Progress in Investigational Agents Targeting Serotonin-6 Receptors for the Treatment of Brain Disorders
Abstract
1. Introduction
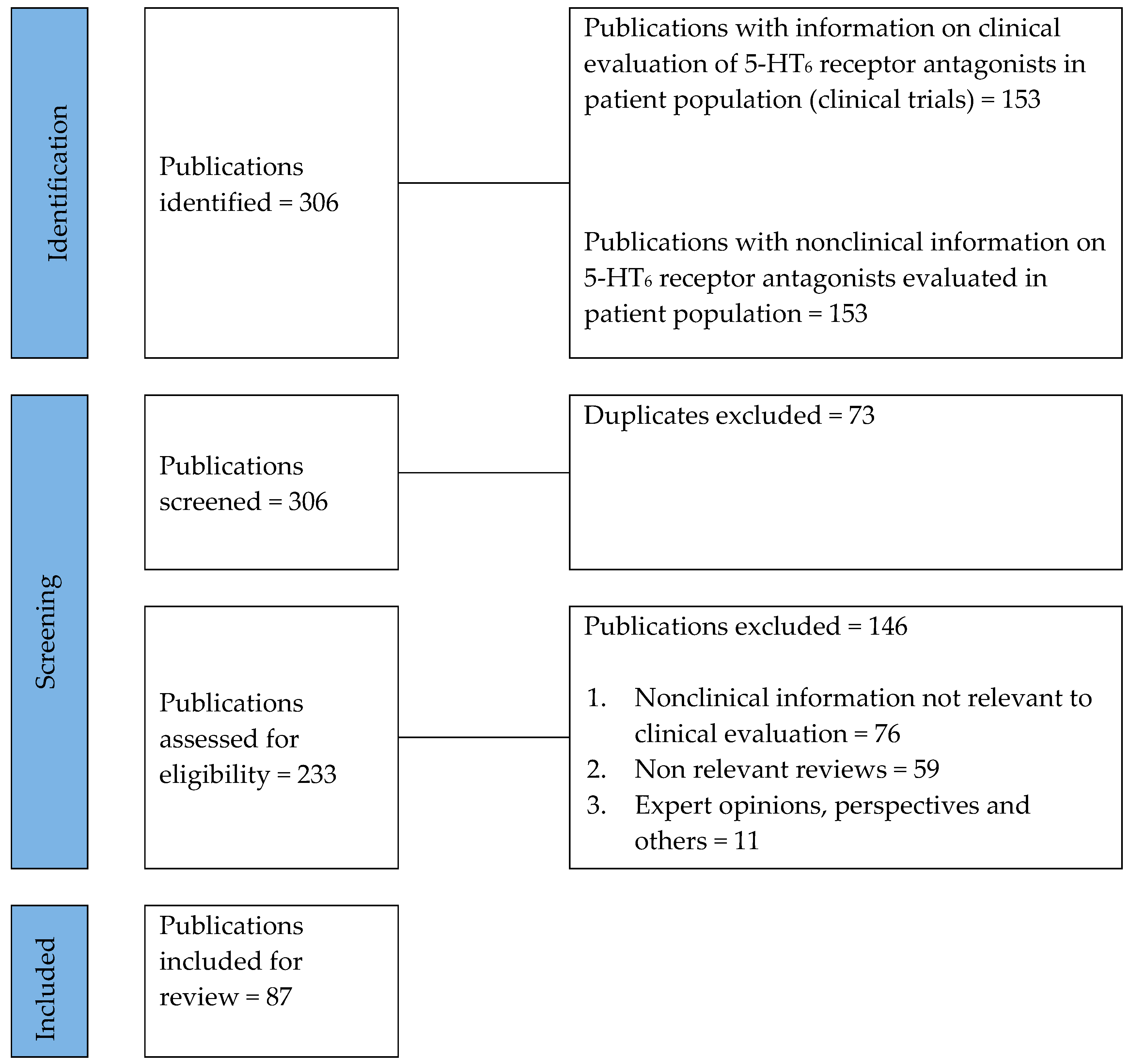
2. Methods
2.1. Literature Search
2.2. Data Extraction
3. Results
3.1. 5-HT6 Receptor and Drug Discovery
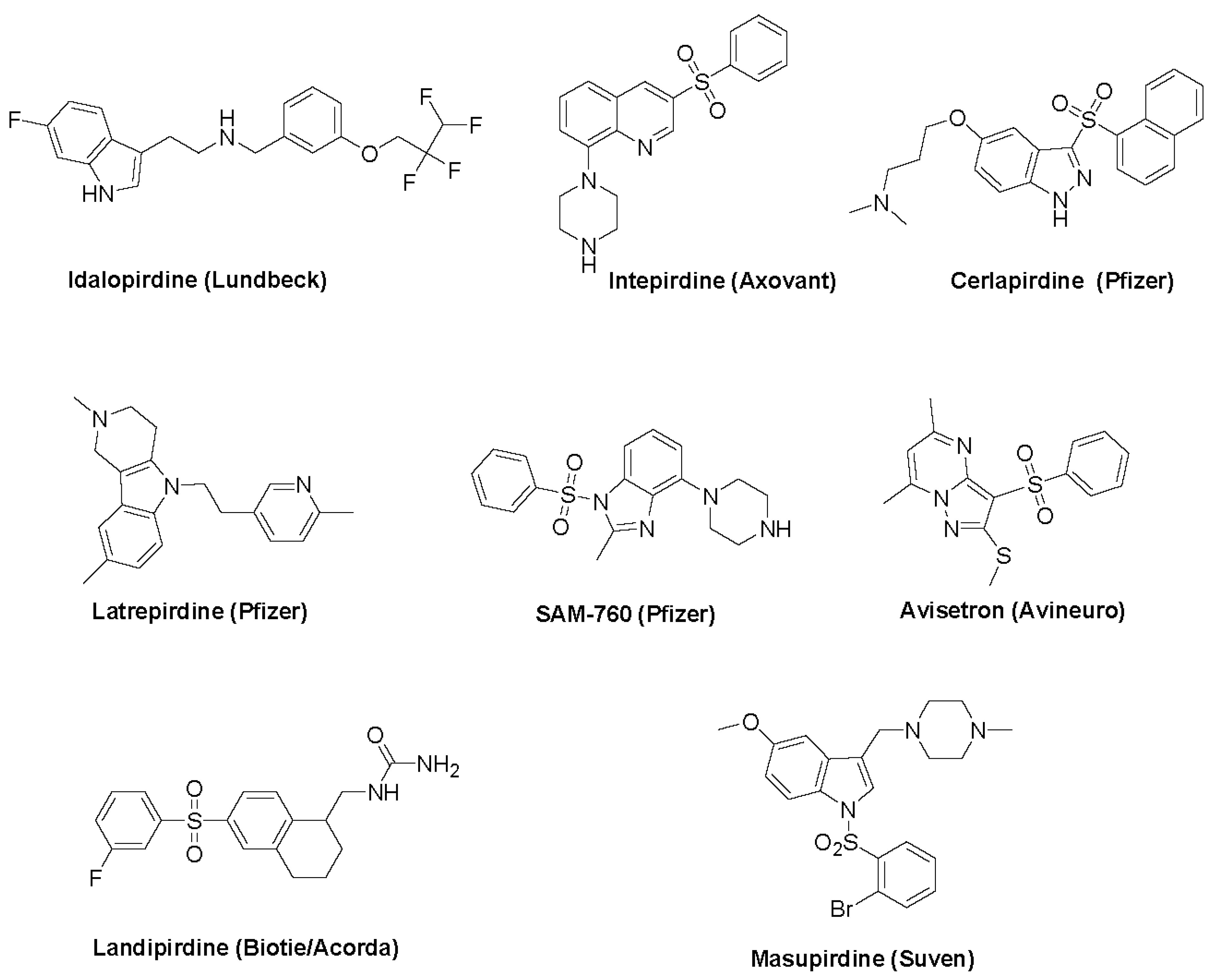
3.2. Avisetron
3.3. Cerlapirdine
3.4. Idalopirdine
3.5. Intepirdine
3.6. Landipirdine
3.7. Latrepirdine
3.8. Masupirdine
3.9. SAM-760
4. Conclusions and Outlook
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Erspamer, V.; Vialli, M. Ricerche sul secreto delle cellule enterocromaffini. Boll. Soc. Med.-Chir. Pavia 1937, 51, 357–363. [Google Scholar]
- Twarog, B.M.; Page, I.H. Serotonin content of some mammalian tissues and urine and a method for its determination. Am. J. Physiol. 1953, 175, 157–161. [Google Scholar] [CrossRef]
- Berger, M.; Gray, J.A.; Roth, B.L. The expanded biology of serotonin. Annu. Rev. Med. 2009, 60, 355–366. [Google Scholar] [CrossRef]
- Bockaert, J.; Roussignol, G.; Becamel, C.; Gavarini, S.; Joubert, L.; Dumuis, A.; Fagni, L.; Marin, P. GPCR-interacting proteins (GIPs): Nature and functions. Biochem. Soc. Trans. 2004, 32, 851–855. [Google Scholar] [CrossRef]
- Millan, M.J.; Marin, P.; Bockaert, J.; Mannoury la Cour, C. signaling at G-protein-coupled serotonin receptors: Recent advances and future research directions. Trends. Pharmacol. Sci. 2008, 29, 454–464. [Google Scholar] [CrossRef]
- Kim, D.Y.; Camilleri, M. Serotonin: A mediator of the brain-gut connection. Am. J. Gastroenterol. 2000, 95, 2698–2709. [Google Scholar] [CrossRef]
- Charnay, Y.; Léger, L. Brain serotonergic circuitries. Dialogues Clin. Neurosci. 2010, 12, 471–487. [Google Scholar] [CrossRef]
- Roth, B.L. Multiple serotonin receptors: Clinical and experimental aspects. Ann. Clin. Psychiatry 1994, 6, 67–78. [Google Scholar] [CrossRef]
- Roth, B.L.; Xia, Z. Molecular and cellular mechanisms for the polarized sorting of serotonin receptors: Relevance for genesis and treatment of psychosis. Crit. Rev. Neurobiol. 2004, 16, 229–236. [Google Scholar] [CrossRef]
- Pithadia, A.B.; Jain, S.M. 5-Hydroxytryptamine Receptor Subtypes and their Modulators with Therapeutic Potentials. J. Clin. Med. Res. 2009, 1, 72–80. [Google Scholar] [CrossRef]
- McCorvy, J.D.; Roth, B.L. Structure and function of serotonin G protein-coupled receptors. Pharmacol. Ther. 2015, 150, 129–142. [Google Scholar] [CrossRef]
- Monsma, F.J., Jr.; Shen, Y.; Ward, R.P.; Hamblin, M.W.; Sibley, D.R. Cloning and expression of a novel serotonin receptor with high affinity for tricyclic psychotropic drugs. Mol. Pharmacol. 1993, 43, 320–327. [Google Scholar]
- Kohen, R.; Metcalf, M.A.; Khan, N.; Druck, T.; Huebner, K.; Lachowicz, J.E.; Meltzer, H.Y.; Sibley, D.R.; Roth, B.L.; Hamblin, M.W. Cloning, characterization, and chromosomal localization of a human 5-HT6 serotonin receptor. J. Neurochem. 1996, 66, 47–56. [Google Scholar] [CrossRef]
- Codony, X.; Burgueño, J.; Ramírez, M.J.; Vela, J.M. 5-HT6 receptor signal transduction second messenger systems. Int. Rev. Neurobiol. 2010, 94, 89–110. [Google Scholar]
- Riccioni, T. 5-HT6 receptor characterization. Int. Rev. Neurobiol. 2010, 94, 67–88. [Google Scholar]
- Woolley, M.L.; Marsden, C.A.; Fone, K.C. 5-ht6 receptors. Curr. Drug Targets CNS Neurol. Disord. 2004, 3, 59–79. [Google Scholar] [CrossRef]
- Brodsky, M.; Lesiak, A.J.; Croicu, A.; Cohenca, N.; Sullivan, J.M.; Neumaier, J.F. 5-HT6 receptor blockade regulates primary cilia morphology in striatal neurons. Brain Res. 2017, 1660, 10–19. [Google Scholar] [CrossRef]
- Gérard, C.; Martres, M.P.; Lefèvre, K.; Miquel, M.C.; Vergé, D.; Lanfumey, L.; Doucet, E.; Hamon, M.; el Mestikawy, S. Immuno-localization of serotonin 5-HT6 receptor-like material in the rat central nervous system. Brain Res. 1997, 746, 207–219. [Google Scholar] [CrossRef]
- Roth, B.L.; Craigo, S.C.; Choudhary, M.S.; Uluer, A.; Monsma, F.J., Jr.; Shen, Y.; Meltzer, H.Y.; Sibley, D.R. Binding of typical and atypical antipsychotic agents to 5-hydroxytryptamine-6 and 5-hydroxytryptamine-7 receptors. J. Pharmacol. Exp. Ther. 1994, 268, 1403–1410. [Google Scholar]
- Bali, A.; Singh, S. Serotonergic 5-HT6 Receptor Antagonists: Heterocyclic Chemistry and Potential Therapeutic Significance. Curr. Top. Med. Chem. 2015, 15, 1643–1662. [Google Scholar] [CrossRef]
- Karila, D.; Freret, T.; Bouet, V.; Boulouard, M.; Dallemagne, P.; Rochais, C. Therapeutic Potential of 5-HT6 Receptor Agonists. J. Med. Chem. 2015, 58, 7901–7912. [Google Scholar] [CrossRef] [PubMed]
