Abstract
Glycosphingolipids comprise a lipid class characterized by the presence of sugar moieties attached to a ceramide backbone. The role of glycosphingolipids in pathophysiology has gained relevance in recent years in parallel with the development of analytical technologies. Within this vast family of molecules, gangliosides modified by acetylation represent a minority. Described for the first time in the 1980s, their relation to pathologies has resulted in increased interest in their function in normal and diseased cells. This review presents the state of the art on 9-O acetylated gangliosides and their link to cellular disorders.
1. Discovery and Chemistry
Glycosphingolipids constitute a subcategory of sphingolipids in which a ceramide backbone is linked to one or more sugar residues. Among glycosphingolipids, gangliosides contain at least one residue of sialic acid, anciently known as neuraminic acid (Figure 1). Gangliosides are subdivided according to the number of sialic acid residues, e.g., monosialylated (GM), disialylated (GD), and trisialylated (GT), and further classified according to the number of neutral sugar residues subtracted from a maximum of five (e.g., GD1 contains four neutral residues, where “1” indicates 5-4 = 1) (Table 1). The sialic acid moiety contained in the ganglioside molecule can present structural modifications, such as acetylation. This modification can be present in other biomolecules containing sialic acid residues, such as glycoproteins.
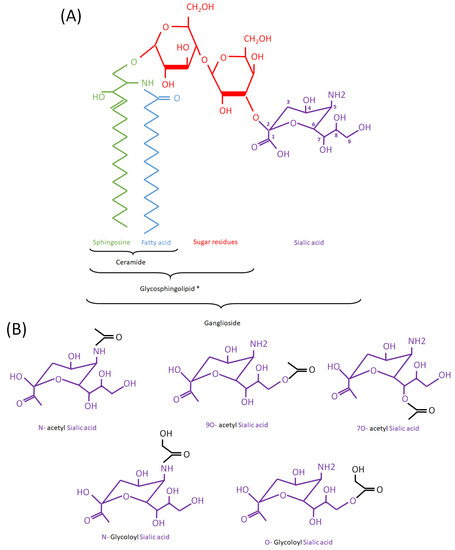
Figure 1.
(Acetyl/Glycoloyl)-Ganglioside structure. (A): Schematic representation of GM3 as an example of ganglioside. * To note: gangliosides are a type of glycosphingolipid but neither gangliosides nor glycosphingolipids are considered as types of ceramides. Ceramide is a structural component of all glycosphingolipids (including gangliosides). Sialic acid carbons are numbered as 1 to 9, starting from the left side of the molecule. (B): Different types of sialic acid modifications in mammalian gangliosides mentioned in the text. N-acetylated (acetyl groups, in black, bound to the N atom) and O-acetylated forms (bound to an O atom) are represented on the upper part. A N-glycoloylated (a glycoloyl group, in black, bound to the N atom) and O-glycoloyl (bound to the O atom) are represented on the lower part.

Table 1.
Main structural characteristics of the gangliosides cited in the text.
1.1. Types of Acetylation and First Findings in Cells
Modifications of sialic acid were first discovered in the secreted products of submandibular glands from cattle [1]. Those include O-glycoloyl, N-glycoloyl, O-acetyl and N-acetyl forms, where glycoloyl and acetyl groups are formed by the hydroxylation and acetylation of sialic acid, respectively (Figure 1). The acetyl and glycoloyl transferase activities necessary to ensure these modifications were found in cytosolic and microsomal extracts from these tissues [2,3]. The O-acetyl transferase reaction conveying the acetyl group to the sialic acid moiety (sialate O-acetyl transferase (SiAOAT) activity) has been recently attributed to the enzyme CASD1 (CAS1 domain containing) by means of genome-editing approaches [4]. This acetylation can be reversed by the 9-O-acetylesterase or sialidase activity (SIAE), found in several microorganisms and mammal brain tissue and resulting in the release of acetyl residues (Figure 2) [5,6,7,8]. Interestingly, the presence of a 9-O acetyl group in sialic acid can have an impact on the activity of sialidases, which remove sialic acid from larger molecules [9].
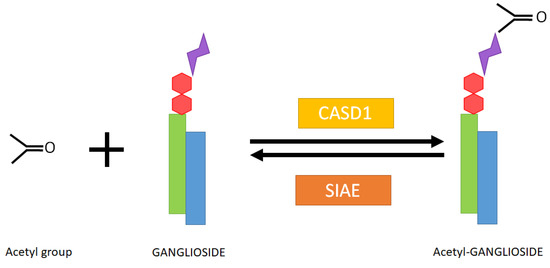
Figure 2.
Enzymatic conversion of a ganglioside to its acetylated form and responsible enzymes in humans. CASD1: CAS1 domain containing (Uniprot ref. Q96PB1). SIAE: Sialate O-acetylesterase (Uniprot ref. Q9HAT2). Green rectangle: sphingoid base. Blue rectangle: Fatty acyl chain. Red hexagons: neutral sugar residues. Purple double triangle: sialic acid residue.
Sialic acid O-acetylation can be present both in proteins and lipids. Membrane-bound acetyl-transferase activity was found to be associated with the modification of endogenous glycoprotein-bound sialic acids, while soluble activity was linked to the modification of exogenous, non-glycosidically bound sialic acids. This finding was further extended to brain tissue from pigs and cows [10]. These first discoveries did not make the distinction between protein-bound and lipid-bound acetylated sialic acids. The first isolation of a ganglioside containing 9-O acetylation was obtained in a mouse brain, within trisialo-ganglioside [11] and tetrasialo-ganglioside [12] structures. This was followed by guinea pig kidney [13], bovine buttermilk [14,15], codfish brain [16,17], rat and equine erythrocytes [18,19], as well as less common species, such as feather starfish [20]. In rat erythrocytes, a combination of thin-layer chromatography, gas chromatography, and an enzymatic treatment with Vibrio cholerae sialidase could identify GD1a (GD1 of the “a” series, bearing 1 sialic acid on the galactose in position II; 0-, b- and c-series bearing 0, 2 and 3, respectively) (Table 1) and not GM1 as the main ganglioside containing this modification. In equine erythrocytes, NMR and fast atom bombardment mass spectrometry (FABMS) could identify 9-O-acetyl-GM3 (9-O-acGM3) [19]. In human tissue, an analysis in a normal thyroid gland resulted in the identification of a potential presence of 9-O acetyl gangliosides, defined as containing alkali-labile sialic acid [21]. Additionally, an antibody claimed to recognize 9-O acetylated GD3 (9-O-acGD3) was able to bind normal human melanocytes [22], and so did another one isolated from melanoma cells [23]. This newly detected form was characterized using NMR and FABMS and further found in other species and tissues, such as rainbow trout, where it accounts for 23% of total gangliosides [24,25]. Finally, an acetylated trisialylated form, 9-O-acGT2, was first identified in cod brain [16].
1.2. Chemical Structure and Interactions
In GD1a, the N-acetylated sialic acid is linked to the outer galactose residue [26]. Conformational studies have been performed through molecular dynamics modeling and NMR on 9-O-acGD1a, concluding that acetylation does not modify the overall conformation of the ganglioside [26]. Specific interaction with a purified IgG fraction from human serum was suggested by the same study. More recently, a study on GM3 indicated that neither 9-O-acetylation nor 9-N-acetylation induces significant conformational changes on dihedral angles or the secondary structure, those being limited to the sialic acid glycerol chain and confirming structural similarities between both forms [27].
Concerning the composition in terms of sphingoid bases and acyl chains, this varies among species and no particular association with 9-O acetylation can be inferred from the scarce data available. Studies made on bovine buttermilk O-acetylated gangliosides have revealed C18-sphingosine as the sphingoid base and C18:0, C22:0, C23:0 and C24:0 as the main fatty acyl chains [15]. In rainbow trout ovarian fluid, the structure differs, as it contains 4-sphingenine as a sphingoid base, and C24:1 among fatty acids [25]. In another fish, mullet milt, 9-O-acGM3 is the predominant, acetylated form, containing mostly C18:1/C16:0 fatty acids [28]. In feather starfish, C16 sphingosine is accompanied by C22:0 or C24:0 as the most common acyl chains in an N-acetylated form [20].
1.3. Enzyme Regulation
Sialic acid O-acetylation appears as a cell-specific and developmentally regulated process. This is based on tightly regulated activity of 9-O-acetyltransferases. Pioneering studies indicate that sialyltransferase action regulates the expression of O-acyltransferases [29]. The cloning of this sialyltransferase (sialate-O-acetyltransferase, CASD1) was an elusive task. In one of the attempts, an open reading frame corresponding to a truncated form of the GC Vitamin-D-binding protein (VDBP) was found specifically responsible for sialic acid 9-O-acetylation of glycoproteins, while a fusion protein between a bacterial tetracycline resistance gene repressor and a sequence of the P3 plasmid (Tetrfusion) was able to acetylate gangliosides [30]. An interesting observation is that the product of O-acetylation makes the sialic acid moiety resistant to sialidase [31], which could have functional implications. Additionally, the natural forms of acetylated GD3, a disialylated ganglioside, present the modification at the terminal sialic acid moiety, as compared to synthetic forms [32]. In another study, it was shown that O-acetyltransferases use preferentially di- and tri-sialogangliosides as substrates rather than mono-sialogangliosides [33]. Acetyltransferase activity on GD3 (9-O-acGD3) is unchanged by the endoplasmic reticulum-to-Golgi transfer stimulator brefeldin A, suggesting that the activity resides in the same Golgi compartment as GD3 synthase, which is not the case for 9-O-acGD2 synthesis [34]. This suggests different compartments and potentially different enzymes for GD3 and GD2 modification. Nevertheless, 9-O-acGD2 can be synthesized either from GD2 by acetylation or from 9-O-acGD3 by glycosylation. It must be noted that the biosynthesis of 9-O-acetylated gangliosides requires a transfer of the acetyl group from acetyl-CoA. The Acatn acetyl-CoA transporter was identified in mice as intervening in this process and being mainly expressed during embryogenesis [35].
The 9-O-acetylation of GD3 has been proposed to be induced in Chinese hamster ovary (CHO) cells by the stable expression of its precursor, GD3, through activation of the Tis21 gene [36]. Moreover, when cells are incubated in the presence of exogenous GD3, cellular 9-O-acGD3 is detected after 6 h and a half-life of 24 h is observed, suggesting the induction of the biosynthetic enzymatic machinery. This process, also reported in human fibroblasts, is inhibited by blocking the clathrin-mediated internalization of GD3 [37]. Conversely, Tis21 does not seem to be involved in the upregulation of 9-O-acGD3 synthesis that occurs in a GM2/GD2 synthase knockout mouse model to compensate for the lack of complex gangliosides [38]. In this model, Vitamin D receptor and acetyl CoA transporter are not upregulated, suggesting an alternative mechanism of synthesis.
