Real-Time Assessment of Intracellular Metabolites in Single Cells through RNA-Based Sensors
Abstract
1. Introduction
2. Cells Sense Intracellular Metabolites and Transduce This Information into Cellular Responses
3. Prokaryotic Cells Utilize RNA to Sense Intracellular Metabolites
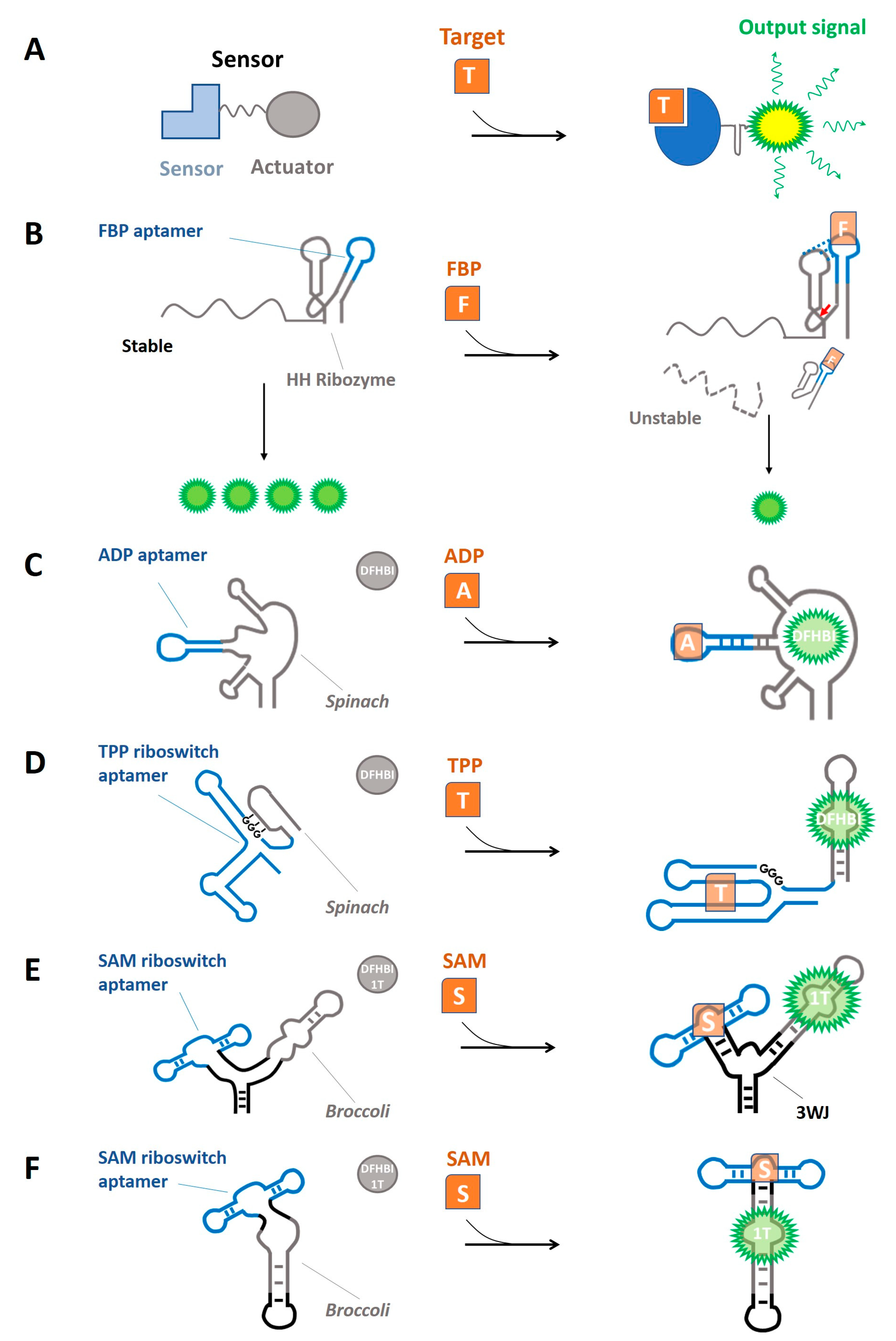
4. Synthetic RNA-Based Sensors for Intracellular Metabolites
| Target Metabolite 1 | Aptamer 2 | Type of Sensor 3 | Output 4 | Cell Type | Reference |
|---|---|---|---|---|---|
| 5-HT | SELEX | Light-up (Broccoli) | GF | Bacteria | Porter et al., 2017 [75] |
| ADP | SELEX | Light-up (Spinach) | GF | Bacteria | Paige et al., 2012 [63] |
| c-AMP-GMP | Natural | Light-up (Spinach) | GF | Bacteria | Kellenberger et al., 2013 [76] |
| c-di-AMP | Natural | Light-up (Spinach2) | GF | Bacteria | Kellenberger et al., 2015 [77] |
| c-di-GMP | Natural | Aptazyme | RF/BF | Mammalian | Xiang et al., 2019 [60] |
| c-di-GMP | Natural | Light-up (Spinach) | GF | Bacteria | Kellenberger et al., 2013 [76] |
| c-di-GMP | Natural | Light-up (Spinach2) | GF | Bacteria | Wang et al., 2016 [78] |
| FBP | SELEX | Aptazyme (HHRz) | GF/RF | Yeast | Ortega et al., 2021 [62] |
| FBP | SELEX | Light-up (Baby Spinach) | GF | Mammalian | Geraci et al., 2022 [79] |
| L-DOPA | SELEX | Light-up (Broccoli) | GF | Bacteria | Porter et al., 2017 [75] |
| (p)ppGpp | Natural | Light-up (Broccoli) | GF | Bacteria | Sun et al., 2021 [80] |
| (p)ppGpp | Natural | BRET (nLuc donor and Pepper acceptor) | GF | Bacteria | Mi et al., 2023 [81] |
| SAH | Natural | Light-up (cpSPinach2) | GF | Bacteria | Su et al., 2016 [82] |
| SAM | Natural | BRET (nLuc donor and Pepper acceptor) | GF | Bacteria | Mi et al., 2023 [81] |
| SAM | Natural | Light-up (Broccoli) | GF | Mammalian | Litke et al., 2019 [66] |
| SAM | Natural | Light-up (Broccoli) | GF | Mammalian | Moon et al., 2021 [65] |
| SAM | Natural | Light-up (Corn) | YF | Mammalian | Kim et al., 2019 [71] |
| SAM | Natural | Light-up (Red Broccoli) | RF | Mammalian | Li et al., 2020 [83] |
| SAM | Natural | Light-up (Spinach) | GF | Bacteria | Paige et al., 2012 [63] |
| SAM | Natural | Light-up (Squash and Broccoli) | YF/GF | Mammalian | Dey et al., 2022 [72] |
| TPP | Natural | Aptazyme | GF | Bacteria | Wieland et al., 2009 [67] |
| TPP | Natural | Aptazyme + Light-up (Broccoli) | GF | Bacteria | You et al. 2019 [74] |
| TPP | Natural | Light-up (Spinach) | GF | Bacteria | You et al., 2015 [64] |
| Xanthine | SELEX | Aptazyme | RF/BF | Mammalian | Xiang et al., 2019 [60] |
5. Development of RNA-Based Sensors for Intracellular Metabolites: A Challenging Endeavor
6. Applications of RNA-Based Sensors for Intracellular Metabolites
7. Outlook and Concluding Remarks
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Costenoble, R.; Picotti, P.; Reiter, L.; Stallmach, R.; Heinemann, M.; Sauer, U.; Aebersold, R. Comprehensive Quantitative Analysis of Central Carbon and Amino-acid Metabolism in Saccharomyces cerevisiae under Multiple Conditions by Targeted Proteomics. Mol. Syst. Biol. 2011, 7, 464. [Google Scholar] [CrossRef]
- Chen, Y.; Nielsen, J. Flux Control through Protein Phosphorylation in Yeast. FEMS Yeast Res. 2016, 16, fow096. [Google Scholar] [CrossRef]
- Kotte, O.; Zaugg, J.B.; Heinemann, M. Bacterial Adaptation through Distributed Sensing of Metabolic Fluxes. Mol. Syst. Biol. 2010, 6, 355. [Google Scholar] [CrossRef]