- Meffre, J.; Chaumont-Dubel, S.; Mannoury la Cour, C.; Loiseau, F.; Watson, D.J.; Dekeyne, A.; Séveno, M.; Rivet, J.M.; Gaven, F.; Déléris, P.; et al. 5-HT(6) receptor recruitment of mTOR as a mechanism for perturbed cognition in schizophrenia. EMBO Mol. Med. 2012, 4, 1043–1056. [Google Scholar] [CrossRef]
- Bockaert, J.; Bécamel, C.; Chaumont-Dubel, S.; Claeysen, S.; Vandermoere, F.; Marin, P. Novel and atypical pathways for serotonin signaling. Fac. Rev. 2021, 10, 52. [Google Scholar] [CrossRef]
- Martin, P.Y.; Doly, S.; Hamieh, A.M.; Chapuy, E.; Canale, V.; Drop, M.; Chaumont-Dubel, S.; Bantreil, X.; Lamaty, F.; Bojarski, A.J.; et al. mTOR activation by constitutively active serotonin6 receptors as new paradigm in neuropathic pain and its treatment. Prog. Neurobiol. 2020, 193, 101846. [Google Scholar] [CrossRef] [PubMed]
- Chaumont-Dubel, S.; Dupuy, V.; Bockaert, J.; Bécamel, C.; Marin, P. The 5-HT6 receptor interactome: New insight in receptor signaling and its impact on brain physiology and pathologies. Neuropharmacology 2020, 172, 107839. [Google Scholar] [CrossRef] [PubMed]
- Vanda, D.; Canale, V.; Chaumont-Dubel, S.; Kurczab, R.; Satała, G.; Koczurkiewicz-Adamczyk, P.; Krawczyk, M.; Pietruś, W.; Blicharz, K.; Pękala, E.; et al. Imidazopyridine-Based 5-HT6 Receptor Neutral Antagonists: Impact of N1-Benzyl and N1-Phenylsulfonyl Fragments on Different Receptor Conformational States. J. Med. Chem. 2021, 64, 1180–1196. [Google Scholar] [CrossRef]
- Drop, M.; Jacquot, F.; Canale, V.; Chaumont-Dubel, S.; Walczak, M.; Satała, G.; Nosalska, K.; Mahoro, G.U.; Słoczyńska, K.; Piska, K.; et al. Neuropathic pain-alleviating activity of novel 5-HT6 receptor inverse agonists derived from 2-aryl-1H-pyrrole-3-carboxamide. Bioorg. Chem. 2021, 115, 105218. [Google Scholar] [CrossRef]
- Yun, H.M.; Kim, S.; Kim, H.J.; Kostenis, E.; Kim, J.I.; Seong, J.Y.; Baik, J.H.; Rhim, H. The novel cellular mechanism of human 5-HT6 receptor through an interaction with Fyn. J. Biol. Chem. 2007, 282, 5496–5505. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, D.H.; Lee, K.H.; Im, S.K.; Hur, E.M.; Chung, K.C.; Rhim, H. Direct interaction and functional coupling between human 5-HT6 receptor and the light chain 1 subunit of the microtubule-associated protein 1B (MAP1B-LC1). PLoS ONE 2014, 9, e91402. [Google Scholar] [CrossRef]
- Yun, H.M.; Baik, J.H.; Kang, I.; Jin, C.; Rhim, H. Physical interaction of Jab1 with human serotonin 6 G-protein-coupled receptor and their possible roles in cell survival. J. Biol. Chem. 2010, 285, 10016–10029. [Google Scholar] [CrossRef]
- Chang, S.D.; Bruchas, M.R. Functional selectivity at GPCRs: New opportunities in psychiatric drug discovery. Neuropsychopharmacology 2014, 39, 248–249. [Google Scholar] [CrossRef]
- Kenakin, T. Functional selectivity and biased receptor signaling. J. Pharmacol. Exp. Ther. 2011, 336, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Canale, V.; Trybała, W.; Chaumont-Dubel, S.; KoczurkiewiczAdamczyk, P.; Satała, G.; Bento, O.; Blicharz-Futera, K.; Bantreil, X.; Pękala, E.; Bojarski, A.J.; et al. 1-(Arylsulfonyl-isoindol-2- yl)piperazines as 5-HT6R Antagonists: Mechanochemical Synthesis, In Vitro Pharmacological Properties and Glioprotective Activity. Biomolecules 2023, 13, 12. [Google Scholar] [CrossRef] [PubMed]
- Kucwaj-Brysz, K.; Baltrukevich, H.; Czarnota, K.; Handzlik, J. Chemical update on the potential for serotonin 5-HT6 and 5-HT7 receptor agents in the treatment of Alzheimer’s disease. Bioorg. Med. Chem. Lett. 2021, 49, 128275. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.G.; Robichaud, A.J. 5-HT6 medicinal chemistry. Int. Rev. Neurobiol. 2010, 94, 1–34. [Google Scholar]
- Grychowska, K.; Satała, G.; Kos, T.; Partyka, A.; Colacino, E.; Chaumont-Dubel, S.; Bantreil, X.; Wesołowska, A.; Pawłowski, M.; Martinez, J.; et al. Novel 1H-Pyrrolo[3,2-c]quinoline Based 5-HT6 Receptor Antagonists with Potential Application for the Treatment of Cognitive Disorders Associated with Alzheimer’s Disease. ACS Chem. Neurosci. 2016, 7, 972–983. [Google Scholar] [CrossRef]
- Wesołowska, A. Potential role of the 5-HT6 receptor in depression and anxiety: An overview of preclinical data. Pharmacol. Rep. 2010, 62, 564–577. [Google Scholar] [CrossRef]
- Woods, S.; Clarke, N.N.; Layfield, R.; Fone, K.C. 5-HT(6) receptor agonists and antagonists enhance learning and memory in a conditioned emotion response paradigm by modulation of cholinergic and glutamatergic mechanisms. Br. J. Pharmacol. 2012, 167, 436–449. [Google Scholar] [CrossRef]
- Ivachtchenko, A.V.; Lavrovsky, Y.; Ivanenkov, Y.A. AVN-211, Novel and Highly Selective 5-HT6 Receptor Small Molecule Antagonist, for the Treatment of Alzheimer’s Disease. Mol. Pharm. 2016, 13, 945–963. [Google Scholar] [CrossRef]
- Comery, T.A.; Aschmies, S.; Haydar, S.; Hughes, Z.; Huselton, C.; Kowal, D.; Kramer, A.; McFarlane, G.; Monaghan, M.; Smith, D.; et al. SAM-531, N,N-dimethyl-3-{[3-(1-naphthylsulfonyl)-1H-indazol-5-yl]oxy} propan-1-amine, a novel serotonin-6 receptor antagonist with preclinical pro-cognitive efficacy. Alzheimers Dement. 2010, 6, S548–S549. [Google Scholar] [CrossRef]
- Arnt, J.; Bang-Andersen, B.; Grayson, B.; Bymaster, F.P.; Cohen, M.P.; DeLapp, N.W.; Giethlen, B.; Kreilgaard, M.; McKinzie, D.L.; Neill, J.C.; et al. Lu AE58054, a 5-HT6 antagonist, reverses cognitive impairment induced by subchronic phencyclidine in a novel object recognition test in rats. Int. J. Neuropsychopharmacol. 2010, 13, 1021–1033. [Google Scholar] [CrossRef] [PubMed]
- Upton, N.; Chuang, T.T.; Hunter, A.J.; Virley, D.J. 5-HT6 receptor antagonists as novel cognitive enhancing agents for Alzheimer’s disease. Neurotherapeutics 2008, 5, 458–469. [Google Scholar] [CrossRef]
- Schaffhauser, H.; Mathiasen, J.R.; Dicamillo, A.; Huffman, M.J.; Lu, L.D.; McKenna, B.A.; Qian, J.; Marino, M.J. Dimebolin is a 5-HT6 antagonist with acute cognition enhancing activities. Biochem. Pharmacol. 2009, 78, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Bachurin, S.; Bukatina, E.; Lermontova, N.; Tkachenko, S.; Afanasiev, A.; Grigoriev, V.; Grigorieva, I.; Ivanov, Y.; Sablin, S.; Zefirov, N. Antihistamine agent Dimebon as a novel neuroprotector and a cognition enhancer. Ann. N. Y. Acad. Sci. 2001, 939, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, Q.; Bezprozvanny, I. Evaluation of Dimebon in cellular model of Huntington’s disease. Mol. Neurodegener. 2008, 3, 15. [Google Scholar] [CrossRef]
- Grigorev, V.V.; Dranyi, O.A.; Bachurin, S.O. Comparative study of action mechanisms of dimebon and memantine on AMPA- and NMDA-subtypes glutamate receptors in rat cerebral neurons. Bull. Exp. Biol. Med. 2003, 136, 474–477. [Google Scholar] [CrossRef] [PubMed]
- Lermontova, N.N.; Redkozubov, A.E.; Shevtsova, E.F.; Serkova, T.P.; Kireeva, E.G.; Bachurin, S.O. Dimebon and tacrine inhibit neurotoxic action of beta-amyloid in culture and block L-type Ca(2+) channels. Bull. Exp. Biol. Med. 2001, 132, 1079–1083. [Google Scholar] [CrossRef]
- Bachurin, S.O.; Shevtsova, E.P.; Kireeva, E.G.; Oxenkrug, G.F.; Sablin, S.O. Mitochondria as a target for neurotoxins and neuroprotective agents. Ann. N. Y. Acad. Sci. 2003, 993, 334–344. [Google Scholar] [CrossRef]
- Nirogi, R.; Shinde, A.; Kambhampati, R.S.; Mohammed, A.R.; Saraf, S.K.; Badange, R.K.; Bandyala, T.R.; Bhatta, V.; Bojja, K.; Reballi, V.; et al. Discovery and Development of 1-[(2-Bromophenyl)sulfonyl]-5-methoxy-3-[(4-methyl-1-piperazinyl)methyl]-1H-indole Dimesylate Monohydrate (SUVN-502): A Novel. ; Potent.; Selective and Orally Active Serotonin 6 (5-HT6) Receptor Antagonist for Potential Treatment of Alzheimer’s Disease. J. Med. Chem. 2017, 60, 1843–1859. [Google Scholar] [PubMed]
- Bell, J.; Baird-Bellaire, S.; Leil, T.; Comery, T.; Plotka, A.; Antinew, J.; Vandal, G.; Chalon, S.; James, K. Single- and multiple-dose safety, tolerability and pharmacokinetics of a Novel 5HT6 receptor full antagonist (SAM-760) for the treatment of the symptoms of Alzheimer’s disease in healthy young adults and elderly subjects. Alzheimers Dement. 2012, 7, S778. [Google Scholar] [CrossRef]
- Comery, T.; Zasadny, K.; Morris, E.; Antinew, J.; Bell, J.; Billing, B.; Boyden, T.; Esterlis, I.; Huang, Y.; Kupiec, J.; et al. Receptor occupancy of the 5-HT6 receptor antagonist SAM-760 in non-human primates and healthy human volunteers. Alzheimers Dement. 2012, 7, S794–S795. [Google Scholar] [CrossRef]
- Schmidt, E.; Areberg, J.; Evans, P.; Zann, V.; Søgaard, B. Assessment of the Contribution of CYP2D6 to the Elimination of Idalopirdine as well as the Absolute Bioavailability Following Multiple Oral Dosing. In Proceedings of the ASCPT 2016 Annual Meeting, San Diego, CA, USA, 8–12 March 2016. [Google Scholar]
- Galimberti, D.; Scarpini, E. Idalopirdine as a treatment for Alzheimer’s disease. Expert Opin. Investig. Drugs 2015, 24, 981–987. [Google Scholar] [CrossRef]
- Arnt, J.; Olsen, C.K. 5-HT6 receptor ligands and their antipsychotic potential. Int. Rev. Neurobiol. 2011, 96, 141–161. [Google Scholar] [PubMed]
- Mørk, A.; Russell, R.V.; de Jong, I.E.; Smagin, G. Effects of the 5-HT6 receptor antagonist idalopirdine on extracellular levels of monoamines.; glutamate and acetylcholine in the rat medial prefrontal cortex. Eur. J. Pharmacol. 2017, 799, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ferris, C.F.; Kulkarni, P.; Yee, J.R.; Nedelman, M.; de Jong, I.E.M. The Serotonin Receptor 6 Antagonist Idalopirdine and Acetylcholinesterase Inhibitor Donepezil Have Synergistic Effects on Brain Activity-A Functional MRI Study in the Awake Rat. Front. Pharmacol. 2017, 8, 279. [Google Scholar] [CrossRef]
- Amat-Foraster, M.; Leiser, S.C.; Herrik, K.F.; Richard, N.; Agerskov, C.; Bundgaard, C.; Bastlund, J.F.; de Jong, I.E.M. The 5-HT6 receptor antagonist idalopirdine potentiates the effects of donepezil on gamma oscillations in the frontal cortex of anesthetized and awake rats without affecting sleep-wake architecture. Neuropharmacology 2017, 113, 45–59. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chuang, A.T.T.; Foley, A.; Pugh, P.L.; Sunter, D.; Tong, X.; Regan, C.; Dawson, L.A.; Medhurst, A.D.; Upton, N. 5-HT6 receptor antagonist SB-742457 as a novel cognitive enhancing agent for Alzheimer’s disease. Alzheimers Dement. 2006, 2, S631–S632. [Google Scholar] [CrossRef]
- Dawson, L.A. The central role of 5-HT6 receptors in modulating brain neurochemistry. Int. Rev. Neurobiol. 2011, 96, 1–26. [Google Scholar]
- Sabbagh, M.N.; Shill, H.A. Latrepirdine, a potential novel treatment for Alzheimer’s disease and Huntington’s chorea. Curr. Opin. Investig. Drugs 2010, 11, 80–91. [Google Scholar]
- Chew, M.L.; Mordenti, J.; Yeoh, T.; Ranade, G.; Qiu, R.; Fang, J.; Liang, Y.; Corrigan, B. Minimization of CYP2D6 Polymorphic Differences and Improved Bioavailability via Transdermal Administration: Latrepirdine Example. Pharm. Res. 2016, 33, 1873–1880. [Google Scholar] [CrossRef]
- Santos, J.; Lobato, L.; Vale, N. Clinical pharmacokinetic study of latrepirdine via in silico sublingual administration. Silico Pharmacol. 2021, 9, 29. [Google Scholar] [CrossRef] [PubMed]
- Giorgetti, M.; Gibbons, J.A.; Bernales, S.; Alfaro, I.E.; Drieu La Rochelle, C.; Cremers, T.; Altar, C.A.; Wronski, R.; Hutter-Paier, B.; Protter, A.A. Cognition-enhancing properties of Dimebon in a rat novel object recognition task are unlikely to be associated with acetylcholinesterase inhibition or N-methyl-D-aspartate receptor antagonism. J. Pharmacol. Exp. Ther. 2010, 333, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Nirogi, R.; Mudigonda, K.; Bhyrapuneni, G.; Muddana, N.R.; Goyal, V.K.; Pandey, S.K.; Palacharla, R.C. Safety, Tolerability and Pharmacokinetics of the Serotonin 5-HT6 Receptor Antagonist, SUVN-502, in Healthy Young Adults and Elderly Subjects. Clin. Drug Investig. 2018, 38, 401–415. [Google Scholar] [CrossRef]
- Nirogi, R.; Abraham, R.; Benade, V.; Medapati, R.B.; Jayarajan, P.; Bhyrapuneni, G.; Muddana, N.; Mekala, V.R.; Subramanian, R.; Shinde, A.; et al. SUVN-502, a novel, potent, pure, and orally active 5-HT6 receptor antagonist: Pharmacological, behavioral, and neurochemical characterization. Behav. Pharmacol. 2019, 30, 16–35. [Google Scholar] [CrossRef] [PubMed]
- Nirogi, R.; Jayarajan, P.; Abraham, R.; Tadiparthi, J.; Benade, V.; Shinde, A.K.; Mohammed, A.R.; Badange, R.K.; Goyal, V.K.; Palacharla, V.R.C.; et al. Effects of Masupirdine (SUVN-502) on Agitation/Aggression and Psychosis in Patients with Probable Alzheimer’s Disease: A Post Hoc Analysis. Alzheimers Dement. 2022, 18, e062695. [Google Scholar] [CrossRef]
- Sawant-Basak, A.; Obach, R.S.; Doran, A.; Lockwood, P.; Schildknegt, K.; Gao, H.; Mancuso, J.; Tse, S.; Comery, T.A. Metabolism of a 5HT6 Antagonist, 2-Methyl-1-(Phenylsulfonyl)-4-(Piperazin-1-yl)-1H-Benzo[d]imidazole (SAM-760): Impact of Sulfonamide Metabolism on Diminution of a Ketoconazole-Mediated Clinical Drug-Drug Interaction. Drug Metab. Dispos. 2018, 46, 934–942. [Google Scholar] [CrossRef]
- Morozova, M.A.; Lepilkina, T.A.; Rupchev, G.E.; Beniashvily, A.G.; Burminskiy, D.S.; Potanin, S.S.; Bondarenko, E.V.; Kazey, V.I.; Lavrovsky, Y.; Ivachtchenko, A.V. Add-on clinical effects of selective antagonist of 5HT6 receptors AVN-211 (CD-008-0173) in patients with schizophrenia stabilized on antipsychotic treatment: Pilot study. CNS Spectr. 2014, 19, 316–323. [Google Scholar] [CrossRef]
- Avineuro Completed Phase II Clinical Study Of AVN-211, A Selective 5-HT6 Receptor Antagonist. Available online: https://www.biospace.com/article/releases/avineuro-completed-phase-ii-clinical-study-of-avn-211-a-selective-5-ht6-receptor-antagonist-/ (accessed on 1 November 2022).
