Optimization of Surface-Engineered Micropatterns on Bacterial Cellulose for Guided Scar-Free Skin Wound Healing
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
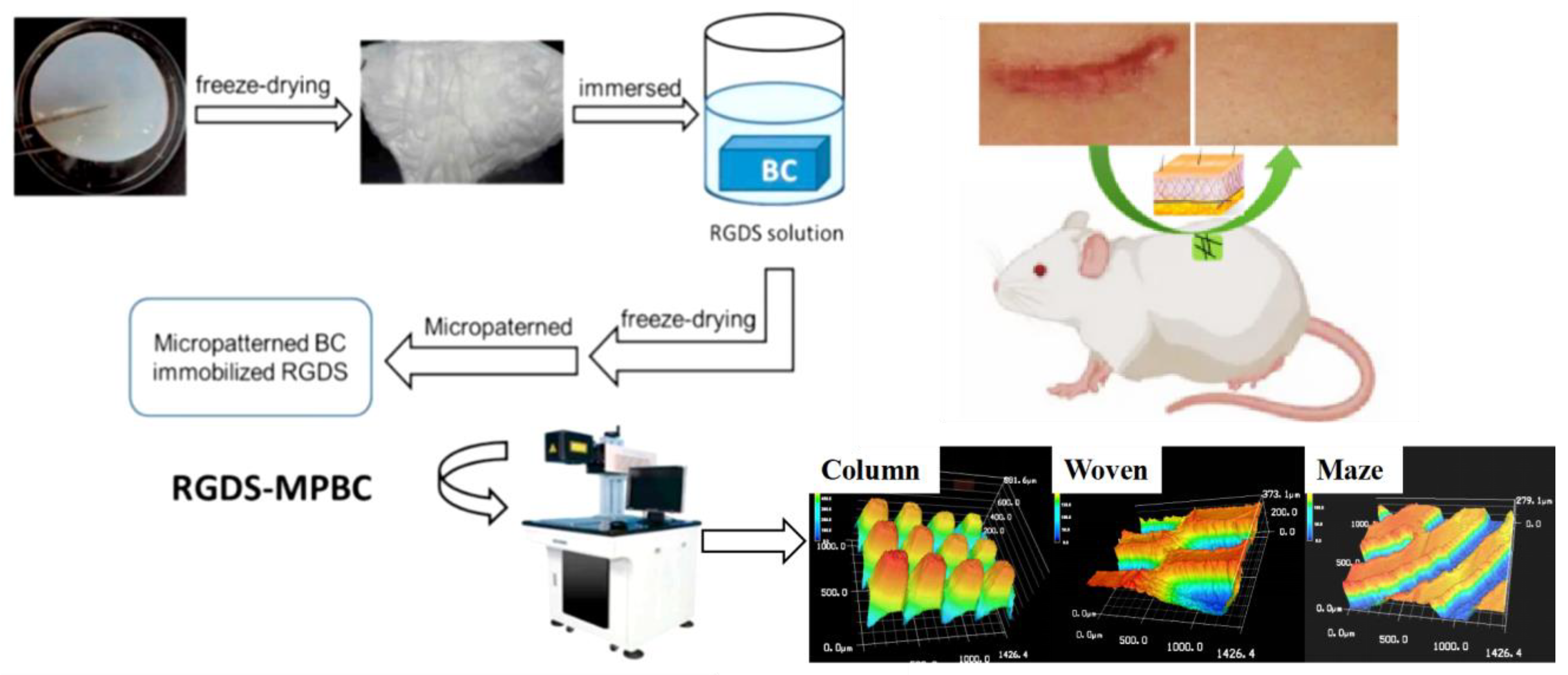
2.2. Preparation of Three Patterns of RGDS-MPBC
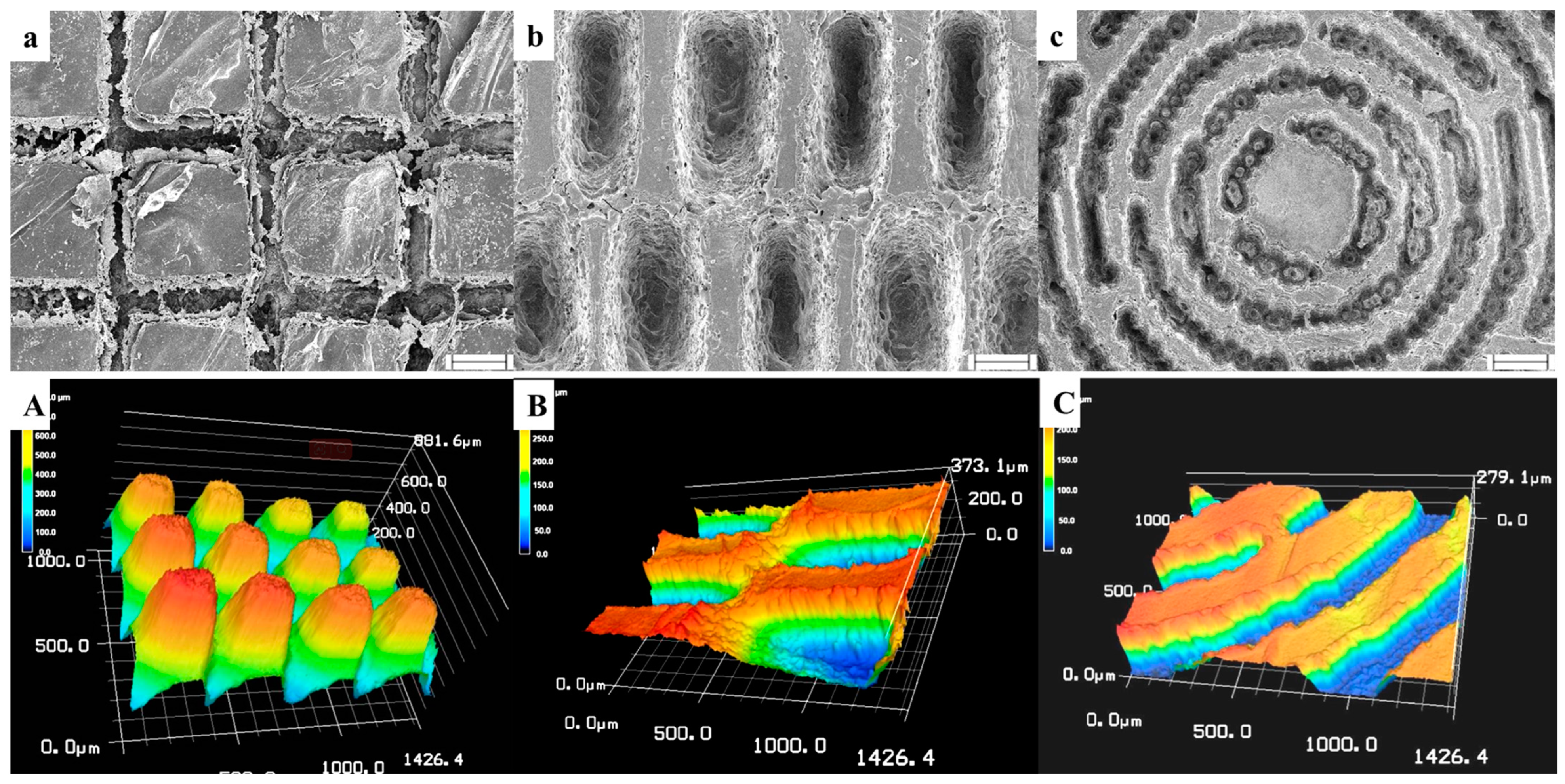
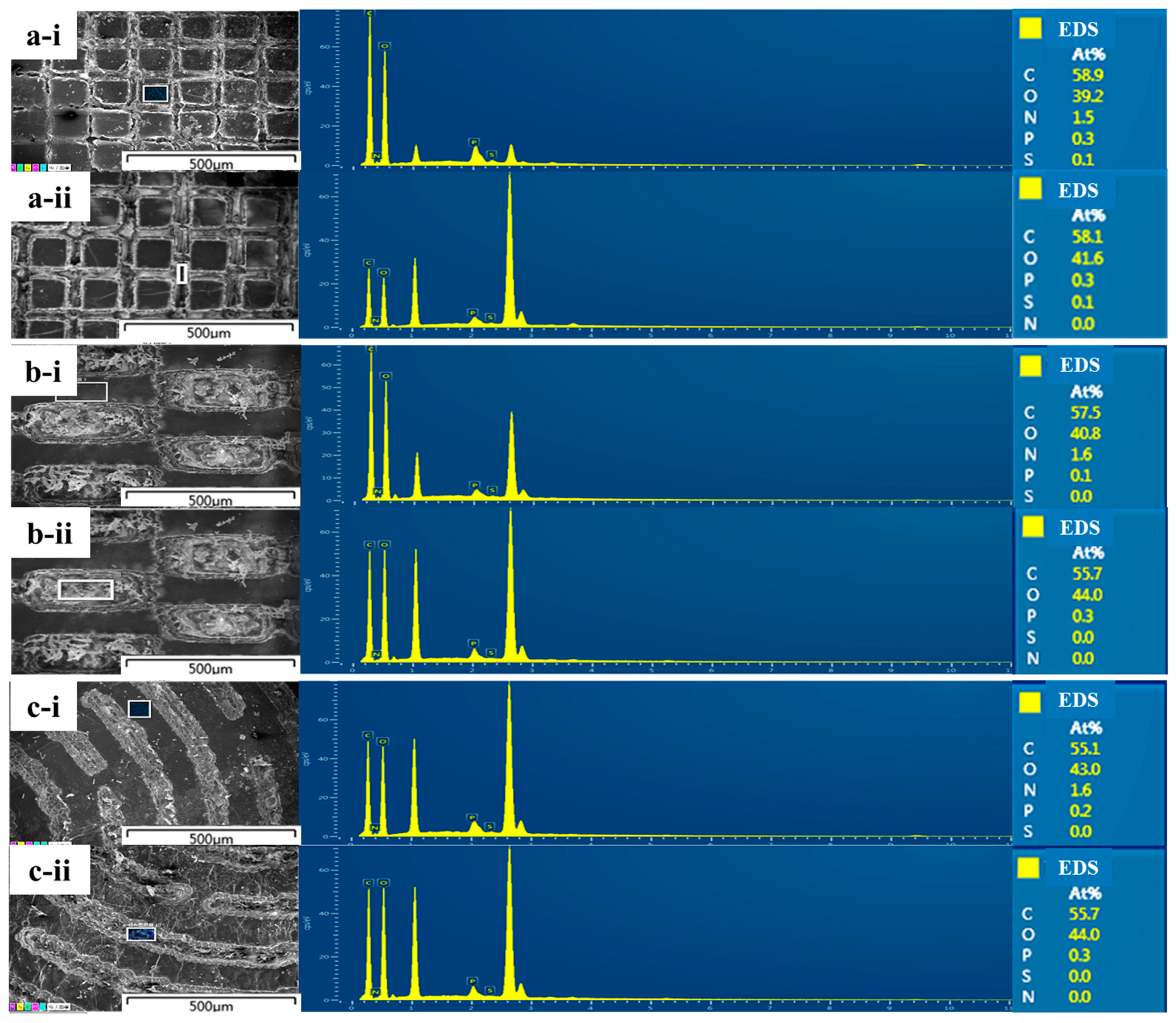
2.3. Characterization of RGDS-MPBC
2.4. Moisture Absorption Test
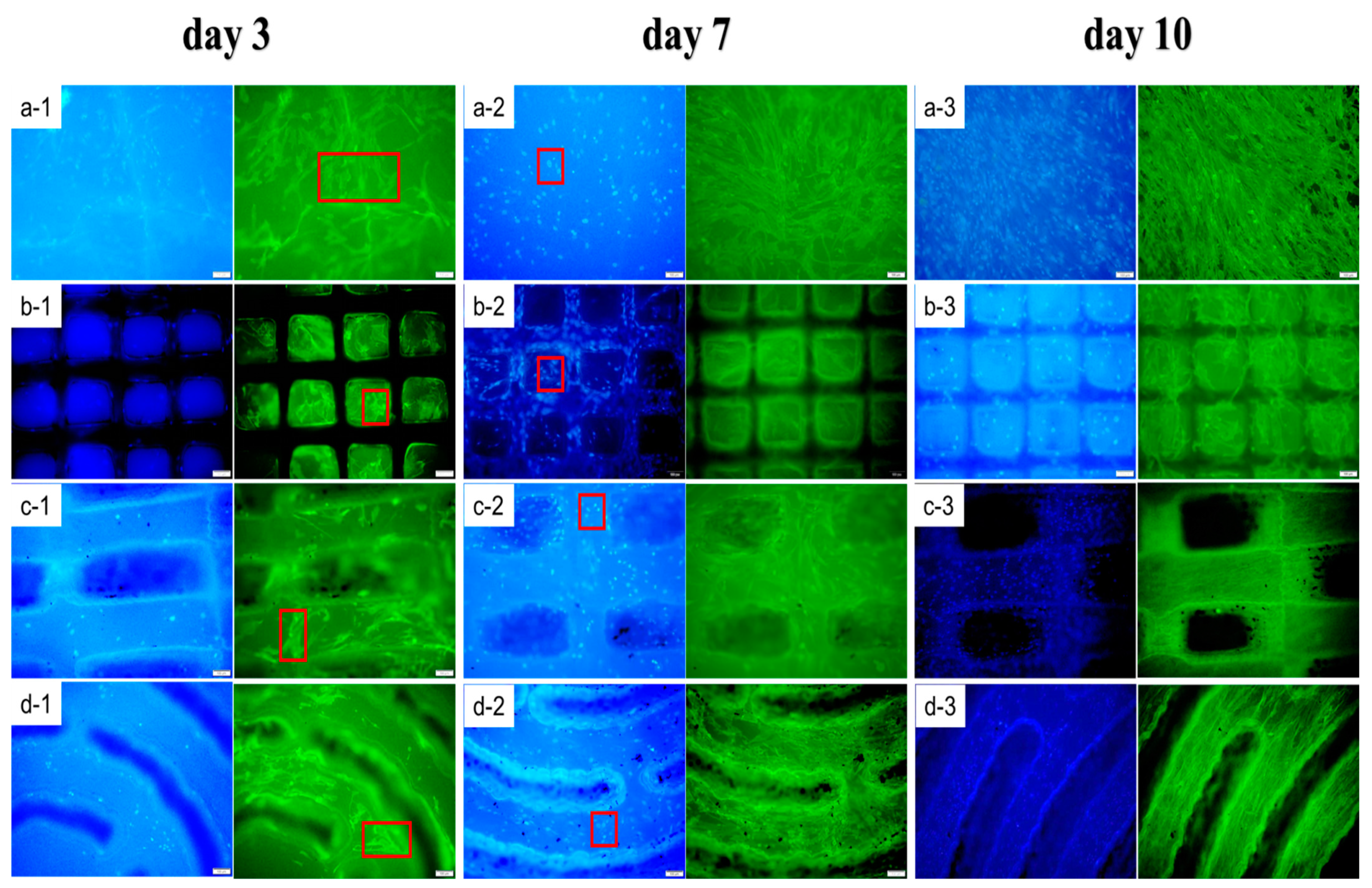
2.5. In-Vitro Study of RGDS-MPBC
2.6. In-Vivo Animal Study
3. Results and Discussion
3.1. Characterization of Different Micropatterns on RGDS-MPBC
3.2. In-Vitro Cell Study
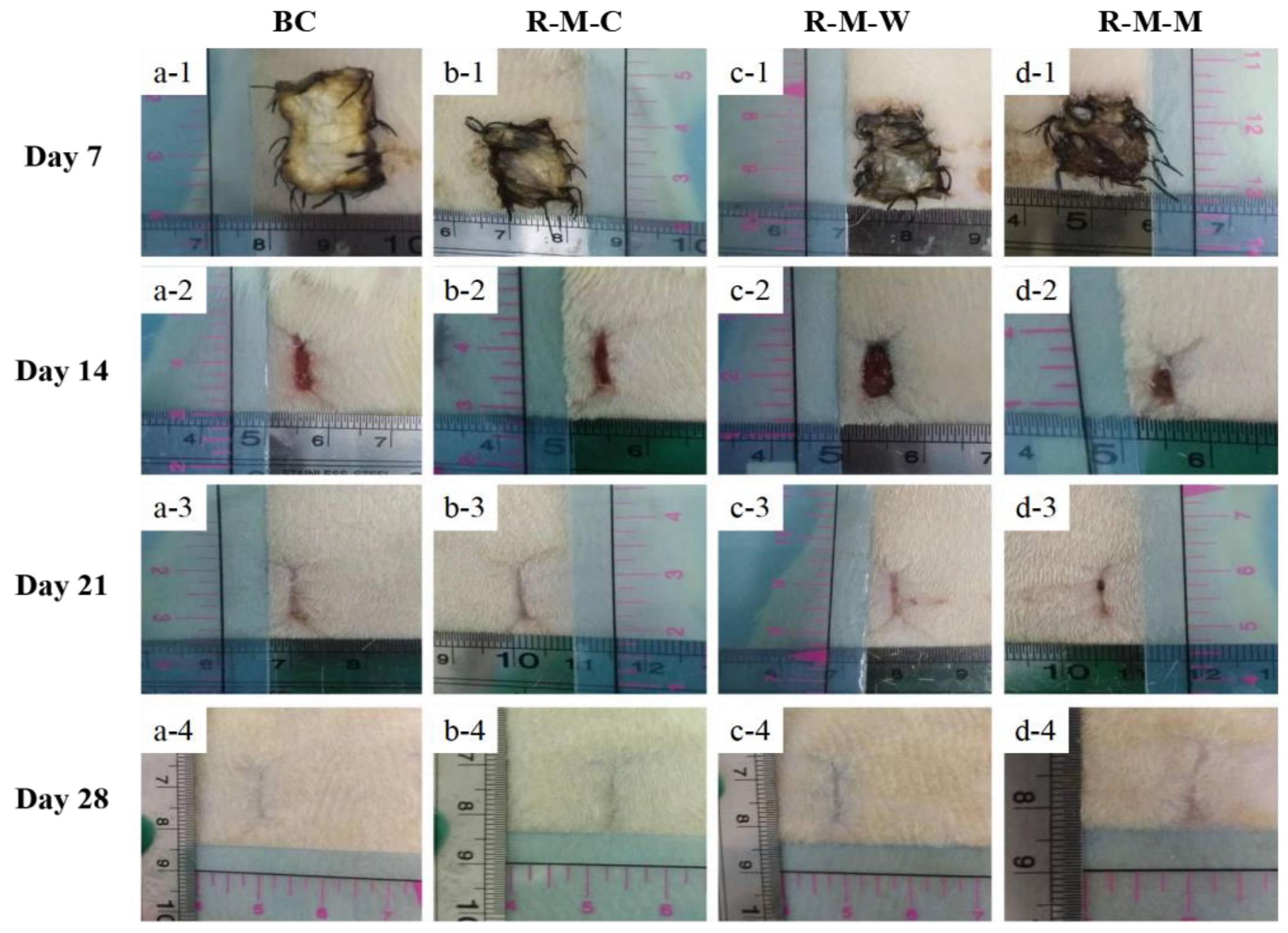
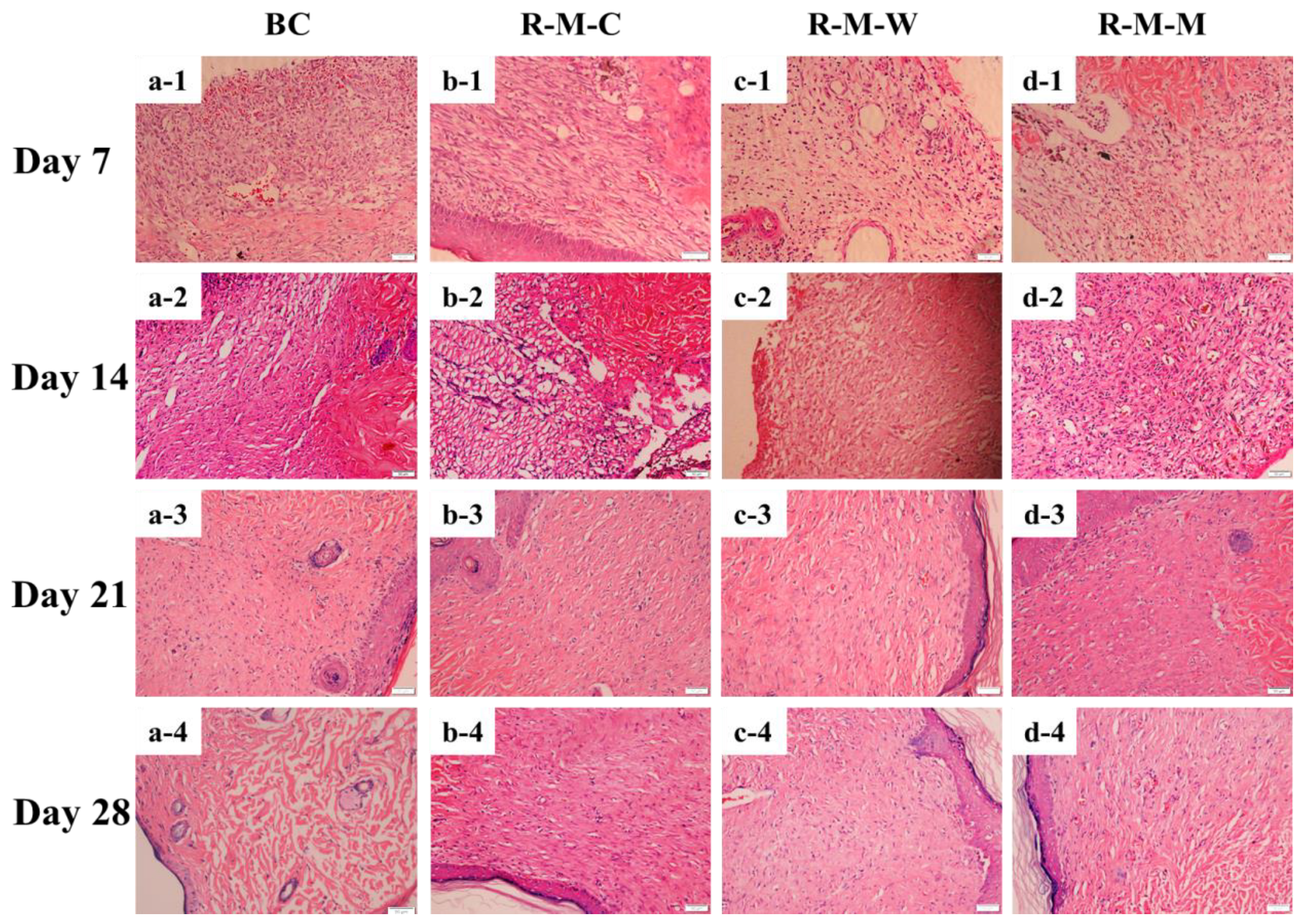
3.3. Evaluation of the RGDS-MPBC in the In-Vivo Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hsu, Y.C.; Li, L.; Fuchs, E. Emerging Interactions between Skin Stem Cells and Their Niches. Nat. Med. 2014, 20, 847–856. [Google Scholar] [CrossRef]
- Thulabandu, V.; Chen, D.; Atit, R.P. Dermal Fibroblast in Cutaneous Development and Healing. Wiley Interdiscip. Rev. Dev. Biol. 2018, 7, e307. [Google Scholar] [CrossRef]
- Dos Reis, G.; Fenili, F.; Gianfelice, A.; Bongiorno, G.; Marchesi, D.; Scopelliti, P.E.; Borgonovo, A.; Podestà, A.; Indrieri, M.; Ranucci, E.; et al. Direct Microfabrication of Topographical and Chemical Cues for the Guided Growth of Neural Cell Networks on Polyamidoamine Hydrogels. Macromol. Biosci. 2010, 10, 842–852. [Google Scholar] [CrossRef]
- Tan, W.; Desai, T.A. Microfluidic Patterning of Cells in Extracellular Matrix Biopolymers: Effects of Channel Size, Cell Type, and Matrix Composition on Pattern Integrity. Tissue Eng. 2003, 9, 255–267. [Google Scholar] [CrossRef]
- Zhang, X.Z.; Ren, Z.Z.; Jia, G.R.; Guo, X.T.; Gong, W.G. Numerical Exploration of Population Transfer of Rydberg-Atom by Single Frequency-Chirped Laser Pulse. Chin. Phys. B 2008, 17, 4476–4480. [Google Scholar] [CrossRef]
