Dietary Capsaicin: A Spicy Way to Improve Cardio-Metabolic Health?
Abstract
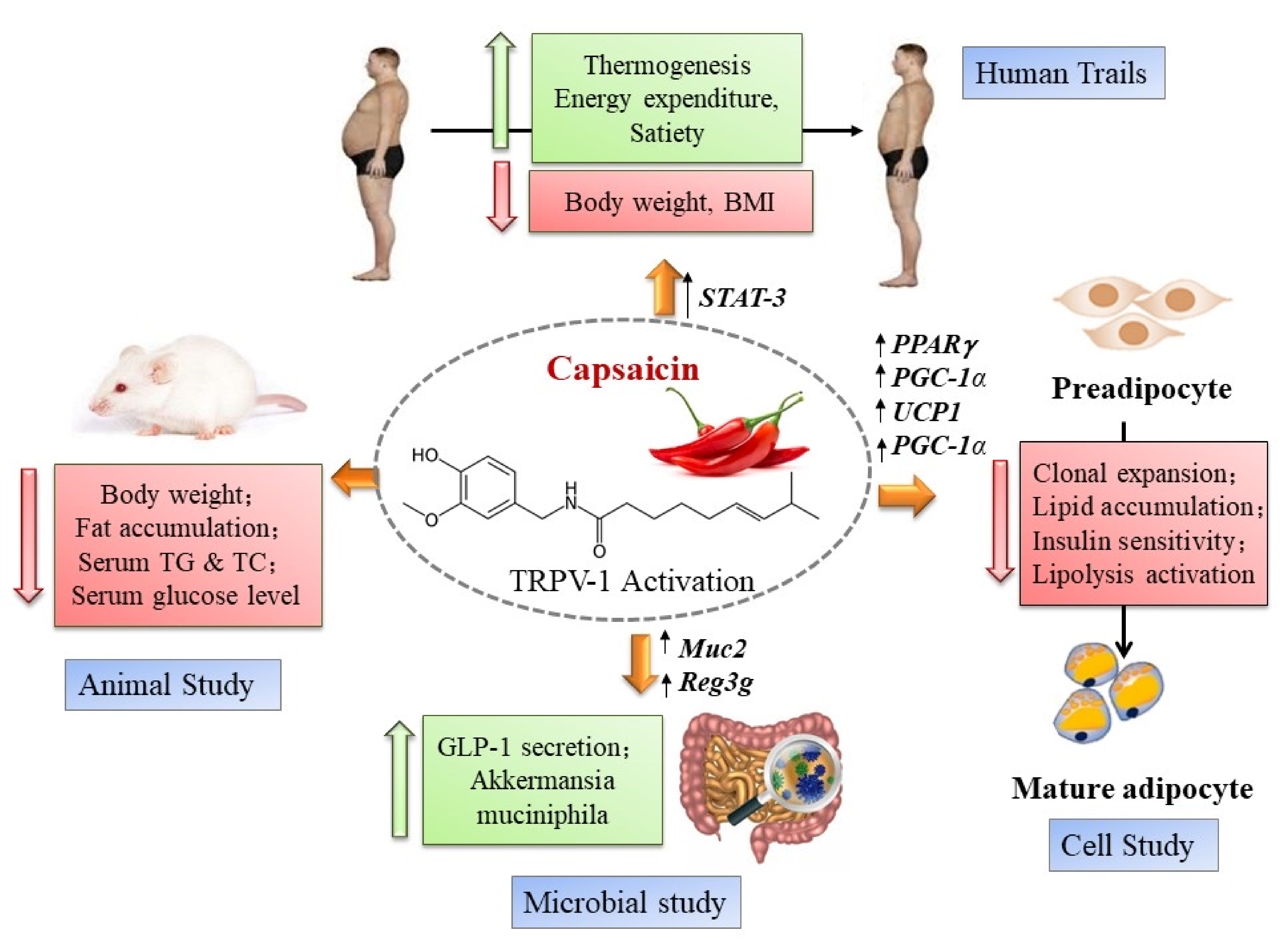
:1. Introduction
2. Dietary Capsaicin in Animal Experiments: Effects on Blood Glucose and Lipid Profile
3. Eating Chili Pepper Makes You Healthy?
4. Clinical Studies with Dietary Capsaicin: Effect on Blood Glucose
5. Clinical Studies with Dietary Capsaicin: Effect on Serum Lipids
6. Dietary Capsaicin and Metabolic Health: Possible Mechanisms of Action
7. Can Capsaicin Prevent or Ameliorate Metabolic Syndrome?
8. Capsaicin, Gut Microbiota, and Metabolic Syndrome
9. Capsaicin and Cardiovascular Disease
10. Conclusions
11. Future Directions
Funding
Data Availability Statement
Conflicts of Interest
References
- Haller, H. Epidemiology and associated risk factors of hyperlipopoteinemia (in German). Z. Gesamte Inn. Med. 1977, 32, 124–128. [Google Scholar] [PubMed]
- Marks, V. The metabolic syndrome. Nurs. Stand. 2003, 17, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Hjermann, I. The metabolic cardiovascular syndrome: Syndrome X, Reaven’s syndrome, insulin resistance syndrome, atherothrombogenic syndrome. J. Cardiovasc. Pharmacol. 1992, 20 (Suppl. 8), S5–S10. [Google Scholar] [CrossRef] [PubMed]
- Horlick, L. Dyslipidemia and metabolic factors in the genesis of heart attack and stroke. Health Rep. 1994, 6, 94–99. [Google Scholar] [PubMed]
- Duell, P.B.; Welty, F.K.; Miller, M.; Chait, A.; Hammond, G.; Ahmad, Z.; Cohen, D.E.; Horton, J.D.; Pressman, G.S.; Toth, P.P.; et al. Nonalcoholic fatty liver disease and cardiovascular risk: A scientific statement from the American Heart Association. Arterioscler. Thromb. Vasc. Biol. 2022, 42, e168–e185. [Google Scholar] [CrossRef]
- Tirandi, A.; Carbone, F.; Montecucco, F.; Liberale, L. The role of metabolic syndrome in sudden cardican death risk: Recent evidence of future directions. Eur. J. Clin. Investig. 2022, 52, e13693. [Google Scholar] [CrossRef]
- Björntorp, P. Abdominal obesity and the metabolic syndrome. Ann. Med. 1992, 24, 465–468. [Google Scholar] [CrossRef]
- Kopelman, P.G.; Albon, L. Obesity, non-insulin-dependent diabetes mellitus and the metabolic syndrome. Br. Med. Bull. 1997, 53, 322–340. [Google Scholar] [CrossRef] [Green Version]
- Obesity Worldwide—Statistics & Facts. Available online: https://www.statista.com/topics/9037/obesity-worldwide/#dossierKeyfigures (accessed on 26 November 2022).
