Apolipoprotein E (ApoE) Rescues the Contractile Smooth Muscle Cell Phenotype in Popliteal Artery Aneurysm Disease
Abstract
1. Introduction
2. Materials and Methods
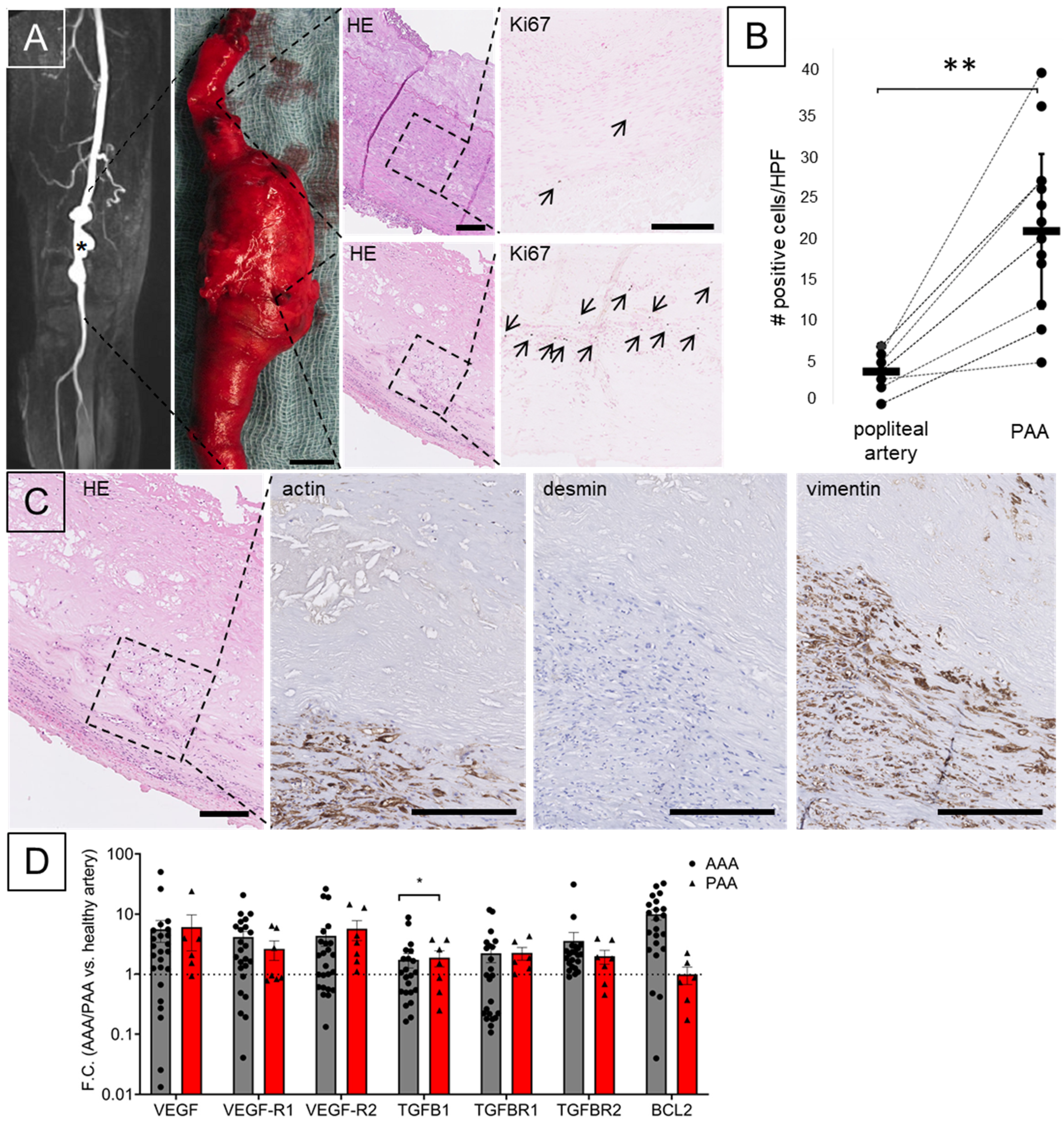
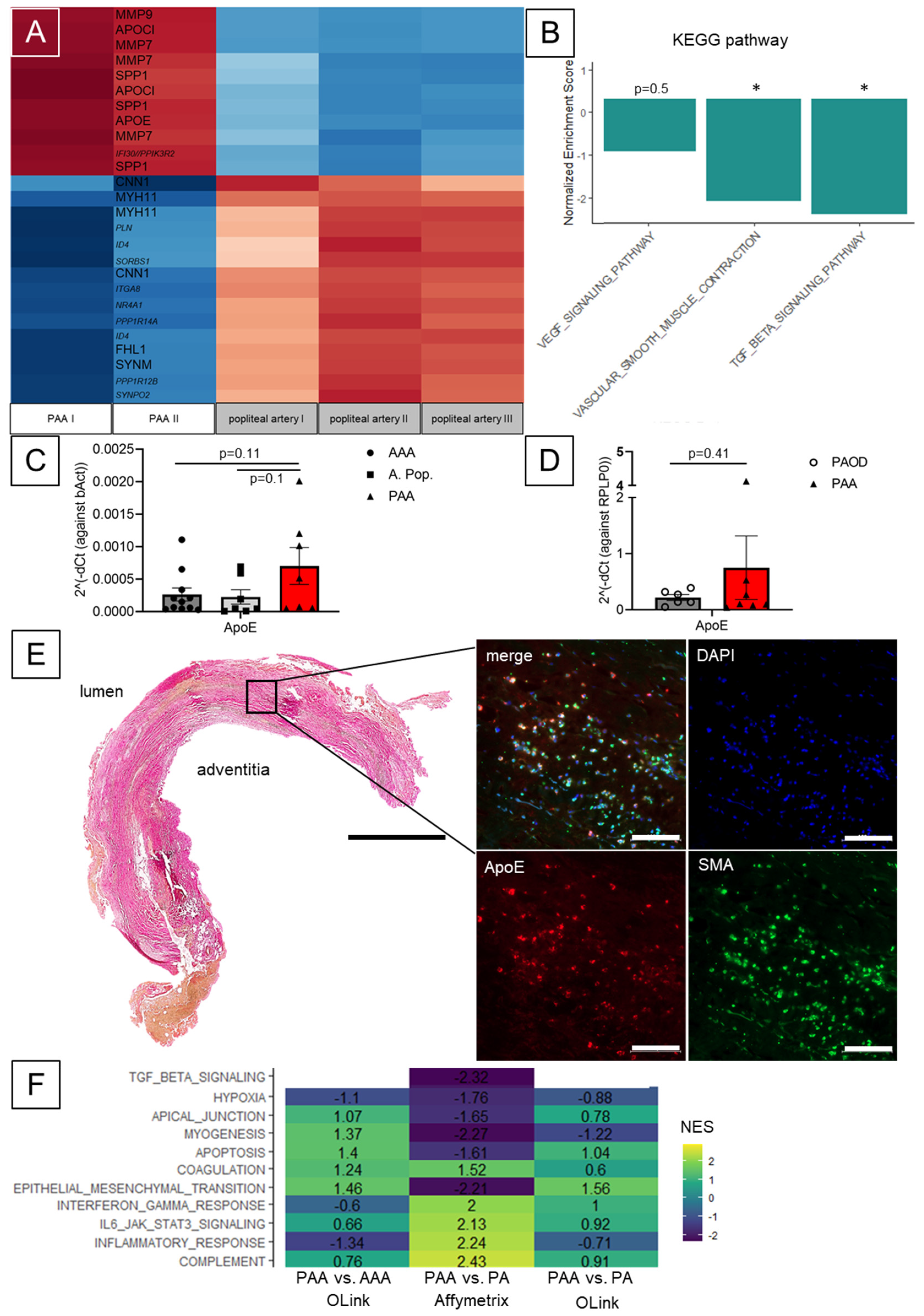
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trickett, J.; Scott, R.; Tilney, H. Screening and management of asymptomatic popliteal aneurysms. J. Med. Screen. 2002, 9, 92–93. [Google Scholar] [CrossRef] [PubMed]
- Tuveson, V.; Löfdahl, H.E.; Hultgren, R. Patients with abdominal aortic aneurysm have a high prevalence of popliteal artery aneurysms. Vasc. Med. 2016, 21, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Ravn, H.; Wanhainen, A.; Bjorck, M. Risk of new aneurysms after surgery for popliteal artery aneurysm. Br. J. Surg. 2008, 95, 571–575. [Google Scholar] [CrossRef] [PubMed]
- Cross, J.; Galland, R. Part One: For the Motion: Asymptomatic Popliteal Artery Aneurysms (less than 3 cm) Should be Treated Conservatively. Eur. J. Vasc. Endovasc. Surg. 2011, 41, 445–448; discussion 449. [Google Scholar] [CrossRef]
- Naazie, I.N.; Arbabi, C.; Moacdieh, M.P.; Hughes, K.; Harris, L.; Malas, M.B. Female sex portends increased risk of major amputation following surgical repair of symptomatic popliteal artery aneurysms. J. Vasc. Surg. 2022, 76, 1030–1036. [Google Scholar] [CrossRef] [PubMed]
- Grip, O.; Mani, K.; Altreuther, M.; Gonçalves, F.B.; Beiles, B.; Cassar, K.; Davidovic, L.; Eldrup, N.; Lattmann, T.; Laxdal, E.; et al. Contemporary Treatment of Popliteal Artery Aneurysms in 14 Countries: A Vascunet Report. Eur. J. Vasc. Endovasc. Surg. 2020, 60, 721–729. [Google Scholar] [CrossRef]
- Cervin, A.; Tjärnström, J.; Ravn, H.; Acosta, S.; Hultgren, R.; Welander, M.; Björck, M. Treatment of Popliteal Aneurysm by Open and Endovascular Surgery: A Contemporary Study of 592 Procedures in Sweden. Eur. J. Vasc. Endovasc. Surg. 2015, 50, 342–350. [Google Scholar] [CrossRef]
- Trinidad-Hernandez, M.; Ricotta, J.J., II; Gloviczki, P.; Kalra, M.; Oderich, G.S.; Duncan, A.A.; Bower, T.C. Results of elective and emergency endovascular repairs of popliteal artery aneurysms. J. Vasc. Surg. 2013, 57, 1299–1305. [Google Scholar] [CrossRef]
- Farber, A.; Angle, N.; Avgerinos, E.; Dubois, L.; Eslami, M.; Geraghty, P.; Haurani, M.; Jim, J.; Ketteler, E.; Pulli, R.; et al. The Society for Vascular Surgery clinical practice guidelines on popliteal artery aneurysms. J. Vasc. Surg. 2021, 75, 109S–120S. [Google Scholar] [CrossRef]
- Nasr, B.; Albert, B.; David, C.H.; da Fonseca, P.M.; Badra, A.; Gouny, P. Exostoses and Vascular Complications in the Lower Limbs: Two Case Reports and Review of the Literature. Ann. Vasc. Surg. 2015, 29, 1315.e7–1315.e14. [Google Scholar] [CrossRef] [PubMed]
- Nora, F.E.; Dahlin, D.C.; Beabout, J.W. Bizarre parosteal osteochondromatous proliferations of the hands and feet. Am. J. Surg. Pathol. 1983, 7, 245–250. [Google Scholar] [CrossRef]
- Stephenson, M.A.; Vlachakis, I.; Valenti, D. Bilateral popliteal artery aneurysms in a young man with Loeys-Dietz syndrome. J. Vasc. Surg. 2012, 56, 486–488. [Google Scholar] [CrossRef]
- Koksoy, C.; Gyedu, A.; Alacayir, I.; Bengisun, U.; Uncu, H.; Anadol, E. Surgical Treatment of Peripheral Aneurysms in Patients with Behcet’s Disease. Eur. J. Vasc. Endovasc. Surg. 2011, 42, 525–530. [Google Scholar] [CrossRef]
- Debasso, R.; Astrand, H.; Bjarnegard, N.; Ryden Ahlgren, A.; Sandgren, T.; Lanne, T. The popliteal artery, an unusual muscular artery with wall properties similar to the aorta: Implications for susceptibility to aneurysm formation? J. Vasc. Surg. 2004, 39, 836–842. [Google Scholar] [CrossRef]
- De Basso, R.; Astrand, H.; Ahlgren, A.R.; Sandgren, T.; Lanne, T. Low wall stress in the popliteal artery: Other mechanisms responsible for the predilection of aneurysmal dilatation? Vasc. Med. 2014, 19, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Busch, A.; Grimm, C.; Hartmann, E.; Paloschi, V.; Kickuth, R.; Lengquist, M.; Otto, C.; Eriksson, P.; Kellersmann, R.; Lorenz, U.; et al. Vessel wall morphology is equivalent for different artery types and localizations of advanced human aneurysms. Histochem. Cell Biol. 2017, 148, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Hussien, H.; Hanemaaijer, R.; Kleemann, R.; Verhaaren, B.F.; van Bockel, J.H.; Lindeman, J.H. The pathophysiology of abdominal aortic aneurysm growth: Corresponding and discordant inflammatory and proteolytic processes in abdominal aortic and popliteal artery aneurysms. J. Vasc. Surg. 2010, 51, 1479–1487. [Google Scholar] [CrossRef] [PubMed]
