2015, 114, 999–1012. [Google Scholar] [CrossRef]
- Younes, R.; LeBlanc, C.A.; Hiram, R. Evidence of Failed Resolution Mechanisms in Arrhythmogenic Inflammation, Fibrosis and Right Heart Disease. Biomolecules 2022, 12, 720. [Google Scholar] [CrossRef] [PubMed]
- Lawler, P.R.; Bhatt, D.L.; Godoy, L.C.; Luscher, T.F.; Bonow, R.O.; Verma, S.; Ridker, P.M. Targeting cardiovascular inflammation: Next steps in clinical translation. Eur. Heart J. 2021, 42, 113–131. [Google Scholar] [CrossRef]
- Avan, A.; Tavakoly Sany, S.B.; Ghayour-Mobarhan, M.; Rahimi, H.R.; Tajfard, M.; Ferns, G. Serum C-reactive protein in the prediction of cardiovascular diseases: Overview of the latest clinical studies and public health practice. J. Cell. Physiol. 2018, 233, 8508–8525. [Google Scholar] [CrossRef]
- Townsend, N.; Kazakiewicz, D.; Lucy Wright, F.; Timmis, A.; Huculeci, R.; Torbica, A.; Gale, C.P.; Achenbach, S.; Weidinger, F.; Vardas, P. Epidemiology of cardiovascular disease in Europe. Nat. Rev. Cardiol. 2022, 19, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.; Malyutina, S.; Kudryavtsev, A.V.; Averina, M.; Bobrova, N.; Boytsov, S.; Brage, S.; Clark, T.G.; Diez Benavente, E.; Eggen, A.E.; et al. Know Your Heart: Rationale, design and conduct of a cross-sectional study of cardiovascular structure, function and risk factors in 4500 men and women aged 35-69 years from two Russian cities, 2015-18. Wellcome Open Res. 2018, 3, 67. [Google Scholar] [CrossRef] [PubMed]
- Lakunchykova, O.; Averina, M.; Wilsgaard, T.; Watkins, H.; Malyutina, S.; Ragino, Y.; Keogh, R.H.; Kudryavtsev, A.V.; Govorun, V.; Cook, S.; et al. Why does Russia have such high cardiovascular mortality rates? Comparisons of blood-based biomarkers with Norway implicate non-ischaemic cardiac damage. J. Epidemiol. Community Health 2020, 74, 698–704. [Google Scholar] [CrossRef]
- Iakunchykova, O.; Averina, M.; Wilsgaard, T.; Malyutina, S.; Kudryavtsev, A.V.; Cook, S.; Wild, S.; Eggen, A.E.; Hopstock, L.A.; Leon, D.A. What factors explain the much higher diabetes prevalence in Russia compared with Norway? Major sex differences in the contribution of adiposity. BMJ Open Diabetes Res. Care 2021, 9, e002021. [Google Scholar] [CrossRef]
- Ruiz-Castell, M.; Le Coroller, G.; Landrier, J.F.; Kerkour, D.; Weber, B.; Fagherazzi, G.; Appenzeller, B.M.R.; Vaillant, M.; Bohn, T. Micronutrients and Markers of Oxidative Stress and Inflammation Related to Cardiometabolic Health: Results from the EHES-LUX Study. Nutrients 2020, 13, 5. [Google Scholar] [CrossRef] [PubMed]
- Jamatia, E.; Lali, P.; Koner, B.C.; Dhanwal, D.K.; Masroor, M.; Krishnamurthy, K.; Singh, A. OLR1 Gene Polymorphism and Oxidized LDL Levels in Metabolic Syndrome in Indian Population. Indian J. Endocrinol. Metab. 2018, 22, 530–534. [Google Scholar] [CrossRef]
- Varbo, A.; Benn, M.; Tybjærg-Hansen, A.; Nordestgaard, B.G. Elevated Remnant Cholesterol Causes Both Low-Grade Inflammation and Ischemic Heart Disease, Whereas Elevated Low-Density Lipoprotein Cholesterol Causes Ischemic Heart Disease Without Inflammation. Circulation 2013, 128, 1298–1309. [Google Scholar] [CrossRef] [PubMed]
- Moens, S.J.B.; Verweij, S.L.; Schnitzler, J.G.; Stiekema, L.C.A.; Bos, M.; Langsted, A.; Kuijk, C.; Bekkering, S.; Voermans, C.; Verberne, H.J.; et al. Remnant Cholesterol Elicits Arterial Wall Inflammation and a Multilevel Cellular Immune Response in Humans. Arter. Thromb. Vasc. Biol. 2017, 37, 969–975. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Chau, L. Fas/Fas ligand-mediated death pathway is involved in oxLDL-induced apoptosis in vascular smooth muscle cells. Am. J. Physiol. Cell Physiol. 2001, 280, C709–C718. [Google Scholar] [CrossRef]
- Ligthart, S.; de Vries, P.; Uitterlinden, A.G.; Hofman, A.; CHARGE Inflammation Working Group; Franco, O.; Chasman, D.I.; Dehghan, A. Pleiotropy among common genetic loci identified for cardiometabolic disorders and C-reactive protein. PLoS ONE 2015, 10, e0118859. [Google Scholar] [CrossRef] [PubMed]
- Si, S.; Li, J.; Tewara, M.A.; Xue, F. Genetically Determined Chronic Low-Grade Inflammation and Hundreds of Health Outcomes in the UK Biobank and the FinnGen Population: A Phenome-Wide Mendelian Randomization Study. Front. Immunol. 2021, 12, 720876. [Google Scholar] [CrossRef]
- Hubacek, J.A.; Peasey, A.; Pikhart, H.; Stavek, P.; Kubinova, R.; Marmot, M.; Bobak, M. APOE polymorphism and its effect on plasma C-reactive protein levels in a large general population sample. Hum. Immunol. 2010, 71, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Averina, M.; Nilssen, O.; Arkhipovsky, V.L.; Kalinin, A.G.; Brox, J. C-reactive protein and alcohol consumption: Is there a U-shaped association? Results from a population-based study in Russia. The Arkhangelsk study. Atherosclerosis 2006, 188, 309–315. [Google Scholar] [CrossRef]
