Matrix Metalloproteinases Contribute to the Calcification Phenotype in Pseudoxanthoma Elasticum
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Cell Culture
2.2. Murine Cell Culture
2.3. Nucleic Acid Isolation
2.4. Gene Expression Analysis
2.5. Enzyme-Linked Immunosorbent Assay
2.6. hMMP12 Immunofluorescent Staining and Fluorescence Microscopy
2.7. Alizarin Red Staining
2.8. ENPP1 Activity Assay
2.9. Statistical Analysis
3. Results
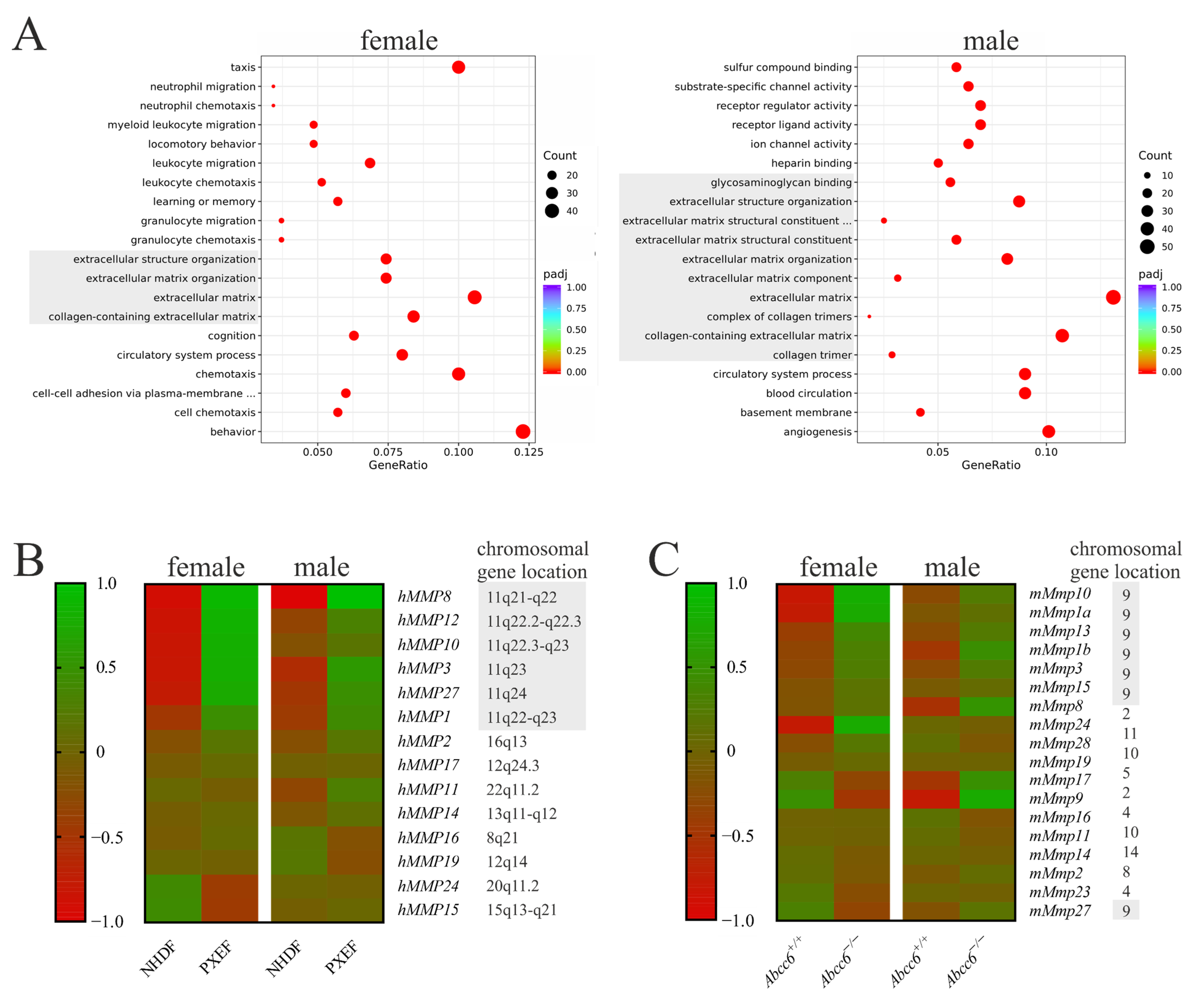
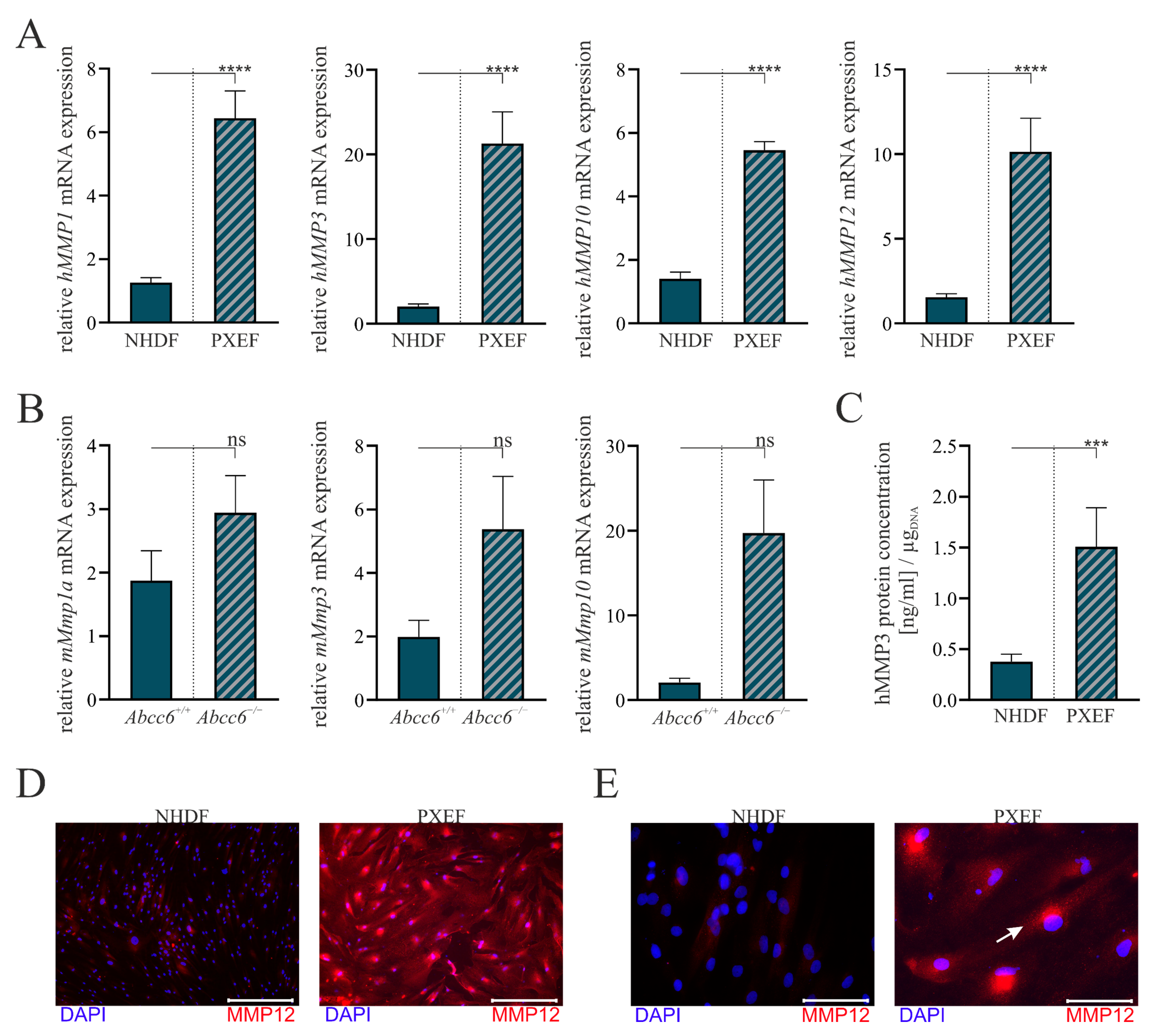
3.1. Upregulated Matrix Metalloproteinases Expression upon ABCC6-Deficiency
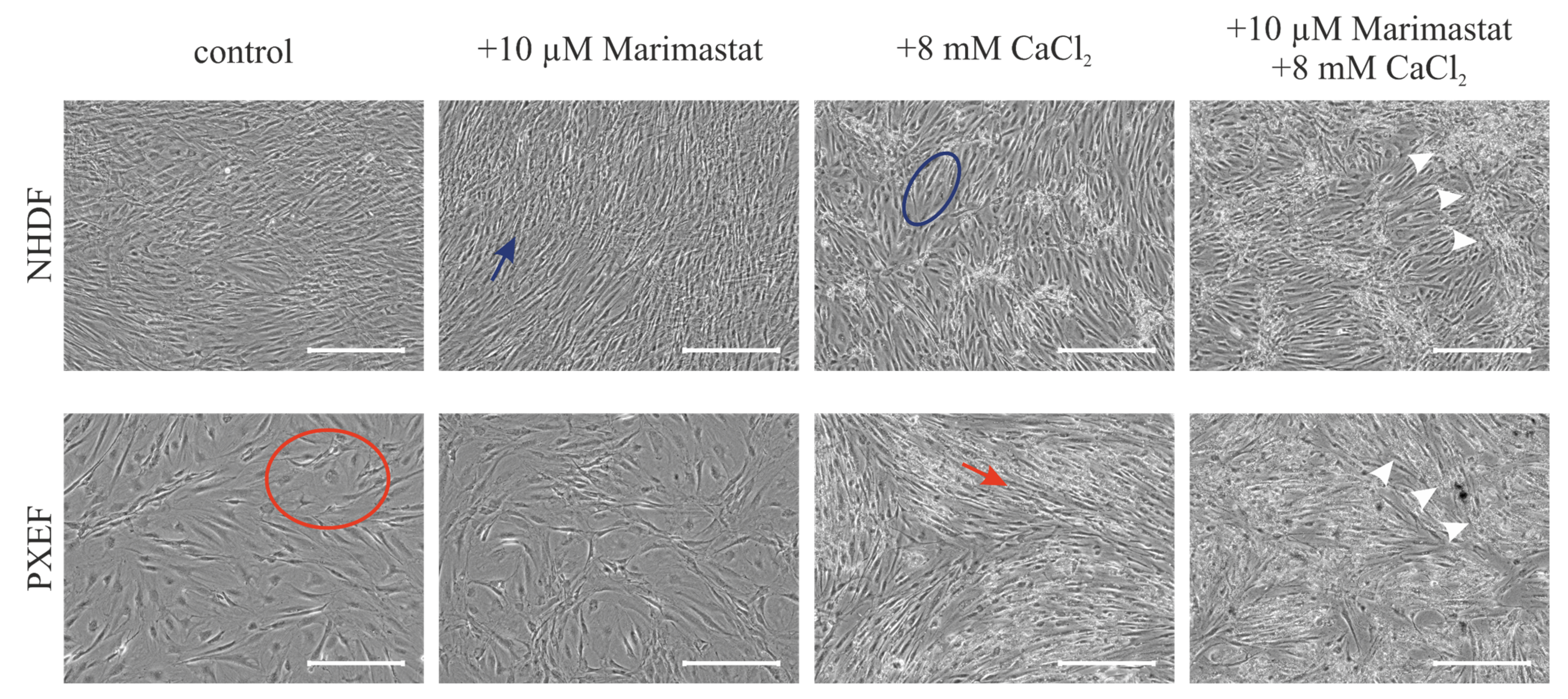
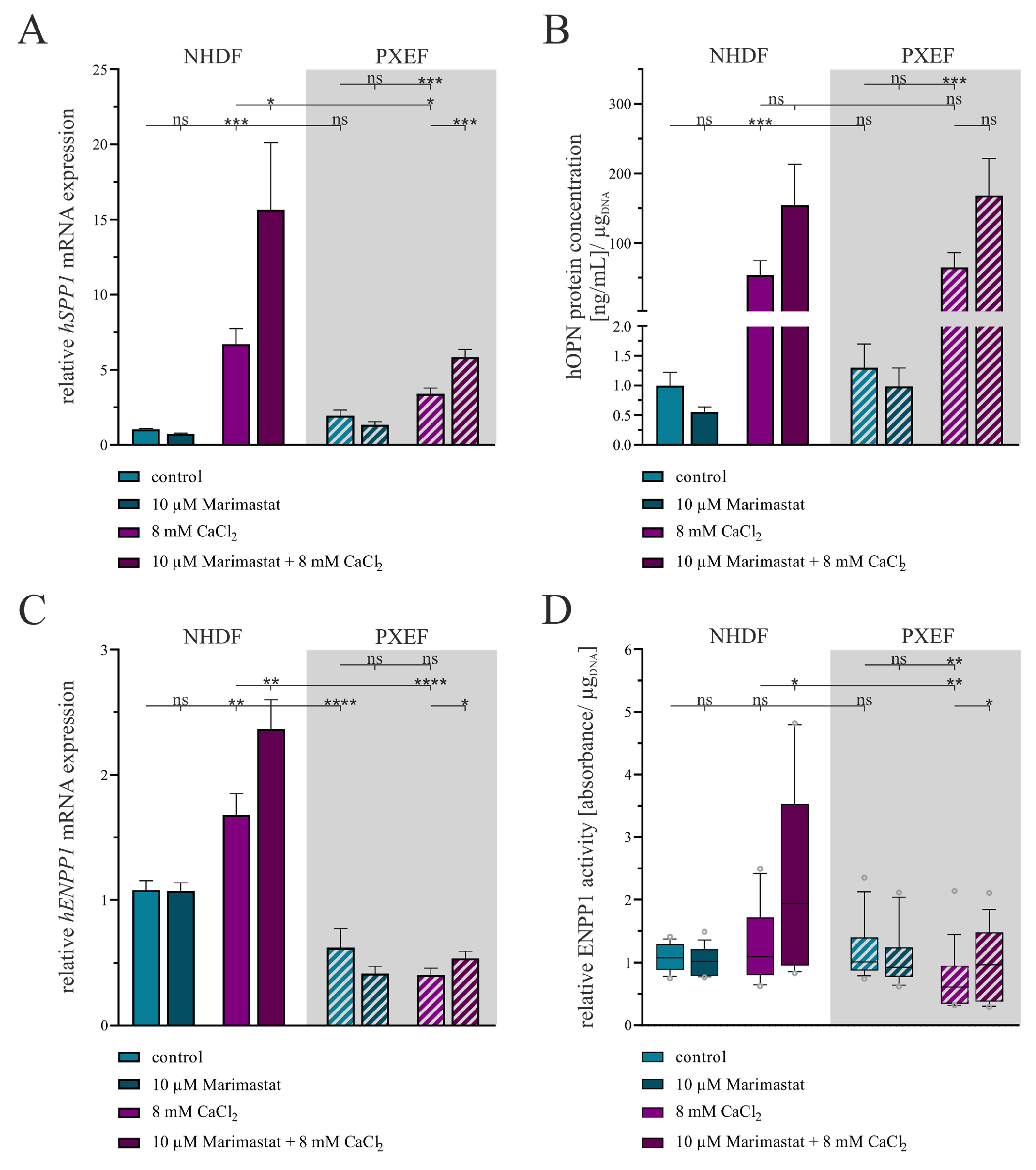
3.2. Correlation of the Calcification Phenotype and Matrix Metalloproteinases
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neldner, K.H. Pseudoxanthoma elasticum. Int. J. Dermatol. 1988, 27, 98–100. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Plomp, A.S.; van Soest, S.; Wijnholds, J.; de Jong, P.T.V.M.; Bergen, A.A.B. Pseudoxanthoma elasticum: A clinical, histopathological, and molecular update. Surv. Ophthalmol. 2003, 48, 424–438. [Google Scholar] [CrossRef] [PubMed]
- Jensen, O.A. Bruch’s membrane in pseudoxanthoma elasticum. Albrecht von Graefes Arch. Klin. Ophthalmol. 1977, 203, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Connor, P.J.; Juergens, J.L.; Perry, H.O.; Hollenhorst, R.W.; Edwards, J.E. Pseudoxanthoma elasticum and angioid streaks: A review of 106 cases. Am. J. Med. 1961, 30, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Laube, S.; Moss, C. Pseudoxanthoma elasticum. Arch. Dis. Child. 2005, 90, 754–756. [Google Scholar] [CrossRef]
- Campens, L.; Vanakker, O.M.; Trachet, B.; Segers, P.; Leroy, B.P.; De Zaeytijd, J.; Voet, D.; De Paepe, A.; De Backer, T.; De Backer, J. Characterization of cardiovascular involvement in pseudoxanthoma elasticum families. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 2646–2652. [Google Scholar] [CrossRef]
- Terkeltaub, R.A. Inorganic pyrophosphate generation and disposition in pathophysiology. Am. J. Physiol. Cell. Physiol. 2001, 281, C1–C11. [Google Scholar] [CrossRef]
- Fleisch, H.; Bisaz, S. Mechanism of calcification: Inhibitory role of pyrophosphate. Nature 1962, 195, 911. [Google Scholar] [CrossRef]
- Hessle, L.; Johnson, K.A.; Anderson, H.C.; Narisawa, S.; Sali, A.; Goding, J.W.; Terkeltaub, R.; Millán, J.L. Tissue-nonspecific alkaline phosphatase and plasma cell membrane glycoprotein-1 are central antagonistic regulators of bone mineralization. Proc. Natl. Acad. Sci. USA 2002, 99, 9445–9449. [Google Scholar] [CrossRef]
- Jansen, R.S.; Küçükosmanoglu, A.; de Haas, M.; Sapthu, S.; Otero, J.A.; Hegman, I.E.M.; Bergen, A.A.B.; Gorgels, T.G.M.F.; Borst, P.; van de Wetering, K. ABCC6 prevents ectopic mineralization seen in pseudoxanthoma elasticum by inducing cellular nucleotide release. Proc. Natl. Acad. Sci. USA 2013, 110, 20206–20211. [Google Scholar] [CrossRef]
