Antimicrobial and Osteogenic Effects of Collagen Membrane Decorated with Chitosan–Nano-Hydroxyapatite
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Material Characterization
2.3. Samples Sterilization
2.4. Isolation, Cultivation, and Characterization of Dental Pulp Stem Cells (DPSCs)
2.5. MTT Assay
2.6. Alkaline Phosphatase (ALP) Activity Assay
2.7. DPSCs Osteodifferentiation
2.8. Antibiofilm Effect
2.8.1. Bacteria Strains and Conditions of Growth
2.8.2. Biofilm Formation
2.8.3. Determination of CFUs of Biofilms Formed on Membranes
2.8.4. Determination of CFUs in Medium Surrounding Membranes
2.8.5. Scanning Electron Microscopy (SEM) for Monomicrobial S. mitis Biofilm Visualization
2.9. Statistical Analysis
3. Results
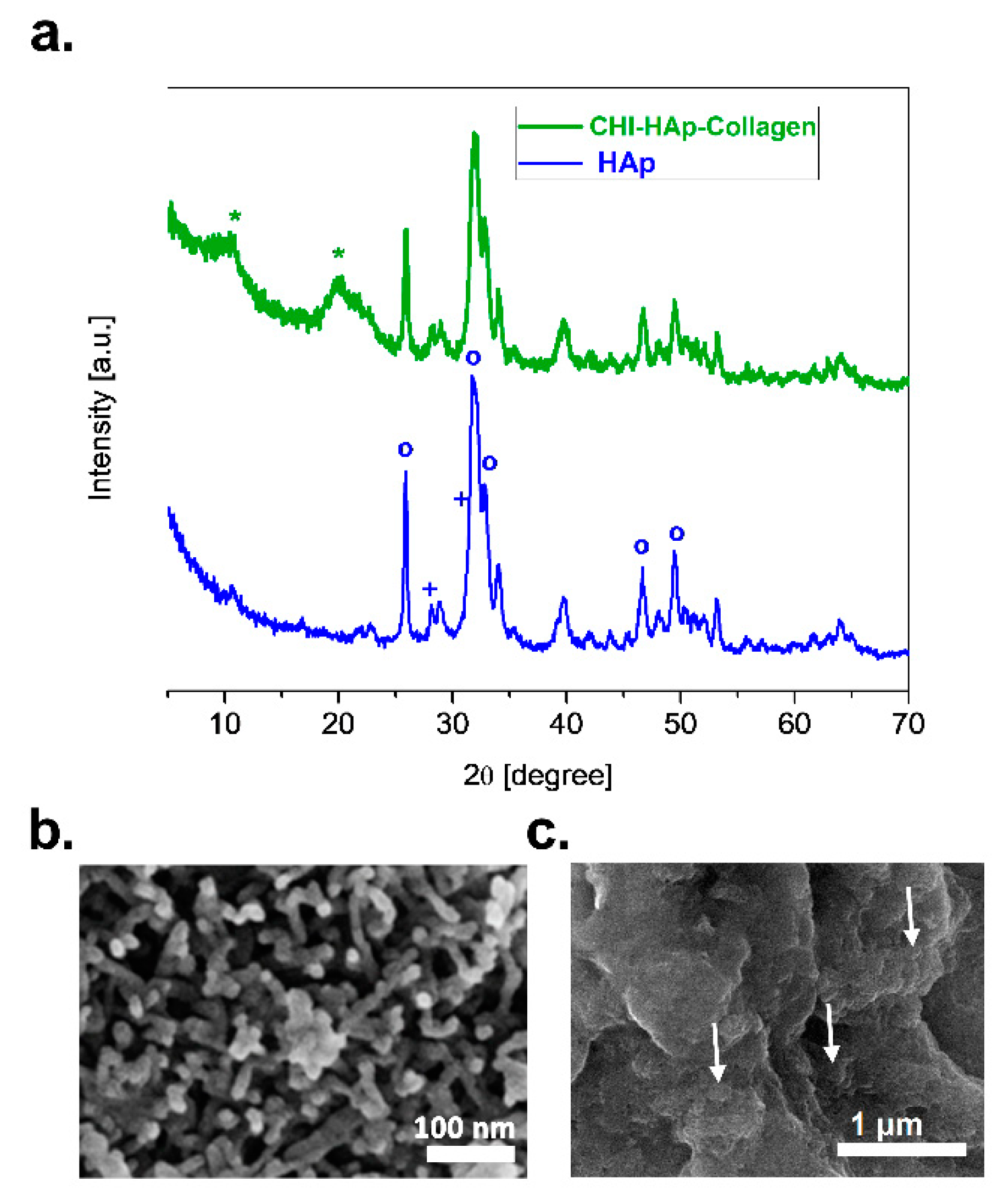
3.1. Characterization of HAp and CHI–HAp–Collagen Membrane
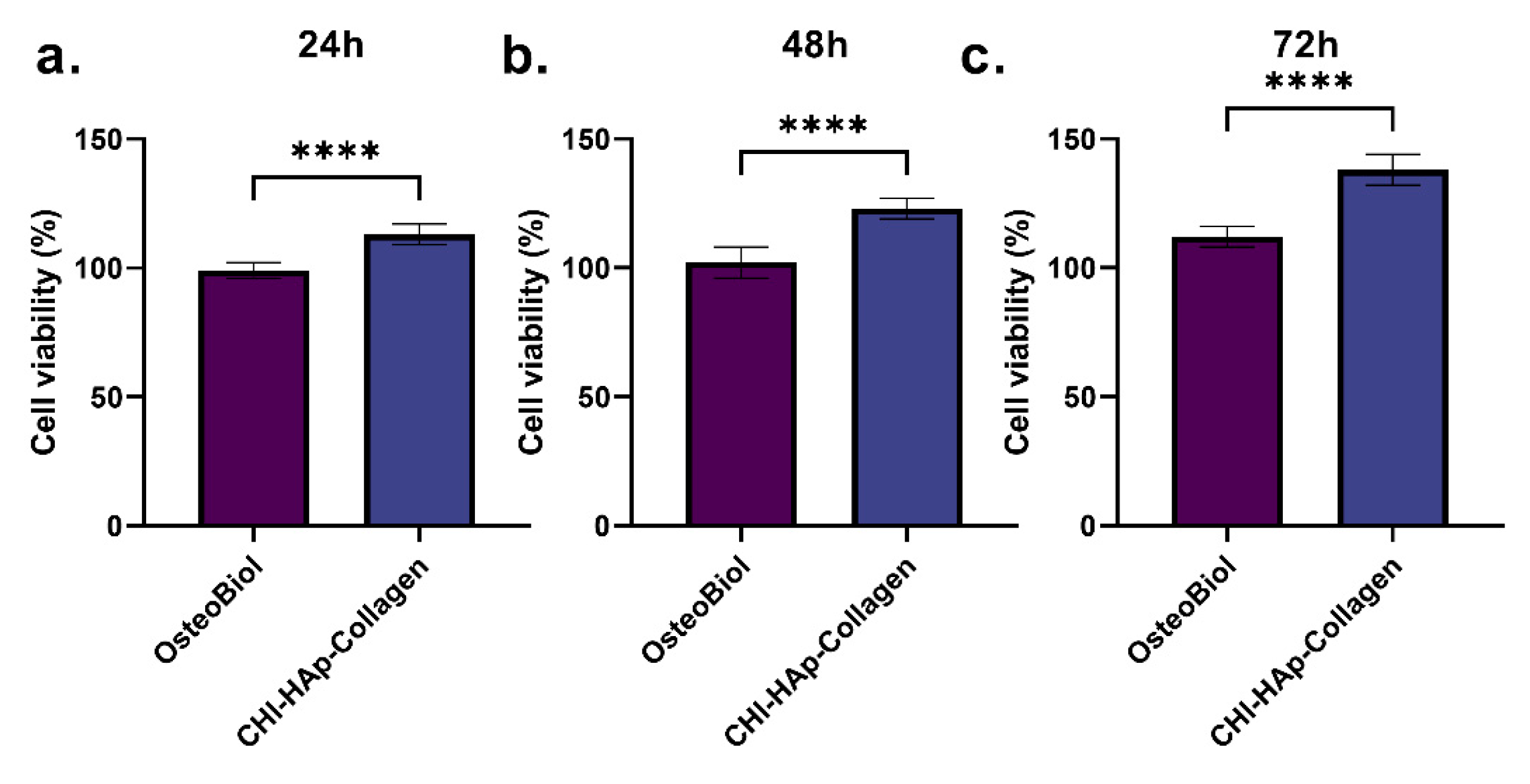
3.2. DPSCs Viability
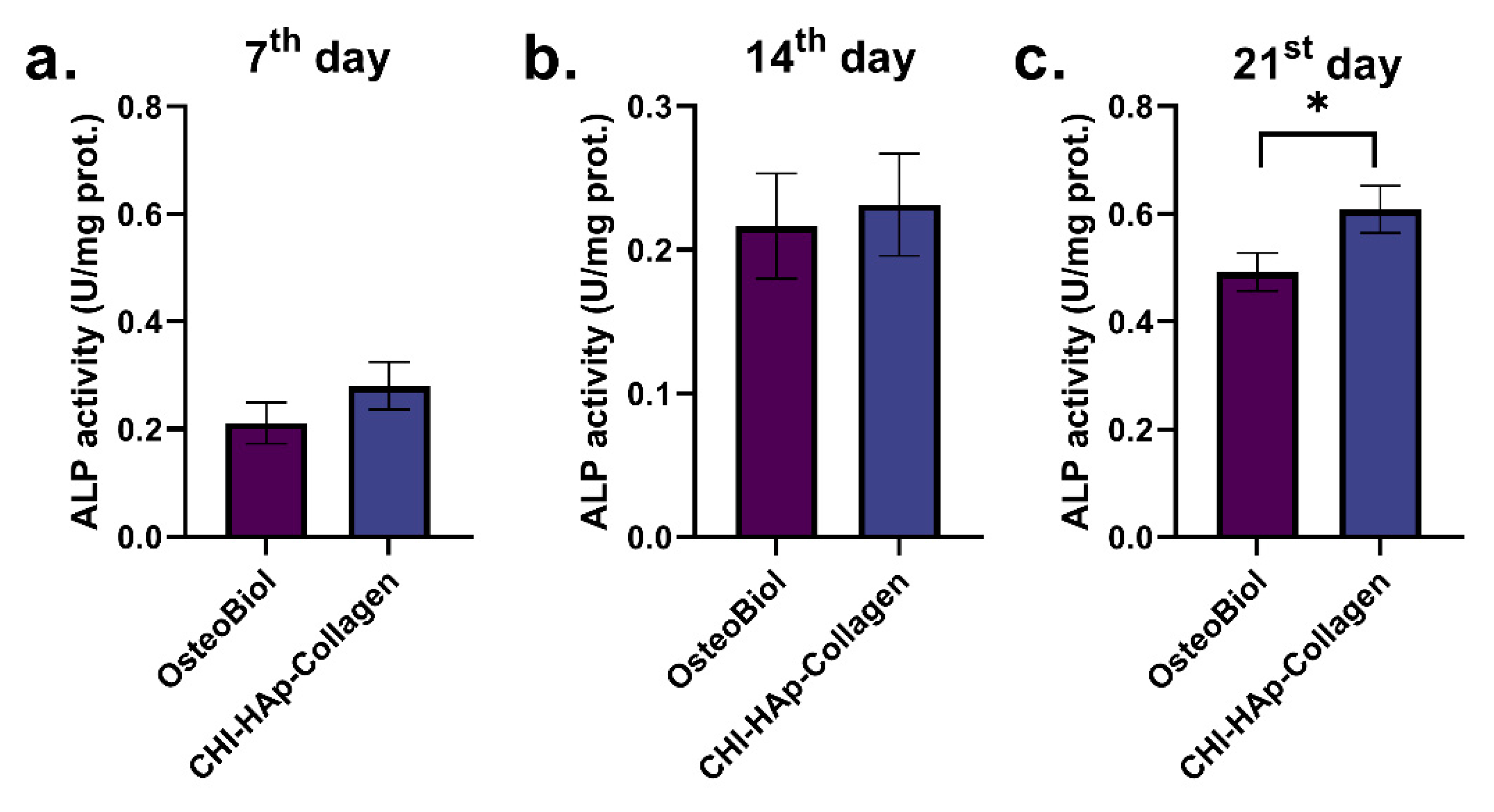
3.3. ALP Activity
3.4. Osteogenic-Related Gene Expression
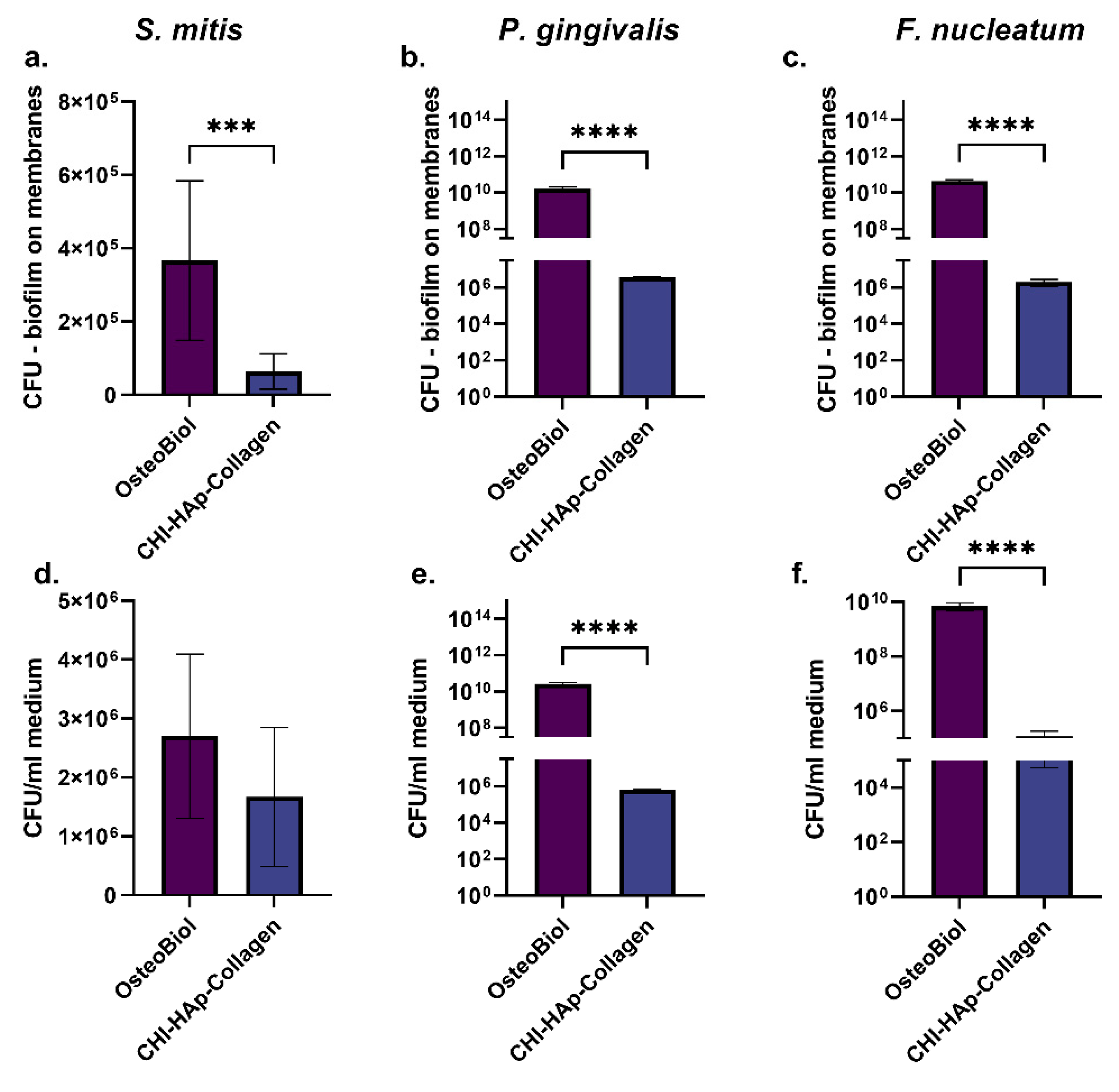
3.5. Colony-Forming Units on Membranes and in Medium around Membranes and SEM Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bottino, M.C.; Thomas, V. Membranes for Periodontal Regeneration—A Materials Perspective. Front. Oral Biol. 2015, 17, 90–100. [Google Scholar]
- Elgali, I.; Omar, O.; Dahlin, C.; Thomsen, P. Guided bone regeneration: Materials and biological mechanisms revisited. Eur. J. Oral. Sci. 2017, 125, 315–337. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, Z.; Qureshi, J.; Alshahrani, A.M.; Nassar, H.; Ikeda, Y.; Glogauer, M.; Ganss, B. Collagen based barrier membranes for periodontal guided bone regeneration applications. Odontology 2017, 105, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Turri, A.; Elgali, I.; Vazirisani, F.; Johansson, A.; Emanuelsson, L.; Dahlin, C.; Thomsen, P.; Omar, O. Guided bone regeneration is promoted by the molecular events in the membrane compartment. Biomaterials 2016, 84, 167–183. [Google Scholar] [CrossRef] [PubMed]
- Sela, M.N.; Kohavi, D.; Krausz, E.; Steinberg, D.; Rosen, G. Enzymatic degradation of collagen-guided tissue regeneration membranes by periodontal bacteria. Clin. Oral Implants Res. 2003, 14, 263–268. [Google Scholar] [CrossRef]
- Toledano, M.; Asady, S.; Toledano-Osorio, M.; García-Godoy, F.; Serrera-Figallo, M.A.; Benítez-García, J.A.; Osorio, R. Differential Biodegradation Kinetics of Collagen Membranes for Bone Regeneration. Polymers 2020, 12, 1290. [Google Scholar] [CrossRef]
