The Constitutive Activity of Spinal 5-HT6 Receptors Contributes to Diabetic Neuropathic Pain in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Animals and Models of Neuropathic Pain
2.3. Behavioral Tests
2.4. Chemicals
2.5. Cell Culture and Transfection
2.6. Western Blot Analysis
2.7. Experimental Design
2.8. Statistical Analysis
3. Results
3.1. Diabetes-Induced Mechanical Hyperalgesia
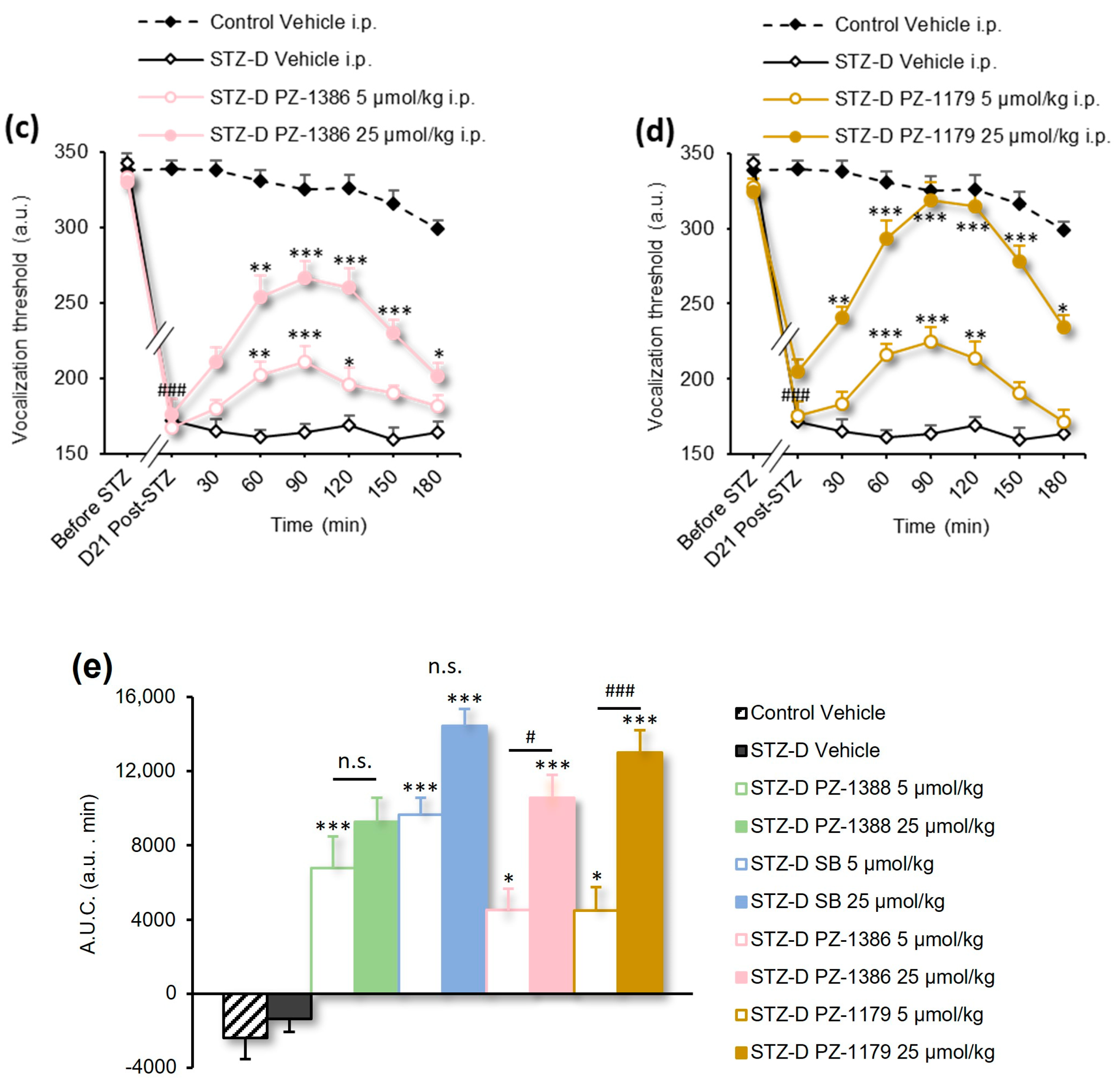
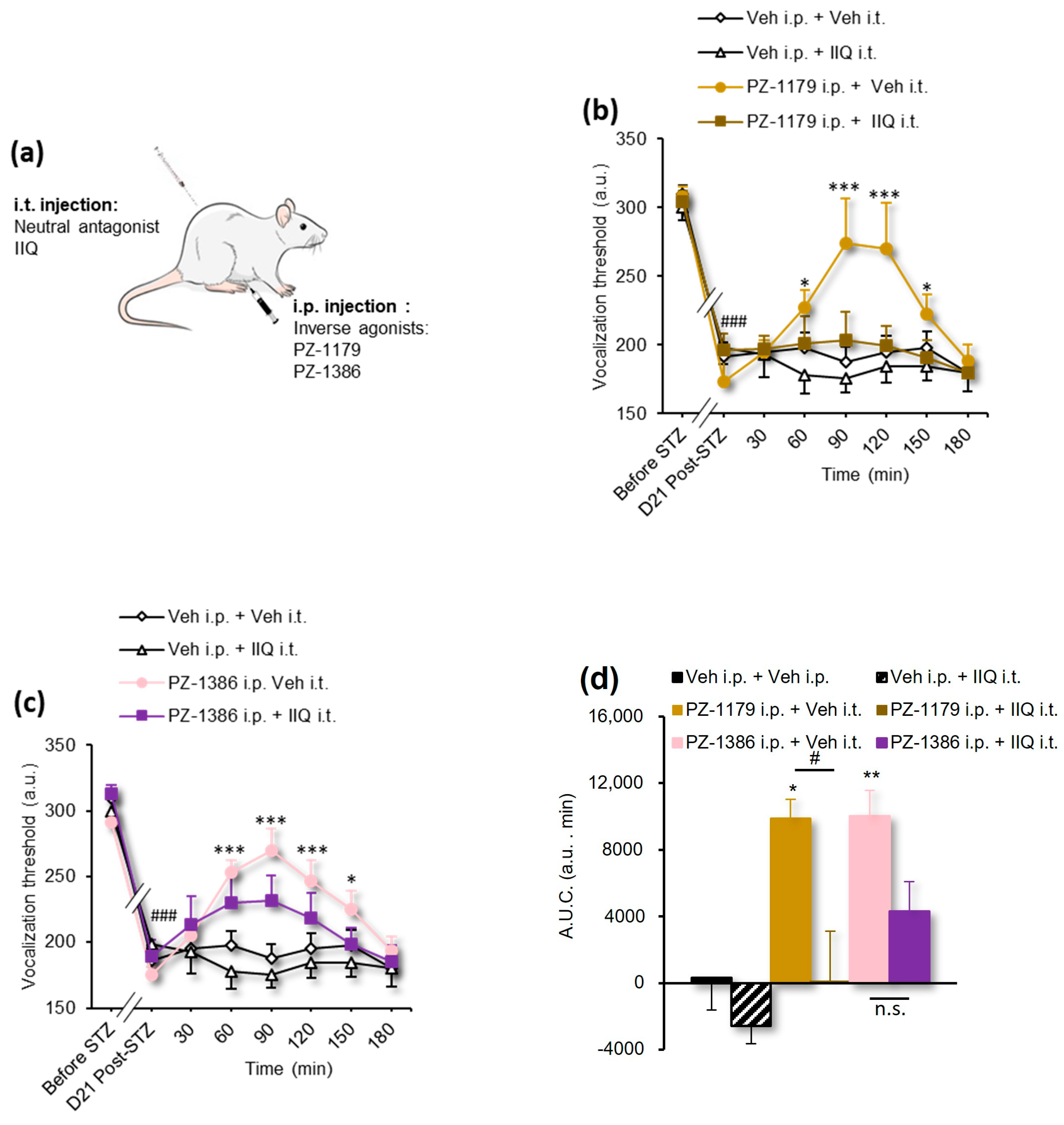
3.2. Serotonin 6 Receptor Blockade Attenuated Mechanical Hyperalgesia in STZ-Induced Diabetic Rats
| Figure | Analysis | Statistics (DFn, DFs) | p Value |
|---|---|---|---|
| Figure 1a–d | 2-way RM ANOVA | F(63, 798) = 12.39 | p < 0.0001 |
| Figure 1e | 1-way ANOVA | F(9, 117) = 24.27 | p < 0.0001 |
