Characterization of Triacylglycerol Estolide Isomers Using High-Resolution Tandem Mass Spectrometry with Nanoelectrospray Ionization
Abstract
1. Introduction
2. Materials and Methods
2.1. Solvents and Additives
2.2. TG-EST Standards
2.3. Mass Spectrometry
3. Results
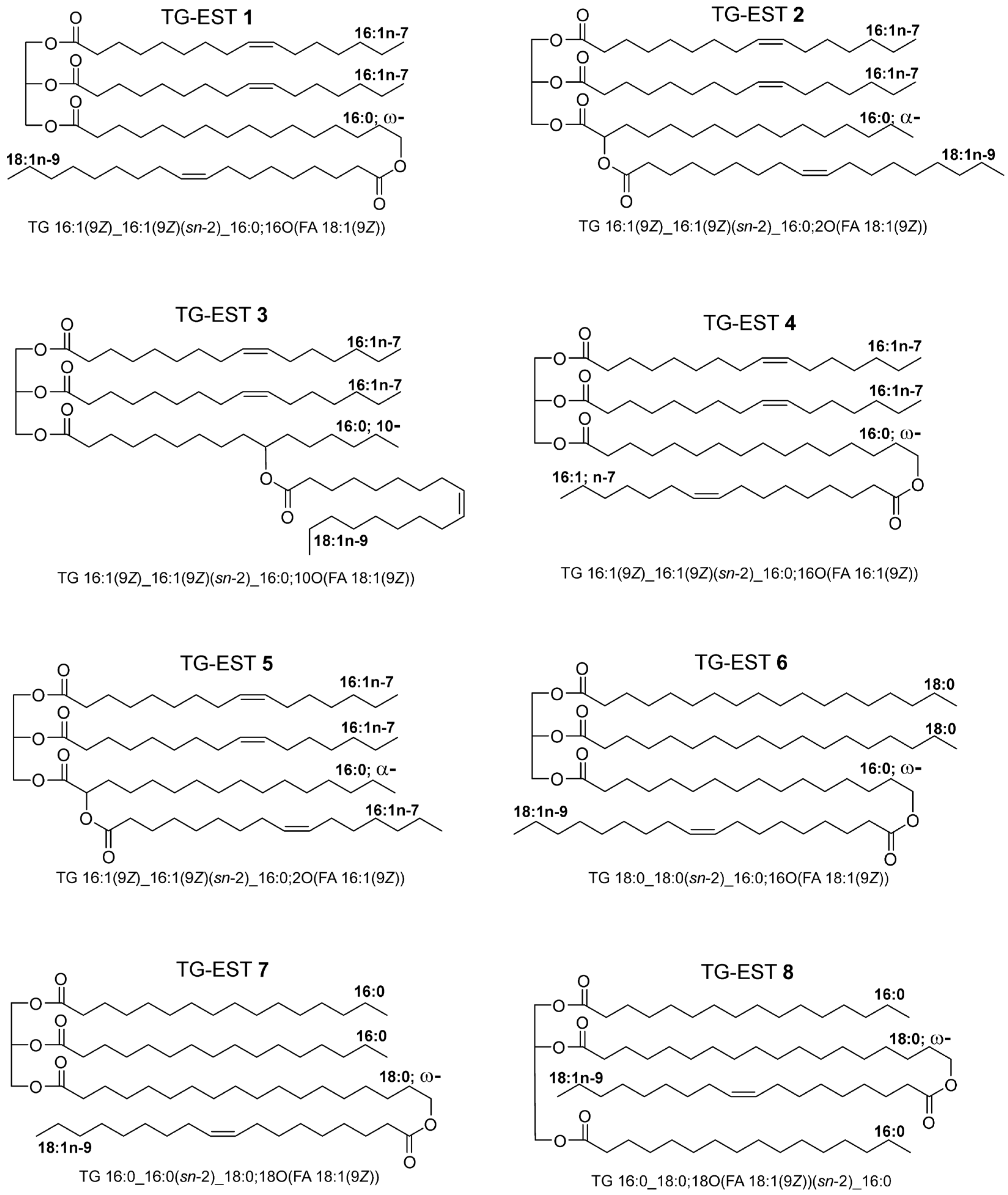
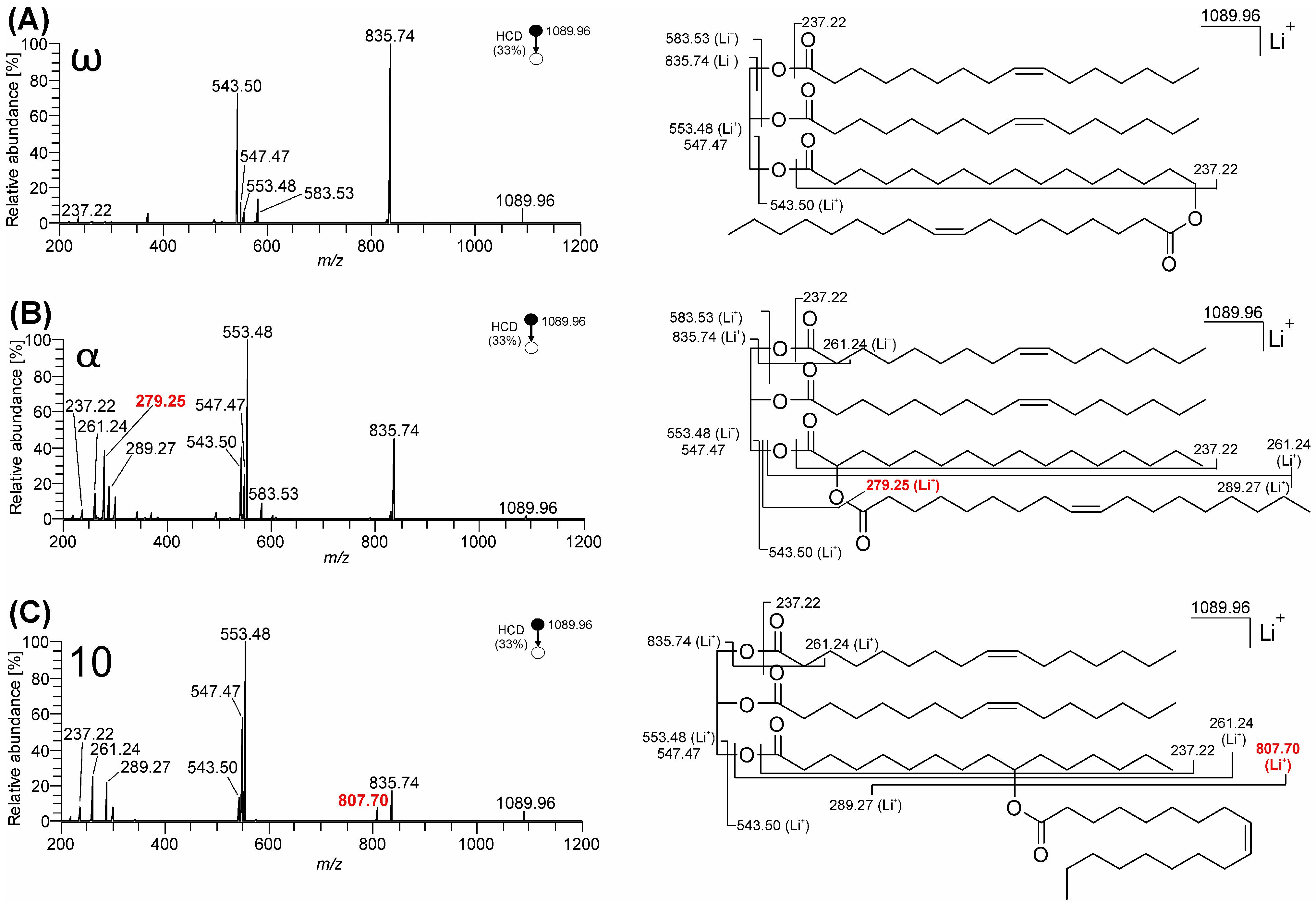
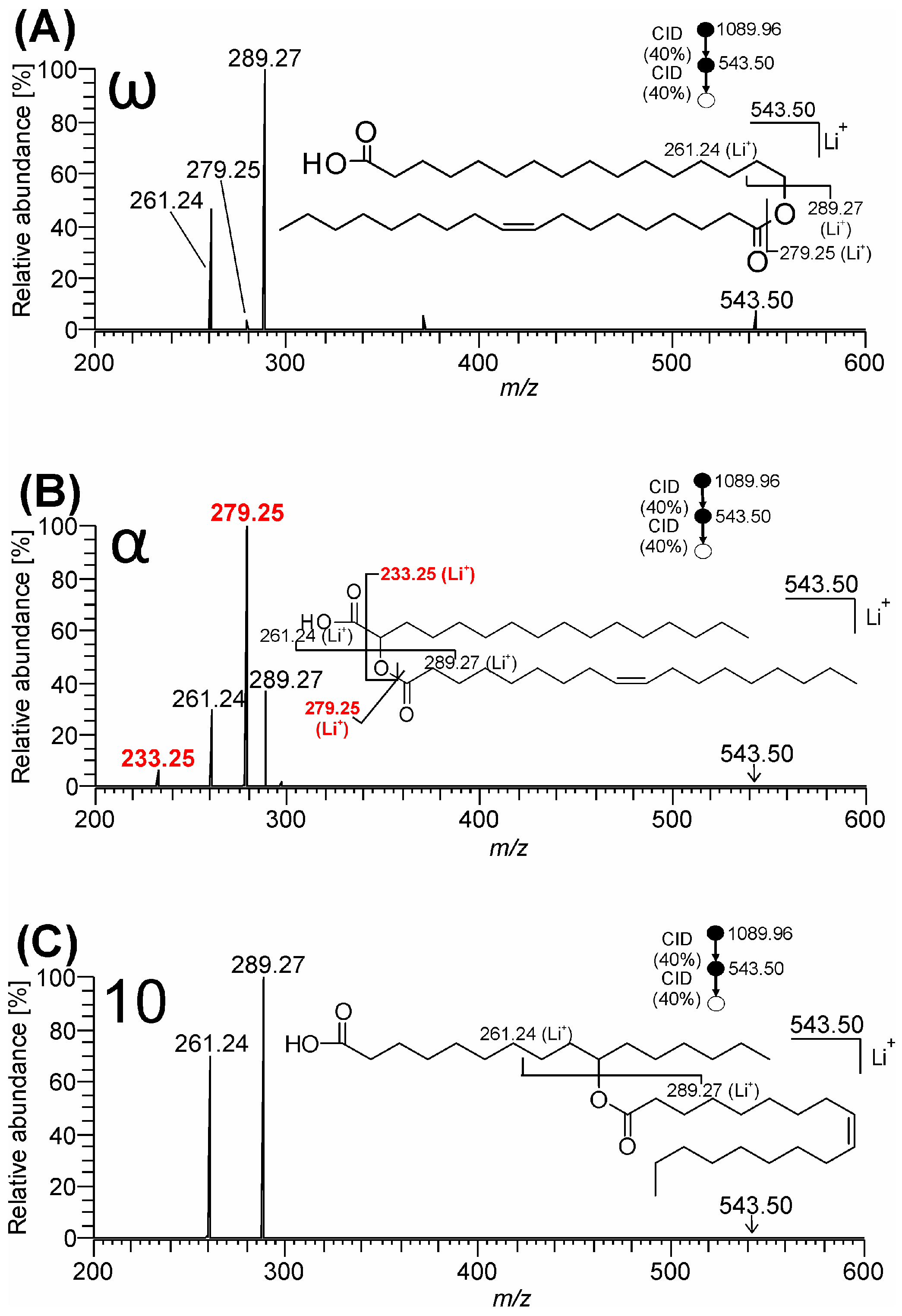
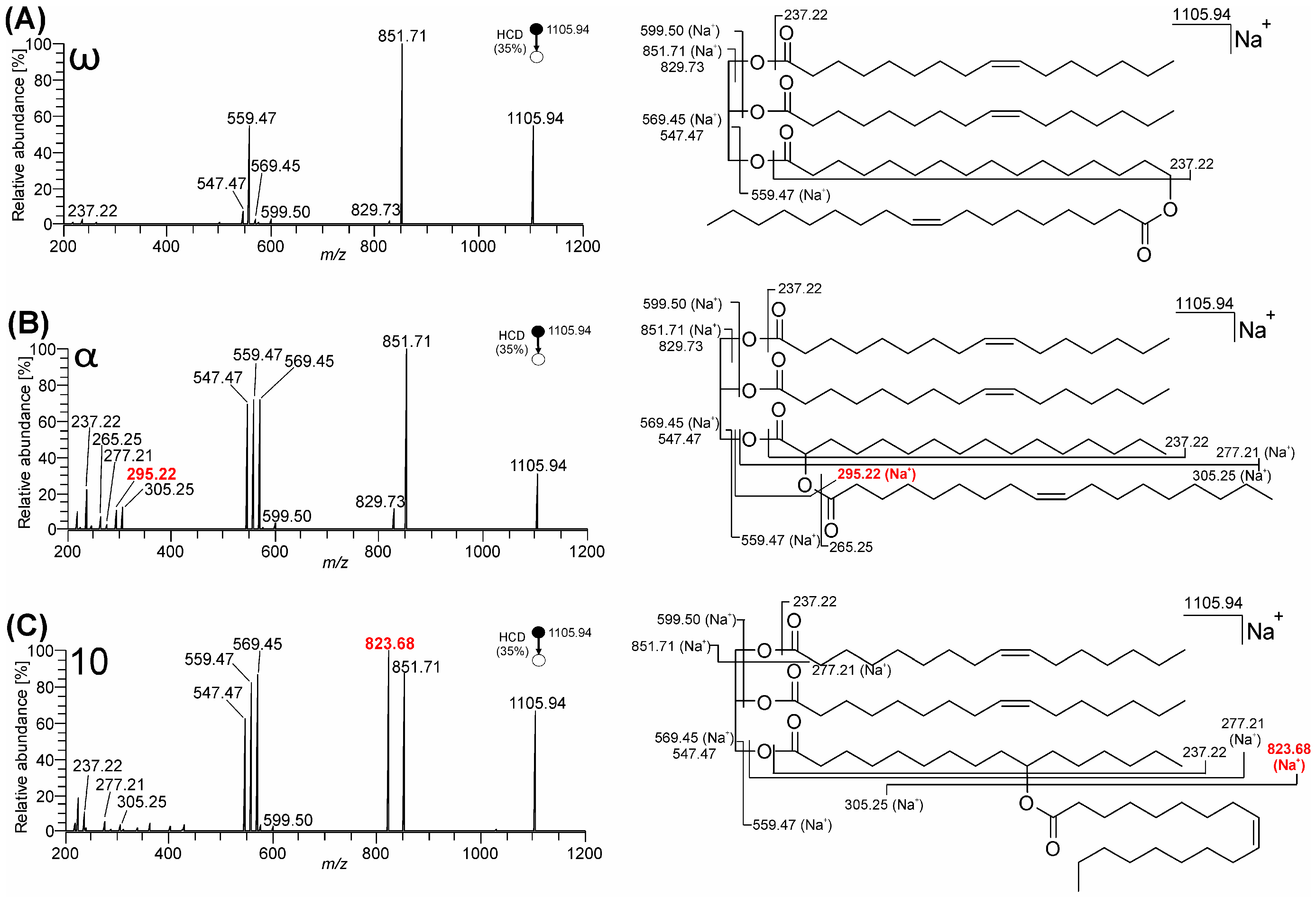
3.1. Mass Spectra of ω-, 10-, and α-Estolide-Branching Regioisomers
3.1.1. Fragmentation of Ammonium Adducts
3.1.2. Fragmentation of Lithium Adducts
3.1.3. Fragmentation of Sodium Adducts
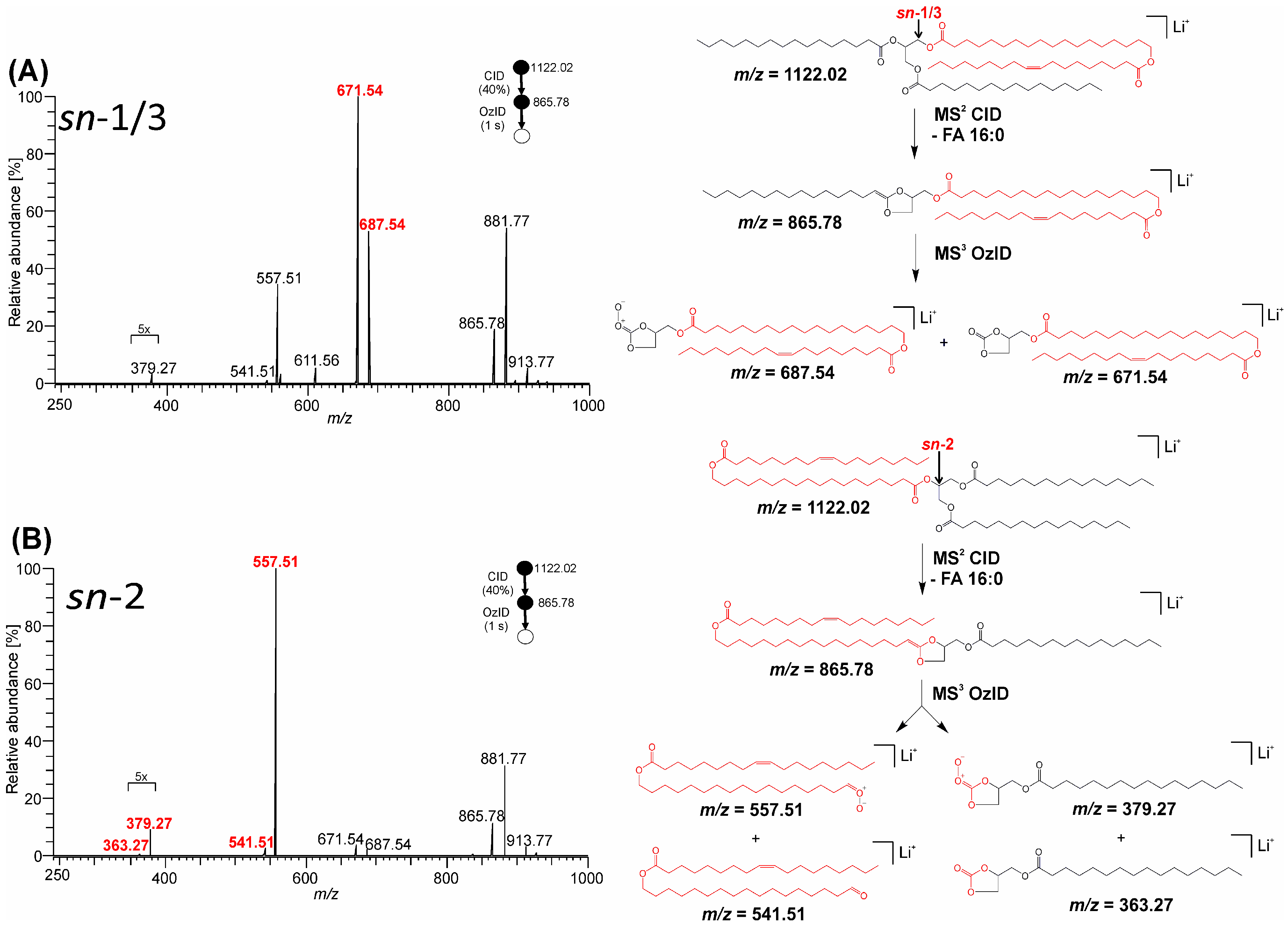
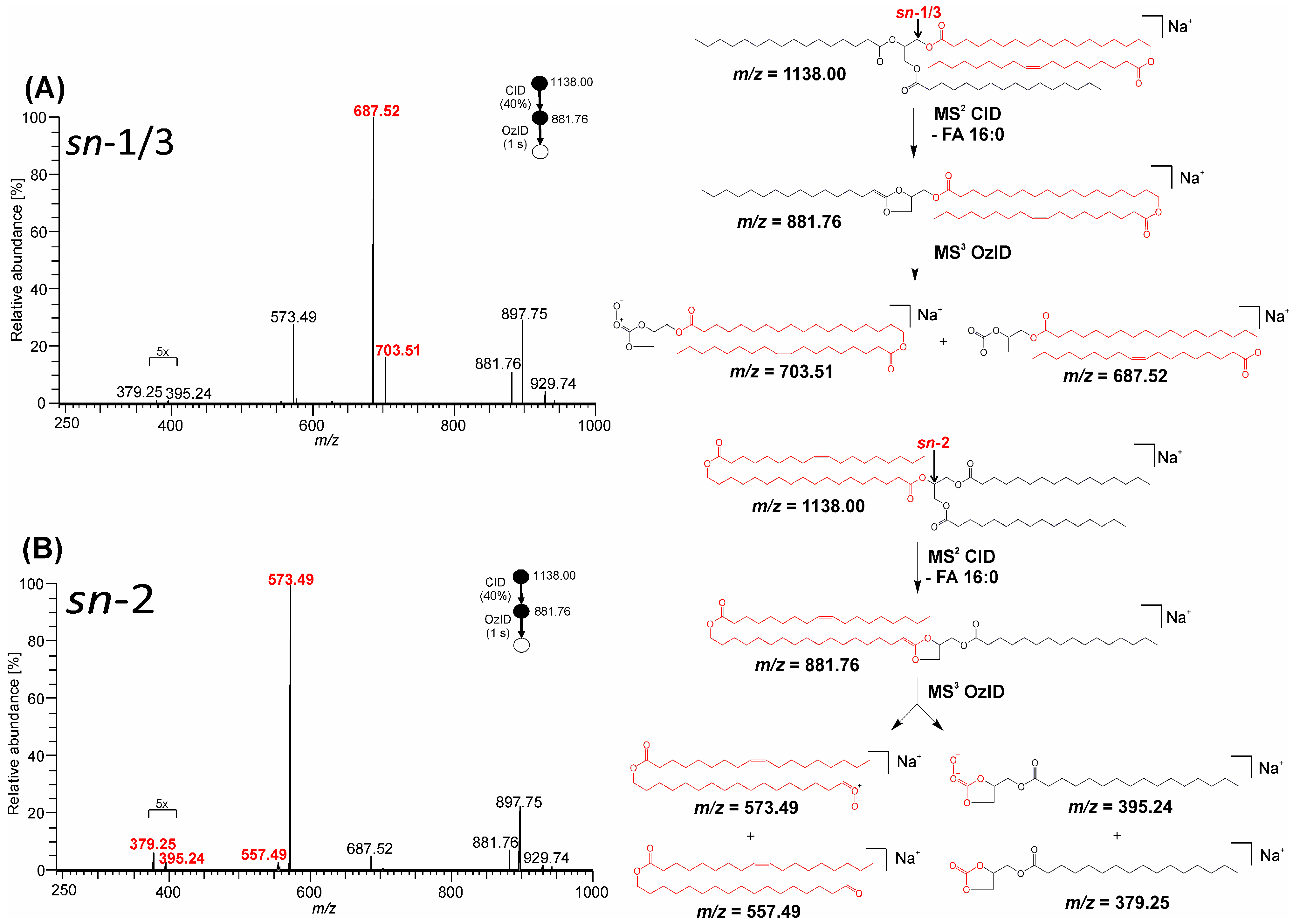
3.2. Mass Spectra of Glycerol sn-Regioisomers
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brejchova, K.; Balas, L.; Paluchova, V.; Brezinova, M.; Durand, T.; Kuda, O. Understanding FAHFAs: From structure to metabolic regulation. Prog. Lipid Res. 2020, 79, 101053. [Google Scholar] [CrossRef] [PubMed]
- Riecan, M.; Paluchova, V.; Lopes, M.; Brejchova, K.; Kuda, O. Branched and linear fatty acid esters of hydroxy fatty acids (FAHFA) relevant to human health. Pharmacol. Ther. 2021, 231, 107972. [Google Scholar] [CrossRef] [PubMed]
- Vavrušová, A.; Vrkoslav, V.; Plavka, R.; Bosáková, Z.; Cvačka, J. Analysis of (O-acyl) alpha-and omega-hydroxy fatty acids in vernix caseosa by high-performance liquid chromatography-Orbitrap mass spectrometry. Anal. Bioanal. Chem. 2020, 412, 2291–2302. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Biresaw, G.; Cermak, S.C.; Isbell, T.A.; Ngo, H.L.; Chen, L.; Durham, A.L. Fatty Acid Estolides: A Review. J. Am. Oil Chem. Soc. 2020, 97, 231–241. [Google Scholar] [CrossRef]
- McLean, S.; Davies, N.W.; Nichols, D.S.; McLeod, B.J. Triacylglycerol estolides, a new class of mammalian lipids, in the paracloacal gland of the brushtail possum (Trichosurus vulpecula). Lipids 2015, 50, 591–604. [Google Scholar] [CrossRef]
- Tan, D.; Ertunc, M.E.; Konduri, S.; Zhang, J.; Pinto, A.M.; Chu, Q.; Kahn, B.B.; Siegel, D.; Saghatelian, A. Discovery of FAHFA-Containing Triacylglycerols and Their Metabolic Regulation. J. Am. Chem. Soc. 2019, 141, 8798–8806. [Google Scholar] [CrossRef]
