Chemistry and Pharmacology of Bergenin or Its Derivatives: A Promising Molecule
Abstract
1. Introduction
| Family | Scientific Name of the Plant | Part of Plant Used | Vernacular Plant Name | Native Location of the Plant | Reference |
|---|---|---|---|---|---|
| Acanthaceae | Gendarussa vulgaris Nees. | Aerial parts | Justicia gendarussa | India, Malaysia | [11] |
| Asclepiadaceae | Streptocaulon griffithii Hook. F. | Root | - | China | [12] |
| Araceae | Arisaema franchetianum Engl. | Tuber | - | Central Africa | [13] |
| Bombaceae | Bombax malabaricum L. | Flowers | Red silk cotton tree | India | [14] |
| Bignoniaceae | Winter bignonia (Ker Gawl.) Miers. | Rhizome | - | India | [15] |
| Caryophyllaceae | Brachystemma calycinum D.Don. | Aerial parts | - | Nepal | [16] |
| Connaraceae | Connarus monocarpus | Root | - | Peninsula of India | [17] |
| Convolvulaceae | Rivea hypocrateriformis (Desr.) Choisy | Stem | - | South Asia | [18] |
| Clusiaceae | Garcinia malaccensis Hook. F. | Stem bark | - | China | [11] |
| Compositae | Pulicaria wightiana C.B.Clarke | Aerial parts | Sonaphuli | India | [19] |
| Capprifoliaceae | Brachystemma calycinum D.Don. | Aerial parts | - | Nepal | [16] |
| Crassulaceae | Crassula ovata cv. Obligua. | Bark | Jade plant | South Africa and Mozambique | [8,20] |
| Rhodiola kirilowii Reg | Leaf | Rose bush | Himalayas | [21] | |
| Dilleniaceae | Doliocarpus dentatus (Aubl.) | Leaves | Thirsty vine | Mexico and tropical America | [22] |
| Dipterocarpaceae | Dipterocarpus grandflorus Blanco | Stem | Keruing Bilinbing | Asia | [2,23] |
| Dryobalonops aroniatica C.F. Gaertn. | Stem bark and heartwood | Borneo camphor | Sumatra, Borneo, Peninsular Malaysia | [2,24] | |
| Hopea utilis (Bedd.) Bole | Leaf | Black kongu | India | [2,25] | |
| Hopea sangal Korth. | Leaf | Mersiput | Singapore | [2,26] | |
| Shorea leprosula PROSEA. | Heartwood | Red meranti | Indonesia | [27] | |
| Shorea robusta Roth. | Leaf and root | Sal | India | [28] | |
| Vatica pauciflora (Korth.) Blume. | Stem bark | - | Malaysia, Sumatera, Thailand and Vietnam | [29,30] | |
| Vatica albiramis Van. | Stem | - | Indonesia | [31] | |
| Vatica bantamensis (Hassk.) Benth. & Hook.F. | Leaf | - | Indonesia | [32] | |
| Vatica diospyroides S. | Stem | - | Malaysia | [33] | |
| Vatica mangachpoi | Leaf | Resak | Malaysia | [34] | |
| Vateria indica C.F. Gaertn. | Leaf, seed, and stem bark | - | Asia | [35] | |
| Ebenaceae | Diospyros sanza-minika PROTA | Wood | Liberia ebony | Africa | [36] |
| Mussaenda erythrophylla Schumach. & Thonn | Aerial parts | Pink mousseenda | Asia | [37] | |
| Ericaceae | Arctostaphylos uva-ursi L. | Leafy shoot | Bearberry | Western America | [38] |
| Euphorbiaceae | Fluggea virosa (willd.) voigt | Aerial parts | Chinese waterberry | Saudi Arabia | [39] |
| Fluggea leucopyrus Willd | Leaf | - | Asia | [40] | |
| Fluggea microcarpa Blume | Leaf | - | Southern Africa | [41] | |
| Fluggea luvangetina | Root | - | Paleotropics | [41] | |
| Fluggea religiosa L. | Bark | - | Paleotropics | [42] | |
| Fluggea virens L. | Bark | - | Paleotropics | [42] | |
| Fluggea glomerata L. | Bark | - | Paleotropics | [42] | |
| Fluggea benghalensis L. | Bark | - | Paleotropics | [42] | |
| Glochidion obovatum Siebold & Zucc. | Leaf | - | Japan | [43] | |
| Glochidion obliquum Siebold & Zucc. | Leaf | - | Japan | [44] | |
| Excoecaria agallocha L. | Leaf | Buta-Buta | Singapore | [45] | |
| Mallotus japonicus Müll. Arg. | Bark and cortex | Akamegashiwa | East Asia | [46] | |
| Mallotus repandus (Rottler) Müll. Arg. | Stem | Liana creeper | Tropical and Subtropical Asia | [47] | |
| Mallotus anisopodus Gagnep. | Aerial parts | - | Vietnam | [48] | |
| Mallotus philippinensis (Lam.) Mull.Arg. | Leaf and stem bark | Kamala tree | Asia and Australia | [49] | |
| Mallotus roxburghianus (Lam.) Müll.Arg. | Leaf | - | India | [50] | |
| Mollotus oppositifolius | Leaf | - | - | [51] | |
| Macaranga peltata (Roxb.) Müll.Arg | Bark | Chandada | India | [2,52] | |
| Phyllanthus columnaris Müll.Arg. | Root bark | - | Andaman Islands | [53] | |
| Phyllanthus flexuosus Müll.Arg. | Stem bark | - | China | [54] | |
| Phyllanthus wightianus Müll.Arg. | Whole plant | - | India | [54] | |
| Securinega virosa (Roxb.) | Leaf | Itachen-gado | Nigeria | [55] | |
| Securinega melanthesoides (F. Muell.) Airy Shaw | Leaf | - | Madagascar and Mascarene Islands | [56,57] | |
| Fabaceae | Peltophorum africanum Sond. | Root | Weeping wattle | Africa | [6] |
| Peltophorum inerme (Roxb.) Náves. | Flower | Yellow cassia | Southeast Asia | [58,59] | |
| Peltophorum pterocarpum | Flower | Yellow-flamboyant | Tropical Southeastem Asia | [60,61] | |
| Peltophorum ferruginium (DC.) Backer | Bark | Yellow Poinciana | Southeast Asia | [2] | |
| Ciser microphyllum | Aerial parts | - | Himalayas | [62] | |
| Teramnus labialis (L.f.) Spreng. | Aerial parts | Blue wiss | Tropical Africa | [63] | |
| Gentianaceae | Tripterospermum chinense (Migo) Harry Sm. | Aerial parts | - | China | [2] |
| Hamamelidaceae | Corylopsis coreana Uyeki | Leaf | - | Eastern Asia | [64] |
| Corylopsis spicata | Bark | Winter Hazel | Japan | [65] | |
| Corylopsis willmottiae | Whole plant | Chinese winter Hazel | China | [2] | |
| Asteraceae | Tridax procumbens L. | Aerial | Bullweed | Tropical Americas | [66] |
| Humiriaceae | Endopleura uchi (Huber.) Cuatrec. | Bark | Uchi | Brazilian Amazon | [67] |
| Humiria balsamifera (Aubl.) A.St.—Hil. | Aerial parts | Umiri de cheiro | Brazilian Amazon | [39,68] | |
| Sacoglottis gabonensis Urb. | Bark | Bitterbark tree | Tropical Africa and South America | [69,70] | |
| Lythraceae | Woodfordia fruticosa (L.) Kurz | Stem | Fire-flame bush | Asia | [71] |
| Lagerstroemia speciosa L. | Flowers | - | - | [14] | |
| Leguminosae | Caesalpinia decapetala (Roth) | Root | - | China | [2] |
| Caesalpinia mimosoides Lam. | Root | - | China | [2] | |
| Caesalpinia pluviosa D | Stem Bark | - | - | [2] | |
| Caesalpinia digyna Rottl. | Root | - | Eastern Himalayas, Assam, and West Bengal | [72] | |
| Cenostigma macrophyllum Tul. | Stem bark | Caneleiro | Brazil | [73] | |
| Cenostigma gardnerianum Tul. | Stem bark | Cinnamon | Brazil | [73] | |
| Pentaclethra macrophylla Benth. | Root | African bean | West and Central Africa | [74,75] | |
| Malvaceae | Brachystenuna calycinum D. Don-GBIF | Root | - | China | [2] |
| Thespesia popunea L. | Bark | - | - | [42] | |
| Moraceae | Ficus racemosa L. | Bark | Red river fig | Australia and tropical Asia | [76] |
| Myrsinaceae | Ardisia crenata Sims. | Root | Coral bush; Blueberry. | East Asia | [77] |
| Ardisia colorata Blume. | Fruit | Marlberry | China | [78] | |
| Ardisia japonica Blume. | Aerial parts | Passion fruit | East China, Japan, and Korea | [79] | |
| Ardisia elliptica Andr. | Root | Duck’s-eyes | West coast of India | [2] | |
| Ardisia punctata (Reinw.) | Root | Common Labisia | Southeast Asia | [2] | |
| Ardisia pusilla A. DC. | Root | Marlberry | Asia | [80] | |
| Ardisia escalloniodes S. and D. | Seed | Marlberry | Asia | [77] | |
| Ardisia compressa (Kunth.) | Seed | Marlberry | Asia | [77] | |
| Ardisia mamillata (Hance.) | Seed | Marlberry | Asia | [77] | |
| Ardisia gigantifolia Stapf. | Root | Marlberry | Asia | [81] | |
| Oleaceae | Olea dioca Roxb. | Flowers | Marlberry | Asia | [14] |
| Pinaceae | Pinus roxburghii Charg. | Leaf | Marlberry | Asia | [82] |
| Ranunculaceae | Cimicifuga foetida L. | Rhizome | - | Europa and Siberia | [2] |
| Pulsatilla koreana Mill. | Root | - | Korea | [2] | |
| Rubiaceae | Wendlandia thyrsoidea (Roth) Steud. | Flowers | - | India | [2] |
| Saxifragaceae | Astilbe chinensis (Maxim.) Engl. | Rhizome | - | Japan | [83,84] |
| Astilbe rivularis Buch. Ham. | Rhizome | River Astilbe | East Asian | [85] | |
| Astilbe myriantha Diels. | Rhizome | Rabbit ear | East Asian | [2,86] | |
| Astilbe thunbergii Miq. | Rhizome | - | Japan | [86] | |
| Bergenia scopulosa | Rhizome | - | China | [2,87] | |
| Bergenia ligulata Wall. | Leaf | - | Central Asia | [87] | |
| Bergenia purpurascens (Hook.f. e Thomson) Engl. | Rhizome | - | Asia | [82] | |
| Bergenia stracheyi (Hook. f. e Thomson) Engl. | Whole plant | - | Central Asia | [88] | |
| Bergenia cordifolia (Haw.) Sternb. | Rhizome | Siberian tea | Central Asia | ||
| Boykinia lycoctonifolia (Maxim) Engl. | Rhizome | - | North America and Asia | [83,84] | |
| Peltiphyllum peltatum L. | Rhizome | Indian-apple | Mato Grosso (Brasil) | [2] | |
| Peltoboykinia watanabei L. | Rhizome | - | Japan | [2] | |
| Rodgersia sambucifolia | Root | Elderberry | China | [2] | |
| Rodgersia pinnata | Rhizome | Bronze Peacock | China | [2] | |
| Rodgersia aesculifolia Bet. | Rhizome | - | Northern China | [1] | |
| Saxifraga crassifolia L. | Leaf | Siberian tea | Central Asia | [1] | |
| Saxifraga melanocentra FRANCH. | Leaf | - | China | [88] | |
| Saxifraga stolonifera Curtis. | Leaf | - | China | [89] | |
| Sapindaceae | Allophylus edulis var. edulis | Leaf | Pigeon fruit | Latin America | [90] |
| Vitaceae | Cissus javana DC. | Root | Rex begonia vine | Thailand | [91] |
2. Materials and Methods
Data Collection
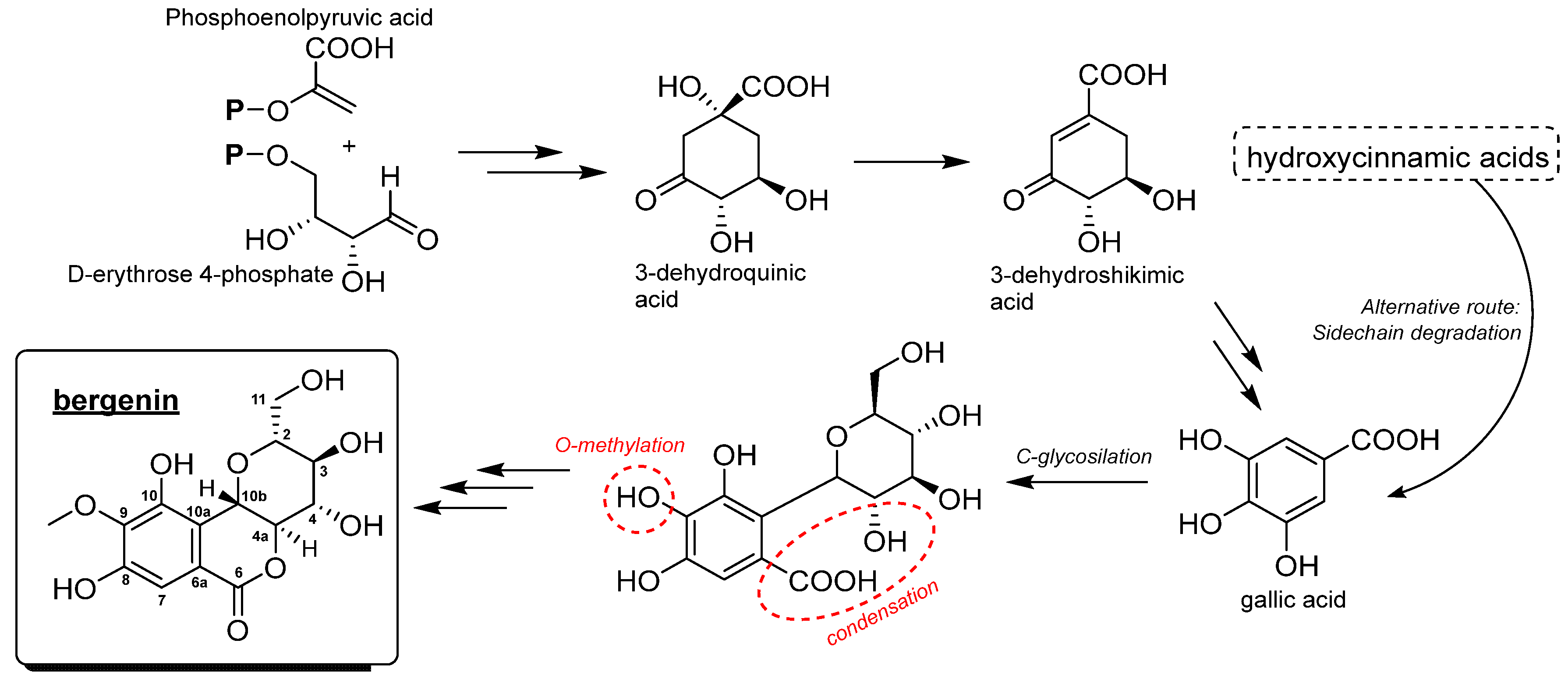
3. Natural Sources of Bergenin
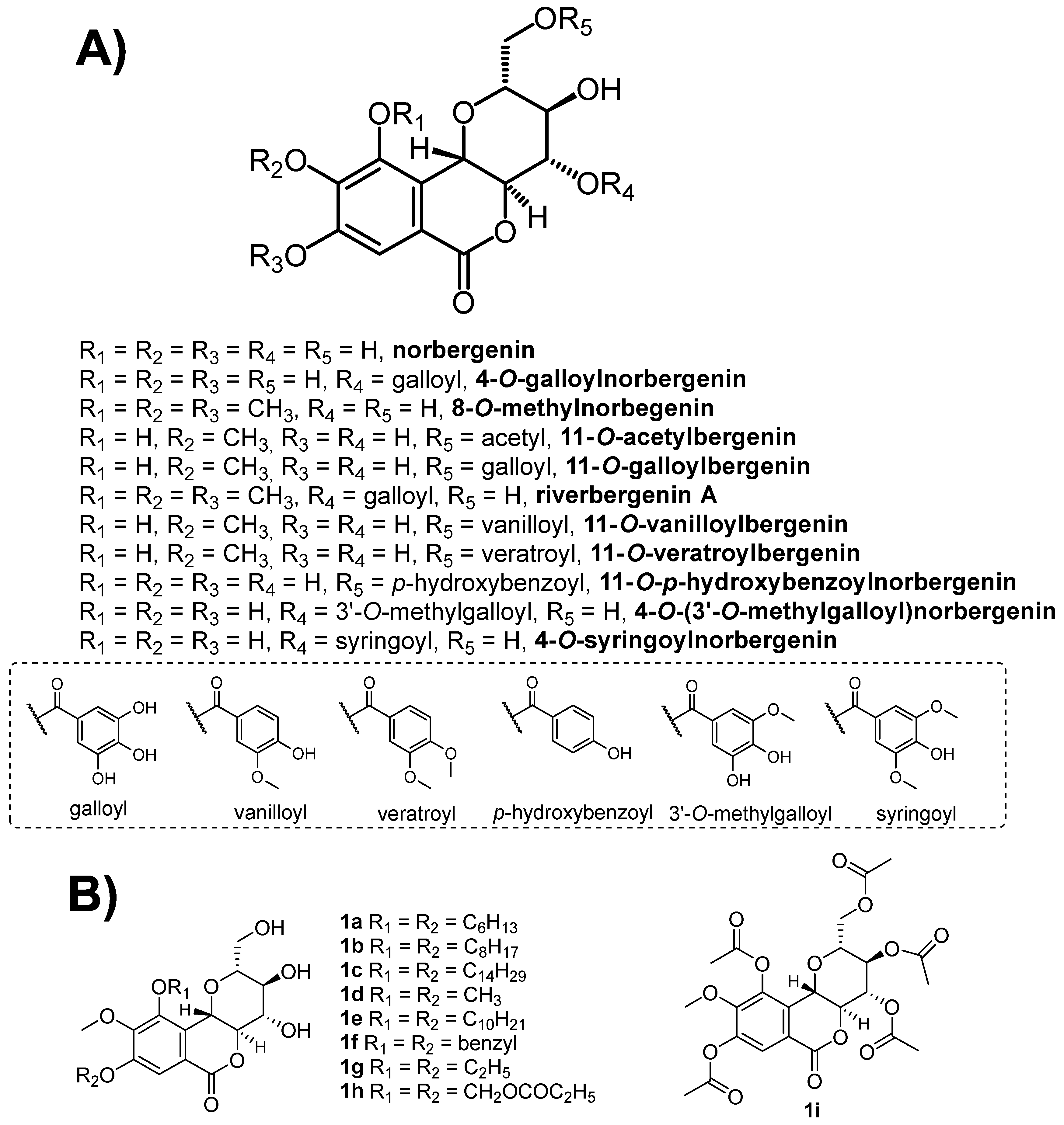
4. Chemical Aspects of Bergenin
5. Biological and Pharmacological Activities of Bergenin
5.1. Antimalarial Activity
| Compound Name | Source of Isolation | Assay Type, IC50 | Cytotoxicity | Reference | |
|---|---|---|---|---|---|
| CL | CV | ||||
| Bergenin | Ethanolic extract of the rhizome of W. bignonia | in vitro, 5.0 µg/mL | HeLa and dermal fibroblasts | NC | [15] |
| Bergenin | Leaves of F. virosa | in vitro, 8.07 µg/mL | Murine intraperitoneal macrophages | NC | [39] |
| Bergenin | Methanolic extract of the stem bark of D. sanza-minika | in vitro, 0.6 µg/mL | ND | ND | [36] |
| Bergenin | Leaves of H. balsamifera | in vitro, SA | ND | ND | [107] |
| Bergenin | Ethanolic extract of the rhizome of B. ciliata | in vitro, 5.0 µg/mL | HeLa and dermal fibroblasts | NC | [15] |
| Bergenin | Rhizome of B. ciliata | in vivo -mice, 50 mg/kg | HeLa and dermal fibroblasts | NC | [15] |
| Bergenin | Leaves of R. aesculifolia | in vitro, 14.1 µg/mL | HeLa and HepG2 | NC | [6] |
| Bergenin | Leaves of R. aesculifolia | in vivo -mice, 800 mg/kg) | HeLa and HepG2 | NC | [6] |
| Bergenin | Roots of B. ligulata | in vitro, 2.41 µg/mL | ND | ND | [105] |
| Bergenin | Whole plant of M. philippensis | in vitro, 6.92 µM | ND | ND | [49] |
| 11-O-Galloylbergenin | Roots of B. ligulata | in vitro, 2.34 µg/mL) | ND | ND | [108] |
| 4-O-(3′-Methylgalloyl)norbergenin | Methanolic extract of the stem bark of D.sanza-minika | in vitro, 0.6 µg/mL | ND | ND | [36] |
| 4-O-Galloylnorbergenin | Methanolic extract of the stem bark of Diospyros sanza-minika | in vitro, 3.9 µg/mL | ND | ND | [36] |
| 11-O-p- Hydroxybenzoylnorbergenin | Methanolic extract of the stem bark of Diospyros sanza-minika | in vitro, 4.9 µg/mL | ND | ND | [36] |
| 11-O-Galloylbergenin | Whole plant of M. philippensis | in vitro, 7.85 µM | ND | ND | [49] |
| Bergenin | Extract of the aerial part of M. erythrophylla | in vitro, 7.43 µg/mL | Raw 264.7 macrophage cells | NC | [37] |
| Bergenin | Leaves of F. virosa | in vivo -mice,100 mg/Kg) | Murine intraperitoneal macrophages | NC | [49] |
| Bergenin | Leaves of H. balsamifera | in vivo -mice, 146.87 mg/kg | ND | ND | [107] |
| Bergenin | Ethanolic extract of the rhizome of W. bignonia | in vivo -mice, 50 mg/kg | HeLa and dermal fibroblasts | NC | [15] |
5.2. Antileishmanial Activity
5.3. Trypanocidal Activity
5.4. Antiviral Activity
5.5. Antibacterial Activity
5.6. Antifungal Activity
5.7. Anti-inflammatory Activity
| Compound Name | Source of Isolation | Type of Assay, IC50 | Cytotoxicity | Reference | |
|---|---|---|---|---|---|
| CL | CV | ||||
| Bergenin | Trunk bark of E. uchi | in vitro, 1.2 µM | NC | NC | [131] |
| Bergenin | Bergenia spp. | in vivo -mice, 50 mg/kg | Raw264.7 cells | NC | [109] |
| Bergenin | NI | in vitro, 50 µM | CCK8Cells | NC | [134] |
| Bergenin | Peltophorum spp. | in vivo -rats, 25 mg/kg | ND | ND | [137] |
| Bergenin | NI | in vitro, 10 µM | Raw264.7 cells | NC | [141] |
| Bergenin | Trunk bark of E. uchi | in vivo -mice, 100 mg/kg | ND | ND | [143] |
| Bergenin | NI | in vivo -rats, 50 mg/kg | ND | ND | [138] |
| Bergenin | Bark, leaf, and branches of E. uchi | in vitro, 1.2 µM | ND | ND | [132] |
| Acetylbergenin | Stem bark of E. uchi | in vivo -rats, 6.8 mg/kg | ND | ND | [144] |
| Bergenin | Stem bark of C. gardnerianum | in vivo -mice,12.5 mg/kg | BALB/c mice splenocytes | NC | [73] |
| Bergenin | Crude extract of M. philippenensis | in vitro, 303.12 µM | NI | NC | [126] |
| Heptylbergenin | Crude extract of M. philippenensis | in vitro, 212.95 µM | NI | NC | [126] |
| Octylbergenin | Crude extract of M. philippenensis | in vitro, 269.99 µM | NI | NC | [126] |
| Ethylbergenin | Crude extract of M. philippenensis | in vitro, 322.09 µM | NI | NC | [126] |
| Propylbergenin | Crude extract of M. philippenensis | in vitro, 303.12 µM | NI | NC | [126] |
| Bergenin | S. stolonifera | in vivo- mice, 100 mg/kg | RAW264.7 cells | NC | [139] |
| Bergenin | S. stolonifera herb | in vitro, 0.1 µM | RAW264.7Cells | NC | [139] |
| Bergenin | NI | in vivo -rats, 87 mg/kg | ND | ND | [147] |
| Bergenin | NI | in vitro, 100 µM | CCK-8 | NC | [135] |
| Bergenin | NI | in vitro, 30 µM | ND | ND | [136] |
| Bergenin | Stem bark of C. gardnerianum | in vivo -rats, 25 mg/kg | Macrophages | NC | [149] |
| Bergenin | Rhizome of Bergenia spp. | in vitro, 7.29 μM | INS-1E rat insulinoma cells | NC | [150] |
| Bergenin | Extract of B. ciliata | in vitro, 12.5 μg/mL | THP-1 | NC | [22] |
| Bergenin | Extract of B. ciliata | in vivo -mice, 100 mg/kg | THP-1 | NC | [22] |
| 11-O-(40-O-Methylgalloyl)-bergenin | Methanolic extract of S. atrata | in vitro, 100 μg | ND | ND | [129] |
| Bergenin | Ethanolic extract of dry leaves of T. procumbens | in vitro, 70.54 µM | ND | ND | [66] |
| Bergenin | Ethanolic extract of dry leaves of T. procumbens | in vivo -rats, 200 mg/kg | ND | ND | [66] |
| 11-O-Galloylbergenin | Ethanolic extract of M. philippinensis | in vivo -rats, 20 mg/kg | LCMK-2 monkey kid-ney epithelial cells and mice hepatocytes | NC | [151] |
| Acetylbergenin | Stem bark of E. uchi | in vivo -rats, 6.8 mg/kg | ND | ND | [152] |
5.8. Antioxidant Activity
| Compound Name | Source of Isolation | Type of Assay + IC50 | Cytotoxicity | Reference | |
|---|---|---|---|---|---|
| CL | CV | ||||
| Bergenin | Roots of B. ligulata | in vitro/DPPH, 100 µg/mL | ND | ND | [105] |
| 11-O-Galloylbergenin | Roots of B. ligulata | in vitro/DPPH, 7.45 µg/mL | ND | ND | [105] |
| Bergenin | Extracts of the aerial parts of B. ligulata | in vitro/DPPH, 54 μg/mL | ND | ND | [108] |
| Hydroxybenzoyl-bergenin | Extracts of the aerial parts of B. ligulata | in vitro/DPPH, 7.45 μg/mL | ND | ND | [108] |
| 11-O-Galloylbergenin | Extracts of the aerial parts of B. ligulata | in vitro/DPPH, 5.39 μg/mL | ND | ND | [108] |
| Bergenin | Stem bark of P. pterocarpum | in vitro/DPPH, 0.96 μg/mL | ND | ND | [154] |
| Bergenin diethyl ether | Stem bark of P. pterocarpum | in vitro/DPPH, 400 μg/mL | ND | ND | [154] |
| Bergenin | Roots of C. digyna | in vitro/DPPH, 165.35 μg/mL | ND | ND | [73] |
| Bergenin | Flowers of P. pterocarpum | in vitro/DPPH, 1.95 μg/mL | ND | ND | [155] |
| Bergenin | Ethanol extract from dried leaves of T. procumbens | in vitro/DPPH, 20.42 μg/mL | ND | ND | [66] |
| Bergenin | Stem bark of M. japonicus | in vitro/DPPH, 951 μM | ND | ND | [46] |
| Bergenin | M. philippensis | in vitro/DPPH, 99.807 μg/mL | ND | ND | [49] |
| 11-O-Galloylbergenin | M. philippensis | in vitro/DPPH, 7.276 μg/mL | ND | ND | [49] |
| Bergenin | Rhizome of B. ciliata | in vitro/DPPH, 100 μg/mL | ND | ND | [157] |
| Bergenin | E. uchi | in vitro/DPPH, 4.02 μg/mL | ND | ND | [158] |
| Bergenin | Rhizome of B. ciliata | in vitro/DPPH, 0.7 mg/g | ND | ND | [156] |
| Bergenin | Aerial parts of T. labialis | in vitro/DPPH, 100 μg/mL | ND | ND | [63] |
| Bergenin | Bark, leaf, and branches of E. uchi | in vitro/DPPH, 24.20 μg/mL | J774 cells of murine macrophages | NC | [168] |
| Bergenin | Ethanol extract from dried leaves of T. procumbens | in vitro/H2O2, 100 μg/mL | ND | ND | [66] |
| Bergenin | Roots of C. digyna | in vitro/H2O2, 32.54 μg/mL | ND | ND | [73] |
| Bergenin | Roots of C. digyna | in vitro/H2O2, 75.06 μg/mL | ND | ND | [73] |
| Bergenin | M. repandus | in vitro/H2O2, 0.25 mg/mL | ND | ND | [47] |
| Bergenin | Roots of C. digyna | in vitro/ABTS, 75.06 μg/mL | ND | ND | [73] |
| Bergenin | Ethanol extract from dried leaves of T. procumbens | in vitro/ABTS, 31.56 μg/mL | ND | ND | [66] |
| Bergenin | M. repandus | in vitro/ABTS, 0.08 mg/mL | ND | ND | [47] |
| Bergenin | M. repandus | in vitro/OH, 0.12 mg/mL | ND | ND | [47] |
| Bergenin | Flowers of P. pterocarpum | in vitro/OH, 8.48 μg/mL | ND | ND | [155] |
| Bergenin | Rhizome of B. crassifolia | in vitro/FRAP, 0.4 mg/g | ND | ND | [156] |
| Bergenin | Rhizome of B. ornata | in vitro/NADH, 1 mg/g | ND | ND | [156] |
| Bergenin | Roots of C. digyna | in vitro/NO, 785.63 μg/mL | ND | ND | [73] |
| Bergenin | M. repandus | in vitro/NO, 0.32 mg/mL | ND | ND | [47] |
| Bergenin | Flowers of P. pterocarpum | in vitro/NO, 2.98 μg/mL | ND | ND | [155] |
| Bergenin | Roots of C. digyna | in vitro/PL, 365.12 μg/mL | ND | ND | [73] |
| Bergenin | NI | in vitro, 75 μM | HepG2 | NC | [161] |
| Bergenin | NI | in vivo -mice, 20 mg/kg | Splenic NK and CTL cells | NC | [163] |
| Bergenin | NI | in vivo -mice, 20 mg/kg | NI | NI | [165] |
| Bergenin | NI | in vivo -mice, 10 mg/kg | HepG2 | NC | [161] |
| Bergenin | Extract of stem bark from S. gabonensis | in vivo -mice, 2.8 mg/100 g | ND | ND | [166] |
| 4-O-p-Hydroxybenzoylnorbergenin | Leaves of D. gilletii | in vitro/DPPH, 8.2 μg/mL | ND | ND | [169] |
| Methylbergenin | Whole plant of A. japonica | in vitro/NO, 38.4 μg/mL | HepG-2 | NC | [170] |
5.9. Antinociceptive Activity
5.10. AntiArthritic Activity
5.11. Antiulcerogenic Activity
5.12. Antidiabetic/Antiobesity Activity
5.13. AntiArrhythmic Activity
5.14. Anticancer Activity
5.15. Hepatoprotective Activity
5.16. Neuroprotective Activity
5.17. Cardioprotective Activity
6. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Okada, T.; Suzuki, T.; Hasobe, S.; Kisara, K. Studies on Bergenin (Report I). Antiulcerogenic activities of Bergenin. Folia Pharmacol. Jpn. 1973, 69, 369–378. [Google Scholar] [CrossRef]
- Bajracharja, G.B. Diversity, pharmacology and synthesis of bergenin and its derivatives: Potential materials for therapeutic usages. Sugar Wounds 2015, 101, 133–152. [Google Scholar] [CrossRef]
- Borges, L.P.; Amorim, V.A. Metabólitos secundários de plantas secondary plant metabolites. Rev. Agrotecnologia. Ipameri 2020, 11, 54–67. [Google Scholar]
- De Sá, B.M.; Estudo da Toxidade Não Clínica do Extrato Hidroetanolico das Cascas do Caule e de Endopleura uchi (Huber) Cuatrec. Dissertação de Mestrado, Programa de Pós-Graduação em Biodiversidade Tropical, Instituto de Pesquisas Cientificas Agropecuária do Amapá. 2014. Available online: http://repositorio.unifap.br:80/jspui/handle/123456789/484 (accessed on 9 August 2022).
- Marx, F.; Andrade, E.H.A.; Zoghbi, M.G.B.; Maia, J.G.S. Studies of edible Amazonian plants. Part 5: Chemical characterization of Amazonian Endopleura uchi fruits. Eur. Food Res. Technol. 2002, 214, 331–334. [Google Scholar] [CrossRef]
- Liang, J.; Li, Y.; Liu, X.; Huang, Y.; Shen, Y.; Wang, J.; Liu, Z.; Zhao, Y. In vivo and in vitro antimalarial activity of bergenin. Biomed. Rep. 2013, 1, 260–264. [Google Scholar] [CrossRef]
- Pu, H.-L.; Huang, X.; Zhao, J.-H.; Hong, A. Bergenin is the Antiarrhythmic Principle of Fluggea virosa. PubMed 2002, 68, 372–374. [Google Scholar] [CrossRef]
- Lee, Y.Y.; Jang, D.S.; Jin, J.L.; Yun-Choi, H.S. Anti-platelet aggregating and anti-oxidative activities of 11-O- (4′-O-methylgalloyl)-bergenin, a new compound isolated from Crassula cv. ‘Himaturi’. Planta Med. 2005, 71, 776–777. [Google Scholar] [CrossRef]
- Rajbhandari, M.; Lalk, M.; Mentel, R.; Lindequist, U. Antiviral Activity and Constituents of the Nepalese Medicinal Plant Astilbe rivularis. Rec. Nat. Prod. 2011, 5, 138–142. [Google Scholar]
- Gao, X.J.; Guo, M.Y.; Wang, T.C.; Cao, Y.G.; Zhang, N.S. Bergenin Plays an Anti-Inflammatory Role via the Modulation of MAPK and NF-κB Signaling Pathways in a Mouse Model of LPS-Induced Mastitis. Inflammation 2015, 38, 1142–1150. [Google Scholar] [CrossRef]
- Lu, S.; Zhang, G. Alkaloids from Gendarussa vulgaris Nees. Nat. Prod. Res. 2008, 22, 1610–1613. [Google Scholar] [CrossRef]
- Zhang, X.-H.; Zhou, T.; Xuan, L.-J. A dipeptide and two glycosides from Streptocaulon griffithii. J. Asian Nat. Prod. Res. 2008, 10, 891–896. [Google Scholar] [CrossRef]
- Su, Y.; Xu, J.-J.; Bi, J.-L.; Wang, Y.-H.; Hu, G.-W.; Yang, J.; Yin, G.-F.; Long, C.-L. Chemical constituents of Arisaema franchetianum tubers. J. Asian Nat. Prod. Res. 2013, 15, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Pavithra, G.M.; Siddiqua, S.; Naik, A.S.; TR, P.K.; Vinayaka, K.S. Antioxidant and antimicrobial activity of flowers of Wendlandia thyrsoidea, Olea dioica, Lagerstroemia speciosa and Bombax malabaricum. J. Appl. Pharmac. Sci. 2013, 3, 114–120. [Google Scholar] [CrossRef]
- Gork, V.; Walter, N.S.; Chauhan, M.; Kaur, M.; Dhingra, N.; Bagai, U.; Kaur, S. Ethanol extract of Bergenia ciliata (Haw.) Sternb. (rhizome) impedes the propagation of the malaria parasite. J. Ethnopharmacol. 2021, 280, 114417. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zeng, L.; Li, X.; Dong, X.; Yan, Y.; Cheng, Y.; Brachystemols, A. three new furan derivatives from Brachystemma calycinum. J. Asian Nat. Prod. Res. 2011, 13, 915–919. [Google Scholar] [CrossRef]
- Aiyar, S.N.; Jain, M.K.; Krishnamurti, M.; Seshadri, T.R. Chemical components of the roots of Connarus monocarpus. Phytochemistry 1964, 3, 335–339. [Google Scholar] [CrossRef]
- Zamarrud, A.I.; Hussain, H.; Ahmad, V.U.; Qaiser, M.; Amyn, A.; Mohammad, F.V. Two new antioxidant bergenin derivatives from the stem of Rivea hypocrateriformis. Fitoterapia 2011, 82, 722–725. [Google Scholar] [CrossRef]
- Venkateswarlu, K.; Satyalakshmi, G.; Suneel, K.; Reddy, T.S.; Raju, T.V.; Das, B.A. benzofuranoid and two clerodane diterpenoids from Pulicaria wightiana. Helv. Chim. Acta 2008, 91, 2081–2088. [Google Scholar] [CrossRef]
- Terracios Suculentas. Crassula ovata. Available online: https:terraciossuculentas.com.br (accessed on 26 June 2022).
- Zhang, S.; Wang, J.; Zhang, H. Chemical constituents of Tibetan medicinal herb Rhodiola kirilowii (Reg.) Reg. Zhongguo Zhong Yao Za Zhi 1991, 16, 483. [Google Scholar]
- Bharate, S.B.; Kumar, V.; Bharate, S.S.; Singh, B.; Singh, G.; Singh, A.; Gupta, M.; Singh, D.; Kumar, A.; Singh, S.; et al. Discovery and potential development of IIIM-160, a Bergenia ciliate-based anti-inflammatory and anti-arthritic botanical drug candidate. J. Integr. Med. 2019, 17, 192–204. [Google Scholar] [CrossRef]
- Flora e Fauna Web. Dipterocarpus grandflorus. Available online: https://www.nparks.gov.sg/florafaunaweb/flora/2/8/2857.Blanco. (accessed on 9 August 2022).
- Flora e Fauna Web. Dryobalanops aromatica C.F. Gaertn. Available online: https://www.nparks.gov.sg/florafaunaweb/flora/2/8/2862 (accessed on 9 August 2022).
- Ashton, P. Hopea utilis. IUCN Red listo f Treatened Species. Available online: https://iucnredlist.org/species/33023 (accessed on 18 June 2022).
- Flora e Fauna Web. Hopea sangal Korth. Available online: https://www.nparks.gov.sg/florafaunaweb/flora/2/9/2961 (accessed on 9 August 2022).
- Carruthers, W.R.; Hay, J.E.; Haynes, L.J. Isolation of bergenin from Shorea leprosula; identity of vakerin and bergenin. Chem. Ind. 1957, 1, 76–77. [Google Scholar]
- Mukherjee, H.; Ojha, D.; Bharitkar, Y.P.; Ghosh, S.; Mondal, S.; Kaity, S.; Dutta, S.; Samanta, A.; Chatterjee, T.K.; Chakrabarti, S.; et al. Evaluation of the wound healing activity of Shorea robusta, an Indian ethnomedicine, and its isolated constituent(s) in topical formulation. J. Ethnopharmacol. 2013, 149, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Tanaka, T.; Iinuma, M.; Iliya, I.; Nakaya, K.; Ali, Z.; Takahashi, Y.; Sawa, R.; Shirataki, Y.; Murata, J.; et al. New resveratrol oligomers in the stem bark of Vatica pauciflora. Tetrahedron 2003, 59, 5347–5363. [Google Scholar] [CrossRef]
- Royal Botanic Gardens. Vatica paucifora (Korth.) Blume. Available online: Https:Powo.science.kew.org/taxon (accessed on 22 June 2022).
- Ito, T.; Hara, Y.; Oyama, M.; Tanaka, T.; Murata, J.; Darnaedi, D.; Linuma, M. Occurrence of bergenin phenylpropanoates in Vatica bantamensis. Phytochem. Lett. 2012, 5, 743–746. [Google Scholar] [CrossRef]
- Seo, E.; Chai, H.; Constant, H.L.; Santisuk, T.; Reutrakul, V.; Beecher, C.W.W.; Farnsworth, N.R.; Cordell, G.A.; Pezzuto, J.M.; Kinghorn, D. Resveratrol tetramers from Vatica diospyroides. J. Org. Chem. 1999, 64, 6976–69783. [Google Scholar] [CrossRef]
- Song, X.; Chen, G.; Song, X.; Han, C.; Chen, S.; Weng, S. Study on the chemical constituents of leaves from Vatica mangachpoi Blanco. Linchan Huaxue Yu Gongye (Chem. Ind. Prod.) 2012, 32, 102–106. [Google Scholar]
- Mo, Z.; Chen, G.; Wang, J.; Wang, T.; Dai, C.; Yuan, Y. The extraction technology of bergenin fromleaf of Vatica mangachapoi Blanco. Shipin Keji (Food Sci. Technol.) 2012, 37, 207–209. [Google Scholar]
- Mishima, S.; Matsumoto, K.; Futamura, Y.; Araki, Y.; Ito, T.; Tanaka, T.; Iinuma, M.; Nozawa, Y.; Akao, Y. Antitumor effect of stilbenoids from Vateria indica against allografted sarcoma S-180 in animal model. J. Exp. Ther. Oncol. 2003, 3, 283–288. [Google Scholar] [CrossRef]
- Tangmouo, J.G.; Ho, R.; Matheeussen, A.; Lannang, A.M.; Komguem, B.B.M. Antimalarial activity of extract and norbergenina derivatives from the stem bark of Diospyros sanza-minika A. Chevalier (Ebenaceae). Phytother. Res. 2010, 24, 1676–1679. [Google Scholar] [CrossRef]
- Bouzeko, I.L.T.; Dongmo, F.L.M.; Ndontsa, B.L.; Ngansop, C.A.N.; Keumoe, R.; Bitchagno, G.T.M.; Jouda, J.B.; Mbouangouere, R.; Tchegnitegni, T.B.; Boyom, F.F.; et al. Chemical constituents of Mussaenda erythrophylla Schumach. & Thonn. (Rubiaceae) and their chemophenetic significance. Biochem. Syst. Ecol. 2021, 98, 1–6. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Chekhirova, G.V. Galloylpicein and other phenolic compounds from Arctostaphylos uva-ursi. Chem. Nat. Prod. 2013, 49, 1–7. [Google Scholar] [CrossRef]
- Singh, S.V.; Manhas, A.; Kumar, Y.; Mishra, S.; Shanker, K.; Khan, F.; Srivastava, K.; Pal, A. Antimalarial activity and safety assessment of Flueggea virosa leaves and its major constituent with special emphasis on their mode of action. Biomed. Pharmacother. 2017, 89, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Bulugahapitiya, V.P.; Munasinghe, M.M.A.B.; Hettihewa, L.M.; Kihara, N. Anti-cancer activity of Fluggea leucopyrus Willd (Katupila) against human ovarian carcinoma and characterization of active compounds. JSc EUSL 2020, 11, 12–26. [Google Scholar] [CrossRef]
- Basile, A.C.; Sertie, J.A.A.; Panizza, S.; Oshiro, T.T.; Azzolini, C.A. Pharmacological assay of Casearia sylvestris. I: Preventive anti-ulcer activity and toxicity of the leaf crude extract. J. Ethnopharmacol. 1990, 30, 185–197. [Google Scholar] [CrossRef]
- Aphale, S.; Pandita, S.; Raima, P.; Mishra, J.N.; Kaul-Ghanekar, R. Phytochemical Standardization of Panchavalkala: An Ayurvedic Formulation and Evaluation of its Anticancer Activity in Cervical Cancer Cell Lines. Pharmacogn. Mag. 2018, 14, 554–560. [Google Scholar]
- Thang, T.D.; Kuo, P.C.; Yu, C.S.; Shen, Y.C.; Hoa, L.T.M.; Thanh, T.V.; Kuo, Y.-H.K.; Yang, M.-L.; Wu, T.-S. Chemical constituents of the leaves of Glochidion obliquum and their bioactivity. Arch. Pharm. Res. 2011, 34, 383–389. [Google Scholar] [CrossRef]
- Takeda, Y.; Mima, C.; Masuda, T.; Hirata, E.; Takushi, A.; Otsuka, H. Glochidioboside, a glucoside of (7S,8R)-dihydrodehydrodiconiferyl alcohol from leaves of Glochidion obovatum. Phytochemistry 1998, 49, 2137–2139. [Google Scholar] [CrossRef]
- Sultana, T.; Mitra, A.K. das S. Evaluation of anti-cancer potential of Excoecaria agallocha (L.) leaf extract on human cervical cancer (SiHa) cell line and assessing the underlying mechanism of action. Future J. Pharm. Sci. 2022, 8, 1–18. [Google Scholar] [CrossRef]
- Takahashi, H.; Kosaka, M.; Watanabe, Y.; Nakade, K.; Fukuyama, Y. Synthesis and Neuroprotective Activity of Bergenin Derivatives with Antioxidant Activity. Bioorganic Med. Chem. 2003, 11, 1781–1788. [Google Scholar] [CrossRef]
- Sriset, Y.; Chatuphonprasert, W.; Jarkamjorn, K. In vitro antioxidant potential of Mallotus repandus (Willd.) Muell. Arg stem extract and its active constituent bergenin. Songklanakarin J. Sci. Technol. 2019, 43, 24–30. [Google Scholar]
- Riviere, C.; Hong, V.N.T.; Hong, Q.T.; Chataigne, G.; Hoai, N.N.; Dejaegher, B.; Tistaert, C.; Kim, T.N.T.; Heyden, Y.V.; Van, M.C.; et al. Mallotus species from Vietnamese mountainous areas: Phytochemistry and pharmacological activities. Phytochem. Ver. 2010, 9, 217–253. [Google Scholar] [CrossRef]
- Khan, H.; Amin, H.; Ullah, A.; Saba, S.; Rafique, J.; Khan, K.; Ahmad, N.; Badshah, S.L. Antioxidant and Antiplasmodial Activities of Bergenin and 11-O-Galloylbergenin Isolated from Mallotus philippensis. Oxidative Med. Cell. Longev. 2016, 1, 1–6. [Google Scholar] [CrossRef]
- Lalhelenmawia, H.; Chidambaran, K.; Brattacharjee, B.B.; Mandal, S.C. Antidiabetic Activity of Mallotus Roxburghianus Leaves in Diabetic Rats Induced by Streptozocin. Pharmacologyonline. 2007, 3, 244–254. [Google Scholar]
- Kabran, F.A.; Okpekon, T.A.; Roblot, F.; Seon-Meniel, B.; Leblanc, K.; Bories, C.; Champy, P.; Yolou, S.F.; Loiseau, P.M.; Djakouré, L.A.; et al. Bioactive phloroglucinols from Mallotus oppositifolius. Fitoterapia 2015, 107, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Flowers of India. Chandada. Available online: http://www.flowersofindia.net/catalog/slides/Chandada.html (accessed on 9 August 2022).
- Tanaka, R.; Matsunaga, S. Triterpene dienols and other constituents from the bark of Phyllanthus flexuosus. Phytochemistry 1988, 27, 2273–2277. [Google Scholar] [CrossRef]
- Priya, O.S.; Viswanathan, M.B.G.; Balakrishna, K.; Venkatesan, M. Chemical constituents and in vitro antioxidant activity of Phyllanthus wightianus. Nat. Prod. Res. 2011, 25, 949–958. [Google Scholar] [CrossRef]
- Sanogo, R.; Vassallo, A.; Malafronte, N.; Imparato, S.; Russo, A.; Piaz, F.D. New phenolic glycosides from Securinega virosa and their antioxidant activity. Nat. Prod. Commun. 2009, 4, 1645–1650. [Google Scholar] [CrossRef]
- Schutz, B.; Orjala, J.; Sticher, O.; Rali, T. Dammarane triterpenes from the leaves of Secur. Melanthesoides. J. Nat. Prod. 1998, 61, 96–98. [Google Scholar] [CrossRef]
- Govaerts, R.; Frodin, D.G.; Radcliffe-Smith, A. World Checklist and Bibliography of Euphorbiaceae (and Pandaceae). Board Trust. R. Bot. Gard. 2000, 4, 1–1622. [Google Scholar]
- Peltophorum inerme. Available online: https://dgnurseries.com/product/peltophorum-inerme-2/ (accessed on 9 August 2022).
- Herabario Virtual Austral Americano Peltophorum inerme. Available online: https://herbariovaa.org/taxa/index.php?tid=44282 (accessed on 9 August 2022).
- Joshi, B.S.; Kamat, V.N. Identity of Peltophorum with bergenin. Nat. Chaften 1969, 56, 89–90. [Google Scholar]
- Peltophorum pterocarpum: Yellow Poinciana. Available online: https://edis.ifas.ufl.edu/publication/ST434 (accessed on 9 August 2022).
- Paulino, C.A.; Carvalho, C.B.; Almeida, B.C.; Chaves, M.H.; Almeida, F.R.C.; Brito, S.M.R.C. The stem bark extracts of Cenostigma macrophyllum attenuates tactile allodynia in streptozotocin-induced diabetic rats. Pharmcae. Biol. 2013, 51, 1243–1248. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, C.; Krishnaraju, V.; Subbaraju, V. Antiinflammatory constituents of Teramnus labialis. Indian J. Pharm. Sci. 2006, 68, 111–114. [Google Scholar] [CrossRef]
- Kim, M.H.; Ha, S.Y.; Oh, M.H.; Kim, H.H.; Kim, S.R.; Lee, M.W. Anti-oxidative and anti-proliferative activity on human prostate cancer cells lines of the phenolic compounds from Corylopsis coreana Uyeki. Molecules 2013, 18, 4876–4886. [Google Scholar] [CrossRef] [PubMed]
- Hattori, S. Corylopsin, a crystalline constituent of the bark of Corylopsis spicata. Acta Phytochim. (Jpn) 1929, 4, 327–341. [Google Scholar]
- Jachak, S.M.; Gautam, R.; Selvam, C.; Madhan, H.; Srivastava, A.; Khan, T. Anti-inflammatory, cyclooxygenase inhibitory and antioxidant activities of standardized extracts of Tridax procumbens L. Fitoterapia 2011, 82, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, S.L.; De Oliveira, V.G.; Yano, T.; Nunomoura, R.C.S. Antimicrobial activity of bergenin from Endopleura uchi (Huber) Cuatrec. Acta Amaz. 2008, 39, 187–192. [Google Scholar] [CrossRef]
- Silva, R.; Oliveira, M.G.M.; Prado, T.C.; Souza, V.C. Humiriaceae in Flora of Brasil. Available online: http://reflora.jbrj.gov.br/reflora/floradobrasil/FB7974 (accessed on 20 August 2022).
- Nwosu, C.O.; Maduka, H.C.C.; Mahe, A.; Adamu, M.; Nwagbara, N.D. Reduction of effects on the haematology of rats infected with Trypanosoma congolense by ethanolic extrat of Sacoglottis gabonensis stem bark. Niger. J. Bot. 2010, 23, 41–54. [Google Scholar]
- Ozouga. Sacoglottis gabunensis. Available online: Https:Tropicaltimber.inf/pt-br/species (accessed on 20 June 2022).
- Patel, D.K.; Patel, K.; Kumar, R.; Gadewar, M.; Tahilyani, V. Pharmacological and analytical aspects of bergenin: A concise report. Asian Pac. J. Trop. Biomed. 2012, 1, 163–167. [Google Scholar] [CrossRef]
- Srinivasan, R.; Chandrasekar, M.J.N.; Nanjan, M.J.; Suresh, B. Antioxidant activity of Caesalpinia digyna root. J. Ethnopharmacol. 2007, 113, 284–291. [Google Scholar] [CrossRef]
- De Oliveira, C.M.S. Potencial Farmacológico da Bergenina para Controle da dor Inflamatória: Um Estudo Pré-Clínico. Dissertação de Mestrado. Curso de Pós-Graduação em Farmácia. Universidade da Bahia. 2010. Available online: https://repositorio.ufba.br/bitstream/ri/22567/1 (accessed on 20 June 2022).
- African plant Pentaclethra macrophylla. Available online: Https:En.m.wikipedia.org/wiki/Pentaclethramacrophylla (accessed on 26 June 2022).
- Gabriel, N.; Folefoc, J.P.B.; Zacharias, T.F.; Bernard, B. Constituents from the roots of Pentaclethra macrophylla. Biochem. Syst. Ecol. 2005, 33, 1280–1282. [Google Scholar]
- Li, R.W.; Leach, D.N.; Myers, S.P.; Lin, G.D.; Leach, G.J.; Waterman, P.G. A new anti-inflammatory glucoside from Ficus racemosa L. Planta Med. 2004, 70, 421–426. [Google Scholar] [PubMed]
- Newell, A.M.B.; Yousef, G.G.; Lila, M.A.; Ramirez-Mares, M.V.; Mejia, E.G.G. Comparative in vitro bioactivities of tea extracts from six species of Ardisia and their effect on growth inhibition of HepG2 cells. J. Ethnopharmacol. 2010, 130, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Sumino, M.; Sekine, T.; Ruangrungsi, N.; Igarashi, K.; Ikegami, F. Ardisiphenols and other antioxidant principles from the fruits of Ardisia colorata. Chem. Pharm. Bull 2002, 50, 1484–1487. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.K.; Gupta, K. Isolation of bergenin from Saxifraga ligulata Wall. J. Indian J. Chem. Soc. 1962, 39, 559–560. [Google Scholar]
- Kobayashi, H.; Mejia, E. The genus Ardisia: A novel source of health-promoting compounds and phytopharmaceuticals. J. Ethnopharmacol. 2005, 96, 347–354. [Google Scholar] [CrossRef]
- Mu, L.M.; Feng, J.Q.; Liu, P. A new bergenin derivative from the rhizome of Ardisia gigantifolia. PMID 2013, 27, 1242–1245. [Google Scholar] [CrossRef]
- Liu, B.; Wang, M.; Wang, X. Phytochemical analysis and antibacterial activity of methanolic extract of Bergenia purpurascens against common respiratory infection causing bacterial species in vitro and in neonatal rats. Microb. Pathog. 2018, 117, 315–319. [Google Scholar] [CrossRef]
- Chen, H.; Chen, T.; Li, J.X.; Xu, Q. Study on constituents in rhizome of Astilbe chinensis. Zhongguo Zhong Yao Za Zhi 2004, 29, 652–654. [Google Scholar]
- Mundo Ecologia. Tudo Sobre a Flor Astilbe; Características, Nome Científico e Fotos. Available online: Https:Mundoecologia.com.br (accessed on 22 June 2022).
- Rajbhandar, M.; Mentel, R.; Jha, P.K.; Chaudhary, R.P.; Bhattarai, S.; Gewali, M.B.; Karmacharya, N.; Hipper, M.; Lindequist, U. Antiviral activity of some plants used in Nepalese traditional medicine. Evid Based Complement Altern. Med. 2009, 6, 517–522. [Google Scholar] [CrossRef]
- Kimura, Y.; Sumiyoshi, M.; Sakanaka, M. Effects of Astilbe thunbergii rhizomes on wound healing: Part 1. Isolation of promotional effectors from Astilbe thunbergii rhizomes on burn wound healing. J. Ethnopharmacol. 2007, 109, 72–77. [Google Scholar] [CrossRef]
- Chauhan, S.K.; Singh, B.; Agrawal, S. Simultaneous determination of bergenin and gallic acid in Bergenia ligulata wall by high-performance thin-layer chromatography. J. AOAC Int. 2000, 83, 1480–1483. [Google Scholar] [CrossRef] [PubMed]
- Zuo, G.Y.; Li, Z.Q.; Chen, L.R.; Xu, X.J. In vitro anti-HCV activities of Saxifraga melanocentra and its related polyphenolic compounds. Antivir. Chem. Chemother. 2005, 16, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Taneyama, M.; Yoshida, S.; Kobayashi, M.; Hasegawa, M. Isolation of norbergenin from Saxifraga stolonifera. Phytochemistry 1983, 22, 1053–1054. [Google Scholar] [CrossRef]
- Hoffmann, B.K.; Lotter, H.; Seligmann, O.; Wagner, H. Antihepatotoxic C-glycosylflavones from the leaves of Allophyllus edulis var. edulis and gracilis. Planta Med. 1992, 58, 544–548. [Google Scholar] [CrossRef] [PubMed]
- San, H.T.; Boonsnongcheep, P.; Putalun, W.; Sritularak, B.; Likhitwitayawuid, K. Bergenin from Cissus javana DC. (Vitaceae) root extractenhances glucose uptake by rat L6 myotubes. Trop. J. Pharm. Res. 2020, 19, 1081–1086. [Google Scholar] [CrossRef]
- Caldas, C.S.; Simone, C.A.; Pereira, M.A.; Malta, V.R.S.; Carvalho, R.L.P.; Da Silva, T.B.C.; Sant´ana, A.E.G.; Conserva, L.M. Bergenin monohydrate, a constituent of Humiria balsamifera, at 120K. Acta Crystallogr. 2002, 58, 609–611. [Google Scholar] [CrossRef]
- Rastogi, S.; Rawat, A.K.S. A comprehensive review on bergenin, a potencial hepatoprotective and antioxidative phytoconstituent. Herba Pol. 2008, 54, 66–79. [Google Scholar]
- Lu, X.; Wang, J. Advances in the study of Bergenia plants. Zhong Yao Cai 2003, 26, 58–60. [Google Scholar]
- Franz, G.; Grün, M. Chemistry, occurrence and biosynthesis of C-glycosyl compounds in plants. Planta Med. 1983, 47, 131–140. [Google Scholar] [CrossRef]
- Taneyama, M.; Yoshida, S. Studies on C-glycosides in higher plants. Bot. Mag. Tokyo 1979, 92, 69–73. [Google Scholar] [CrossRef]
- Rai, M.; Rai, A.; Mori, T.; Nakabayashi, R.; Yamamoto, M.; Nakamura, M.; Suzuki, H.; Saito, K.; Yamazaki, M. Gene-Metabolite Network Analysis Revealed Tissue-Specific Accumulation of Therapeutic Metabolites in Mallotus japonicus. Int. J. Mol. Sci. 2021, 22, 8835. [Google Scholar] [CrossRef] [PubMed]
- Saijo, R.; Nonaka, G.I.; Nishioka, I. Gallic acid esters of bergenin and norbergenin from Mallotus japonicus. Phytochemistry 1990, 29, 267–270. [Google Scholar] [CrossRef]
- Feng, W.S.; Li, Z.; Zheng, X.K.; Li, Y.J.; Su, F.Y.; Zhang, Y.L. Chemical constituents of Saxifraga stolonifera (L.). Meeb. Yao Xue Xue Bao (Acta Pharm. Sin.) 2010, 45, 742–746. [Google Scholar]
- Wang, G.C.; Liang, J.P.; Wang, Y.; Li, Q.; Ye, W.C. Chemical constituents from Flueggea virosa. Chin. J. Nat. Med. 2008, 6, 251–253. [Google Scholar] [CrossRef]
- Jia, Z.; Mitsunaga, K.; Koike, K.; Ohmoto, T. New bergenin derivatives from Ardisia crenata. Nat. Med. 1995, 49, 187–189. [Google Scholar]
- Tangmouo, J.G.; Ho, R.; Lannang, A.M.; Konguem, J.; Lontsi, A.T.; Lontsi, D.; Hostettmann, K. Norbergenin derivatives from the stem bark of Diospyros sanza-minika (Ebenaceae) and their radical scavenging activity. Phytochem. Lett. 2009, 2, 192–195. [Google Scholar] [CrossRef]
- Kaur, R.; Kaur, S. Evaluation of in vitro and in vivo antileishmanial potential of bergenin rich Bergenia ligulata (Wall.) Engl. root extract against visceral leishmaniasis in inbred BALB/c mice through immunomodulation. J. Tradit. Complement. Med. 2018, 8, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Almeida, L.C.; Santos, E.; Santana, C.S.; Araújo, J.S.C.; Taranto, A.G.; Leite, F.H.A. Revisão de literatura sobre o mecanismo de ação da Artemisinina e dos endoperóxidos antimaláricos—parte II. Rev. Textura 2016, 9, 15–24. [Google Scholar] [CrossRef]
- Uddin, G.; Sadat, A.; Siddiqui, B. Comparative Antioxidant and Antiplasmodial Activities of 11-O-Galloylbergenin and Bergenin Isolated from Bergenia ligulata. Trop Biomed. 2014, 31, 143–148. [Google Scholar] [PubMed]
- Picote, S.; Bienvenu, A. Plasmodium. In Encyclopedia of Infection and Immunity; Elsevier: Amsterdam, The Netherlands, 2022; Volume 2, pp. 655–665. [Google Scholar] [CrossRef]
- Da Silva, T.B.C.; Alves, V.L.; Mendonça, L.V.H.; Conserva, L.M.; Da Rocha, E.M.M.; Lemos, R.P.L. Chemical Constituents and Preliminary Antimalarial Activity of Humiria balsamifera. Pharm. Biol. 2004, 42, 94–97. [Google Scholar] [CrossRef]
- Sadat, A.; Uddin, G.; Alam, M.; Ahmad, A.; Siddiqui, S. Structure activity relationship of bergenin, p-hydroxybenzoyl bergenin, 11-O-galloylbergenin as potent antioxidant and urease inhibitor isolated from Bergenia ligulata. Nat. Prod. Res. 2015, 29, 2291–2294. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Yu, Z.; Wang, L.; Yuan, T.; Wang, X.; Zhang, X.; Wang, J.; Lv, Y.; Du, G. The natural product bergenin ameliorates lipopolysaccharide-induced acute lung injury by inhibiting NF-kappaB activation. J. Ethnopharmacol. 2017, 200, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Melos, J.L.R.; Echevarria, A. Sistemas Enzimáticos de Tripanossomatídeos como Potenciais Alvos Quimioterápicos. Rev. Virtual Quim 2012, 4, 374–392. [Google Scholar]
- Nyunt, K.S.; Elkhateeb, A.; Tosa, Y.; Nabata, K.; Katakura, K.; Matsuura, H. Isolation of Antitrypanosomal Compounds from Vitis repens, a Medicinal Plant of Myanmar. Nat. Prod. Commun. 2012, 7, 609–610. [Google Scholar] [CrossRef]
- Tumová, L.; Hendrychová, H.; Vokurková, D. Immunostimulant Activity of Bergenia Extracts. Pharmacogn. Mag. 2017, 14, 328–332. [Google Scholar] [CrossRef]
- Horna, J.C.C. Biological Activities and Chemical Content of Glycyrrhiza species. Ph.D. Thesis, Faculty of Pharmacy, Hradec Kralove, Charles University, Staré MěSto, Czech Republic, 2010. [Google Scholar]
- Sastry, B.S.; Vykuntam, U.; Rao, E. Chemical examination of the aerial parts of Astilbe rivularis. Indian Drugs 1987, 24, 354–359. [Google Scholar]
- Hegde, V.R.; Pu, H.; Patel, M.; Das, P.R.; Butkiewicz, N.; Arreaza, G.; Gullo, V.P.; Chan, T.M. Two antiviral compounds from the plant Stylogne cauliflora as inhibitors of HCV NS3 protease. Bioorganic Med. Chem. Lett. 2003, 13, 2925–2928. [Google Scholar] [CrossRef]
- Piacente, S.; Pizza, C.; De Tommasi, N.; Mahmood, N. Constituents of Ardisia japonica and Their in Vitro Anti-HIV Activity. J. Nat. Prod. 1996, 59, 565–569. [Google Scholar] [CrossRef]
- Bessong, P.O.; Obi, C.L.; Andreola, M.L.; Rojas, L.B.; Pouysegu, L.; Igumbor, E.; Meyer, J.J.; Quideau, S.; Litvak, S. Evaluation of selected South African medicinal plants for inhibitory properties against human immunodeficiency virus type 1 reverse transcriptase and integrase. J. Ethnopharmacol. 2005, 99, 83–91. [Google Scholar] [CrossRef]
- Seo, J.-H.; Kim, J.-E.; Shim, J.-H.; Yoon, G.; Bang, M.-A.; Bae, C.-S.; Lee, K.-J.; Park, D.-H.; Cho, S.-S. HPLC Analysis, Optimization of Extraction Conditions and Biological Evaluation of Corylopsis coreana Uyeki Flos. Molecules 2016, 21, 94. [Google Scholar] [CrossRef] [PubMed]
- Rolta, R.; Goyal, M.; Sharma, D.; Bharaj, D.; Salaria, D.; Upadhyay, N.K.; Lal, U.R.; Dev, K.; Sourirajan, A. Bioassay Guided Fractionation of Phytocompounds from Bergenia ligulata: A synergistic approach to treat drug resistant bacterial and fungal pathogens. Pharmacol. Res. Mod. Chin. Med. 2022, 3, 1–11. [Google Scholar] [CrossRef]
- Adhikary, P.; Roshan, K.C.; Kayastha, D.; Thapa, D.; Shrestha, R.; Shrestha, T.M.; Gyawali, R. In Vitro Evaluation of Antimicrobial and Cytotoxic Potential of Dry Rhizome Extract of Astilbe Rivulari. Int. J. Pharmacogn. Phytochem. Res. 2012, 4, 122–126. [Google Scholar]
- Abbas, T.; Bhatti, A.A.; Saeed, A.; Tasleem, F.; Azhar, I.; Mehmood, Z.A. In vitro antibacterial activity of copper pod/yellow flame tree or peelagulmohar (Peltophorum roxburghii). Int. J. Curr. Res. 2015, 7, 14634–14639. [Google Scholar]
- Neto, O.C.S.; Teodoro, M.T.F.; Do Nascimento, B.O.; Cardoso, K.V.; Silva, E.O.; David, J.M.; David, J.P. Bergenin of Peltophorum dubium (Fabaceae) Roots and Its Bioactive Semi-Synthetic Derivatives. J. Braz. Chem. Soc. 2020, 31, 2644–2650. [Google Scholar] [CrossRef]
- Nyemb, J.N.; Djankou, M.T.; Talla, E.; Tchinda, A.T.; Ngoudjou, D.T.; Iqbai, J.; Mbafor, J.T. Antimicrobial, α-Glucosidase and Alkaline Phosphatase Inhibitory Activities of Bergenin, The Major Constituent of Cissus populnea Roots. Med. Chem. 2018, 8, 426–430. [Google Scholar] [CrossRef]
- Chauke, A.M.; Shai, L.J.; Mphahlele, P.M.; Mogale, M.A. Radical scavenging activity of selected medicinal plants from limpopo province of south Africa. Afr. J. Tradit. Complement. Altern. Med. 2012, 9, 426–430. [Google Scholar] [CrossRef]
- Raj, M.K.; Duraipandiyan, V.; Agustin, P.; Ignacimuthu, S. Antimicrobial activity of bergenin isolated from Peltophorum pterocarpum DC. Flowers. Asian Pac. J. Trop. Biomed. 2012, 1, 901–904. [Google Scholar] [CrossRef]
- Shah, M.R.; Arfan, M.; Amin, H.; Hussain, Z.; Qadir, M.I.; Choudhary, M.I.; VanDerveer, D.; Mesaik, M.A.; Soomro, S.; Jabeen, A.; et al. Synthesis of new bergenin derivatives as potent inhibitors of inflammatory mediators NO and TNF-α. Bioorganic Med. Chem. Lett. 2012, 22, 2744–2747. [Google Scholar] [CrossRef]
- De Freitas, F.A.; Araújo, R.C.; Soares, E.R.; Nunomura, R.C.S.; Da Silva, F.M.A.; Da Silva, S.R.S.; De Souza, A.Q.L.; De Souza, A.D.L.; Franco-Montalbán, F.; Acho, L.D.R.; et al. Biological evaluation and quantitative analysis of antioxidante compounds in pulps of the Amazonian fruits bacuri (Platonia insignis Mart.), ing_a (Inga edulis Mart.), and uchi (Sacoglottis uchi Huber) by UHPLC-ESI-MS/MS. J. Food Biochem. 2018, 42, 1–10. [Google Scholar] [CrossRef]
- Kumar, S.; Sharma, C.; Kaushik, S.R.; Kulshreshtha, A.; Chaturvedi, S.; Nanda, R.K.; Bhaskar, A.; Chattopadhyay, G.D.; Das, G.; Dwivwdi, V.P. The phytochemical bergenin as an adjunct immunotherapy for tuberculosis in mice. J. Biol. Chem. 2019, 294, 8555–8563. [Google Scholar] [CrossRef]
- Li, G.; Fang, Y.; Ma, Y.; Dawa, Y.; Wang, Q.; Gan, J.; Dang, J. Screening and Isolation of Potential Anti-Inflammatory Compounds from Saxifraga atrata via Affinity Ultrafiltration-HPLC and Multi-Target Molecular Docking Analyses. Nutrients 2022, 14, 2405. [Google Scholar] [CrossRef]
- Dias, E.d.J.S.; Filho, A.J.C.; Carneiro, F.J.C.; Da Rocha, C.Q.; Da Silva, L.C.N.; Santos, J.C.B.; Barros, T.F.; Santos, D.M. Antimicrobial Activity of Extracts from the Humiria balsamifera (Aubl). Plants 2021, 10, 1479. [Google Scholar] [CrossRef]
- Nunomura, R.C.S.; Oliveira, V.G.; Da Silva, S.L.; Nunomura, S.M. Characterization of Bergenin in Endopleura uchi Bark and its Anti-Inflammatory Activity. Braz. Chem. Soc. 2009, 20, 1060–1064. [Google Scholar] [CrossRef]
- De Oliveira, V.G.; Nunumura, R.C.S.; Nunumura, S.M. Estudo fitoquímico e de atividade biológica de Endopleura uchi. Universidade Federal do Amazonas—Instituto de Pesquisas da Amazonas. Química de Produtos Naturais. Available online: http://www.sbpcnet.org.br/livro/61ra/resumos/resumos/6902.htm (accessed on 5 September 2022).
- Larsen, A.K.; Skladanowski, A.; Bojanowski, K. The roles of DNA topoisomerase II during the cell cycle, Prog. Cell Cycle Res. 1996, 2, 229–239. [Google Scholar]
- Ye, X.; Ning, H. Bergenin attenuates TNF-α-induced oxidative stress and inflammation in HaCaT cells by activating Nrf2 pathway and inhibiting NF-κB pathway. Trop. J. Pharm. Res. 2022, 21, 1209–1213. [Google Scholar] [CrossRef]
- Zhou, B.X.; Wu, B.; Chen, M.; Chen, C.; Gao, Y.; Li, D.; Huang, D.; Chen, Z.; Zhao, X.; Huang, Q.; et al. Bergenin-activated SIRT1 inhibits TNF-α-induced proinflammatory response by blocking the NF-κB signaling pathway. Pulm. Pharmacol. Ther. 2020, 62, 101921. [Google Scholar]
- Jung, J.; Lim, E.; Kim, S.; Jung, M.; Oh, S. Practical Synthesis and Biological Evaluation of Bergenin Analogs. Chem. Biol. Drug Des. 2011, 109, 2270–2277. [Google Scholar] [CrossRef]
- De Oliveira, G.A.L.; De la Lastra, C.A.; Rosillo, M.A.; Martinez, M.L.C.; Sánchez-Hidalgo, M.; Medeiros, J.V.R.; Villegas, I. Preventive effect of bergenin against the development of TNBS-induced acute colitis in rats is associated with inflammatory mediators inhibition and NLRP3/ASC inflammasome signaling pathways. Chem. Biol. Interact. 2019, 297, 25–33. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S. NF-kB signaling in inflammation. Signal Transduct. Snd Target. Ther. 2017, 2, 1–9. [Google Scholar]
- Wang, K.; Li, Y.; Lv, Q.; Li, X.; Dai, Y.; Wei, Z. Bergenin, Acting as an Agonist of PPARγ, Ameliorates Experimental Colitis in Mice through Improving Expression of SIRT1, and Therefore Inhibiting NF-κB-Mediated Macrophage Activation. Front. Pharm. 2018, 8, 981. [Google Scholar] [CrossRef]
- Glezer, I.; Marcourakis, T.; Avella, M.; Gorenstein, C.; Scavone, C. O factor de transcricao NF-kapaB nos mecanismos moleculares de acção de psicofármacos. Rev Braz. J. Psychiatry 2000, 22, 26–30. [Google Scholar] [CrossRef]
- Tang, Q.; Wang, Q.; Sun, Z.; Kang, S.; Fan, Y.; Hao, Z. Bergenin Monohydrate Attenuates Inflammatory Response via MAPK and NF-κB Pathways Against Klebsiella pneumonia Infection. Front. Pharmacol. 2021, 12, 651–664. [Google Scholar] [CrossRef]
- Dutra, R.C.; Tavares, C.Z.; Ferraz, S.O.; Sousa, O.V.; Pimenta, D.S. Investigação das atividades analgésica e anti-inflamatória do extrato metanolico dos rizomas de Echinodorus grandiflorus. Ver. Bras. Farm. 2006, 16, 469–474. [Google Scholar] [CrossRef]
- Souza, M.K.J.; Soares, G.L.; Guilhon-Simplicio, F.; Perez, A.C.; Moura, C.C.V. Pharmacological evaluation of the antinociceptive and antiinflammatory activity of the species Endopleura Uchi. Enciclopedia Biosf. 2021, 18, 1–12. [Google Scholar] [CrossRef]
- Borges, J.C.M. Acetilbergenina: Obtenção e Avaliação das Atividades Antinociceptiva e Anti-Inflamatória. Dissertação de Mestrado. Programa de Pós-Graduação em Ciências Farmacêuticas. Instituto de Ciências da saúde. Universidade Federal do Pará. 2010. Available online: http://repositorio.ufpa.br:8080/jspui/handle/2011/5642 (accessed on 10 September 2022).
- Cunha, T.M.; Verri-Jr, W.A.; Silva, J.S.; Poole, S.; Cunha, F.Q.; Ferreira, S.H. A cascade of cytokines mediates mechanical inflamatory hypemociception in mice. Proc. Natl. Acad. Sci. USA 2005, 102, 1755–1760. [Google Scholar] [CrossRef]
- Verri-Jr, W.A.; Cunha, T.M.; Parada, C.A.; Poole, S.; Cunha, F.Q.; Ferreira, S.H. Hypemociceptive role of cytokines and chemokines: Targets for analgesic drug development? Pharmacol. Ther. 2006, 112, 116–138. [Google Scholar] [CrossRef]
- Ren, X.; Ma, S.; Wang, J.; Tian, S.; Fu, X.; Liu, X.; Li, Z.; Zhao, B.; Wang, X. Comparative effects of dexamethasone and bergenin on chronic bronchitis and their anti-inflammatory mechanisms based on NMR metabolomics. Mol. BioSystems 2016, 12, 1938–1947. [Google Scholar] [CrossRef]
- Sartori, T. Influência dos Aminoácidos de Cadeia Ramificada Sobre Aspectos Imunoregulatórios das Células Tronco Mesenquimais. Tese de Doutorado do Programa de Pós-Graduação em Farmácia. Universidade de São Paulo. 2020. Available online: https://www.teses.usp.br/teses/disponiveis/9/9142/tde-29062021-174755/pt-br.php (accessed on 25 September 2022). [CrossRef]
- Villareal, C.F.; Santos, D.S.; Lauria, P.S.S.; Gama, K.B.; Espírito-Santo, R.F.; Juiz, P.J.L.; Alves, C.Q.; David, J.M.; Soares, M.B.P. Bergenin Reduces Experimental Painful Diabetic Neuropathy by Restoring Redox and Immune Homeostasis in the Nervous System. In. J. Mol. Sci 2020, 21, 4850. [Google Scholar] [CrossRef]
- Rajput, S.A.; Mirza, M.R.; Choudhary, M.I. Bergenin protects pancreatic beta cells against cytokine-induced apoptosis in INS-1E cells. PLoS ONE 2020, 12, e0241349. [Google Scholar] [CrossRef]
- Arfan, M.; Amin, H.; Khan, N.; Khan, I.; Saeed, M.; Khan, M.A.; Rehman, F.U. Analgesic and anti-inflammatory activities of 11-O-galloylbergenin. Ethnopharmacol. Commun. 2010, 131, 502–504. [Google Scholar] [CrossRef]
- Borges, C.M.J.; Aguiar, R.W.S.; Filho, H.S.R.; Guilhom, G.S.P. Anti-inflammatory and non ulcerogenic activities of acetylbergenin. Afr. J. Pharm. Pharmacol. 2017, 11, 402–410. [Google Scholar]
- Huang, D.J.; Ou, B.X.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agrick. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Htwe, M.M.; Kyaw, Z.T.A.; Ngwe, H. A Study on Antioxidant Activity of Bergenin and its Derivative from the Bark of Peltophorum pterocarpum (DC.) K. Heyne (Pan-mèzali). 3rd Myanmar Korea Conf. Res. J. 2020, 3, 1667–1674. [Google Scholar]
- Subramanian, R.; Subbramaniyan, P.; Raj, V. Isolation of bergenin from Peltophorum pterocarpum flowers and its bioactivity. J. Basic Appl. Sci. 2015, 4, 256–261. [Google Scholar] [CrossRef]
- Hendrychova, H.; Martins, J.; Tumova, L.; Kcevar-Glavac, N. Bergenin Content and Free Radical Scavenging Activity of Bergenia Extracts. Nat. Prod. Commun. 2014, 10, 1273–1275. [Google Scholar] [CrossRef]
- Ravikanth, K.; Mehra, S.; Ganguly, B.; Sapra, S. Bergenin: Isolation from aqueous extract of Bergenia ciliata, antioxidant activity and in silico studies. Innov. Pharm. Pharm. 2020, 8, 10–14. [Google Scholar]
- Tacon, L.A.; Freitas, L.A.P. Box-Behnken design to study the bergenin content and antioxidant activity of Endopleura uchi bark extracts obtained by dynamic maceration. Rev. Bras. De Farmacogn. 2012, 23, 65–71. [Google Scholar] [CrossRef]
- Garcia, E.J.; Oldoni, T.L.C.; De Alencar, S.M.; Reis, A.; Loguercio, A.D.; Grande, R.H.M. Antioxidant activity by DPPY assay of potencial solution to be applied on bleached teeth. Braz. Dent. J. 2012, 23, 22–27. [Google Scholar] [CrossRef]
- Kinzler, K.W.; Vogelstein, B. The GLI gene encodes a nuclear protein, which binds specific sequences in the human genome. Mol. Cell Biol. 1990, 10, 634–642. [Google Scholar] [CrossRef]
- Sriset, Y.; Chatuphonprasert, W.; Jarukamjorn, K. Bergenin Attenuates Sodium Selenite-Induced Hepatotoxicity via Improvement of Hepatic Oxidant-Antioxidant Balance in HepG2 Cells and ICR Mice. J. Biol. Act. Prod. Nat. 2021, 11, 97–115. [Google Scholar] [CrossRef]
- Akak, C.M.; Nkengfack, A.E.; Tu, P. Norbergenin Derivatives from Diospyros crassiflora (Ebenaceae). Nat. Prod. Commun. 2013, 8, 1575–1578. [Google Scholar] [CrossRef] [PubMed]
- Qi, Q.; Dong, Z.; Sun, Y.; Li, S.; Zhao, Z. Protective Effect of Bergenin against Cyclophosphamide-Induced Immunosuppression by Immunomodulatory Effect and Antioxidation in Balb/c Mice. Molecules 2018, 23, 2668. [Google Scholar] [CrossRef]
- Lima, G.P.C.; Superóxido Dismutase (SOD). Universidade Estadual Paulista: Laboratório de Química e Bioquímica Vegetal—LQBV 2017, 1–3. Available online: https://www.ibb.unesp.br (accessed on 1 October 2022).
- Yun, J.; Lee, Y.; Yun, K.; Oh, S. Bergenin decreases the morphine-induced physical dependence via antioxidative activity in mice. Arch. Pharm. Res. 2015, 38, 1248–1254. [Google Scholar] [CrossRef] [PubMed]
- Maduka, H.C.C.; Okoye, Z.S.C. The effect of Sacoglottis gabonensis stem bark extract, a Nigerian alcoholic beverage additive on the natural antioxidant defences during 2,4-dinitrophenyl hydrazine-induced membrane peroxidation in vivo. Vasc. Pharmmacology 2002, 39, 21–31. [Google Scholar] [CrossRef]
- Maduka, H.C.C.; Okoye, Z.S.C. Bergenin, an alcoholic beverage additive from Sacoglottis gabonensis as an antioxidant protectors of mammalian erythroytes against lysis by peroxylradicals. J. Med. Sci. 2000, 9, 88–92. [Google Scholar]
- Muniz, M.P.; Nunomura, S.M.; Lima, E.S.; De Almeida, P.D.O.; Nunomura, C.S. Quantification of bergenin, antioxidant activity and nitric oxide inhibition from bark, leaf and twig of Endopleura uchi. Quim. Nova 2020, 43, 413–418. [Google Scholar] [CrossRef]
- Tameye, N.S.J.; Akak, C.M.; Happi, G.M.; Frese, M.; Stammler, H.-G.; Neumann, B.; Lenta, B.N.; Sewald, N.; Nkengfack, A.E. Antioxidant norbergenin derivatives from the leaves of Diospyros gilletii De Wild (Ebenaceae). Phytochem. Lett. 2020, 36, 63–67. [Google Scholar] [CrossRef]
- Yu, K.-Y.; Wu, W.; Li, S.-Z.; Dou, L.-L.; Liu, L.-L.; Li, P.; Liu, E.-H. A new compound, methylbergenin along with eight known compounds with cytotoxicity and anti-inflammatory activity from Ardisia japônica. Nat. Prod. Res. 2017, 31, 2581–2586. [Google Scholar] [CrossRef]
- Branquinho, L.S.; Verdan, M.H.; Silva-Filho, S.E.; Oliveira, R.J.; Cardoso, C.A.L.; Arena, A.C.; Kassuya, C.A.L. Antiarthritic and Antinociceptive Potential of Ethanolic Extract from Leaves of Doliocarpus dentatus (Aubl.) Standl. in Mouse Model. Pharmacogn. Res. 2021, 13, 28–33. [Google Scholar]
- Aggarwal, D.; Gautam, D.; Sharma, M.; Singla, S.K. Bergenin attenuates renal injury by reversing mitochondrial dysfunction in ethylene glycol induced hyperoxaluric rat model. Eur. J. Pharmacol. 2016, 791, 611–621. [Google Scholar] [CrossRef]
- Borges, J.C.M.; Filho, H.d.S.R.; Gulhon, G.M.S.P.; Carvalho, J.C.T.; Santos, L.S.; Sousa, P.J.C. Antinociceptive Activity of Acetylbergenin in Mice. Lat. Am. J. Pharm 2011, 30, 1303–1308. [Google Scholar]
- Jain, S.K.; Singh, S.; Khajuria, A.; Guru, S.K.; Joshi, P.; Meena, S.; Nadkarni, J.R.; Singh, A.; Bharate, S.S.; Bhushan, S. Pyrano-isochromanones as IL-6 Inhibitors: Synthesis, in Vitro and in Vivo Antiarthritic Activity. J. Med. Chem. 2014, 57, 7085–7097. [Google Scholar] [CrossRef]
- Nazir, N.; Koul, S.; Qurishi, M.A.; Taneja, S.C.; Ahmad, S.S.; Bani, S.; Qazi, G.N. Immunomodulatory effect of bergenin and norbergenin against adjuvant-induced arthritis—A flow cytometric study. J. Ethnopharmacol. 2007, 112, 401–405. [Google Scholar] [CrossRef]
- El-hawary, S.S.; Mohammed, R.; Abouzid, S.; Ali, Z.Y.; Elwekeel, A. Anti-arthritic activity of 11-O-(4’-O-methyl galloyl)-bergenin and Crassula capitella extract in rats. J. Pharm. Pharmacol. 2016, 68, 834–844. [Google Scholar] [CrossRef]
- Zhang, Y.-H.; Fang, L.-H.; Lee, M.-H.; Ku, B.-S. Short communication: In vitro Inhibitory Effects of Bergenin and Norbergenin on Bovine Adrenal Tyrosine Hydroxylase. Phytother. Res 2003, 17, 967–969. [Google Scholar] [CrossRef]
- Abe, K.; Sakai, K.; Uchida, M. Effects of bergenin on experimental ulcers--prevention of stress induced ulcers in rats. Gen. Pharmacol. 1980, 11, 361–368. [Google Scholar] [CrossRef]
- Kumar, R.; Patel, D.K.; Prasad, S.K.; Sairam, K.; Hemalatha, S. Antidiabetic activity of alcoholic root extract of Caesalpinia digyna in streptozotocin-nicotinamide induced diabetic rats. Asian Pac. J. Trop. Biomed. 2012, 1, S934–S940. [Google Scholar] [CrossRef]
- Inthongkaew, P.; Chatsumpun, N.; Supasuteekul, C.; Kitisripanya, T.; Putalun, W.; Likhitwitayawuid, K.; Sritularak, B. α- Glucosidase and pancreatic lipase inhibitory activities and glucose uptake stimulatory effect of phenolic compounds from Dendrobium formosum. Rev. Bras. Farm. 2017, 27, 480–487. [Google Scholar] [CrossRef]
- Ambika, S.; Saravanan, R. Antihyperglycemic and antihyperlipidemic effect of bergenin on C57BL/6J mice with high fat-diet induced type 2 diabetes. J. Pharm. Res. 2016, 10, 126–132. [Google Scholar]
- Veerapur, V.P.; Prabhakar, K.R.; Thippeswamy, B.S.; Bansal, P.; Srinivasan, K.K.; Unnikrishnan, M.K. Antidiabetic effect of Ficus racemosa Linn. stem bark in high-fat diet and low-dose streptozotocin-induced type 2 diabetic rats: A mechanistic study. Food Chem. 2012, 132, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Qiao, S.; Liu, R.; Lv, C.; Miao, Y.; Yue, M.; Tao, Y.; Wei, Z.; Xia, Y.X.; Dai, Y. Bergenin impedes the generation of extracellular matrix in glomerular mesangial cells and ameliorates diabetic nephropathy in mice by inhibiting oxidative stress via the mTOR/β-TrcP/Nrf2 pathway. Free Radic. Biol. Med. 2019, 145, 118–135. [Google Scholar] [CrossRef] [PubMed]
- Riris, I.D.; Napitupulu, M.A. Antidiabetic activity of methoxy bergenin isolated from ethanol extrat of raru stem bark (Vatica pauciflora Blume) in alloxan induced diabetic wistar rats. Asian J. Chem. 2017, 29, 870–874. [Google Scholar] [CrossRef]
- Kumar, T.V.; Tiwari, A.K.; Robinson, A.; Babu, K.S.; Kumar, R.S.C.; Kumar, D.A.; Zehra, A.; Rao, M. Synthesis and antiglycation potentials of bergenin derivatives. Bioorganic Med. Chem. Lett. 2011, 21, 4928–4931. [Google Scholar] [CrossRef]
- Habtemariam, S.; Cowley, R.A. Antioxidant and Anti-a-glucosidase Compounds from the Rhizome of Peltiphyllum peltatum (Torr.) Engl. Phytother. Res. 2012, 26, 1656–1660. [Google Scholar] [CrossRef]
- Filho, A.L.; Lorga, A.M.; Lopes, A.N.G.; De Paola, A.A.V.; Da Costa, A.N.; Péres, A.K.; Grupi, C.J.; Halperin, C.; Moreira, D.A.R.; Sousa, E.A.; et al. Diretriz de fibrilação atrial. Arq. Bras. Cardiol. 2003, 81, 1–23. [Google Scholar] [CrossRef]
- Konoshima, T.; Konishi, T.; Takasaki, M.; Yamazoe, K.; Tokuda, H. Antitumor-promoting activity of the diterpene from Excoecaria agallocha II. Biol. Pharm. Bull. 2001, 24, 1440–1442. [Google Scholar] [CrossRef]
- Gao, X.; Wang, Y.; Zhang, J.; Lin, L.; Yao, Q.; Xiang, G. Bergenin suppresses the growth of colorectal cancer cells by inhibiting PI3K/AKT/mTOR signaling pathway. Trop. J. Pharm. Res. 2017, 16, 2307–2313. [Google Scholar] [CrossRef]
- Marmol, I.; Sanchez-de-Diego, C.; Dieste, A.P.; Cerrada, E.; Yoldi, M.J.R. Colorectal Carcinoma: A General Overview and Future Perspectives in Colorectal Cancer. Int. J. Mol. Sci. 2017, 18, 197. [Google Scholar] [CrossRef]
- Courtney, K.D.; Corcoran, R.B.; Engelman, J.A. The PI3K pathway as drug target in human cancer. J. Clin. Oncol. 2010, 28, 1075–1083. [Google Scholar] [CrossRef]
- Hennessy, B.T.; Smith, D.L.; Ram, P.T.; Lu, Y.; Mills, G.B. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat. Rev. Drug Discov. 2005, 4, 988–1004. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.; Zhou, Y.; Song, X. In vitro inhibitory effects of bergenin on human liver cytochrome P450 enzymes. Pharm. Biol. 2018, 56, 620–625. [Google Scholar] [CrossRef]
- Leão, R.; Apolónio, J.D.; Lee, D.; Figueiredo, A.; Tabori, U.; Castelo-Branco, P. Mechanisms of human telomerase reverse transcriptase (hTERT) regulation: Clinical impacts in cancer. J. Biomed. Sci. 2018, 25, 1–12. [Google Scholar] [CrossRef]
- Hettihewa, L.M.; Munasinghe, M.M.A.B.; Bulugahapitiya, V.B.; Kihara, N. Dose dependent anti proliferative and cytotoxic effects of Flueggea leucopyrus Willd against human ovarian carcinoma; MTS and human telomerase enzyme inhibition. EJBPS 2015, 2, 14–18. [Google Scholar]
- Chen, Z.; Liu, Y.-M.; Yang, S.; Song, B.-A.; Xu, G.-F.; Bhadury, P.S.; Jin, L.-H.; Hu, D.-Y.; Liu, F.; Xue, W.; et al. Studies on the chemical constituents and anticancer activity of Saxifraga stolonifera (L) Meeb. Bioorganic Med. Chem. 2008, 16, 1337–1344. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Pei, S.; Ju, W.; Jia, M.; Tian, D.; Tang, Y.; Mao, G. Synthesis and in vitro and in vivo antitumour activity study of 11-hydroxyl esterified bergenin/cinnamic acid hybrids. Eur. J. Med. Chem. 2017, 133, 319–328. [Google Scholar] [CrossRef]
- Liu, D.; Yang, P.; Zhang, Y. Water-soluble extract of Saxifraga stolonifera has anti-tumor effects on Lewis lung carcinoma-bearing mice. Bioorganic Med. Chem. Lett. 2016, 26, 4671–4678. [Google Scholar] [CrossRef]
- Yan, D.-B.; Zhang, D.-P.; Li, M.; Liu, W.-Y.; Feng, F.; Di, B.; Guo, Q.-L.; Xie, N. Synthesis and cytotoxic activity of 3, 4, 11-trihydroxyl modified derivatives of bergenin. Chin. J. Nat. Med. 2014, 12, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zhu, X.; Bao, H.; Shek, T.W.; Huang, Z.; Wang, Y.; Wu, X.; Wu, Y.; Chang, Z.; Wu, S.; et al. Genetic and clonal dissection of osteosarcoma progression and lung metastasis. Int. J. Cancer 2018, 143, 1134–1142. [Google Scholar] [CrossRef]
- Kim, H.; Lim, H.; Chung, M.; Kim, Y.C. Antihepatotoxic activity of bergenin, the major constituent of Mallotus japonicus, on carbon tetrachloride-intoxicated hepatocytes. J. Ethnopharmacol. 2000, 69, 79–83. [Google Scholar] [CrossRef]
- Berry, M.N.; Friend, D.S. High-yield preparation of isolated rat liver parenchymal cells. J. Cell Biol. 1969, 43, 506–520. [Google Scholar] [CrossRef]
- Recknagel, R.O. A new direction in the study of carbon tetrachloride hepatotoxicity. Life Sci. 1983, 33, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Recknagel, R.O.; Glende, E.A.; Hruszkewycz, A.M., Jr. Free Radicals in Biology; Academic Press: Cambridge, MA, USA, 1976; Volume 3, pp. 97–132. [Google Scholar]
- Boyer, T.D.; Vessey, D.A.; Holcomb, C.; Saley, N. Studies of the relationship between the catalytic activity and binding of non-substrate ligands by the glutathione S-transferases. Biochem. J. 1984, 217, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; Kim, H.; Choi, H.; Oh, S.; Choi, J. Hepatoprotective effects of bergenin, a major constituent of Mallotus japonicus, on carbon tetrachloride-intoxicated rats. J. Ethnopharmacol. 2000, 72, 469–474. [Google Scholar] [CrossRef]
- Rong-Hua, P.; Hong-Mei, H.; Yue, D.; Yu-Feng, X. Comparative pharmacokinetics of bergenin, a main active constituent of Saxifraga stolonifera Curt., in normal and hepatic injury rats after oral administration. Chin. J. Nat. Med. 2016, 14, 776–782. [Google Scholar] [CrossRef]
- Tasleem, F.; Mahmood, S.B.Z.; Imam, S.; Hameed, N.; Jafrey, R.; Azhar, I.; Gulzar, R.; Mahmoo, Z.A. Hepatoprotective effect of Peltophorum pterocarpum leaves extracts and pure compound against carbon tetra chloride induced liver injury in rats. Med. Res. Arch. 2017, 5, 1–14. [Google Scholar]
- Mondal, M.; Hossain, M.M.; Hasan, M.R.; Tarun, M.T.I.; Islam, M.A.F.; Choudhuri, M.S.K.; Islam, M.T.; Mubarak, M. Hepatoprotective and Antioxidant Capacity of Mallotus repandus Ethyl Acetate Stem Extract against D Galactosamine-Induced Hepatotoxicity in Rats. ACS Omega 2020, 5, 6523–6531. [Google Scholar] [CrossRef]
- Lim, H.K.; Kim, H.S.; Choi, H.S.; Oh, S.; Jang, C.G.; Shoi, J.; Kim, S.H.; Chang, M.J. Effects of acetylbergenin against d-galactosamine-induced hepatotoxicity in rats. Pharmacol. Res. 2000, 42, 472–474. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T.; Haq, I.U.; Khan, T.; Mahnashi, M.H.; Alasmary, M.Y.; Almedhesh, S.A.; Shehri, H.A.; Alshahrani, M.A.; Shah, A.J. Bergenin from Bergenia Species Produces a Protective Response against Myocardial Infarction in Rats. Processes 2022, 10, 1403. [Google Scholar] [CrossRef]
- Suzuki, S.; Okuse, Y.; Kawase, M.; Takiguchi, M.; Fukuyama, Y.; Takahashi, H.; Sato, M. A Norbergenin Derivative Inhibits Neuronal Cell Damage Induced by Tunicamycin. Biol. Pharm. Bull 2006, 29, 1335–1338. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salimo, Z.M.; Yakubu, M.N.; da Silva, E.L.; de Almeida, A.C.G.; Chaves, Y.O.; Costa, E.V.; da Silva, F.M.A.; Tavares, J.F.; Monteiro, W.M.; de Melo, G.C.; et al. Chemistry and Pharmacology of Bergenin or Its Derivatives: A Promising Molecule. Biomolecules 2023, 13, 403. https://doi.org/10.3390/biom13030403
Salimo ZM, Yakubu MN, da Silva EL, de Almeida ACG, Chaves YO, Costa EV, da Silva FMA, Tavares JF, Monteiro WM, de Melo GC, et al. Chemistry and Pharmacology of Bergenin or Its Derivatives: A Promising Molecule. Biomolecules. 2023; 13(3):403. https://doi.org/10.3390/biom13030403
Chicago/Turabian StyleSalimo, Zeca M., Michael N. Yakubu, Emanuelle L. da Silva, Anne C. G. de Almeida, Yury O. Chaves, Emmanoel V. Costa, Felipe M. A. da Silva, Josean F. Tavares, Wuelton M. Monteiro, Gisely C. de Melo, and et al. 2023. "Chemistry and Pharmacology of Bergenin or Its Derivatives: A Promising Molecule" Biomolecules 13, no. 3: 403. https://doi.org/10.3390/biom13030403
APA StyleSalimo, Z. M., Yakubu, M. N., da Silva, E. L., de Almeida, A. C. G., Chaves, Y. O., Costa, E. V., da Silva, F. M. A., Tavares, J. F., Monteiro, W. M., de Melo, G. C., & Koolen, H. H. F. (2023). Chemistry and Pharmacology of Bergenin or Its Derivatives: A Promising Molecule. Biomolecules, 13(3), 403. https://doi.org/10.3390/biom13030403