- Ozcelik, H.; Hindie, M.; Hasan, A.; Engin, N.; Cell, V.; Barthes, J.; Özçelik, H.; Hindié, M.; Ndreu-halili, A.; Vrana, N.E. Cell Microenvironment Engineering and Monitoring for Tissue Engineering and Regenerative Medicine: The Citation Accessed Citable Link Cell Microenvironment Engineering and Monitoring for Tissue Engineering and Regenerative Medicine: The Recent Advances. BioMed Res. Int. 2014, 2014, 921905. [Google Scholar]
- Théry, M. Micropatterning as a Tool to Decipher Cell Morphogenesis and Functions. J. Cell Sci. 2010, 123, 4201–4213. [Google Scholar] [CrossRef]
- Martínez, E.; Engel, E.; Planell, J.A.; Samitier, J. Effects of Artificial Micro- and Nano-Structured Surfaces on Cell Behaviour. Ann. Anat. 2009, 191, 126–135. [Google Scholar] [CrossRef]
- Teixeira, A.I.; Abrams, G.A.; Bertics, P.J.; Murphy, C.J.; Nealey, P.F. Epithelial Contact Guidance on Well-Defined Micro- and Nanostructured Substrates. J. Cell Sci. 2003, 116, 1881–1892. [Google Scholar] [CrossRef]
- Li, F.; Li, B.; Wang, Q.M.; Wang, J.H.C. Cell Shape Regulates Collagen Type I Expression in Human Tendon Fibroblasts. Cell Motil. Cytoskelet. 2008, 65, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Newaz, G. A Comprehensive Review of Surface Modification for Neural Cell Adhesion and Patterning. J. Biomed. Mater. Res. Part A 2010, 93, 1209–1224. [Google Scholar] [CrossRef]
- Pot, S.A.; Liliensiek, S.J.; Myrna, K.E.; Bentley, E.; Jester, J.V.; Nealey, P.F.; Murphy, C.J. Nanoscale Topography-Induced Modulation of Fundamental Cell Behaviors of Rabbit Corneal Keratocytes, Fibroblasts, and Myofibroblasts. Investig. Ophthalmol. Vis. Sci. 2010, 51, 1373–1381. [Google Scholar] [CrossRef]
- Fu, R.; Liu, Q.; Song, G.; Baik, A.; Hu, M.; Sun, S.; Guo, X.E.; Long, M.; Huo, B. Spreading Area and Shape Regulate Apoptosis and Differentiation of Osteoblasts. Biomed. Mater. 2013, 8, 055005. [Google Scholar] [CrossRef]