- Litsios, A.; Ortega, A.D.; Wit, E.C.; Heinemann, M. Metabolic-Flux Dependent Regulation of Microbial Physiology. Curr. Opin. Microbiol. 2017, 42, 71–78. [Google Scholar] [CrossRef]
- Niedenführ, S.; Wiechert, W.; Nöh, K. How to Measure Metabolic Fluxes: A Taxonomic Guide for 13C Fluxomics. Curr. Opin. Biotechnol. 2015, 34, 82–90. [Google Scholar] [CrossRef]
- Zhang, J.; Jensen, M.K.; Keasling, J.D. Development of Biosensors and Their Application in Metabolic Engineering. Curr. Opin. Chem. Biol. 2015, 28, 1–8. [Google Scholar] [CrossRef]
- Shi, S.; Xie, Y.; Wang, G.; Luo, Y. Metabolite-Based Biosensors for Natural Product Discovery and Overproduction. Curr. Opin. Biotechnol. 2022, 75, 102699. [Google Scholar] [CrossRef]
- Sourjik, V.; Berg, H.C. Functional Interactions between Receptors in Bacterial Chemotaxis. Nature 2004, 428, 437–441. [Google Scholar] [CrossRef]
- Ozcan, S.; Dover, J.; Rosenwald, A.G.; Wölfl, S.; Johnston, M. Two Glucose Transporters in Saccharomyces cerevisiae Are Glucose Sensors That Generate a Signal for Induction of Gene Expression. Proc. Natl. Acad. Sci. USA 1996, 93, 12428–12432. [Google Scholar] [CrossRef]
- Huberts, D.H.E.W.; Niebel, B.; Heinemann, M. A Flux-Sensing Mechanism Could Regulate the Switch between Respiration and Fermentation. FEMS Yeast Res. 2012, 12, 118–128. [Google Scholar] [CrossRef]
- Chantranupong, L.; Wolfson, R.L.; Sabatini, D.M. Nutrient-Sensing Mechanisms across Evolution. Cell 2015, 161, 67–83. [Google Scholar] [CrossRef]
- Ferenci, T. Sensing Nutrient Levels in Bacteria. Nat. Chem. Biol. 2007, 3, 607–608. [Google Scholar] [CrossRef]
- Leigh, J.A.; Dodsworth, J.A. Nitrogen Regulation in Bacteria and Archaea. Annu. Rev. Microbiol. 2007, 61, 349–377. [Google Scholar] [CrossRef]
- Lane, N.; Martin, W. The Energetics of Genome Complexity. Nature 2010, 467, 929–934. [Google Scholar] [CrossRef]
- Dalebroux, Z.D.; Swanson, M.S. PpGpp: Magic beyond RNA Polymerase. Nat. Rev. Microbiol. 2012, 10, 203–212. [Google Scholar] [CrossRef]
- Kochanowski, K.; Volkmer, B.; Gerosa, L.; Haverkorn van Rijsewijk, B.R.; Schmidt, A.; Heinemann, M. Functioning of a Metabolic Flux Sensor in Escherichia coli. Proc. Natl. Acad. Sci. USA 2013, 110, 1130–1135. [Google Scholar] [CrossRef]
- Görke, B.; Stülke, J. Carbon Catabolite Repression in Bacteria: Many Ways to Make the Most out of Nutrients. Nat. Rev. Microbiol. 2008, 6, 613–624. [Google Scholar] [CrossRef]
- Jault, J.-M.; Fieulaine, S.; Nessler, S.; Gonzalo, P.; Di Pietro, A.; Deutscher, J.; Galinier, A. The HPr Kinase from Bacillus subtilis Is a Homo-Oligomeric Enzyme Which Exhibits Strong Positive Cooperativity for Nucleotide and Fructose 1,6-Bisphosphate Binding. J. Biol. Chem. 2000, 275, 1773–1780. [Google Scholar] [CrossRef]
- Schumacher, M.A.; Allen, G.S.; Diel, M.; Seidel, G.; Hillen, W.; Brennan, R.G. Structural Basis for Allosteric Control of the Transcription Regulator CcpA by the Phosphoprotein HPr-Ser46-P. Cell 2004, 118, 731–741. [Google Scholar] [CrossRef]
- Doan, T.; Aymerich, S. Regulation of the Central Glycolytic Genes in Bacillus subtilis: Binding of the Repressor CggR to Its Single DNA Target Sequence Is Modulated by Fructose-1,6-Bisphosphate. Mol. Microbiol. 2003, 47, 1709–1721. [Google Scholar] [CrossRef]
