Gut Micro- and Mycobiota in Preeclampsia: Bacterial Composition Differences Suggest Role in Pathophysiology
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Definitions
2.3. Study Cohort and Questionnaire
2.4. Sample Collection
2.5. Protein Extraction and Calprotectin ELISA
2.6. Reference Values
2.7. DNA Extraction, 16S rRNA, and ITS1 Gene Amplicon Sequencing
2.8. Sequence Data Processing
2.9. Statistical Analyses
3. Results
3.1. Patient Characteristics and Questionnaire
3.2. Oral Glucose Tolerance Test at Gestational Week 28
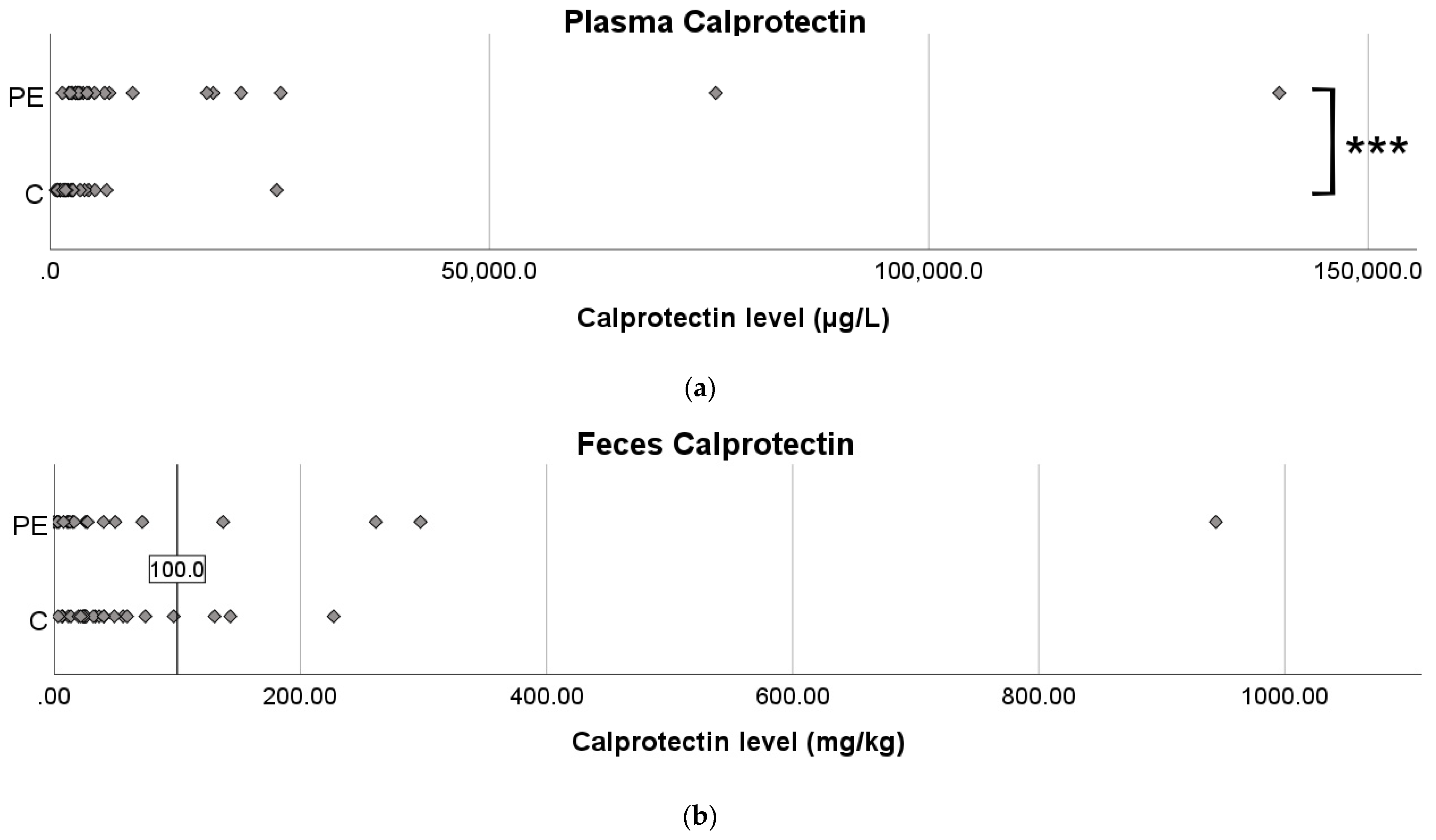
3.3. Calprotectin Levels in Plasma and Feces
3.4. Micro- and Mycobiomes Based on 16S and ITS rRNA Gene Sequencing
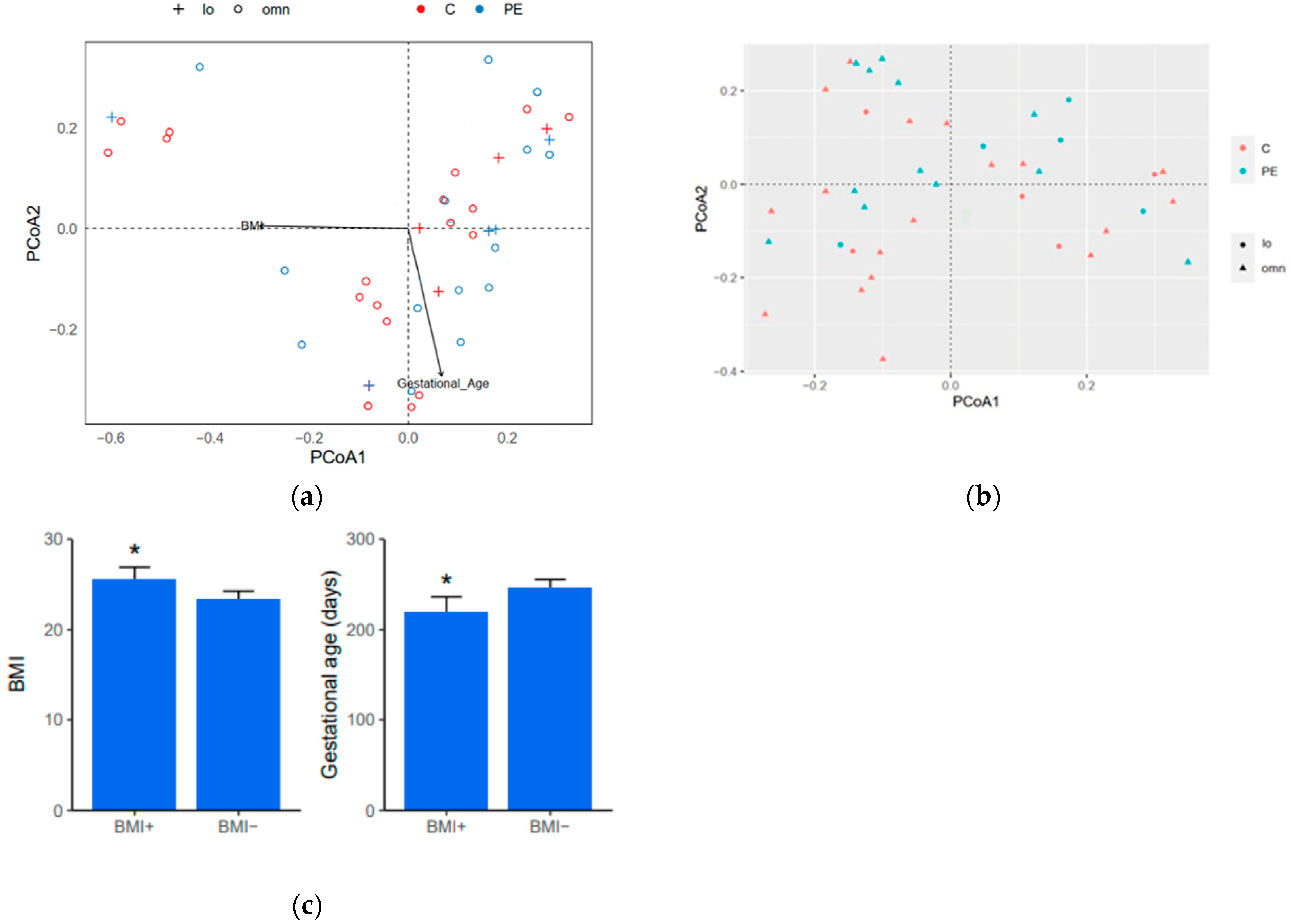
3.5. Diversity Indices
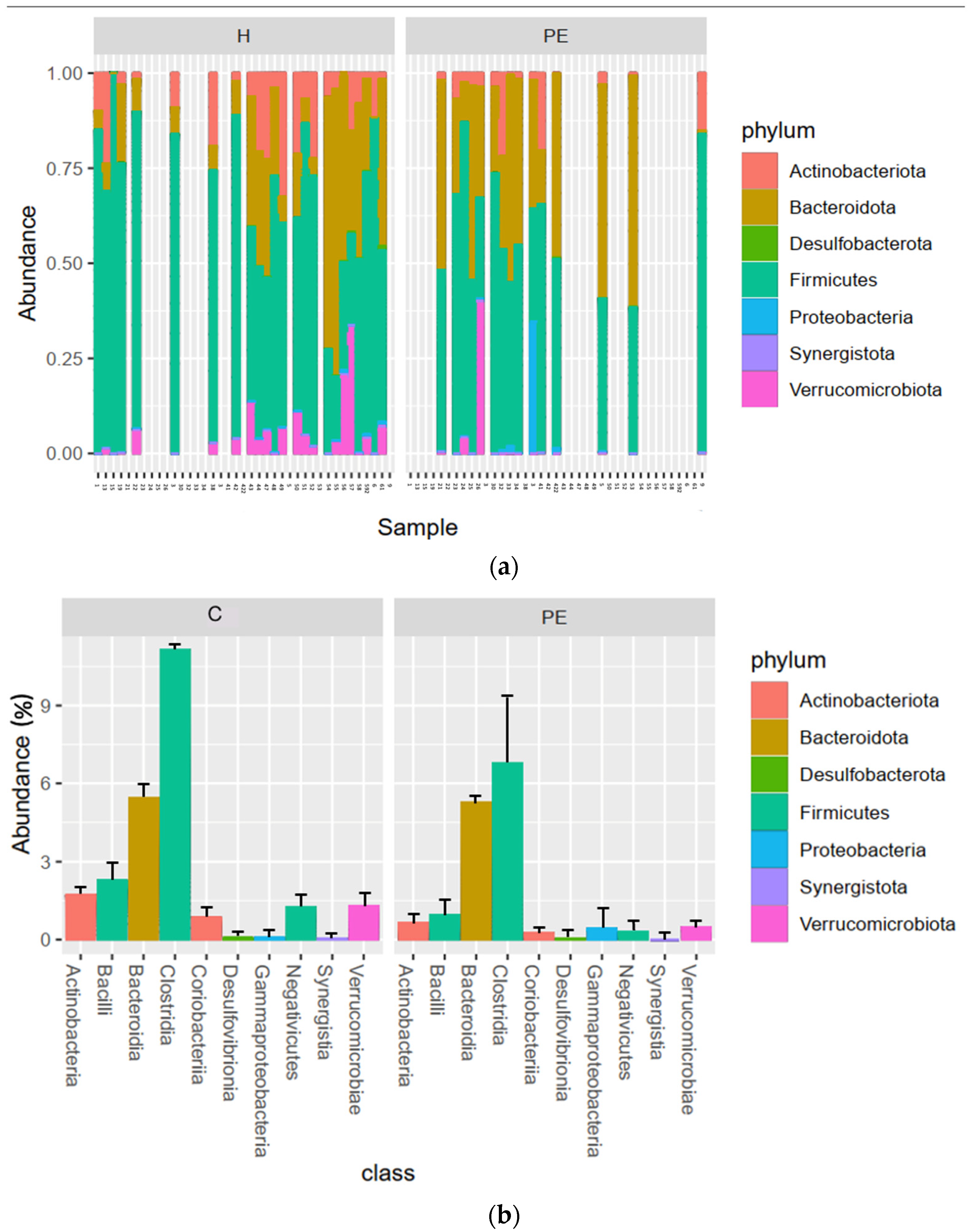
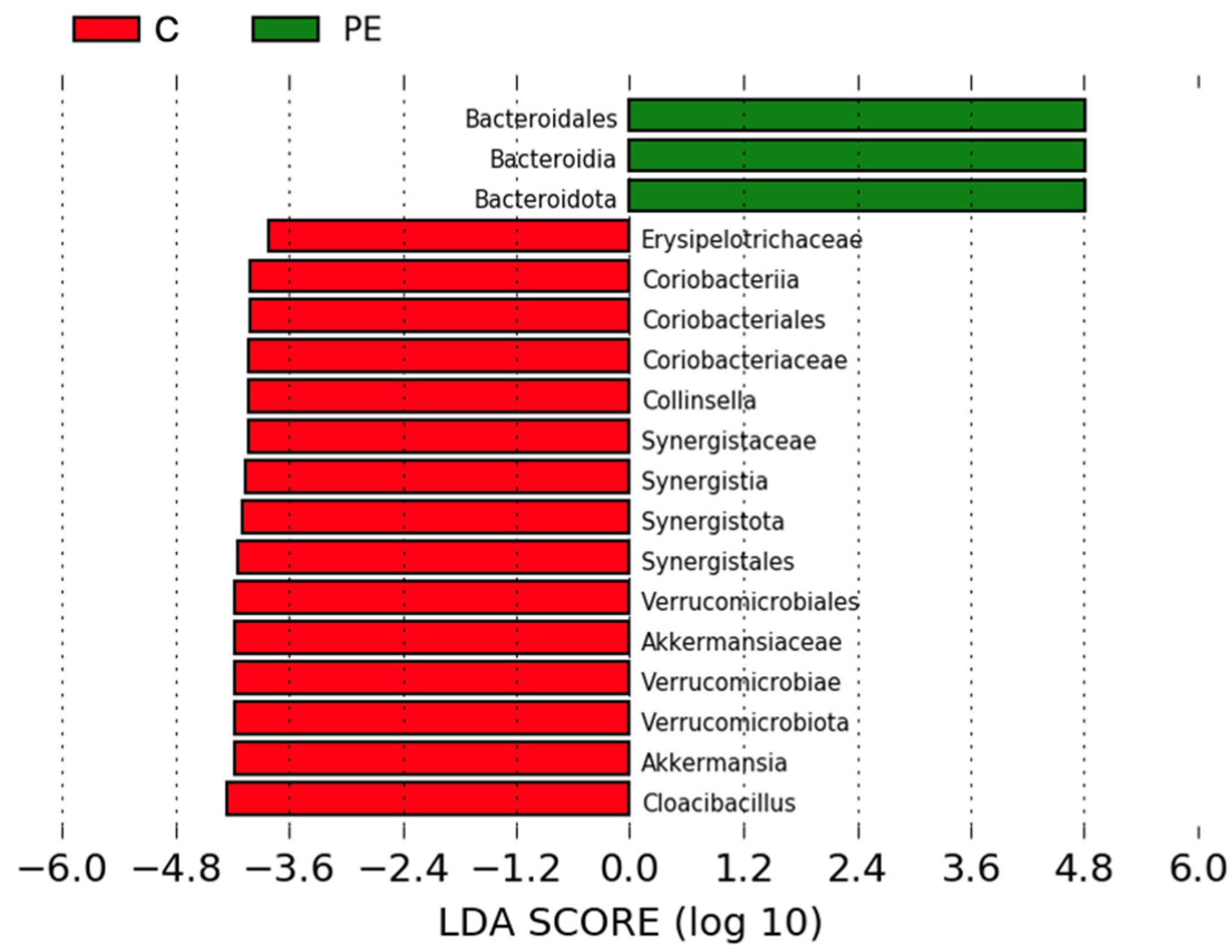
3.6. Gut Bacterial Microbiome Composition
3.7. Gut Mycobiome Composition
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Steegers, E.A.P.; Chappell, L.C.; Cluver, C.A.; Kingdom, J.; Tong, S. Pre-eclampsia. Lancet 2010, 376, 631–644. [Google Scholar]
- Brown, M.A.; Magee, L.A.; Kenny, L.C.; Karumanchi, S.A.; McCarthy, F.P.; Saito, S.; Hall, D.R.; Warren, C.E.; Adoyi, G.; Ishaku, S. The hypertensive disorders of pregnancy: ISSHP classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens. 2018, 13, 291–310. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.; Lemoine, E.; Granger, J.P.; Karumanchi, A. Preeclampsia: Pathophysiology, Challenges, and Perspectives. Circ Res. 2019, 124, 1094–1112. [Google Scholar] [PubMed]
- Bellamy, L.; Casas, J.-P.; Hingorani, A.D.; Williams, D.J. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: Systematic review and meta-analysis. BMJ 2007, 335, 974. [Google Scholar] [CrossRef]
- Redman, C.W.; Staff, A.C.; Roberts, J.M. Syncytiotrophoblast stress in preeclampsia: The convergence point for multiple pathways. Am. J. Obstet. Gynecol. 2021, 226, S907–S927. [Google Scholar] [CrossRef]