- Busch, A.; Pauli, J.; Winski, G.; Bleichert, S.; Chernogubova, E.; Metschl, S.; Winter, H.; Trenner, M.; Wiegering, A.; Otto, C.; et al. Lenvatinib halts aortic aneurysm growth by restoring smooth muscle cell contractility. J. Clin. Investig. 2021, 6, e140364. [Google Scholar] [CrossRef] [PubMed]
- King, K.E.; Iyemere, V.P.; Weissberg, P.L.; Shanahan, C.M. Kruppel-like factor 4 (KLF4/GKLF) is a target of bone morphogenetic proteins and transforming growth factor beta 1 in the regulation of vascular smooth muscle cell phenotype. J. Biol. Chem. 2003, 278, 11661–11669. [Google Scholar] [CrossRef]
- Riches-Suman, K.; Clark, E.; Helliwell, R.J.; Angelini, T.G.; Hemmings, K.; Bailey, M.A.; Bridge, K.I.; Scott, D.J.A.; Porter, K.E. Progressive Development of Aberrant Smooth Muscle Cell Phenotype in Abdominal Aortic Aneurysm Disease. J. Vasc. Res. 2018, 55, 35–46. [Google Scholar] [CrossRef]
- Moehle, C.W.; Bhamidipati, C.M.; Alexander, M.R.; Mehta, G.S.; Irvine, J.N.; Salmon, M.; Upchurch, G.R.; Kron, I.L.; Owens, G.K.; Ailawadi, G. Bone marrow–derived MCP1 required for experimental aortic aneurysm formation and smooth muscle phenotypic modulation. J. Thorac. Cardiovasc. Surg. 2011, 142, 1567–1574. [Google Scholar] [CrossRef]
- Jacob, T.; Schutzer, R.; Hingorani, A.; Ascher, E. Differential expression of YAMA/CPP-32 by T lymphocytes in popliteal artery aneurysm. J. Surg. Res. 2003, 112, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Hurks, R.; Kropman, R.H.; Pennekamp, C.W.; Hoefer, I.E.; de Vries, J.-P.P.; Pasterkamp, G.; Vink, A.; Moll, F.L. Popliteal artery aneurysms differ from abdominal aortic aneurysms in cellular topography and inflammatory markers. J. Vasc. Surg. 2014, 60, 1514–1519. [Google Scholar] [CrossRef] [PubMed]
- Busch, A.; Hartmann, E.; Grimm, C.; Ergün, S.; Kickuth, R.; Otto, C.; Kellersmann, R.; Lorenz, U. Heterogeneous histomorphology, yet homogeneous vascular smooth muscle cell dedifferentiation, characterize human aneurysm disease. J. Vasc. Surg. 2017, 66, 1553–1564.e6. [Google Scholar] [CrossRef]