- Brejchova, K.; Radner, F.P.W.; Balas, L.; Paluchova, V.; Cajka, T.; Chodounska, H.; Kudova, E.; Schratter, M.; Schreiber, R.; Durand, T.; et al. Distinct roles of adipose triglyceride lipase and hormone-sensitive lipase in the catabolism of triacylglycerol estolides. Proc. Natl. Acad. Sci. USA 2021, 118, e2020999118. [Google Scholar] [CrossRef]
- Paluchova, V.; Oseeva, M.; Brezinova, M.; Cajka, T.; Bardova, K.; Adamcova, K.; Zacek, P.; Brejchova, K.; Balas, L.; Chodounska, H.; et al. Lipokine 5-PAHSA Is Regulated by Adipose Triglyceride Lipase and Primes Adipocytes for De Novo Lipogenesis in Mice. Diabetes 2020, 69, 300–312. [Google Scholar] [CrossRef]
- Brezinova, M.; Cajka, T.; Oseeva, M.; Stepan, M.; Dadova, K.; Rossmeislova, L.; Matous, M.; Siklova, M.; Rossmeisl, M.; Kuda, O. Exercise training induces insulin-sensitizing PAHSAs in adipose tissue of elderly women. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2020, 1865, 158576. [Google Scholar] [CrossRef]
- Saponara, E.; Penno, C.; Orsini, V.; Wang, Z.-Y.; Fischer, A.; Aebi, A.; Matadamas-Guzman, M.L.; Brun, V.; Fischer, B.; Brousseau, M.; et al. Loss of Hepatic Leucine-Rich Repeat-Containing G-protein Coupled Receptors 4 and 5 Promotes Nonalcoholic Fatty Liver Disease. Am. J. Pathol. 2023, 193, 161–181. [Google Scholar] [CrossRef]
- Brejchova, K.; Paluchova, V.; Brezinova, M.; Cajka, T.; Balas, L.; Durand, T.; Krizova, M.; Stranak, Z.; Kuda, O. Triacylglycerols containing branched palmitic acid ester of hydroxystearic acid (PAHSA) are present in the breast milk and hydrolyzed by carboxyl ester lipase. Food Chem. 2022, 388, 132983. [Google Scholar] [CrossRef]
- Sprecher, H.W.; Maier, R.; Barber, M.; Holman, R.T. Structure of an optically active allene-containing tetraester triglyceride isolated from the seed oil of Sapium sebiferum. Biochemistry 1965, 4, 1856–1863. [Google Scholar] [CrossRef]
- Mikolajczak, K.L.; Smith, C.R., Jr.; Wolff, I.A. Glyceride structure of Cardamine impatiens L. Seed oil. Lipids 1968, 3, 215–220. [Google Scholar] [CrossRef]
- Christie, W.W. The glyceride structure of sapium sebiferum seed oil. Biochim. Biophys. Acta (BBA)-Lipids Lipid Metab. 1969, 187, 1–5. [Google Scholar] [CrossRef]