Reports on pharmacological agents exerting an impact on these synthesis reactions are scarce. In one of the few examples, it has been shown that salicylate leads to the deacetylation of gangliosides [39]. Additionally, cytidinmonophosphate-sialic acid and acetyl-CoA inhibit in vitro sialyl transferase activity [40].
In addition to enzyme activity, the regulation of enzyme expression must be considered. To date, no precise regulatory mechanisms for CASD1 or SIAE expression based on experimental evidence have been published. Nevertheless, their promoters are defined in the Ensembl database and several transcription factor binding sites have been confirmed in numerous cell lines via ChIP-seq within the ENCODE project (Tables S1 and S2). In addition, both promoters contain a CpG island (108 CpG in the CASD1 promoter and 50 CpG in the SIAE promoter) (Figure S1). Interestingly, SIAE mRNA transcriptional variant 2 sequence starts upstream from its CpG island location, maybe as part of a mechanism to avoid silencing by methylation. Although the regulatory landscape of these two genes currently remains unknown, according to the Protein Atlas, endocrine tissues present the highest CASD1 mRNA expression, followed by the eye and digestive tract, while the protein has been found in high abundance also in the brain, pancreas, reproductive tissues, bone marrow and lymphoid tissues [https://www.proteinatlas.org/ENSG00000127995-CASD1/tissue (accessed on 3 May 2023)]. SIAE mRNA shows the highest expression level in the gastrointestinal tract, while the highest protein expression corresponds to the brain, endocrine tissue, urinary system, male tissues and bone marrow and lymphoid tissues [https://www.proteinatlas.org/ENSG00000110013-SIAE/tissue (accessed on 3 May 2023)].
1.4. Methodological Points
Early studies and many of the follow up works have been based on the detection of this type of modified ganglioside using monoclonal antibodies in combination with thin-layer chromatography (TLC) or immunohistochemistry (IHC). The so-called JONES, VIM-2 [41], 13A and 27A [42], UM4D4 [43], CDW60 [44], and MT6004 [45] antibodies have been shown to detect 9-O-acGD3, while the SGR37 monoclonal antibody distinctly detects the de-N-acetyl form of GD3 [46]. It must be pointed out, though, that targeting lipid antigens in IHC can be seriously impacted by the use of organic solvents for fixation and deparaffination, such as acetone and xylol, respectively. Special care must be taken, as an incorrect fixation protocol is likely to induce artifactual results [47].
The specific binding of influenza C virus has also been considered as the basis of detection methods. This microorganism presents a higher affinity for 9-O-ac and a lower affinity for 7-O-ac glycoconjugates [48,49], regardless of the nature of the core moiety (lipid or protein). Virus binding is also able to discriminate monoacetylated sialic acids from polyacetylated [48]. As a consequence, recombinant soluble influenza C hemagglutinin has been used to characterize 9-O-acetyl sialylation [50]. Other molecules recognizing 9-acetylated sialic acid and displaying a specificity for gangliosides are monocyte ficolins, highly conserved oligomeric lectins involved in innate immunity [51].
As explained above, chemical characterization has been mainly based on NMR and FABMS. Finally, the evaluation of sialyl transferase and SIAE enzymatic activities has added a functional dimension to some studies [52].
2. 9-O Acetylation of Gangliosides in Pathophysiology
2.1. In Cell Physiology
2.1.1. Embryogenesis
Human embryonic stem cells present a high abundance of 9-O-acGD3 that generally decreases alongside differentiation [53,54]. A particular type of cancer cell (NTERA-2, a human embryonic carcinoma line) has been used to study the ontogeny of glycolipids in association with cell differentiation during embryonic development. In this model, ganglio-series, including 9-O-ac forms, replaced globo-series (glycosphingolipids containing at least two neutral sugar residues and no sialic acid) when differentiation was induced with retinoic acid [55].
These molecules have been mainly studied in the context of nervous system development. In particular, the presence of 9-O-acGD3 has been shown in neuroepithelial precursor cells [56]. An antigen expressed during neural development was identified as 9-O-acGD3 [57]. In developing rat retina, the pattern of 9-O-acGD3 and that of its precursor GD3 were determined by the reactivity to several monoclonal antibodies (JONES, R24). The two patterns differed; in the case of the 9-O acetylated form, a rise was found between day E15 and postnatal day 2, with a pronounced drop between postnatal days 2 and 4 [58]. 9-O-acGD3 has also been found in primary cultures of both neurons and glia (reviewed in [59]). In freshly dissociated retinal cells, 9-O-acGD3 was found to be present on amacrine photoreceptors and in ganglion cells [58]. In a chick embryo, a monoclonal antibody (8A2) allowed detecting 9-O-ac gangliosides in the optic fiber layer of the central retina [60]. Another study based on monoclonal antibody staining and on sialidase sensitivity concluded that a 9-O-ac form of GT3 (ganglioside C series) was also increased in rat cerebral cortex at day 14 of gestation, then progressively decreased and was absent in adult rats [61], along with its 9-O-acGD3 counterpart [62].
In the developing rat nervous system, acetylated gangliosides have been associated with regions characterized by cell migration [63], such as the olfactory epithelium, where they are involved in the formation of the mature olfactory bulb [64] and the hippocampus [65]. They were detected in relation to the cell stream migrating from the lateral ventricle rostral subventricular zone to the olfactory bulb, suggesting a function in cell migration [66]. These gangliosides were also isolated from 10-day embryonic chicken brain [67]. Concerning their cellular function, there is evidence that 9-O acetylated gangliosides play a role in the extension of growth cones in neurites [68], along with a regulation of the microfilament and microtubular structure of their cytoskeleton, probably modulating cell motility [69]. The same authors found 9-O-acGD3 localized to contact points of neural growth cones, associated with beta-1-integrin and vinculin [70].
The functional relevance during embryogenesis of the 9-O acetylation of sialic acid was studied through the generation of a transgenic mouse model overexpressing the sialic acid-specific acetylesterase of influenza C virus under the control of the metallothionein promoter [71]. This resulted in an arrest of development at the 2-cell stage. Using the phenylethanolamine-N-methyltransferase promoter, the authors induced expression in the retina and adrenal gland, leading to an impaired morphology and function of these organs.
2.1.2. Postnatal Nervous System
The nervous system is generally rich in gangliosides, including 9-O-acGD3. In a mouse model constitutively knocked out for GM2/GD2 synthase, the lack of complex gangliosides is compensated by an accumulation of the precursors, namely GM3 and GD3, in nervous tissue [72]. This accumulation also includes 9-O-acGD3, suggesting that this molecule can take over some of the functions of the absent glycosphingolipids [38]. In postnatal rat retina, a dorsal–ventral gradient of 9-O-acGD3 has been reported, an observation based on the JONES monoclonal antibody [58], as well as in the adult olfactory bulb, but at lower levels than in the developing nervous system [66]. In a chicken, 9-O acetylated gangliosides were no longer detected in the adult in the central optic fiber. In contrast, they would remain in the inner and outer plexiform layer, and in the outer nuclear layer [60]. Likewise, 9-O-ac gangliosides have been found to be absent in rat adult hippocampus [65]. In primary cell cultures from the retina, they are present in the retinal ganglion but not in Muller cells [60]. In the rat subventricular zone, the presence of 9-O-acGD3 has been demonstrated from neural stem and progenitor cells to the adult brain [73]. To add insight into the subcellular distribution of these molecules, in olfactory ensheathing glia from rats, 9-O-acGD3 has been identified in membrane rafts [74].
With respect to the potential function of these molecules in the nervous system, in cerebellar astroglia isolated from rats, JONES staining was found in the contact sites of migrating granule cells and in radial glia when cultured in the presence of neurons [63,75]. Another study suggested a role in the regulation of both neuronophilic and gliophilic migration [76]. Staining is also present in neurons and glia involved in the axonal regeneration of the sciatic nerve in adult rats [77], which is defective in GD3 synthase knockout mice [78]. The same antibody blocks migration in a dose-dependent manner, adding evidence to the participation of 9-O-acetyl gangliosides in granule cell migration [75,79] through a calcium-signaling mechanism involving PY2 receptors [80]. Anti-9-O-acGD3 antibody-based inhibition of olfactory ensheathing glia migration has been observed in organotypical cultures [81]; the inhibition of neuronal migration has been shown in vivo in normal mice [82,83] and confirmed through videomicroscopy [84], while migration was also blocked by a broad inhibitor of ganglioside synthesis (D-threo-1-phenyl-2-palmitoylamino-3-pyrrolidino-1-propanol, an inhibitor of the ganglioside precursor glucosylceramide) [84]. However, the fact that antibody-based inhibition also occurs in GD3 synthase knockout mice, which are not supposed to contain the acetylated derivative, suggests that the antibody inhibits migration through an alternative mechanism, while it also raises questions on its specificity [83]. Nevertheless, sciatic regeneration is perturbed in this mouse model and rescued by the administration of exogenous GD3, which supports a genuine role for downstream-generated gangliosides [78].
9-O-acetylated glycolipids have been detected in mammalian cerebellar Purkinje cells [85], where they occupy the rostral lobes in mice [86]. They mostly mark the late-onset sagittal banding patterns [87]. Interestingly, in the so-called nervous mutation model of mouse Purkinje cells, the surviving mutant cells in the cerebellum correspond to those positive for 9-O-acetylated gangliosides [86], mainly corresponding to 9-O-acGD3 [88].
2.1.3. Immune System
Some glycolipid antigens at the surface of T lymphocytes were initially recognized by monoclonal antibodies and defined as CDw60. These molecules have been shown to induce costimulatory signals. The CDw60 antigen, also recognized by influenza C virus glycoprotein, was characterized as 9-O-acGD3 [89]. T lymphocytes (mostly CD4+) and granulocytes present high amounts of this CD60 antigen, in contrast to the low levels present in B cells, thymus cells and monocytes [90]. It was estimated that about 25% of peripheral T cells present a surface localization of CD60, while roughly all T cells express modest amounts intracellularly in Golgi vesicles [91]. In an early report, a subtype of CD8+ T cells, also expressing CD60—a so-called a T helper CD8+ CD60+ subset—was claimed to provide help to B cells, while CD8+ CD60- suppressed B cell differentiation. Both populations produced IL-2 equally, but CD60+ would secrete more IL-4 and less interferon gamma [92]. In spite of the low levels initially reported, CD60 has been proposed as an activation marker of human B cells, as peripheral and tonsillar B cells become CD60+ when activated by phorbol esters [93]. It must be pointed out that another acetylated form of GD3, 7-O-acGD3, was also found in human leukocytes, recognized by a specific monoclonal antibody that induced cell proliferation [94]. T cell receptor (TCR) activation results in the decreased presence of detectable 9-O-acetyl sialic acid at the surface of T cells, but this is mostly due to decreased sialomucins, which also contain this residue, and not necessarily to gangliosides [50]. In peripheral blood mononuclear cells (PBMC), treatment with a monoclonal antibody targeting 9-O-acGD3, but not with another one against non-acetylated GD3, was able to induce phosphorylation of the spleen tyrosine kinase (Syk, p72), involved in T and B cell receptor signal transduction, resulting in phosphoinositide mobilization and cell proliferation [95].