- Brosens, I.; Puttemans, P.; Benagiano, G. Placental bed research: I. The placental bed: From spiral arteries remodeling to the great obstetrical syndromes. Am. J. Obstet. Gynecol. 2019, 221, 437–456. [Google Scholar] [CrossRef] [PubMed]
- Brosens, J.J.; Pijnenborg, R.; Brosens, I.A. The myometrial junctional zone spiral arteries in normal and abnormal pregnancies: A review of the literature. Am. J. Obstet. Gynecol. 2002, 187, 1416–1423. [Google Scholar]
- Erlandsson, L.; Masoumi, Z.; Hansson, L.R.; Hansson, S.R. The roles of free iron, heme, haemoglobin, and the scavenger proteins haemopexin and alpha-1-microglobulin in preeclampsia and fetal growth restriction. J. Intern. Med. 2021, 290, 952–968. [Google Scholar]
- Redman, C.W.; Sargent, I.L.; Staff, A.C. IFPA Senior Award Lecture: Making sense of pre-eclampsia—Two placental causes of preeclampsia? Placenta 2014, 35 (Suppl. S35), S20–S25. [Google Scholar]
- Roberts, J.; Hubel, C. The Two Stage Model of Preeclampsia: Variations on the Theme. Placenta 2009, 30, 32–37. [Google Scholar] [CrossRef]
- Aggarwal, R.; Jain, A.K.; Mittal, P.; Kohli, M.; Jawanjal, P.; Rath, G. Association of pro- and anti-inflammatory cytokines in preeclampsia. J. Clin. Lab. Anal. 2019, 33, e22834. [Google Scholar] [PubMed]
- Rezniczek, G.A.; Förster, C.; Hilal, Z.; Westhoff, T.; Tempfer, C.B. Calprotectin in pregnancy and pregnancy-associated diseases: A systematic review and prospective cohort study. Arch. Gynecol. Obstet. 2019, 299, 1567–1577. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.R.; Anim-Nyame, N.; Johnson, P.; Sooranna, S.R.; Steer, P.J. Does endothelial cell activation occur with intrauterine growth restriction? Bjog 2002, 109, 836–839. [Google Scholar] [CrossRef] [PubMed]
- Ehrchen, J.M.; Sunderkotter, C.; Foell, D.; Vogl, T.; Roth, J. The endogenous Toll-like receptor 4 agonist S100A8/S100A9 (calprotectin) as innate amplifier of infection, autoimmunity, and cancer. J. Leukoc. Biol. 2009, 86, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Vogl, T.; Tenbrock, K.; Ludwig, S.; Leukert, N.; Ehrhardt, C.; Zoelen, M.A.D.V.; Nacken, W.; Foell, D.; van der Poll, T.; Sorg, C.; et al. Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat. Med. 2007, 13, 1042–1049. [Google Scholar] [CrossRef]