- Bley Folly, B.; Ortega, A.D.; Hubmann, G.; Bonsing-Vedelaar, S.; Wijma, H.J.; van der Meulen, P.; Milias-Argeitis, A.; Heinemann, M. Assessment of the Interaction between the Flux-Signaling Metabolite Fructose-1,6-Bisphosphate and the Bacterial Transcription Factors CggR and Cra. Mol. Microbiol. 2018, 109, 278–290. [Google Scholar] [CrossRef]
- Ludwig, H.; Homuth, G.; Schmalisch, M.; Dyka, F.M.; Hecker, M.; Stülke, J. Transcription of Glycolytic Genes and Operons in Bacillus subtilis: Evidence for the Presence of Multiple Levels of Control of the GapA Operon. Mol. Microbiol. 2001, 41, 409–422. [Google Scholar] [CrossRef]
- Colombo, S.; Ronchetti, D.; Thevelein, J.M.; Winderickx, J.; Martegani, E. Activation State of the Ras2 Protein and Glucose-Induced Signaling in Saccharomyces cerevisiae. J. Biol. Chem. 2004, 279, 46715–46722. [Google Scholar] [CrossRef]
- Peeters, K.; Van Leemputte, F.; Fischer, B.; Bonini, B.M.; Quezada, H.; Tsytlonok, M.; Haesen, D.; Vanthienen, W.; Bernardes, N.; Gonzalez-Blas, C.B.; et al. Fructose-1,6-Bisphosphate Couples Glycolytic Flux to Activation of Ras. Nat. Commun. 2017, 8, 922. [Google Scholar] [CrossRef]
- Zhang, C.-S.; Hawley, S.A.; Zong, Y.; Li, M.; Wang, Z.; Gray, A.; Ma, T.; Cui, J.; Feng, J.-W.; Zhu, M.; et al. Fructose-1,6-Bisphosphate and Aldolase Mediate Glucose Sensing by AMPK. Nature 2017, 548, 112–116. [Google Scholar] [CrossRef]
- Etzel, M.; Mörl, M. Synthetic Riboswitches—From Plug and Pray towards Plug and Play. Biochemistry 2017, 56, 1181–1198. [Google Scholar] [CrossRef]
- Winkler, W.C. Metabolic Monitoring by Bacterial MRNAs. Arch. Microbiol. 2005, 183, 151–159. [Google Scholar] [CrossRef]
- Serganov, A.; Patel, D.J. Metabolite Recognition Principles and Molecular Mechanisms Underlying Riboswitch Function. Annu. Rev. Biophys. 2012, 41, 343–370. [Google Scholar] [CrossRef]
- Klähn, S.; Bolay, P.; Wright, P.R.; Atilho, R.M.; Brewer, K.I.; Hagemann, M.; Breaker, R.R.; Hess, W.R. A Glutamine Riboswitch Is a Key Element for the Regulation of Glutamine Synthetase in Cyanobacteria. Nucleic Acids Res. 2018, 46, 10082–10094. [Google Scholar] [CrossRef]
- Weinberg, Z.; Wang, J.X.; Bogue, J.; Yang, J.; Corbino, K.; Moy, R.H.; Breaker, R.R. Comparative Genomics Reveals 104 Candidate Structured RNAs from Bacteria, Archaea, and Their Metagenomes. Genome Biol. 2010, 11, R31. [Google Scholar] [CrossRef]
- Breaker, R.R. The Biochemical Landscape of Riboswitch Ligands. Biochemistry 2022, 61, 137–149. [Google Scholar] [CrossRef]
- Nelson, J.W.; Breaker, R.R. The Lost Language of the RNA World. Sci. Signal. 2017, 10, eaam8812. [Google Scholar] [CrossRef]
- Breaker, R.R. Riboswitches and the RNA World. Cold Spring Harb. Perspect. Biol. 2012, 4, a003566. [Google Scholar] [CrossRef]
- Serganov, A.; Nudler, E. A Decade of Riboswitches. Cell 2013, 152, 17–24. [Google Scholar] [CrossRef]
- Winkler, W.C.; Nahvi, A.; Roth, A.; Collins, J.A.; Breaker, R.R. Control of Gene Expression by a Natural Metabolite-Responsive Ribozyme. Nature 2004, 428, 281–286. [Google Scholar] [CrossRef]
- Ramesh, A.; Winkler, W.C. Metabolite-Binding Ribozymes. Biochim. Biophys. Acta Gene Regul. Mech. 2014, 1839, 989–994. [Google Scholar] [CrossRef]
- Klein, D.J.; Ferré-D’Amaré, A.R. Structural Basis of GlmS Ribozyme Activation by Glucosamine-6-Phosphate. Science 2006, 313, 1752–1756. [Google Scholar] [CrossRef]