- Phillips, B.E.; Smith, C.R., Jr. Glycerides of Monnina emarginata seed oil. Biochim. Biophys. Acta (BBA)-Lipids Lipid Metab. 1970, 218, 71–82. [Google Scholar] [CrossRef]
- Kleiman, R.; Spencer, G.F.; Earle, F.R.; Nieschlag, H.J.; Barclay, A.S. Tetra-acid triglycerides containing a new hydroxy eicosadienoyl moiety in Lesquerella auriculata seed oil. Lipids 1972, 7, 660–665. [Google Scholar] [CrossRef]
- Payne-Wahl, K.; Plattner, R.D.; Spencer, G.F.; Kleiman, R. Separation of tetra-, penta-, and hexaacyl triglycerides by high performance liquid chromatography. Lipids 1979, 14, 601–605. [Google Scholar] [CrossRef]
- Plattner, R.D.; Payne-Wahl, K.; Tjarks, L.W.; Kleiman, R. Hydroxy acids and estolide triglycerides of Heliophila amplexicaulis L.f. Seed oil. Lipids 1979, 14, 576–579. [Google Scholar] [CrossRef]
- Madrigal, R.V.; Smith, C.R., Jr. Estolide triglycerides of Trewia nudiflora seed oil. Lipids 1982, 17, 650–655. [Google Scholar] [CrossRef]
- Payne-Wahl, K. Quantitation of estolide triglycerides in sapium seeds by high performance liquid chromatography with infrared detection. J. Am. Oil Chem. Soc. 1983, 60, 1011–1012. [Google Scholar] [CrossRef]
- Aitzetmüller, K.; Xin, Y.; Werner, G.; Grönheim, M. High-performance liquid chromatographic investigations of stillingia oil. J. Chromatogr. A 1992, 603, 165–173. [Google Scholar] [CrossRef]
- Hayes, D.G.; Kleiman, R.; Phillips, B.S. The triglyceride composition, structure, and presence of estolides in the oils of Lesquerella and related species. J. Am. Oil Chem. Soc. 1995, 72, 559–569. [Google Scholar] [CrossRef]
- Hayes, D.G.; Kleiman, R.; Weisleder, D.; Adlof, R.O.; Cuperus, F.P.; Derksen, J.T.P. Occurrence of estolides in processed Dimorphotheca pluvialis seed oil. Ind. Crops Prod. 1995, 4, 295–301. [Google Scholar] [CrossRef]
- Spitzer, V.; Tomberg, W.; Pohlentz, G. Structure analysis of an allene-containing estolide tetraester triglyceride in the seed oil of Sebastiana commersoniana (Euphorbiaceae). Lipids 1997, 32, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-T.; Arcinas, A.; Harden, L.R.; Fagerquist, C.K. Identification of (12-Ricinoleoylricinoleoyl)diricinoleoylglycerol, an Acylglycerol Containing Four Acyl Chains, in Castor (Ricinus communis L.) Oil by LC-ESI-MS. J. Agric. Food Chem. 2006, 54, 3498–3504. [Google Scholar] [CrossRef]
