Organismal Roles of Hsp90
Abstract
1. Introduction
2. Hsp90 in Organismal Development and Evolution
2.1. Development
2.2. Hsp90 as a Capacitor of Organismal Evolution
3. Hsp90-Dependent Regulation of Organismal Proteostasis, Stress and Aging
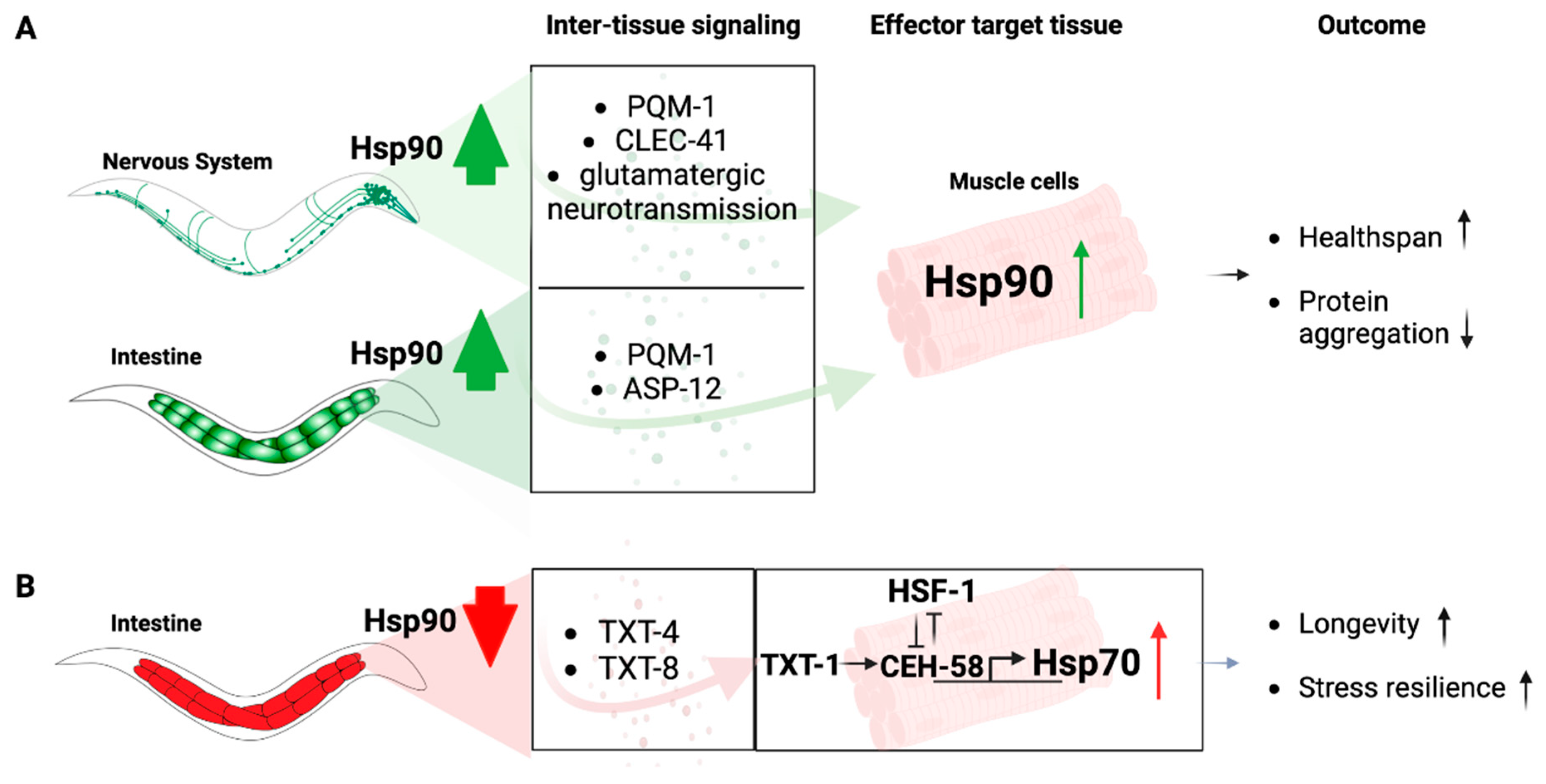
3.1. Hsp90 in the Regulation of Cell Nonautonomous Stress Signaling
3.2. Hsp90-Dependent Regulation of Lifespan and Aging
4. Pathogen Response and Innate Immunity
5. Neuronal Signaling and Behavior
6. Outlook and Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johnson, J.L. Evolution and Function of Diverse Hsp90 Homologs and Cochaperone Proteins. Biochim. Biophys. Acta 2012, 1823, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Whitesell, L.; Lindquist, S.L. HSP90 and the Chaperoning of Cancer. Nat. Rev. Cancer 2005, 5, 761–772. [Google Scholar] [CrossRef] [PubMed]
- Picard, D. Chaperoning Steroid Hormone Action. Trends Endocrinol. Metab. 2006, 17, 229–235. [Google Scholar] [CrossRef]
- Picard, D.; Khursheed, B.; Garabedian, M.J.; Fortin, M.G.; Lindquist, S.; Yamamoto, K.R. Reduced Levels of Hsp90 Compromise Steroid Receptor Action in Vivo. Nature 1990, 348, 166–168. [Google Scholar] [CrossRef] [PubMed]
- Echeverria, P.C.; Picard, D. Molecular Chaperones, Essential Partners of Steroid Hormone Receptors for Activity and Mobility. Biochim. Biophys. Acta 2010, 1803, 641–649. [Google Scholar] [CrossRef]
- Riggs, D.L.; Roberts, P.J.; Chirillo, S.C.; Cheung-Flynn, J.; Prapapanich, V.; Ratajczak, T.; Gaber, R.; Picard, D.; Smith, D.F. The Hsp90-Binding Peptidylprolyl Isomerase FKBP52 Potentiates Glucocorticoid Signaling in Vivo. EMBO J. 2003, 22, 1158–1167. [Google Scholar] [CrossRef] [PubMed]
- Sahasrabudhe, P.; Rohrberg, J.; Biebl, M.M.; Rutz, D.A.; Buchner, J. The Plasticity of the Hsp90 Co-Chaperone System. Mol. Cell 2017, 67, 947–961.e5. [Google Scholar] [CrossRef] [PubMed]
- Prodromou, C.; Bjorklund, D.M. Advances towards Understanding the Mechanism of Action of the Hsp90 Complex. Biomolecules 2022, 12, 600. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.L. Mutations in Hsp90 Cochaperones Result in a Wide Variety of Human Disorders. Front. Mol. Biosci. 2021, 8, 787260. [Google Scholar] [CrossRef]
- Mollapour, M.; Tsutsumi, S.; Truman, A.W.; Xu, W.; Vaughan, C.K.; Beebe, K.; Konstantinova, A.; Vourganti, S.; Panaretou, B.; Piper, P.W.; et al. Threonine 22 Phosphorylation Attenuates Hsp90 Interaction with Cochaperones and Affects Its Chaperone Activity. Mol. Cell 2011, 41, 672–681. [Google Scholar] [CrossRef]
- Woodford, M.R.; Truman, A.W.; Dunn, D.M.; Jensen, S.M.; Cotran, R.; Bullard, R.; Abouelleil, M.; Beebe, K.; Wolfgeher, D.; Wierzbicki, S.; et al. Mps1 Mediated Phosphorylation of Hsp90 Confers Renal Cell Carcinoma Sensitivity and Selectivity to Hsp90 Inhibitors. Cell Rep. 2016, 14, 872–884. [Google Scholar] [CrossRef] [PubMed]
- Mollapour, M.; Bourboulia, D.; Beebe, K.; Woodford, M.R.; Polier, S.; Hoang, A.; Chelluri, R.; Li, Y.; Guo, A.; Lee, M.-J.; et al. Asymmetric Hsp90 N Domain SUMOylation Recruits Aha1 and ATP-Competitive Inhibitors. Mol. Cell 2014, 53, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Backe, S.J.; Sager, R.A.; Woodford, M.R.; Makedon, A.M.; Mollapour, M. Post-Translational Modifications of Hsp90 and Translating the Chaperone Code. J. Biol. Chem. 2020, 295, 11099–11117. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.M.U.; Roe, S.M.; Vaughan, C.K.; Meyer, P.; Panaretou, B.; Piper, P.W.; Prodromou, C.; Pearl, L.H. Crystal Structure of an Hsp90–Nucleotide–P23/Sba1 Closed Chaperone Complex. Nature 2006, 440, 1013–1017. [Google Scholar] [CrossRef] [PubMed]
- Hawle, P.; Siepmann, M.; Harst, A.; Siderius, M.; Reusch, H.P.; Obermann, W.M.J. The Middle Domain of Hsp90 Acts as a Discriminator between Different Types of Client Proteins. Mol. Cell. Biol. 2006, 26, 8385–8395. [Google Scholar] [CrossRef]
- Hawle, P.; Horst, D.; Bebelman, J.P.; Yang, X.X.; Siderius, M.; van der Vies, S.M. Cdc37p Is Required for Stress-Induced High-Osmolarity Glycerol and Protein Kinase C Mitogen-Activated Protein Kinase Pathway Functionality by Interaction with Hog1p and Slt2p (Mpk1p). Eukaryot. Cell 2007, 6, 521–532. [Google Scholar] [CrossRef]
- Vaughan, C.K.; Gohlke, U.; Sobott, F.; Good, V.M.; Ali, M.M.U.; Prodromou, C.; Robinson, C.V.; Saibil, H.R.; Pearl, L.H. Structure of an Hsp90-Cdc37-Cdk4 Complex. Mol. Cell 2006, 23, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Meyer, P.; Prodromou, C.; Liao, C.; Hu, B.; Roe, S.M.; Vaughan, C.K.; Vlasic, I.; Panaretou, B.; Piper, P.W.; Pearl, L.H. Structural Basis for Recruitment of the ATPase Activator Aha1 to the Hsp90 Chaperone Machinery. EMBO J. 2004, 23, 1402–1410. [Google Scholar] [CrossRef] [PubMed]
- Lotz, G.P.; Lin, H.; Harst, A.; Obermann, W.M.J. Aha1 Binds to the Middle Domain of Hsp90, Contributes to Client Protein Activation, and Stimulates the ATPase Activity of the Molecular Chaperone. J. Biol. Chem. 2003, 278, 17228–17235. [Google Scholar] [CrossRef]
- Pearl, L.H.; Prodromou, C. Structure and Mechanism of the Hsp90 Molecular Chaperone Machinery. Annu. Rev. Biochem. 2006, 75, 271–294. [Google Scholar] [CrossRef]
- Maiti, S.; Picard, D. Cytosolic Hsp90 Isoform-Specific Functions and Clinical Significance. Biomolecules 2022, 12, 1166. [Google Scholar] [CrossRef] [PubMed]
- Biebl, M.M.; Buchner, J. Structure, Function, and Regulation of the Hsp90 Machinery. Cold Spring Harb. Perspect. Biol. 2019, 11, a034017. [Google Scholar] [CrossRef] [PubMed]
- Mankovich, A.G.; Freeman, B.C. Regulation of Protein Transport Pathways by the Cytosolic Hsp90s. Biomolecules 2022, 12, 1077. [Google Scholar] [CrossRef] [PubMed]
- McClellan, A.J.; Xia, Y.; Deutschbauer, A.M.; Davis, R.W.; Gerstein, M.; Frydman, J. Diverse Cellular Functions of the Hsp90 Molecular Chaperone Uncovered Using Systems Approaches. Cell 2007, 131, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Echtenkamp, F.J.; Gvozdenov, Z.; Adkins, N.L.; Zhang, Y.; Lynch-Day, M.; Watanabe, S.; Peterson, C.L.; Freeman, B.C. Hsp90 and P23 Molecular Chaperones Control Chromatin Architecture by Maintaining the Functional Pool of the RSC Chromatin Remodeler. Mol. Cell 2016, 64, 888–899. [Google Scholar] [CrossRef]
- DeZwaan, D.C.; Toogun, O.A.; Echtenkamp, F.J.; Freeman, B.C. The Hsp82 Molecular Chaperone Promotes a Switch between Unextendable and Extendable Telomere States. Nat. Struct. Mol. Biol. 2009, 16, 711–716. [Google Scholar] [CrossRef]
- Sawarkar, R.; Paro, R. Hsp90@chromatin.Nucleus: An Emerging Hub of a Networker. Trends Cell Biol. 2013, 23, 193–201. [Google Scholar] [CrossRef]
- Antonova, A.; Hummel, B.; Khavaran, A.; Redhaber, D.M.; Aprile-Garcia, F.; Rawat, P.; Gundel, K.; Schneck, M.; Hansen, E.C.; Mitschke, J.; et al. Heat-Shock Protein 90 Controls the Expression of Cell-Cycle Genes by Stabilizing Metazoan-Specific Host-Cell Factor HCFC1. Cell Rep. 2019, 29, 1645–1659.e9. [Google Scholar] [CrossRef]
- Gvozdenov, Z.; Bendix, L.D.; Kolhe, J.; Freeman, B.C. The Hsp90 Molecular Chaperone Regulates the Transcription Factor Network Controlling Chromatin Accessibility. J. Mol. Biol. 2019, 431, 4993–5003. [Google Scholar] [CrossRef]
- Muise-Helmericks, R.C.; Grimes, H.L.; Bellacosa, A.; Malstrom, S.E.; Tsichlis, P.N.; Rosen, N. Cyclin D Expression Is Controlled Post-Transcriptionally via a Phosphatidylinositol 3-Kinase/Akt-Dependent Pathway *. J. Biol. Chem. 1998, 273, 29864–29872. [Google Scholar] [CrossRef]
- Bedin, M.; Gaben, A.-M.; Saucier, C.; Mester, J. Geldanamycin, an Inhibitor of the Chaperone Activity of HSP90, Induces MAPK-Independent Cell Cycle Arrest. Int. J. Cancer 2004, 109, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Mahony, D.; Parry, D.A.; Lees, E. Active Cdk6 Complexes Are Predominantly Nuclear and Represent Only a Minority of the Cdk6 in T Cells. Oncogene 1998, 16, 603–611. [Google Scholar] [CrossRef]
- Stepanova, L.; Leng, X.; Parker, S.B.; Harper, J.W. Mammalian P50Cdc37 Is a Protein Kinase-Targeting Subunit of Hsp90 That Binds and Stabilizes Cdk4. Genes Dev. 1996, 10, 1491–1502. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, M.J.; Jimenez, J. Genetic Interactions between Hsp90 and the Cdc2 Mitotic Machinery in the Fission Yeast Schizosaccharomyces Pombe. Mol. Gen. Genet 1999, 261, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Aligue, R.; Akhavan-Niak, H.; Russell, P. A Role for Hsp90 in Cell Cycle Control: Wee1 Tyrosine Kinase Activity Requires Interaction with Hsp90. EMBO J. 1994, 13, 6099–6106. [Google Scholar] [CrossRef] [PubMed]
- Goes, F.S.; Martin, J. Hsp90 Chaperone Complexes Are Required for the Activity and Stability of Yeast Protein Kinases Mik1, Wee1 and Swe1. Eur. J. Biochem. 2001, 268, 2281–2289. [Google Scholar] [CrossRef] [PubMed]
- Mollapour, M.; Tsutsumi, S.; Neckers, L. Hsp90 Phosphorylation, Wee1 and the Cell Cycle. Cell Cycle 2010, 9, 2310–2316. [Google Scholar] [CrossRef] [PubMed]
- Fortugno, P.; Beltrami, E.; Plescia, J.; Fontana, J.; Pradhan, D.; Marchisio, P.C.; Sessa, W.C.; Altieri, D.C. Regulation of Survivin Function by Hsp90. Proc. Natl. Acad. Sci. USA 2003, 100, 13791–13796. [Google Scholar] [CrossRef]
- Lange, B.M.H.; Rebollo, E.; Herold, A.; González, C. Cdc37 Is Essential for Chromosome Segregation and Cytokinesis in Higher Eukaryotes. EMBO J. 2002, 21, 5364–5374. [Google Scholar] [CrossRef]
- Jameel, A.; Skilton, R.A.; Campbell, T.A.; Chander, S.K.; Coombes, R.C.; Luqmani, Y.A. Clinical and Biological Significance of HSP89 Alpha in Human Breast Cancer. Int. J. Cancer 1992, 50, 409–415. [Google Scholar] [CrossRef]
- Whitesell, L.; Mimnaugh, E.G.; De Costa, B.; Myers, C.E.; Neckers, L.M. Inhibition of Heat Shock Protein HSP90-Pp60v-Src Heteroprotein Complex Formation by Benzoquinone Ansamycins: Essential Role for Stress Proteins in Oncogenic Transformation. Proc. Natl. Acad. Sci. USA 1994, 91, 8324–8328. [Google Scholar] [CrossRef] [PubMed]
- Neckers, L. Hsp90 Inhibitors as Novel Cancer Chemotherapeutic Agents. Trends Mol. Med. 2002, 8, S55–S61. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Liu, Y. Recent Advances in the Discovery of Novel HSP90 Inhibitors: An Update from 2014. Curr. Drug Targets 2020, 21, 302–317. [Google Scholar] [CrossRef]
- Voss, A.K.; Thomas, T.; Gruss, P. Mice Lacking HSP90beta Fail to Develop a Placental Labyrinth. Development 2000, 127, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Grad, I.; Cederroth, C.R.; Walicki, J.; Grey, C.; Barluenga, S.; Winssinger, N.; Massy, B.D.; Nef, S.; Picard, D. The Molecular Chaperone Hsp90α Is Required for Meiotic Progression of Spermatocytes beyond Pachytene in the Mouse. PLoS ONE 2010, 5, e15770. [Google Scholar] [CrossRef] [PubMed]
- Du, S.J.; Li, H.; Bian, Y.; Zhong, Y. Heat-Shock Protein 90α1 Is Required for Organized Myofibril Assembly in Skeletal Muscles of Zebrafish Embryos. Proc. Natl. Acad. Sci. USA 2008, 105, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Gaiser, A.M.; Kaiser, C.J.O.; Haslbeck, V.; Richter, K. Downregulation of the Hsp90 System Causes Defects in Muscle Cells of Caenorhabditis Elegans. PLoS ONE 2011, 6, e25485. [Google Scholar] [CrossRef]
- Frumkin, A.; Dror, S.; Pokrzywa, W.; Bar-Lavan, Y.; Karady, I.; Hoppe, T.; Ben-Zvi, A. Challenging Muscle Homeostasis Uncovers Novel Chaperone Interactions in Caenorhabditis Elegans. Front. Mol. Biosci. 2014, 1, 21. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, K.; Maiti, S.; Zahoran, S.; Weidenauer, L.; Hany, D.; Wider, D.; Bernasconi, L.; Quadroni, M.; Collart, M.; Picard, D. Translational Reprogramming in Response to Accumulating Stressors Ensures Critical Threshold Levels of Hsp90 for Mammalian Life. Nat. Commun. 2022, 13, 6271. [Google Scholar] [CrossRef] [PubMed]
- Eckl, J.; Sima, S.; Marcus, K.; Lindemann, C.; Richter, K. Hsp90-Downregulation Influences the Heat-Shock Response, Innate Immune Response and Onset of Oocyte Development in Nematodes. PLoS ONE 2017, 12, e0186386. [Google Scholar] [CrossRef]
- Inoue, T.; Hirata, K.; Kuwana, Y.; Fujita, M.; Miwa, J.; Roy, R.; Yamaguchi, Y. Cell Cycle Control by Daf-21/Hsp90 at the First Meiotic Prophase/Metaphase Boundary during Oogenesis in Caenorhabditis Elegans. Dev. Growth Differ. 2006, 48, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Tewari, M.; Hu, P.J.; Ahn, J.S.; Ayivi-Guedehoussou, N.; Vidalain, P.-O.; Li, S.; Milstein, S.; Armstrong, C.M.; Boxem, M.; Butler, M.D.; et al. Systematic Interactome Mapping and Genetic Perturbation Analysis of a C. Elegans TGF-β Signaling Network. Mol. Cell 2004, 13, 469–482. [Google Scholar] [CrossRef]
- Murakami, M.; Koga, M.; Ohshima, Y. DAF-7/TGF-Beta Expression Required for the Normal Larval Development in C. Elegans Is Controlled by a Presumed Guanylyl Cyclase DAF-11. Mech. Dev. 2001, 109, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Birnby, D.A.; Link, E.M.; Vowels, J.J.; Tian, H.; Colacurcio, P.L.; Thomas, J.H. A Transmembrane Guanylyl Cyclase (DAF-11) and Hsp90 (DAF-21) Regulate a Common Set of Chemosensory Behaviors in Caenorhabditis Elegans. Genetics 2000, 155, 85–104. [Google Scholar] [CrossRef] [PubMed]
- Vowels, J.J.; Thomas, J.H. Multiple Chemosensory Defects in Daf-11 and Daf-21 Mutants of Caenorhabditis Elegans. Genetics 1994, 138, 303–316. [Google Scholar] [CrossRef]
- Greenwald, I.; Kovall, R. Notch Signaling: Genetics and Structure. WormBook 2013, 1–28. [Google Scholar] [CrossRef]
- Qiao, L.; Lissemore, J.L.; Shu, P.; Smardon, A.; Gelber, M.B.; Maine, E.M. Enhancers of Glp-1, a Gene Required for Cell-Signaling in Caenorhabditis Elegans, Define a Set of Genes Required for Germline Development. Genetics 1995, 141, 551–569. [Google Scholar] [CrossRef] [PubMed]
- Lissemore, J.L.; Connors, E.; Liu, Y.; Qiao, L.; Yang, B.; Edgley, M.L.; Flibotte, S.; Taylor, J.; Au, V.; Moerman, D.G.; et al. The Molecular Chaperone HSP90 Promotes Notch Signaling in the Germline of Caenorhabditis Elegans. G3 (Bethesda) 2018, 8, 1535–1544. [Google Scholar] [CrossRef]
- Borkovich, K.A.; Farrelly, F.W.; Finkelstein, D.B.; Taulien, J.; Lindquist, S. Hsp82 Is an Essential Protein That Is Required in Higher Concentrations for Growth of Cells at Higher Temperatures. Mol. Cell. Biol. 1989, 9, 3919–3930. [Google Scholar] [CrossRef]
- Cutforth, T.; Rubin, G.M. Mutations in Hsp83 and Cdc37 Impair Signaling by the Sevenless Receptor Tyrosine Kinase in Drosophila. Cell 1994, 77, 1027–1036. [Google Scholar] [CrossRef]
- Yue, L.; Karr, T.L.; Nathan, D.F.; Swift, H.; Srinivasan, S.; Lindquist, S. Genetic Analysis of Viable Hsp90 Alleles Reveals a Critical Role in Drosophila Spermatogenesis. Genetics 1999, 151, 1065–1079. [Google Scholar] [CrossRef]
- van der Straten, A.; Rommel, C.; Dickson, B.; Hafen, E. The Heat Shock Protein 83 (Hsp83) Is Required for Raf-Mediated Signalling in Drosophila. EMBO J. 1997, 16, 1961–1969. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, S.L.; Lindquist, S. Hsp90 as a Capacitor for Morphological Evolution. Nature 1998, 396, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Queitsch, C.; Sangster, T.A.; Lindquist, S. Hsp90 as a Capacitor of Phenotypic Variation. Nature 2002, 417, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Rohner, N.; Jarosz, D.F.; Kowalko, J.E.; Yoshizawa, M.; Jeffery, W.R.; Borowsky, R.L.; Lindquist, S.; Tabin, C.J. Cryptic Variation in Morphological Evolution: HSP90 as a Capacitor for Loss of Eyes in Cavefish. Science 2013, 342, 1372–1375. [Google Scholar] [CrossRef]
- Yeyati, P.L.; Bancewicz, R.M.; Maule, J.; van Heyningen, V. Hsp90 Selectively Modulates Phenotype in Vertebrate Development. PLoS Genet 2007, 3, e43. [Google Scholar] [CrossRef]
- Burga, A.; Casanueva, M.O.; Lehner, B. Predicting Mutation Outcome from Early Stochastic Variation in Genetic Interaction Partners. Nature 2011, 480, 250–253. [Google Scholar] [CrossRef]
- Casanueva, M.O.; Burga, A.; Lehner, B. Fitness Trade-Offs and Environmentally Induced Mutation Buffering in Isogenic C. Elegans. Science 2012, 335, 82–85. [Google Scholar] [CrossRef]
- Karras, G.I.; Yi, S.; Sahni, N.; Fischer, M.; Xie, J.; Vidal, M.; D’Andrea, A.D.; Whitesell, L.; Lindquist, S. HSP90 Shapes the Consequences of Human Genetic Variation. Cell 2017, 168, 856–866.e12. [Google Scholar] [CrossRef]
- Hummel, B.; Hansen, E.C.; Yoveva, A.; Aprile-Garcia, F.; Hussong, R.; Sawarkar, R. The Evolutionary Capacitor HSP90 Buffers the Regulatory Effects of Mammalian Endogenous Retroviruses. Nat. Struct. Mol. Biol. 2017, 24, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Sollars, V.; Lu, X.; Xiao, L.; Wang, X.; Garfinkel, M.D.; Ruden, D.M. Evidence for an Epigenetic Mechanism by Which Hsp90 Acts as a Capacitor for Morphological Evolution. Nat. Genet 2003, 33, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Anckar, J.; Sistonen, L. Regulation of HSF1 Function in the Heat Stress Response: Implications in Aging and Disease. Annu. Rev. Biochem. 2011, 80, 1089–1115. [Google Scholar] [CrossRef]
- Zou, L.; Wu, D.; Zang, X.; Wang, Z.; Wu, Z.; Chen, D. Construction of a Germline-Specific RNAi Tool in C. Elegans. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, D.; Jones, L.M.; Good, S.; Miles, J.; Vijayabaskar, M.S.; Aston, R.; Smith, C.E.; Westhead, D.R.; van Oosten-Hawle, P. A PQM-1-Mediated Response Triggers Transcellular Chaperone Signaling and Regulates Organismal Proteostasis. Cell Rep. 2018, 23, 3905–3919. [Google Scholar] [CrossRef] [PubMed]
- Somogyvári, M.; Gecse, E.; Sőti, C. DAF-21/Hsp90 Is Required for C. Elegans Longevity by Ensuring DAF-16/FOXO Isoform A Function. Sci. Rep. 2018, 8, 12048. [Google Scholar] [CrossRef]
- Maman, M.; Marques, F.C.; Volovik, Y.; Dubnikov, T.; Bejerano-Sagie, M.; Cohen, E. A Neuronal GPCR Is Critical for the Induction of the Heat Shock Response in the Nematode C. Elegans. J. Neurosci. 2013, 33, 6102–6111. [Google Scholar] [CrossRef]
- Tatum, M.C.; Ooi, F.K.; Chikka, M.R.; Chauve, L.; Martinez-Velazquez, L.A.; Steinbusch, H.W.M.; Morimoto, R.I.; Prahlad, V. Neuronal Serotonin Release Triggers the Heat Shock Response in C. Elegans in the Absence of Temperature Increase. Curr. Biol. 2015, 25, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Prahlad, V.; Cornelius, T.; Morimoto, R.I. Regulation of the Cellular Heat Shock Response in Caenorhabditis Elegans by Thermosensory Neurons. Science 2008, 320, 811–814. [Google Scholar] [CrossRef]
- Gildea, H.K.; Frankino, P.A.; Tronnes, S.U.; Pender, C.L.; Durieux, J.; Dishart, J.G.; Choi, H.O.; Hunter, T.D.; Cheung, S.S.; Frakes, A.E.; et al. Glia of C. Elegans Coordinate a Protective Organismal Heat Shock Response Independent of the Neuronal Thermosensory Circuit. Sci. Adv. 2022, 8, eabq3970. [Google Scholar] [CrossRef]
- Sugi, T.; Nishida, Y.; Mori, I. Regulation of Behavioral Plasticity by Systemic Temperature Signaling in Caenorhabditis Elegans. Nat. Neurosci. 2011, 14, 984–992. [Google Scholar] [CrossRef]
- Jing, R.; Duncan, C.B.; Duncan, S.A. A Small-Molecule Screen Reveals That HSP90β Promotes the Conversion of Induced Pluripotent Stem Cell-Derived Endoderm to a Hepatic Fate and Regulates HNF4A Turnover. Development 2017, 144, 1764–1774. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chauve, L.; Phelps, G.; Brielmann, R.M.; Morimoto, R.I. E2F Coregulates an Essential HSF Developmental Program That Is Distinct from the Heat-Shock Response. Genes Dev. 2016, 30, 2062–2075. [Google Scholar] [CrossRef]
- Morton, E.A.; Lamitina, T. Caenorhabditis Elegans HSF-1 Is an Essential Nuclear Protein That Forms Stress Granule-like Structures Following Heat Shock. Aging Cell 2013, 12, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Abane, R.; Mezger, V. Roles of Heat Shock Factors in Gametogenesis and Development. FEBS J. 2010, 277, 4150–4172. [Google Scholar] [CrossRef] [PubMed]
- Edwards, S.L.; Erdenebat, P.; Morphis, A.C.; Kumar, L.; Wang, L.; Chamera, T.; Georgescu, C.; Wren, J.D.; Li, J. Insulin/IGF-1 Signaling and Heat Stress Differentially Regulate HSF1 Activities in Germline Development. Cell. Rep. 2021, 36, 109623. [Google Scholar] [CrossRef] [PubMed]
- Miles, J.; Oosten-Hawle, P. van Tissue-Specific RNAi Tools to Identify Components for Systemic Stress Signaling. JoVE (J. Vis. Exp. ) 2020, 159, e61357. [Google Scholar] [CrossRef]
- Miles, J.; Townend, S.; Smith, W.; Westhead, D.R.; van Oosten-Hawle, P. Transcellular Chaperone Signaling Is an Intercellular Stress-Response Distinct from the HSF-1 Mediated HSR. bioRxiv 2022, 2022.03.17.484707. [Google Scholar]
- van Oosten-Hawle, P.; Porter, R.S.; Morimoto, R.I. Regulation of Organismal Proteostasis by Transcellular Chaperone Signaling. Cell 2013, 153, 1366–1378. [Google Scholar] [CrossRef]
- Fawcett, T.W.; Sylvester, S.L.; Sarge, K.D.; Morimoto, R.I.; Holbrook, N.J. Effects of Neurohormonal Stress and Aging on the Activation of Mammalian Heat Shock Factor 1. J. Biol. Chem. 1994, 269, 32272–32278. [Google Scholar] [CrossRef] [PubMed]
- Liao, D.-F.; Jin, Z.-G.; Baas, A.S.; Daum, G.; Gygi, S.P.; Aebersold, R.; Berk, B.C. Purification and Identification of Secreted Oxidative Stress-Induced Factors from Vascular Smooth Muscle Cells *. J. Biol. Chem. 2000, 275, 189–196. [Google Scholar] [CrossRef]
- Bhatia, A.; O’Brien, K.; Guo, J.; Lincoln, V.; Kajiwara, C.; Chen, M.; Woodley, D.T.; Udono, H.; Li, W. Extracellular and Non-Chaperone Function of Heat Shock Protein-90α Is Required for Skin Wound Healing. J. Invest. Derm. 2018, 138, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Sahu, D.; Tsen, F. Secreted Heat Shock Protein-90 (Hsp90) in Wound Healing and Cancer. Biochim. Et Biophys. Acta (BBA)—Mol. Cell Res. 2012, 1823, 730–741. [Google Scholar] [CrossRef] [PubMed]
- Jay, D.; Luo, Y.; Li, W. Extracellular Heat Shock Protein-90 (EHsp90): Everything You Need to Know. Biomolecules 2022, 12, 911. [Google Scholar] [CrossRef] [PubMed]
- Janssens, G.E.; Lin, X.-X.; Millan-Ariño, L.; Kavšek, A.; Sen, I.; Seinstra, R.I.; Stroustrup, N.; Nollen, E.A.A.; Riedel, C.G. Transcriptomics-Based Screening Identifies Pharmacological Inhibition of Hsp90 as a Means to Defer Aging. Cell Rep. 2019, 27, 467–480.e6. [Google Scholar] [CrossRef]
- Hsu, A.-L.; Murphy, C.T.; Kenyon, C. Regulation of Aging and Age-Related Disease by DAF-16 and Heat-Shock Factor. Science 2003, 300, 1142–1145. [Google Scholar] [CrossRef]
- Douglas, P.M.; Baird, N.A.; Simic, M.S.; Uhlein, S.; McCormick, M.A.; Wolff, S.C.; Kennedy, B.K.; Dillin, A. Heterotypic Signals from Neural HSF-1 Separate Thermotolerance from Longevity. Cell Rep. 2015, 12, 1196–1204. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.F.; Morimoto, R.I. Regulation of Longevity in Caenorhabditis Elegans by Heat Shock Factor and Molecular Chaperones. Mol. Biol. Cell 2004, 15, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Luo, J.; Jiang, P.; Li, Y.; Wang, Q.; Bai, Y.; Chen, J.; Wang, J.; Zhang, J. Inhibition of Embryonic HSP 90 Function Promotes Variation of Cold Tolerance in Zebrafish. Front. Genet. 2020, 11, 541944. [Google Scholar] [CrossRef] [PubMed]
- Horikawa, M.; Sural, S.; Hsu, A.-L.; Antebi, A. Co-Chaperone P23 Regulates C. Elegans Lifespan in Response to Temperature. PLoS Genet 2015, 11, e1005023. [Google Scholar] [CrossRef] [PubMed]
- Freeman, B.C.; Felts, S.J.; Toft, D.O.; Yamamoto, K.R. The P23 Molecular Chaperones Act at a Late Step in Intracellular Receptor Action to Differentially Affect Ligand Efficacies. Genes Dev. 2000, 14, 422–434. [Google Scholar] [CrossRef]
- Nguyen, M.T.; Somogyvári, M.; Sőti, C. Hsp90 Stabilizes SIRT1 Orthologs in Mammalian Cells and C. Elegans. Int. J. Mol. Sci. 2018, 19, 3661. [Google Scholar] [CrossRef] [PubMed]
- Hubert, D.A.; Tornero, P.; Belkhadir, Y.; Krishna, P.; Takahashi, A.; Shirasu, K.; Dangl, J.L. Cytosolic HSP90 Associates with and Modulates the Arabidopsis RPM1 Disease Resistance Protein. EMBO J. 2003, 22, 5679–5689. [Google Scholar] [CrossRef] [PubMed]
- Sangster, T.A.; Queitsch, C. The HSP90 Chaperone Complex, an Emerging Force in Plant Development and Phenotypic Plasticity. Curr. Opin. Plant Biol. 2005, 8, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Aballay, A. Heat-Shock Transcription Factor (HSF)-1 Pathway Required for Caenorhabditis Elegans Immunity. Proc. Natl. Acad. Sci. USA 2006, 103, 13092–13097. [Google Scholar] [CrossRef]
- Singh, V.; Aballay, A. Heat Shock and Genetic Activation of HSF-1 Enhance Immunity to Bacteria. Cell Cycle 2006, 5, 2443–2446. [Google Scholar] [CrossRef] [PubMed]
- Wojda, I.; Kowalski, P. Galleria Mellonella Infected with Bacillus Thuringiensis Involves Hsp90. Open Life Sci. 2013, 8, 561–569. [Google Scholar] [CrossRef]
- Oura, J.; Tamura, Y.; Kamiguchi, K.; Kutomi, G.; Sahara, H.; Torigoe, T.; Himi, T.; Sato, N. Extracellular Heat Shock Protein 90 Plays a Role in Translocating Chaperoned Antigen from Endosome to Proteasome for Generating Antigenic Peptide to Be Cross-Presented by Dendritic Cells. Int. Immunol. 2011, 23, 223–237. [Google Scholar] [CrossRef]
- Murshid, A.; Gong, J.; Calderwood, S.K. Heat Shock Protein 90 Mediates Efficient Antigen Cross Presentation through the Scavenger Receptor Expressed by Endothelial Cells-I. J. Immunol. 2010, 185, 2903–2917. [Google Scholar] [CrossRef] [PubMed]
- Murshid, A.; Gong, J.; Calderwood, S.K. Hsp90–Peptide Complexes Stimulate Antigen Presentation through the Class II Pathway after Binding Scavenger Receptor SREC-I. Immunobiology 2014, 219, 924–931. [Google Scholar] [CrossRef] [PubMed]
- Graner, M.W. Chapter Eight - HSP90 and Immune Modulation in Cancer. In Advances in Cancer Research; Hsp90 in Cancer: Beyond the Usual Suspects; Isaacs, J., Whitesell, L., Eds.; Academic Press: Cambridge, MA, USA, 2016; Volume 129, pp. 191–224. [Google Scholar]
- Anderson, I.; Low, J.S.; Weston, S.; Weinberger, M.; Zhyvoloup, A.; Labokha, A.A.; Corazza, G.; Kitson, R.A.; Moody, C.J.; Marcello, A.; et al. Heat Shock Protein 90 Controls HIV-1 Reactivation from Latency. Proc. Natl. Acad. Sci. USA 2014, 111, E1528–E1537. [Google Scholar] [CrossRef]
- Henderson, B.; Calderwood, S.K.; Coates, A.R.M.; Cohen, I.; van Eden, W.; Lehner, T.; Pockley, A.G. Caught with Their PAMPs Down? The Extracellular Signalling Actions of Molecular Chaperones Are Not Due to Microbial Contaminants. Cell Stress Chaperones 2010, 15, 123–141. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Feinbaum, R.; Alloing, G.; Emerson, F.E.; Garsin, D.A.; Inoue, H.; Tanaka-Hino, M.; Hisamoto, N.; Matsumoto, K.; Tan, M.-W.; et al. A Conserved P38 MAP Kinase Pathway in Caenorhabditis Elegans Innate Immunity. Science 2002, 297, 623–626. [Google Scholar] [CrossRef] [PubMed]
- Su, B.; Karin, M. Mitogen-Activated Protein Kinase Cascades and Regulation of Gene Expression. