Combined In Vivo Microdialysis and PET Studies to Validate [11C]Yohimbine Binding as a Marker of Noradrenaline Release
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Setup
2.3. Tomography
2.4. PET Data Analysis
2.5. HPLC Analysis of Dialysates
2.6. Statistical Analysis
2.7. Verification of the Probe Location and Histology
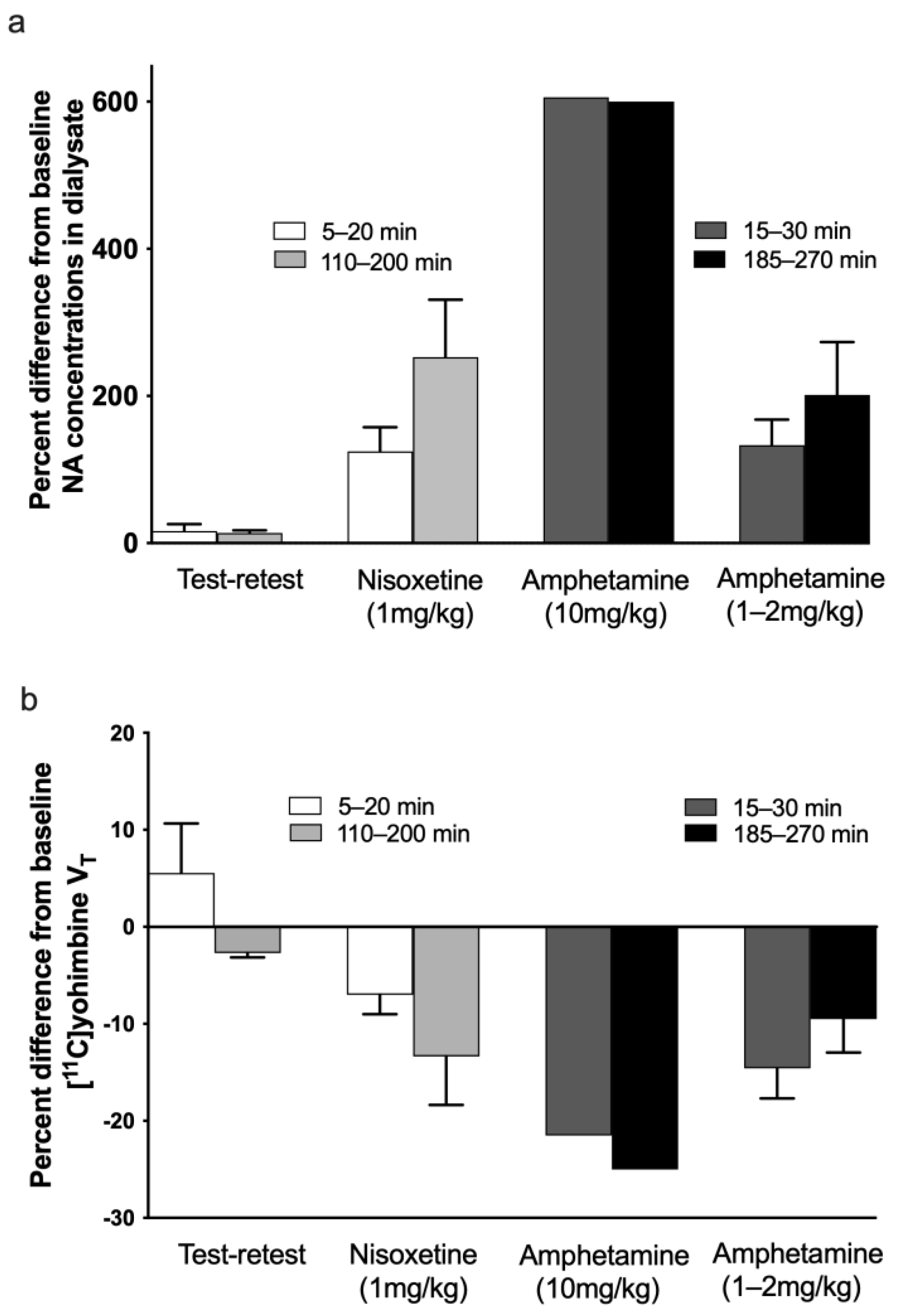
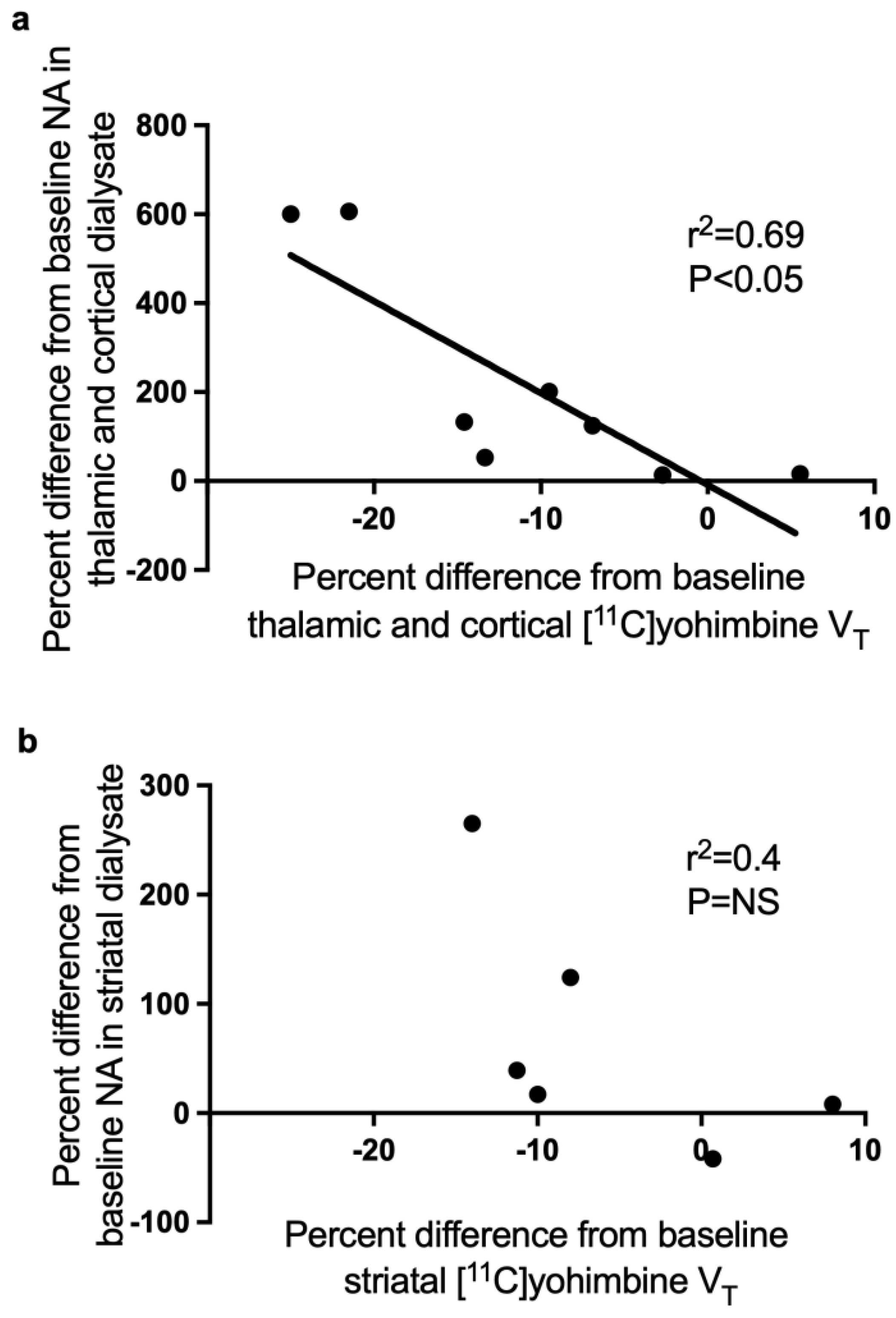
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Itoi, K.; Sugimoto, N. The Brainstem Noradrenergic Systems in Stress, Anxiety and Depression. J. Neuroendocr. 2010, 22, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Rommelfanger, K.; Weinshenker, D. Norepinephrine: The redheaded stepchild of Parkinson’s disease. Biochem. Pharmacol. 2007, 74, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Cumming, P.; Wong, D.F.; Dannals, R.F.; Gillings, N.; Hilton, J.; Scheffel, U.; Gjedde, A. The Competition Between Endogenous Dopamine and Radioligands for Specific Binding to Dopamine Receptors. Ann. N. Y. Acad. Sci. 2002, 965, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Ginovart, N. Imaging the Dopamine System with In Vivo [11C]raclopride Displacement Studies: Understanding the True Mechanism. Mol. Imaging Biol. 2005, 7, 45–52. [Google Scholar] [CrossRef]
- Laruelle, M.; Abi-Dargham, A.; van Dyck, C.H.; Gil, R.; D’Souza, C.D.; Erdos, J.; McCance, E.; Rosenblatt, W.; Fingado, C.; Zoghbi, S.S.; et al. Single photon emission computerized tomography imaging of amphetamine-induced dopamine release in drug-free schizophrenic subjects. Proc. Natl. Acad. Sci. USA 1996, 93, 9235–9240. [Google Scholar] [CrossRef]
- Koepp, M.J.; Gunn, R.N.; Lawrence, A.D.; Cunningham, V.J.; Dagher, A.; Jones, T.; Brooks, D.J.; Bench, C.J.; Grasby, P.M. Evidence for striatal dopamine release during a video game. Nature 1998, 393, 266–268. [Google Scholar] [CrossRef]
- Piccini, P.; Brooks, D.; Björklund, A.; Gunn, R.; Grasby, P.M.; Rimoldi, O.; Brundin, P.; Hagell, P.; Rehncrona, S.; Widner, H.; et al. Dopamine release from nigral transplants visualized in vivo in a Parkinson’s patient. Nat. Neurosci. 1999, 2, 1137–1140. [Google Scholar] [CrossRef]