- Lin, J.-T.; Arcinas, A. Regiospecific Identification of 2-(12-Ricinoleoylricinoleoyl)-1,3-diricinoleoyl-sn-glycerol in Castor (Ricinus communis L.) Oil by ESI-MS4. J. Agric. Food Chem. 2008, 56, 3616–3622. [Google Scholar] [CrossRef]
- Lin, J.-T.; Chen, G.Q. Identification of Minor Acylglycerols Less Polar than Triricinolein in Castor Oil by Mass Spectrometry. J. Am. Oil Chem. Soc. 2012, 89, 1773–1784. [Google Scholar] [CrossRef]
- Lin, J.-T.; Chen, G.Q. Ratios of Regioisomers of Minor Acylglycerols Less Polar than Triricinolein in Castor Oil Estimated by Mass Spectrometry. J. Am. Oil Chem. Soc. 2012, 89, 1785–1792. [Google Scholar] [CrossRef]
- Zhang, H.; Olson, D.J.H.; Van, D.; Purves, R.W.; Smith, M.A. Rapid identification of triacylglycerol-estolides in plant and fungal oils. Ind. Crops Prod. 2012, 37, 186–194. [Google Scholar] [CrossRef]
- Lin, J.-T.; Chen, G.Q. Identification of Tetraacylglycerols in Lesquerella Oil by Electrospray Ionization Mass Spectrometry of the Lithium Adducts. J. Am. Oil Chem. Soc. 2013, 90, 1831–1836. [Google Scholar] [CrossRef]
- Smith, M.A.; Zhang, H.; Forseille, L.; Purves, R.W. Characterization of novel triacylglycerol estolides from the seed oil of Mallotus philippensis and Trewia nudiflora. Lipids 2013, 48, 75–85. [Google Scholar] [CrossRef]
- Lin, J.-T.; Chen, G. Quantification of the molecular species of tetraacylglycerols in lesquerella (Physaria fendleri) Oil by HPLC and MS. Enliven Bio Anal. Tech. 2014, 1, 1–5. [Google Scholar]
- Zhang, H.; Smith, M.A.; Purves, R.W. Optimization of triacylglycerol-estolide analysis by matrix-assisted laser desorption/ionization-mass spectrometry. J. Am. Oil Chem. Soc. 2014, 91, 905–915. [Google Scholar] [CrossRef]
- Smith, M.A.; Zhang, H. Apocynaceae seed lipids: Characterization and occurrence of isoricinoleic acid and triacylglycerol estolides. J. Am. Oil Chem. Soc. 2016, 93, 105–114. [Google Scholar] [CrossRef]