- Omar, O.; Elgali, I.; Dahlin, C.; Thomsen, P. Barrier membranes: More than the barrier effect? J. Clin. Periodontol. 2019, 46 (Suppl. 21), 103–123. [Google Scholar] [CrossRef]
- Wessing, B.; Lettner, S.; Zechner, W. Guided Bone Regeneration with Collagen Membranes and Particulate Graft Materials: A Systematic Review and Meta-Analysis. Int. J. Oral Maxillofac. Implants 2018, 33, 87–100. [Google Scholar] [CrossRef]
- Chaushu, G.; Mardinger, O.; Peleg, M.; Ghelfan, O.; Nissan, J. Analysis of Complications following Augmentation with Cancellous Block Allografts. J. Periodontol. 2010, 81, 1759–1764. [Google Scholar] [CrossRef]
- Becker, W.; Dahlin, C.; Becker, B.E.; Lekholm, U.; van Steenberghe, D.; Higuchi, K.; Kultje, C. The use of e-PTFE barrier membranes for bone promotion around titanium implants placed into extraction sockets: A prospective multicenter study. Int. J. Oral Maxillofac. Implants. 1994, 9, 31–40. [Google Scholar]
- Zucchelli, G.; Sforza, N.M.; Clauser, C.; Cesari, C.; de Sanctis, M. Topical and Systemic Antimicrobial Therapy in Guided Tissue Regeneration. J. Periodontol. 1999, 70, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Morrow, B.R.; Jefferson, M.M.; Li, F.; Hong, L. Antibacterial Collagen Composite Membranes Containing Minocycline. J. Pharm. Sci. 2021, 110, 2177–2184. [Google Scholar] [CrossRef]
- Xue, J.; Shi, R.; Niu, Y.; Gong, M.; Coates, P.; Crawford, A.; Chen, D.; Tian, W.; Zhang, L. Fabrication of drug-loaded anti-infective guided tissue regeneration membrane with adjustable biodegradation property. Colloids Surf. B Biointerfaces 2015, 135, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Suchý, T.; Šupová, M.; Sauerová, P.; Kalbáčová, M.H.; Klapková, E.; Pokorný, M.; Horný, L.; Závora, J.; Ballay, R.; Denk, F.; et al. Evaluation of collagen/hydroxyapatite electrospun layers loaded with vancomycin, gentamicin and their combination: Comparison of release kinetics, antimicrobial activity and cytocompatibility. Eur. J. Pharm. Biopharm. 2019, 140, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Bai, T.; Wang, L.; Cheng, Y.; Xia, D.; Yu, S.; Zheng, Y. Biomimetic AgNPs@antimicrobial peptide/silk fibroin coating for infection-trigger antibacterial capability and enhanced osseointegration. Bioact. Mater. 2023, 20, 64–80. [Google Scholar] [CrossRef]