- Iakunchykova, O.; Averina, M.; Kudryavtsev, A.V.; Wilsgaard, T.; Soloviev, A.; Schirmer, H.; Cook, S.; Leon, D.A. Evidence for a Direct Harmful Effect of Alcohol on Myocardial Health: A Large Cross-Sectional Study of Consumption Patterns and Cardiovascular Disease Risk Biomarkers from Northwest Russia, 2015 to 2017. J. Am. Heart Assoc. 2020, 9, e014491. [Google Scholar] [CrossRef] [PubMed]
- Custodero, C.; Mankowski, R.T.; Lee, S.A.; Chen, Z.; Wu, S.; Manini, T.M.; Hincapie Echeverri, J.; Sabba, C.; Beavers, D.P.; Cauley, J.A.; et al. Evidence-based nutritional and pharmacological interventions targeting chronic low-grade inflammation in middle-age and older adults: A systematic review and meta-analysis. Ageing Res. Rev. 2018, 46, 42–59. [Google Scholar] [CrossRef] [PubMed]
- Davillas, A.; Benzeval, M.; Kumari, M. Socio-economic inequalities in C-reactive protein and fibrinogen across the adult age span: Findings from Understanding Society. Sci. Rep. 2017, 7, 2641. [Google Scholar] [CrossRef] [PubMed]
- Maharani, A. Socio-economic inequalities in C-reactive protein levels: Evidence from longitudinal studies in England and Indonesia. Brain Behav. Immun. 2019, 82, 122–128. [Google Scholar] [CrossRef]
- Villegas, R.; Xiang, Y.B.; Cai, H.; Elasy, T.; Cai, Q.; Zhang, X.; Fazio, S.; Linton, M.F.; Li, H.; Xu, W.H.; et al. Lifestyle determinants of C-reactive protein in middle-aged, urban Chinese men. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 223–230. [Google Scholar] [CrossRef]
- Iakunchykova, O.; Schirmer, H.; Leong, D.; Malyutina, S.; Ryabikov, A.; Averina, M.; Kudryavtsev, A.; Kornev, M.; Voronina, E.; Paramonov, A.; et al. Heavy alcohol drinking and subclinical echocardiographic abnormalities of structure and function. Open Heart 2021, 8, e001457. [Google Scholar] [CrossRef]
- El Assar, M.; Alvarez-Bustos, A.; Sosa, P.; Angulo, J.; Rodriguez-Manas, L. Effect of Physical Activity/Exercise on Oxidative Stress and Inflammation in Muscle and Vascular Aging. Int. J. Mol. Sci. 2022, 23, 8713. [Google Scholar] [CrossRef]
- Metsios, G.S.; Moe, R.H.; Kitas, G.D. Exercise and inflammation. Best Pract. Res. Clin. Rheumatol. 2020, 34, 101504. [Google Scholar] [CrossRef]
- Niemela, O.; Nivukoski, U.; Bloigu, A.; Bloigu, R.; Aalto, M.; Laatikainen, T. Laboratory test based assessment of WHO alcohol risk drinking levels. Scand. J. Clin. Lab. Investig. 2019, 79, 58–64. [Google Scholar] [CrossRef]
- Derella, C.C.; Tingen, M.S.; Blanks, A.; Sojourner, S.J.; Tucker, M.A.; Thomas, J.; Harris, R.A. Smoking cessation reduces systemic inflammation and circulating endothelin-1. Sci. Rep. 2021, 11, 24122. [Google Scholar] [CrossRef]
- EurWORK. Coding and Classification Standards. Available online: www.eurofound.europa.eu/surveys/ewcs/2005/classification (accessed on 7 February 2023).
- Babor, T.F.; Higgins-Biddle, J.C.; Saunders, J.B.; Monteiro, M.G. AUDIT: The Alcohol Use Disorders Identification Test: Guidelines for Use in Primary Health Care, 2nd ed.; World Health Ogranization: Geneva, Switzerland, 2001; p. 40. [Google Scholar]
- InterAct, C.; Peters, T.; Brage, S.; Westgate, K.; Franks, P.W.; Gradmark, A.; Tormo Diaz, M.J.; Huerta, J.M.; Bendinelli, B.; Vigl, M.; et al. Validity of a short questionnaire to assess physical activity in 10 European countries. Eur. J. Epidemiol. 2012, 27, 15–25. [Google Scholar] [CrossRef]
- Toft, U.; Kristoffersen, L.H.; Lau, C.; Borch-Johnsen, K.; Jorgensen, T. The Dietary Quality Score: Validation and association with cardiovascular risk factors: The Inter99 study. Eur. J. Clin. Nutr. 2007, 61, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Sahu, S.; Chawla, R.; Uppal, B. Comparison of two methods of estimation of low density lipoprotein cholesterol, the direct versus friedewald estimation. Indian J. Clin. Biochem. 2005, 20, 54–61. [Google Scholar] [CrossRef]
- ATC; WHOCC. WHOCC—ATC/DDD Index. Available online: https://www.whocc.no/atc_ddd_index/ (accessed on 3 February 2023).
- WHO. WHO Collaborating Centre for Drug Statistics Methodology. Available online: https://www.whocc.no/ (accessed on 3 February 2023).