| Figure 2b,c | 2-way RM ANOVA | F(35, 273) = 2.812 | p < 0.0001 |
| Figure 2d | 1-way ANOVA | F(5, 39) = 8.252 | p < 0.0001 |
| Figure 3a | 2-way RM ANOVA | F(24, 160) = 3.399 | p < 0.0001 |
| Figure 3b | 1-way ANOVA | F(3, 20) = 16.11 | p < 0.0001 |
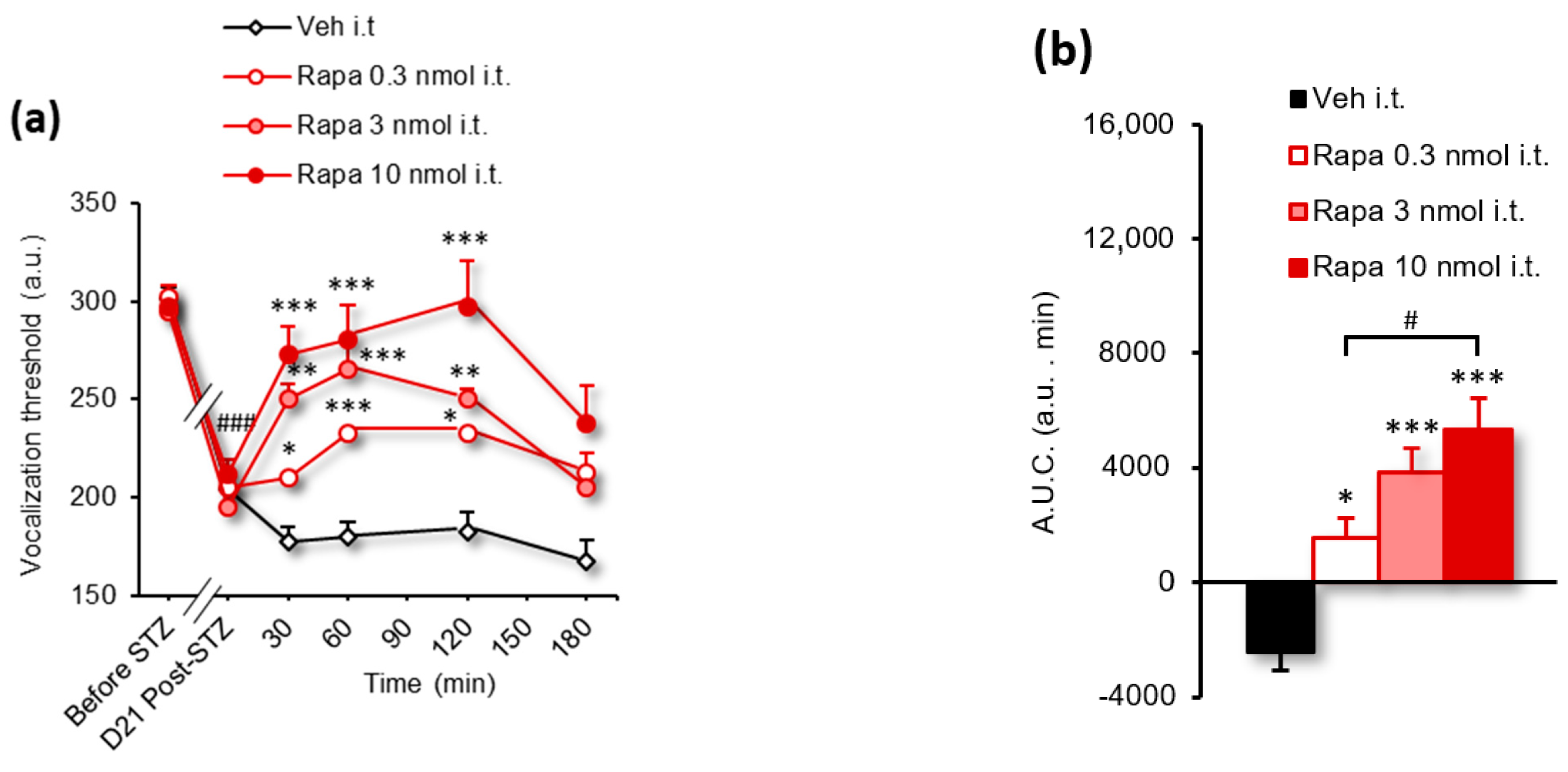
| Figure 4a | 2-way RM ANOVA | F(14, 119) = 8.237 | p < 0.0001 |
| Figure 4b | 1-way ANOVA | F(2, 17) = 11.23 | p = 0.0008 |
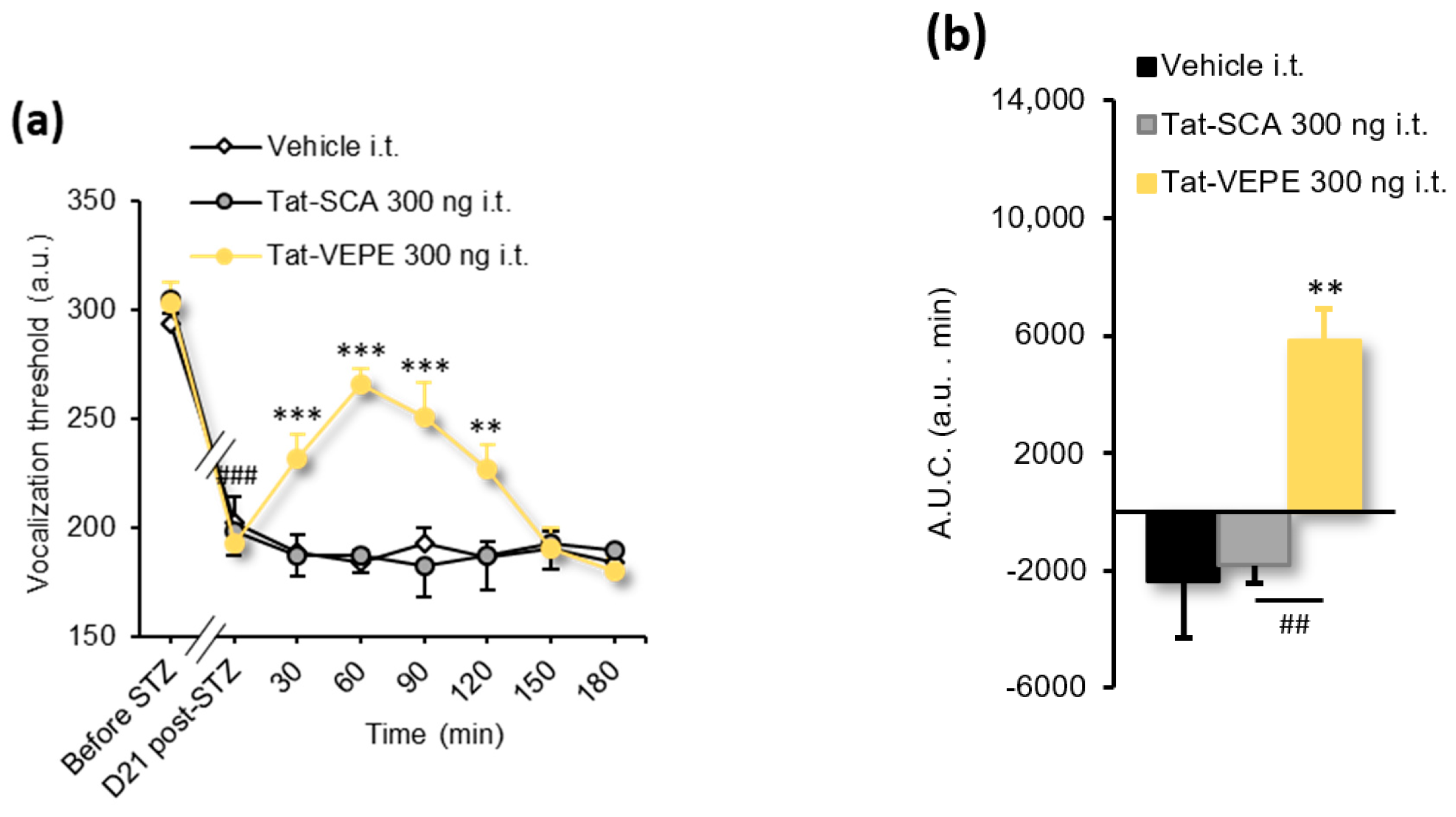
| Figure 5a | 1-way ANOVA | F(2, 40) = 6.181 | p = 0.0046 |
| Figure 5b,c | 1-way ANOVA | F(3, 27) = 5.497 | p = 0.0044 |
3.3. Rapamycin and Tat-VEPE Attenuated Hyperalgesia Induced by Diabetes
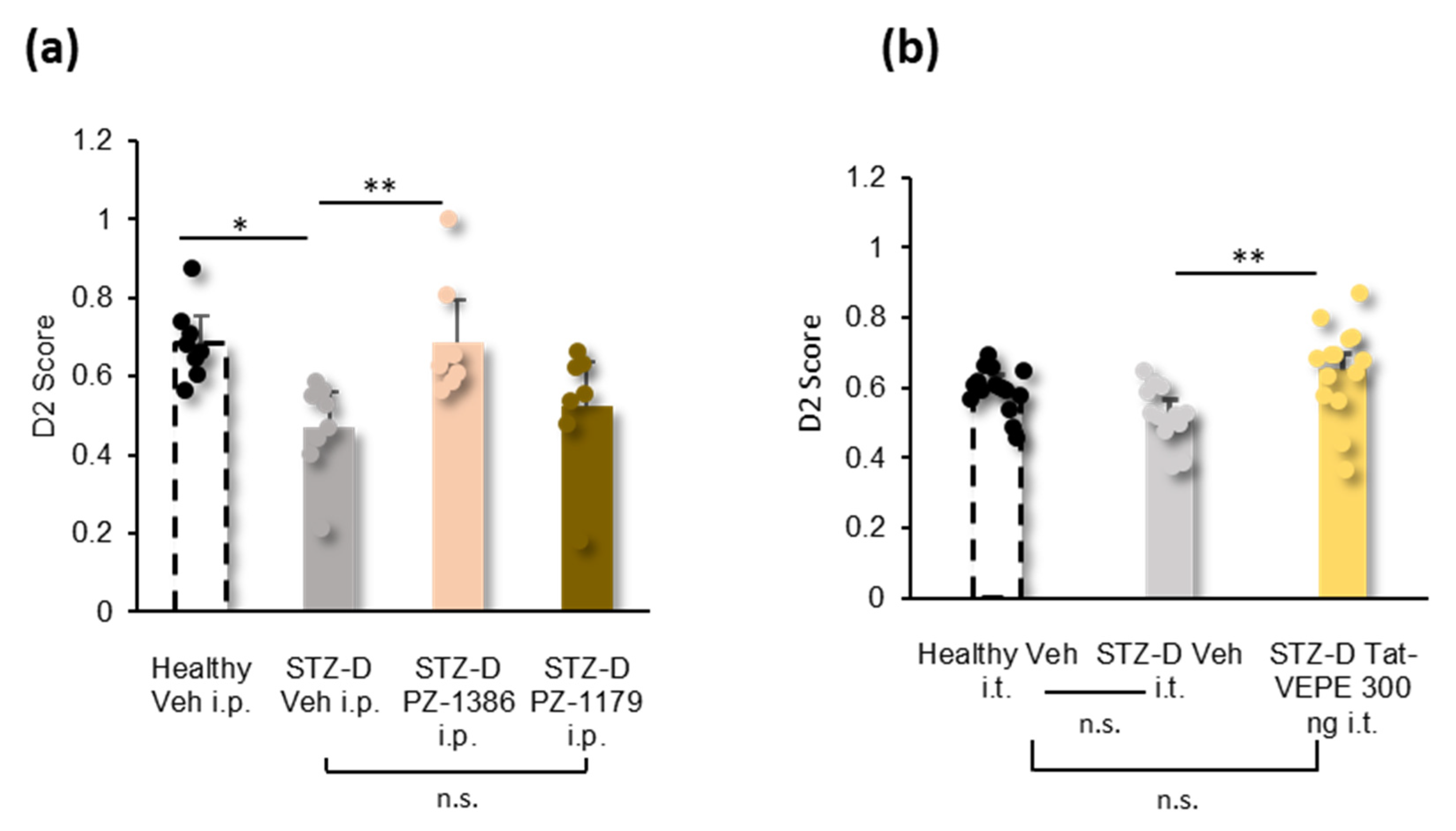
3.4. Effect of 5-HT6 Receptor Ligands and Tat-VEPE on Co-Morbid Cognitive Symptoms
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021.