- Sánchez-Tévar, A.M.; García-Fernández, M.; Murcia-Casas, B.; Rioja-Villodres, J.; Carrillo, J.L.; Camacho, M.; Van Gils, M.; Sánchez-Chaparro, M.A.; Vanakker, O.; Valdivielso, P. Plasma inorganic pyrophosphate and alkaline phosphatase in patients with pseudoxanthoma elasticum. Ann. Transl. Med. 2019, 7, 798. [Google Scholar] [CrossRef]
- Pomozi, V.; Julian, C.B.; Zoll, J.; Pham, K.; Kuo, S.; Tőkési, N.; Martin, L.; Váradi, A.; Le Saux, O. Dietary pyrophosphate modulates calcification in a mouse model of pseudoxanthoma elasticum: Implication for treatment of patients. J. Investig. Dermatol. 2019, 139, 1082–1088. [Google Scholar] [CrossRef] [PubMed]
- Boraldi, F.; Annovi, G.; Bartolomeo, A.; Quaglino, D. Fibroblasts from patients affected by pseudoxanthoma elasticum exhibit an altered PPi metabolism and are more r to pro-calcifying stimuli. J. Dermatol. Sci. 2014, 74, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Dabisch-Ruthe, M.; Kuzaj, P.; Götting, C.; Knabbe, C.; Hendig, D. Pyrophosphates as a major inhibitor of matrix calcification in pseudoxanthoma elasticum. J. Dermatol. Sci. 2014, 75, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Sundberg, J.P.; Levine, M.A.; Terry, S.F.; Uitto, J. The effects of bisphosphonates on ectopic soft tissue mineralization caused by mutations in the ABCC6 gene. Cell Cycle 2015, 14, 1082–1089. [Google Scholar] [CrossRef]
- Shimada, B.K.; Pomozi, V.; Zoll, J.; Kuo, S.; Martin, L.; Le Saux, O. ABCC6, Pyrophosphate and ectopic calcification: Therapeutic Solutions. Int. J. Mol. Sci. 2021, 22, 4555. [Google Scholar] [CrossRef] [PubMed]
- Addison, W.N.; Azari, F.; Sørensen, E.S.; Kaartinen, M.T.; McKee, M.D. Pyrophosphate inhibits mineralization of osteoblast cultures by binding to mineral, up-regulating osteopontin, and inhibiting alkaline phosphatase activity. J. Biol. Chem. 2007, 282, 15872–15883. [Google Scholar] [CrossRef]
- Harmey, D.; Hessle, L.; Narisawa, S.; Johnson, K.A.; Terkeltaub, R.; Millán, J.L. Concerted regulation of inorganic pyrophosphate and osteopontin by Akp2, Enpp1, and Ank: An integrated model of the pathogenesis of mineralization disorders. Am. J. Pathol. 2004, 164, 1199–1209. [Google Scholar] [CrossRef]
- Denhardt, D.T.; Guo, X. Osteopontin: A protein with diverse functions. FASEB J. 1993, 7, 1475–1482. [Google Scholar] [CrossRef]
- Butler, W.T. The nature and significance of osteopontin. Connect. Tissue Res. 1989, 23, 123–136. [Google Scholar] [CrossRef]