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C., Jr.; et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef] [PubMed]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B. The PHQ-9: Validity of a brief depression severity measure. J. Gen. Intern. Med. 2001, 16, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Spitzer, R.L.; Kroenke, K.; Williams, J.B.; Lowe, B. A brief measure for assessing generalized anxiety disorder: The GAD-7. Arch. Intern. Med. 2006, 166, 1092–1097. [Google Scholar] [CrossRef] [PubMed]
- Evstifeeva, S.E.; Shalnova, S.A.; Kutsenko, V.A.; Yarovaya, E.B.; Balanova, Y.A.; Imaeva, A.E.; Kapustina, A.V.; Muromtseva, G.A.; Maksimov, S.A.; Karamnova, N.S.; et al. Association of high-sensitivity C-reactive protein with fatal and non-fatal cardiovascular events in working-age people: Data from the ESSE-RF study. Russ. J. Cardiol. 2021, 26, 4399. [Google Scholar] [CrossRef]
- Polyakova, O.A.; Kirichenko, A.A.; Borodin, I.A. Levels of high-sensitivity C-reactive protein in young and middle-aged individuals and their association with hypertension. Med. Alph. 2021, 1, 44–48. [Google Scholar] [CrossRef]
- Sevostyanova, E.V.; Nikolaev, Y.A.; Mitrofanov, I.M.; Polyakov, V.Y. C-reactive protein as an indicator of polymorbidity in patients with arterial hypertension. Sib. Sci. Med. J. 2022, 42, 58–64. [Google Scholar] [CrossRef]
- Mirolyubova, O.A.; Kudryavtsev, A.V.; Semchyugova, E.O.; Malyutina, S.K.; Ryabikov, A.N. C-reactive protein and its associations with cardiometabolic risk factors and echocardiographic indicators of heart failure: Results of “Know your heart” study in Arkhangelsk. Kardiologiia 2020, 60, 68–75. [Google Scholar] [CrossRef]
- Shah, T.; Newcombe, P.; Smeeth, L.; Addo, J.; Casas, J.P.; Whittaker, J.; Miller, M.A.; Tinworth, L.; Jeffery, S.; Strazzullo, P.; et al. Ancestry as a determinant of mean population C-reactive protein values: Implications for cardiovascular risk prediction. Circ. Cardiovasc. Genet. 2010, 3, 436–444. [Google Scholar] [CrossRef]
- Anand, S.S.; Razak, F.; Yi, Q.; Davis, B.; Jacobs, R.; Vuksan, V.; Lonn, E.; Teo, K.; McQueen, M.; Yusuf, S. C-reactive protein as a screening test for cardiovascular risk in a multiethnic population. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1509–1515. [Google Scholar] [CrossRef]
- Tang, Y.B.; Huo, J.S.; Huang, J.; Li, H.; Piao, J.H.; Sun, J.; Wang, L.J. Distribution of High-sensitivity C-reactive Protein Status in an Urban Population in China. Biomed. Environ. Sci. 2020, 33, 396–402. [Google Scholar] [PubMed]
- Ong, K.L.; Allison, M.A.; Cheung, B.M.; Wu, B.J.; Barter, P.J.; Rye, K.A. Trends in C-reactive protein levels in US adults from 1999 to 2010. Am. J. Epidemiol. 2013, 177, 1430–1442. [Google Scholar] [CrossRef] [PubMed]
- Georgakis, M.K.; Malik, R.; Richardson, T.G.; Howson, J.M.M.; Anderson, C.D.; Burgess, S.; Hovingh, G.K.; Dichgans, M.; Gill, D. Associations of genetically predicted IL-6 signaling with cardiovascular disease risk across population subgroups. BMC Med. 2022, 20, 245. [Google Scholar] [CrossRef] [PubMed]
- Minciullo, P.L.; Catalano, A.; Mandraffino, G.; Casciaro, M.; Crucitti, A.; Maltese, G.; Morabito, N.; Lasco, A.; Gangemi, S.; Basile, G. Inflammaging and Anti-Inflammaging: The Role of Cytokines in Extreme Longevity. Arch. Immunol. Ther. Exp. 2016, 64, 111–126. [Google Scholar] [CrossRef]
- Lee, Y.J.; Lee, J.H.; Shin, Y.H.; Kim, J.K.; Lee, H.R.; Lee, D.C. Gender difference and determinants of C-reactive protein level in Korean adults. Clin. Chem. Lab. Med. 2009, 47, 863–869. [Google Scholar] [CrossRef]
- Meysamie, A.; Ghodsi, S.; Ghalehtaki, R.; Esteghamati, A.; Asgari, F.; Gouya, M.M. Distributions of High-Sensitivity C-Reactive Protein, Total Cholesterol-HDL Ratio and 10-Year Cardiovascular Risk: National Population-Based Study. Acta Med. Iran. 2017, 55, 218–227. [Google Scholar]
- Lau, E.S.; Paniagua, S.M.; Guseh, J.S.; Bhambhani, V.; Zanni, M.V.; Courchesne, P.; Lyass, A.; Larson, M.G.; Levy, D.; Ho, J.E. Sex Differences in Circulating Biomarkers of Cardiovascular Disease. J. Am. Coll. Cardiol. 2019, 74, 1543–1553. [Google Scholar] [CrossRef]
- Lew, J.; Sanghavi, M.; Ayers, C.R.; McGuire, D.K.; Omland, T.; Atzler, D.; Gore, M.O.; Neeland, I.; Berry, J.D.; Khera, A.; et al. Sex-Based Differences in Cardiometabolic Biomarkers. Circulation 2017, 135, 544–555. [Google Scholar] [CrossRef]
- Ujcic-Voortman, J.K.; Baan, C.A.; Verhoeff, A.P.; Krol, A.; Seidell, J.C. Ethnic differences in systemic inflammation: An investigation of C-reactive protein levels among Moroccan, Turkish and Dutch groups in the Netherlands. Atherosclerosis 2011, 218, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Kottilil, S.; Mathur, P. The influence of inflammation on cardiovascular disease in women. Front. Glob. Womens Health 2022, 3, 979708. [Google Scholar] [CrossRef] [PubMed]