- Lin, J.-T.; Chen, G.Q. Structural characteristics of the molecular species of tetraacylglycerols in lesquerella (Physaria fendleri) oil elucidated by mass spectrometry. Biocatal. Agric. Biotechnol. 2017, 10, 167–173. [Google Scholar] [CrossRef]
- Romsdahl, T.; Shirani, A.; Minto, R.E.; Zhang, C.; Cahoon, E.B.; Chapman, K.D.; Berman, D. Nature-Guided Synthesis of Advanced Bio-Lubricants. Sci. Rep. 2019, 9, 11711. [Google Scholar] [CrossRef]
- Matsuzawa, Y.; Higashi, Y.; Takano, K.; Takahashi, M.; Yamada, Y.; Okazaki, Y.; Nakabayashi, R.; Saito, K.; Tsugawa, H. Food Lipidomics for 155 Agricultural Plant Products. J. Agric. Food Chem. 2021, 69, 8981–8990. [Google Scholar] [CrossRef]
- Liu, Y.; Tu, X.; Lin, L.; Du, L.; Feng, X. Analysis of Lipids in Pitaya Seed Oil by Ultra-Performance Liquid Chromatography-Time-of-Flight Tandem Mass Spectrometry. Foods 2022, 11, 2988. [Google Scholar] [CrossRef]
- Morris, L.J.; Hall, S.W. The structure of the glycerides of ergot oils. Lipids 1966, 1, 188–196. [Google Scholar] [CrossRef]
- Batrakov, S.G.; Tolkachev, O.N. The structures of triacylglycerols from sclerotia of the rye ergot Claviceps purpurea (Fries) Tul. Chem. Phys. Lipids 1997, 86, 1–12. [Google Scholar] [CrossRef]
- Lucejko, J.J.; La Nasa, J.; Porta, F.; Vanzetti, A.; Tanda, G.; Mangiaracina, C.F.; Corretti, A.; Colombini, M.P.; Ribechini, E. Long-lasting ergot lipids as new biomarkers for assessing the presence of cereals and cereal products in archaeological vessels. Sci. Rep. 2018, 8, 3935. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Santoro, A.; Hofer, P.; Tan, D.; Oberer, M.; Nelson, A.T.; Konduri, S.; Siegel, D.; Zechner, R.; Saghatelian, A.; et al. ATGL is a biosynthetic enzyme for fatty acid esters of hydroxy fatty acids. Nature 2022, 606, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Nelson, A.T.; Kolar, M.J.; Chu, Q.; Syed, I.; Kahn, B.B.; Saghatelian, A.; Siegel, D. Stereochemistry of Endogenous Palmitic Acid Ester of 9-Hydroxystearic Acid and Relevance of Absolute Configuration to Regulation. J. Am. Chem. Soc. 2017, 139, 4943–4947. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, S.; Okahashi, N.; Tsugawa, H.; Ogata, Y.; Ikeda, K.; Suda, W.; Arita, M. Elucidation of Gut Microbiota-Associated Lipids Using LC-MS/MS and 16S rRNA Sequence Analyses. iScience 2020, 23, 101841. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.C.; Mitchell, T.W.; Harman, D.G.; Deeley, J.M.; Nealon, J.R.; Blanksby, S.J. Ozone-Induced Dissociation: Elucidation of Double Bond Position within Mass-Selected Lipid Ions. Anal. Chem. 2008, 80, 303–311. [Google Scholar] [CrossRef]
- Brown, S.H.J.; Mitchell, T.W.; Blanksby, S.J. Analysis of unsaturated lipids by ozone-induced dissociation. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2011, 1811, 807–817. [Google Scholar] [CrossRef]
- Poad, B.L.J.; Marshall, D.L.; Harazim, E.; Gupta, R.; Narreddula, V.R.; Young, R.S.E.; Duchoslav, E.; Campbell, J.L.; Broadbent, J.A.; Cvačka, J.; et al. Combining Charge-Switch Derivatization with Ozone-Induced Dissociation for Fatty Acid Analysis. J. Am. Soc. Mass Spectrom. 2019, 30, 2135–2143. [Google Scholar] [CrossRef]
- Pham, H.T.; Maccarone, A.T.; Thomas, M.C.; Campbell, J.L.; Mitchell, T.W.; Blanksby, S.J. Structural characterization of glycerophospholipids by combinations of ozone-and collision-induced dissociation mass spectrometry: The next step towards “top-down” lipidomics. Analyst 2014, 139, 204–214. [Google Scholar] [CrossRef]
- Marshall, D.L.; Saville, J.T.; Maccarone, A.T.; Ailuri, R.; Kelso, M.J.; Mitchell, T.W.; Blanksby, S.J. Determination of ester position in isomeric (O-acyl)-hydroxy fatty acids by ion trap mass spectrometry. Rapid Commun. Mass Spectrom. 2016, 30, 2351–2359. [Google Scholar] [CrossRef]
- Marshall, D.L.; Pham, H.T.; Bhujel, M.; Chin, J.S.R.; Yew, J.Y.; Mori, K.; Mitchell, T.W.; Blanksby, S.J. Sequential Collision- and Ozone-Induced Dissociation Enables Assignment of Relative Acyl Chain Position in Triacylglycerols. Anal. Chem. 2016, 88, 2685–2692. [Google Scholar] [CrossRef]
- Kozlowski, R.L.; Mitchell, T.W.; Blanksby, S.J. Separation and Identification of Phosphatidylcholine Regioisomers by Combining Liquid Chromatography with a Fusion of Collision-and Ozone-Induced Dissociation. Eur. J. Mass Spectrom. 2015, 21, 191–200. [Google Scholar] [CrossRef]
- Liebisch, G.; Fahy, E.; Aoki, J. Update on LIPID MAPS classification, nomenclature, and shorthand notation for MS-derived lipid structures. J. Lipid Res. 2020, 61, 1539–1555. [Google Scholar] [CrossRef]