- Bédard, A.S.V.; Hien, E.D.M.; Lafontaine, D.A. Riboswitch Regulation Mechanisms: RNA, Metabolites and Regulatory Proteins. Biochim. Biophys. Acta Gene Regul. Mech. 2020, 1863, 194501. [Google Scholar] [CrossRef]
- Winkler, W.; Nahvi, A.; Breaker, R.R. Thiamine Derivatives Bind Messenger RNAs Directly to Regulate Bacterial Gene Expression. Nature 2002, 419, 952–956. [Google Scholar] [CrossRef]
- Mironov, A.S.; Gusarov, I.; Rafikov, R.; Lopez, L.E.; Shatalin, K.; Kreneva, R.A.; Perumov, D.A.; Nudler, E. Sensing Small Molecules by Nascent RNA: A Mechanism to Control Transcription in Bacteria. Cell 2002, 111, 747–756. [Google Scholar] [CrossRef]
- Ceres, P.; Garst, A.D.; Marcano-Velázquez, J.G.; Batey, R.T. Modularity of Select Riboswitch Expression Platforms Enables Facile Engineering of Novel Genetic Regulatory Devices. ACS Synth. Biol. 2013, 2, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Ceres, P.; Trausch, J.J.; Batey, R.T. Engineering Modular “ON” RNA Switches Using Biological Components. Nucleic Acids Res. 2013, 41, 10449–10461. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Breaker, R.R. Rational Design of Allosteric Ribozymes. Chem. Biol. 1997, 4, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Sassanfar, M.; Szostak, J.W. An RNA Motif That Binds ATP. Nature 1993, 364, 550–553. [Google Scholar] [CrossRef] [PubMed]
- Soukup, G.A.; Breaker, R.R. Engineering Precision RNA Molecular Switches. Proc. Natl. Acad. Sci. USA 1999, 96, 3584–3589. [Google Scholar] [CrossRef] [PubMed]
- Jenison, R.D.; Gill, S.C.; Pardi, A.; Polisky, B. High-Resolution Molecular Discrimination by RNA. Science 1994, 263, 1425–1429. [Google Scholar] [CrossRef]
- Suess, B.; Hanson, S.; Berens, C.; Fink, B.; Schroeder, R.; Hillen, W. Conditional Gene Expression by Controlling Translation with Tetracycline-Binding Aptamers. Nucleic Acids Res. 2003, 31, 1853–1858. [Google Scholar] [CrossRef]
- Berschneider, B.; Wieland, M.; Rubini, M.; Hartig, J.S. Small-Molecule-Dependent Regulation of Transfer RNA in Bacteria. Angew. Chem. Int. Ed. 2009, 48, 7564–7567. [Google Scholar] [CrossRef]
- Wieland, M.; Berschneider, B.; Erlacher, M.D.; Hartig, J.S. Aptazyme-Mediated Regulation of 16S Ribosomal RNA. Chem. Biol. 2010, 17, 236–242. [Google Scholar] [CrossRef]
- Wieland, M.; Hartig, J.S. Improved Aptazyme Design and In Vivo Screening Enable Riboswitching in Bacteria. Angew. Chem. Int. Ed. 2008, 47, 2604–2607. [Google Scholar] [CrossRef]
- Bayer, T.S.; Smolke, C.D. Programmable Ligand-Controlled Riboregulators of Eukaryotic Gene Expression. Nat. Biotechnol. 2005, 23, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Ausländer, S.; Ketzer, P.; Hartig, J.S. A Ligand-Dependent Hammerhead Ribozyme Switch for Controlling Mammalian Gene Expression. Mol. Biosyst. 2010, 6, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Jensen, M.C.; Smolke, C.D. Genetic Control of Mammalian T-Cell Proliferation with Synthetic RNA Regulatory Systems. Proc. Natl. Acad. Sci. USA 2010, 107, 8531–8536. [Google Scholar] [CrossRef] [PubMed]
- Win, M.N.; Smolke, C.D. A Modular and Extensible RNA-Based Gene-Regulatory Platform for Engineering Cellular Function. Proc. Natl. Acad. Sci. USA 2007, 104, 14283–14288. [Google Scholar] [CrossRef]