- Pergialiotis, V.; Prodromidou, A.; Pappa, E.; Vlachos, G.D.; Perrea, D.N.; Papantoniou, N. An evaluation of calprotectin as serum marker of preeclampsia: A systematic review of observational studies. Inflamm. Res. 2016, 65, 95–102. [Google Scholar] [CrossRef]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fva, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar]
- Cani, P.D.; Osto, M.; Geurts, L.; Everard, A. Involvement of gut microbiota in the development of low-grade inflammation and type 2 diabetes associated with obesity. Gut Microbes 2012, 3, 279–288. [Google Scholar] [CrossRef]
- Saad, M.J.A.; Santos, A.; Prada, P.O. Linking Gut Microbiota and Inflammation to Obesity and Insulin Resistance. Physiology 2016, 31, 283–293. [Google Scholar] [CrossRef]
- Jose, P.A.; Raj, D. Gut microbiota in hypertension. Curr. Opin. Nephrol. Hypertens. 2015, 24, 403–409. [Google Scholar] [CrossRef]
- Power, S.E.; O’Toole, P.W.; Stanton, C.; Ross, R.P.; Fitzgerald, G. Intestinal microbiota, diet and health. Br. J. Nutr. 2014, 111, 387–402. [Google Scholar] [PubMed]
- O’Hara, A.M.; Shanahan, F. The gut flora as a forgotten organ. EMBO Rep. 2006, 7, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Chen, Y.; Zhou, Q.; Wang, C.; Chen, L.; Di, W.; Zhang, Y. Short-chain fatty acids accompanying changes in the gut microbiome contribute to the development of hypertension in patients with preeclampsia. Clin. Sci. 2020, 134, 289–302. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; He, Z.; Chen, W.; Holzman, I.R.; Lin, J. Effects of Butyrate on Intestinal Barrier Function in a Caco-2 Cell Monolayer Model of Intestinal Barrier. Pediatr. Res. 2007, 61, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Schwiertz, A.; Taras, D.; Schäfer, K.; Beijer, S.; Bos, N.A.; Donus, C.; Hardt, P.D. Microbiota and SCFA in Lean and Overweight Healthy Subjects. Obesity 2010, 18, 190–195. [Google Scholar] [CrossRef]