- Linnet, J.; Møller, A.; Peterson, E.; Gjedde, A.; Doudet, D. Dopamine release in ventral striatum during Iowa Gambling Task performance is associated with increased excitement levels in pathological gambling. Addiction 2011, 106, 383–390. [Google Scholar] [CrossRef]
- Abi-Dargham, A.; Kegeles, L.S.; Zea-Ponce, Y.; Mawlawi, O.; Martinez, D.; Mitropoulou, V.; O’Flynn, K.; Koenigsberg, H.W.; Van Heertum, R.; Cooper, T.; et al. Striatal amphetamine-induced dopamine release in patients with schizotypal personality disorder studied with single photon emission computed tomography and [123I]iodobenzamide. Biol. Psychiatry 2004, 55, 1001–1006. [Google Scholar] [CrossRef]
- Jakobsen, S.; Pedersen, K.; Smith, D.F.; Jensen, S.B.; Munk, O.L.; Cumming, P. Detection of alpha2-adrenergic receptors in brain of living pig with 11C-yohimbine. J. Nucl. Med. 2006, 47, 2008–2015. [Google Scholar]
- Smith, D.F.; Dyve, S.; Minuzzi, L.; Jakobsen, S.; Munk, O.L.; Marthi, K.; Cumming, P. Inhibition of [11C]mirtazapine binding by α2-adrenoceptor antagonists studied by positron emission tomography in living porcine brain. Synapse 2006, 59, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Strazielle, C.; Lalonde, R.; Hebert, C.; Reader, T.A. Regional brain distribution of noradrenaline uptake sites, and of alpha1-alpha2- and beta-adrenergic receptors in pcd mutant mice: A quantitative autoradiographic study. Neuroscience 1999, 94, 287–304. [Google Scholar] [CrossRef] [PubMed]
- Young, W.S., 3rd; Kuhar, M.J. Noradrenergic alpha 1 and alpha 2 receptors: Light microscopic autoradiographic localization. Proc. Natl. Acad. Sci. USA 1980, 77, 1696–1700. [Google Scholar] [CrossRef]
- Schwarz, L.A.; Luo, L. Organization of the Locus Coeruleus-Norepinephrine System. Curr. Biol. 2015, 25, R1051–R1056. [Google Scholar] [CrossRef] [PubMed]