- Babiskin, A.H.; Smolke, C.D. Engineering Ligand-Responsive RNA Controllers in Yeast through the Assembly of RNase III Tuning Modules. Nucleic Acids Res. 2011, 39, 5299–5311. [Google Scholar] [CrossRef]
- Weigand, J.E.; Suess, B. Tetracycline Aptamer-Controlled Regulation of Pre-MRNA Splicing in Yeast. Nucleic Acids Res. 2007, 35, 4179–4185. [Google Scholar] [CrossRef]
- Beisel, C.L.; Chen, Y.Y.; Culler, S.J.; Hoff, K.G.; Smolke, C.D. Design of Small Molecule-Responsive MicroRNAs Based on Structural Requirements for Drosha Processing. Nucleic Acids Res. 2011, 39, 2981–2994. [Google Scholar] [CrossRef]
- Liang, J.C.; Chang, A.L.; Kennedy, A.B.; Smolke, C.D. A High-Throughput, Quantitative Cell-Based Screen for Efficient Tailoring of RNA Device Activity. Nucleic Acids Res. 2012, 40, e154. [Google Scholar] [CrossRef]
- Townshend, B.; Kennedy, A.B.; Xiang, J.S.; Smolke, C.D. High-Throughput Cellular RNA Device Engineering. Nat. Methods 2015, 12, 989–994. [Google Scholar] [CrossRef]
- Xiang, J.S.; Kaplan, M.; Dykstra, P.; Hinks, M.; McKeague, M.; Smolke, C.D. Massively Parallel RNA Device Engineering in Mammalian Cells with RNA-Seq. Nat. Commun. 2019, 10, 4327. [Google Scholar] [CrossRef]
- Galloway, K.E.; Franco, E.; Smolke, C.D. Dynamically Reshaping Signaling Networks to Program Cell Fate via Genetic Controllers. Science 2013, 341, 1235005. [Google Scholar] [CrossRef] [PubMed]
- Ortega, A.D.; Takhaveev, V.; Vedelaar, S.R.; Long, Y.; Mestre-Farràs, N.; Incarnato, D.; Ersoy, F.; Olsen, L.F.; Mayer, G.; Heinemann, M. A Synthetic RNA-Based Biosensor for Fructose-1,6-Bisphosphate That Reports Glycolytic Flux. Cell Chem. Biol. 2021, 28, 1554–1568.e8. [Google Scholar] [CrossRef] [PubMed]
- Paige, J.S.; Nguyen-Duc, T.; Song, W.; Jaffrey, S.R. Fluorescence Imaging of Cellular Metabolites with RNA. Science 2012, 335, 1194. [Google Scholar] [CrossRef] [PubMed]
- You, M.; Litke, J.L.; Jaffrey, S.R. Imaging Metabolite Dynamics in Living Cells Using a Spinach-Based Riboswitch. Proc. Natl. Acad. Sci. USA 2015, 112, E2756–E2765. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.D.; Wu, J.; Dey, S.K.; Litke, J.L.; Li, X.; Kim, H.; Jaffrey, S.R. Naturally Occurring Three-Way Junctions Can Be Repurposed as Genetically Encoded RNA-Based Sensors. Cell Chem. Biol. 2021, 28, 1569–1580. [Google Scholar] [CrossRef] [PubMed]
- Litke, J.L.; Jaffrey, S.R. Highly Efficient Expression of Circular RNA Aptamers in Cells Using Autocatalytic Transcripts. Nat. Biotechnol. 2019, 37, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Wieland, M.; Benz, A.; Klauser, B.; Hartig, J.S. Artificial Ribozyme Switches Containing Natural Riboswitch Aptamer Domains. Angew. Chem. Int. Ed. 2009, 48, 2715–2718. [Google Scholar] [CrossRef]
- Jaffrey, S.R. RNA-Based Fluorescent Biosensors for Detecting Metabolites In Vitro and in Living Cells. Adv. Pharmacol. 2018, 82, 187–203. [Google Scholar]
- Paige, J.S.; Wu, K.Y.; Jaffrey, S.R. RNA Mimics of Green Fluorescent Protein. Science 2011, 333, 642–646. [Google Scholar] [CrossRef]
- Filonov, G.S.; Moon, J.D.; Svensen, N.; Ja, S.R. Broccoli: Rapid Selection of an RNA Mimic of Green Fluorescent Protein by Fluorescence-Based Selection and Directed Evolution. J. Am. Chem. Soc. 2014, 136, 16299–16308. [Google Scholar] [CrossRef]