- Machara, A.; Křivánek, J.; Dolejšová, K.; Havlíčková, J.; Bednárová, L.; Hanus, R.; Kyjaková, P. Identification and Enantiodivergent Synthesis of (5Z,9S)-Tetradec-5-en-9-olide, a Queen-Specific Volatile of the Termite Silvestritermes minutus. J. Nat. Prod. 2018, 81, 2266–2274. [Google Scholar] [CrossRef]
- Tupec, M.; Culka, M.; Machara, A.; Macháček, S.; Bím, D.; Svatoš, A.; Rulíšek, L.; Pichová, I. Understanding desaturation/hydroxylation activity of castor stearoyl Δ9-Desaturase through rational mutagenesis. Comput. Struct. Biotechnol. J. 2022, 20, 1378–1388. [Google Scholar] [CrossRef]
- Poad, B.L.; Young, R.S.; Marshall, D.L.; Trevitt, A.J.; Blanksby, S.J. Accelerating Ozonolysis Reactions Using Supplemental RF-Activation of Ions in a Linear Ion Trap Mass Spectrometer. Anal. Chem. 2022, 94, 3897–3903. [Google Scholar] [CrossRef]
- McAnoy, A.M.; Wu, C.C.; Murphy, R.C. Direct qualitative analysis of triacylglycerols by electrospray mass spectrometry using a linear ion trap. J. Am. Soc. Mass Spectrom. 2005, 16, 1498–1509. [Google Scholar] [CrossRef]
- Dorschel, C.A. Characterization of the TAG of peanut oil by electrospray LC-MS-MS. J. Am. Oil Chem. Soc. 2002, 79, 749–753. [Google Scholar] [CrossRef]
- Murphy, R.C. Tandem Mass Spectrometry of Lipids: Molecular Analysis of Complex Lipids; Royal Society of Chemistry: Cambridge, UK, 2015. [Google Scholar]
- Hsu, F.F.; Turk, J. Structural characterization of triacylglycerols as lithiated adducts by electrospray ionization mass spectrometry using low-energy collisionally activated dissociation on a triple stage quadrupole instrument. J. Am. Soc. Mass Spectrom. 1999, 10, 587–599. [Google Scholar] [CrossRef]
- Tran, A.; Wan, L.; Xu, Z.; Haro, J.M.; Li, B.; Jones, J.W. Lithium Hydroxide Hydrolysis Combined with MALDI TOF Mass Spectrometry for Rapid Sphingolipid Detection. J. Am. Soc. Mass Spectrom. 2021, 32, 289–300. [Google Scholar] [CrossRef]
- Hsu, F.-F.; Bohrer, A.; Turk, J. Formation of lithiated adducts of glycerophosphocholine lipids facilitates their identification by electrospray ionization tandem mass spectrometry. J. Am. Soc. Mass Spectrom. 1998, 9, 516–526. [Google Scholar] [CrossRef]
