Diffuse Myocardial Fibrosis on Cardiac Magnetic Resonance Imaging Is Related to Galectin-3 and Predicts Outcome in Heart Failure
Abstract
1. Introduction
2. Methods
2.1. Animal Studies
2.1.1. Mouse Model
2.1.2. Immunohistochemistry
2.1.3. Quantitative Polymerase Chain Reaction
2.2. Human Studies
2.2.1. Patient Cohort
2.2.2. CMR Protocol
2.2.3. LGE Analysis
2.2.4. T1 Measurements
2.2.5. Biochemical Measurements
2.3. Statistics
3. Results
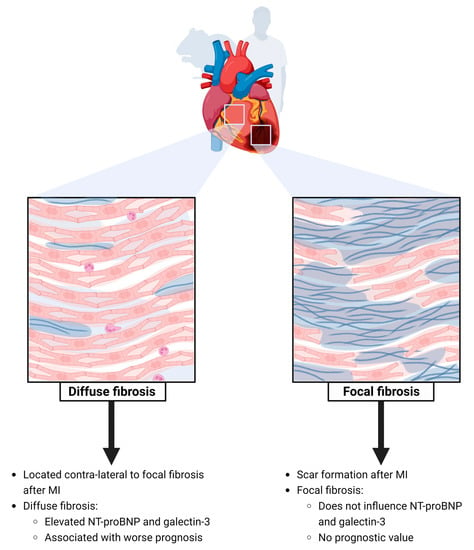
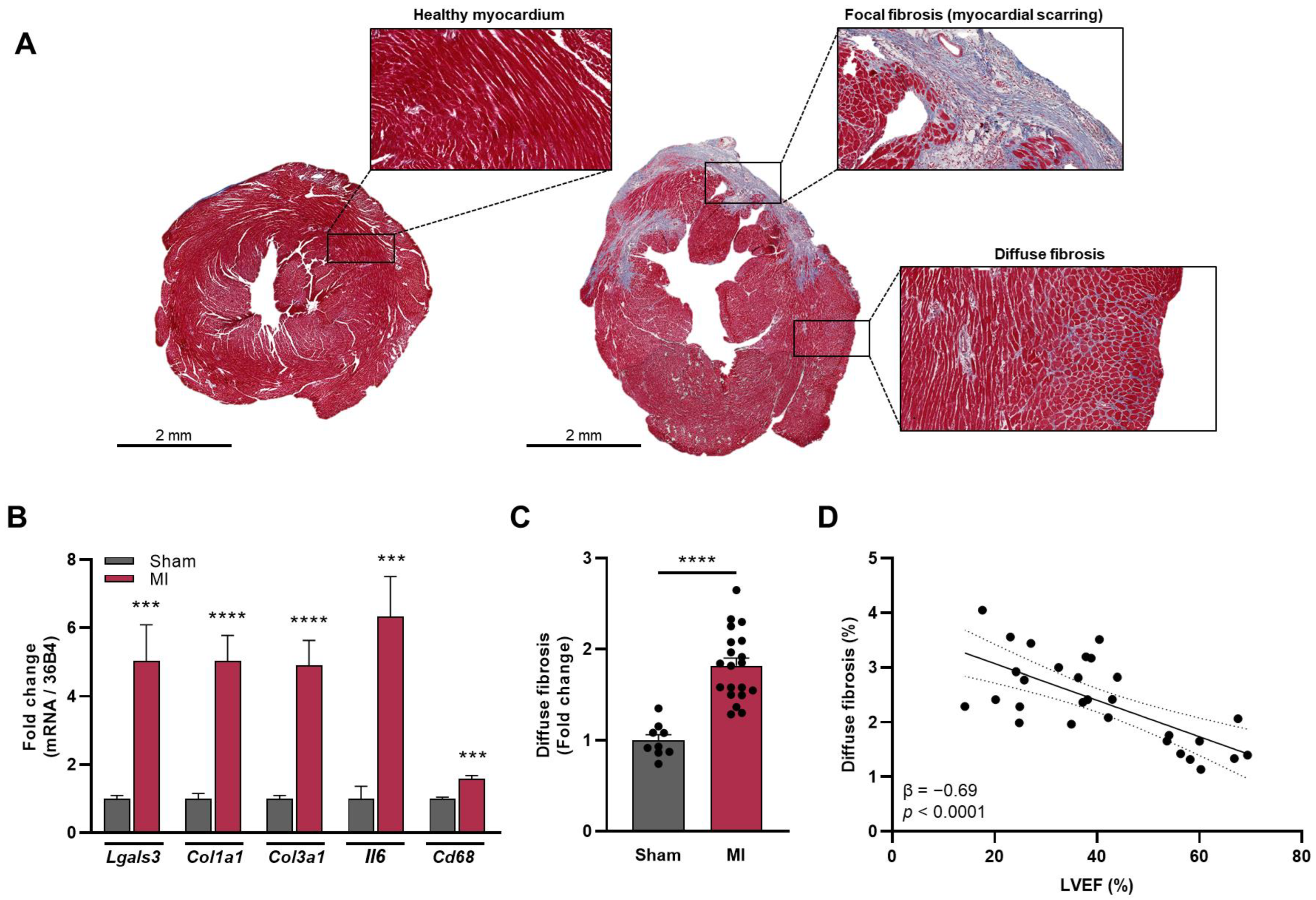
3.1. Presence of Focal Myocardial Fibrosis after MI
3.2. Presence of Diffuse Fibrosis of the Non-Infarcted Myocardium
3.3. LGE Positive vs. LGE Negative and Cardiac Biomarkers
3.4. Diffuse Fibrosis of the Non-Infarcted Myocardium and Cardiac Biomarkers
3.5. Presence of Focal Fibrosis as A Determinant of Outcome
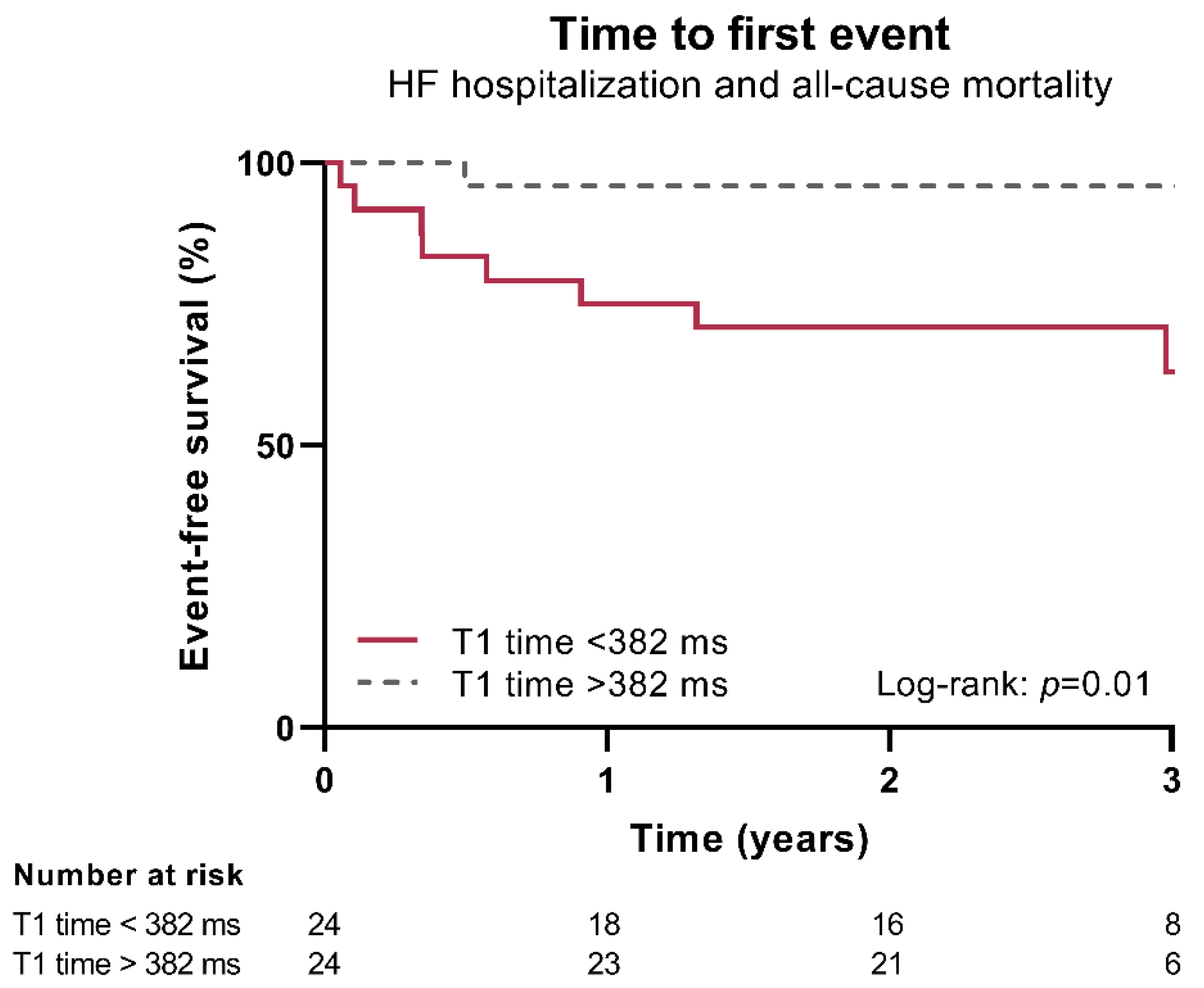
3.6. Presence of Diffuse Fibrosis of the Non-Infarcted Myocardium as A Determinant of Outcome
4. Discussion
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Frantz, S.; Hundertmark, M.J.; Schulz-Menger, J.; Bengel, F.M.; Bauersachs, J. Left ventricular remodelling post-myocardial infarction: Pathophysiology, imaging, and novel therapies. Eur. Heart J. 2022, 43, 2549–2561. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.; Azevedo, C.; Cheng, A.; Gupta, S.; Bluemke, D.; Foo, T.; Gerstenblith, G.; Weiss, R.G.; Marbán, E.; Tomaselli, G.F.; et al. Infarct tissue heterogeneity by magnetic resonance imaging identifies enhanced cardiac arrhythmia susceptibility in patients with left ventricular dysfunction. Circulation 2007, 115, 2006–2014. [Google Scholar] [CrossRef]
- Gulati, A.; Jabbour, A.; Ismail, T.; Guha, K.; Khwaja, J.; Raza, S.; Morarji, K.; Brown, T.D.; Ismail, N.A.; Dweck, M.R.; et al. Association of fibrosis with mortality and sudden cardiac death in patients with nonischemic dilated cardiomyopathy. JAMA 2013, 309, 896–908. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Weiss, R.; Thiemann, D.; Kitagawa, K.; Schmidt, A.; Dalal, D.; Lai, S.; Bluemke, D.A.; Gerstenblith, G.; Marbán, E.; et al. Late gadolinium enhancement by cardiovascular magnetic resonance heralds an adverse prognosis in nonischemic cardiomyopathy. J. Am. Coll. Cardiol. 2008, 51, 2414–2421. [Google Scholar] [CrossRef]