- Kim, H.; Jaffrey, S.R. A Fluorogenic RNA-Based Sensor Activated by Metabolite-Induced RNA Dimerization. Cell Chem. Biol. 2019, 26, 1725–1731.e6. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.K.; Filonov, G.S.; Olarerin-George, A.O.; Jackson, B.T.; Finley, L.W.S.; Jaffrey, S.R. Repurposing an Adenine Riboswitch into a Fluorogenic Imaging and Sensing Tag. Nat. Chem. Biol. 2022, 18, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Klauser, B.; Atanasov, J.; Siewert, L.K.; Hartig, J.S. Ribozyme-Based Aminoglycoside Switches of Gene Expression Engineered by Genetic Selection in S. Cerevisiae. ACS Synth. Biol. 2015, 4, 516–525. [Google Scholar] [CrossRef] [PubMed]
- You, M.; Litke, J.L.; Wu, R.; Jaffrey, S.R. Detection of Low-Abundance Metabolites in Live Cells Using an RNA Integrator. Cell Chem. Biol. 2019, 26, 471–481.e3. [Google Scholar] [CrossRef]
- Porter, E.B.; Polaski, J.T.; Morck, M.M.; Batey, R.T. Recurrent RNA Motifs as Scaffolds for Genetically Encodable Small-Molecule Biosensors. Nat. Chem. Biol. 2017, 13, 295–301. [Google Scholar] [CrossRef]
- Kellenberger, C.A.; Wilson, S.C.; Sales-Lee, J.; Hammond, M.C. RNA-Based Fluorescent Biosensors for Live Cell Imaging of Second Messengers Cyclic Di-GMP and Cyclic AMP-GMP. J. Am. Chem. Soc. 2013, 135, 4906–4909. [Google Scholar] [CrossRef]
- Kellenberger, C.A.; Chen, C.; Whiteley, A.T.; Portnoy, D.A.; Hammond, M.C. RNA-Based Fluorescent Biosensors for Live Cell Imaging of Second Messenger Cyclic Di-AMP. J. Am. Chem. Soc. 2015, 137, 6432–6435. [Google Scholar] [CrossRef]
- Wang, X.C.; Wilson, S.C.; Hammond, M.C. Next-Generation RNA-Based Fluorescent Biosensors Enable Anaerobic Detection of Cyclic Di-GMP. Nucleic Acids Res. 2016, 44, e139. [Google Scholar] [CrossRef]
- Geraci, I.; Autour, A.; Pietruschka, G.; Shiian, A.; Borisova, M.; Mayer, C.; Ryckelynck, M.; Mayer, G. Fluorogenic RNA-Based Biosensor to Sense the Glycolytic Flux in Mammalian Cells. ACS Chem. Biol. 2022, 17, 1164–1173. [Google Scholar] [CrossRef]
- Sun, Z.; Wu, R.; Zhao, B.; Zeinert, R.; Chien, P.; You, M. Live-Cell Imaging of Guanosine Tetra- and Pentaphosphate (p)PpGpp with RNA-Based Fluorescent Sensors. Angew. Chem. Int. Ed. 2021, 60, 24070–24074. [Google Scholar] [CrossRef]
- Mi, L.; Yu, Q.; Karunanayake Mudiyanselage, A.P.K.K.; Wu, R.; Sun, Z.; Zheng, R.; Ren, K.; You, M. Genetically Encoded RNA-Based Bioluminescence Resonance Energy Transfer (BRET) Sensors. ACS Sens. 2023, 8, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Hickey, S.F.; Keyser, S.G.L.; Hammond, M.C. In Vitro and in Vivo Enzyme Activity Screening via RNA-Based Fluorescent Biosensors for S-Adenosyl- l -Homocysteine (SAH). J. Am. Chem. Soc. 2016, 138, 7040–7047. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Mo, L.; Litke, J.L.; Dey, S.K.; Suter, S.R.; Jaffrey, S.R. Imaging Intracellular S-Adenosyl Methionine Dynamics in Live Mammalian Cells with a Genetically Encoded Red Fluorescent RNA-Based Sensor. J. Am. Chem. Soc. 2020, 142, 14117–14124. [Google Scholar] [CrossRef] [PubMed]
- Coimbatore Narayanan, B.; Westbrook, J.; Ghosh, S.; Petrov, A.I.; Sweeney, B.; Zirbel, C.L.; Leontis, N.B.; Berman, H.M. The Nucleic Acid Database: New Features and Capabilities. Nucleic Acids Res. 2014, 42, D114–D122. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, Z.; Lünse, C.E.; Corbino, K.A.; Ames, T.D.; Nelson, J.W.; Roth, A.; Perkins, K.R.; Sherlock, M.E.; Breaker, R.R. Detection of 224 Candidate Structured RNAs by Comparative Analysis of Specific Subsets of Intergenic Regions. Nucleic Acids Res. 2017, 45, 10811–10823. [Google Scholar] [CrossRef] [PubMed]