- Landau, A.M.; Dyve, S.; Jakobsen, S.; Alstrup, A.K.; Gjedde, A.; Doudet, D.J. Acute Vagal Nerve Stimulation Lowers α2 Adrenoceptor Availability: Possible Mechanism of Therapeutic Action. Brain Stimul. 2015, 8, 702–707. [Google Scholar] [CrossRef]
- Loughlin, S.E.; Foote, S.L.; Fallon, J.H. Locus coeruleus projections to cortex: Topography, morphology and collateralization. Brain Res. Bull. 1982, 9, 287–294. [Google Scholar] [CrossRef]
- Dorr, A.E.; Debonnel, G. Effect of Vagus Nerve Stimulation on Serotonergic and Noradrenergic Transmission. J. Pharmacol. Exp. Ther. 2006, 318, 890–898. [Google Scholar] [CrossRef]
- Groves, D.A.; Bowman, E.; Brown, V. Recordings from the rat locus coeruleus during acute vagal nerve stimulation in the anaesthetised rat. Neurosci. Lett. 2005, 379, 174–179. [Google Scholar] [CrossRef]
- Manta, S.; Dong, J.; Debonnel, G.; Blier, P. Enhancement of the function of rat serotonin and norepinephrine neurons by sustained vagus nerve stimulation. J. Psychiatry Neurosci. 2009, 34, 272–280. [Google Scholar]
- Collins, L.; Boddington, L.; Steffan, P.J.; McCormick, D. Vagus nerve stimulation induces widespread cortical and behavioral activation. Curr. Biol. 2021, 31, 2088–2098.e3. [Google Scholar] [CrossRef]
- Roosevelt, R.W.; Smith, D.C.; Clough, R.W.; Jensen, R.A.; Browning, R.A. Increased extracellular concentrations of norepinephrine in cortex and hippocampus following vagus nerve stimulation in the rat. Brain Res. 2006, 1119, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Phan, J.-A.; Landau, A.M.; Wong, D.F.; Jakobsen, S.; Nahimi, A.; Doudet, D.J.; Gjedde, A. Quantification of [11C]yohimbine Binding to α2 Adrenoceptors in Rat Brain in vivo. J. Cereb. Blood Flow Metab. 2015, 35, 501–511. [Google Scholar] [CrossRef]
- Landau, A.M.; Doudet, R.J.; Jakobsen, S. Amphetamine challenge decreases yohimbine binding to α2 adrenoceptors in Landrace pig brain. Psychopharmacology 2012, 222, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Watanabeab, H.; Andersen, F.L.; Simonsen, C.Z.; Evans, S.M.; Gjedde, A.; Cumming, P. MR-Based Statistical Atlas of the Göttingen Minipig Brain. Neuroimage 2001, 14, 1089–1096. [Google Scholar] [CrossRef] [PubMed]