- Ghavimi, M.A.; Shahabadi, A.B.; Jarolmasjed, S.; Memar, M.Y.; Dizaj, S.M.; Sharifi, S. Nanofibrous asymmetric collagen/curcumin membrane containing aspirin-loaded PLGA nanoparticles for guided bone regeneration. Sci. Rep. 2020, 10, 18200. [Google Scholar] [CrossRef]
- Chen, P.; Wu, Z.; Leung, A.; Chen, X.; Landao-Bassonga, E.; Gao, J.; Chen, L.; Zheng, M.; Yao, F.; Yang, H.; et al. Fabrication of a silver nanoparticle-coated collagen membrane with anti-bacterial and anti-inflammatory activities for guided bone regeneration. Biomed. Mater. 2018, 13, 065014. [Google Scholar] [CrossRef]
- Qasim, S.B.; Najeeb, S.; Delaine-Smith, R.M.; Rawlinson, A.; Ur Rehman, I. Potential of electrospun chitosan fibers as a surface layer in functionally graded GTR membrane for periodontal regeneration. Dent. Mater. Off Publ. Acad. Dent. Mater. 2017, 33, 71–83. [Google Scholar] [CrossRef]
- Pakravan, M.; Heuzey, M.C.; Ajji, A. Core–Shell Structured PEO-Chitosan Nanofibers by Coaxial Electrospinning. Biomacromolecules 2012, 13, 412–421. [Google Scholar] [CrossRef]
- Ma, S.; Adayi, A.; Liu, Z.; Li, M.; Wu, M.; Xiao, L.; Sun, Y.; Cai, Q.; Yang, X.; Zhang, X.; et al. Asymmetric Collagen/chitosan Membrane Containing Minocycline-loaded Chitosan Nanoparticles for Guided Bone Regeneration. Sci. Rep. 2016, 6, 31822. [Google Scholar] [CrossRef]
- Behring, J.; Junker, R.; Walboomers, X.F.; Chessnut, B.; Jansen, J.A. Toward guided tissue and bone regeneration: Morphology, attachment, proliferation, and migration of cells cultured on collagen barrier membranes. A systematic review. Odontology 2008, 96, 1–11. [Google Scholar] [CrossRef]
- Furuhata, M.; Takayama, T.; Yamamoto, T.; Ozawa, Y.; Senoo, M.; Ozaki, M.; Yamano, S.; Sato, S. Real-time assessment of guided bone regeneration in critical size mandibular bone defects in rats using collagen membranes with adjunct fibroblast growth factor-2. J. Dent. Sci. 2021, 16, 1170–1181. [Google Scholar] [CrossRef] [PubMed]
- Jo, J.Y.; Jeong, S.I.; Shin, Y.M.; Kang, S.S.; Kim, S.E.; Jeong, C.M.; Huh, J.B. Sequential delivery of BMP-2 and BMP-7 for bone regeneration using a heparinized collagen membrane. Int. J. Oral Maxillofac. Surg. 2015, 44, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Wang, Y.; Wang, Y.; Yang, R.; Liu, L.; Rung, S.; Xiang, L.; Wu, Y.; Du, S.; Man, Y.; et al. Evaluation of epigallocatechin-3-gallate (EGCG) modified collagen in guided bone regeneration (GBR) surgery and modulation of macrophage phenotype. Mater. Sci. Eng. C 2019, 99, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Song, J.H.; Kim, H.E.; Kim, H.W. Collagen-apatite nanocomposite membranes for guided bone regeneration. J. Biomed. Mater. Res. B Appl. Biomater. 