- Brewer, K.I.; Gaffield, G.J.; Puri, M.; Breaker, R.R. DIMPL: A Bioinformatics Pipeline for the Discovery of Structured Noncoding RNA Motifs in Bacteria. Bioinformatics 2022, 38, 533–535. [Google Scholar] [CrossRef] [PubMed]
- McCown, P.J.; Corbino, K.A.; Stav, S.; Sherlock, M.E.; Breaker, R.R. Riboswitch Diversity and Distribution. RNA 2017, 23, 995–1011. [Google Scholar] [CrossRef]
- Tapsin, S.; Sun, M.; Shen, Y.; Zhang, H.; Lim, X.N.; Susanto, T.T.; Yang, S.L.; Zeng, G.S.; Lee, J.; Lezhava, A.; et al. Genome-Wide Identification of Natural RNA Aptamers in Prokaryotes and Eukaryotes. Nat. Commun. 2018, 9, 1289. [Google Scholar] [CrossRef]
- Dar, D.; Shamir, M.; Mellin, J.R.; Koutero, M.; Stern-Ginossar, N.; Cossart, P.; Sorek, R. Term-Seq Reveals Abundant Ribo-Regulation of Antibiotics Resistance in Bacteria. Science 2016, 352, aad9822. [Google Scholar] [CrossRef]
- Balaratnam, S.; Rhodes, C.; Bume, D.D.; Connelly, C.; Lai, C.C.; Kelley, J.A.; Yazdani, K.; Homan, P.J.; Incarnato, D.; Numata, T.; et al. A Chemical Probe Based on the PreQ1 Metabolite Enables Transcriptome-Wide Mapping of Binding Sites. Nat. Commun. 2021, 12, 5856. [Google Scholar] [CrossRef]
- McKeague, M.; Derosa, M.C. Challenges and Opportunities for Small Molecule Aptamer Development. J. Nucleic Acids 2012, 2012, 748913. [Google Scholar] [CrossRef] [PubMed]
- McKeague, M.; Wong, R.S.; Smolke, C.D. Opportunities in the Design and Application of RNA for Gene Expression Control. Nucleic Acids Res. 2016, 44, 2987–2999. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Pfeiffer, F.; Mayer, G.; Schrøder, T.D.; Özalp, V.C.; Olsen, L.F. Selection of Aptamers for Metabolite Sensing and Construction of Optical Nanosensors. Methods Mol. Biol. 2016, 1380, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Kiga, D.; Futamura, Y.; Sakamoto, K.; Yokoyama, S. An RNA Aptamer to the Xanthine/Guanine Base with a Distinctive Mode of Purine Recognition. Nucleic Acids Res. 1998, 26, 1755–1760. [Google Scholar] [CrossRef]
- Filonov, G.S.; Kam, C.W.; Song, W.; Jaffrey, S.R. In-Gel Imaging of RNA Processing Using Broccoli Reveals Optimal Aptamer Expression Strategies. Chem. Biol. 2015, 22, 649–660. [Google Scholar] [CrossRef]
- Strack, R.L.; Disney, M.D.; Jaffrey, S.R. A Superfolding Spinach2 Reveals the Dynamic Nature of Trinucleotide Repeat-Containing RNA. Nat. Methods 2013, 10, 1219–1224. [Google Scholar] [CrossRef]
- Weigand, J.E.; Sanchez, M.; Gunnesch, E.; Zeiher, S.; Schroeder, R.; Suess, B. Screening for Engineered Neomycin Riboswitches That Control Translation Initiation. RNA 2008, 14, 89–97. [Google Scholar] [CrossRef]
- Groher, F.; Bofill-Bosch, C.; Schneider, C.; Braun, J.; Jager, S.; Geißler, K.; Hamacher, K.; Suess, B. Riboswitching with Ciprofloxacin—Development and Characterization of a Novel RNA Regulator. Nucleic Acids Res. 2018, 46, 2121–2132. [Google Scholar] [CrossRef]
- Boussebayle, A.; Torka, D.; Ollivaud, S.; Braun, J.; Bofill-Bosch, C.; Dombrowski, M.; Groher, F.; Hamacher, K.; Suess, B. Next-Level Riboswitch Development—Implementation of Capture-SELEX Facilitates Identification of a New Synthetic Riboswitch. Nucleic Acids Res. 2019, 47, 4883–4895. [Google Scholar] [CrossRef]