- Walker, E.R.; Frederickson, R.G.; Mayes, M.D. The mineralization of elastic fibers and alterations of extracellular matrix in pseudoxanthoma elasticum: Ultrastructure, immunocytochemistry, and X-ray analysis. Arch. Dermatol. 1989, 125, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Contri, M.B.; Boraldi, F.; Taparelli, F.; De Paepe, A.; Ronchetti, I.P. Matrix proteins with high affinity for calcium ions are associated with mineralization within the elastic fibers of pseudoxanthoma elasticum dermis. Am. J. Pathol. 1996, 148, 569–577. [Google Scholar] [PubMed]
- Otkjær-Nielsen, A.; Johnson, E.; Hentzer, B.; Danielsen, L.; Carlsen, F. Apatite crystals in pseudoxanthoma elasticum: A combined study using electron microscopy and selected area diffraction analysis. J. Investig. Dermatol. 1977, 69, 376–378. [Google Scholar] [CrossRef] [PubMed]
- Tiozzo Costa, R.; Contri, M.B.; Cingi, M.R.; Pasquali Ronchetti, I.; Salvini, R.; Rindi, S.; De Luca, G. Pseudoxanthoma elasticum (PXE): Ultrastructural and biochemical study on proteoglycan and proteoglycan-associated material produced by skin fibroblasts in vitro. Coll. Relat. Res. 1988, 8, 49–64. [Google Scholar] [CrossRef]
- Gheduzzi, D.; Sammarco, R.; Quaglino, D.; Bercovitch, L.; Terry, S.; Taylor, W.; Ronchetti, I.P. Extracutaneous ultrastructural alterations in pseudoxanthoma elasticum. Ultrastruct. Pathol. 2003, 27, 375–384. [Google Scholar] [CrossRef]
- Jackson, B.C.; Nebert, D.W.; Vasiliou, V. Update of human and mouse matrix metalloproteinase families. Hum. Genom. 2010, 4, 194. [Google Scholar] [CrossRef]
- Nagase, H.; Visse, R.; Murphy, G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc. Res. 2006, 69, 562–573. [Google Scholar] [CrossRef]
- Diekmann, U.; Zarbock, R.; Hendig, D.; Szliska, C.; Kleesiek, K.; Götting, C. Elevated circulating levels of matrix metalloproteinases MMP-2 and MMP-9 in pseudoxanthoma elasticum patients. J. Mol. Med. 2009, 87, 965–970. [Google Scholar] [CrossRef]
- Quaglino, D.; Sartor, L.; Garbisa, S.; Boraldi, F.; Croce, A.; Passi, A.; De Luca, G.; Tiozzo, R.; Pasquali-Ronchetti, I. Dermal fibroblasts from pseudoxanthoma elasticum patients have raised MMP-2 degradative potential. Biochim. Biophys. Acta 2005, 1741, 42–47. [Google Scholar] [CrossRef]
- Ringpfeil, F.; McGuigan, K.; Fuchsel, L.; Kozic, H.; Larralde, M.; Lebwohl, M.; Uitto, J. Pseudoxanthoma elasticum is a recessive disease characterized by compound heterozygosity. J. Investig. Dermatol. 2006, 126, 782–786. [Google Scholar] [CrossRef]
- Bergen, A.A.B.; Plomp, A.S.; Schuurman, E.J.; Terry, S.; Breuning, M.; Dauwerse, H.; Swart, J.; Kool, M.; van Soest, S.; Baas, F.; et al. Mutations in ABCC6 cause pseudoxanthoma elasticum. Nat. Genet. 2000, 25, 228–231. [Google Scholar] [CrossRef] [PubMed]
- Pseudoxanthoma Elasticum and ABCC6. ClinVar. NCBI. Available online: https://www.ncbi.nlm.nih.gov/clinvar/?term=pseudoxanthoma+elasticum+abcc6 (accessed on 9 December 2022).