Following subsequent studies, CD60 was subdivided into CD60a (GD3), CD60b (the O-acetylated form), and CD60c (the N-acetylated form) [96]. The CD60b form was found present in tonsillar B cells in the activated germinal center, colocalizing in lipid rafts with Syk and Lyn, in line with previous results [93,95]. Hence, B cells can be costimulated by anti-CD60b and anti-IgM/IL-4. Extrafollicular T cells also present with CD60b and can be costimulated with anti-CD60 and phytohemagglutinin (PHA). Conversely, anti-CD60c—recognizing the N-acetylated form—has been found to be sufficient to induce proliferation [96]. In a thorough study on the presence of the three CD60 forms during the differentiation of T cells and B cells, CD4+ cells showed the strongest, and CD8+ cells the weakest, presence of CD60b at the surface in thymocytes. Both T and B cells presented CD60b staining in a patchy fashion as compared to the other forms. Interestingly, subcellular distribution studies following biochemical methods showed 9-O-acGD3 mainly localized to non-raft microdomains in T cells and to raft microdomains in B cells [45].
2.1.4. Hematopoiesis
In human bone marrow, erythroid progenitors are rich in 9-O-acGD3, but the molecule is progressively lost during maturation, becoming proapoptotic in mature erythrocytes [97]. The presence of 9-O-acGD3 in lymphoid and erythroid cells is reviewed in [98].
2.1.5. Kidney
Cultured visceral glomerular epithelial cells, podocytes, contain the specific epitope 9-O-acGD3 recognized by several monoclonal antibodies, such as 13A and 27A. The latter could immunoprecipitate with a noncharacterized podocyte protein [42]. This epitope was found by the 27A antibody to colocalize in podocyte lipid rafts with nephrin, a protein present in the slit diaphragm, a structure responsible for the podocyte intercellular interaction and a main constituent of the glomerular filtration barrier. These seminal works indicate the importance of this modified ganglioside in the physiology and function of the glomerular barrier [99].
2.2. In Cell Pathology—Diseases
2.2.1. Cancer
The 9-O-acetylation of gangliosides has been extensively associated with cancer, and even considered as a marker of cell and tissue growth [100]. Very early studies on melanoma cells found in extracts a thin-layer chromatography band comigrating with 9-O-acetylated gangliosides [101]. It was estimated that 10% of gangliosides in melanoma cells presented this modification. These modified sialic acids, independently of their associated moiety—either protein or sphingolipid—were recognized by a monoclonal antibody prepared against the rat brain tumor cell line B49. In another study, chromatographic comigration with GD3 was found in cell extracts after isolation with a monoclonal antibody derived from the immunization of mice with WM164 melanoma cells [23]. It was estimated that all nevus cell lines and one third of melanoma cell lines were positive to an antibody detecting this modification, which was also found in lymphocytes infiltrating 30% of tumors. Ever since, 9-O-acGD3 has been considered as a melanoma antigen [57,102,103,104], as has 9-O-acGD2 [105]. When evaluating different stages of Bomirski melanomas, 9-O-acGD3 was found increased in the amelanotic, fast-growing stage, as compared with the slow-growing, highly differentiated forms [106], suggesting a role for the molecule in cell growth. Its presence in nodular melanoma has been found to be greater than in metastatic acral lentiginous melanoma [107]. However, it has not been found present in uveal melanoma [108,109], which may indicate that the acetylated varieties are characteristic of metastatic forms (cutaneous) as compared with non-metastatic (uveal). Interestingly, while other gangliosides, such as GD2 and GD3, have been found to be increased in the serum of melanoma patients, this is not the case for 9-O-acGD3 [110].
In hamster melanoma, the O-acetylated form of GD3 was characterized as 7-O instead of the human 9-O. The structure of the former is not very different from that of buttermilk ganglioside, as it contains C18:0 sphingosine and a slightly different fatty acid composition: C16:0, C18:0, C20:0, C22:0 and C24:0 [111]. In human melanoma, a quite-high presence of C24:1 has been reported in both the 9-O-acGD3 and the GD3 precursor [23,112]. Melanoma cells also display de-N-acGD3 (resulting from the loss of the 5-N-acetyl group), with an intracellular and non-lysosomal distribution [46]. In this case, the main esterifying fatty acids are C16:0 and C18:0 [112].
In mouse erythroleukemia cells, 9-O-acGD3 is also present but not detectable at the surface, where 9-O-acetyl sialic acid is associated with sialomucins [113]. In lymphoblasts from acute lymphoblastic leukemia patients, 9-O-acGD3 levels are increased [114]. Increased SiAOAT enzymatic activity was detected in the microsomes of these cells. The activity was found to be higher at diagnosis and decreased in remission, whereas SIAE activity is down in the cytosol and in lysosomes [40,52]. In Sézary syndrome, a very aggressive leukemic form of cutaneous T cell lymphoma, circulating levels of CD60b (9-O-acGD3)-positive T cells were found to be associated with a poor prognosis [115].
9-O acGD3, along with other gangliosides, has been proposed as a marker of several neuroectodermal cancers. For example, it was detected in basal cell carcinoma cells and found to be dramatically increased as compared to normal epidermis or dermis [116,117]. It has been suggested as a marker of small-cell lung cancer [118]. Studies in breast tissue have demonstrated the presence of CD60 antigen in the Golgi apparatus of normal ductal cells, and increased in atypical hyperplasia and other benign lesions, as well as in mammary carcinoma cells [119]. In well-differentiated and invasive duct carcinoma, the antigen, identified as 9-O-acGD3, was found mostly present at the surface, with decreased presence in nondifferentiated carcinomas [119]. In some breast cancer cell lines (Hs 578T and SUM159PT), 9-O-acGD2 but not 9-O-acGD3 has been identified [120], and CASD1 has been demonstrated as the enzyme responsible for its synthesis [121]. Both GD3 and 9-O-acGD3 were detected and increased in 13 neural tumor cell lines [122] and in glioblastoma, where a critical ratio between the two forms promoting tumor survival was established [123]. As a consequence of all these findings, the presence of acetylated gangliosides in blood as cancer biomarkers has been considered and specific testing by liquid chromatography–mass spectrometry on dry blood samples has been developed [124].
The link between the 9-O-acetylation of gangliosides and cancer is underlined by its effect on apoptosis. GD3 is considered as a proapoptotic agent, at least in vitro, while its 9-O acetylated form is shown as antiapoptotic [39,125,126]. The presence of 9-O-acGD3 in Jurkat and Molt-4 cells prevents cell death induced by proapoptotic agents such as N-acetyl sphingosine and daunorubicin [39]. Lymphoblasts from lymphoblastic leukemia patients accumulate 9-O-acGD3 in mitochondrial membranes [114]. Unlike GD3, exogenous 9-O-acGD3 prevents mitochondrial membrane depolarization, cytochrome C release and caspase activation in lymphoblasts [114]. Interestingly, 9-O-acGD1, also known as neurostatin, has antiproliferative effects on astrocytoma cells [127] and synthetic forms have been produced and approved as anticancer drugs [128]. The potential regulation of apoptosis by acetylated gangliosides (CD60) has been addressed in lymphocytes [96]. However, a hematopoiesis study conducted on human bone marrow revealed a proapoptotic impact of 9-O-acGD3 on mature erythrocytes, in contrast to its effect on lymphoblasts [97].
9-O acetyl-GD3 was consequently proposed as a potential target for immunotherapy [129,130]. The antibody response to injection in melanoma patients of 9-O-acGD3 extracted from buttermilk was studied, but the reactivity was not found antigen specific [131], which underlies the problem of the low immunogenicity of the molecule. This was improved by combining the antigen with very-low-density lipoproteins and enhanced by IL-2, which could be used as adjuvants [132]. 9-N-acGD2, used as a stable surrogate of 9-O-acGD2, has been also used as antigen, in this case conjugated with the carrier bacteriophage Qbeta, eliciting a strong and long lasting immune response in dog [133]. Interestingly, a high titer of anti-9-O-acGD3 antibodies has been found in the serum of medulloblastoma patients [122]. Finally, in glioblastoma cells, several strategies based on hemagglutinin esterase cleavage of the acetyl group have been explored [123].
2.2.2. Infection
Influenza C virus is known to infect cells through binding to N-acetyl-9-O-acetyl sialic acid, an ability that is shared with bovine coronavirus [134,135]. Treatment of cells with 9-O acetylesterase confers resistance to infection, which is reversed by treating cells with ganglioside preparations from bovine brain containing 9-O acetylated forms, suggesting 9-O-acetylated gangliosides as potential receptors for this pathogen [5]. Binding to 9-O-acGD1a has been demonstrated [136]. Conversely, influenza C virus is able to slowly hydrolyze in vitro 9-O-acGD1a [7] and 9-O-acGT3 [137], since the hemagglutinin encoded by the viral genome possesses a 9-O-acetyl sialic-acid-specific acetyl esterase activity [71]. Another pathogen, Mycobacterium leprae, invades Schwann cells with the help of endogenous 9-O-acGD3, which is also upregulated upon infection. Immunoblocking of the ganglioside reduces the demyelinization effect of the bacterium [138].
2.2.3. Autoimmune Diseases
9-O-acGD1b has been associated with Guillain–Barré syndrome, an autoimmune disorder characterized by the presence of anti-glycolipid antibodies in the blood. The serum of a subset of patients reacts with this modified ganglioside, along with the non-acetylated form, and with GM1, as found using ELISA and thin-layer chromatography immunostaining [139].
Psoriatic basal and suprabasal keratinocytes express 9-O-acGD3 at the surface, and the extent of expression is increased when these cells are subjected to material secreted by T cells isolated from the same lesions, suggesting that soluble factors secreted by T cells are responsible for this effect. In the same context, IL-4 and IL-13 induced the upregulation and interferon gamma downregulation of the ganglioside, while the upregulation effect was reduced by an anti-IL-13 antibody [43].
2.2.4. Toxicology
Lead exposure has been associated with increased detection of several gangliosides in kidney, including 9-O-acGD3 in glomeruli, using monoclonal antibodies and confirmed with thin-layer chromatography [44]. This was suggested by the authors of the work to constitute a marker of lead exposure and to be associated with a dysregulation of apoptosis, in that high levels of 9-O-acGD3 in glomeruli were correlated with a lower number of apoptotic cells in the kidney.
3. Concluding Remarks: From Controversy to Future Prospects
The fact that detection systems target the acetylated sialic acid moiety, present in both gangliosides and glycoproteins, leads to the ambiguous interpretation of many results in the absence of further biochemical characterization. Thus, a thorough study on the expression of CD60 antigen in T cells and melanoma cells led to the conclusion that it corresponds mostly to a glycoprotein marker in the former and a glycolipid in the latter [140]. Another example of this ambiguity is the reported recognition by the JONES antibody of β1-integrin in mouse cerebellum [83], which compromises some conclusions based on this particular tool. Considering these constraints, mass spectrometry emerges as the most reliable approach to search for the distribution and biological effects of 9-O-acetylated gangliosides.