- Kilian, K.A.; Bugarija, B.; Lahn, B.T.; Mrksich, M. Geometric Cues for Directing the Differentiation of Mesenchymal Stem Cells. Proc. Natl. Acad. Sci. USA 2010, 107, 4872–4877. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.; Slater, J.H.; Culver, J.C.; Dickinson, M.E.; West, J.L. Biomimetic Surface Patterning Promotes Mesenchymal Stem Cell Differentiation. ACS Appl. Mater. Interfaces 2016, 8, 21883–21892. [Google Scholar] [CrossRef]
- Liu, H.; Hu, Y.; Zhu, Y.; Wu, X.; Zhou, X.; Pan, H.; Chen, S.; Tian, P. A Simultaneous Grafting/Vinyl Polymerization Process Generates a Polycationic Surface for Enhanced Antibacterial Activity of Bacterial Cellulose. Int. J. Biol. Macromol. 2020, 143, 224–234. [Google Scholar] [CrossRef]
- Hu, Y.; Catchmark, J.M. In Vitro Biodegradability and Mechanical Properties of Bioabsorbable Bacterial Cellulose Incorporating Cellulases. Acta Biomater. 2011, 7, 2835–2845. [Google Scholar] [CrossRef]
- Hu, Y.; Catchmark, J.M. Formation and Characterization of Spherelike Bacterial Cellulose Particles Produced by Acetobacter Xylinum JCM 9730 Strain. Biomacromolecules 2010, 11, 1727–1734. [Google Scholar] [CrossRef]
- Adiguzel, Z.; Sagnic, S.A.; Aroguz, A.Z. Preparation and Characterization of Polymers Based on PDMS and PEG-DMA as Potential Scaffold for Cell Growth. Mater. Sci. Eng. C 2017, 78, 942–948. [Google Scholar] [CrossRef] [PubMed]
- Knoblich, J.A. Asymmetric Cell Division: Recent Developments and Their Implications for Tumour Biology. Nat. Rev. Mol. Cell Biol. 2010, 11, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Mancini, F.; Bauleo, A.; Cole, J.; Lui, F.; Porro, C.A.; Haggard, P.; Iannetti, G.D. Whole-Body Mapping of Spatial Acuity for Pain and Touch. Ann. Neurol. 2014, 75, 917–924. [Google Scholar] [CrossRef] [PubMed]
- Hutchens, S.A.; Benson, R.S.; Evans, B.R.; O’Neill, H.M.; Rawn, C.J. Biomimetic Synthesis of Calcium-Deficient Hydroxyapatite in a Natural Hydrogel. Biomaterials 2006, 27, 4661–4670. [Google Scholar] [CrossRef]