2007, 83B, 248–257. [Google Scholar] [CrossRef]
- Pandey, A.; Midha, S.; Sharma, R.K.; Maurya, R.; Nigam, V.K.; Ghosh, S.; Balani, K. Antioxidant and antibacterial hydroxyapatite-based biocomposite for orthopedic applications. Mater. Sci. Eng. C 2018, 88, 13–24. [Google Scholar] [CrossRef]
- Kalantari, E.; Naghib, S.M.; Iravani, N.J.; Esmaeili, R.; Naimi-Jamal, M.R.; Mozafari, M. Biocomposites based on hydroxyapatite matrix reinforced with nanostructured monticellite (CaMgSiO4) for biomedical application: Synthesis, characterization, and biological studies. Mater. Sci. Eng. C 2019, 105, 109912. [Google Scholar] [CrossRef]
- Epple, M.; Ganesan, K.; Heumann, R.; Klesing, J.; Kovtun, A.; Neumann, S.; Sokolova, V. Application of calcium phosphate nanoparticles in biomedicine. J. Mater. Chem. 2009, 20, 18–23. [Google Scholar] [CrossRef]
- Yazdani, J.; Ahmadian, E.; Sharifi, S.; Shahi, S.; Dizaj, S.M. A short view on nanohydroxyapatite as coating of dental implants. Biomed. Pharmacother. 2018, 105, 553–557. [Google Scholar] [CrossRef]
- Yunoki, S.; Ikoma, T.; Monkawa, A.; Ohta, K.; Kikuchi, M.; Marukawa, E.; Sotome, S.; Shinomiya, K.; Tanaka, J. Fabrication of three-dimensional porous hydroxyapatite/collagen composite with rubber-like elasticity. Mater. Sci. Eng. C 2007. [Google Scholar] [CrossRef]
- Becerra, J.; Rodriguez, M.; Leal, D.; Noris-Suarez, K.; Gonzalez, G. Chitosan-collagen-hydroxyapatite membranes for tissue engineering. J. Mater. Sci. Mater. Med. 2022, 33, 18. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, S.; Chawla, A.; Vairamani, M.; Sastry, T.P.; Subramanian, K.S.; Selvamurugan, N. Scaffolds containing chitosan, gelatin and graphene oxide for bone tissue regeneration in vitro and in vivo. Int. J. Biol. Macromol. 2017, 104, 1975–1985. [Google Scholar] [CrossRef] [PubMed]
- Vukajlovic, D.; Parker, J.; Bretcanu, O.; Novakovic, K. Chitosan based polymer/bioglass composites for tissue engineering applications. Mater. Sci. Eng. C 2019, 96, 955–967. [Google Scholar] [CrossRef] [PubMed]