- Beck, K.; Hayashi, K.; Dang, K.; Hayashi, M.; Boyd, C.D. Analysis of ABCC6 (MRP6) in normal human tissues. Histochem. Cell. Biol. 2005, 123, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Le Saux, O.; Bunda, S.; VanWart, C.M.; Douet, V.; Got, L.; Martin, L.; Hinek, A. Serum factors from pseudoxanthoma elasticum patients alter elastic fiber formation in vitro. J. Investig. Dermatol. 2006, 126, 1497–1505. [Google Scholar] [CrossRef]
- Kuzaj, P.; Kuhn, J.; Michalek, R.D.; Karoly, E.D.; Faust, I.; Dabisch-Ruthe, M.; Knabbe, C.; Hendig, D. Large-scaled metabolic profiling of human dermal fibroblasts derived from pseudoxanthoma elasticum patients and healthy controls. PLoS ONE 2014, 9, e108336. [Google Scholar] [CrossRef] [PubMed]
- Tiemann, J.; Wagner, T.; Lindenkamp, C.; Plümers, R.; Faust, I.; Knabbe, C.; Hendig, D. Linking ABCC6 deficiency in primary human dermal fibroblasts of PXE patients to P21-mediated premature cellular senescence and the development of a proinflammatory secretory phenotype. Int. J. Mol. Sci. 2020, 21, 9665. [Google Scholar] [CrossRef] [PubMed]
- Gorgels, T.G.M.F.; Hu, X.; Scheffer, G.L.; van der Wal, A.C.; Toonstra, J.; de Jong, P.T.V.M.; van Kuppevelt, T.H.; Levelt, C.N.; de Wolf, A.; Loves, W.J.P.; et al. Disruption of Abcc6 in the mouse: Novel insight in the pathogenesis of pseudoxanthoma elasticum. Hum. Mol. Genet. 2005, 14, 1763–1773. [Google Scholar] [CrossRef]
- Hendig, D.; Langmann, T.; Kocken, S.; Zarbock, R.; Szliska, C.; Schmitz, G.; Kleesiek, K.; Götting, C. Gene expression profiling of ABC transporters in dermal fibroblasts of pseudoxanthoma elasticum patients identifies new candidates involved in PXE pathogenesis. Lab. Investig. 2008, 88, 1303–1315. [Google Scholar] [CrossRef]
- Scott, K.A.; Wood, E.J.; Karran, E.H. A matrix metalloproteinase inhibitor which prevents fibroblast-mediated collagen lattice contraction. FEBS Lett. 1998, 441, 137–140. [Google Scholar] [CrossRef]
- Lugo-Cintrón, K.M.; Gong, M.M.; Ayuso, J.M.; Tomko, L.A.; Beebe, D.J.; Virumbrales-Muñoz, M.; Ponik, S.M. Breast fibroblasts and ECM components modulate breast cancer cell migration through the secretion of MMPs in a 3D microfluidic co-culture model. Cancers 2020, 12, 1173. [Google Scholar] [CrossRef]
- Neidhart, M.; Seemayer, C.A.; Hummel, K.M.; Michel, B.A.; Gay, R.E.; Gay, S. Functional characterization of adherent synovial fluid cells in rheumatoid arthritis: Destructive potential in vitro and in vivo. Arthritis Rheum. 2003, 48, 1873–1880. [Google Scholar] [CrossRef]
- Dabisch-Ruthe, M. Charakterisierung des Pyrophosphatmetabolismus bei pseudoxanthoma elasticum; Universität Bielefeld: Bielefeld, Germany, 2014. [Google Scholar]
- Takashima, A. Establishment of fibroblast cultures. Curr. Protoc. Cell. Biol. 1998, 2.1, 2.1.1–2.1.12. [Google Scholar] [CrossRef] [PubMed]