- Picheth, G.F.; Pirich, C.L.; Sierakowski, M.R.; Woehl, M.A.; Sakakibara, C.N.; de Souza, C.F.; Martin, A.A.; da Silva, R.; de Freitas, R.A. Bacterial Cellulose in Biomedical Applications: A Review. Int. J. Biol. Macromol. 2017, 104, 97–106. [Google Scholar] [CrossRef]
- Wang, J.; Tavakoli, J.; Tang, Y. Bacterial Cellulose Production, Properties and Applications with Different Culture Methods—A Review. Carbohydr. Polym. 2019, 219, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Catchmark, J.M.; Vogler, E.A. Factors Impacting the Formation of Sphere-like Bacterial Cellulose Particles and Their Biocompatibility for Human Osteoblast Growth. Biomacromolecules 2013, 14, 3444–3452. [Google Scholar] [CrossRef] [PubMed]
- Ode Boni, B.O.; Lamboni, L.; Bakadia, B.M.; Hussein, S.A.; Yang, G. Combining Silk Sericin and Surface Micropatterns in Bacterial Cellulose Dressings to Control Fibrosis and Enhance Wound Healing. Eng. Sci. 2020, 10, 68–77. [Google Scholar] [CrossRef]
- Schroeder, A.; Blum, J.; Mayer, J.; Karamuk, E.; Wintermantel, E. Effect of Scaffold Pore Size on Cell Behaviour in Vitro. Biomed. Tech. 1996, 41, 436–437. [Google Scholar] [CrossRef]
- Navya, P.V.; Gayathri, V.; Samanta, D.; Sampath, S. Bacterial Cellulose: A Promising Biopolymer with Interesting Properties and Applications. Int. J. Biol. Macromol. 2022, 220, 435–461. [Google Scholar] [CrossRef]
- Hu, Y.; Catchmark, J.M.; Zhu, Y.; Abidi, N.; Zhou, X.; Wang, J.; Liang, N. Engineering of Porous Bacterial Cellulose toward Human Fibroblasts Ingrowth for Tissue Engineering. J. Mater. Res. 2014, 760, 2682–2693. [Google Scholar] [CrossRef]
- Cheng, M.; Deng, J.; Yang, F.; Gong, Y.; Zhao, N.; Zhang, X. Study on Physical Properties and Nerve Cell Affinity of Composite Films from Chitosan and Gelatin Solutions. Biomaterials 2003, 24, 2871–2880. [Google Scholar] [CrossRef]