- Kranjac, A.W.; Kranjac, D.; Lounsbury, O. Deconstructing sex differences in C-reactive protein trends over time. Am. J. Hum. Biol. 2022, 34, e23705. [Google Scholar] [CrossRef]
- Wood, W.G.; Ludemann, J.; Mitusch, R.; Heinrich, J.; Maass, R.; Frick, U. Evaluation of a sensitive immunoluminometric assay for the determination of C-reactive protein (CRP) in serum and plasma and the establishment of reference ranges for different groups of subjects. Clin. Lab. 2000, 46, 131–140. [Google Scholar]
- Choi, J.; Joseph, L.; Pilote, L. Obesity and C-reactive protein in various populations: A systematic review and meta-analysis. Obes. Rev. 2013, 14, 232–244. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.L.; Lin, Y.; Chen, J.Y. The Association between High-Sensitivity C-Reactive Protein and Metabolic Syndrome in an Elderly Population Aged 50 and Older in a Community Receiving Primary Health Care in Taiwan. Int. J. Environ. Res. Public Health 2022, 19, 13111. [Google Scholar] [CrossRef] [PubMed]
- Artemniak-Wojtowicz, D.; Kucharska, A.M.; Pyrzak, B. Obesity and chronic inflammation crosslinking. Cent. Eur. J. Immunol. 2020, 45, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Kholmatova, K.; Krettek, A.; Leon, D.A.; Malyutina, S.; Cook, S.; Hopstock, L.A.; Løvsletten, O.; Kudryavtsev, A.V. Obesity Prevalence and Associated Socio-Demographic Characteristics and Health Behaviors in Russia and Norway. Int. J. Environ. Res. Public Health 2022, 19, 9428. [Google Scholar] [CrossRef]
- Berger, E.; Castagne, R.; Chadeau-Hyam, M.; Bochud, M.; d’Errico, A.; Gandini, M.; Karimi, M.; Kivimaki, M.; Krogh, V.; Marmot, M.; et al. Multi-cohort study identifies social determinants of systemic inflammation over the life course. Nat. Commun. 2019, 10, 773. [Google Scholar] [CrossRef]
- Bonaccio, M.; Di Castelnuovo, A.; Pounis, G.; De Curtis, A.; Costanzo, S.; Persichillo, M.; Cerletti, C.; Donati, M.B.; de Gaetano, G.; Iacoviello, L.; et al. Relative contribution of health-related behaviours and chronic diseases to the socioeconomic patterning of low-grade inflammation. Int. J. Public Health 2017, 62, 551–562. [Google Scholar] [CrossRef]
- Trias-Llimos, S.; Cook, S.; Eggen, A.E.; Kudryavtsev, A.V.; Malyutina, S.; Shkolnikov, V.M.; Leon, D.A. Socioeconomic inequalities in physiological risk biomarkers and the role of lifestyles among Russians aged 35-69 years. Int. J. Equity Health 2022, 21, 51. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, M.; Cui, Y.; Zhu, Z.; Chen, J.; Zeng, F.; Gao, M.; Li, Y.; Huang, F.; Chen, H. Increased risk of testosterone deficiency is associated with the systemic immune-inflammation index: A population-based cohort study. Front. Endocrinol. 2022, 13, 974773. [Google Scholar] [CrossRef] [PubMed]
- Stewart, S.H.; Mainous, A.G., 3rd; Gilbert, G. Relation between alcohol consumption and C-reactive protein levels in the adult US population. J. Am. Board Fam. Pract. 2002, 15, 437–442. [Google Scholar] [PubMed]
- Esser, N.; Legrand-Poels, S.; Piette, J.; Scheen, A.J.; Paquot, N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res. Clin. Pract. 2014, 105, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, J.L.; Brown, M.S. A century of cholesterol and coronaries: From plaques to genes to statins. Cell 2015, 161, 161–172. [Google Scholar] [CrossRef]
- Yang, X.; Li, Y.; Li, Y.; Ren, X.; Zhang, X.; Hu, D.; Gao, Y.; Xing, Y.; Shang, H. Oxidative Stress-Mediated Atherosclerosis: Mechanisms and Therapies. Front. Physiol. 2017, 8, 600. [Google Scholar] [CrossRef]
- Ragino, Y.I.; Baum, V.A.; Polonskaya, Y.V.; Sadovski, E.V.; Baum, S.R.; Nikitin, Y.P. Relationship between oxidized fibrinogen and hemostasis disturbances and endothelial dysfunction in myocardial infarction. Bull. Exp. Biol. Med. 2008, 145, 412–414. [Google Scholar] [CrossRef]
- Holvoet, P.; Lee, D.H.; Steffes, M.; Gross, M.; Jacobs, D.R., Jr. Association between circulating oxidized low-density lipoprotein and incidence of the metabolic syndrome. JAMA 2008, 299, 2287–2293. [Google Scholar] [CrossRef]
- Hurtado-Roca, Y.; Bueno, H.; Fernandez-Ortiz, A.; Ordovas, J.M.; Ibanez, B.; Fuster, V.; Rodriguez-Artalejo, F.; Laclaustra, M. Oxidized LDL Is Associated with Metabolic Syndrome Traits Independently of Central Obesity and Insulin Resistance. Diabetes 2017, 66, 474–482. [Google Scholar] [CrossRef]