- Lamarque, G.; Viton, C.; Domard, A. Comparative Study of the First Heterogeneous Deacetylation of α- and β-Chitins in a Multistep Process. Biomacromolecules 2004, 5, 992–1001. [Google Scholar] [CrossRef]
- Teng, S.H.; Lee, E.J.; Wang, P.; Shin, D.S.; Kim, H.E. Three-layered membranes of collagen/hydroxyapatite and chitosan for guided bone regeneration. J. Biomed. Mater. Res. B Appl. Biomater. 2008, 87, 132–138. [Google Scholar] [CrossRef] [PubMed]
- OsteoBiol ®. Available online: https://www.osteobiol.com/clinical-indication/soft_tissue_augmentation/derma-o14.html (accessed on 24 January 2023).
- Ignjatović, N.L.; Liu, C.Z.; Czernuszka, J.T.; Uskoković, D.P. Micro- and nano-injectable composite biomaterials containing calcium phosphate coated with poly(dl-lactide-co-glycolide). Acta Biomater. 2007, 3, 927–935. [Google Scholar] [CrossRef] [PubMed]
- de Marco, P.; Zara, S.; de Colli, M.; Radunovic, M.; Lazović, V.; Ettorre, V.; di Crescenzo, A.; Piattelli, A.; Cataldi, A.; Fontana, A. Graphene oxide improves the biocompatibility of collagen membranes in an in vitro model of human primary gingival fibroblasts. Biomed. Mater. 2017, 12, 055005. [Google Scholar] [CrossRef]
- Vukovic, M.; Lazarevic, M.; Mitic, D.; Karisik, M.J.; Ilic, B.; Andric, M.; Jevtic, B.; Roganovic, J.; Milasin, J. Acetylsalicylic-acid (ASA) regulation of osteo/odontogenic differentiation and proliferation of human dental pulp stem cells (DPSCs) in vitro. Arch. Oral Biol. 2022, 144, 105564. [Google Scholar] [CrossRef]
- Kamiloglu, S.; Sari, G.; Ozdal, T.; Capanoglu, E. Guidelines for cell viability assays. Food Front. 2020, 1, 332–349. [Google Scholar] [CrossRef]
- Stojkovska, J.; Kostić, D.; Jovanović, Ž.; Vukašinović-Sekulić, M.; Mišković-Stanković, V.; Obradović, B. A comprehensive approach to in vitro functional evaluation of Ag/alginate nanocomposite hydrogels. Carbohydr. Polym. 2014, 111, 305–314. [Google Scholar] [CrossRef]
- Bellamy, L.J. The Infra-Red Spectra of Complex Molecules, 1st ed.; Springer: Dordrecht, The Netherlands, 2013. [Google Scholar]
- Mucha, M.; Pawlak, A. Complex study on chitosan degradability. Polimery 2002, 47, 509–516. [Google Scholar] [CrossRef]
- Ignjatović, N.; Djurić, S.V.; Mitić, Ž.; Janković, D.; Uskoković, D. Investigating an organ-targeting platform based on hydroxyapatite nanoparticles using a novel in situ method of radioactive 125Iodine labeling. Mater. Sci. Eng. C 2014, 43, 439–446. [Google Scholar] [CrossRef]