- Isolauri, E.; Sütas, Y.; Kankaanpää, P.; Arvilommi, H.; Salminen, S. Probiotics: Effects on immunity. Am. J. Clin. Nutr. 2001, 73, 444s–450s. [Google Scholar] [CrossRef]
- Blackwood, B.P.; Yuan, C.Y.; Wood, D.R.; Nicolas, J.D.; Grothaus, J.S.; Hunter, C.J. Probiotic Lactobacillus Species Strengthen Intestinal Barrier Function and Tight Junction Integrity in Experimental Necrotizing Enterocolitis. J. Probiot. Health 2017, 5, 159. [Google Scholar] [CrossRef]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef]
- Underhill, D.M.; Iliev, I.D. The mycobiota: Interactions between commensal fungi and the host immune system. Nat. Rev. Immunol. 2014, 14, 405–416. [Google Scholar] [CrossRef]
- Li, X.V.; Leonardi, I.; Iliev, I.D. Gut Mycobiota in Immunity and Inflammatory Disease. Immunity 2019, 50, 1365–1379. [Google Scholar] [CrossRef]
- Mar Rodríguez, M.; Pérez, D.; Javier Chaves, F.; Esteve, E.; Marin-Garcia, P.; Xifra, G.; Vendrell, J.; Jové, M.; Pamplona, R.; Ricart, W.; et al. Obesity changes the human gut mycobiome. Sci. Rep. 2015, 5, 14600. [Google Scholar] [CrossRef]
- Ott, S.J.; Kühbacher, T.; Musfeldt, M.; Rosenstiel, P.; Hellmig, S.; Rehman, A.; Drews, O.; Weichert, W.; Timmis, K.N.; Schreiber, S. Fungi and inflammatory bowel diseases: Alterations of composition and diversity. Scand. J. Gastroenterol. 2008, 43, 831–841. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, C.; Tang, C.; He, Q.; Li, N.; Li, J. Dysbiosis of Gut Fungal Microbiota is Associated With Mucosal Inflammation in Crohn’s Disease. J. Clin. Gastroenterol. 2014, 48, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Koren, O.; Goodrich, J.K.; Cullender, T.C.; Spor, A.; Laitinen, K.; Bäckhed, H.K.; Gonzalez, A.; Werner, J.J.; Angenent, L.T.; Knight, R.; et al. Host Remodeling of the Gut Microbiome and Metabolic Changes during Pregnancy. Cell 2012, 150, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhya, I.; Hansen, R.; El-Omar, E.M.; Hold, G.L. IBD-what role do Proteobacteria play? Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Barbour, L.A.; McCurdy, C.E.; Hernandez, T.L.; Kirwan, J.P.; Catalano, P.M.; Friedman, J.E. Cellular Mechanisms for Insulin Resistance in Normal Pregnancy and Gestational Diabetes. Diabetes Care 2007, 30 (Suppl. S2), S112–S119. [Google Scholar] [CrossRef]