- Elowitz, M.; Levine, A.; Siggia, E.; Swain, P. Stochastic Gene Expression in a Single Cell. Science 2002, 297, 1183–1187. [Google Scholar] [CrossRef]
- Cox, R.S.; Dunlop, M.J.; Elowitz, M.B. A Synthetic Three-Color Scaffold for Monitoring Genetic Regulation and Noise. J. Biol. Eng. 2010, 4, 10. [Google Scholar] [CrossRef]
- Michener, J.J.K.; Thodey, K.; Liang, J.J.C.; Smolke, C.D.C. Applications of Genetically-Encoded Biosensors for the Construction and Control of Biosynthetic Pathways. Metab. Eng. 2012, 14, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Michener, J.K.; Smolke, C.D. High-Throughput Enzyme Evolution in Saccharomyces cerevisiae Using a Synthetic RNA Switch. Metab. Eng. 2012, 14, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.; Pellaux, R.; Potot, S.; Becker, K.; Hohmann, H.-P.; Panke, S.; Held, M. Optimization of a Whole-Cell Biocatalyst by Employing Genetically Encoded Product Sensors inside Nanolitre Reactors. Nat. Chem. 2015, 7, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Rugbjerg, P.; Sommer, M.O.A. Overcoming Genetic Heterogeneity in Industrial Fermentations. Nat. Biotechnol. 2019, 37, 869–876. [Google Scholar] [CrossRef]
- Deparis, Q.; Claes, A.; Foulquié-Moreno, M.R.; Thevelein, J.M. Engineering Tolerance to Industrially Relevant Stress Factors in Yeast Cell Factories. FEMS Yeast Res. 2017, 17, fox036. [Google Scholar] [CrossRef]
- Torello Pianale, L.; Rugbjerg, P.; Olsson, L. Real-Time Monitoring of the Yeast Intracellular State During Bioprocesses with a Toolbox of Biosensors. Front. Microbiol. 2022, 12, 802169. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Seo, S.W.; Jang, S.; Shin, S.-I.I.; Lim, C.H.; Roh, T.-Y.Y.; Jung, G.Y. Synthetic RNA Devices to Expedite the Evolution of Metabolite-Producing Microbes. Nat. Commun. 2013, 4, 1413. [Google Scholar] [CrossRef]
- Kim, J.; DeBerardinis, R.J. Mechanisms and Implications of Metabolic Heterogeneity in Cancer. Cell Metab. 2019, 30, 434–446. [Google Scholar] [CrossRef]
- Win, M.N.; Smolke, C.D. Higher-Order Cellular Information Processing with Synthetic RNA Devices. Science 2008, 322, 456–460. [Google Scholar] [CrossRef]
- Sharma, V.; Nomura, Y.; Yokobayashi, Y. Engineering Complex Riboswitch Regulation by Dual Genetic Selection. J. Am. Chem. Soc. 2008, 130, 16310–16315. [Google Scholar] [CrossRef] [PubMed]
- Sherlock, M.E.; Sudarsan, N.; Stav, S.; Breaker, R.R. Tandem Riboswitches Form a Natural Boolean Logic Gate to Control Purine Metabolism in Bacteria. eLife 2018, 7, e33908. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortega, A.D. Real-Time Assessment of Intracellular Metabolites in Single Cells through RNA-Based Sensors. Biomolecules 2023, 13, 765. https://doi.org/10.3390/biom13050765
Ortega AD. Real-Time Assessment of Intracellular Metabolites in Single Cells through RNA-Based Sensors. Biomolecules. 2023; 13(5):765. https://doi.org/10.3390/biom13050765
Chicago/Turabian StyleOrtega, Alvaro Darío. 2023. "Real-Time Assessment of Intracellular Metabolites in Single Cells through RNA-Based Sensors" Biomolecules 13, no. 5: 765. https://doi.org/10.3390/biom13050765
APA StyleOrtega, A. D. (2023). Real-Time Assessment of Intracellular Metabolites in Single Cells through RNA-Based Sensors. Biomolecules, 13(5), 765. https://doi.org/10.3390/biom13050765