- Gregory, C.A.; Grady Gunn, W.; Peister, A.; Prockop, D.J. An alizarin red-based assay of mineralization by adherent cells in culture: Comparison with Cetylpyridinium chloride extraction. Anal. Biochem. 2004, 329, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Lau, W.M.; Doucet, M.; Stadel, R.; Huang, D.; Weber, K.L.; Kominsky, S.L. Enpp1: A potential facilitator of breast cancer bone metastasis. PLoS ONE 2013, 8, e66752. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Faghankhani, M.; Cao, Y.; Uitto, J.; Li, Q. Molecular genetics and modifier genes in pseudoxanthoma elasticum, a heritable multisystem ectopic mineralization disorder. J. Investig. Dermatol. 2021, 141, 1148–1156. [Google Scholar] [CrossRef]
- Pomozi, V.; Le Saux, O.; Brampton, C.; Apana, A.; Iliás, A.; Szeri, F.; Martin, L.; Monostory, K.; Paku, S.; Sarkadi, B.; et al. ABCC6 is a basolateral plasma membrane protein. Circ. Res. 2013, 112, e148–e151. [Google Scholar] [CrossRef]
- Klement, J.F.; Matsuzaki, Y.; Jiang, Q.-J.; Terlizzi, J.; Choi, H.Y.; Fujimoto, N.; Li, K.; Pulkkinen, L.; Birk, D.E.; Sundberg, J.P.; et al. Targeted ablation of the Abcc6 gene results in ectopic mineralization of connective tissues. Mol. Cell. Biol. 2005, 25, 8299–8310. [Google Scholar] [CrossRef]
- Li, Q.; van de Wetering, K.; Uitto, J. Pseudoxanthoma elasticum as a paradigm of heritable ectopic mineralization disorders: Pathomechanisms and treatment development. Am. J. Pathol. 2019, 189, 216–225. [Google Scholar] [CrossRef]
- Quaglino, D.; Boraldi, F.; Barbieri, D.; Croce, A.; Tiozzo, R.; Pasquali Ronchetti, I. Abnormal phenotype of in vitro dermal fibroblasts from patients with pseudoxanthoma elasticum (PXE). Biochim. Biophys. Acta Mol. Basis Dis. 2000, 1501, 51–62. [Google Scholar] [CrossRef]
- Brampton, C.; Pomozi, V.; Chen, L.-H.; Apana, A.; McCurdy, S.; Zoll, J.; Boisvert, W.A.; Lambert, G.; Henrion, D.; Blanchard, S.; et al. ABCC6 deficiency promotes dyslipidemia and atherosclerosis. Sci. Rep. 2021, 11, 3881. [Google Scholar] [CrossRef]
- Plümers, R.; Osterhage, M.R.; Lindenkamp, C.; Knabbe, C.; Hendig, D. Targeting ABCC6 in mesenchymal stem cells: Impairment of mature adipocyte lipid homeostasis. Int. J. Mol. Sci. 2022, 23, 9218. [Google Scholar] [CrossRef]
- Tiemann, J.; Wagner, T.; Vanakker, O.M.; van Gils, M.; Cabrera, J.-L.B.; Ibold, B.; Faust, I.; Knabbe, C.; Hendig, D. Cellular and molecular biomarkers indicate premature aging in pseudoxanthoma elasticum patients. Aging Dis. 2019, 11, 536–546. [Google Scholar] [CrossRef] [PubMed]
- Miglionico, R.; Ostuni, A.; Armentano, M.F.; Milella, L.; Crescenzi, E.; Carmosino, M.; Bisaccia, F. ABCC6 knockdown in HepG2 cells induces a senescent-like cell phenotype. Cell. Mol. Biol. Lett. 2017, 22, 7. [Google Scholar] [CrossRef]