- Lak, A.; Mazloumi, M.; Mohajerani, M.S.; Zanganeh, S.; Shayegh, M.R.; Kajbafvala, A.; Arami, H.; Sadrnezhaad, S.K. Rapid Formation of Mono-Dispersed Hydroxyapatite Nanorods with Narrow-Size Distribution via Microwave Irradiation. J. Am. Ceram. Soc. 2008, 91, 3580–3584. [Google Scholar] [CrossRef]
- Liu, C.-Y.; Matsusaki, M.; Akashi, M. Cell effects on the formation of collagen triple helix fibers inside collagen gels or on cell surfaces. Polym. J. 2015, 47, 391–399. [Google Scholar] [CrossRef]
- Sun, T.W.; Zhu, Y.J.; Chen, F. Hydroxyapatite nanowire/collagen elastic porous nanocomposite and its enhanced performance in bone defect repair. RSC Adv. 2018, 8, 26218–26229. [Google Scholar] [CrossRef] [PubMed]
- Shaghiera, A.D.; Widiyanti, P.; Yusuf, H. Synthesis and Characterization of Injectable Hydrogels with Varying Collagen–Chitosan–Thymosin β4 Composition for Myocardial Infarction Therapy. J. Funct. Biomater. 2018, 9, 33. [Google Scholar] [CrossRef]
- Przekora, A.; Ginalska, G. In vitro evaluation of the risk of inflammatory response after chitosan/HA and chitosan/β-1,3-glucan/HA bone scaffold implantation. Mater. Sci. Eng. C 2016, 61, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Ching, H.S.; Luddin, N.; Rahman, I.A.; Ponnuraj, K.T. Expression of Odontogenic and Osteogenic Markers in DPSCs and SHED: A Review. Curr. Stem Cell Res. Ther. 2017, 12, 71–79. [Google Scholar] [CrossRef]
- Liu, M.; Goldman, G.; MacDougall, M.; Chen, S. BMP Signaling Pathway in Dentin Development and Diseases. Cells 2022, 11, 2216. [Google Scholar] [CrossRef]
- Huang, X.; Chen, X.; Chen, H.; Xu, D.; Lin, C.; Peng, B. Rho/Rho-associated protein kinase signaling pathway-mediated downregulation of runt-related transcription factor 2 expression promotes the differentiation of dental pulp stem cells into odontoblasts. Exp. Ther. Med. 2018, 15, 4457–4464. [Google Scholar] [CrossRef]
- Bruderer, M.; Richards, R.G.; Alini, M.; Stoddart, M.J. Role and regulation of RUNX2 in osteogenesis. Eur. Cell Mater. 2014, 28, 269–286. [Google Scholar] [CrossRef] [PubMed]
- Croes, M.; van der Wal, B.C.H.; Vogely, H.C. Impact of Bacterial Infections on Osteogenesis: Evidence From In Vivo Studies. J. Orthop. Res. 2019, 37, 2067–2076. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Lei, C.; Meng, L.; Wang, C.; Song, Y. Chitosan as a barrier membrane material in periodontal tissue regeneration. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100B, 1435–1443. [Google Scholar] [CrossRef]