- Bollen, P.J.A.; Hansen, A.K.; Alstrup, A.K.O. The Laboratory Swine, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Bjarkam, C.; Cancian, G.; Larsen, M.; Rosendahl, F.; Ettrup, K.; Zeidler, D.; Blankholm, A.; Østergaard, L.; Sunde, N.; Sørensen, J. A MRI-compatible stereotaxic localizer box enables high-precision stereotaxic procedures in pigs. J. Neurosci. Methods 2004, 139, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Glud, A.N.; Bech, J.; Tvilling, L.; Zaer, H.; Orlowski, D.; Fitting, L.M.; Ziedler, D.; Geneser, M.; Sangill, R.; Alstrup, A.K.O.; et al. A fiducial skull marker for precise MRI-based stereotaxic surgery in large animal models. J. Neurosci. Methods 2017, 285, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, C.; Brennum, L.T.; Stórustovu, S.; Kreilgård, M.; Mørk, A. Depression and poor sleep: The effect of monoaminergic antidepressants in a pre-clinical model in rats. Pharmacol. Biochem. Behav. 2007, 86, 468–476. [Google Scholar] [CrossRef]
- Wegener, G.; Linnet, K.; Rosenberg, R.; Mørk, A. The effect of acute citalopram on extracellular 5-HT levels is not augmented by lithium: An in vivo microdialysis study. Brain Res. 2000, 871, 338–342. [Google Scholar] [CrossRef]
- Cornil, C.A.; Ball, G.F. Interplay among catecholamine systems: Dopamine binds to α2-adrenergic receptors in birds and mammals. J. Comp. Neurol. 2008, 511, 610–627. [Google Scholar] [CrossRef]
- Zhang, W.; Klimek, V.; Farley, J.T.; Zhu, M.Y.; A Ordway, G. alpha2C adrenoceptors inhibit adenylyl cyclase in mouse striatum: Potential activation by dopamine. J. Pharmacol. Exp. Ther. 1999, 289, 1286–1292. [Google Scholar]
- Félix, B.; Léger, M.-E.; Albe-Fessard, D.; Marcilloux, J.-C.; Rampin, O.; Laplace, J.-P.; Duclos, A.; Fort, F.; Gougis, S.; Costa, M.; et al. Stereotaxic atlas of the pig brain. Brain Res. Bull. 1999, 49, 1–137. [Google Scholar] [CrossRef] [PubMed]