- Chong, M.S.; Hester, J. Diabetic Painful Neuropathy: Current and Future Treatment Options. Drugs 2007, 67, 569–585. [Google Scholar] [CrossRef] [PubMed]
- Holt, R.I.G. (Ed.) Textbook of Diabetes, 4th ed.; Wiley-Blackwell: Chichester, West Sussex, UK; Hoboken, NJ, USA, 2010; ISBN 978-1-4051-9181-4. [Google Scholar]
- Jensen, T.S.; Baron, R.; Haanpaa, M.; Kalso, E.; Loeser, J.D.; Rice, A.S.; Treede, R.D. A New Definition of Neuropathic Pain. Pain 2011, 152, 2204–2205. [Google Scholar] [CrossRef] [PubMed]
- Tesfaye, S.; Boulton, A.J.M.; Dyck, P.J.; Freeman, R.; Horowitz, M.; Kempler, P.; Lauria, G.; Malik, R.A.; Spallone, V.; Vinik, A.; et al. Diabetic Neuropathies: Update on Definitions, Diagnostic Criteria, Estimation of Severity, and Treatments. Diabetes Care 2010, 33, 2285–2293. [Google Scholar] [CrossRef]
- Bouhassira, D.; Letanoux, M.; Hartemann, A. Chronic Pain with Neuropathic Characteristics in Diabetic Patients: A French Cross-Sectional Study. PLoS ONE 2013, 8, e74195. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.-W.; Lovblom, L.E.; Cardinez, M.; Weisman, A.; Farooqi, M.A.; Halpern, E.M.; Boulet, G.; Eldelekli, D.; Lovshin, J.A.; Lytvyn, Y.; et al. Neuropathy and Presence of Emotional Distress and Depression in Longstanding Diabetes: Results from the Canadian Study of Longevity in Type 1 Diabetes. J. Diabetes Complicat. 2017, 31, 1318–1324. [Google Scholar] [CrossRef]
- Jain, A.; Sharmab, R.; Yadavc, N.; Chaudhary, P.; Jainc, G.; Maanju, M. Quality of Life and Its Association with Insomnia and Clinical Variables in Type 2 Diabetes. J. Egypt Public Health Assoc. 2017, 92, 52–59. [Google Scholar] [CrossRef]
- Moheet, A.; Mangia, S.; Seaquist, E.R. Impact of Diabetes on Cognitive Function and Brain Structure. Ann. N. Y. Acad. Sci. 2015, 1353, 60–71. [Google Scholar] [CrossRef]
- Galer, B.S.; Gianas, A.; Jensen, M.P. Painful Diabetic Polyneuropathy: Epidemiology, Pain Description, and Quality of Life. Diabetes Res. Clin. Pract. 2000, 47, 123–128. [Google Scholar] [CrossRef]
- Geelen, C.C.; Smeets, R.J.E.M.; Schmitz, S.; van den Bergh, J.P.; Goossens, M.E.J.B.; Verbunt, J.A. Anxiety Affects Disability and Quality of Life in Patients with Painful Diabetic Neuropathy. Eur. J. Pain 2017, 21, 1632–1641. [Google Scholar] [CrossRef]
- Finnerup, N.B.; Attal, N.; Haroutounian, S.; McNicol, E.; Baron, R.; Dworkin, R.H.; Gilron, I.; Haanpaa, M.; Hansson, P.; Jensen, T.S.; et al. Pharmacotherapy for Neuropathic Pain in Adults: A Systematic Review and Meta-Analysis. Lancet Neurol. 2015, 14, 162–173. [Google Scholar] [CrossRef]
- Tesfaye, S.; Sloan, G.; Petrie, J.; White, D.; Bradburn, M.; Julious, S.; Rajbhandari, S.; Sharma, S.; Rayman, G.; Gouni, R.; et al. Comparison of Amitriptyline Supplemented with Pregabalin, Pregabalin Supplemented with Amitriptyline, and Duloxetine Supplemented with Pregabalin for the Treatment of Diabetic Peripheral Neuropathic Pain (OPTION-DM): A Multicentre, Double-Blind, Randomised Crossover Trial. Lancet 2022, 400, 680–690. [Google Scholar] [CrossRef] [PubMed]
- Rosenberger, D.C.; Blechschmidt, V.; Timmerman, H.; Wolff, A.; Treede, R.-D. Challenges of Neuropathic Pain: Focus on Diabetic Neuropathy. J. Neural. Transm. 2020, 127, 589–624. [Google Scholar] [CrossRef] [PubMed]
- Abraira, V.E.; Kuehn, E.D.; Chirila, A.M.; Springel, M.W.; Toliver, A.A.; Zimmerman, A.L.; Orefice, L.L.; Boyle, K.A.; Bai, L.; Song, B.J.; et al. The Cellular and Synaptic Architecture of the Mechanosensory Dorsal Horn. Cell 2017, 168, 295–310.e19. [Google Scholar] [CrossRef]