- Morozova, M.; Burminskiy, D.; Rupchev, G.; Lepilkina, T.; Potanin, S.; Beniashvili, A.; Lavrovsky, Y.; Vostokova, N.; Ivaschenko, A. 5-HT6 Receptor Antagonist as an Adjunct Treatment Targeting Residual Symptoms in Patients with Schizophrenia: Unexpected Sex-Related Effects (Double-Blind Placebo-Controlled Trial). J. Clin. Psychopharmacol. 2017, 37, 169–175. [Google Scholar] [CrossRef]
- Brisard, C.; Safirstein, B.; Booth, K.; Hua, L.; Brault, Y.; Raje, S.; Leventer, S. Safety, tolerability, and preliminary efficacy of SAM-531, a 5HT-6 antagonist, in subjects with mild-to-moderate Alzheimer’s disease: Results from a phase 2a study. Alzheimers Dement. 2010, 6, S311. [Google Scholar] [CrossRef]
- Study Comparing 3 Dosage Levels Of SAM-531 In Outpatients With Mild To Moderate Alzheimer Disease. Available online: https://clinicaltrials.gov/ct2/show/NCT00895895 (accessed on 1 November 2022).
- H. Lundbeck A/S, DK. Methods of treating Alzheimer’s disease and pharmaceutical compositions thereof. WO2014037532.
- Saegis Pharmaceuticals, Inc. Completes Phase IIa Clinical Study Of SGS518. Available online: https://www.biospace.com/article/releases/saegis-pharmaceuticals-inc-completes-phase-iia-clinical-study-of-sgs518-/ (accessed on 1 November 2022).
- Wilkinson, D.; Windfeld, K.; Colding-Jørgensen, E. Safety and efficacy of idalopirdine, a 5-HT6 receptor antagonist, in patients with moderate Alzheimer’s disease (LADDER): A randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol. 2014, 13, 1092–1099. [Google Scholar] [CrossRef]
- Atri, A.; Frölich, L.; Ballard, C.; Tariot, P.N.; Molinuevo, J.L.; Boneva, N.; Windfeld, K.; Raket, L.L.; Cummings, J.L. Effect of Idalopirdine as Adjunct to Cholinesterase Inhibitors on Change in Cognition in Patients with Alzheimer Disease: Three Randomized Clinical Trials. JAMA 2018, 319, 130–142. [Google Scholar] [CrossRef] [PubMed]
- Maher-Edwards, G.; Zvartau-Hind, M.; Hunter, A.J.; Gold, M.; Hopton, G.; Jacobs, G.; Davy, M.; Williams, P. Double-blind, controlled phase II study of a 5-HT6 receptor antagonist, SB-742457, in Alzheimer’s disease. Curr. Alzheimer Res. 2010, 7, 374–385. [Google Scholar] [CrossRef] [PubMed]
- Maher-Edwards, G.; Dixon, R.; Hunter, J.; Gold, M.; Hopton, G.; Jacobs, G.; Hunter, J.; Williams, P. SB-742457 and donepezil in Alzheimer disease: A randomized.; placebo-controlled study. Int. J. Geriatr. Psychiatry 2011, 26, 536–544. [Google Scholar] [CrossRef]
- Maher-Edwards, G.; Watson, C.; Ascher, J.; Barnett, C.; Boswell, D.; Davies, J.; Fernandez, M.; Kurz, A.; Zanetti, O.; Safirstein, B.; et al. Two randomized controlled trials of SB742457 in mild-to-moderate Alzheimer’s disease. Alzheimers Dement. 2015, 1, 23–36. [Google Scholar] [CrossRef]
- Lang, F.M.; Mo, Y.; Sabbagh, M.; Solomon, P.; Boada, M.; Jones, R.W.; Frisoni, G.B.; Grimmer, T.; Dubois, B.; Harnett, M.; et al. Intepirdine as adjunctive therapy to donepezil for mild-to-moderate Alzheimer’s disease: A randomized.; placebo-controlled.; phase 3 clinical trial (MINDSET). Alzheimers Dement. 2021, 7, e12136. [Google Scholar] [CrossRef]
- Lang, F.M.; Kwon, D.Y.; Aarsland, D.; Boeve, B.; Tousi, B.; Harnett, M.; Mo, Y.; Noel Sabbagh, M. An international.; randomized.; placebo-controlled.; phase 2b clinical trial of intepirdine for dementia with Lewy bodies (HEADWAY-DLB). Alzheimers Dement. 2021, 7, e12171. [Google Scholar] [CrossRef]
- Fernandez, H.H. SYN120 (a dual 5-HT6/5-HT2A antagonist) study to evaluate safety, tolerability, and efficacy in Parkinson’s disease dementia (SYNAPSE): Phase 2a study results. Neurology 2019, 92, S4.005. [Google Scholar]
- Morozova, M.A.; Beniashvili, A.G.; Lepilkina, T.A.; Rupchev, G.E. Double-blind placebo-controlled randomized efficacy and safety trial of add-on treatment of dimebon plus risperidone in schizophrenic patients during transition from acute psychotic episode to remission. Psychiatr. Danub. 2012, 24, 159–166. [Google Scholar]
- Doody, R.S.; Gavrilova, S.I.; Sano, M.; Thomas, R.G.; Aisen, P.S.; Bachurin, S.O.; Seely, L.; Hung, D.; dimebon investigators. Effect of dimebon on cognition, activities of daily living, behaviour, and global function in patients with mild-to-moderate Alzheimer’s disease: A randomised, double-blind, placebo-controlled study. Lancet 2008, 372, 207–215. [Google Scholar] [CrossRef]
- Pfizer And Medivation Announce Results From Two Phase 3 Studies In Dimebon (latrepirdine*) Alzheimer’s Disease Clinical Development Program. Available online: https://www.pfizer.com/news/press-release/press-release-detail/pfizer_and_medivation_announce_results_from_two_phase_3_studies_in_dimebon_latrepirdine_alzheimer_s_disease_clinical_development_program (accessed on 1 November 2022).
- Nirogi, R.; Ieni, J.; Goyal, V.K.; Ravula, J.; Jetta, S.; Shinde, A.; Jayarajan, P.; Benade, V.; Palacharla, V.R.C.; Dogiparti, D.K.; et al. Effect of masupirdine (SUVN-502) on cognition in patients with moderate Alzheimer’s disease: A randomized, double-blind, phase 2, proof-of-concept study. Alzheimers Dement. 2022, 8, e12307. [Google Scholar] [CrossRef]
- Nirogi, R.; Goyal, V.K.; Benade, V.; Subramanian, R.; Ravula, J.; Jetta, S.; Shinde, A.; Pandey, S.K.; Jayarajan, P.; Jasti, V.; et al. Effect of Concurrent Use of Memantine on the Efficacy of Masupirdine (SUVN-502): A Post Hoc Analysis of a Phase 2 Randomized Placebo-Controlled Study. Neurol. Ther. 2022, 11, 1583–1594. [Google Scholar] [CrossRef] [PubMed]
- Nirogi, R.; Jayarajan, P.; Benade, V.; Shinde, A.; Goyal, V.K.; Jetta, S.; Ravula, J.; Abraham, R.; Grandhi, V.R.; Subramanian, R.; et al. Potential beneficial effects of masupirdine (SUVN-502) on agitation/aggression and psychosis in patients with moderate Alzheimer’s disease: Exploratory post hoc analyses. Int. J. Geriatr. Psychiatry 2022, 37, 10.1002/gps.5813. [Google Scholar] [CrossRef]
- Masupirdine for the Treatment of Agitation in Dementia of the Alzheimer’s Type. Available online: https://clinicaltrials.gov/ct2/show/NCT05397639 (accessed on 1 November 2022).
- Fullerton, T.; Binneman, B.; David, W.; Delnomdedieu, M.; Kupiec, J.; Lockwood, P.; Mancuso, J.; Miceli, J.; Bell, J. A Phase 2 clinical trial of PF-05212377 (SAM-760) in subjects with mild to moderate Alzheimer’s disease with existing neuropsychiatric symptoms on a stable daily dose of donepezil. Alzheimers. Res. Ther. 2018, 10, 38. [Google Scholar] [CrossRef] [PubMed]
- Atri, A. The Alzheimer’s Disease Clinical Spectrum: Diagnosis and Management. Med. Clin. N. Am. 2019, 103, 263–293. [Google Scholar] [CrossRef]
- Bartus, R.T.; Dean, R.L., 3rd; Beer, B.; Lippa, A.S. The cholinergic hypothesis of geriatric memory dysfunction. Science 1982, 217, 408–414. [Google Scholar] [CrossRef]
- Bourson, A.; Borroni, E.; Austin, R.H.; Monsma, F.J., Jr.; Sleight, A.J. Determination of the role of the 5-ht6 receptor in the rat brain: A study using antisense oligonucleotides. J. Pharmacol. Exp. Ther. 1995, 274, 173–180. [Google Scholar] [PubMed]
- Mitchell, E.S. 5-HT6 receptor ligands as antidementia drugs. Int. Rev. Neurobiol. 2011, 96, 163–187. [Google Scholar]
- Rossé, G.; Schaffhauser, H. 5-HT6 receptor antagonists as potential therapeutics for cognitive impairment. Curr. Top. Med. Chem. 2010, 10, 207–221. [Google Scholar] [CrossRef]
- Johnson, C.N.; Ahmed, M.; Miller, N.D. 5-HT6 receptor antagonists: Prospects for the treatment of cognitive disorders including dementia. Curr. Opin. Drug. Discov. Devel. 2008, 11, 642–654. [Google Scholar]
- Iarkov, A.; Mendoza, C.; Echeverria, V. Cholinergic Receptor Modulation as a Target for Preventing Dementia in Parkinson’s Disease. Front. Neurosci. 2021, 15, 665820. [Google Scholar] [CrossRef]
- Mori, E.; Ikeda, M.; Kosaka, K. Donepezil-DLB Study Investigators. Donepezil for dementia with Lewy bodies: A randomized.; placebo-controlled trial. Ann. Neurol. 2012, 72, 41–52. [Google Scholar] [CrossRef]
- Müller, M.L.; Bohnen, N.I. Cholinergic dysfunction in Parkinson’s disease. Curr. Neurol. Neurosci. Rep. 2013, 13, 377. [Google Scholar] [CrossRef]
- Assal, F.; Cummings, J.L. Neuropsychiatric symptoms in the dementias. Curr. Opin. Neurol. 2002, 15, 445–450. [Google Scholar] [CrossRef]
- Porsteinsson, A.P.; Antonsdottir, I.M. Neuropsychiatric Symptoms in Dementia: A Cause or Consequence? Am. J. Psychiatry 2015, 172, 410–411. [Google Scholar] [CrossRef]
- Cerejeira, J.; Lagarto, L.; Mukaetova-Ladinska, E.B. Behavioral and psychological symptoms of dementia. Front. Neurol. 2012, 3, 73. [Google Scholar] [CrossRef]
- Radue, R.; Walaszek, A.; Asthana, S. Neuropsychiatric symptoms in dementia. Handb. Clin. Neurol. 2019, 167, 437–454. [Google Scholar]
- Khoo, S.A.; Chen, T.Y.; Ang, Y.H.; Yap, P. The impact of neuropsychiatric symptoms on caregiver distress and quality of life in persons with dementia in an Asian tertiary hospital memory clinic. Int. Psychogeriatr. 2013, 25, 1991–1999. [Google Scholar] [CrossRef]
- Mukherjee, A.; Biswas, A.; Roy, A.; Biswas, S.; Gangopadhyay, G.; Das, S.K. Behavioural and Psychological Symptoms of Dementia: Correlates and Impact on Caregiver Distress. Dement. Geriatr. Cogn. Dis. Extra 2017, 7, 354–365. [Google Scholar] [CrossRef]
- Rogers, J.; Polhamus, D.; Lockwood, P.; Brault, Y.; Desmet, A.; Ito, K.; Romero, K.; Qiu, R.; Corrigan, B.; Gillespie, W.; et al. Model-based analysis to support strategic decision-making: A case study from the development of a 5HT6 antagonist for the treatment of Alzheimer’s disease. Alzheimers Dement. 2012, 8, P585. [Google Scholar] [CrossRef]
- Atri, A.; Tong, G.; Isojarvi, J.; Odergren, T. A 5-HT6 antagonist as adjunctive therapy to cholinesterase inhibitors in patients with mild to-moderate Alzheimer’s disease: Idalopirdine in phase III. Am. J. Geriatr. Psychiatry 2016, 24, S147–S148. [Google Scholar] [CrossRef]
- Parker, C.A.; Rabiner, E.A.; Gunn, R.N.; Searle, G.; Martarello, L.; Comley, R.A.; Davy, M.; Wilson, A.A.; Houle, S.; Mizrahi, R.; et al. Human Kinetic Modeling of the 5HT6 PET Radioligand 11C-GSK215083 and Its Utility for Determining Occupancy at Both 5HT6 and 5HT2A Receptors by SB742457 as a Potential Therapeutic Mechanism of Action in Alzheimer Disease. J. Nucl. Med. 2015, 56, 1901–1909. [Google Scholar] [CrossRef][Green Version]
- Weintraub, D. Impact of SYN120 (a Dual 5-HT6/5-HT2A antagonist) on psychiatric symptoms in patients with Parkinson’s disease dementia: Results from a Phase 2a Study. Mov. Disord. 2018, 33, S105. [Google Scholar]
- Lockwood, P.; Bell, J.; Chen, I.; Miceli, J.; Macci, K.; Van Winkle, J.; Planeta, B.; Henry, S.; Nabulsi, N.; Carson, R. 5HT2a receptor occupancy (RO) in healthy subjects determined by positron emission tomography (PET) following single-dose administration of SAM760 (PF-05212377). Clin. Pharmacol. Ther. 2015, 97, S21. [Google Scholar]
- Lacroix, L.P.; Dawson, L.A.; Hagan, J.J.; Heidbreder, C.A. 5-HT6 receptor antagonist SB-271046 enhances extracellular levels of monoamines in the rat medial prefrontal cortex. Synapse 2004, 51, 158–164. [Google Scholar] [CrossRef]
| Agents | In-Vitro Profile | ||
|---|---|---|---|
| 5-HT6 Receptors | Other Serotonergic Receptors | Other Receptors | |
| Avisetron (AVN-211) | Ki 1.09 nM Kb 0.83–1.97 nM [39] | 5-HT1A IC50 > 1000 nM; 5-HT1B IC50 > 1000 nM; 5-HT2A IC50 > 1000 nM; 5-HT2B IC50 96 nM; 5-HT2C IC50 > 1000 nM; 5-HT3 IC50 > 1000 nM; 5-HT4 IC50 > 1000 nM; 5-HT7 IC50 >1000 nM [39] | 5000-fold selectivity over 65 other receptors, enzymes, and ion channels [39] |
| Cerlapirdine (SAM-531 or WAY-262531) | Ki 1.3 nM [40] | 5-HT2B Ki 440 nM; 5-HT7 Ki 881 nM; 5-HT1B IC50 > 1000 nM; 5-HT1D IC50 > 1000 nM; 5-HT2A IC50 > 1000 nM; 5-HT2C IC50 > 1000 nM [40] | Not reported |
| Idalopirdine (LY 483518 or SGS518 or Lu AE58054) | Ki 0.83 nM; EC50 25 nM; Kb 4.9 nM [41] | 5-HT1A Ki 2300 nM; 5-HT1B Ki > 10,000 nM; 5-HT1D Ki 2600 nM; 5-HT1E Ki > 4600 nM; 5-HT1F Ki 2400 nM; 5-HT2A Ki 83 nM; 5-HT2B Ki > 4100 nM; 5-HT2C Ki 250 nM; 5-HT3 IC50 > 10,000 nM; 5-HT4e IC50 > 10,000 nM; 5-HT7 Ki > 10,000 nM [41] | >50-fold selectivity over 100 other receptors, enzymes, and ion channels, except for adrenergic receptors (α1A Ki 21 nM; α1B Ki 22 nM) [41] |
| Intepirdine (SB-742457 or RVT-101) | Ki 0.23 nM [42] | 5-HT2A Ki 10 nM [42] | >100-fold selectivity over other receptors, enzymes, and ion channels [42] |
| Landipirdine (SYN-120) | Not reported | ||
| Latrepirdine (Dimebon) | Ki 26 nM; Kb 26 nM [43] | Not reported | Weak inhibitor of cholinesterase, N-methyl-D-aspartate and voltage-gated calcium channels and weak modulator of the mitochondrial permeability transition pore [44,45,46,47,48] |
| Masupirdine (SUVN-502) | Ki 2.04 nM Kb 2.6 nM [49] | 5-HT1A Ki 7020 nM; 5-HT1B IC50 > 10,000 nM; 5-HT1D IC50 > 10,000 nM; 5-HT2A Ki 2514 nM; 5-HT2C Ki > 1000 nM; 5-HT4B Ki 4166 nM; 5-HT5A IC50 > 10,000 nM; 5-HT7 IC50 > 10,000 nM [49] | >500-fold selectivity over 100 other targets that includes receptors, ion channels, enzymes, peptides, growth factors, steroids, immunological factors, second messengers, and prostaglandins except for dopamine receptor (D3 Ki 616 nM) and adrenergic receptors (α2A Ki 2570 nM; α2C Kb 619 nM) [49] |
| SAM-760 (WYE-103760 or PF-05212377) | Ki 0.53 nM; IC50 0.76 nM [50,51] | Not reported | |
| Agents | Oral Bioavailability (%) | CYP Isoform Involved in the Metabolism | Active Metabolite | Drug Interaction Liability | In-Vivo Profile |
|---|---|---|---|---|---|
| Avisetron | 5.73 [39] | Not reported | Inhibitor of CYP 2B6, 2C9, 2C19 [39] | Attenuated the memory deficits induced by MK-801, and scopolamine in the object recognition task, passive avoidance task, and Morris water maze task [39] | |
| Cerlapirdine | 24 [40] | Not reported | None [40] | Attenuated the memory deficits induced by MK-801, scopolamine, combined scopolamine and MK-801 treatment in the object recognition task [40] | |
| Idalopirdine | 60 # [52] | CYP3A4 and CYP2D6 [53] | Not reported | Low [52] | Improved cognition in phencyclidine-challenged rats; modulated dopamine, norepinephrine and glutamate neurotransmitters in brain; potentiated the effects of donepezil on neuronal oscillations, acetylcholine modulation and blood oxygen level-dependent functional signaling [54,55,56,57] |
| Intepirdine | 76 [42] | Not reported | Attenuated the memory deficits caused by scopolamine in the object recognition task and passive avoidance task and reversed the memory deficit associated with senile dementia; enhanced medial prefrontal cortex cholinergic neurotransmission as standalone and add-on treatment to donepezil [58,59] | ||
| Landipirdine | Not reported | ||||
| Latrepirdine | 53 [60]; 5 to 6% in extensive CYP2D6 metabolizers and 45% in poor CYP2D6 metabolizers * [61] | CYP2D6 [62] | Not reported | Enhanced memory in the social recognition task and object recognition task [43,63] | |
| Masupirdine | 24.9 [49] | CYP3A4 [64] | Yes [64] | No $ [64] | Attenuated scopolamine, MK-801 and ageing associated memory deficits; potentiated the effects of donepezil on neuronal oscillations, and acetylcholine modulation; potentiated the effects of memantine on acetylcholine modulation; potentiated the effects of donepezil and memantine on cognition in the Morris water maze task, neuronal oscillations, and acetylcholine modulation; reduced aggression-like behaviors in resident intruder task and dominance levels in the dominant–submissive assay; Modulated cortical dopamine and norepinephrine [65,66] |
| SAM-760 | Not reported | CYP3A4/5 [67] | Not reported | No [67] | Attenuated the memory deficits induced by MK-801, scopolamine, combined scopolamine and MK-801 treatment in the object recognition task [50,51] |
| Agents | Indication | NCT Number | Endpoint | Outcome |
|---|---|---|---|---|
| Avisetron | Schizophrenia (Pilot study) [68,69] | Not available | Key Endpoints: PANSS, CAT | As augmentation therapy, avisetron treatment-related benefits were observed in PANSS total scores, PANSS positive subscores and CAT |
| Schizophrenia (Phase 2 study) [70] | Not available | Primary: Change from baseline in the PANSS total scores Other Key Endpoints: Change from baseline in the CGI-S, CGI-I, NSA-16, PSPS, CogFu, BACS and CPT | As augmentation therapy, a trend towards avisetron treatment-related benefits were observed in PANSS total score, PANSS positive subscores and PANSS general psychopathology scale after 6 weeks of treatment; No notable effects on CGI-S, CGI-I, NSA, PSPS, CogFu, BACS and CPT | |
| Cerlapirdine | Alzheimer’s disease (Pilot study) [71] | NCT00481520 | Key Endpoints: MMSE, ADAS-Cog and CANTAB | Trend towards improvement was observed with cerlapirdine treatment on the ADAS-Cog 11 and CANTAB at the end of 4 weeks |
| Alzheimer’s disease (Phase 2 study) [72] | NCT00895895 | Primary: Change from baseline in the ADAS-Cog 11 total scores Other Key Endpoints: Change from baseline in the ADCS-CGIC, CANTAB and NPI-12 | No beneficial effects of cerlapirdine were observed at the end of 24 weeks on any of the studied endpoints | |
| Idalopirdine | Schizophrenia (Pilot study) [73] | Not available | Safety, tolerability, pharmacokinetics and pharmacodynamics (cognitive changes assessed using BACS) | Safe and well-tolerated as standalone treatment for 14 days; Idalopirdine treatment was associated with dose-dependent pattern of improvement in the BACS endpoint; No effect in the placebo treated group |
| Schizophrenia (Phase 2 study) [74] | NCT00810667 | Primary: Change in PANSS total scores Other Key Endpoints: Neurocognitive performance using the BACS | As augmentation therapy, no change was observed in the PANSS total scores or BACS scores or PANSS cognitive subscale scores as compared to placebo after 12 weeks of treatment | |
| Alzheimer’s disease (Phase 2 study) [75] | NCT01019421 | Primary: Change from baseline in the ADAS-Cog 11 Other Key Endpoints: Change from baseline in the ADCS-ADL, ADCS-CGIC, MMSE and NPI-12 | As augmentation therapy, significant improvements in ADAS-Cog 11 scores were observed as compared to placebo after 24 weeks of treatment; Parallel trend towards improvement in ADCS-ADL and ADCS-CGIC; Improvements in anxiety and hallucinations domains of the NPI-12 scale in a post hoc analysis | |
| Alzheimer’s disease (Phase 3 studies) [76] | NCT01955161, NCT02006641, and NCT02006654 | Primary: Change from baseline in the ADAS-Cog Other Key Endpoints: Change from baseline in the ADCS-ADL, ADCS-CGIC, MMSE and NPI-12 | As augmentation therapy, no significant improvements in ADAS-Cog 11 scores as compared to placebo after 24 weeks of treatment; Similar observations were noted in other endpoints | |