- Mokkala, K.; Röytiö, H.; Munukka, E.; Pietilä, S.; Ekblad, U.; Rönnemaa, T.; Eerola, E.; Laiho, A.; Laitinen, K. Gut Microbiota Richness and Composition and Dietary Intake of Overweight Pregnant Women Are Related to Serum Zonulin Concentration, a Marker for Intestinal Permeability. J. Nutr. 2016, 146, 1694–1700. [Google Scholar] [CrossRef] [PubMed]
- Ahmadian, E.; Saadat, Y.R.; Khatibi, S.M.H.; Nariman-Saleh-Fam, Z.; Bastami, M.; Vahed, F.Z.; Ardalan, M.; Vahed, S.Z. Pre-Eclampsia: Microbiota possibly playing a role. Pharmacol. Res. 2020, 155, 104692. [Google Scholar] [CrossRef]
- Liu, J.; Yang, H.; Yin, Z.; Jiang, X.; Zhong, H.; Qiu, D.; Zhu, F.; Li, R. Remodeling of the gut microbiota and structural shifts in Preeclampsia patients in South China. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 36, 713–719. [Google Scholar] [CrossRef]
- Lv, L.-J.; Li, S.-H.; Li, S.-C.; Zhong, Z.-C.; Duan, H.-L.; Tian, C.; Li, H.; He, W.; Chen, M.-C.; He, T.-W.; et al. Early-Onset Preeclampsia Is Associated With Gut Microbial Alterations in Antepartum and Postpartum Women. Front. Cell Infect. Microbiol. 2019, 9, 224. [Google Scholar] [CrossRef]
- Wang, J.; Gu, X.; Yang, J.; Wei, Y.; Zhao, Y. Gut Microbiota Dysbiosis and Increased Plasma LPS and TMAO Levels in Patients With Preeclampsia. Front. Cell Infect. Microbiol. 2019, 9, 409. [Google Scholar] [CrossRef]
- Chen, X.; Li, P.; Liu, M.; Zheng, H.; He, Y.; Chen, M.-X.; Tang, W.; Yue, X.; Huang, Y.; Zhuang, L.; et al. Gut dysbiosis induces the development of pre-eclampsia through bacterial translocation. Gut 2020, 69, 513–522. [Google Scholar] [CrossRef]
- Gomez-Arango, L.F.; Barrett, H.L.; McIntyre, H.D.; Callaway, L.K.; Morrison, M.; Nitert, M.D. Increased Systolic and Diastolic Blood Pressure Is Associated with Altered Gut Microbiota Composition and Butyrate Production in Early Pregnancy. Hypertension 2016, 68, 974–981. [Google Scholar] [CrossRef]