- Meffre, J.; Chaumont-Dubel, S.; Mannoury la Cour, C.; Loiseau, F.; Watson, D.J.; Dekeyne, A.; Seveno, M.; Rivet, J.M.; Gaven, F.; Deleris, P.; et al. 5-HT(6) Receptor Recruitment of MTOR as a Mechanism for Perturbed Cognition in Schizophrenia. EMBO Mol. Med. 2012, 4, 1043–1056. [Google Scholar] [CrossRef]
- Duhr, F.; Déléris, P.; Raynaud, F.; Séveno, M.; Morisset-Lopez, S.; Mannoury la Cour, C.; Millan, M.J.; Bockaert, J.; Marin, P.; Chaumont-Dubel, S. Cdk5 Induces Constitutive Activation of 5-HT6 Receptors to Promote Neurite Growth. Nat. Chem. Biol. 2014, 10, 590–597. [Google Scholar] [CrossRef] [PubMed]
- De Deurwaerdère, P.; Bharatiya, R.; Chagraoui, A.; Di Giovanni, G. Constitutive Activity of 5-HT Receptors: Factual Analysis. Neuropharmacology 2020, 168, 107967. [Google Scholar] [CrossRef]
- Kohen, R.; Fashingbauer, L.A.; Heidmann, D.E.A.; Guthrie, C.R.; Hamblin, M.W. Cloning of the Mouse 5-HT6 Serotonin Receptor and Mutagenesis Studies of the Third Cytoplasmic Loop. Mol. Brain Res. 2001, 90, 110–117. [Google Scholar] [CrossRef]
- Deraredj Nadim, W.; Chaumont-Dubel, S.; Madouri, F.; Cobret, L.; De Tauzia, M.-L.; Zajdel, P.; Bénédetti, H.; Marin, P.; Morisset-Lopez, S. Physical Interaction between Neurofibromin and Serotonin 5-HT6 Receptor Promotes Receptor Constitutive Activity. Proc. Natl. Acad. Sci. USA 2016, 113, 12310–12315. [Google Scholar] [CrossRef]
- Martin, P.-Y.; Doly, S.; Hamieh, A.M.; Chapuy, E.; Canale, V.; Drop, M.; Chaumont-Dubel, S.; Bantreil, X.; Lamaty, F.; Bojarski, A.J.; et al. MTOR Activation by Constitutively Active Serotonin6 Receptors as New Paradigm in Neuropathic Pain and Its Treatment. Prog. Neurobiol. 2020, 193, 101846. [Google Scholar] [CrossRef]
- Drop, M.; Jacquot, F.; Canale, V.; Chaumont-Dubel, S.; Walczak, M.; Satała, G.; Nosalska, K.; Mahoro, G.U.; Słoczyńska, K.; Piska, K.; et al. Neuropathic Pain-Alleviating Activity of Novel 5-HT6 Receptor Inverse Agonists Derived from 2-Aryl-1H-Pyrrole-3-Carboxamide. Bioorganic Chem. 2021, 115, 105218. [Google Scholar] [CrossRef]
- Drop, M.; Canale, V.; Chaumont-Dubel, S.; Kurczab, R.; Satała, G.; Bantreil, X.; Walczak, M.; Koczurkiewicz-Adamczyk, P.; Latacz, G.; Gwizdak, A.; et al. 2-Phenyl-1 H -Pyrrole-3-Carboxamide as a New Scaffold for Developing 5-HT6 Receptor Inverse Agonists with Cognition-Enhancing Activity. ACS Chem. Neurosci. 2021, 12, 1228–1240. [Google Scholar] [CrossRef] [PubMed]
- Saxton, R.A.; Sabatini, D.M. MTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef] [PubMed]
- Courteix, C.; Bardin, M.; Chantelauze, C.; Lavarenne, J.; Eschalier, A. Study of the Sensitivity of the Diabetes-Induced Pain Model in Rats to a Range of Analgesics. Pain 1994, 57, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Courteix, C.; Eschalier, A.; Lavarenne, J. Streptozocin-Induced Diabetic Rats: Behavioural Evidence for a Model of Chronic Pain. Pain 1993, 53, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Rafiey, M.; Nosrati, R.; Babaei, P. Protective Effect of MiR-34c Antagomir against STZ-Induced Memory Impairment by Targeting MTOR and PSD-95 in the Hippocampus of Rats. Neurosci. Lett. 2022, 789, 136881. [Google Scholar] [CrossRef]
- He, W.-Y.; Zhang, B.; Zhao, W.-C.; He, J.; Wang, Y.; Zhang, L.; Xiong, Q.-M.; Wang, H.-B. MTOR Activation Due to APPL1 Deficiency Exacerbates Hyperalgesia via Rab5/Akt and AMPK Signaling Pathway in Streptozocin-Induced Diabetic Rats. Mol. Pain 2019, 15, 174480691988064. [Google Scholar] [CrossRef]