Some points raised by previous works will need to be clarified, while others are as yet unexplored. For example, a basic question is the relationship between the cell cycle and 9-O-acetylated ganglioside synthesis. Another one is the subcellular distribution of these molecules. Previous studies have shown their presence in mitochondria, at the plasma membrane surface in and out of raft-like membrane microdomains; yet, to date, little is known about their function in these compartments. Conversely, their presence in the nucleus has not been explored.
Regarding the likely abundance of 9-O-ac gangliosides in membrane raft-like microdomains, a potential function as entry points to viral particles could be hypothesized. It has been shown that the sialic acid moieties of gangliosides, by means of their negative charge, determine the electrostatic potential and thereby impact the interaction of viruses, such as SARS-CoV-2 with host cells [141,142]. Interestingly, SARS-CoV-2 spike protein binds preferentially to 9-N-ac and 9-O-ac sialic acid [143]. It is tempting to hypothesize that the 9-O-acetylation of gangliosides changes the dynamics of virus–raft interaction and eventually virus entry. Whether this is the case and whether the mechanism involves a receptor-like or a change in electrostatic interaction remain to be clarified.
While a reasonable body of knowledge has been gathered for 9-O-acetylated gangliosides in the context of cancer, an aspect that has been insufficiently addressed is their implications in other pathologies, especially those accounting for alterations in lipid metabolism (i.e., cardiovascular disease, type 2 diabetes mellitus, and nonalcoholic fatty liver disease) or lipid storage disorders. Likewise, the presence of 9-O-acetylated gangliosides in circulating macromolecular structures, such as lipoproteins or extracellular vesicles, is currently unexplored (apart from the enhanced immunogenicity of 9-O-acGD3 when adsorbed onto very-low-density lipoproteins [132]).
Finally, in light of the available data summarized in this review (Table 2), a question arises on the levels of 9-O-ac gangliosides found in physiological and pathological conditions. As suggested by several studies, these molecules play a key role in cell survival and cell mobility. These two properties are relevant to cancer cells to avoid immune defense mechanisms and to propagate throughout the body. This would explain why some 9-O-ac gangliosides are overabundant in cancer cells, hereby displaying potential as cancer biomarkers. Nevertheless, these roles are also important in other cells in physiological conditions. Consequently, 9-O-ac gangliosides are not exclusive to cancer cells and their role as cancer biomarkers can be contested. For example, melanocytes increase their 9-O-acGD3 content during carcinogenesis. However, other cells in physiological conditions (e.g., podocytes, neuroblast cells, and lymphocytes) have been proven to contain the same molecule, which somehow represents a paradox. It can be hypothesized that their physiological/pathological role in cells depends on a combination of at least two parameters, namely abundance (as shown in [104]) and subcellular location. An additional parameter would be the ratio between 9-O-ac and nonacetylated counterparts [39,125,126], or between different types of acetylated forms (i.e., 9-O-ac, 7-O-ac, and N-ac). Even the fatty acyl chain esterifying the ceramide moiety could play a part [20,25,28]. This requires a global analysis of all ganglioside forms, and further underlines the importance of mass-spectrometry-based methods.

Table 2.
Synthesis of reported observations involving different 9-O-acetyl gangliosides in physiological and pathological conditions.
In conclusion, the results so far point towards a relevant role of 9-O-ac gangliosides in many tissues and cellular mechanisms. Nevertheless, the available information is highly fragmented and further systematic research will be necessary to pursue the understanding of this fascinating puzzle.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biom13050827/s1, Figure S1: Alignment of CASD1 and SIAE (B) NCBI transcript variants to human genome and its CpG Islands; Table S1: CASD1 promoter transcription factor binding sites; Table S2: SIAE promoter transcription factor binding sites.
Author Contributions
Conceptualization, L.V.H.-M., D.S. and M.O; writing—original draft preparation, L.V.H.-M. and M.O.; writing—review and editing, L.V.H.-M., D.S. and M.O. All authors have read and agreed to the published version of the manuscript.
Funding
Luis V Herrera-Marcos’s contract has been funded by the European Union’s NextGenerationEU budget through the Spanish University Ministry program Margarita Salas.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data contained in the supplementary material have been obtained from the Ensembl database (https://www.ensembl.org/Homo_sapiens/Regulation/Summary?db=core;fdb=funcgen;g=ENSG00000127995;r=7:94509219-94557019;rf=ENSR00000215297; https://www.ensembl.org/Homo_sapiens/Regulation/Summary?db=core;fdb=funcgen;g=ENSG00000110013;r=11:124633113-124695707;rf=ENSR00000046429); the UCSC genome database (http://genome.ucsc.edu/cgi-bin/hgTracks?db=hg19&lastVirtModeType=default&lastVirtModeExtraState=&virtModeType=default&virtMode=0&nonVirtPosition=&position=chr7%3A94139170%2D94186328&hgsid=1620065055_IR0bazPQThDkcqjiqyjTZvZZi1oC); and the Human Protein Atlas (https://www.proteinatlas.org/ENSG00000127995-CASD1/tissue; https://www.proteinatlas.org/ENSG00000110013-SIAE/tissue).
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| 7-O-ac: | 7-O-acetylated |
| 9-N-ac: | 9-N-acetylated |
| 9-O-ac: | 9-O-acetylated |
| 9-O-acLD1: | disialosyl-lacto-N-neotetraosylceramide (LD1) |
| CASD1: | CAS1 domain containing |
| CDw60 = CD60: | 9-O-acetylated GD3 antigen |
| CD60a: | GD3 (non-acetylated) antigen |
| CD60b: | 9-O-acGD3 antigen |
| CD60c: | 7-O-acGD3 antigen |
| CHE-FcD = hemagglutinin Esterase of Influenzavirus C fused to the carboxyl end with human IgG1 Fc region treated with diisopropylfluorophosphate to eradicate its esterase activity. | |
| CM: | confocal microscopy |
| FABMS: | fast atom bombardment mass spectrometry |
| IEM: | immunoelectron Microscopy. |
| IF: | Immunofluorescence |
| IHC: | Immunohistochemistry |
| IP: | Immunoprecipitation |
| N-ac: | N-acetylation |
| NMR: | nuclear magnetic resonance |
| PHA: | Phytohemagglutinin |
| SIAE: | sialate O-acetylesterase |
| SiAOAT: | sialate O-acetyltransferase |
| TLC: | thin-layer chromatography |
References
- Buscher, H.P.; Casals-Stenzel, J.; Schaufer, R. New Sialic Acids. Identification of N-Glycoloyl-O-Acetylneuraminic Acids and N-Acetyl-O-Glycoloylneuraminic Acids by Improved Methods for Detection of N-Acyl and O-Acyl Groups and by Gas-Liquid Chromatography. Eur. J. Biochem. 1974, 50, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Oehler, C.; Kopitz, J.; Cantz, M. Substrate Specificity and Inhibitor Studies of a Membrane-Bound Ganglioside Sialidase Isolated from Human Brain Tissue. Biol. Chem. 2002, 383, 1735–1742. [Google Scholar] [CrossRef] [PubMed]
- Corfield, A.P.; Ferreira do Amaral, C.; Wember, M.; Schauer, R. The Metabolism of O-Acyl-N-Acylneuraminic Acids. Biosynthesis of O-Acylated Sialic Acids in Bovine and Equine Submandibular Glands. Eur. J. Biochem. 1976, 68, 597–610. [Google Scholar] [CrossRef]
- Baumann, A.-M.T.; Bakkers, M.J.G.; Buettner, F.F.R.; Hartmann, M.; Grove, M.; Langereis, M.A.; de Groot, R.J.; Mühlenhoff, M. 9-O-Acetylation of Sialic Acids Is Catalysed by CASD1 via a Covalent Acetyl-Enzyme Intermediate. Nat. Commun. 2015, 6, 7673. [Google Scholar] [CrossRef] [PubMed]
- Herrler, G.; Klenk, H.D. The Surface Receptor Is a Major Determinant of the Cell Tropism of Influenza C Virus. Virology 1987, 159, 102–108. [Google Scholar] [CrossRef]
- Teufel, M.; Roggentin, P.; Schauer, R. Properties of Sialidase Isolated from Actinomyces Viscosus DSM 43798. Biol. Chem. Hoppe Seyler 1989, 370, 435–443. [Google Scholar] [CrossRef]
- Schauer, R.; Reuter, G.; Stoll, S.; Posadas del Rio, F.; Herrler, G.; Klenk, H.D. Isolation and Characterization of Sialate 9(4)-O-Acetylesterase from Influenza C Virus. Biol. Chem. Hoppe Seyler 1988, 369, 1121–1130. [Google Scholar] [CrossRef]
- Hasegawa, A.; Ogawa, H.; Ishida, H.; Kiso, M. Synthesis of an S-(Alpha-Sialosyl)-(2----9)-O-(Alpha-Sialosyl)-(2----3’)-Beta-Lactos Ylceramide. Carbohydr. Res. 1992, 224, 175–184. [Google Scholar] [CrossRef]