- Jin, J.; Gao, L.; Zou, X.; Zhang, Y.; Zheng, Z.; Zhang, X.; Li, J.; Tian, Z.; Wang, X.; Gu, J.; et al. Gut Dysbiosis Promotes Preeclampsia by Regulating Macrophages and Trophoblasts. Circ. Res. 2022, 131, 492–506. [Google Scholar] [CrossRef]
- Altemani, F.; Barrett, H.L.; Gomez-Arango, L.; Josh, P.; McIntyre, H.D.; Callaway, L.K.; Morrison, M.; Tyson, G.W.; Nitert, M.D. Pregnant women who develop preeclampsia have lower abundance of the butyrate-producer Coprococcus in their gut microbiota. Pregnancy Hypertens. 2021, 23, 211–219. [Google Scholar] [CrossRef]
- Brantsaeter, A.L.; Myhre, R.; Haugen, M.; Myking, S.; Sengpiel, V.; Magnus, P.; Jacobsson, B.; Meltzer, H.M. Intake of Probiotic Food and Risk of Preeclampsia in Primiparous Women: The Norwegian Mother and Child Cohort Study. Am. J. Epidemiol. 2011, 174, 807–815. [Google Scholar] [CrossRef]
- Nordqvist, M.; Jacobsson, B.; Brantsæter, A.-L.; Myhre, R.; Nilsson, S.; Sengpiel, V. Timing of probiotic milk consumption during pregnancy and effects on the incidence of preeclampsia and preterm delivery: A prospective observational cohort study in Norway. BMJ Open 2018, 8, e018021. [Google Scholar] [CrossRef]
- Miao, T.; Yu, Y.; Sun, J.; Ma, A.; Yu, J.; Cui, M.; Yang, L.; Wang, H. Decrease in abundance of bacteria of the genus Bifidobacterium in gut microbiota may be related to pre-eclampsia progression in women from East China. Food Nutr. Res. 2021, 65. [Google Scholar] [CrossRef]
- Takahashi, S.; Tomita, J.; Nishioka, K.; Hisada, T.; Nishijima, M. Development of a Prokaryotic Universal Primer for Simultaneous Analysis of Bacteria and Archaea Using Next-Generation Sequencing. PLoS ONE 2014, 9, e105592. [Google Scholar] [CrossRef]
- Illumina. 16S Metagenomic Sequencing Library Preparation. 2014. Available online: http://emea.support.illumina.com/downloads/16s_metagenomic_sequencing_library_preparation.html# (accessed on 31 December 2022).