- Inyang, K.E.; Szabo-Pardi, T.; Wentworth, E.; McDougal, T.A.; Dussor, G.; Burton, M.D.; Price, T.J. The Antidiabetic Drug Metformin Prevents and Reverses Neuropathic Pain and Spinal Cord Microglial Activation in Male but Not Female Mice. Pharmacol. Res. 2019, 139, 1–16. [Google Scholar] [CrossRef]
- Cao, X.-J.; Wu, R.; Qian, H.-Y.; Chen, X.; Zhu, H.-Y.; Xu, G.-Y.; Sun, Y.-Z.; Zhang, P.-A. Metformin Attenuates Diabetic Neuropathic Pain via AMPK/NF-ΚB Signaling Pathway in Dorsal Root Ganglion of Diabetic Rats. Brain Res. 2021, 1772, 147663. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Animal Research: Reporting in Vivo Experiments: The ARRIVE Guidelines. Br. J. Pharmacol. 2010, 160, 1577–1579. [Google Scholar] [CrossRef]
- Kim, S.H.; Chung, J.M. An Experimental Model for Peripheral Neuropathy Produced by Segmental Spinal Nerve Ligation in the Rat. Pain 1992, 50, 355–363. [Google Scholar] [CrossRef]
- Ling, B.; Authier, N.; Balayssac, D.; Eschalier, A.; Coudore, F. Behavioral and Pharmacological Description of Oxaliplatin-Induced Painful Neuropathy in Rat. Pain 2007, 128, 225–234. [Google Scholar] [CrossRef]
- Randall, L.O.; Selitto, J.J. A Method for Measurement of Analgesic Activity on Inflamed Tissue. Arch. Int. Pharm. Therm. 1957, 111, 409–419. [Google Scholar]
- Chaplan, S.R.; Bach, F.W.; Pogrel, J.W.; Chung, J.M.; Yaksh, T.L. Quantitative Assessment of Tactile Allodynia in the Rat Paw. J. Neurosci. Methods 1994, 53, 55–63. [Google Scholar] [CrossRef]
- Dixon, W.J. Efficient Analysis of Experimental Observations. Annu. Rev. Pharmacol. Toxicol. 1980, 20, 441–462. [Google Scholar] [CrossRef]
- Ennaceur, A.; Delacour, J. A New One-Trial Test for Neurobiological Studies of Memory in Rats. 1: Behavioral Data. Behav. Brain Res. 1988, 31, 47–59. [Google Scholar] [CrossRef]
- Grychowska, K.; Satała, G.; Kos, T.; Partyka, A.; Colacino, E.; Chaumont-Dubel, S.; Bantreil, X.; Wesołowska, A.; Pawłowski, M.; Martinez, J.; et al. Novel 1 H -Pyrrolo[3,2- c ]Quinoline Based 5-HT 6 Receptor Antagonists with Potential Application for the Treatment of Cognitive Disorders Associated with Alzheimer’s Disease. ACS Chem. Neurosci. 2016, 7, 972–983. [Google Scholar] [CrossRef]
- Rondón, L.J.; Farges, M.C.; Davin, N.; Sion, B.; Privat, A.M.; Vasson, M.P.; Eschalier, A.; Courteix, C. L-Arginine Supplementation Prevents Allodynia and Hyperalgesia in Painful Diabetic Neuropathic Rats by Normalizing Plasma Nitric Oxide Concentration and Increasing Plasma Agmatine Concentration. Eur. J. Nutr. 2018, 57, 2353–2363. [Google Scholar] [CrossRef]
- Vanda, D.; Canale, V.; Chaumont-Dubel, S.; Kurczab, R.; Satała, G.; Koczurkiewicz-Adamczyk, P.; Krawczyk, M.; Pietruś, W.; Blicharz, K.; Pękala, E.; et al. Imidazopyridine-Based 5-HT 6 Receptor Neutral Antagonists: Impact of N 1 -Benzyl and N 1 -Phenylsulfonyl Fragments on Different Receptor Conformational States. J. Med. Chem. 2021, 64, 1180–1196. [Google Scholar] [CrossRef]
- Zajdel, P.; Marciniec, K.; Satała, G.; Canale, V.; Kos, T.; Partyka, A.; Jastrzębska-Więsek, M.; Wesołowska, A.; Basińska-Ziobroń, A.; Wójcikowski, J.; et al. N 1-Azinylsulfonyl-1 H-Indoles: 5-HT6 Receptor Antagonists with Procognitive and Antidepressant-Like Properties. ACS Med. Chem. Lett. 2016, 7, 618–622. [Google Scholar] [CrossRef]
- Courteix, C.; Eschalier, A.; Mallet, C. L’évaluation de La Douleur Chez l’animal de Laboratoire. Douleur Analg. 2021, 34, 114–122. [Google Scholar] [CrossRef]
- Wattiez, A.-S.; Dupuis, A.; Courteix, C. Le rat STZ-diabétique: Modèle adapté à l’étude de la neuropathie diabétique douloureuse ? Douleur Analg. 2012, 25, 38–45. [Google Scholar] [CrossRef]