- Program, B.E.; Program, B.E. Matrix Stiffness Modulates Mesenchymal Stem Cell Sensitivity to Geometric Asymmetry Signals. Ann. Biomed. Eng. 2018, 46, 888–898. [Google Scholar] [CrossRef]
- Fang, B.; Wan, Y.Z.; Tang, T.T.; Gao, C.; Dai, K.R. Proliferation and Osteoblastic Differentiation of Human Bone Marrow Stromal Cells on Hydroxyapatite/Bacterial Cellulose Nanocomposite Scaffolds. Tissue Eng. Part A 2009, 15, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Hartgerink, J.D.; Beniash, E.; Stupp, S.I. Self-Assembly and Mineralization of Peptide-Amphiphile Nanofibers. Science 2001, 294, 1684–1688. [Google Scholar] [CrossRef] [PubMed]
- Fricain, J.C.; Granja, P.L.; Barbosa, M.A.; De Jéso, B.; Barthe, N.; Baquey, C. Cellulose Phosphates as Biomaterials. In Vivo Biocompatibility Studies. Biomaterials 2002, 23, 971–980. [Google Scholar] [CrossRef]
- Dorairajan, A.; Reddy, R.M.; Krikler, S. Outcome of Acetabular Revision Using an Uncemented Hydroxyapatite-Coated Component: Two- to Five-Year Results and Review. J. Arthroplast. 2005, 20, 209–218. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, X.C.; Zhang, S.; Li, D. A Numerical Fluid-Solid Coupling Model for the Dynamics of Ships in Atrocious Sea Conditions. J. Algorithms Comput. Technol. 2015, 9, 163–175. [Google Scholar] [CrossRef]
- Ghasemi-Mobarakeh, L.; Prabhakaran, M.P.; Morshed, M.; Nasr-Esfahani, M.H.; Ramakrishna, S. Electrospun Poly(ε-Caprolactone)/Gelatin Nanofibrous Scaffolds for Nerve Tissue Engineering. Biomaterials 2008, 29, 4532–4539. [Google Scholar] [CrossRef] [PubMed]
- Jing, W.; Chunxi, Y.; Yizao, W.; Honglin, L.; Fang, H.; Kerong, D.; Yuan, H. Laser Patterning of Bacterial Cellulose Hydrogel and Its Modification with Gelatin and Hydroxyapatite for Bone Tissue Engineering. Soft Mater. 2013, 11, 173–180. [Google Scholar] [CrossRef]