- Mansyur, M.A.; Bakri, S.; Patellongi, I.J.; Rahman, I.A. The association between metabolic syndrome components, low-grade systemic inflammation and insulin resistance in non-diabetic Indonesian adolescent male. Clin. Nutr. ESPEN 2020, 35, 69–74. [Google Scholar] [CrossRef]
- Quispe, R.; Martin, S.S.; Michos, E.D.; Lamba, I.; Blumenthal, R.S.; Saeed, A.; Lima, J.; Puri, R.; Nomura, S.; Tsai, M.; et al. Remnant cholesterol predicts cardiovascular disease beyond LDL and ApoB: A primary prevention study. Eur. Heart J. 2021, 42, 4324–4332. [Google Scholar] [CrossRef] [PubMed]
- Jaishankar, T.; Shivasekar, M.; Vinodhini, V.M. Assessment of Remnant Lipoprotein Cholesterol and Oxidized Low density Lipoprotein Associated with Low-grade Inflammation in Coronary Heart Disease Subjects of Young South Indian Population. J. Assoc. Physicians India 2022, 70, 11–12. [Google Scholar] [PubMed]
- Galmes, S.; Cifre, M.; Palou, A.; Oliver, P.; Serra, F. A Genetic Score of Predisposition to Low-Grade Inflammation Associated with Obesity May Contribute to Discern Population at Risk for Metabolic Syndrome. Nutrients 2019, 11, 298. [Google Scholar] [CrossRef] [PubMed]
- Sesso, H.D.; Wang, L.; Buring, J.E.; Ridker, P.M.; Gaziano, J.M. Comparison of interleukin-6 and C-reactive protein for the risk of developing hypertension in women. Hypertension 2007, 49, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhao, L.; Zhou, X.; Meng, X.; Zhou, X. Role of inflammation, immunity, and oxidative stress in hypertension: New insights and potential therapeutic targets. Front. Immunol. 2022, 13, 1098725. [Google Scholar] [CrossRef]
- Hage, F.G. C-reactive protein and hypertension. J. Hum. Hypertens. 2014, 28, 410–415. [Google Scholar] [CrossRef]
- Wang, C.H.; Li, S.H.; Weisel, R.D.; Fedak, P.W.; Dumont, A.S.; Szmitko, P.; Li, R.K.; Mickle, D.A.; Verma, S. C-reactive protein upregulates angiotensin type 1 receptors in vascular smooth muscle. Circulation 2003, 107, 1783–1790. [Google Scholar] [CrossRef]
- Kusche-Vihrog, K.; Urbanova, K.; Blanque, A.; Wilhelmi, M.; Schillers, H.; Kliche, K.; Pavenstadt, H.; Brand, E.; Oberleithner, H. C-reactive protein makes human endothelium stiff and tight. Hypertension 2011, 57, 231–237. [Google Scholar] [CrossRef]
- Latkin, C.A.; Edwards, C.; Davey-Rothwell, M.A.; Tobin, K.E. The relationship between social desirability bias and self-reports of health, substance use, and social network factors among urban substance users in Baltimore, Maryland. Addict. Behav. 2017, 73, 133–136. [Google Scholar] [CrossRef]
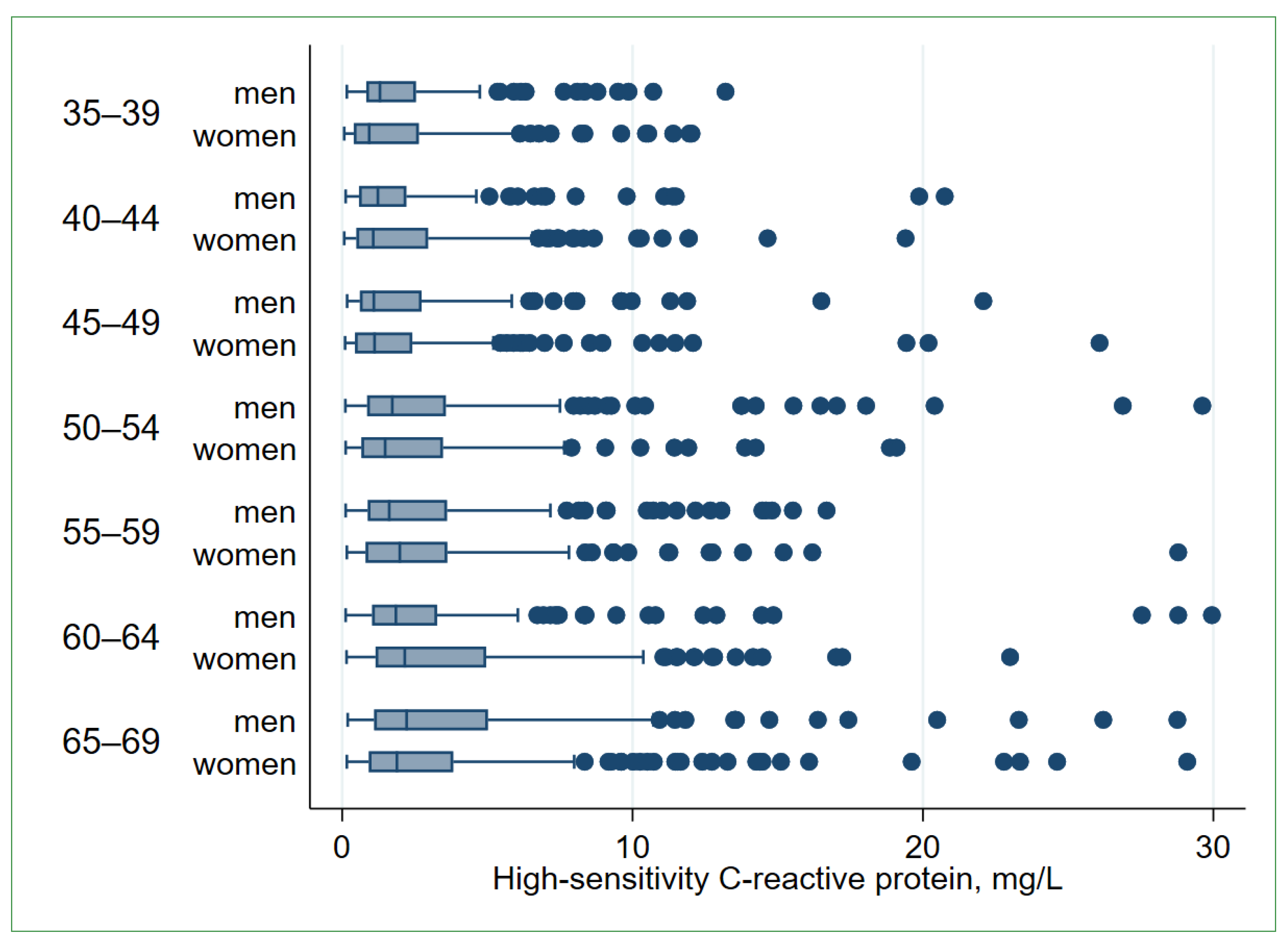

| Men | p-Value * | Women | p-Value * | |||
|---|---|---|---|---|---|---|
| LGSI− | LGSI+ | LGSI− | LGSI+ | |||
| n | 546 | 308 | 752 | 448 | ||
| Socio-economic characteristics | ||||||
| Age, years, M ± SD | 52.8 ± 9.5 | 55.0 ± 9.5 | 0.001 | 52.4 ± 10.0 | 55.2 ± 9.4 | <0.001 |
| Higher education, Abs (%) | 205 (37.6) | 96 (31.2) | 0.061 | 325 (43.2) | 136 (30.4) | <0.001 |
| Being married, Abs (%) | 437 (80.0) | 226 (73.4) | 0.025 | 380 (50.5) | 221 (49.3) | 0.697 |
| In regular paid work, Abs (%) | 379 (69.4) | 178 (57.8) | 0.001 | 505 (67.2) | 262 (58.5) | 0.002 |
| Occupation category, Abs (%) | 0.141 | 0.024 | ||||
| - High-skilled white-collar (ISCO 1–3) | 201 (36.8) | 94 (30.5) | 346 (46.0) | 170 (38.0) | ||
| - Low-skilled white-collar (ISCO 4–5) | 42 (7.7) | 18 (5.8) | 246 (32.7) | 154 (34.4) | ||
| - High-skilled blue-collar (ISCO 6–7) | 119 (21.8) | 76 (24.7) | 46 (6.1) | 38 (8.5) | ||
| - Low-skilled blue-collar (ISCO 8–9) | 184 (33.7) | 120 (39.0) | 114 (15.2) | 86 (19.2) | ||
| Poor financial situation a, Abs (%) | 55 (10.1) | 38 (12.3) | 0.308 | 127 (16.9) | 75 (16.7) | 0.947 |
| Housing characteristics | ||||||
| Shared flat, house, or hostel, Abs (%) | 13 (2.4) | 13 (4.2) | 0.133 | 28 (3.7) | 22 (4.9) | 0.319 |
| No hot water amenities, Abs (%) | 49 (9.0) | 35 (11.4) | 0.260 | 74 (9.8) | 54 (12.1) | 0.230 |
| No central heating, Abs (%) | 33 (6.0) | 20 (6.5) | 0.794 | 50 (6.7) | 39 (8.7) | 0.189 |
| Household size, members, Me (Q1; Q3) | 3.0 (2.0; 4.0) | 3.0 (2.0; 4.0) | 0.247 | 2.0 (2.0; 4.0) | 2.0 (2.0; 3.0) | 0.227 |
| Dwelling size per member, m2, Me (Q1; Q3) | 20.0 (14.7; 28.0) | 19.8 (14.7; 27.3) | 0.900 | 20.7 (15.0; 30.3) | 21.0 (15.1; 29.8) | 0.982 |
| Lifestyle characteristics | ||||||
| Smoking, Abs (%) | 151 (27.7) | 126 (40.9) | <0.001 | 105 (14.0) | 62 (13.8) | 0.690 |
| Physical inactivity b, Abs (%) | 81 (14.8) | 49 (15.9) | 0.675 | 119 (15.8) | 85 (19.0) | 0.160 |
| Unhealthy diet c, Abs (%) | 26 (4.8) | 16 (5.2) | 0.779 | 36 (4.8) | 20 (4.5) | 0.798 |