- Hunter, C.D.; Khanna, N.; Richards, M.R.; Rezaei Darestani, R.; Zou, C.; Klassen, J.S.; Cairo, C.W. Human Neuraminidase Isoenzymes Show Variable Activities for 9-O-Acetyl-Sialoside Substrates. ACS Chem. Biol. 2018, 13, 922–932. [Google Scholar] [CrossRef]
- Haverkamp, J.; Veh, R.W.; Sander, M.; Schauer, R.; Kamerling, J.P.; Vliegenthart, J.G. Demonstration of 9-O-Acetyl-N-Acetylneuraminic Acid in Brain Gangliosides from Various Vertebrates Including Man. Hoppe Seylers Z Physiol. Chem. 1977, 358, 1609–1612. [Google Scholar] [CrossRef]
- Ghidoni, R.; Sonnino, S.; Tettamanti, G.; Baumann, N.; Reuter, G.; Schauer, R. Isolation and Characterization of a Trisialoganglioside from Mouse Brain, Containing 9-O-Acetyl-N-Acetylneuraminic Acid. J. Biol. Chem. 1980, 255, 6990–6995. [Google Scholar] [CrossRef]
- Chigorno, V.; Sonnino, S.; Ghidoni, R.; Tettamanti, G. Isolation and Characterization of a Tetrasialoganglioside from Mouse Brain, Containing 9-O-Acetyl,N-Acetylneuraminic Acid. Neurochem. Int. 1982, 4, 531–539. [Google Scholar] [CrossRef]
- Hirabayashi, Y.; Li, Y.T.; Li, S.C. Occurrence of a New Hematoside in the Kidney of Guinea Pig. FEBS Lett. 1983, 161, 127–130. [Google Scholar] [CrossRef]
- Bonafede, D.M.; Macala, L.J.; Constantine-Paton, M.; Yu, R.K. Isolation and Characterization of Ganglioside 9-O-Acetyl-GD3 from Bovine Buttermilk. Lipids 1989, 24, 680–684. [Google Scholar] [CrossRef]
- Ren, S.; Scarsdale, J.N.; Ariga, T.; Zhang, Y.; Klein, R.A.; Hartmann, R.; Kushi, Y.; Egge, H.; Yu, R.K. O-Acetylated Gangliosides in Bovine Buttermilk. Characterization of 7-O-Acetyl, 9-O-Acetyl, and 7,9-Di-O-Acetyl GD3. J. Biol. Chem. 1992, 267, 12632–12638. [Google Scholar] [CrossRef]
- Waki, H.; Masuzawa, A.; Kon, K.; Ando, S. A New O-Acetylated Trisialoganglioside, 9-O-Acetyl GT2, in Cod Brain. J. Biochem. 1993, 114, 459–462. [Google Scholar] [CrossRef]
- Waki, H.; Murata, A.; Kon, K.; Maruyama, K.; Kimura, S.; Ogura, H.; Ando, S. Isolation and Characterization of a Trisialyllactosylceramide, GT3, Containing an O-Acetylated Sialic Acid in Cod Fish Brain. J. Biochem. 1993, 113, 502–507. [Google Scholar] [CrossRef]
- Gowda, D.C.; Reuter, G.; Shukla, A.K.; Schauer, R. Identification of a Disialoganglioside (GD1a) Containing Terminal N-Acetyl-9-O-Acetylneuraminic Acid in Rat Erythrocytes. Hoppe Seylers Z Physiol. Chem. 1984, 365, 1247–1253. [Google Scholar] [CrossRef]
- Yachida, Y.; Tsuchihashi, K.; Gasa, S. Characterization of Novel Mono-O-Acetylated GM3s Containing 9-O-Acetyl Sialic Acid and 6-O-Acetyl Galactose in Equine Erythrocytes. Glycoconj. J. 1996, 13, 225–233. [Google Scholar] [CrossRef]
- Inagaki, M.; Shiizaki, M.; Hiwatashi, T.; Miyamoto, T.; Higuchi, R. Constituents of Crinoidea. 5. Isolation and Structure of a New Glycosyl Inositolphosphoceramide-Type Ganglioside from the Feather Star Comanthina Schlegeli. Chem. Pharm. Bull. 2007, 55, 1649–1651. [Google Scholar] [CrossRef]
- Svennerholm, L. Gangliosides of Human Thyroid Gland. Biochim. Biophys. Acta 1985, 835, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Herlyn, M.; Thurin, J.; Balaban, G.; Bennicelli, J.L.; Herlyn, D.; Elder, D.E.; Bondi, E.; Guerry, D.; Nowell, P.; Clark, W.H. Characteristics of Cultured Human Melanocytes Isolated from Different Stages of Tumor Progression. Cancer Res. 1985, 45, 5670–5676. [Google Scholar] [PubMed]
- Thurin, J.; Herlyn, M.; Hindsgaul, O.; Strömberg, N.; Karlsson, K.A.; Elder, D.; Steplewski, Z.; Koprowski, H. Proton NMR and Fast-Atom Bombardment Mass Spectrometry Analysis of the Melanoma-Associated Ganglioside 9-O-Acetyl-GD3. J. Biol. Chem. 1985, 260, 14556–14563. [Google Scholar] [CrossRef] [PubMed]
- Ostrander, G.K.; Bozlee, M.; Fukuda, M.; Dell, A.; Thomas-Oates, J.E.; Levery, S.B.; Eaton, H.L.; Hakomori, S.; Holmes, E.H. Isolation and Characterization of the Major Glycosphingolipids from the Liver of the Rainbow Trout (Oncorhynchus mykiss): Identification of an Abundant Source of 9-O-Acetyl GD3. Arch. Biochem. Biophys. 1991, 284, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Kitajima, K.; Inoue, S.; Muto, Y.; Kasama, T.; Handa, S.; Inoue, Y. Structure of Novel Gangliosides, Deaminated Neuraminic Acid (KDN)-Containing Glycosphingolipids, Isolated from Rainbow Trout Ovarian Fluid. Biochemistry 1993, 32, 9221–9229. [Google Scholar] [CrossRef]
- Siebert, H.C.; von der Lieth, C.W.; Dong, X.; Reuter, G.; Schauer, R.; Gabius, H.J.; Vliegenthart, J.F. Molecular Dynamics-Derived Conformation and Intramolecular Interaction Analysis of the N-Acetyl-9-O-Acetylneuraminic Acid-Containing Ganglioside GD1a and NMR-Based Analysis of Its Binding to a Human Polyclonal Immunoglobulin G Fraction with Selectivity for O-Acetylated Sialic Acids. Glycobiology 1996, 6, 561–572. [Google Scholar] [CrossRef]
- Li, W.; Battistel, M.D.; Reeves, H.; Oh, L.; Yu, H.; Chen, X.; Wang, L.-P.; Freedberg, D.I. A Combined NMR, MD and DFT Conformational Analysis of 9-O-Acetyl Sialic Acid-Containing GM3 Ganglioside Glycan and Its 9-N-Acetyl Mimic. Glycobiology 2020, 30, 787–801. [Google Scholar] [CrossRef]
- Zhu, J.; Li, Y.T.; Li, S.C.; Cole, R.B. Structural Characterization of Gangliosides Isolated from Mullet Milt Using Electrospray Ionization-Tandem Mass Spectrometry. Glycobiology 1999, 9, 985–993. [Google Scholar] [CrossRef]
- Shi, W.X.; Chammas, R.; Varki, A. Linkage-Specific Action of Endogenous Sialic Acid O-Acetyltransferase in Chinese Hamster Ovary Cells. J. Biol. Chem. 1996, 271, 15130–15138. [Google Scholar] [CrossRef]
- Shi, W.X.; Chammas, R.; Varki, A. Induction of Sialic Acid 9-O-Acetylation by Diverse Gene Products: Implications for the Expression Cloning of Sialic Acid O-Acetyltransferases. Glycobiology 1998, 8, 199–205. [Google Scholar] [CrossRef]
- Corfield, A.P.; Sander-Wewer, M.; Veh, R.W.; Wember, M.; Schauer, R. The Action of Sialidases on Substrates Containing O-Acetylsialic Acids. Biol. Chem. Hoppe Seyler 1986, 367, 433–439. [Google Scholar] [CrossRef]
- Ritter, G.; Boosfeld, E.; Markstein, E.; Yu, R.K.; Ren, S.L.; Stallcup, W.B.; Oettgen, H.F.; Old, L.J.; Livingston, P.O. Biochemical and Serological Characteristics of Natural 9-O-Acetyl GD3 from Human Melanoma and Bovine Buttermilk and Chemically O-Acetylated GD3. Cancer Res. 1990, 50, 1403–1410. [Google Scholar]
- Manzi, A.E.; Sjoberg, E.R.; Diaz, S.; Varki, A. Biosynthesis and Turnover of O-Acetyl and N-Acetyl Groups in the Gangliosides of Human Melanoma Cells. J. Biol. Chem. 1990, 265, 13091–13103. [Google Scholar] [CrossRef]
- Sjoberg, E.R.; Varki, A. Kinetic and Spatial Interrelationships between Ganglioside Glycosyltransferases and O-Acetyltransferase(s) in Human Melanoma Cells. J. Biol. Chem. 1993, 268, 10185–10196. [Google Scholar] [CrossRef]
- Bora, R.S.; Kanamori, A.; Hirabayashi, Y. Cloning and Characterization of a Putative Mouse Acetyl-CoA Transporter CDNA. Gene 1999, 238, 455–462. [Google Scholar] [CrossRef]
- Satake, H.; Chen, H.Y.; Varki, A. Genes Modulated by Expression of GD3 Synthase in Chinese Hamster Ovary Cells. Evidence That the Tis21 Gene Is Involved in the Induction of GD3 9-O-Acetylation. J. Biol. Chem. 2003, 278, 7942–7948. [Google Scholar] [CrossRef]
- Chen, H.Y.; Challa, A.K.; Varki, A. 9-O-Acetylation of Exogenously Added Ganglioside GD3. The GD3 Molecule Induces Its Own O-Acetylation Machinery. J. Biol. Chem. 2006, 281, 7825–7833. [Google Scholar] [CrossRef]
- Furukawa, K.; Aixinjueluo, W.; Kasama, T.; Ohkawa, Y.; Yoshihara, M.; Ohmi, Y.; Tajima, O.; Suzumura, A.; Kittaka, D.; Furukawa, K. Disruption of GM2/GD2 Synthase Gene Resulted in Overt Expression of 9-O-Acetyl GD3 Irrespective of Tis21. J. Neurochem. 2008, 105, 1057–1066. [Google Scholar] [CrossRef]
- Kniep, B.; Kniep, E.; Ozkucur, N.; Barz, S.; Bachmann, M.; Malisan, F.; Testi, R.; Rieber, E.P. 9-O-Acetyl GD3 Protects Tumor Cells from Apoptosis. Int. J. Cancer 2006, 119, 67–73. [Google Scholar] [CrossRef]
- Mandal, C.; Srinivasan, G.V.; Chowdhury, S.; Chandra, S.; Mandal, C.; Schauer, R.; Mandal, C. High Level of Sialate-O-Acetyltransferase Activity in Lymphoblasts of Childhood Acute Lymphoblastic Leukaemia (ALL): Enzyme Characterization and Correlation with Disease Status. Glycoconj. J. 2009, 26, 57–73. [Google Scholar] [CrossRef]