- Beckwith, K.M.; Sikorski, P. Patterned Cell Arrays and Patterned Co-Cultures on Polydopamine-Modified Poly(Vinyl Alcohol) Hydrogels. Biofabrication 2013, 5, 045009. [Google Scholar] [CrossRef] [PubMed]
- Thakar, R.G.; Cheng, Q.; Patel, S.; Chu, J.; Nasir, M.; Liepmann, D.; Komvopoulos, K.; Li, S. Cell-Shape Regulation of Smooth Muscle Cell Proliferation. Biophys. J. 2009, 96, 3423–3432. [Google Scholar] [CrossRef]
- Cheng, C.M.; LeDuc, P.R. Micropatterning Polyvinyl Alcohol as a Biomimetic Material through Soft Lithography with Cell Culture. Mol. BioSystems 2006, 2, 299–304. [Google Scholar] [CrossRef]
- Park, T.H.; Shuler, M.L. Integration of Cell Culture and Microfabrication Technology. Biotechnol. Prog. 2003, 19, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hu, X.; Dulinska-Molak, I.; Kawazoe, N.; Yang, Y.; Chen, G. Discriminating the Independent Influence of Cell Adhesion and Spreading Area on Stem Cell Fate Determination Using Micropatterned Surfaces. Sci. Rep. 2016, 6, 1–13. [Google Scholar] [CrossRef]
- Wang, X.; Song, W.; Kawazoe, N.; Chen, G. The Osteogenic Differentiation of Mesenchymal Stem Cells by Controlled Cell-Cell Interaction on Micropatterned Surfaces. J. Biomed. Mater. Res. Part A 2013, 101, 3388–3395. [Google Scholar] [CrossRef] [PubMed]
- Ki, C.S.; Baek, D.H.; Gang, K.D.; Lee, K.H.; Um, I.C.; Park, Y.H. Characterization of Gelatin Nanofiber Prepared from Gelatin-Formic Acid Solution. Polymer 2005, 46, 5094–5102. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, H.; Zhou, X.; Pan, H.; Wu, X.; Abidi, N.; Zhu, Y.; Wang, J. Surface Engineering of Spongy Bacterial Cellulose via Constructing Crossed Groove/Column Micropattern by Low-Energy CO2 Laser Photolithography toward Scar-Free Wound Healing. Mater. Sci. Eng. C 2019, 99, 333–343. [Google Scholar] [CrossRef]
- Park, J.A.; Lee, H.R.; Park, S.Y.; Jung, S. Self-Organization of Fibroblast-Laden 3D Collagen Microstructures from Inkjet-Printed Cell Patterns. Adv. Biosyst. 2020, 4, 1900280. [Google Scholar] [CrossRef]