| Hazardous drinking d, Abs (%) | 141 (25.8) | 106 (34.4) | 0.008 | 18 (2.4) | 11 (2.5) | 0.946 |
| Men | p-Value * | Women | p-Value * | |||
|---|---|---|---|---|---|---|
| LGSI− | LGSI+ | LGSI− | LGSI+ | |||
| n | 546 | 308 | 752 | 448 | ||
| Abs (%) | Abs (%) | |||||
| Cardiometabolic characteristics | ||||||
| Dyslipidemia a | 460 (84.3) | 278 (90.3) | 0.014 | 605 (80.5) | 405 (90.4) | <0.001 |
| Hypertension b | 324 (59.3) | 223 (72.4) | <0.001 | 327 (43.5) | 281 (62.7) | <0.001 |
| Diabetes c | 31 (5.7) | 26 (8.4) | 0.120 | 50 (6.7) | 61 (13.6) | 0.001 |
| Abdominal obesity d | 248 (45.4) | 194 (63.0) | <0.001 | 390 (51.9) | 381 (85.0) | <0.001 |
| Self-reported diseases | ||||||
| Cardiovascular diseases e | 96 (17.6) | 89 (28.9) | <0.001 | 158 (21.0) | 132 (29.5) | 0.001 |
| Pulmonary diseases f | 53 (9.7) | 40 (13.0) | 0.140 | 115 (15.3) | 100 (22.3) | 0.002 |
| Neoplasms g | 14 (2.6) | 8 (2.6) | 0.976 | 48 (6.4) | 36 (8.0) | 0.278 |
| Kidney diseases g | 63 (11.5) | 43 (14.0) | 0.303 | 168 (22.3) | 91 (20.3) | 0.409 |
| Liver diseases g | 80 (14.7) | 42 (13.6) | 0.684 | 139 (18.5) | 81 (18.1) | 0.861 |
| Joint diseases h | 93 (17.0) | 70 (22.7) | 0.042 | 186 (24.7) | 162 (36.2) | <0.001 |
| Mental health | ||||||
| Depression i | 118 (21.6) | 75 (24.4) | 0.358 | 275 (36.6) | 161 (35.9) | 0.826 |
| Anxiety j | 70 (12.8) | 40 (13.0) | 0.944 | 190 (25.3) | 108 (24.1) | 0.653 |
| Model 1 | Model 2 | Model 3 | Model 4 | |
|---|---|---|---|---|
| OR (95% CI) | ||||
| Socio-demographic characteristics | ||||
| Age, years | 1.03 (1.02; 1.04) | 1.02 (1.01; 1.04) | 1.00 (0.99; 1.02) | 1.00 (0.99; 1.01) |
| Sex, woman | 1.06 (0.88; 1.27) | 1.07 (0.73; 1.57) | 0.59 (0.36; 0.96) | 0.55 (0.33; 0.89) |
| Higher education | 0.70 (0.57; 0.84) | 0.78 (0.61; 1.01) | 0.87 (0.67; 1.12) | 0.88 (0.68; 1.14) |
| Being married | 0.86 (0.71; 1.03) | 0.70 (0.50; 0.98) | 0.64 (0.45; 0.90) | 0.62 (0.44; 0.88) |
| In regular paid work | 0.81 (0.65; 1.00) | 0.85 (0.68; 1.06) | 0.83 (0.66; 1.04) | 0.85 (0.68; 1.07) |
| Occupation category | ||||
| - High-skilled white-collar (ISCO 1–3) | Reference | Reference | Reference | Reference |
| - Low-skilled white-collar (ISCO 4–5) | 1.27 (1.00; 1.61) | 1.05 (0.79; 1.38) | 1.02 (0.76; 1.36) | 1.02 (0.76; 1.37) |
| - High-skilled blue-collar (ISCO 6–7) | 1.39 (1.05; 1.84) | 1.13 (0.81; 1.59) | 1.14 (0.81; 1.61) | 1.13 (0.80; 1.60) |
| - Low-skilled blue-collar (ISCO 8–9) | 1.34 (1.06; 1.69) | 1.09 (0.81; 1.46) | 1.04 (0.77; 1.40) | 1.04 (0.77; 1.41) |
| Lifestyle characteristics | ||||
| Smoking | 1.44 (1.16; 1.79) | 1.65 (1.22; 2.24) | 1.94 (1.42; 2.66) | 1.92 (1.40; 2.63) |
| Hazardous drinking | 1.46 (1.12; 1.90) | 1.42 (1.06; 1.91) | 1.28 (0.95; 1.73) | 1.28 (0.94; 1.73) |
| Cardiometabolic characteristics | ||||
| Dyslipidemia a | 1.78 (1.34; 2.37) | 1.48 (1.09; 2.01) | 1.46 (1.07; 1.98) | |
| Hypertension b | 1.76 (1.43; 2.16) | 1.28 (1.02; 1.60) | 1.25 (1.00; 1.57) | |
| Diabetes c | 1.61 (1.16; 2.23) | 1.25 (0.89; 1.76) | 1.19 (0.85; 1.69) | |
| Abdominal obesity d | 3.09 (2.52; 3.78) | 2.15 (1.58; 2.91) | 2.13 (1.57; 2.89) | |
| Self-reported diseases | ||||
| Cardiovascular diseases e | 1.42 (1.13; 1.78) | 1.24 (0.97; 1.58) | ||
| Pulmonary diseases f | 1.44 (1.12; 1.84) | 1.37 (1.05; 1.79) | ||
| Joint diseases g | 1.44 (1.17; 1.77) | 1.23 (0.98; 1.54) | ||
| Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | Model 1 | Model 2 | Model 3 | Model 4 | |
| OR (95% CI) | OR (95% CI) | |||||||
| Socio-demographic characteristics | ||||||||
| Age, years | 1.02 (1.01; 1.04) | 1.02 (1.01; 1.04) | 1.02 (1.00; 1.04) | 1.01 (0.99; 1.03) | 1.02 (1.01; 1.04) | 1.02 (1.01; 1.04) | 0.99 (0.98; 1.01) | 0.99 (0.97; 1.01) |
| Higher education | 0.78 (0.58; 1.05) | 0.94 (0.63; 1.41) | 0.96 (0.63; 1.46) | 0.96 (0.63; 1.46) | 0.64 (0.50; 0.83) | 0.68 (0.49; 0.95) | 0.75 (0.53; 1.06) | 0.75 (0.53; 1.06) |
| Being married | 0.63 (0.45; 0.88) | 0.68 (0.48; 0.96) | 0.60 (0.42; 0.85) | 0.59 (0.41; 0.84) | 1.01 (0.80; 1.28) | 1.01 (0.80; 1.29) | 1.00 (0.78; 1.29) | 1.01 (0.78; 1.30) |
| In regular paid work | 0.70 (0.50; 0.96) | 0.74 (0.53; 1.03) | 0.72 (0.51; 1.01) | 0.75 (0.53; 1.06) | 0.91 (0.68; 1.21) | 0.95 (0.71; 1.27) | 0.91 (0.67; 1.24) | 0.93 (0.69; 1.27) |
| Occupation category | ||||||||
| - High-skilled white-collar (ISCO 1–3) | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| - Low-skilled white-collar (ISCO 4–5) | 0.97 (0.53; 1.78) | 0.87 (0.46; 1.64) | 0.84 (0.44; 1.61) | 0.89 (0.46; 1.72) | 1.30 (0.99; 1.71) | 1.05 (0.76; 1.46) | 1.00 (0.71; 1.41) | 0.99 (0.70; 1.40) |