- Crimarco, A.; Landry, M.J.; Gardner, C.D. Ultra-processed foods, weight gain, and co-morbidity risk. Curr. Obes. Rep. 2022, 11, 80–92. [Google Scholar] [CrossRef]
- Salzberg, L. Risk factors and lifestyle interventions. Prim. Care 2022, 49, 201–212. [Google Scholar] [CrossRef]
- Moore, J.X.; Chaudhary, N.; Akinyemiju, T. Metabolic syndrome prevalence by Race, Ethnicity and Sexz in the United States; National Health and Nutrition Examination Survey, 1988–2012. Prev. Chronic Dis. 2017, 14, 161287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lakka, H.-M.; Laaksonen, D.E.; Lakka, T.A.; Niskanen, L.K.; Kumpusalo, E.; Tuomilehto, J.; Salonen, J.K. The metabolic syndrome and total and cardiovascular disease mortality in middle.aged men. J. Am. Med. Assoc. 2002, 288, 2709–2716. [Google Scholar] [CrossRef] [Green Version]
- Guembe, M.J.; Fernandez-Lazaro, C.I.; Sayon-Orea, C.; Toledo, E.; Moreno-Iribas, C.; for the RIVANA Study Investigators. Risk for cardiovascular disease associated with metabolic syndrome and its components: A 13-year prospective study in the RIVANA cohort. Cardiovasc. Diabetol. 2020, 19, 195. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Li, J.T.; Sun, P.; Wang, L.-L.; Sun, L.-Z.; Pang, S.-G. Effects of lifestyle interventions on glucose regulation and diabetes risk in adults with impaired glucose tolerance or prediabetes; a meta-analysis. Arch. Endocrinol. Metab. 2022, 66, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Porte, D., Jr. Mechanism for hyperglycemia in the metabolic syndrome. Ann. N. Y. Acad. Sci. 1999, 892, 73–83. [Google Scholar] [CrossRef]
- Ruotolo, G.; Howard, B.V. Dyslipidemia of the metabolic syndrome. Curr. Cardiol. Rep. 2002, 4, 494–500. [Google Scholar] [CrossRef]
- Alkhatib, D.H.; Jaleel, A.; Tariq, M.N.M.; Feehan, J.; Apostolopoulos, V.; Ismail, L.C.; Stojanovska, L.; Al Dhaheri, A.S. The role of bioactive compounds from dietary spices in the management of metabolic syndrome: An overview. Nutrients 2021, 14, 175. [Google Scholar] [CrossRef]
- Pastor, R.; Bouzas, C.; Tur, J.A. Beneficial effects of dietary supplementation with olive oil, oleic acid, or hydroxytyrosol in metabolic syndrome: Systematic review and meta-analysis. Free Radic. Biol. Med. 2021, 172, 372–385. [Google Scholar] [CrossRef]
- Marrone, G.; Guerriero, C.; Palazzetti, D.; Lido, P.; Marolla, A.; Di Daniele, F.; Noce, A. Vegan diet health benefits in metabolic syndrome. Nutrients 2021, 13, 817. [Google Scholar] [CrossRef]
- Chopan, M.; Littenberg, B. The association of hot red chili pepper consumption and mortality: A large population-based cohort study. PLoS ONE 2017, 12, e0169876. [Google Scholar] [CrossRef]
- Lv, J.; Qi, L.; Yu, C.; Yang, L.; Guo, Y.; Chen, Y.; Bian, Z.; Sun, D.; Du, J.; Ge, P.; et al. Consumption of spicy foods and total and cause specific mortality: Population based cohort study. Br. Med. J. 2015, 351, h3942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonaccio, M.; Di Castelnuovo, A.; Costanzo, S.; Ruggiero, E.; De Curtis, A.; Persichillo, M.; Tabolacci, C.; Facchiano, F.; Cerletti, C.; Donati, M.B.; et al. Chili pepper consumption and mortality in Italian adults. J. Am. Coll. Cardiol. 2019, 74, 3139–3149. [Google Scholar] [CrossRef] [PubMed]
- Ofori-Asenso, R.; Mohsenpourm, M.A.; Nouri, M.; Faghih, S.; Liew, D.; Mazidi, M. Association of spicy chilli food consumption with cardiovascular and all-cause mortality: A meta-analysis of prospective cohort studies. Angiology 2021, 72, 625–632. [Google Scholar] [CrossRef] [PubMed]
- How to Live Longer: The Anti-Inflammatory Fruit That May Significantly Promote Longevity. Available online: https://www.express.co.uk/life-style/health/1630817/how-to-live-longer-chilli-pepper-extends-lifespan (accessed on 26 November 2022).
- Buck, S.H.; Burks, T.F. The neuropharmacology of capsaicin: Review of some recent observations. Pharmacol. Rev. 1986, 38, 179–226. [Google Scholar]
- Holzer, P. Capsaicin: Cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacol. Rev. 1991, 43, 143–201. [Google Scholar]
- Szallasi, A.; Blumberg, P.M. Vanilloid (capsaicin) receptors and mechanisms. Pharmacol. Rev. 1999, 51, 159–212. [Google Scholar]
- Szolcsányi, J. Forty years in capsaicin research for sensory pharmacology and physiology. Neuropeptides 2004, 38, 377–384. [Google Scholar] [CrossRef]
- Lee, T.S. Physiological gustatory sweating in a warm climate. J. Physiol. 1954, 124, 528–542. [Google Scholar] [CrossRef]
- Bosland, P.W. Hot stuff—Do people living in hot climates like their food spicy or not? Temperature 2016, 3, 41–42. [Google Scholar] [CrossRef] [Green Version]
- Monsereenusorn, Y.; Glinsukon, T. Inhibitory effect of capsaicin on intestional glucose absorption in vitro. Food Cosmet. Toxicol. 1978, 16, 469–473. [Google Scholar] [CrossRef]
- Udupihille, M. The effect of capsaicin on the small intestinal absorption of glucose and alanine in the rat. Indian J. Physiol. Pharmacol. 1993, 37, 59–62. [Google Scholar] [PubMed]
- Golynski, M.; Balicki, I.; Lutnicki, K.; Smiech, A.; Adamek, L.; Szczepanik, M.; Wilkolek, P.; Brodzki, A.; Adaszek, L. Systemic and local effects of intragastric administration of the habanero fruit (Capsicum chinense Jacquin c.v.) in rats. J. Physiol. Pharmacol. 2015, 66, 259–265. [Google Scholar] [PubMed]
- Baskaran, P.; Krishman, V.; Fettel, K.; Gao, P.; Zhu, Z.; Ren, J.; Thyagarajan, B. TRPV1 activation counters diet-induced obesity through sirtuin-1 activation and PRDM-16 deacylation in brown adipose tissue. Int. J. Obes. 2017, 41, 739–749. [Google Scholar] [CrossRef]
- Hui, S.; Huang, L.; Wang, X.; Zhu, X.; Zhou, M.; Chen, M.; Yi, L.; Mi, M. Capsaicin improves glucose homeostasis by enhancing glucagon-like peptide-1 secretion through the regulation of bile acid metabolism via the remodeling of the gut microbiota in male mice. FASEB J. 2020, 34, 8558–8573. [Google Scholar] [CrossRef] [PubMed]
- Hirotani, Y.; Fukamachi, J.; Ueyama, R.; Urashima, Y.; Ikeda, K. Effects of capsaicin coadministered with eicosapentaenoic acid on obesity-related dysregulation of high-fat-fed mice. Biol. Pharm. Bull. 2017, 40, 1581–1585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bliss, E.S. Capsaicin in Attenuating Metabolic Syndrome. Bachelor’s Thesis, University of Southern Queenslads, Darling Heights, Australia, 2017. Available online: http://eprints.usq.edu.au/id/eprint/33742 (accessed on 26 November 2022).