- Young, R.S.E.; Flakelar, C.L.; Narreddula, V.R.; Jekimovs, L.J.; Menzel, J.P.; Poad, B.L.J.; Blanksby, S.J. Identification of Carbon-Carbon Double Bond Stereochemistry in Unsaturated Fatty Acids by Charge-Remote Fragmentation of Fixed-Charge Derivatives. Anal. Chem. 2022, 94, 16180–16188. [Google Scholar] [CrossRef] [PubMed]
- Buenger, E.W.; Reid, G.E. Shedding light on isomeric FAHFA lipid structures using 213 nm ultraviolet photodissociation mass spectrometry. Eur. J. Mass Spectrom. 2020, 26, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Herrera, L.C.; Potvin, M.A.; Melanson, J.E. Quantitative analysis of positional isomers of triacylglycerols via electrospray ionization tandem mass spectrometry of sodiated adducts. Rapid Commun. Mass Spectrom. 2010, 24, 2745–2752. [Google Scholar] [CrossRef] [PubMed]
- Laakso, P. Mass spectrometry of triacylglycerols. Eur. J. Lipid Sci. Technol. 2002, 104, 43–49. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cudlman, L.; Machara, A.; Vrkoslav, V.; Polášek, M.; Bosáková, Z.; Blanksby, S.J.; Cvačka, J. Characterization of Triacylglycerol Estolide Isomers Using High-Resolution Tandem Mass Spectrometry with Nanoelectrospray Ionization. Biomolecules 2023, 13, 475. https://doi.org/10.3390/biom13030475
Cudlman L, Machara A, Vrkoslav V, Polášek M, Bosáková Z, Blanksby SJ, Cvačka J. Characterization of Triacylglycerol Estolide Isomers Using High-Resolution Tandem Mass Spectrometry with Nanoelectrospray Ionization. Biomolecules. 2023; 13(3):475. https://doi.org/10.3390/biom13030475
Chicago/Turabian StyleCudlman, Lukáš, Aleš Machara, Vladimír Vrkoslav, Miroslav Polášek, Zuzana Bosáková, Stephen J. Blanksby, and Josef Cvačka. 2023. "Characterization of Triacylglycerol Estolide Isomers Using High-Resolution Tandem Mass Spectrometry with Nanoelectrospray Ionization" Biomolecules 13, no. 3: 475. https://doi.org/10.3390/biom13030475
APA StyleCudlman, L., Machara, A., Vrkoslav, V., Polášek, M., Bosáková, Z., Blanksby, S. J., & Cvačka, J. (2023). Characterization of Triacylglycerol Estolide Isomers Using High-Resolution Tandem Mass Spectrometry with Nanoelectrospray Ionization. Biomolecules, 13(3), 475. https://doi.org/10.3390/biom13030475