- van Woerden, G.; van Veldhuisen, D.J.; Gorter, T.M.; Willems, T.P.; van Empel, V.P.M.; Peters, A.; Pundziute, G.; op den Akker, J.W.; Rienstra, M.; Westenbrink, B.D. The clinical and prognostic value of late Gadolinium enhancement imaging in heart failure with mid-range and preserved ejection fraction. Heart Vessels 2022, 37, 273–281. [Google Scholar] [CrossRef]
- Fraccarollo, D.; Berger, S.; Galuppo, P.; Kneitz, S.; Hein, L.; Schütz, G.; Frantz, S.; Ertl, G.; Bauersachs, J. Deletion of Cardiomyocyte Mineralocorticoid Receptor Ameliorates Adverse Remodeling After Myocardial Infarction. Circulation 2011, 123, 400–408. [Google Scholar] [CrossRef]
- Taylor, A.J.; Salerno, M.; Dharmakumar, R.; Jerosch-Herold, M. T1 Mapping: Basic Techniques and Clinical Applications. JACC Cardiovasc. Imaging 2016, 9, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Meijers, W.C.; Maglione, M.; Bakker, S.J.L.; Oberhuber, R.; Kieneker, L.M.; de Jong, S.; Haubner, B.J.; Nagengast, W.B.; Lyon, A.R.; van der Vegt, B.; et al. Heart failure stimulates tumor growth by circulating factors. Circulation 2018, 138, 678–691. [Google Scholar] [CrossRef]
- Gehlken, C.; Screever, E.M.; Suthahar, N.; van der Meer, P.; Westenbrink, B.; Coster, J.; van Veldhuisen, D.J.; de Boer, R.A.; Meijers, W.C. Left atrial volume and left ventricular mass indices in heart failure with preserved and reduced ejection fraction. ESC Heart Fail. 2021, 8, 2458–2466. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Edelman, R.; Hesselink, J.; Zlatkin, M.; Crues, J. Clinical Magnetic Resonance Imaging, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Messroghli, D.R.; Radjenovic, A.; Kozerke, S.; Higgins, D.M.; Sivananthan, M.U.; Ridgway, J.P. Modified Look-Locker Inversion Recovery (MOLLI) for High-Resolution T 1 Mapping of the Heart. Magn. Reson. Med. 2004, 52, 141–146. [Google Scholar] [CrossRef]