- Saikali, S.; Meurice, P.; Sauleau, P.; Eliat, P.-A.; Bellaud, P.; Randuineau, G.; Vérin, M.; Malbert, C.-H. A three-dimensional digital segmented and deformable brain atlas of the domestic pig. J. Neurosci. Methods 2010, 192, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Bjarkam, C.R.; Glud, A.N.; Orlowski, D.; Sørensen, J.C.H.; Palomero-Gallagher, N. The telencephalon of the Göttingen minipig, cytoarchitecture and cortical surface anatomy. Brain Struct. Funct. 2017, 222, 2093–2114. [Google Scholar] [CrossRef]
- Zhang, S.; Zhen, J.; Reith, M.E.; Dutta, A.K. Structural requirements for 2,4- and 3,6-disubstituted pyran biomimetics of cis-(6-benzhydryl-piperidin-3-yl)-benzylamine compounds to interact with monoamine transporters. Bioorg. Med. Chem. 2004, 12, 6301–6315. [Google Scholar] [CrossRef]
- Gallezot, J.-D.; Weinzimmer, D.; Nabulsi, N.; Lin, S.-F.; Fowles, K.; Sandiego, C.; McCarthy, T.J.; Maguire, R.P.; Carson, R.E.; Ding, Y.-S. Evaluation of [11C]MRB for assessment of occupancy of norepinephrine transporters: Studies with atomoxetine in non-human primates. Neuroimage 2011, 56, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, R.; Okumura, M.; Ito, H.; Seki, C.; Takahashi, H.; Takano, H.; Nakao, R.; Suzuki, K.; Okubo, Y.; Halldin, C.; et al. Quantitative Analysis of Norepinephrine Transporter in the Human Brain Using PET with (S,S)-18F-FMeNER-D2. J. Nucl. Med. 2008, 49, 1270–1276. [Google Scholar] [CrossRef] [PubMed]
- Lehto, J.; Johansson, J.; Vuorilehto, L.; Luoto, P.; Arponen, E.; Scheinin, H.; Rouru, J.; Scheinin, M. Sensitivity of [11C]ORM-13070 to increased extracellular noradrenaline in the CNS—A PET study in human subjects. Psychopharmacology 2015, 232, 4169–4178. [Google Scholar] [CrossRef] [PubMed]
- Kadhim, A.S.A.; Sossi, V.; Doudet, D.J. (University of British Columbia, Vancouver, BC, Canada); Jakobsen, S. (Aarhus University, Aarhus, Denmark). Displacement Studies of Yohimbine Binding in Non-Human Primates. Personal communication, 2016. [Google Scholar]
- Laurencin, C.; Lancelot, S.; Gobert, F.; Redouté, J.; Mérida, I.; Iecker, T.; Liger, F.; Irace, Z.; Greusard, E.; Lamberet, L.; et al. Modeling [11C]yohimbine PET human brain kinetics with test-retest reliability, competition sensitivity studies and search for a suitable reference region. Neuroimage 2021, 240, 118328. [Google Scholar] [CrossRef]