- Illumina. Funfal Metagenomic Sequencing Demonstrate Protocol. 2019. Available online: https://emea.support.illumina.com/content/dam/illumina-support/documents/documentation/chemistry_documentation/metagenomic/fungal-metagenomic-demonstrated-protocol-1000000064940-01.pdf (accessed on 31 December 2022).
- Yang, H.; Guo, R.; Li, S.; Liang, F.; Tian, C.; Zhao, X.; Long, Y.; Liu, F.; Jiang, M.; Zhang, Y.; et al. Systematic analysis of gut microbiota in pregnant women and its correlations with individual heterogeneity. Biofilms Microbiomes 2020, 6, 1–12. [Google Scholar] [CrossRef]
- Black, K.D.; Horowitz, J.A. Inflammatory Markers and Preeclampsia: A Systematic Review. Nurs. Res. 2018, 67, 242–251. [Google Scholar]
- Cotechini, T.; Komisarenko, M.; Sperou, A.; Macdonald-Goodfellow, S.; Adams, M.A.; Graham, C.H. Inflammation in rat pregnancy inhibits spiral artery remodeling leading to fetal growth restriction and features of preeclampsia. J. Exp. Med. 2014, 211, 165–179. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, J.; Bao, J.; Li, X.; Ye, A.; Zhang, G.; Liu, H. Activation of the cholinergic anti-inflammatory pathway by nicotine ameliorates lipopolysaccharide-induced preeclampsia-like symptoms in pregnant rats. Placenta 2017, 49, 23–32. [Google Scholar] [CrossRef]
- Collado, M.C.; Derrien, M.; Isolauri, E.; de Vos, W.M.; Salminen, S. Intestinal Integrity and Akkermansia muciniphila, a Mucin-Degrading Member of the Intestinal Microbiota Present in Infants, Adults, and the Elderly. Appl. Environ. Microbiol. 2007, 73, 7767–7770. [Google Scholar] [CrossRef]
- Derrien, M.; Vaughan, E.E.; Plugge, C.M.; de Vos, W.M. Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. Int. J. Syst. Evol. Microbiol. 2004, 54, 1469–1476. [Google Scholar] [CrossRef]
- Shin, N.R.; Lee, J.C.; Lee, H.Y.; Kim, M.S.; Whon, T.W.; Lee, M.S.; Bae, J.W. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut 2014, 63, 727–735. [Google Scholar] [CrossRef]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef]
- Ganesh, B.P.; Klopfleisch, R.; Loh, G.; Blaut, M. Commensal Akkermansia muciniphila Exacerbates Gut Inflammation in Salmonella Typhimurium-Infected Gnotobiotic Mice. PLoS ONE 2013, 8, e74963. [Google Scholar] [CrossRef]
- Bawah, A.T.; Tornyi, H.; Seini, M.M.; Ngambire, L.T.; Yeboah, F.A. Zonulin as marker of pregnancy induced hypertension: A case control study. Clin. Hypertens. 2020, 26, 1–7. [Google Scholar] [CrossRef]
- Depommier, C.; Everard, A.; Druart, C.; Plovier, H.; Van Hul, M.; Vieira-Silva, S.; Falony, G.; Raes, J.; Maiter, D.; Delzenne, N.M.; et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: A proof-of-concept exploratory study. Nat. Med. 2019, 25, 1096–1103. [Google Scholar] [CrossRef]
- Ganesan, A.; Chaussonnerie, S.; Tarrade, A.; Dauga, C.; Bouchez, T.; Pelletier, E.; Le Paslier, D.; Sghir, A. Cloacibacillus evryensis gen. nov., sp. nov., a novel asaccharolytic, mesophilic, amino-acid-degrading bacterium within the phylum ‘Synergistetes’, isolated from an anaerobic sludge digester. Int. J. Syst. Evol. Microbiol. 2008, 58, 2003–2012. [Google Scholar] [CrossRef]
- Looft, T.; Levine, U.Y.; Stanton, T.B. Cloacibacillus porcorum sp. nov., a mucin-degrading bacterium from the swine intestinal tract and emended description of the genus Cloacibacillus. Int. J. Syst. Evol. Microbiol. 2013, 63, 1960–1966. [Google Scholar] [CrossRef]
- Vandeputte, D.; Falony, G.; Vieira-Silva, S.; Tito, R.Y.; Joossens, M.; Raes, J. Stool consistency is strongly associated with gut microbiota richness and composition, enterotypes and bacterial growth rates. Gut 2016, 65, 57–62. [Google Scholar] [CrossRef]