- Zarbock, R.; Hendig, D.; Szliska, C.; Kleesiek, K.; Götting, C. Analysis of MMP2 promoter polymorphisms in patients with pseudoxanthoma elasticum. Clin. Chim. Acta 2010, 411, 1487–1490. [Google Scholar] [CrossRef] [PubMed]
- Pendás, A.M.; Santamaría, I.; Alvarez, M.V.; Pritchard, M.; López-Otín, C. Fine physical mapping of the human matrix metalloproteinase genes clustered on chromosome 11q22.3. Genomics 1996, 37, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Butler, G.S.; Overall, C.M. Updated Biological Roles for Matrix Metalloproteinases and New “Intracellular” Substrates Revealed by Degradomics. Biochemistry 2009, 48, 10830–10845. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Boyd, D.D. Regulation of matrix metalloproteinase gene expression. J. Cell. Physiol. 2007, 211, 19–26. [Google Scholar] [CrossRef]
- Angel, P.; Szabowski, A.; Schorpp-Kistner, M. Function and regulation of AP-1 subunits in skin physiology and pathology. Oncogene 2001, 20, 2413–2423. [Google Scholar] [CrossRef]
- Benbow, U.; Brinckerhoff, C.E. The AP-1 site and MMP gene regulation: What is all the fuss about? Matrix Biol. 1997, 15, 519–526. [Google Scholar] [CrossRef]
- Liacini, A.; Sylvester, J.; Li, W.Q.; Zafarullah, M. Inhibition of interleukin-1-stimulated MAP kinases, activating protein-1 (AP-1) and nuclear factor kappa B (NF-ΚB) transcription factors down-regulates matrix metalloproteinase gene expression in articular chondrocytes. Matrix Biol. 2002, 21, 251–262. [Google Scholar] [CrossRef]
- De Vilder, E.Y.G.; Martin, L.; Lefthériotis, G.; Coucke, P.; Van Nieuwerburgh, F.; Vanakker, O.M. Rare modifier variants alter the severity of cardiovascular disease in pseudoxanthoma elasticum: Identification of novel candidate modifier genes and disease pathways through mixture of effects analysis. Front. Cell. Dev. Biol. 2021, 9, 612581. [Google Scholar] [CrossRef]
- Kaden, J.J.; Dempfle, C.-E.; Grobholz, R.; Tran, H.-T.; Kılıç, R.; Sarıkoç, A.; Brueckmann, M.; Vahl, C.; Hagl, S.; Haase, K.K.; et al. Interleukin-1 beta promotes matrix metalloproteinase expression and cell proliferation in calcific aortic valve stenosis. Atherosclerosis 2003, 170, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Roach, H.I.; Yamada, N.; Cheung, K.S.C.; Tilley, S.; Clarke, N.M.P.; Oreffo, R.O.C.; Kokubun, S.; Bronner, F. Association between the abnormal expression of matrix-degrading enzymes by human osteoarthritic chondrocytes and demethylation of specific CpG sites in the promoter regions. Arthritis Rheum. 2005, 52, 3110–3124. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Corriere, M.A.; Matrisian, L.M.; Guzman, R.J. Matrix metalloproteinase inhibition attenuates aortic calcification. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1510–1516. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.X.; O’Neill, K.D.; Chen, X.; Kiattisunthorn, K.; Gattone, V.H.; Moe, S.M. Activation of arterial matrix metalloproteinases leads to vascular calcification in chronic kidney disease. Am. J. Nephrol. 2011, 34, 211–219. [Google Scholar] [CrossRef]