- Logan, J.; Ding, Y.-S.; Lin, K.-S.; Pareto, D.; Fowler, J.; Biegon, A. Modeling and analysis of PET studies with norepinephrine transporter ligands: The search for a reference region. Nucl. Med. Biol. 2005, 32, 531–542. [Google Scholar] [CrossRef]
- Pérez-Santos, I.; Palomero-Gallagher, N.; Zilles, K.; Cavada, C. Distribution of the Noradrenaline Innervation and Adrenoceptors in the Macaque Monkey Thalamus. Cereb. Cortex 2021, 31, 4115–4139. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Landau, A.M.; Jakobsen, S.; Thomsen, M.B.; Alstrup, A.K.O.; Orlowski, D.; Jacobsen, J.; Wegener, G.; Mørk, A.; Sørensen, J.C.H.; Doudet, D.J. Combined In Vivo Microdialysis and PET Studies to Validate [11C]Yohimbine Binding as a Marker of Noradrenaline Release. Biomolecules 2023, 13, 674. https://doi.org/10.3390/biom13040674
Landau AM, Jakobsen S, Thomsen MB, Alstrup AKO, Orlowski D, Jacobsen J, Wegener G, Mørk A, Sørensen JCH, Doudet DJ. Combined In Vivo Microdialysis and PET Studies to Validate [11C]Yohimbine Binding as a Marker of Noradrenaline Release. Biomolecules. 2023; 13(4):674. https://doi.org/10.3390/biom13040674
Chicago/Turabian StyleLandau, Anne Marlene, Steen Jakobsen, Majken Borup Thomsen, Aage Kristian Olsen Alstrup, Dariusz Orlowski, Jan Jacobsen, Gregers Wegener, Arne Mørk, Jens Christian Hedemann Sørensen, and Doris J. Doudet. 2023. "Combined In Vivo Microdialysis and PET Studies to Validate [11C]Yohimbine Binding as a Marker of Noradrenaline Release" Biomolecules 13, no. 4: 674. https://doi.org/10.3390/biom13040674
APA StyleLandau, A. M., Jakobsen, S., Thomsen, M. B., Alstrup, A. K. O., Orlowski, D., Jacobsen, J., Wegener, G., Mørk, A., Sørensen, J. C. H., & Doudet, D. J. (2023). Combined In Vivo Microdialysis and PET Studies to Validate [11C]Yohimbine Binding as a Marker of Noradrenaline Release. Biomolecules, 13(4), 674. https://doi.org/10.3390/biom13040674