| - High-skilled blue-collar (ISCO 6–7) | 1.33 (0.91; 1.95) | 1.08 (0.67; 1.72) | 1.12 (0.69; 1.81) | 1.12 (0.69; 1.82) | 1.60 (1.00; 2.57) | 1.26 (0.76; 2.10) | 1.15 (0.67; 1.98) | 1.12 (0.65; 1.93) |
| - Low-skilled blue-collar (ISCO 8–9) | 1.33 (0.95; 1.87) | 1.10 (0.70; 1.71) | 1.05 (0.66; 1.66) | 1.07 (0.67; 1.69) | 1.39 (0.99; 1.95) | 1.09 (0.73; 1.62) | 0.99 (0.65; 1.50) | 0.99 (0.65; 1.50) |
| Lifestyle characteristics | ||||||||
| Smoking | 1.88 (1.40; 2.53) | 1.68 (1.24; 2.29) | 1.97 (1.43; 2.72) | 1.96 (1.42; 2.70) | 1.11 (0.78; 1.56) | 1.02 (0.72; 1.46) | 0.99 (0.68; 1.45) | 0.95 (0.65; 1.39) |
| Hazardous drinking | 1.70 (1.24; 2.33) | 1.50 (1.09; 2.08) | 1.45 (1.04; 2.02) | 1.46 (1.04; 2.04) | 1.24 (0.58; 2.67) | 1.07 (0.48; 2.35) | 0.75 (0.33; 1.71) | 0.74 (0.33; 1.69) |
| Cardiometabolic characteristics | ||||||||
| Dyslipidemia a | 1.63 (1.05; 2.55) | 1.56 (0.98; 2.50) | 1.52 (0.95; 2.44) | 1.90 (1.30; 2.78) | 1.45 (0.97; 2.17) | 1.45 (0.97; 2.18) | ||
| Hypertension a | 1.61 (1.17; 2.22) | 1.42 (1.01; 2.00) | 1.39 (0.99; 1.97) | 1.95 (1.48; 2.56) | 1.19 (0.88; 1.60) | 1.16 (0.86; 1.57) | ||
| Diabetes a | 1.31 (0.75; 2.27) | 1.06 (0.59; 1.91) | 1.04 (0.57; 1.87) | 1.79 (1.19; 2.69) | 1.39 (0.91; 2.12) | 1.33 (0.86; 2.04) | ||
| Abdominal obesity a | 2.00 (1.50; 2.67) | 2.17 (1.58; 2.96) | 2.13 (1.56; 2.92) | 5.02 (3.70; 6.82) | 4.38 (3.16; 6.06) | 4.38 (3.16; 6.07) | ||
| Self-reported diseases | ||||||||
| Cardiovascular diseases a | 1.66 (1.16; 2.37) | 1.47 (1.01; 2.15) | 1.27 (0.95; 1.70) | 1.06 (0.77; 1.46) | ||||
| Pulmonary diseases a | 1.34 (0.86; 2.08) | 1.23 (0.78; 1.96) | 1.47 (1.09; 1.99) | 1.49 (1.07; 2.07) | ||||
| Joint diseases a | 1.32 (0.92; 1.87) | 1.22 (0.83; 1.78) | 1.50 (1.15; 1.96) | 1.28 (0.96; 1.69) | ||||
| Total Sample | Men | Women | ||
|---|---|---|---|---|
| OR (95% CI) a | p-Value for Interaction b | OR (95% CI) c | OR (95% CI) c | |
| Age, years | 1.01 (1.00; 1.02) | 1.02 (1.00; 1.04) | 1.00 (0.99; 1.02) | |
| Sex, woman | 0.97 (0.66; 1.42) | |||
| Being married | 0.62 (0.44; 0.87) | 0.017 | 0.63 (0.44; 0.89) | 1.03 (0.80; 1.31) |
| Smoking | 1.96 (1.44; 2.67) | 0.004 | 1.89 (1.39; 2.58) | 0.97 (0.67; 1.40) |
| Hazardous drinking d | 1.43 (1.06; 1.93) | 1.55 (1.12; 2.14) | 1.07 (0.47; 2.39) | |
| Metabolic syndrome e | 2.32 (1.90; 2.84) | 1.82 (1.33; 2.49) | 2.83 (2.16; 3.70) | |
| Cardiovascular diseases f | 1.22 (0.97; 1.55) | 1.50 (1.03; 2.18) | 1.07 (0.79; 1.46) | |
| Pulmonary diseases g | 1.36 (1.05; 1.77) | 1.28 (0.81; 2.02) | 1.42 (1.04; 1.95) | |
| Unadjusted Comparisons of Participants Divided by LGSI Status | Age- and Sex-Adjusted Associations with ln-Transformed hs-CRP a | ||||
|---|---|---|---|---|---|
| LGSI− (n = 1298) | LGSI+ (n = 756) | ||||
| M ± SD | p-Value * | β | p-Value | ||
| Lipid profiles | |||||
| Total cholesterol, mmol/L | 5.34 ± 1.53 | 5.53 ± 1.15 | <0.001 | 0.107 | <0.001 |
| HDL-C, mmol/L | 1.51 ± 0.37 | 1.39 ± 0.35 | <0.001 | −0.208 | <0.001 |
| LDL-C, mmol/L | 3.58 ± 0.88 | 3.76 ± 0.91 | <0.001 | 0.136 | <0.001 |
| Triglycerides, mmol/L b | 1.33 ± 0.97 | 1.70 ± 1.14 | <0.001 | 0.265 | <0.001 |
| Non-HDL cholesterol, mmol/L | 3.83 ± 1.03 | 4.13 ± 1.11 | <0.001 | 0.175 | <0.001 |
| Remnant cholesterol, mmol/L | 0.57 ± 0.31 | 0.73 ± 0.37 | <0.001 | 0.247 | <0.001 |
| Apolipoprotein A-1 (Apo A-1), g/L | 1.40 ± 0.23 | 1.36 ± 0.23 | <0.001 | −0.136 | <0.001 |
| Apolipoprotein B (Apo B), g/L | 0.93 ± 0.22 | 1.00 ± 0.24 | <0.001 | 0.195 | <0.001 |
| Apo B/Apo A-1 ratio | 0.69 ± 0.21 | 0.76 ± 0.22 | <0.001 | 0.226 | <0.001 |
| Lipoprotein(a), mg/dl b | 21.1 ± 27.7 | 22.1 ± 27.6 | 0.434 | 0.032 | 0.141 |
| Blood pressure | |||||
| Systolic blood pressure, mm Hg | 129.9 ± 19.6 | 134.9 ± 20.3 | <0.001 | 0.174 | <0.001 |
| Diastolic blood pressure, mm Hg | 82.1 ± 11.5 | 85.0 ± 11.4 | <0.001 | 0.198 | <0.001 |
| Blood sugar | |||||
| HbA1c, % | 5.45 ± 0.58 | 5.65 ± 0.88 | <0.001 | 0.149 | <0.001 |
| Anthropometry | |||||
| Body Mass Index | 26.1 ± 4.4 | 29.7 ± 5.3 | <0.001 | 0.406 | <0.001 |
| Waist circumference, cm | 86.8 ± 12.4 | 96.3 ± 13.0 | <0.001 | 0.460 | <0.001 |
| Hip circumference, cm | 100.4 ± 8.3 | 106.5 ± 10.8 | <0.001 | 0.354 | <0.001 |
| Waist-to-hip ratio | 0.86 ± 0.09 | 0.90 ± 0.08 | <0.001 | 0.473 | <0.001 |
| Medication | Abs (%) | ||||
| Statins | 125 (9.6) | 81 (10.7) | 0.430 | −0.025 | 0.263 |
| Antihypertensives | 428 (33.0) | 362 (47.9) | <0.001 | 0.155 | <0.001 |
| Antidiabetics | 56 (4.3) | 49 (6.5) | 0.031 | 0.050 | 0.025 |