- Fang, Z.; Li, L.; Zhao, J.; Zhang, H.; Lee, Y.-K.; Lu, W.; Chen, W. Bifidobacteria adolescentis regulated immune responses and gut microbial composition to alleviate DNFB-induced atopic dermatitis in mice. Eur. J. Nutr. 2020, 59, 3069–3081. [Google Scholar] [CrossRef]
- Konieczna, P.; Groeger, D.; Ziegler, M.; Frei, R.; Frestl, R.; Fergus, S.; Quigley, E.M.M.; Kiely, B.; Akdis, C.A.; O’Mahony, L. Bifidobacterium infantis 35624 administration induces Foxp3 T regulatory cells in human peripheral blood: Potential role for myeloid and plasmacytoid dendritic cells. Gut 2012, 61, 354–366. [Google Scholar] [CrossRef]
- Santner-Nanan, B.; Peek, M.J.; Khanam, R.; Richarts, L.; Zhu, E.; Groth, B.F.D.S.; Nanan, R. Systemic Increase in the Ratio between Foxp3+ and IL-17-Producing CD4+ T Cells in Healthy Pregnancy but Not in Preeclampsia. J. Immunol. 2009, 183, 7023–7030. [Google Scholar] [CrossRef]
- Nilsson, R.H.; Ryberg, M.; Kristiansson, E.; Abarenkov, K.; Larsson, K.-H.; Koljalg, U. Taxonomic reliability of DNA sequences in public sequence databases: A fungal perspective. PLoS ONE 2006, 1, e59. [Google Scholar]

| Parameter | Controls (C) | Preeclampsia (PE) | p-Value |
|---|---|---|---|
| N = | 29 | 24 | |
| Age (years) | 31 (28–34.5) | 30.5 (28–33.75) | 0.632 |
| Pre-pregnancy BMI (kg/m2) | 23.7 (22.1–24.9) | 22.7 (20.8–25.0) 2 | 0.233 |
| Nulliparous (%) | 65.50% | 87.5% | 0.055 |
| Gestational age at inclusion (weeks + days) | 36 + 1 (32 + 1–40 + 0) | 35 + 0 (32 + 4–37 + 0) | 0.221 |
| Gestational age at birth (weeks + days) | 40 + 1 (39 + 0–41 + 1) | 36 + 0 (33 + 5–37 + 2) | <0.001 *** |
| Birth weight (g) | 3680 (3252–4012) | 2490 (1850–2796) | <0.001 *** |
| Sex of fetus (% boys/% girls) | 55.2%/44.8% | 58.3%/41.7% | 0.819 |
| Smoker (% no/% yes/% quit before pregnancy) | 93.1%/0%/6.9% | 87.0%/4.3%/8.7% 3 | 0.514 |
| Diet (% vegan/% lacto-ovo vegetarian/% omnivorous) | 3.4%/20.7%/75.9% | 0%/20.8%/79.2 % | 0.731 |
| Probiotic food items consumed > 1 time/week | 2 (1.5–2) | 2 (2–2) | 0.327 |
| Gestational week 28 OGTT 2 h plasma glucose (mmol/L) | 6.65 (6.13–7.15) | 6.10 (5.10–6.70) 4 | 0.030 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meijer, S.; Pasquinelli, E.; Renzi, S.; Lavasani, S.; Nouri, M.; Erlandsson, L.; Cavalieri, D.; Hansson, S.R. Gut Micro- and Mycobiota in Preeclampsia: Bacterial Composition Differences Suggest Role in Pathophysiology. Biomolecules 2023, 13, 346. https://doi.org/10.3390/biom13020346
Meijer S, Pasquinelli E, Renzi S, Lavasani S, Nouri M, Erlandsson L, Cavalieri D, Hansson SR. Gut Micro- and Mycobiota in Preeclampsia: Bacterial Composition Differences Suggest Role in Pathophysiology. Biomolecules. 2023; 13(2):346. https://doi.org/10.3390/biom13020346
Chicago/Turabian StyleMeijer, Sofie, Elena Pasquinelli, Sonia Renzi, Shahram Lavasani, Mehrnaz Nouri, Lena Erlandsson, Duccio Cavalieri, and Stefan R. Hansson. 2023. "Gut Micro- and Mycobiota in Preeclampsia: Bacterial Composition Differences Suggest Role in Pathophysiology" Biomolecules 13, no. 2: 346. https://doi.org/10.3390/biom13020346
APA StyleMeijer, S., Pasquinelli, E., Renzi, S., Lavasani, S., Nouri, M., Erlandsson, L., Cavalieri, D., & Hansson, S. R. (2023). Gut Micro- and Mycobiota in Preeclampsia: Bacterial Composition Differences Suggest Role in Pathophysiology. Biomolecules, 13(2), 346. https://doi.org/10.3390/biom13020346