- Ehara, T.; Kameyama, A.; Yamada, Y.; Ishida, H.; Kiso, M.; Hasegawa, A. Total Synthesis of VIM-2 Ganglioside Isolated from Human Chronic Myelogenous Leukemia Cells. Carbohydr. Res. 1996, 281, 237–252. [Google Scholar] [CrossRef] [PubMed]
- Coers, W.; Reivinen, J.; Miettinen, A.; Huitema, S.; Vos, J.T.; Salant, D.J.; Weening, J.J. Characterization of a Rat Glomerular Visceral Epithelial Cell Line. Exp. Nephrol. 1996, 4, 184–192. [Google Scholar] [PubMed]
- Skov, L.; Chan, L.S.; Fox, D.A.; Larsen, J.K.; Voorhees, J.J.; Cooper, K.D.; Baadsgaard, O. Lesional Psoriatic T Cells Contain the Capacity to Induce a T Cell Activation Molecule CDw60 on Normal Keratinocytes. Am. J. Pathol. 1997, 150, 675–683. [Google Scholar] [PubMed]
- Aguilar, R.P.; Genta, S.; Sánchez, S. Renal Gangliosides Are Involved in Lead Intoxication. J. Appl. Toxicol. 2008, 28, 122–131. [Google Scholar] [CrossRef]
- Wipfler, D.; Srinivasan, G.V.; Sadick, H.; Kniep, B.; Arming, S.; Willhauck-Fleckenstein, M.; Vlasak, R.; Schauer, R.; Schwartz-Albiez, R. Differentially Regulated Expression of 9-O-Acetyl GD3 (CD60b) and 7-O-Acetyl-GD3 (CD60c) during Differentiation and Maturation of Human T and B Lymphocytes. Glycobiology 2011, 21, 1161–1172. [Google Scholar] [CrossRef]
- Chammas, R.; Sonnenburg, J.L.; Watson, N.E.; Tai, T.; Farquhar, M.G.; Varki, N.M.; Varki, A. De-N-Acetyl-Gangliosides in Humans: Unusual Subcellular Distribution of a Novel Tumor Antigen. Cancer Res. 1999, 59, 1337–1346. [Google Scholar]
- Schwarz, A.; Futerman, A.H. Determination of the Localization of Gangliosides Using Anti-Ganglioside Antibodies: Comparison of Fixation Methods. J. Histochem. Cytochem. 1997, 45, 611–618. [Google Scholar] [CrossRef]
- Harms, G.; Reuter, G.; Corfield, A.P.; Schauer, R. Binding Specificity of Influenza C-Virus to Variably O-Acetylated Glycoconjugates and Its Use for Histochemical Detection of N-Acetyl-9-O-Acetylneuraminic Acid in Mammalian Tissues. Glycoconj. J. 1996, 13, 621–630. [Google Scholar] [CrossRef]
- Hubl, U.; Ishida, H.; Kiso, M.; Hasegawa, A.; Schauer, R. Studies on the Specificity and Sensitivity of the Influenza C Virus Binding Assay for 9-O-Acetylated Sialic Acids and Its Application to Human Melanomas. J. Biochem. 2000, 127, 1021–1031. [Google Scholar] [CrossRef]
- Krishna, M.; Varki, A. 9-O-Acetylation of Sialomucins: A Novel Marker of Murine CD4 T Cells That Is Regulated during Maturation and Activation. J. Exp. Med. 1997, 185, 1997–2013. [Google Scholar] [CrossRef]
- Gout, E.; Garlatti, V.; Smith, D.F.; Lacroix, M.; Dumestre-Pérard, C.; Lunardi, T.; Martin, L.; Cesbron, J.-Y.; Arlaud, G.J.; Gaboriaud, C.; et al. Carbohydrate Recognition Properties of Human Ficolins: Glycan Array Screening Reveals the Sialic Acid Binding Specificity of M-Ficolin. J. Biol. Chem. 2010, 285, 6612–6622. [Google Scholar] [CrossRef]
- Mandal, C.; Mandal, C.; Chandra, S.; Schauer, R.; Mandal, C. Regulation of O-Acetylation of Sialic Acids by Sialate-O-Acetyltransferase and Sialate-O-Acetylesterase Activities in Childhood Acute Lymphoblastic Leukemia. Glycobiology 2012, 22, 70–83. [Google Scholar] [CrossRef]
- Draper, J.S.; Pigott, C.; Thomson, J.A.; Andrews, P.W. Surface Antigens of Human Embryonic Stem Cells: Changes upon Differentiation in Culture. J. Anat. 2002, 200, 249–258. [Google Scholar] [CrossRef]
- Azevedo-Pereira, R.L.; Morrot, A.; Machado, G.S.; Paredes, B.D.; de Carvalho Rodrigues, D.; de Carvalho, A.C.C.; Mendez-Otero, R. Expression of Ganglioside 9-O Acetyl GD3 in Undifferentiated Embryonic Stem Cells. Cell Biol. Int. 2015, 39, 121–127. [Google Scholar] [CrossRef]
- Fenderson, B.A.; Andrews, P.W.; Nudelman, E.; Clausen, H.; Hakomori, S. Glycolipid Core Structure Switching from Globo- to Lacto- and Ganglio-Series during Retinoic Acid-Induced Differentiation of TERA-2-Derived Human Embryonal Carcinoma Cells. Dev. Biol. 1987, 122, 21–34. [Google Scholar] [CrossRef]
- Yanagisawa, M.; Taga, T.; Nakamura, K.; Ariga, T.; Yu, R.K. Characterization of Glycoconjugate Antigens in Mouse Embryonic Neural Precursor Cells. J. Neurochem. 2005, 95, 1311–1320. [Google Scholar] [CrossRef]
- Blum, A.S.; Barnstable, C.J. O-Acetylation of a Cell-Surface Carbohydrate Creates Discrete Molecular Patterns during Neural Development. Proc. Natl. Acad. Sci. USA 1987, 84, 8716–8720. [Google Scholar] [CrossRef]
- Sparrow, J.R.; Barnstable, C.J. A Gradient Molecule in Developing Rat Retina: Expression of 9-O-Acetyl GD3 in Relation to Cell Type, Developmental Age, and GD3 Ganglioside. J. Neurosci. Res. 1988, 21, 398–409. [Google Scholar] [CrossRef]
- Mendez-Otero, R.; Santiago, M.F. Functional Role of a Specific Ganglioside in Neuronal Migration and Neurite Outgrowth. Braz. J. Med. Biol. Res. 2003, 36, 1003–1013. [Google Scholar] [CrossRef]
- Drazba, J.; Pierce, M.; Lemmon, V. Studies of the Developing Chick Retina Using Monoclonal Antibody 8A2 That Recognizes a Novel Set of Gangliosides. Dev. Biol. 1991, 145, 154–163. [Google Scholar] [CrossRef]
- Hirabayashi, Y.; Hirota, M.; Suzuki, Y.; Matsumoto, M.; Obata, K.; Ando, S. Developmentally Expressed O-Acetyl Ganglioside GT3 in Fetal Rat Cerebral Cortex. Neurosci. Lett. 1989, 106, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Ji, L.; Kurono, S.; Fujita, S.C.; Furuya, S.; Hirabayashi, Y. Developmentally Regulated O-Acetylated Sialoglycans in the Central Nervous System Revealed by a New Monoclonal Antibody 493D4 Recognizing a Wide Range of O-Acetylated Glycoconjugates. Glycoconj. J. 1997, 14, 847–857. [Google Scholar] [CrossRef] [PubMed]
- Mendez-Otero, R.; Constantine-Paton, M. Granule Cell Induction of 9-O-Acetyl Gangliosides on Cerebellar Glia in Microcultures. Dev. Biol. 1990, 138, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Mendez-Otero, R.; Ramon-Cueto, A. Expression of 9-O-Acetylated Gangliosides during Development of the Rat Olfactory System. Neuroreport 1994, 5, 1755–1759. [Google Scholar] [CrossRef]
- Mello, L.E.; Mendez-Otero, R. Expression of 9-O-Acetylated Gangliosides in the Rat Hippocampus. Neurosci. Lett. 1996, 213, 17–20. [Google Scholar] [CrossRef]
- Mendez-Otero, R.; Cavalcante, L.A. Expression of 9-O-Acetylated Gangliosides Is Correlated with Tangential Cell Migration in the Rat Brain. Neurosci. Lett. 1996, 204, 97–100. [Google Scholar] [CrossRef]
- Dubois, C.; Manuguerra, J.C.; Hauttecoeur, B.; Maze, J. Monoclonal Antibody A2B5, Which Detects Cell Surface Antigens, Binds to Ganglioside GT3 (II3 (NeuAc)3LacCer) and to Its 9-O-Acetylated Derivative. J. Biol. Chem. 1990, 265, 2797–2803. [Google Scholar] [CrossRef]
- Mendez-Otero, R.; Friedman, J.E. Role of Acetylated Gangliosides on Neurite Extension. Eur. J. Cell Biol. 1996, 71, 192–198. [Google Scholar]
- Araujo, H.; Menezes, M.; Mendez-Otero, R. Blockage of 9-O-Acetyl Gangliosides Induces Microtubule Depolymerization in Growth Cones and Neurites. Eur. J. Cell Biol. 1997, 72, 202–213. [Google Scholar]
- Negreiros, E.M.A.; Leão, A.C.M.; Santiago, M.F.; Mendez-Otero, R. Localization of Ganglioside 9-O-Acetyl GD3 in Point Contacts of Neuronal Growth Cones. J. Neurobiol. 2003, 57, 31–37. [Google Scholar] [CrossRef]
- Varki, A.; Hooshmand, F.; Diaz, S.; Varki, N.M.; Hedrick, S.M. Developmental Abnormalities in Transgenic Mice Expressing a Sialic Acid-Specific 9-O-Acetylesterase. Cell 1991, 65, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Takamiya, K.; Yamamoto, A.; Furukawa, K.; Yamashiro, S.; Shin, M.; Okada, M.; Fukumoto, S.; Haraguchi, M.; Takeda, N.; Fujimura, K.; et al. Mice with Disrupted GM2/GD2 Synthase Gene Lack Complex Gangliosides but Exhibit Only Subtle Defects in Their Nervous System. Proc. Natl. Acad. Sci. USA 1996, 93, 10662–10667. [Google Scholar] [CrossRef]
- Gubert, F.; Zaverucha-do-Valle, C.; Furtado, M.; Pimentel-Coelho, P.M.; Mortari, N.; Leão, A.C.M.; Hayashi, E.A.; Nobrega, A.; Mendez-Otero, R.; Santiago, M.F. CD60b: Enriching Neural Stem/Progenitor Cells from Rat Development into Adulthood. Stem Cells Int. 2017, 2017, 5759490. [Google Scholar] [CrossRef]
- Campos, F.S.O.; Piña-Rodrigues, F.M.; Reis, A.; Atella, G.C.; Mermelstein, C.S.; Allodi, S.; Cavalcante, L.A. Lipid Rafts from Olfactory Ensheathing Cells: Molecular Composition and Possible Roles. Cell. Mol. Neurobiol. 2021, 41, 525–536. [Google Scholar] [CrossRef]
- Santiago, M.F.; Berredo-Pinho, M.; Costa, M.R.; Gandra, M.; Cavalcante, L.A.; Mendez-Otero, R. Expression and Function of Ganglioside 9-O-Acetyl GD3 in Postmitotic Granule Cell Development. Mol. Cell. Neurosci. 2001, 17, 488–499. [Google Scholar] [CrossRef]