| Men | Women | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted Comparisons of Participants Divided by LGSI Status | Age-Adjusted Associations with ln-Transformed hs-CRP a | Unadjusted Comparisons of Participants Divided by LGSI Status | Age-Adjusted Associations with ln-Transformed hs-CRP a | |||||||
| LGSI− (n = 546) | LGSI + (n = 308) | LGSI− (n = 752) | LGSI + (n = 448) | |||||||
| M ± SD | p-Value * | β | p-Value | Mean ± SD | p-Value * | β | p-Value | |||
| Lipid profiles | ||||||||||
| Total cholesterol, mmol/L | 5.26 ± 1.03 | 5.27 ± 1.09 | 0.913 | 0.056 | 0.099 | 5.39 ± 1.07 | 5.70 ± 1.19 | <0.001 | 0.133 | <0.001 |
| HDL-C, mmol/L | 1.35 ± 0.33 | 1.28 ± 0.34 | 0.003 | −0.140 | <0.001 | 1.62 ± 0.37 | 1.47 ± 0.34 | <0.001 | −0.228 | <0.001 |
| LDL-C, mmol/L | 3.60 ± 0.86 | 3.62 ± 0.89 | 0.682 | 0.077 | 0.024 | 3.57 ± 0.90 | 3.86 ± 0.92 | <0.001 | 0.169 | <0.001 |
| Triglycerides, mmol/L b | 1.53 ± 1.15 | 1.73 ± 1.13 | <0.001 | 0.166 | <0.001 | 1.18 ± 0.77 | 1.69 ± 1.15 | <0.001 | 0.335 | <0.001 |
| Non-HDL cholesterol, mmol/L | 3.91 ± 1.02 | 3.99 ± 1.07 | 0.282 | 0.102 | 0.003 | 3.77 ± 1.03 | 4.23 ± 1.12 | <0.001 | 0.221 | <0.001 |
| Remnant cholesterol, mmol/L | 0.63 ± 0.34 | 0.73 ± 0.36 | <0.001 | 0.171 | <0.001 | 0.52 ± 0.28 | 0.73 ± 0.38 | <0.001 | 0.298 | <0.001 |
| Apolipoprotein A-1 (Apo A-1), g/L | 1.31 ± 0.21 | 1.28 ± 0.22 | 0.016 | −0.125 | <0.001 | 1.46 ± 0.23 | 1.41 ± 0.22 | <0.001 | −0.132 | <0.001 |
| Apolipoprotein B (Apo B), g/L | 0.95 ± 0.22 | 0.98 ± 0.23 | 0.070 | 0.130 | <0.001 | 0.92 ± 0.22 | 1.02 ± 0.24 | <0.001 | 0.234 | <0.001 |
| Apo B/Apo A-1 ratio | 0.74 ± 0.21 | 0.79 ± 0.23 | 0.003 | 0.171 | <0.001 | 0.64 ± 0.19 | 0.74 ± 0.21 | <0.001 | 0.258 | <0.001 |
| Lipoprotein(a), mg/dl b | 21.5 ± 28.5 | 21.7 ± 27.7 | 0.482 | 0.012 | 0.735 | 20.9 ± 27.1 | 22.4 ± 27.6 | 0.077 | 0.045 | 0.115 |
| Blood pressure | ||||||||||
| Systolic blood pressure, mm Hg | 136.8 ± 18.8 | 139.5 ± 19.2 | 0.048 | 0.118 | 0.001 | 125.0 ± 18.7 | 131.7 ± 20.4 | <0.001 | 0.199 | <0.001 |
| Diastolic blood pressure, mm Hg | 86.1 ± 11.2 | 87.9 ± 11.1 | 0.025 | 0.160 | <0.001 | 79.3 ± 10.9 | 83.0 ± 11.2 | <0.001 | 0.210 | <0.001 |
| Blood glucose level | ||||||||||
| HbA1c, % | 5.46 ± 0.59 | 5.60 ± 0.85 | 0.007 | 0.114 | 0.001 | 5.44 ± 0.57 | 5.68 ± 0.90 | <0.001 | 0.170 | <0.001 |
| Anthropometry | ||||||||||
| Body Mass Index | 26.7 ± 4.0 | 28.3 ± 4.6 | <0.001 | 0.243 | <0.001 | 25.7 ± 4.7 | 30.7 ± 5.5 | <0.001 | 0.498 | <0.001 |
| Waist circumference, cm | 93.1 ± 10.6 | 98.5 ± 12.3 | <0.001 | 0.292 | <0.001 | 82.2 ± 11.5 | 94.9 ± 13.2 | <0.001 | 0.530 | <0.001 |
| Hip circumference, cm | 99.9 ± 7.1 | 102.1 ± 8.4 | <0.001 | 0.179 | <0.001 | 100.8 ± 9.0 | 109.6 ± 11.2 | <0.001 | 0.433 | <0.001 |
| Waist-to-hip ratio | 0.93 ± 0.06 | 0.96 ± 0.06 | <0.001 | 0.334 | <0.001 | 0.81 ± 0.07 | 0.86 ± 0.07 | <0.001 | 0.400 | <0.001 |
| Medication | Abs (%) | Abs (%) | ||||||||
| Statins | 49 (9.0) | 39 (12.7) | 0.089 | 0.019 | 0.585 | 76 (10.1) | 42 (9.4) | 0.681 | −0.055 | 0.060 |
| Antihypertensives | 177 (32.4) | 137 (44.5) | <0.001 | 0.109 | 0.003 | 251 (33.4) | 225 (50.2) | <0.001 | 0.185 | <0.001 |
| Antidiabetics | 18 (3.3) | 15 (4.9) | 0.252 | 0.040 | 0.251 | 38 (5.1) | 34 (7.6) | 0.074 | 0.052 | 0.071 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mirolyubova, O.; Kholmatova, K.; Postoeva, A.; Kostrova, G.; Malyutina, S.; Kudryavtsev, A.V. Socio-Demographic, Lifestyle, and Cardiometabolic Characteristics Associated with Low-Grade Systemic Inflammation in Russian Adult Population. Biomolecules 2023, 13, 835. https://doi.org/10.3390/biom13050835
Mirolyubova O, Kholmatova K, Postoeva A, Kostrova G, Malyutina S, Kudryavtsev AV. Socio-Demographic, Lifestyle, and Cardiometabolic Characteristics Associated with Low-Grade Systemic Inflammation in Russian Adult Population. Biomolecules. 2023; 13(5):835. https://doi.org/10.3390/biom13050835
Chicago/Turabian StyleMirolyubova, Olga, Kamila Kholmatova, Anna Postoeva, Galina Kostrova, Sofia Malyutina, and Alexander V. Kudryavtsev. 2023. "Socio-Demographic, Lifestyle, and Cardiometabolic Characteristics Associated with Low-Grade Systemic Inflammation in Russian Adult Population" Biomolecules 13, no. 5: 835. https://doi.org/10.3390/biom13050835
APA StyleMirolyubova, O., Kholmatova, K., Postoeva, A., Kostrova, G., Malyutina, S., & Kudryavtsev, A. V. (2023). Socio-Demographic, Lifestyle, and Cardiometabolic Characteristics Associated with Low-Grade Systemic Inflammation in Russian Adult Population. Biomolecules, 13(5), 835. https://doi.org/10.3390/biom13050835