- Wattiez, A.-S.; Barrière, D.A. Rodent Models of Painful Diabetic Neuropathy: What Can We Learn from Them? J. Diabetes Metab. 2012, S5, 008. [Google Scholar] [CrossRef]
- Wang-Fischer, Y.; Garyantes, T. Improving the Reliability and Utility of Streptozotocin-Induced Rat Diabetic Model. J. Diabetes Res. 2018, 2018, 8054073. [Google Scholar] [CrossRef]
- Handwerker, H.O.; Arendt-Nielsen, L. (Eds.) Pain Models: Translational Relevance and Applications; IASP Press: Washington, DC, USA, 2013; ISBN 978-0-931092-94-7. [Google Scholar]
- Sari, C.C.; Gunduz, O.; Ulugol, A. Spinal Serotonin and 5HT6 Receptor Levels During Development of Neuropathy and Influence of Blockade of These Receptors on Thermal Hyperalgesia in Diabetic Mice. Drug Res. 2019, 69, 428–433. [Google Scholar] [CrossRef]
- Godinez-Chaparro, B.; Lopez-Santillan, F.J.; Orduna, P.; Granados-Soto, V. Secondary Mechanical Allodynia and Hyperalgesia Depend on Descending Facilitation Mediated by Spinal 5-HT(4), 5-HT(6) and 5-HT(7) Receptors. Neuroscience 2012, 222, 379–391. [Google Scholar] [CrossRef]
- Pineda-Farias, J.B.; Barragán-Iglesias, P.; Valdivieso-Sánchez, A.; Rodríguez-Silverio, J.; Flores-Murrieta, F.J.; Granados-Soto, V.; Rocha-González, H.I. Spinal 5-HT 4 and 5-HT 6 Receptors Contribute to the Maintenance of Neuropathic Pain in Rats. Pharmacol. Rep. 2017, 69, 916–923. [Google Scholar] [CrossRef]
- Yamamoto, H.; Shimoshige, Y.; Yamaji, T.; Murai, N.; Aoki, T.; Matsuoka, N. Pharmacological Characterization of Standard Analgesics on Mechanical Allodynia in Streptozotocin-Induced Diabetic Rats. Neuropharmacology 2009, 57, 403–408. [Google Scholar] [CrossRef]
- Finnerup, N.B.; Haroutounian, S.; Baron, R.; Dworkin, R.H.; Gilron, I.; Haanpaa, M.; Jensen, T.S.; Kamerman, P.R.; McNicol, E.; Moore, A.; et al. Neuropathic Pain Clinical Trials: Factors Associated with Decreases in Estimated Drug Efficacy. Pain 2018, 159, 2339–2346. [Google Scholar] [CrossRef]
- He, W.; Zhang, B.; Zhao, W.; He, J.; Zhang, L.; Xiong, Q.; Wang, J.; Wang, H. Contributions of MTOR Activation-Mediated Upregulation of Synapsin II and Neurite Outgrowth to Hyperalgesia in STZ-Induced Diabetic Rats. ACS Chem. Neurosci. 2019, 10, 2385–2396. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mokhtar, N.; Drop, M.; Jacquot, F.; Lamoine, S.; Chapuy, E.; Prival, L.; Aissouni, Y.; Canale, V.; Lamaty, F.; Zajdel, P.; et al. The Constitutive Activity of Spinal 5-HT6 Receptors Contributes to Diabetic Neuropathic Pain in Rats. Biomolecules 2023, 13, 364. https://doi.org/10.3390/biom13020364
Mokhtar N, Drop M, Jacquot F, Lamoine S, Chapuy E, Prival L, Aissouni Y, Canale V, Lamaty F, Zajdel P, et al. The Constitutive Activity of Spinal 5-HT6 Receptors Contributes to Diabetic Neuropathic Pain in Rats. Biomolecules. 2023; 13(2):364. https://doi.org/10.3390/biom13020364
Chicago/Turabian StyleMokhtar, Nazarine, Marcin Drop, Florian Jacquot, Sylvain Lamoine, Eric Chapuy, Laetitia Prival, Youssef Aissouni, Vittorio Canale, Frédéric Lamaty, Paweł Zajdel, and et al. 2023. "The Constitutive Activity of Spinal 5-HT6 Receptors Contributes to Diabetic Neuropathic Pain in Rats" Biomolecules 13, no. 2: 364. https://doi.org/10.3390/biom13020364
APA StyleMokhtar, N., Drop, M., Jacquot, F., Lamoine, S., Chapuy, E., Prival, L., Aissouni, Y., Canale, V., Lamaty, F., Zajdel, P., Marin, P., Doly, S., & Courteix, C. (2023). The Constitutive Activity of Spinal 5-HT6 Receptors Contributes to Diabetic Neuropathic Pain in Rats. Biomolecules, 13(2), 364. https://doi.org/10.3390/biom13020364