- Basalyga, D.M.; Simionescu, D.T.; Xiong, W.; Baxter, B.T.; Starcher, B.C.; Vyavahare, N.R. Elastin degradation and calcification in an abdominal aorta injury model. Circulation 2004, 110, 3480–3487. [Google Scholar] [CrossRef]
- Nollet, L.; Van Gils, M.; Willaert, A.; Coucke, P.J.; Vanakker, O.M. Minocycline attenuates excessive DNA damage response and reduces ectopic calcification in pseudoxanthoma elasticum. J. Investig. Dermatol. 2022, 142, 1629–1638.e6. [Google Scholar] [CrossRef]
- Sparano, J.A.; Bernardo, P.; Stephenson, P.; Gradishar, W.J.; Ingle, J.N.; Zucker, S.; Davidson, N.E. Randomized phase III trial of Marimastat versus placebo in patients with metastatic breast cancer who have responding or stable disease after first-line chemotherapy: Eastern Cooperative Oncology Group Trial E2196. J. Clin. Oncol. 2004, 22, 4683–4690. [Google Scholar] [CrossRef]
- Rasmussen, H.S.; McCann, P.P. Matrix metalloproteinase inhibition as a novel anticancer strategy: A review with special focus on Batimastat and Marimastat. Pharmacol. Ther. 1997, 75, 69–75. [Google Scholar] [CrossRef]
- Durham, A.L.; Speer, M.Y.; Scatena, M.; Giachelli, C.M.; Shanahan, C.M. Role of Smooth Muscle Cells in Vascular Calcification: Implications in Atherosclerosis and Arterial Stiffness. Cardiovascular Research 2018, 114, 590–600. [Google Scholar] [CrossRef]
- Johnson, J.L.; Dwivedi, A.; Somerville, M.; George, S.J.; Newby, A.C. Matrix Metalloproteinase (MMP)-3 Activates MMP-9 Mediated Vascular Smooth Muscle Cell Migration and Neointima Formation in Mice. Arterioscler. Thromb. Vasc. Biol. 2011, 31, e35–e44. [Google Scholar] [CrossRef]
- Hendig, D.; Arndt, M.; Szliska, C.; Kleesiek, K.; Götting, C. SPP1 Promoter polymorphisms: Identification of the first modifier gene for pseudoxanthoma elasticum. Clin. Chem. 2007, 53, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Ronchetti, I.; Boraldi, F.; Annovi, G.; Cianciulli, P.; Quaglino, D. Fibroblast involvement in soft connective tissue calcification. Front. Genet. 2013, 4, 22. [Google Scholar] [CrossRef] [PubMed]
- Sage, A.P.; Tintut, Y.; Demer, L.L. Regulatory mechanisms in vascular calcification. Nat. Rev. Cardiol. 2010, 7, 528–536. [Google Scholar] [CrossRef] [PubMed]


| Sample ID | Gender | Age [year] | Biopsy Source | ABCC6 Genotype | Genotype Status | |
|---|---|---|---|---|---|---|
| PXEF | ||||||
| P265F | female | 62 | neck | c.1132C>T (p.Q378X) | c.3421C>T (p.R1141X) | cht |
| P3M | male | 57 | neck | c.3421C>T (p.R1141X) | c.3883-6G>A (SSM) | cht |
| P128M | male | 51 | neck | c.3769_3770insC (p.L1259fsX1277) | c.3769_3770insC (p.L1259fsX1277) | hm |