- Costa, E.M.; Silva, S.; Pina, C.; Tavaria, F.K.; Pintado, M.M. Evaluation and insights into chitosan antimicrobial activity against anaerobic oral pathogens. Anaerobe 2012, 18, 305–309. [Google Scholar] [CrossRef]
- Li, B.; Xia, X.; Guo, M.; Jiang, Y.; Li, Y.; Zhang, Z.; Liu, S.; Li, H.; Liang, C.; Wang, H. Biological and antibacterial properties of the micro-nanostructured hydroxyapatite/chitosan coating on titanium. Sci. Rep. 2019, 9, 14052. [Google Scholar] [CrossRef]
- Perinelli, D.R.; Fagioli, L.; Campana, R.; Lam, J.K.W.; Baffone, W.; Palmieri, G.F.; Casettari, L.; Bonacucina, G. Chitosan-based nanosystems and their exploited antimicrobial activity. Eur. J. Pharm. Sci. 2018, 117, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhuang, S. Antibacterial activity of chitosan and its derivatives and their interaction mechanism with bacteria: Current state and perspectives. Eur. Polym. J. 2020, 138, 109984. [Google Scholar] [CrossRef]
- Martinez, L.R.; Mihu, M.R.; Tar, M.; Cordero, R.J.B.; Han, G.; Friedman, A.J.; Friedman, J.M.; Nosanchuk, J.D. Demonstration of Antibiofilm and Antifungal Efficacy of Chitosan against Candidal Biofilms, Using an In Vivo Central Venous Catheter Model. J. Infect Dis. 2010, 201, 1436–1440. [Google Scholar] [CrossRef] [PubMed]
- No, H.K.; Park, N.Y.; Lee, S.H.; Meyers, S.P. Antibacterial activity of chitosans and chitosan oligomers with different molecular weights. Int. J. Food Microbiol. 2002, 74, 65–72. [Google Scholar] [CrossRef]
- Chung, Y.-C.; Su, Y.-P.; Chen, C.-C.; Jia, G.; Wang, H.-L.; Wu, J.C.G.; Lin, J.-G. Relationship between antibacterial activity of chitosan and surface characteristics of cell wall. Acta Pharmacol. Sin. 2004, 25, 932–936. [Google Scholar]
- Raafat, D.; Sahl, H.G. Chitosan and its antimicrobial potential—A critical literature survey. Microb. Biotechnol. 2009, 2, 186–201. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.Y.; Zhu, J.F. Study on antimicrobial activity of chitosan with different molecular weights. Carbohydr. Polym. 2003, 54, 527–530. [Google Scholar] [CrossRef]
- Jakubovics, N.S.; Yassin, S.A.; Rickard, A.H. Community interactions of oral streptococci. Adv. Appl. Microbiol. 2014, 87, 43–110. [Google Scholar] [PubMed]