- Mun, J.-M.; Ok, H.M.; Kwon, O. Corn gluten hydrolysate and capsaicin have complimentary actions on body weight reduction and lipid-related genes in diet-induced obese rats. Nutr. Res. 2014, 34, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Song, J.-X.; Ren, H.; Gao, Y.-F.; Lee, C.-Y.; Li, S.-F.; Zhang, F.; Li, L.; Chen, H. Dietary capsaicin improves glucose homeostasis and alters gut microbiota in obese diabetic ob/ob mice. Front. Physiol. 2017, 8, 602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okumara, T.; Tsukui, T.; Hosokawa, M.; Miyashita, K. Effects of caffeine and capsaicin on the blood glucose levels of obese/diabetic KK-A(y) mice. J. Oleo Sci. 2012, 61, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.-H.; Tsuyoshi, G.; Ngoc, H.L.; Kim, H.-M.; Tu, T.H.; Noh, H.-J.; Kim, C.-S.; Choe, S.-Y.; Kawada, T.; Yoo, H.; et al. Dietary capsaicin attenuates metabolic dysregulation in genetically obese diabetic mice. J. Med. Food 2011, 14, 310–315. [Google Scholar] [CrossRef] [PubMed]
- da Silva Tremarin, C.; Rabello Casali, K.; Meurer, L.; D’Agord Schaan, B. Capsaicin-induced metabolic and cardiovascular autonomic improvement in an animal model of metabolic syndrome. Br. J. Nutr. 2014, 111, 207–214. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Ma, X.; Zhang, L.; Sun, H.; Liu, X. Capsaicin reduces blood glucose by increasing insulin levels and glycogen content better than capsiate in streptozotocin-induced diabetic rats. J. Agric. Food Chem. 2017, 65, 2323–2330. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Tang, L.; Xu, F.; Hui, Y.; Lu, H.; Liu, X. TRPV1 receptor-mediated hypoglycemic mechanism of capsaicin in streptozotocin-induced diabetic rats. Front. Nutr. 2021, 8, 750355. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.Y.; Kim, Y.S.; Ryu, S.Y.; Cha, M.-R.; Yon, G.-H.; Yang, H.J.; Kim, M.J.; Kang, S.; Park, S. Capsiate improves glucose metabolism by improving insulin sensitivity better than capsaicin in diabetic rats. J. Nutr. Biochem. 2013, 24, 1078–1085. [Google Scholar] [CrossRef] [PubMed]
- Tolan, I.; Ragoobirsingh, D.; Morrison, E.Y. The effect of capsaicin on blood glucose, plasma insulin levels and insulin binding in dog models. Phytother. Res. 2001, 15, 391–394. [Google Scholar] [CrossRef]
- Seyithanoglu, M.; Iyidogan, Y.Ö.; Dogru-Abbasoglu, S.; Tanrikulu-Kücük, S.; Kocak, H.; Behan-Özdas, S.; Kocak-Toker, N. The effect of dietary curcumin and capsaicin on hepatic fetuin-A expression and fat accumulation in rats fed high-fat diet. Arch. Physiol. Biochem. 2016, 122, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ding, Y.; Song, J.; Kan, J. Hypolipidemic effect and mechanism of paprika seed oil on Sprague-Dawley rats. J. Sci. Food Agric. 2017, 97, 4242–4249. [Google Scholar] [CrossRef] [PubMed]
- Negulesco, J.A.; Lohse, C.-L.; Hrabovsky, E.E.; Boggs, M.T.; Davis, D.H. Dihydrocapsaicin protects against serum hyperlipidemia in guinea pigs fed a cholesterol-enriched diet. Artery 1989, 16, 174–188. [Google Scholar]
- Yang, S.; Liu, L.; Meng, L.; Hu, X. Capsaicin is beneficial to hyperlipidemia, oxidative stress, endothelial dysfunction, and atherosclerosis in Guinea pigs fed on high-fat diet. Chem. Biol. Interact. 2019, 297, 1–7. [Google Scholar] [CrossRef]
- Negulesco, J.A.; Noel, S.A.; Newman, H.A.; Naber, E.C.; Bhat, H.B.; Witiak, D.T. Effects of pure capsaicinoid (capsaicin and dihydrocapsaicin) on plasma lipid and lipoprotein concentrations of turkey poults. Atherosclerosis 1987, 64, 85–90. [Google Scholar] [CrossRef]
- Szolcsányi, J.; Sann, H.; Pierau, F.-K. Nociception in pigeons is not impaired by capsaicin. Pain 1986, 27, 247–260. [Google Scholar] [CrossRef]
- Jordt, S.E.; Julius, D. Molecular basis for the species-specific sensitivity to “hot” chili peppers. Cell 2002, 108, 421–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, Y.; Cohen, B.E.; Chuang, H.-H. A single TRPV1 amino acid controls species sensitivity to capsaicin. Sci. Rep. 2020, 10, 8038. [Google Scholar] [CrossRef] [PubMed]
- Ao, Z.; Huang, Z.; Liu, H. Spicy food and chili peppers and multiple health outcomes: Umbrella review. Mol. Nutr. Food Res. 2022, e2200167. [Google Scholar] [CrossRef]
- Tewksbury, J.J.; Nabhan, G.P. Seed dispersal. Directed deterrence by capsaicin in chilies. Nature 2001, 412, 403–404. [Google Scholar] [CrossRef] [PubMed]
- Andelt, W.F.; Burnham, K.P. Effectiveness of capsaicin and bitrex repellents for deterring browsing by captive mule deer. J. Wildl. Manag. 2018, 58, 330–334. [Google Scholar] [CrossRef]
- Romanovsky, A.A. Protecting western redcedar from deer-browsing—With a passing reference to TRP channels. Temperature 2015, 2, 142–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fitzgerald, C.S.; Curtis, P.D.; Richmond, M.E.; Dunn, J.A. Effectiveness of Capsaicin as a Repellent to Birdseed Consumption by Gray Squirrels. USDA National Wildlife Research Center Symposia. 1995. Available online: https://digitalcommons.unl.edu/nwrcrepellants/16 (accessed on 26 November 2022).
- Szallasi, A. Some like it hot (even more so in the tropics): Puzzle with no solution. Temperature 2016, 3, 54–55. [Google Scholar] [CrossRef] [Green Version]
- Byrnes, N.K.; Hayes, J.E. Personality factors predict spicy food liking and intake. Food Qual. Prefer. 2013, 28, 213–221. [Google Scholar] [CrossRef] [Green Version]
- Byrnes, N.K.; Hayes, J.E. Behavioral measures of risk tasking, sensation seeking and sensitivity to reward may reflect different motivation for spicy food liking and consumption. Appetite 2016, 103, 411–422. [Google Scholar] [CrossRef] [Green Version]
- Goldman, J.G. Culinary Masochism: The Pain of Peppers. 2010. Available online: https://blogs.scientificamerican.com/thoughtful-animal/culinary-masochism-the-pain-of-peppers/ (accessed on 26 November 2022).