- Pelisek, J.; Hegenloh, R.; Bauer, S.; Metschl, S.; Pauli, J.; Glukha, N.; Busch, A.; Reutersberg, B.; Kallmayer, M.; Trenner, M.; et al. Biobanking: Objectives, Requirements, and Future Challenges-Experiences from the Munich Vascular Biobank. J. Clin. Med. 2019, 8, 251. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Assarsson, E.; Lundberg, M.; Holmquist, G.; Björkesten, J.; Thorsen, S.B.; Ekman, D.; Eriksson, A.; Dickens, E.R.; Ohlsson, S.; Edfeldt, G.; et al. Homogenous 96-Plex PEA Immunoassay Exhibiting High Sensitivity, Specificity, and Excellent Scalability. PLoS ONE 2014, 9, e95192. [Google Scholar] [CrossRef] [PubMed]
- Lind, L.; Ärnlöv, J.; Lindahl, B.; Siegbahn, A.; Sundström, J.; Ingelsson, E. Use of a proximity extension assay proteomics chip to discover new biomarkers for human atherosclerosis. Atherosclerosis 2015, 242, 205–210. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S.; Kawashima, S.; Nakaya, A. The KEGG databases at GenomeNet. Nucleic Acids Res. 2002, 30, 42–46. [Google Scholar] [CrossRef]
- Liberzon, A.; Birger, C.; Thorvaldsdottir, H.; Ghandi, M.; Mesirov, J.P.; Tamayo, P. The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst. 2015, 1, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Fabregat, A.; Sidiropoulos, K.; Viteri, G.; Forner, O.; Marin-Garcia, P.; Arnau, V.; D’eustachio, P.; Stein, L.; Hermjakob, H. Reactome pathway analysis: A high-performance in-memory approach. BMC Bioinform. 2017, 18, 142. [Google Scholar] [CrossRef]
- Sachdeva, J.; Mahajan, A.; Cheng, J.; Baeten, J.T.; Lilly, B.; Kuivaniemi, H.; Hans, C.P. Smooth muscle cell-specific Notch1 haploinsufficiency restricts the progression of abdominal aortic aneurysm by modulating CTGF expression. PLoS ONE 2017, 12, e0178538. [Google Scholar] [CrossRef]
- Rijbroek, A.; Moll, F.; Dijk, H.; Meijer, R.; Jansen, J. Inflammation of the abdominal aortic aneurysm wall. Eur. J. Vasc. Surg. 1994, 8, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Shiwani, H.; Baxter, P.; Taylor, E.; Bailey, M.A.; Scott, D.J.A. Modelling the growth of popliteal artery aneurysms. Br. J. Surg. 2018, 105, 1749–1752. [Google Scholar] [CrossRef]
- Wågsäter, D.; Ravn, H.; Wanhainen, A.; Isaksson, H.; Björck, M. Circulating microRNA in patients with popliteal and multiple artery aneurysms. JVS Vasc. Sci. 2021, 2, 129–135. [Google Scholar] [CrossRef]
- Busch, A.; Busch, M.; Scholz, C.-J.; Kellersmann, R.; Otto, C.; Chernogubova, E.; Maegdefessel, L.; Zernecke, A.; Lorenz, U. Aneurysm miRNA Signature Differs, Depending on Disease Localization and Morphology. Int. J. Mol. Sci. 2016, 17, 81. [Google Scholar] [CrossRef] [PubMed]
- Serra, R.; Grande, R.; Montemurro, R.; Butrico, L.; Caliò, F.G.; Mastrangelo, D.; Scarcello, E.; Gallelli, L.; Buffone, G.; de Franciscis, S. The role of matrix metalloproteinases and neutrophil gelatinase-associated lipocalin in central and peripheral arterial aneurysms. Surgery 2015, 157, 155–162. [Google Scholar] [CrossRef]
- Kershen, L.M.; Schucany, W.G.; Gilbert, N.F. Nora’s lesion: Bizarre parosteal osteochondromatous proliferation of the tibia. Proc. Bayl. Univ. Med. Cent. 2012, 25, 369–371. [Google Scholar] [CrossRef]
- Faggioli, G.L.; Gargiulo, M.; Bertoni, F.; Bacchini, P.; Gessaroli, M.; Stella, A. Parietal inflammatory infiltrate in peripheral aneurysms of atherosclerotic origin. J. Cardiovasc. Surg. 1992, 33, 331–336. [Google Scholar]
- Akamatsu, D.; Fujishima, F.; Sato, A.; Goto, H.; Watanabe, T.; Hashimoto, M.; Shimizu, T.; Sugawara, H.; Miura, T.; Zukeran, T.; et al. Inflammatory Popliteal Aneurysm. Ann. Vasc. Surg. 2011, 25, 698.e13–698.e16. [Google Scholar] [CrossRef]
- Busch, A.; Bleichert, S.; Ibrahim, N.; Wortmann, M.; Eckstein, H.-H.; Brostjan, C.; Wagenhäuser, M.U.; Goergen, C.J.; Maegdefessel, L. Translating mouse models of abdominal aortic aneurysm to the translational needs of vascular surgery. JVS Vasc. Sci. 2021, 2, 219–234. [Google Scholar] [CrossRef]
- Johnson, S.C.; Dong, X.; Vijg, J.; Suh, Y. Genetic evidence for common pathways in human age-related diseases. Aging Cell 2015, 14, 809–817. [Google Scholar] [CrossRef] [PubMed]
- Aboyans, V.; Ricco, J.B.; Bartelink, M.E.L.; Bjorck, M.; Brodmann, M.; Cohnert, T.; Collet, J.P.; Czerny, M.; De Carlo, M.; Debus, S.; et al. Editor’s Choice—2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS). Eur. J. Vasc. Endovasc. Surg. 2018, 55, 305–368. [Google Scholar] [CrossRef] [PubMed]
- Palazzuoli, A.; Gallotta, M.; Guerrieri, G.; Quatrini, I.; Franci, B.; Campagna, M.S.; Neri, E.; Benvenuti, A.; Sassi, C.; Nuti, R. Prevalence of risk factors, coronary and systemic atherosclerosis in abdominal aortic aneurysm: Comparison with high cardiovascular risk population. Vasc. Health Risk Manag. 2008, 4, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Ketelhuth, D.F.; Hansson, G.K. Adaptive Response of T and B Cells in Atherosclerosis. Circ. Res. 2016, 118, 668–678. [Google Scholar] [CrossRef]
- Sakalihasan, N.; Michel, J.B.; Katsargyris, A.; Kuivaniemi, H.; Defraigne, J.O.; Nchimi, A.; Powell, J.T.; Yoshimura, K.; Hultgren, R. Abdominal aortic aneurysms. Nat. Rev. Dis. Prim. 2018, 4, 34. [Google Scholar] [CrossRef]
- Michel, J.-B.; Martin-Ventura, J.-L.; Egido, J.; Sakalihasan, N.; Treska, V.; Lindholt, J.; Allaire, E.; Thorsteinsdottir, U.; Cockerill, G.; Swedenborg, J.; et al. Novel aspects of the pathogenesis of aneurysms of the abdominal aorta in humans. Cardiovasc. Res. 2011, 90, 18–27. [Google Scholar] [CrossRef]
- Ruddy, J.M.; Jones, J.A.; Spinale, F.G.; Ikonomidis, J.S. Regional heterogeneity within the aorta: Relevance to aneurysm disease. J. Thorac. Cardiovasc. Surg. 2008, 136, 1123–1130. [Google Scholar] [CrossRef]
- Lindsay, M.E.; Dietz, H.C. Lessons on the pathogenesis of aneurysm from heritable conditions. Nature 2011, 473, 308–316. [Google Scholar] [CrossRef]
- Rickel, A.P.; Sanyour, H.J.; Kinser, C.; Khatiwada, N.; Vogel, H.; Hong, Z. Exploring the difference in the mechanics of vascular smooth muscle cells from wild-type and apolipoprotein-E knockout mice. Am. J. Physiol. Physiol. 2022, 323, C1393–C1401. [Google Scholar] [CrossRef]
- Gomez, D.; Owens, G.K. Smooth muscle cell phenotypic switching in atherosclerosis. Cardiovasc. Res. 2012, 95, 156–164. [Google Scholar] [CrossRef]
- Schwartz, S.M.; Virmani, R.; Majesky, M.W. An update on clonality: What smooth muscle cell type makes up the atherosclerotic plaque? F1000Research 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Jacob, T.; Hingorani, A.; Ascher, E. Examination of the Apoptotic Pathway and Proteolysis in the Pathogenesis of Popliteal Artery Aneurysms. Eur. J. Vasc. Endovasc. Surg. 2001, 22, 77–85. [Google Scholar] [CrossRef]
- Jacob, T.; Ascher, E.; Hingorani, A.; Gunduz, Y.; Kallakuri, S. Initial Steps in the Unifying Theory of the Pathogenesis of Artery Aneurysms. J. Surg. Res. 2001, 101, 37–43. [Google Scholar] [CrossRef]