- Blank, E.; Grischke, J.; Winkel, A.; Eberhard, J.; Kommerein, N.; Doll, K.; Yang, I.; Stiesch, M. Evaluation of biofilm colonization on multi-part dental implants in a rat model. BMC Oral Health 2021, 21, 313. [Google Scholar] [CrossRef] [PubMed]



| Product Name | Sequences (5′→3′) | |
|---|---|---|
| ALP | Forward Reverse | CCACGTCTTCACATTTGGTG ATGGCAGTGAAGGGCTTCTT |
| BMP2 | Forward Reverse | CACTGTGCGCAGCTTCC CCTCCGTGGGGATAGAACTT |
| OCN | Forward Reverse | TTGGACACAAAGGCTGCAC CTCACACTCCTCGCCCTATT |
| RUNX2 | Forward Reverse | ACAAACAACCACAGAACCACAAGT GTCTCGGTGGCTGGTAGTGA |
| GAPDH | Forward Reverse | TCATGACCACAGTCCATGCCATCA CCCTGTTGCTGTAGCCAAATTCGT |
| Reference Strain | Medium | Agar | Temperature | Incubation Time | Conditions |
|---|---|---|---|---|---|
| Porphyromonas gingivalis | Schaedler broth with hemin and vitamin K1 * | Brucella agar with 5% sheep blood *** | 37 °C | 5 days | Anaerobic |
| Fusobacterium nucleatum | Schaedler broth with hemin and vitamin K1* | Brucella agar with 5% sheep blood *** | 37 °C | 5 days | Anaerobic |
| Streptococcus mitis | Brain heart infusion (BHI) broth** | Columbia agar with 5% sheep blood **** | 37 °C | 48 h | Anaerobic |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazarevic, M.; Petrovic, S.; Pierfelice, T.V.; Ignjatovic, N.; Piattelli, A.; Vlajic Tovilovic, T.; Radunovic, M. Antimicrobial and Osteogenic Effects of Collagen Membrane Decorated with Chitosan–Nano-Hydroxyapatite. Biomolecules 2023, 13, 579. https://doi.org/10.3390/biom13040579
Lazarevic M, Petrovic S, Pierfelice TV, Ignjatovic N, Piattelli A, Vlajic Tovilovic T, Radunovic M. Antimicrobial and Osteogenic Effects of Collagen Membrane Decorated with Chitosan–Nano-Hydroxyapatite. Biomolecules. 2023; 13(4):579. https://doi.org/10.3390/biom13040579
Chicago/Turabian StyleLazarevic, Milos, Sanja Petrovic, Tania Vanessa Pierfelice, Nenad Ignjatovic, Adriano Piattelli, Tamara Vlajic Tovilovic, and Milena Radunovic. 2023. "Antimicrobial and Osteogenic Effects of Collagen Membrane Decorated with Chitosan–Nano-Hydroxyapatite" Biomolecules 13, no. 4: 579. https://doi.org/10.3390/biom13040579
APA StyleLazarevic, M., Petrovic, S., Pierfelice, T. V., Ignjatovic, N., Piattelli, A., Vlajic Tovilovic, T., & Radunovic, M. (2023). Antimicrobial and Osteogenic Effects of Collagen Membrane Decorated with Chitosan–Nano-Hydroxyapatite. Biomolecules, 13(4), 579. https://doi.org/10.3390/biom13040579