- Hellström, M.; Hellström, S.; Engström-Laurent, A.; Bertheim, U. The Structure of the Basement Membrane Zone Differs between Keloids, Hypertrophic Scars and Normal Skin: A Possible Background to an Impaired Function. J. Plast. Reconstr. Aesthetic Surg. 2014, 67, 1564–1572. [Google Scholar] [CrossRef]
- Naylor, E.C.; Watson, R.E.B.; Sherratt, M.J. Molecular Aspects of Skin Ageing. Maturitas 2011, 69, 249–256. [Google Scholar] [CrossRef]
- Strydom, H.; Maltha, J.C.; Kuijpers-Jagtman, A.M.; Von Den Hoff, J.W. The Oxytalan Fibre Network in the Periodontium and Its Possible Mechanical Function. Arch. Oral Biol. 2012, 57, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.; Geyer, S.; Weninger, W.; Guimberteau, J.C.; Wong, J.K. The Dynamic Anatomy and Patterning of Skin. Exp. Dermatol. 2016, 25, 92–98. [Google Scholar] [CrossRef] [PubMed]
- van Gaal, R.C.; Buskermolen, A.B.C.; Ippel, B.D.; Fransen, P.P.K.H.; Zaccaria, S.; Bouten, C.V.C.; Dankers, P.Y.W. Functional Peptide Presentation on Different Hydrogen Bonding Biomaterials Using Supramolecular Additives. Biomaterials 2019, 224, 119466. [Google Scholar] [CrossRef]
- Zhou, S.; Hokugo, A.; McClendon, M.; Zhang, Z.; Bakshi, R.; Wang, L.; Segovia, L.A.; Rezzadeh, K.; Stupp, S.I.; Jarrahy, R. Bioactive Peptide Amphiphile Nanofiber Gels Enhance Burn Wound Healing. Burns 2019, 45, 1112–1121. [Google Scholar] [CrossRef] [PubMed]
- Kambe, Y. Functionalization of Silk Fibroin-Based Biomaterials for Tissue Engineering. Polym. J. 2021, 53, 1345–1351. [Google Scholar] [CrossRef]
- Adamczak, M.; Scislowska-Czarnecka, A.; Genet, M.J.; Dupont-Gillain, C.C.; Pamula, E. Surface Characterization, Collagen Adsorption and Cell Behaviour on Poly(L-Lactide-Co-Glycolide). Acta Bioeng. Biomech. 2011, 13, 63–75. [Google Scholar] [PubMed]
- Bauer, S.; Park, J.; von der Mark, K.; Schmuki, P. Improved Attachment of Mesenchymal Stem Cells on Super-Hydrophobic TiO2 Nanotubes. Acta Biomater. 2008, 4, 1576–1582. [Google Scholar] [CrossRef] [PubMed]