- Qin, Y.; Ran, L.; Wang, J.; Yu, L.; Lang, H.D.; Wang, X.-L.; Mi, M.-T.; Zhu, J.-D. Capsaicin supplementation improved risk factors of coronary heart disease in individuals with low HDL-C levels. Nutrients 2017, 9, 1037. [Google Scholar] [CrossRef] [Green Version]
- Foshati, S.; Moradi, S.; Tavassoly, M.; Rouhani, M.H. Short- and long-term effects of capsaicin supplementation on glycemic control: A systematic review and meta-analysis of controlled trials. Food Funct. 2021, 12, 5236–5246. [Google Scholar] [CrossRef]
- Kroff, J.; Hume, D.J.; Pienaar, P.; Tucker, R.; Lambert, E.V.; Rae, D.E. The metabolic effects of commercially available chicken peri-peri (African bird’s eye chilli) meal in overweight individuals. Br. J. Nutr. 2015, 117, 635–644. [Google Scholar] [CrossRef] [Green Version]
- Chaiyasit, K.; Khovidhunkit, W.; Wittayalertpanya, S. Pharmacokinetic and the effect of capsaicin in Capsicum frutescens on decreasing plasma glucose level. J. Med. Assoc. Thai 2009, 92, 108–113. [Google Scholar]
- Dömötör, A.; Szolcsányi, J.; Mózsik, G. Capsaicin and glucose absorption and utilization in healthy human subjects. Eur. J. Pharmacol. 2006, 534, 280–283. [Google Scholar] [CrossRef]
- Van Schaik, L.; Kettele, C.; Green, R.; Wundersitz, D.; Gordon, B.; Irving, H.R.; Rathler, J.A. Both caffeine and Capsicum annuum fruit powder lower blood glucose levels and increase brown adipose tissue temperature in healthy adult males. Front. Physiol. 2022, 13, 870154. [Google Scholar] [CrossRef]
- Yuan, L.J.; Qin, Y.; Wang, L.; Zheng, Y.; Chang, H.; Wang, J.; Wang, B.; Wan, J.; Chen, S.H.; Zhang, Q.Y.; et al. Capsaicin-containing chili improved postprandial hyperglycemia, hyperinsulinemia, and fasting lipid disorders in women with gestational diabetes mellitus and lowered the incidence of large-for-gestational-age newborns. Clin. Nutr. 2016, 35, 388–393. [Google Scholar] [CrossRef]
- Ahuja, K.D.K.; Ball, M.J. Effects of daily ingestion of chilli on serum lipoprotein oxidation in adult men and women. Br. J. Nutr. 2006, 96, 239–242. [Google Scholar] [CrossRef] [Green Version]
- Kick Start Your Calorie Burn. Available online: https://www.capsimax.com (accessed on 26 November 2022).
- Urbina, S.L.; Roberts, M.D.; Kephart, W.C.; Villa, K.B.; Santos, E.N.; Olivencia, A.M.; Bennett, H.M.; Lara, M.D.; Foster, C.A.; Purpura, M.; et al. Effects of twelve weeks of capsaicinoi supplementation on body composition, appetie and self-reported caloric intake in overweight individuals. Appetite 2017, 113, 264–273. [Google Scholar] [CrossRef]
- Kelava, L.; Nemeth, D.; Hegyi, P.; Keringer, P.; Kovacs, D.K.; Balasko, M.; Solymar, M.; Pakai, E.; Rumbus, Z.; Garami, A. Dietary supplementation of transient receptor potential vanilloid-1 channel agonists reducesn serum total cholesterol level: A meta-analysis of controlled human trials. Crit. Rev. Food Sci. Nutr. 2022, 62, 7025–7035. [Google Scholar] [CrossRef]
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature 1997, 389, 816–824. [Google Scholar] [CrossRef]
- Zhang, L.L.; Liu, D.Y.; Ma, L.Q.; Luo, Z.D.; Cao, T.B.; Zhong, J.; Yan, Z.C.; Wang, L.J.; Zhao, Z.G.; Zhu, S.J.; et al. Activation of ntransient receptor potential vanilloid type-1 channel prevents adipogenesis and obesity. Circ. Res. 2007, 100, 1063–1070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xin, H.; Tanaka, H.; Yamaguchi, M.; Takemori, S.; Nakamura, A.; Kohama, K. Vanilloid receptor is expressed in the sarcoplasmic reticulum of rat skeletal muscle. Biochem. Biophys. Res. Commun. 2005, 332, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Akiba, Y.; Kato, S.; Katsube, K.; Nakamura, M.; Takeuchi, K.; Ishii, H.; Hibi, T. Transient receptor potential vanilloid subfamily 1 expressed in pancreatic islet beta cells modulates insulin secretion in rats. Biochem. Biophys. Res. Commun. 2004, 321, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Rychkov, G.Y.; Barritt, G.J. Expression and function of TRP channels in liver cells. Adv. Exp. Med. Biol. 2011, 704, 667–686. [Google Scholar]
- Razavi, R.; Chan, Y.; Afifiyan, F.N.; Liu, X.J.; Wan, X.; Yantha, J.; Tsui, H.; Tang, L.; Tsai, S.; Santamaria, P. TRPV1+ sensory neurons control β cell stress and islet inflammation in autoimmune diabetes. Cell 2006, 127, 1123–1135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gram, D.X.; Ahren, B.; Nagy, I.; Olsen, U.B.; Brand, C.L.; Sundler, F.; Tabanera, R.; Svendsen, O.; Carr, R.D.; Santha, P.; et al. Capsaicin-sensitive sensory fibers in the islets of Langerhans contribute to defective insulin secretion in Zucker diabetic rat, an animal model for some aspects of human type 2 diabetes. Eur. J. Neurosci. 2007, 25, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Wanner, S.P.; Garami, A.; Romanovsky, A.A. Hyperactive when young and overweight when aged: Connecting the dots in the story about locomotor activity, body mass, and aging in Trpv1 knockout mice. Aging 2011, 3, 450–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, B.; Ma, S.; Wang, D.H. TRPV1 mediates glucose-induced insulin secretion through releasing neuropeptides. In Vivo 2019, 33, 1431–1437. [Google Scholar] [CrossRef] [Green Version]
- Lee, E.; Jung, D.Y.; Kim, J.H.; Patel, P.R.; Hu, X.; Lee, Y.; Azuma, Y.; Wang, H.-F.; Tsitsilianos, N.; Shafiq, U.; et al. Transient receptor potential vanilloid type-1 channel regulates diet-induced obesity, insulin resistance, and leptin resistance. FASEB J. 2015, 29, 3189–3192. [Google Scholar] [CrossRef] [Green Version]
- Guillot, E.; Coste, A.; Angel, I. Involvement of capsaicin-sensitive nerves in the regulation of glucose tolerance in diabetic rats. Life Sci. 1996, 59, 969–977. [Google Scholar] [CrossRef]
- Gram, D.X.; Hansen, A.J.; Wilken, M.; Elm, T.; Svendsen, O.; Carr, R.D.; Ahrén, B.; Brand, C.L. Plasma calcitonin gene-related peptide is increased prior to obesity, and sensory nerve desensitization by capsaicin improves oral glucose tolerance in obese Zucker rats. Eur. J. Endocrinol. 2005, 153, 963–969. [Google Scholar] [CrossRef] [Green Version]
- Gram, D.X.; Hansen, A.J.; Deacon, C.F.; Brand, C.L.; Ribel, U.; Wilken, M.; Carr, R.D.; Svendsen, O.; Ahren, B. Sensory nerve desensitization by resiniferatoxin improves glucose tolerance and increases secretion in Zucker diabetic fatty rats and is associated with reduced plasma activity of dipeptidyl peptidase IV. Eur. J. Pharmacol. 2005, 509, 211–217. [Google Scholar] [CrossRef] [PubMed]
- van de Wall, E.; Gram, D.X.; Strubbe, J.H.; Scheurink, A.J.W.; Koolhaas, J.M. Ablation of capsaicin-sensitive afferent nerves affects insulin response during an intravenous glucose tolerance test. Life Sci. 2005, 77, 1283–1292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahrén, B. Sensory nerves contribute to insulin secretion by glucagon-like peptide-1 in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 286, R269–R272. [Google Scholar] [CrossRef]
- Fernadez-Real, J.M.; Ricart, E. Insulin resistance and chronic cardiovascular inflammatory syndrome. Endocr. Rev. 2003, 24, 278–301. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, M.I.; Duncan, B.B. Diabesity; an inflammatory condition. Clin. Chem. Lab. Med. 2003, 41, 1120–1130. [Google Scholar] [CrossRef]
- Ma, X.; Nan, F.; Liang, H.; Shu, P.; Fan, X.; Song, X.; Hou, Y.; Zhang, D. Excessive intake of sugar: An accomplice of inflammation. Front. Immunol. 2022, 13, 988481. [Google Scholar] [CrossRef]
- Reaven, G.M. Banting lecture 1988. Role of insulin resistance inn human disease. Diabetes 1988, 37, 1595–1607. [Google Scholar] [CrossRef]
- Lu, M.; Chen, C.; Lan, Y.; Xiao, J.; Li, R.; Huang, J.; Huang, Q.; Cao, Y.; Ho, C.-T. Capsaicin—The major bioactive ingredient in chilli peppers: Bio-efficacy and delivery systems. Food Funct. 2020, 11, 2848–2860. [Google Scholar] [CrossRef]
- Gram, D.X.; Holst, J.J.; Szallasi, A. TRPV1: A potential therapeutic target in type-2 diabetes and comorbidities? Trends Mol. Med. 2017, 23, 1002–1013. [Google Scholar] [CrossRef]
- Szallasi, A. Capsaicin for weight control: “Exercise in a pill” (or just another fad)? Pharmaceuticals 2022, 15, 851. [Google Scholar] [CrossRef] [PubMed]
- Suri, A.; Szallasi, A. The emerging role of TRPV1 in diabetes and obesity. Trends Pharmacol. Sci. 2008, 29, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S.; Shargill, N.S.; Spiegelman, P.S. Adipose expression of tumor necrosis factor-α: Direct role in obesity-linked insulin resistance. Science 1993, 259, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S. Inflammation, metaflammation, and immunometabolic disorders. Nature 2017, 542, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Sánchez, A.; Madrigal-Santillán, E.; Bautista, M.; Esquivel-Soto, J.; Morales-González, A.; Esquivel-Chirino, C.; Durante-Montiel, I.; Sánchez-Rivera, G.; Valadez-Vega, C.; Morales-González, J.A. Inflammation, oxidative stress, and obesity. Int. J. Mol. Sci. 2021, 12, 3117. [Google Scholar] [CrossRef] [Green Version]
- Juhan-Vague, I.; Alessi, M.C. PAI-1, obesity, insulin resistance and risk of cardiovascular events. Thromb. Haemost. 1997, 78, 656–660. [Google Scholar] [CrossRef]
- Sell, H.; Dietze-Schroeder, D.; Kaiser, U.; Eckel, J. Monocyte chemotactic protein-1 is a potential player in the negative cross-talk between adipose tissue and skeletal muscle. Endocinology 2006, 147, 2458–2467. [Google Scholar] [CrossRef] [Green Version]
- Zelissen, P.M.; Koppeschaar, H.P.; Lips, C.J.; Hackeng, W.H. Calcitonin gene-related peptide in human obesity. Peptides 1991, 12, 861–863. [Google Scholar] [CrossRef]
- Suresh, D.; Srinivasan, K. Tissue distribution and elimination of capsaicin, piperine and curcumin following oral intake in rats. Indian J. Med. Res. 2010, 131, 682–691. [Google Scholar]
- Reilly, C.A.; Ehlhardt, W.J.; Jackson, D.A.; Kulanthaivel, P.; Mulib, A.E.; Espina, R.J.; Moody, D.E.; Crouch, D.J.; Yost, G.S. Metabolism of capsaicin by cytochrome P450 produces novel dehydrogenated metabolites and decreased cytotoxicity to lung and liver cells. Chem. Res. Toxicol. 2003, 16, 336–349. [Google Scholar] [CrossRef]
- Sun, H.; Wang, H.; Liu, H.; Zhang, X.; Wu, B. Glucuronidation of capsaicin by liver microsomes and expressed UGT enzymes: Reaction kinetics, contribution of individual enzymes, and marked species differences. Exp. Opin. Drug Metab. Toxicol. 2014, 10, 1325–1336. [Google Scholar] [CrossRef] [PubMed]
- Donnerer, J.; Amann, R.; Schuligoi, R.; Lembeck, F. Absorption and metabolism of capsaicinoids following intragastric administration in rats. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1990, 342, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Molnár, J.; Makara, G.; György, L.; Unyi, G. The bronchoconstrictor action of capsaicin in the guinea pig. Acta Physiol. Hung. 1969, 36, 413–420. [Google Scholar] [PubMed]
- Chahl, L.; Lynch, A.M. The acute effects of capsaicin on the cardiovascular system. Acta Physiol. Hung. 1987, 69, 413–419. [Google Scholar]
- Clifford, P.S.; Litzow, J.T.; Coon, R.L. Pulmonary pressor reflex elicited by capsaicin in conscious intact and lung-denervated dogs. Am. J. Physiol. 1987, 252, R394–R397. [Google Scholar]
- Kaczynska, K.; Szereda-Przestaszewska, M. Respiratory effects of capsaicin occur beyond the lung vagi of anesthesized rats. Acta Neurobiol. Exp. 2000, 60, 159–165. [Google Scholar]
- Borges, J.; Sautier, C.; Krebs-Drouot, L.; Henry, P.; Paysant, F.; Scolan, V. Death and non-lethal weapons: A case of homicide by penetrating injury without projectile. Forensic Sci. Int. 2022, 337, 111374. [Google Scholar] [CrossRef]
- Busker, R.W.; van Helden, H.P. Toxicological evaluation of pepper spray as a possible weapon for the Dutch police force: Risk assessment and efficacy. Am. J. Forensic Med. Pathol. 1998, 19, 309–316. [Google Scholar] [CrossRef]
- Haar, R.J.; Iacopino, V.; Ranadive, N.; Weiser, S.D.; Dandu, M. Health impacts of chemical irritants used for crowd control: A systematic review of the injuries and deaths caused by tear gas and pepper spray. BMC Public Health 2017, 17, 831. [Google Scholar] [CrossRef] [Green Version]
- Dabke, K.; Hendrick, G.; Devkota, S. The gut microbiome and metabolic syndrome. J. Clin. Investig. 2019, 129, 4050–4057. [Google Scholar] [CrossRef]
- Wu, D.; Wang, H.; Xie, L.; Hu, F. Cross-talk between gut microbiota and adipose tissue in obesity and related metabolic disorders. Front. Endocrinol. 2022, 13, 908868. [Google Scholar] [CrossRef] [PubMed]
- Michels, N.; Zouiouich, S.; Vanderbauwhede, B.; Vanacker, J.; Indave Ruiz, B.I. Huybrechts, I. Human microbiome and metabolic health: An overview of systematic reviews. Obes. Rev. 2022, 23, e13409. [Google Scholar] [CrossRef]
- Carrizales-Sánchez, A.K.; Garcia-Cayuela, T.; Hernández-Brenes, C.; Senes-Guerrero, C. Gut microbiota associations with metabolic syndrome and relevance of its study in pediatric subjects. Gut Microbes 2021, 13, 1960135. [Google Scholar] [CrossRef] [PubMed]
- Dang, G.; Wu, W.; Zhang, H.; Everaert, N. A new paradigm for a simple chemical: Butyrate and immune regulation. Food Funct. 2021, 12, 12181–12193. [Google Scholar] [CrossRef]
- Arora, T.; Sharma, R.; Frost, G. Propionate, Anti-obesity and satiety enhancing factor? Appetite 2011, 56, 511–515. [Google Scholar] [CrossRef]
- Perry, R.J.; Peng, L.; Barry, N.A.; Cline, G.W.; Zhang, D.; Cardone, R.L.; Petersen, K.F.; Kibbey, R.G.; Goodman, A.L.; Shulman, G.I. Acetate mediates a microbiome-brain-beta-cell axis to promote metabolic syndrome. Nature 2016, 534, 213–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandes, J.; Su, W.; Rahat-Rozenbloom, S.; Wolever, T.M.S.; Comelli, E.M. Adiposity, gut microbiota and faecal short chain fatty acids and linked in adult humans. Nutr. Diabetes 2014, 4, e121. [Google Scholar] [CrossRef] [Green Version]
- Alhabeeb, H.; AlFaiz, A.; Kutbi, E.; AlShahrani, D.; Alsuhail, A.; AlRajhi, S.; Alotaibi, N.; Alotaibi, K.; AlAmri, S.; Alghamdi, S.; et al. Gut hormones in health and obesity: The upcoming role of short chain fatty acids. Nutrients 2021, 13, 481. [Google Scholar] [CrossRef]
- Davis, C.D. The gut microbiome and its role in obesity. Nutr. Today 2016, 51, 167–174. [Google Scholar] [CrossRef] [Green Version]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial ecology—Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef]
- Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Cheng, J.; Duncan, A.E.; Kau, A.L.; Griffin, N.W.; Lombard, V.; Henrissat, B.; Bain, J.R.; et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 2013, 341, 1241214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellekilde, M.; Selfjord, E.; Larsen, C.S.; Jakesevic, M.; Rune, I.; Tranberg, B.; Vogensen, F.K.; Nielsen, D.S.; Bahl, M.L.; Lichtm, T.R.; et al. Transfer of gut microbiota from lean and obese mice to antibiotic-treated mice. Sci. Rep. 2014, 4, 5922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Napolitano, M.; Covasa, M. Microbiota transplant in the treatment of obesity and diabetes: Current and future perspectives. Front. Microbiol. 2020, 11, 590370. [Google Scholar] [CrossRef] [PubMed]
- Fecal Microbiota Transplant (FMT) to Induce Weight Loss in Obese Subjects. Available online: https://clinicaltrials.gov/ct2/show/NCT037894.61 (accessed on 26 November 2022).
- Mocanu, V.; Zhang, Z.; Deehan, E.C.; Kao, D.H.; Hotte, N.; Karmali, S.; Birch, D.W.; Samarasinghe, K.K.; Walter, J.; Madsen, K.L. Fecal microbial transplantation and fiber supplementation in patient with severe obesity and metabolic syndrome: A randomized double-blind, placebo-controlled phase-2 trial. Nat. Med. 2021, 27, 1272–1279. [Google Scholar] [CrossRef]
- Xiang, Q.; Tang, X.; Cui, S.; Zhang, Q.; Liu, X.; Zhao, J.; Zhang, H.; Mao, B.; Chen, W. Capsaicin, the spicy ingredient in chili peppers: Effects on gastrointestinal tract and composition of gut microbiota at various dosages. Foods 2022, 11, 686. [Google Scholar] [CrossRef]
- Rosca, A.E.; Iesanu, M.I.; Zahiu, C.D.M.; Voiculescu, S.E.; Paslaru, A.C.; Zagrean, A.-M. Capsaicin and gut microbiota in health and disease. Molecules 2020, 25, 5681. [Google Scholar] [CrossRef]
- Kang, C.; Wang, B.; Kaliannan, K.; Wang, X.; Lang, H.; Hui, S.; Huang, L.; Zhang, Y.; Zhou, M.; Chen, M.; et al. Gut microbiota mediates the protective effects of dietary capsaicin against chronic low-grade inflammation and associated obesity by high-fat diet. mBio 2017, 8, e00470. [Google Scholar] [CrossRef] [Green Version]
- de Paula Menezes, R.; de Souza Bessa, M.A.; de Paula Siqueira, C.; Teixeira, S.C.; Ferro, E.A.; Martinbs, M.M.; Cunha, L.C.S.; Gomes Martins, C.H. Antimicrobial, antivirulence and antiparasitic potential of Capsicum chinense Jacq, extracts and their isolated compound capsaicin. Antibiotics 2022, 11, 1154. [Google Scholar] [CrossRef]
- Omolo, M.A.; Wong, Z.-Z.; Mergen, A.K.; Hastings, J.C.; Le, N.C.; Reiland, H.A.; Case, K.A.; Baumler, D.J. Antimicrobial properties of chili peppers. J. Infect. Dis. Ther. 2014, 2, 145. [Google Scholar] [CrossRef]
- Omolo, M.A.; Wong, Z.-Z.; Borth, W.G.; Hedblom, G.A.; Dev, K.; Baumler, D.J. Comparative analysis of capsaicin in twenty-nine varieties of unexplored Capsicum and its antimicrobial activity against bacterial and fungal pathogens. J. Med. Plants Res. 2018, 12, 544–556. [Google Scholar]
- Billing, J.; Sherman, P.W. Antimicrobial functions of spices: Why some like it hot. Q. Rev. Biol. 1998, 73, 3–49. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Kumar, V.; Mahajan, N.; Kaur, J.; Devi, K.; Dharavath, R.N.; Singh, R.P.; Kondepudi, K.K.; Bishnoi, M. Mucin secretory action of capsaicin prevents high fat diet-induced gut barrier dysfunction in C57BL/6 mice colon. Biomed. Pharmacother. 2022, 145, 112452. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Depommier, C.; Derrien, M.; Everard, A.; de Vos, W.M. Akkermansia muciniphila: Paradigm for next-generation beneficial microorganisms. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 625–637. [Google Scholar] [CrossRef] [PubMed]
- Natividad, J.M.; Lamas, B.; Pham, H.P.; Michel, M.L.; Rainteau, D.; Bridonneau, C.; da Costa, G.; van Hylckama Vlieg, J.; Sovran, B.; Chamignon, C.; et al. Bilophila wadsworhia aggrevates high fat diet induced metabolic dysfunctions in mice. Nat. Commun. 2018, 9, 2802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneeberger, M.; Everard, A.; Gómez-Valades, A.G.; Matamoros, S.; Ramirez, S.; Delzenne, N.M.; Gomis, R.; Claret, M.; Cani, P.D. Akkermansia muciniphila inversely correlates with the onset of inflammation, altered adipose tissue metabolism and metabolic disorder during obesity in mice. Sci. Rep. 2015, 5, 16643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, W.; Shen, M.; Zhao, X.; Zhu, H.; Yang, Y.; Lu, S.; Tan, Y.; Li, G.; Li, M.; Wang, J.; et al. Anti-obesity effect of capsaicin in mice fed high-fat diet is associated with an increase in population of the gut bacterium Akkermansia muciniphila. Front. Microbiol. 2017, 8, 272. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Tang, C.; Tang, Y.; Yin, H.; Liu, X. Capsaicin has an anti-obesity effect through alterations in gut microbiota populations and short fatty chain fatty acid concentrations. Food Nutr. Res. 2020, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uysal, K.T.; Wiesbrock, S.M.; Marino, M.E.; Hotamisligil, G.S. Protection from obesity-induced insulin resistance in mice lacking TNFα function. Nature 1997, 389, 610–614. [Google Scholar] [CrossRef]
- Hotamisligil, G.S.; Arner, P.; Caro, J.F.; Atkinson, R.L.; Spiegelman, B.M. Increased adipose tissue expression of tumor necrosis factor-α in human obesity and insulin resistance. J. Clin. Investig. 1995, 95, 2409–2415. [Google Scholar] [CrossRef]
- Osaka, T.; Moriyama, E.; Arai, S.; Date, Y.; Yagi, J.; Kikuchi, J.; Tsuneda, S. Meta-analysis of fecal microbiota and metabolites in experimental colitic mice during the inflammatory and healing phases. Nutrients 2017, 9, 1329. [Google Scholar] [CrossRef] [Green Version]
- Hersoug, L.-G.; Moller, P.; Loft, S. Role of microbiota-derived lipopolysaccharide in adipose tissue inflammation, adipocyte size and pyroptosis during obesity. Nutr. Res. Rev. 2018, 31, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Kim, C.S.; Han, I.S.; Kawada, T.; Yu, R. Capsaicin, a spicy component of hot peppers, modulates adipokine gene expression and protein release from obese-mouse adipose tissues and isolated adipocytes, and suppresses the inflammatory responses of adipose tissue macrophages. FEBS Lett. 2007, 581, 4389–4396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, W.H.W.; Wang, Z.; Levison, B.S.; Koeth, R.A.; Britt, E.B.; Fu, X.; Wu, Y.; Hazen, S.L. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N. Engl. J. Med. 2013, 368, 1575–1584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romano, K.A.; Vivas, E.D.; Amador-Noguez, D.; Rey, F. Intestinal microbiota composition modulates choline bioavailability from diet and accumulation of the proatherogenic metabolite trimethyl-N-oxide. mBio 2015, 6, e02481-14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Randrianarisoa, E.; Lehn-Stefan, A.; Wang, X.; Hoene, M.; Peter, A.; Heinzmann, S.S.; Zhao, X.; Königsrainer, I.; Königsrainer, A.; Balletshofer, B.; et al. Relationship of serum trimethylamine N-oxide /TMAO) levels with early atherosclerosis in humans. Sci. Rep. 2016, 6, 26745. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Cheang, W.S.; Wang, X.; Lei, L.; Liu, Y.; Ma, K.Y.; Zheng, F.; Huang, Y.; Chen, Z.-Y. Capsaicinoids but not their analogue capsinoids lowe plasma cholesterol and posesses beneficial vascular activity. J. Agric. Food Chem. 2014, 62, 8415–8420. [Google Scholar] [CrossRef]
- Liang, Y.T.; Tian, X.Y.; Chen, J.N.; Peng, C.; Ma, K.Y.; Zuo, Y.; Jiao, R.; Lu, Y.; Huang, Y.; Chen, Z.Y. Capsaicinoids lower plasma cholesterol and improve endothelial function in hamsters. Eur. J. Nutr. 2013, 52, 379–388. [Google Scholar] [CrossRef]
- Hu, Y.W.; Ma, X.; Huang, J.L.; Mao, X.R.; Yang, J.Y.; Zhao, J.Y.; Li, S.F.; Qiu, Y.R.; Yang, J.; Zheng, L.; et al. Dihydrocapsaicin attenuates plaque formation through a PPARγ/LXRα pathway in apoE (-/-) mice fed high-fat/high-cholesterol diet. PLoS ONE 2013, 8, e66876. [Google Scholar] [CrossRef] [Green Version]
- Golech, A.A.; McCarron, R.M.; Chen, Y.; Bembry, J.; Lenz, F.; Mechoulam, R.; Shohami, E.; Spatz, M. Human brain endothelium: Coexpression and function of vanilloid and endocannabinoid receptors. Brain Res. Mol. Brain Res. 2004, 132, 87–92. [Google Scholar] [CrossRef]
- Poblete, I.M.; Orliac, M.L.; Briones, R.; Adler-Graschinsky, E.; Huidobro-Toro, J.P. Anandamide elicits an acute release of nitric oxide through endothelial TRPV1 receptor activation in the rat arterial mesenteric bed. J. Physiol. 2005, 568, 539–551. [Google Scholar] [CrossRef]
- Ives, S.J.; Park, S.Y.; Kwon, O.S.; Gifford, J.R.; Andtbacka, R.H.; Hyngstrom, J.R.; Richardson, R.S. TRPV1 channels in human skeletal muscle feed arteries: Implications for vascular function. Exp. Physiol. 2017, 102, 1245–1258. [Google Scholar] [CrossRef] [PubMed]
- Phan, T.X.; Ton, H.T.; Gulyás, H.; Pórszász, R.; Tóth, A.; Russo, R.; Kay, M.W.; Sahibzada, N.; Ahern, G.P. TRPV1 in arteries enables a rapid myogenic tone. J. Physiol. 2022, 600, 1651–1666. [Google Scholar] [CrossRef] [PubMed]
- Torres-Narváez, J.C.; Mondragón, L.V.; Valera López, E.; Pèrez-Torres, I.; Diaz Juárez, J.A.; Suárez, J.; Hernández, G.P. Role of the transient receptor potential vanilloid typr 1 receptor and stretch-activated ion channels in nitric oxide release from endothelial cells of the aorta and heart in rats. Exp. Clin. Cardiol. 2012, 17, 89–94. [Google Scholar]
- Yang, D.; Luo, Z.; Ma, S.; Wong, W.T.; Ma, L.; Zhong, J.; He, H.; Zhao, Z.; Cao, T.; Yan, Y.; et al. Activation of TRPV1 by dietary capsaicin improves endothelium-dependent vasorelaxation and prevents hypertension. Cell Metab. 2010, 12, 130–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, L.; Zhong, J.; Zhao, Z.; Luo, Z.; Ma, S.; Sun, J.; He, H.; Zhu, T.; Liu, D.; Zhu, Z.; et al. Activation of TRPV1 reduces vascular lipid accumulation and attenuates atherosclerosis. Cardiovasc. Res. 2011, 92, 504–513. [Google Scholar] [CrossRef] [Green Version]
- Rosa, A.; Deiana, M.; Casu, V.; Paccagnini, S.; Appendino, G.; Ballero, M.; Dessi, M.A. Antioxidant activity of capsinoids. J. Agric. Food Chem. 2002, 50, 7396–7401. [Google Scholar] [CrossRef]
- Palma, J.M.; Terán, F.; Contreras-Ruiz, A.; Rodriguez-Ruiz, M.; Corpas, F.J. Antioxidant profile of pepper (Capsicum annuum L.) fruits containing diverse levels of capsaicinoids. Antioxidants 2020, 9, 878. [Google Scholar] [CrossRef]
- Chaudray, A.; Gour, J.K.; Rizvi, S.I. Capsaicin has potent anti-oxidative effects in vivo through a mechanism which is non-receptor mediated. Arch. Physiol. Biochem. 2022, 128, 141–147. [Google Scholar] [CrossRef]
- Luqman, S.; Rizvi, S.I. Protection of lipid peroxidation and carbonyl formation in proteins by capsaicin in human erythrocytes subjected to oxidative stress. Phytother. Res. 2006, 20, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Lisco, A.M.; Rudebush, T.L.; Yu, L.; Gao, L.; Kitzerow, O.; Zucker, I.H.; Wang, H.-J. Identification of cardiac expression pattern of transient receptor potential vanilloid type 1 (TRPV1) receptor using a transgenic reporter mouse model. Neurosci. Lett. 2020, 737, 135320. [Google Scholar] [CrossRef]
- Jia, X.; Yu, T.; Xiao, C.; Sheng, D.; Yang, M.; Cheng, Q.; Wu, J.; Lian, T.; Zhao, Y.; Zhang, S. Expression of transient receptor potential vanilloid genes and proteins in the diabetic heart. Mol. Biol. Rep. 2021, 48, 1217–1223. [Google Scholar] [CrossRef] [PubMed]
- Ferdinandy, P.; Jancsó, G. Capsaicin-sensitive sensory nerves in myocardial ischemia-reperfusion injury and ischemic stress adaptation: Role of nitric oxide and calcitonin gene-related peptide. NeuroImmune Biol. 2009, 8, 267–288. [Google Scholar]
- Vemula, P.; Gautam, B.; Abela, G.S.; Wang, D.H. Myocardial ischemia/reperfusion injury: Potential of TRPV1 agonists as cardioprotective agents. Cardiovasc. Hematol. Disord. Drug Targets 2014, 14, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Zhu, F.; Zhang, Y.; Duan, L.; Lei, H.; Huang, W. Transient receptor potential vanilloid subtype 1 inhibits inflammation and apoptosis via the release of calcitonin gene-related peptide in the heart after myocardial infarction. Cardiology 2016, 134, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, D.H. TRPV1 gene knockout impairs postischemic recovery in isolated perfused heart in mice. Circulation 2005, 112, 3617–3623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, W.; Rubinstein, J.; Prieto, A.O.; Thang, L.V.; Wang, D.H. Transient receptor potential vanilloid gene deletion exacerbates inflammation and atypical cardiac remodeling after myocardial infarction. Hypertension 2009, 53, 243–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, R.L.; Guo, Z.; Wang, L.L.; Wu, J. Degeneration of capsaicin-sensitive sensory nerves enhances acute myocardial infarction in rats. Int. J. Cardiol. 2012, 160, 41–47. [Google Scholar] [CrossRef]
- Fragasso, G.; Palloshi, A.; Piatti, P.M.; Monti, L.; Rossetti, E.; Setola, E.; Montano, C.; Bassanelli, G.; Calori, G.; Margonato, A. Nitric-oxide mediated effects pf transdermal capsaicin patches on the ischemic threshold in patients with stable coronary disease. J. Cardiovasc. Pharmacol. 2004, 44, 340–347. [Google Scholar] [CrossRef]
- Akcay, A.B.; Ozcan, T.; Seyis, S.; Acele, A. Coronary vasospasm and acute myocardial infarction induced by a topical capsaicin patch. Turk. Kardiyol. Dern. Ars. 2009, 37, 497–500. [Google Scholar]
- Sogut, O.; Kaya, H.; Gokdemir, M.T.; Sezen, Y. Acute myocardial infarction and coronary vasospasm associated with ingestion of cayenne pepper in a 25-year-old male. Int. J. Emerg. Med. 2012, 5, 5. [Google Scholar] [CrossRef] [Green Version]
- Panchal, S.K.; Bliss, E.; Brown, L. Capsaicin and metabolic syndrome. Nutrients 2018, 10, 630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCarty, M.F.; DiNicolantonio, J.J.; O’Keefe, J.H. Capsaicin may have important potential for promoting vascular and metabolic health. Open Heart 2015, 2, e000262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kröll, F.; Karlsson, J.A.; Lundberg, J.M.; Persson, C.G. Albumin protects against capsaicin- and adenosine-induced bronchoconstriction and reduces overflow of calcitonin gene-related peptide from guinea pig lung. Acta Physiol. Scand. 1990, 139, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Szallasi, A.; Cortright, D.N.; Blum, D.; Eid, S.R. The vanilloid receptor TRPV1: 10 years from channel cloning to antagonist proof-of-concept. Nat. Rev. Drug Discov. 2007, 6, 357–372. [Google Scholar] [CrossRef]
- Aghazadeh Tabrizi, M.; Baraldi, P.G.; Baraldi, S.; Gessi, S.; Merighi, S.; Borea, P.A. Medicinal chemistry, pharmacology and clinical implications of TRPV1 receptor antagonists. Med. Res. Rev. 2017, 37, 936–983. [Google Scholar] [CrossRef]
- Gao, M.; Wang, Y.; Qiao, Z.; Yan, L. A patent review of transient receptor potential vanilloid type 1 modulators (2014–present). Expert Opin. Ther. Pat. 2021, 31, 169–187. [Google Scholar] [CrossRef]
- Koivisto, A.-P.; Belvisi, M.; Gaudet, R.; Szallasi, A. Advances in TRP channel drug discovery: From target validation to clinical studies. Nat. Rev. Drug. Discov. 2022, 21, 41–59. [Google Scholar] [CrossRef]
- Gram, D.X.; Fribo, J.; Nagy, I.; Gotfredsen, C.; Charrua, A.; Hansen, J.B.; Hansen, A.J.; Szallasi, A. TRPV1 antagonists as novel anti-diabetic agents: Regulation of oral glucose tolerance and insulin secretion through a reduction of low-grade inflammation? Med. Sci. 2019, 7, 82. [Google Scholar] [CrossRef]
- Available online: https://www.pilapharma.com/clinical-development/ (accessed on 26 November 2022).
| Heading | High-Carbohydrate/Fat Diet (HCD) | HCD + Capsaicin (7.3 mg/kg/day) | p Value |
|---|---|---|---|
| energy intake, kJ/day | 725 + 17 | 584 + 14 | <0.0001 |
| total fat mass, g | 170.5 + 17.9 | 126.5 + 11.6 | 0.0688 |
| BMI, g/cm2 | 0.86 + 0.02 | 0.73 + 0.01 | <0.0001 |
| weight gain, g | 276 + 36 | 186 + 9 | 0.0056 |
| abdominal circumference, cm | 24.3 + 0.5 | 19.0 + 0.2 | <0.0001 |
| visceral fat, % | 11.33+ 0.48 | 8.42 + 0.46 | <0.0001 |
| Heading | Regular Diet | Capsaicin | p Value |
|---|---|---|---|
| food intake, g/24 h | 19.9 + 4.7 | 22.7 + 7.6 | 0.494 |
| total cholesterol, mmol/L | 1.4 + 0.5 | 1.2 + 0.5 | 0.433 |
| LDL-cholesterol, mmol/L | 0.6 + 0.5 | 0.5 + 0.3 | 0.435 |
| HDL-cholesterol, mmol/L | 0.5 + 0.3 | 0.5 + 0.2 | 0.179 |
| mean arterial pressure, mmHg | 152 + 28 | 148 + 26 | 0.123 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szallasi, A. Dietary Capsaicin: A Spicy Way to Improve Cardio-Metabolic Health? Biomolecules 2022, 12, 1783. https://doi.org/10.3390/biom12121783
Szallasi A. Dietary Capsaicin: A Spicy Way to Improve Cardio-Metabolic Health? Biomolecules. 2022; 12(12):1783. https://doi.org/10.3390/biom12121783
Chicago/Turabian StyleSzallasi, Arpad. 2022. "Dietary Capsaicin: A Spicy Way to Improve Cardio-Metabolic Health?" Biomolecules 12, no. 12: 1783. https://doi.org/10.3390/biom12121783
APA StyleSzallasi, A. (2022). Dietary Capsaicin: A Spicy Way to Improve Cardio-Metabolic Health? Biomolecules, 12(12), 1783. https://doi.org/10.3390/biom12121783