| Intepirdine | Alzheimer’s disease (Phase 2 study) [77] | NCT00224497 | Primary: Change from baseline in the ADAS-Cog 11 scores and CIBIC+ Other Key Endpoints: Change from baseline in the MMSE and NPI-12 | Significant improvement in the CIBIC+ and trend in the ADAS-Cog 11 scores was observed with 24 weeks of intepirdine treatment |
| Alzheimer’s disease (Phase 2 studies) [78,79] | NCT00348192 and NCT00708552 | Primary: Change from baseline in the ADAS-Cog 11 scores and CIBIC+ Other Key Endpoints: Change from baseline in the MMSE and ADCS-ADL | No significant effect was observed on the CIBIC+ or ADAS-Cog 11 scores at the end of 24 weeks of intepirdine treatment | |
| Alzheimer’s disease (Phase 2 study) [79] | NCT00710684 | Primary: Change from baseline in the ADAS-Cog 11 scores and CDR-SB Other Key Endpoints: Change from baseline in the MMSE and ADCS-ADL | As an add-on therapy to donepezil, beneficial effects of intepirdine were observed in the ADAS-Cog 11 scores at the end of 24 weeks and the effects were noted up to 48 weeks; No notable effects were observed on the CDR-SB | |
| Alzheimer’s disease (Phase 3 study) [80] | NCT02585934 | Primary: Change from baseline in the ADAS-Cog 11 scores and ADCS-ADL Other Key Endpoints: Change from baseline in the NPI-12 | As an add-on therapy to donepezil, no beneficial effects of intepirdine were observed in the ADAS-Cog 11 or ADCS-ADL scores at the end of 24 weeks | |
| Dementia with Lewy bodies (Phase 2 study) [81] | NCT02669433 | Primary: Change from baseline in the UPDRS–III total scores Other Key Endpoints: Change from baseline in the ADAS-Cog 11 and CIBIC+ | No beneficial effects of intepirdine were observed in the UPDRS–III total score at the end of 24 weeks | |
| Landipirdine | Parkinson’s disease dementia (Phase 2 study) [82] | NCT02258152 | Primary: Change from baseline in the CDRCOA total scores Other Key Endpoints: Change from baseline in the ADCS-CGIC, MoCA and NPI-12 | No beneficial effects of landipirdine were observed as an add-on treatment to cholinesterase inhibitor after 16 weeks of treatment; Post hoc analysis suggested beneficial effects on apathy, anxiety, and irritability/lability |
| Latrepirdine | Schizophrenia (Phase 2 study) [83] | Not available | Key Endpoints: PANSS, CGI-S and NSA-16 | As an add-on therapy, no beneficial effect of latrepirdine was observed in the PANSS total or sub scale scores; Latrepirdine showed statistically significant improvement in the NSA-16 scale |
| Alzheimer’s disease (Pilot study) [44] | Not available | Key Endpoint: Bukatina scale | Treatment with latrepirdine was associated with improvements in cognitive function and reduction of NPS | |
| Alzheimer’s disease (Phase 2 study) [84] | NCT00377715 | Primary: Change from baseline in the ADAS-Cog 11 scores Other Key Endpoints: Change from baseline in the MMSE, ADCS-ADL and NPI-12 | Significant improvement was observed on the ADAS-Cog 11, MMSE, ADCS-ADL and NPI-12 after 24 weeks of treatment | |
| Alzheimer’s disease (Phase 3 studies) [85] | NCT00675623 and NCT00829374 | Primary: Change from baseline in the ADAS-Cog 11 scores and CIBIC+ (NCT00675623) or change from baseline in the ADAS-Cog 11 scores and ADCS-ADL (NCT00829374) Other Key Endpoints: Change from baseline in the MMSE, ADCS-ADL and NPI-12 | No significant effect of latrepirdine treatment was observed as standalone or add-on to donepezil after 26 or 52 weeks of treatment | |
| Masupirdine | Alzheimer’s disease (Phase 2 study) [86,87,88] | NCT02580305 | Primary: Change from baseline in the ADAS-Cog 11 total scores Other Key Endpoints: Change from baseline in the ADCS-ADL, MMSE, CDR-SB and NPI-12 | No beneficial effects of masupirdine were observed as an add-on treatment to donepezil and memantine in the ADAS-Cog 11 after 26 weeks of treatment; Post hoc analysis suggested potential impact of memantine on the efficacy, and potential beneficial effects on agitation/aggression and psychosis |
| Alzheimer’s disease Agitation (Potentially pivotal study) [89] | NCT05397639 | Primary: Change from baseline in the CMAI items scores aligning to the International Psychogeriatric Association agitation criteria domains Other Key Endpoints: Change from baseline in the modified ADCS-CGI-C, MMSE and ADAS-Cog 11 | Study in progress | |
| SAM-760 | Alzheimer’s disease (Phase 2 study) [90] | NCT01712074 | Primary: Change from baseline in the ADAS-Cog 13 total scores Other Key Endpoints: Change from baseline in the COWAT, CFT and NPI-12 | Trial was stopped after a futility analysis; No beneficial effect of SAM-760 treatment was observed after 12 weeks of treatment |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nirogi, R.; Jayarajan, P.; Shinde, A.; Mohammed, A.R.; Grandhi, V.R.; Benade, V.; Goyal, V.K.; Abraham, R.; Jasti, V.; Cummings, J. Progress in Investigational Agents Targeting Serotonin-6 Receptors for the Treatment of Brain Disorders. Biomolecules 2023, 13, 309. https://doi.org/10.3390/biom13020309
Nirogi R, Jayarajan P, Shinde A, Mohammed AR, Grandhi VR, Benade V, Goyal VK, Abraham R, Jasti V, Cummings J. Progress in Investigational Agents Targeting Serotonin-6 Receptors for the Treatment of Brain Disorders. Biomolecules. 2023; 13(2):309. https://doi.org/10.3390/biom13020309
Chicago/Turabian StyleNirogi, Ramakrishna, Pradeep Jayarajan, Anil Shinde, Abdul Rasheed Mohammed, Venkata Ramalingayya Grandhi, Vijay Benade, Vinod Kumar Goyal, Renny Abraham, Venkat Jasti, and Jeffrey Cummings. 2023. "Progress in Investigational Agents Targeting Serotonin-6 Receptors for the Treatment of Brain Disorders" Biomolecules 13, no. 2: 309. https://doi.org/10.3390/biom13020309
APA StyleNirogi, R., Jayarajan, P., Shinde, A., Mohammed, A. R., Grandhi, V. R., Benade, V., Goyal, V. K., Abraham, R., Jasti, V., & Cummings, J. (2023). Progress in Investigational Agents Targeting Serotonin-6 Receptors for the Treatment of Brain Disorders. Biomolecules, 13(2), 309. https://doi.org/10.3390/biom13020309