- Holloway, S. Skin Considerations for Older Adults with Wounds. Br. J. Community Nurs. 2019, 24, S15–S19. [Google Scholar] [CrossRef]
- Shariatinia, Z. Carboxymethyl Chitosan: Properties and Biomedical Applications. Int. J. Biol. Macromol. 2018, 120, 1406–1419. [Google Scholar] [CrossRef]
- Yoshimitsu, Z.; Nakajima, A.; Watanabe, T.; Hashimoto, K. Effects of Surface Structure on the Hydrophobicity and Sliding Behavior of Water Droplets. Langmuir 2002, 18, 5818–5822. [Google Scholar] [CrossRef]
- Komai, Y.; Ushiki, T. The Three-Dimensional Organisation of Collagen Fibrils in the Human Cornea and Sclera. Investig. Ophthalmol. Vis. Sci. 1991, 32, 2244–2258. [Google Scholar]
- Sorg, H.; Tilkorn, D.J.; Hager, S.; Hauser, J.; Mirastschijski, U. Skin Wound Healing: An Update on the Current Knowledge and Concepts. Eur. Surg. Res. 2017, 58, 81–94. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Hu, Y.; Wu, X.; Hu, R.; Liu, Y. Optimization of Surface-Engineered Micropatterns on Bacterial Cellulose for Guided Scar-Free Skin Wound Healing. Biomolecules 2023, 13, 793. https://doi.org/10.3390/biom13050793
Liu H, Hu Y, Wu X, Hu R, Liu Y. Optimization of Surface-Engineered Micropatterns on Bacterial Cellulose for Guided Scar-Free Skin Wound Healing. Biomolecules. 2023; 13(5):793. https://doi.org/10.3390/biom13050793
Chicago/Turabian StyleLiu, Haiyan, Yang Hu, Xiuping Wu, Rong Hu, and Yingyu Liu. 2023. "Optimization of Surface-Engineered Micropatterns on Bacterial Cellulose for Guided Scar-Free Skin Wound Healing" Biomolecules 13, no. 5: 793. https://doi.org/10.3390/biom13050793
APA StyleLiu, H., Hu, Y., Wu, X., Hu, R., & Liu, Y. (2023). Optimization of Surface-Engineered Micropatterns on Bacterial Cellulose for Guided Scar-Free Skin Wound Healing. Biomolecules, 13(5), 793. https://doi.org/10.3390/biom13050793