- Screever, E.M.; van der Wal, M.H.L.; van Veldhuisen, D.J.; Jaarsma, T.; Koops, A.; van Dijk, K.S.; Warink-Riemersma, J.; Coster, J.E.; Westenbrink, B.D.; van der Meer, P.; et al. Comorbidities complicating heart failure: Changes over the last 15 years. Clin. Res. Cardiol. 2022, 112, 123–133. [Google Scholar] [CrossRef]
- Zile, M.R.; Baicu, C.F.; Ikonomidis, J.S.; Stroud, R.E.; Nietert, P.J.; Bradshaw, A.D.; Slater, R.; Palmer, B.M.; Van Buren, P.; Meyer, M.; et al. Myocardial stiffness in patients with heart failure and a preserved ejection fraction: Contributions of collagen and titin. Circulation 2015, 131, 1247–1259. [Google Scholar] [CrossRef] [PubMed]
- van der Velde, A.R.; Meijers, W.C.; Ho, J.E.; Brouwers, F.P.; Rienstra, M.; Bakker, S.J.L.; Kobold, A.C.M.; van Veldhuisen, D.J.; van Gilst, W.H.; van der Harst, P.; et al. Serial galectin-3 and future cardiovascular disease in the general population. Heart 2016, 102, 1134–1141. [Google Scholar] [CrossRef]
- Meijers, W.C.; Januzzi, J.L.; Defilippi, C.; Adourian, A.S.; Shah, S.J.; van Veldhuisen, D.J.; De Boer, R.A. Elevated plasma galectin-3 is associated with near-term rehospitalization in heart failure: A pooled analysis of 3 clinical trials. Am. Heart J. 2014, 167, 853–860. [Google Scholar] [CrossRef]
- Meijers, W.C.; de Boer, R.A.; van Veldhuisen, D.J.; Jaarsma, T.; Hillege, H.L.; Maisel, A.S.; Di Somma, S.; Voors, A.A.; Peacock, W.F. Biomarkers and low risk in heart failure. Data from COACH and TRIUMPH. Eur. J. Heart Fail. 2015, 17, 1271–1282. [Google Scholar] [CrossRef] [PubMed]
- de Boer, R.A.; Voors, A.A.; Muntendam, P.; van Gilst, W.H.; van Veldhuisen, D.J. Galectin-3: A novel mediator of heart failure development and progression. Eur. J. Heart Fail. 2009, 11, 811–817. [Google Scholar] [CrossRef]
- Henderson, N.C.; Mackinnon, A.C.; Farnworth, S.L.; Poirier, F.; Russo, F.P.; Iredale, J.P.; Haslett, C.; Simpson, K.J.; Sethi, T. Galectin-3 regulates myofibroblast activation and hepatic fibrosis. Proc. Natl. Acad. Sci. USA 2006, 103, 5060–5065. [Google Scholar] [CrossRef] [PubMed]
- Meijers, W.C.; van der Velde, A.R.; Pascual-Figal, D.A.; de Boer, R.A. Galectin-3 and post-myocardial infarction cardiac remodeling. Eur. J. Pharmacol. 2015, 763, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Rubinshtein, R.; Glockner, J.F.; Ommen, S.R.; Araoz, P.A.; Ackerman, M.J.; Sorajja, P.; Bos, J.M.; Tajik, A.J.; Valeti, U.S.; Nishimura, R.A.; et al. Characteristics and clinical significance of late gadolinium enhancement by contrast-enhanced magnetic resonance imaging in patients with hypertrophic cardiomyopathy. Circ. Heart Fail. 2010, 3, 51–58. [Google Scholar] [CrossRef]
- Assomull, R.G.; Prasad, S.K.; Lyne, J.; Smith, G.; Burman, E.D.; Khan, M.; Sheppard, M.N.; Poole-Wilson, P.A.; Pennell, D.J. Cardiovascular magnetic resonance, fibrosis, and prognosis in dilated cardiomyopathy. J. Am. Coll. Cardiol. 2006, 48, 1977–1985. [Google Scholar] [CrossRef]
- Puntmann, V.O.; Carr-White, G.; Jabbour, A.; Yu, C.Y.; Gebker, R.; Kelle, S.; Hinojar, R.; Doltra, A.; Varma, N.; Child, N.; et al. T1-Mapping and Outcome in Nonischemic Cardiomyopathy All-Cause Mortality and Heart Failure. JACC Cardiovasc. Imaging 2016, 9, 40–50. [Google Scholar] [CrossRef]
- Meijers, W.C.; López-Andrés, N.; de Boer, R.A. Galectin-3, Cardiac Function, and Fibrosis. Am. J. Pathol. 2016, 186, 2232–2234. [Google Scholar] [CrossRef]
- Puntmann, V.O.; Carr-White, G.; Jabbour, A.; Yu, C.Y.; Gebker, R.; Kelle, S.; Rolf, A.; Zitzmann, S.; Peker, E.; D’Angelo, T.; et al. Native T1 and ECV of Noninfarcted Myocardium and Outcome in Patients with Coronary Artery Disease. J. Am. Coll. Cardiol. 2018, 71, 766–778. [Google Scholar] [CrossRef]
- Marques, M.; Weinberg, R.; Kapoor, S.; Ostovaneh, M.; Kato, Y.; Liu, C.-Y.; Shea, S.; McClelland, R.L.; Post, W.S.; Bluemke, D.A.; et al. Myocardial fibrosis by T1 mapping magnetic resonance imaging predicts incident cardiovascular events and all-cause mortality: The Multi-Ethnic Study of Atherosclerosis. Eur. Heart J. Cardiovasc. Imaging 2022, 23, 1407–1416. [Google Scholar] [CrossRef]
- O’Hara, R.P.; Binka, E.; Prakosa, A.; Zimmerman, S.L.; Cartoski, M.J.; Abraham, M.R.; Lu, D.Y.; Boyle, P.M.; Trayanova, N.A. Personalized computational heart models with T1-mapped fibrotic remodeling predict sudden death risk in patients with hypertrophic cardiomyopathy. Elife 2022, 11, e73325. [Google Scholar] [CrossRef]