- Wilson, W.; Anderton, M.; Choke, E.; Dawson, J.; Loftus, I.; Thompson, M. Elevated Plasma MMP1 and MMP9 are Associated with Abdominal Aortic Aneurysm Rupture. Eur. J. Vasc. Endovasc. Surg. 2008, 35, 580–584. [Google Scholar] [CrossRef]
- Petsophonsakul, P.; Furmanik, M.; Forsythe, R.; Dweck, M.; Schurink, G.W.; Natour, E.; Reutelingsperger, C.; Jacobs, M.; Mees, B.; Schurgers, L. Role of Vascular Smooth Muscle Cell Phenotypic Switching and Calcification in Aortic Aneurysm Formation. Arter. Thromb. Vasc. Biol. 2019, 39, 1351–1368. [Google Scholar] [CrossRef]
- Paloschi, V.; Sabater-Lleal, M.; Middelkamp, H.; Vivas, A.; Johansson, S.; van der Meer, A.; Tenje, M.; Maegdefessel, L. Organ-on-a-chip technology: A novel approach to investigate cardiovascular diseases. Cardiovasc. Res. 2021, 117, 2742–2754. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pauli, J.; Reisenauer, T.; Winski, G.; Sachs, N.; Chernogubova, E.; Freytag, H.; Otto, C.; Reeps, C.; Eckstein, H.-H.; Scholz, C.-J.; et al. Apolipoprotein E (ApoE) Rescues the Contractile Smooth Muscle Cell Phenotype in Popliteal Artery Aneurysm Disease. Biomolecules 2023, 13, 1074. https://doi.org/10.3390/biom13071074
Pauli J, Reisenauer T, Winski G, Sachs N, Chernogubova E, Freytag H, Otto C, Reeps C, Eckstein H-H, Scholz C-J, et al. Apolipoprotein E (ApoE) Rescues the Contractile Smooth Muscle Cell Phenotype in Popliteal Artery Aneurysm Disease. Biomolecules. 2023; 13(7):1074. https://doi.org/10.3390/biom13071074
Chicago/Turabian StylePauli, Jessica, Tessa Reisenauer, Greg Winski, Nadja Sachs, Ekaterina Chernogubova, Hannah Freytag, Christoph Otto, Christian Reeps, Hans-Henning Eckstein, Claus-Jürgen Scholz, and et al. 2023. "Apolipoprotein E (ApoE) Rescues the Contractile Smooth Muscle Cell Phenotype in Popliteal Artery Aneurysm Disease" Biomolecules 13, no. 7: 1074. https://doi.org/10.3390/biom13071074
APA StylePauli, J., Reisenauer, T., Winski, G., Sachs, N., Chernogubova, E., Freytag, H., Otto, C., Reeps, C., Eckstein, H.-H., Scholz, C.-J., Maegdefessel, L., & Busch, A. (2023). Apolipoprotein E (ApoE) Rescues the Contractile Smooth Muscle Cell Phenotype in Popliteal Artery Aneurysm Disease. Biomolecules, 13(7), 1074. https://doi.org/10.3390/biom13071074