- Miyakoshi, L.M.; Mendez-Otero, R.; Hedin-Pereira, C. The 9-O-Acetyl GD3 Gangliosides Are Expressed by Migrating Chains of Subventricular Zone Neurons in Vitro. Braz. J. Med. Biol. Res. 2001, 34, 669–673. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ribeiro-Resende, V.T.; Oliveira-Silva, A.; Ouverney-Brandão, S.; Santiago, M.F.; Hedin-Pereira, C.; Mendez-Otero, R. Ganglioside 9-O-Acetyl GD3 Expression Is Upregulated in the Regenerating Peripheral Nerve. Neuroscience 2007, 147, 97–105. [Google Scholar] [CrossRef]
- Ribeiro-Resende, V.T.; Araújo Gomes, T.; de Lima, S.; Nascimento-Lima, M.; Bargas-Rega, M.; Santiago, M.F.; Reis, R.A.d.M.; de Mello, F.G. Mice Lacking GD3 Synthase Display Morphological Abnormalities in the Sciatic Nerve and Neuronal Disturbances during Peripheral Nerve Regeneration. PLoS ONE 2014, 9, e108919. [Google Scholar] [CrossRef]
- Santiago, M.F.; Liour, S.S.; Mendez-Otero, R.; Yu, R.K. Glial-Guided Neuronal Migration in P19 Embryonal Carcinoma Stem Cell Aggregates. J. Neurosci. Res. 2005, 81, 9–20. [Google Scholar] [CrossRef]
- Santiago, M.F.; Scemes, E. Neuroblast Migration and P2Y(1) Receptor Mediated Calcium Signalling Depend on 9-O-Acetyl GD3 Ganglioside. ASN Neuro 2012, 4, 357–369. [Google Scholar] [CrossRef]
- Santos-Silva, A.; Piña-Rodrigues, F.M.; Mermelstein, C.D.S.; Allodi, S.; Barradas, P.C.; Cavalcante, L.A. A Role for Gangliosides and Β1-Integrin in the Motility of Olfactory Ensheathing Glia. J. Anat. 2019, 235, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Santiago, M.F.; Costa, M.R.; Mendez-Otero, R. Immunoblockage of 9-O-Acetyl GD3 Ganglioside Arrests the in Vivo Migration of Cerebellar Granule Neurons. J. Neurosci. 2004, 24, 474–478. [Google Scholar] [CrossRef]
- Yang, C.-R.; Liour, S.S.; Dasgupta, S.; Yu, R.K. Inhibition of Neuronal Migration by JONES Antibody Is Independent of 9-O-Acetyl GD3 in GD3-Synthase Knockout Mice. J. Neurosci. Res. 2007, 85, 1381–1390. [Google Scholar] [CrossRef]
- Miyakoshi, L.M.; Todeschini, A.R.; Mendez-Otero, R.; Hedin-Pereira, C. Role of the 9-O-Acetyl GD3 in Subventricular Zone Neuroblast Migration. Mol. Cell. Neurosci. 2012, 49, 240–249. [Google Scholar] [CrossRef]
- Leclerc, N.; Schwarting, G.A.; Herrup, K.; Hawkes, R.; Yamamoto, M. Compartmentation in Mammalian Cerebellum: Zebrin II and P-Path Antibodies Define Three Classes of Sagittally Organized Bands of Purkinje Cells. Proc. Natl. Acad. Sci. USA 1992, 89, 5006–5010. [Google Scholar] [CrossRef]
- Edwards, M.A.; Crandall, J.E.; Leclerc, N.; Yamamoto, M. Effects of Nervous Mutation on Purkinje Cell Compartments Defined by Zebrin II and 9-O-Acetylated Gangliosides Expression. Neurosci. Res. 1994, 19, 167–174. [Google Scholar] [CrossRef]
- Baader, S.L.; Vogel, M.W.; Sanlioglu, S.; Zhang, X.; Oberdick, J. Selective Disruption of “Late Onset” Sagittal Banding Patterns by Ectopic Expression of Engrailed-2 in Cerebellar Purkinje Cells. J. Neurosci. 1999, 19, 5370–5379. [Google Scholar] [CrossRef]
- Yamamoto, M.; Schwarting, G.A.; Crandall, J.E. Altered 9-O Acetylation of Disialogangliosides in Cerebellar Purkinje Cells of the Nervous Mutant Mouse. Brain Res. 1994, 662, 223–232. [Google Scholar] [CrossRef]
- Zimmer, G.; Suguri, T.; Reuter, G.; Yu, R.K.; Schauer, R.; Herrler, G. Modification of Sialic Acids by 9-O-Acetylation Is Detected in Human Leucocytes Using the Lectin Property of Influenza C Virus. Glycobiology 1994, 4, 343–349. [Google Scholar] [CrossRef]
- Kniep, B.; Flegel, W.A.; Northoff, H.; Rieber, E.P. CDw60 Glycolipid Antigens of Human Leukocytes: Structural Characterization and Cellular Distribution. Blood 1993, 82, 1776–1786. [Google Scholar] [CrossRef]
- Lünsdorf, H.; Kniep, E.; Kniep, B. Immunocytochemical Localization of CDw60 Antigens on Human Peripheral T Cells. Carbohydr. Res. 2000, 329, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Rieber, E.P.; Rank, G. CDw60: A Marker for Human CD8+ T Helper Cells. J. Exp. Med. 1994, 179, 1385–1390. [Google Scholar] [CrossRef] [PubMed]
- Vater, M.; Kniep, B.; Gross, H.J.; Claus, C.; Dippold, W.; Schwartz-Albiez, R. The 9-O-Acetylated Disialosyl Carbohydrate Sequence of CDw60 Is a Marker on Activated Human B Lymphocytes. Immunol. Lett. 1997, 59, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Kniep, B.; Claus, C.; Peter-Katalinic, J.; Monner, D.A.; Dippold, W.; Nimtz, M. 7-O-Acetyl-GD3 in Human T-Lymphocytes Is Detected by a Specific T-Cell-Activating Monoclonal Antibody. J. Biol. Chem. 1995, 270, 30173–30180. [Google Scholar] [CrossRef]
- Reivinen, J.; Holthöfer, H.; Miettinen, A. Tyrosine Phosphorylation of P72syk Induced by Anti-9-O-Acetyl GD3 Antibodies in Human Peripheral Blood Mononuclear Cells. Scand. J. Immunol. 1998, 48, 615–622. [Google Scholar] [CrossRef]
- Erdmann, M.; Wipfler, D.; Merling, A.; Cao, Y.; Claus, C.; Kniep, B.; Sadick, H.; Bergler, W.; Vlasak, R.; Schwartz-Albiez, R. Differential Surface Expression and Possible Function of 9-O- and 7-O-Acetylated GD3 (CD60 b and c) during Activation and Apoptosis of Human Tonsillar B and T Lymphocytes. Glycoconj. J. 2006, 23, 627–638. [Google Scholar] [CrossRef]
- Mukherjee, K.; Chowdhury, S.; Mondal, S.; Mandal, C.; Chandra, S.; Bhadra, R.K.; Mandal, C. 9-O-Acetylated GD3 Triggers Programmed Cell Death in Mature Erythrocytes. Biochem. Biophys. Res. Commun. 2007, 362, 651–657. [Google Scholar] [CrossRef]
- Mukherjee, K.; Chowdhury, S.; Mondal, S.; Mandal, C.; Chandra, S.; Mandal, C. 9-O-Acetyl GD3 in Lymphoid and Erythroid Cells. Adv. Exp. Med. Biol. 2011, 705, 317–334. [Google Scholar] [CrossRef]
- Simons, M.; Schwarz, K.; Kriz, W.; Miettinen, A.; Reiser, J.; Mundel, P.; Holthöfer, H. Involvement of Lipid Rafts in Nephrin Phosphorylation and Organization of the Glomerular Slit Diaphragm. Am. J. Pathol. 2001, 159, 1069–1077. [Google Scholar] [CrossRef]
- Kohla, G.; Stockfleth, E.; Schauer, R. Gangliosides with O-Acetylated Sialic Acids in Tumors of Neuroectodermal Origin. Neurochem. Res. 2002, 27, 583–592. [Google Scholar] [CrossRef]
- Cheresh, D.A.; Varki, A.P.; Varki, N.M.; Stallcup, W.B.; Levine, J.; Reisfeld, R.A. A Monoclonal Antibody Recognizes an O-Acylated Sialic Acid in a Human Melanoma-Associated Ganglioside. J. Biol. Chem. 1984, 259, 7453–7459. [Google Scholar] [CrossRef]
- Herlyn, M.; Rodeck, U.; Mancianti, M.; Cardillo, F.M.; Lang, A.; Ross, A.H.; Jambrosic, J.; Koprowski, H. Expression of Melanoma-Associated Antigens in Rapidly Dividing Human Melanocytes in Culture. Cancer Res. 1987, 47, 3057–3061. [Google Scholar]
- Berd, D.; Herlyn, M.; Koprowski, H.; Mastrangelo, M.J. Flow Cytometric Determination of the Frequency and Heterogeneity of Expression of Human Melanoma-Associated Antigens. Cancer Res. 1989, 49, 6840–6844. [Google Scholar]
- Hamilton, W.B.; Helling, F.; Lloyd, K.O.; Livingston, P.O. Ganglioside Expression on Human Malignant Melanoma Assessed by Quantitative Immune Thin-Layer Chromatography. Int. J. Cancer 1993, 53, 566–573. [Google Scholar] [CrossRef]
- Sjoberg, E.R.; Manzi, A.E.; Khoo, K.H.; Dell, A.; Varki, A. Structural and Immunological Characterization of O-Acetylated GD2. Evidence That GD2 Is an Acceptor for Ganglioside O-Acetyltransferase in Human Melanoma Cells. J. Biol. Chem. 1992, 267, 16200–16211. [Google Scholar] [CrossRef]
- Ren, S.L.; Slominski, A.; Yu, R.K. Glycosphingolipids in Bomirski Transplantable Melanomas in Hamsters. Cancer Res. 1989, 49, 7051–7056. [Google Scholar]
- Kageshita, T.; Nakamura, T.; Yamada, M.; Kuriya, N.; Arao, T.; Ferrone, S. Differential Expression of Melanoma Associated Antigens in Acral Lentiginous Melanoma and in Nodular Melanoma Lesions. Cancer Res. 1991, 51, 1726–1732. [Google Scholar]
- Natali, P.G.; Bigotti, A.; Nicotra, M.R.; Nardi, R.M.; Delovu, A.; Segatto, O.; Ferrone, S. Analysis of the Antigenic Profile of Uveal Melanoma Lesions with Anti-Cutaneous Melanoma-Associated Antigen and Anti-HLA Monoclonal Antibodies. Cancer Res. 1989, 49, 1269–1274. [Google Scholar]
- Kanda, S.; Cochran, A.J.; Lee, W.R.; Morton, D.L.; Irie, R.F. Variations in the Ganglioside Profile of Uveal Melanoma Correlate with Cytologic Heterogeneity. Int. J. Cancer 1992, 52, 682–687. [Google Scholar] [CrossRef]
- Sela, B.A.; Iliopoulos, D.; Guerry, D.; Herlyn, D.; Koprowski, H. Levels of Disialogangliosides in Sera of Melanoma Patients Monitored by Sensitive Thin-Layer Chromatography and Immunostaining. J. Natl. Cancer Inst. 1989, 81, 1489–1492. [Google Scholar] [CrossRef]
- Ren, S.; Ariga, T.; Scarsdale, J.N.; Zhang, Y.; Slominski, A.; Livingston, P.O.; Ritter, G.; Kushi, Y.; Yu, R.K. Characterization of a Hamster Melanoma-Associated Ganglioside Antigen as 7-O-Acetylated Disialoganglioside GD3. J. Lipid Res. 1993, 34, 1565–1572. [Google Scholar] [CrossRef] [PubMed]
- Popa, I.; Pons, A.; Mariller, C.; Tai, T.; Zanetta, J.-P.; Thomas, L.; Portoukalian, J. Purification and Structural Characterization of De-N-Acetylated Form of GD3 Ganglioside Present in Human Melanoma Tumors. Glycobiology 2007, 17, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.X.; Chammas, R.; Varki, A. Regulation of Sialic Acid 9-O-Acetylation during the Growth and Differentiation of Murine Erythroleukemia Cells. J. Biol. Chem. 1996, 271, 31517–31525. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, K.; Chava, A.K.; Mandal, C.; Dey, S.N.; Kniep, B.; Chandra, S.; Mandal, C. O-Acetylation of GD3 Prevents Its Apoptotic Effect and Promotes Survival of Lymphoblasts in Childhood Acute Lymphoblastic Leukaemia. J. Cell. Biochem. 2008, 105, 724–734. [Google Scholar] [CrossRef]
- Scala, E.; Abeni, D.; Pomponi, D.; Narducci, M.G.; Lombardo, G.A.; Mari, A.; Frontani, M.; Picchio, M.C.; Pilla, M.A.; Caprini, E.; et al. The Role of 9-O-Acetylated Ganglioside D3 (CD60) and {alpha}4{beta}1 (CD49d) Expression in Predicting the Survival of Patients with Sezary Syndrome. Haematologica 2010, 95, 1905–1912. [Google Scholar] [CrossRef][Green Version]
- Paller, A.S.; Arnsmeier, S.L.; Robinson, J.K.; Bremer, E.G. Alteration in Keratinocyte Ganglioside Content in Basal Cell Carcinomas. J. Investig. Dermatol. 1992, 98, 226–232. [Google Scholar] [CrossRef]
- Fahr, C. Detection of Sialic Acids and Gangliosides with Special Reference to 9-O-Acetylated Species in Basaliomas and Normal Human Skin. J. Investig. Dermatol. 2001, 116, 254–260. [Google Scholar] [CrossRef]
- Fuentes, R.; Allman, R.; Mason, M.D. Ganglioside Expression in Lung Cancer Cell Lines. Lung Cancer 1997, 18, 21–33. [Google Scholar] [CrossRef]
- Gocht, A.; Rutter, G.; Kniep, B. Changed Expression of 9-O-Acetyl GD3 (CDw60) in Benign and Atypical Proliferative Lesions and Carcinomas of the Human Breast. Histochem. Cell Biol. 1998, 110, 217–229. [Google Scholar] [CrossRef]
- Cavdarli, S.; Dewald, J.H.; Yamakawa, N.; Guérardel, Y.; Terme, M.; Le Doussal, J.-M.; Delannoy, P.; Groux-Degroote, S. Identification of 9-O-Acetyl-N-Acetylneuraminic Acid (Neu5,9Ac2) as Main O-Acetylated Sialic Acid Species of GD2 in Breast Cancer Cells. Glycoconj. J. 2019, 36, 79–90. [Google Scholar] [CrossRef]
- Cavdarli, S.; Schröter, L.; Albers, M.; Baumann, A.-M.; Vicogne, D.; Le Doussal, J.-M.; Mühlenhoff, M.; Delannoy, P.; Groux-Degroote, S. Role of Sialyl-O-Acetyltransferase CASD1 on GD2 Ganglioside O-Acetylation in Breast Cancer Cells. Cells 2021, 10, 1468. [Google Scholar] [CrossRef]
- Ariga, T.; Suetake, K.; Nakane, M.; Kubota, M.; Usuki, S.; Kawashima, I.; Yu, R.K. Glycosphingolipid Antigens in Neural Tumor Cell Lines and Anti-Glycosphingolipid Antibodies in Sera of Patients with Neural Tumors. Neurosignals 2008, 16, 226–234. [Google Scholar] [CrossRef]
- Birks, S.M.; Danquah, J.O.; King, L.; Vlasak, R.; Gorecki, D.C.; Pilkington, G.J. Targeting the GD3 Acetylation Pathway Selectively Induces Apoptosis in Glioblastoma. Neuro Oncol. 2011, 13, 950–960. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Y.; Zheng, Q.; Li, J. Analysis of O-Acetylated Sialic Acids in Dried Blood Spots. Anal. Chem. 2019, 91, 2744–2751. [Google Scholar] [CrossRef]
- Malisan, F.; Franchi, L.; Tomassini, B.; Ventura, N.; Condò, I.; Rippo, M.R.; Rufini, A.; Liberati, L.; Nachtigall, C.; Kniep, B.; et al. Acetylation Suppresses the Proapoptotic Activity of GD3 Ganglioside. J. Exp. Med. 2002, 196, 1535–1541. [Google Scholar] [CrossRef]
- Giussani, P.; Tringali, C.; Riboni, L.; Viani, P.; Venerando, B. Sphingolipids: Key Regulators of Apoptosis and Pivotal Players in Cancer Drug Resistance. Int. J. Mol. Sci. 2014, 15, 4356–4392. [Google Scholar] [CrossRef]
- Romero-Ramírez, L.; Nieto-Sampedro, M. Inhibiting Human Astrocytoma Growth: Structure-Activity Relationships in Neurostatin Related Glycolipids. J. Med. Chem. 2004, 47, 4983–4984. [Google Scholar] [CrossRef]
- Valle-Argos, B.; Gómez-Nicola, D.; Nieto-Sampedro, M. Synthesis and Characterization of Neurostatin-Related Compounds with High Inhibitory Activity of Glioma Growth. Eur. J. Med. Chem. 2010, 45, 2034–2043. [Google Scholar] [CrossRef][Green Version]
- Zhang, S.; Helling, F.; Lloyd, K.O.; Livingston, P.O. Increased Tumor Cell Reactivity and Complement-Dependent Cytotoxicity with Mixtures of Monoclonal Antibodies against Different Gangliosides. Cancer Immunol. Immunother. 1995, 40, 88–94. [Google Scholar] [CrossRef]
- Zhang, S.; Cordon-Cardo, C.; Zhang, H.S.; Reuter, V.E.; Adluri, S.; Hamilton, W.B.; Lloyd, K.O.; Livingston, P.O. Selection of Tumor Antigens as Targets for Immune Attack Using Immunohistochemistry: I. Focus on Gangliosides. Int. J. Cancer 1997, 73, 42–49. [Google Scholar] [CrossRef]
- Ritter, G.; Ritter-Boosfeld, E.; Adluri, R.; Calves, M.; Ren, S.; Yu, R.K.; Oettgen, H.F.; Old, L.J.; Livingston, P.O. Analysis of the Antibody Response to Immunization with Purified O-Acetyl GD3 Gangliosides in Patients with Malignant Melanoma. Int. J. Cancer 1995, 62, 668–672. [Google Scholar] [CrossRef] [PubMed]
- Dumontet, C.; Rebbaa, A.; Portoukalian, J. Very Low Density Lipoproteins and Interleukin 2 Enhance the Immunogenicity of 9-O-Acetyl-GD3 Ganglioside in BALB/c Mice. J. Immunol. Methods 1997, 206, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Ye, J.; DeLaitsch, A.T.; Rashidijahanabad, Z.; Lang, S.; Kakeshpour, T.; Zhao, Y.; Ramadan, S.; Saavedra, P.V.; Yuzbasiyan-Gurkan, V.; et al. Chemoenzymatic Synthesis of 9NHAc-GD2 Antigen to Overcome the Hydrolytic Instability of O-Acetylated-GD2 for Anticancer Conjugate Vaccine Development. Angew. Chem. Int. Ed. Engl. 2021, 60, 24179–24188. [Google Scholar] [CrossRef] [PubMed]
- Schultze, B.; Zimmer, G.; Herrler, G. Virus Entry into a Polarized Epithelial Cell Line (MDCK): Similarities and Dissimilarities between Influenza C Virus and Bovine Coronavirus. J. Gen. Virol. 1996, 77 Pt 10, 2507–2514. [Google Scholar] [CrossRef]
- Li, Z.; Lang, Y.; Liu, L.; Bunyatov, M.I.; Sarmiento, A.I.; de Groot, R.J.; Boons, G.-J. Synthetic O-Acetylated Sialosides Facilitate Functional Receptor Identification for Human Respiratory Viruses. Nat. Chem. 2021, 13, 496–503. [Google Scholar] [CrossRef]
- Zimmer, G.; Reuter, G.; Schauer, R. Use of Influenza C Virus for Detection of 9-O-Acetylated Sialic Acids on Immobilized Glycoconjugates by Esterase Activity. Eur. J. Biochem. 1992, 204, 209–215. [Google Scholar] [CrossRef]
- Manuguerra, J.C.; DuBois, C.; Hannoun, C. Analytical Detection of 9(4)-O-Acetylated Sialoglycoproteins and Gangliosides Using Influenza C Virus. Anal. Biochem. 1991, 194, 425–432. [Google Scholar] [CrossRef]
- Ribeiro-Resende, V.T.; Ribeiro-Guimarães, M.L.; Lemes, R.M.R.; Nascimento, I.C.; Alves, L.; Mendez-Otero, R.; Pessolani, M.C.V.; Lara, F.A. Involvement of 9-O-Acetyl GD3 Ganglioside in Mycobacterium Leprae Infection of Schwann Cells. J. Biol. Chem. 2010, 285, 34086–34096. [Google Scholar] [CrossRef]
- Hitoshi, S.; Kusunoki, S.; Kon, K.; Chiba, A.; Waki, H.; Ando, S.; Kanazawa, I. A Novel Ganglioside, 9-O-Acetyl GD1b, Is Recognized by Serum Antibodies in Guillain-Barré Syndrome. J. Neuroimmunol. 1996, 66, 95–101. [Google Scholar] [CrossRef]
- Fox, D.A.; He, X.; Abe, A.; Hollander, T.; Li, L.L.; Kan, L.; Friedman, A.W.; Shimizu, Y.; Shayman, J.A.; Kozarsky, K. The T Lymphocyte Structure CD60 Contains a Sialylated Carbohydrate Epitope That Is Expressed on Both Gangliosides and Glycoproteins. Immunol. Investig. 2001, 30, 67–85. [Google Scholar] [CrossRef]
- Fantini, J.; Azzaz, F.; Chahinian, H.; Yahi, N. Electrostatic Surface Potential as a Key Parameter in Virus Transmission and Evolution: How to Manage Future Virus Pandemics in the Post-COVID-19 Era. Viruses 2023, 15, 284. [Google Scholar] [CrossRef]
- Sun, X.-L. The Role of Cell Surface Sialic Acids for SARS-CoV-2 Infection. Glycobiology 2021, 31, 1245–1253. [Google Scholar] [CrossRef]
- Oh, L.; Varki, A.; Chen, X.; Wang, L.-P. SARS-CoV-2 and MERS-CoV Spike Protein Binding Studies Support Stable Mimic of Bound 9-O-Acetylated Sialic Acids. Molecules 2022, 27, 5322. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).