- Lewis, G.; Dodd, S.; Clayton, D.; Bedson, E.; Eccleson, H.; Schelbert, E.; Naish, J.H.; Jimenez, B.D.; Williams, S.G.; Cunnington, C.; et al. Pirfenidone in heart failure with preserved ejection fraction: A randomized phase 2 trial. Nat. Med. 2021, 27, 1477–1482. [Google Scholar] [CrossRef]
- Lau, E.; Liu, E.; Paniagua, S.; Sarma, A.; Zampierollo, G.; López, B.; Díez, J.; Wang, T.J.; Ho, J.E. Galectin-3 Inhibition with Modified Citrus Pectin in Hypertension. JACC Basic to Transl. Sci. 2021, 6, 12–21. [Google Scholar] [CrossRef]
- Yu, L.; Ruifrok, W.P.T.; Meissner, M.; Bos, E.M.; van Goor, H.; Sanjabi, B.; van der Harst, P.; Pitt, B.; Goldstein, I.J.; Koerts, J.A.; et al. Genetic and pharmacological inhibition of galectin-3 prevents cardiac remodeling by interfering with myocardial fibrogenesis. Circ. Heart Fail 2013, 6, 107–117. [Google Scholar] [CrossRef]
- Pitt, B.; Zannad, F.; Remme, W.; Cody, R.; Castaigne, A.; Perez, A.; Palensky, J.; Wittes, J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N. Engl. J. Med. 1999, 341, 709–717. [Google Scholar] [CrossRef]
- Moschetti, K.; Muzzarelli, S.; Pinget, C.; Wagner, A.; Pilz, G.; Wasserfallen, J.B.; Schulz-Menger, J.; Nothnagel, D.; Dill, T.; Frank, H.; et al. Cost evaluation of cardiovascular magnetic resonance versus coronary angiography for the diagnostic work-up of coronary artery disease: Application of the European Cardiovascular Magnetic Resonance registry data to the German, United Kingdom, Swiss, and United States health care systems. J. Cardiovasc. Magn. Reason. 2012, 14, 35. [Google Scholar]

| Characteristics | Total Cohort n = 159 | LGE Negative n = 98 | LGE Positive n = 61 | p-Value |
|---|---|---|---|---|
| Age (y), mean (SD) | 59 (14) | 58 (14) | 60 (13) | 0.40 |
| Female sex, n (%) | 66 (42) | 48 (49) | 18 (30) | 0.015 |
| SBP (mmHg), mean (SD) | 120 (20) | 122 (20) | 116 (21) | 0.075 |
| DBP (mmHg), mean (SD) | 73 (11) | 74 (12) | 71 (10) | 0.081 |
| BMI (kg/m2), mean (SD) | 28 (5) | 28 (6) | 27 (4) | 0.063 |
| Heart failure history, n (%) | ||||