| P255F | female | 48 | arm | c.3421C>T (p.R1141X) | c.2787+1G>T | cht |
| P308M | male | 42 | n. s. | c.3421C>T (p.R1141X) | c.-90ins14 | cht |
| NHDF | ||||||
| F63A (AG12786) | female | 63 | arm | - | - | wt |
| M57A (AG13145) | male | 57 | arm | - | - | wt |
| M52A (AG11482) | male | 52 | arm | - | - | wt |
| F48A (AG14284) | female | 48 | arm | - | - | wt |
| M42A (AG06307) | male | 42 | arm | - | - | wt |
| Gene | 5′-3′ Primer Sequences | TA (°C) | Efficiency | Product Size (bp) |
|---|---|---|---|---|
| hENPP1 | AATGCCCCTTTGGACATC CCCGTAACTCACTTTGGT | 59 | 1.72 | 151 |
| hGAPDH | AGGTCGGAGTCAACGGAT TCCTGGAAGATGGTGATG | 63 | 1.84 | 223 |
| hHMBS | CTGCCAGAGAAGAGTGTG AGCTGTTGCCAGGATGAT | 63 | 1.92 | 165 |
| hMMP1 | AGAAACACAAGAGCAAGATGTG TGGCGTGTAATTTTCAATCCTGT | 63 | 1.85 | 298 |
| hMMP3 | GCCATCTCTTCCTTCAGGCG CCTAGGGTGTGGATGCCTCT | 63 | 1.85 | 141 |
| hMMP10 | GCATTCAGTCTCTCTACGGACC AAAAACGGTGTCCCTGCTGT | 66 | 2.00 | 287 |
| hMMP12 | CACATTCAGGAGGCACAAAC ATTTCCCACGGTAGTGACAG | 59 | 1.82 | 275 |
| hRPL13 | CGGAAGGTGGTGGTCGTA CTCGGGAAGGGTTGGTGT | 63 | 1.87 | 115 |
| hSDHA | AACTCGCTCTTGGACCTG GAGTCGCAGTTCCGATGT | 63 | 1.93 | 177 |
| hSPP1 | TGATGACCATGTGGACAG ACCATTCAACTCCTCGCT | 59 | 1.70 | 322 |
| Gene | 5′-3′ Primer Sequences | TA (°C) | Efficiency | Product Size (bp) |
|---|---|---|---|---|
| mEif3a | GAGTATCAGGAGCGAGTCAAG CCTCTCATCATCCCGAGTTTC | 59 | 1.72 | 151 |
| mGapdh | GCATCTTGGGCTACACTGAGG GGGTGGTCCAGGGTTTCTTAC | 59 | 1.92 | 211 |
| mHprt | GTTGGATACAGGCCAGAC GCCACAGGACTAGAACAC | 59 | 1.93 | 226 |
| mMmp1a | GTTGGAGCAGGCAGGAAGGAGG ACCCCACACCTGGGCTTCTT | 68 | 2.00 | 328 |
| mMmp3 | CCCCTGATGTCCTCGTGGTA GTGCCCTCGTATAGCCCAGA | 66 | 2.00 | 150 |
| mMmp10 | GTACCTTCCCAGGTTCGCCA GGGTAAAAGTCTCCGTGTTCTCC | 66 | 2.00 | 217 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plümers, R.; Lindenkamp, C.; Osterhage, M.R.; Knabbe, C.; Hendig, D. Matrix Metalloproteinases Contribute to the Calcification Phenotype in Pseudoxanthoma Elasticum. Biomolecules 2023, 13, 672. https://doi.org/10.3390/biom13040672
Plümers R, Lindenkamp C, Osterhage MR, Knabbe C, Hendig D. Matrix Metalloproteinases Contribute to the Calcification Phenotype in Pseudoxanthoma Elasticum. Biomolecules. 2023; 13(4):672. https://doi.org/10.3390/biom13040672
Chicago/Turabian StylePlümers, Ricarda, Christopher Lindenkamp, Michel Robin Osterhage, Cornelius Knabbe, and Doris Hendig. 2023. "Matrix Metalloproteinases Contribute to the Calcification Phenotype in Pseudoxanthoma Elasticum" Biomolecules 13, no. 4: 672. https://doi.org/10.3390/biom13040672
APA StylePlümers, R., Lindenkamp, C., Osterhage, M. R., Knabbe, C., & Hendig, D. (2023). Matrix Metalloproteinases Contribute to the Calcification Phenotype in Pseudoxanthoma Elasticum. Biomolecules, 13(4), 672. https://doi.org/10.3390/biom13040672