| Coronary artery disease | 40 (25) | 8 (8) | 32 (52) | <0.001 |
| Hypertension | 64 (40) | 40 (41) | 24 (39) | 0.85 |
| Atrial fibrillation | 42 (26) | 29 (30) | 13 (21) | 0.25 |
| NYHA class, n (%) | 0.33 | |||
| I | 33 (21) | 22 (22) | 11 (18) | |
| II | 90 (57) | 51 (52) | 39 (64) | |
| III | 36 (22) | 25 (26) | 11 (18) | |
| Medication, n (%) | ||||
| β-blocker | 145 (91) | 88 (90) | 57 (93) | 0.43 |
| ACEi/ARB | 138 (87) | 84 (86) | 54 (89) | 0.61 |
| Diuretic | 105 (66) | 65 (66) | 40 (66) | 0.92 |
| Aldosterone antagonist | 73 (46) | 37 (38) | 36 (59) | 0.009 |
| Laboratory measurements | ||||
| eGFR (mL/min/1.73 m2), mean (SD) | 72 (30) | 72 (31) | 71 (29) | 0.78 |
| NT-proBNP (ng/L), median [IQR] | 753 [235–2137] | 606 [172–1929] | 1082 [381–2530] | 0.052 |
| Galectin-3 (μg/L), median [IQR] | 16.6 [13.1–24.1] | 16.7 [12.8–22.9] | 16.6 [13.5–27.5] | 0.49 |
| CRP (mg/L), median [IQR] | 4.0 [1.6–7.1] | 4.3 [1.7–7.4] | 3.1 [1.3–6.2] | 0.42 |
| Sodium (mmol/L), mean (SD) | 140 (3) | 141 (3) | 139 (3) | 0.016 |
| CMR parameters | ||||
| LVEF (%), median [IQR] | 38 [29–45] | 37 [25–48] | 35 [28–47] | 0.77 |
| Post-contrast T1 time (ms), median [IQR] | 390 (62) | 405 [372–436] | 382 [347–426] | 0.13 |
| LVEDV (mL), mean (SD) | 232 (77) | 231 (76) | 234 (80) | 0.83 |
| LVESV (mL), mean (SD) | 152 (73) | 150 (70) | 156 (78) | 0.59 |
| LVSV (mL), mean (SD) | 79 (28) | 80 (29) | 78 (26) | 0.68 |
| Characteristics | T1 Time < 382 ms n = 24 | T1 Time > 382 ms n = 24 | p-Value |
|---|---|---|---|
| Age (y), mean (SD) | 62 (12) | 56 (14) | 0.11 |
| Female sex, n (%) | 11 (46) | 5 (21) | 0.066 |
| Heart failure history, n (%) | |||
| Coronary artery disease | 11 (46) | 10 (42) | 0.77 |
| Hypertension | 9 (38) | 8 (33) | 0.76 |
| Atrial fibrillation | 5 (21) | 4 (17) | 0.71 |
| NYHA class, n (%) | 0.28 | ||
| I | 4 (17) | 6 (25) | |
| II | 14 (58) | 16 (67) | |
| III | 6 (25) | 2 (8) | |
| Medication, n (%) | |||
| β-blocker | 22 (92) | 22 (92) | 1.00 |
| ACEi/ARB | 22 (92) | 24 (100) | 0.15 |
| Diuretic | 18 (75) | 13 (54) | 0.13 |
| Aldosterone antagonist | 15 (63) | 14 (58) | 0.77 |
| Laboratory measurements | |||
| NT-proBNP (ng/L), median [IQR] | 1611 [1066–4406] | 453 [323–1557] | 0.009 |
| Galectin-3 (μg/L), median [IQR] | 20.3 [16.3–32.2] | 14.5 [12.6–20.0] | 0.011 |
| Creatinine (μmol/L), median [IQR] | 100 [84–135] | 89 [74–99] | 0.18 |
| eGFR (mL/min/1.73 m2), mean (SD) | 61 [41–79] | 84 [62–98] | 0.008 |
| CMR parameters | |||
| LVEF (%), median [IQR] | 33 [22–36] | 33 [23–45] | 0.35 |
| Post-contrast T1 time (ms), median [IQR] | 347 [303–365] | 426 [410–443] | <0.001 |
| LVEDV (mL/m2), median [IQR] | 135 [100–149] | 132 [103–159] | 0.51 |
| LVESV (mL/m2), median [IQR] | 92 [66–113] | 93 [62–115] | 0.85 |
| LV mass (g/m2), median [IQR] | 53 [44–78] | 59 [53–77] | 0.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Screever, E.M.; Gorter, T.M.; Willems, T.P.; Aboumsallem, J.P.; Suthahar, N.; Mahmoud, B.; van Veldhuisen, D.J.; de Boer, R.A.; Meijers, W.C. Diffuse Myocardial Fibrosis on Cardiac Magnetic Resonance Imaging Is Related to Galectin-3 and Predicts Outcome in Heart Failure. Biomolecules 2023, 13, 410. https://doi.org/10.3390/biom13030410
Screever EM, Gorter TM, Willems TP, Aboumsallem JP, Suthahar N, Mahmoud B, van Veldhuisen DJ, de Boer RA, Meijers WC. Diffuse Myocardial Fibrosis on Cardiac Magnetic Resonance Imaging Is Related to Galectin-3 and Predicts Outcome in Heart Failure. Biomolecules. 2023; 13(3):410. https://doi.org/10.3390/biom13030410
Chicago/Turabian StyleScreever, Elles M., Thomas M. Gorter, Tineke P. Willems, Joseph Pierre Aboumsallem, Navin Suthahar, Belend Mahmoud, Dirk J. van Veldhuisen, Rudolf A. de Boer, and Wouter C. Meijers. 2023. "Diffuse Myocardial Fibrosis on Cardiac Magnetic Resonance Imaging Is Related to Galectin-3 and Predicts Outcome in Heart Failure" Biomolecules 13, no. 3: 410. https://doi.org/10.3390/biom13030410
APA StyleScreever, E. M., Gorter, T. M., Willems, T. P., Aboumsallem, J. P., Suthahar, N., Mahmoud, B., van Veldhuisen, D. J., de Boer, R. A., & Meijers, W. C. (2023). Diffuse Myocardial Fibrosis on Cardiac Magnetic Resonance Imaging Is Related to Galectin-3 and Predicts Outcome in Heart Failure. Biomolecules, 13(3), 410. https://doi.org/10.3390/biom13030410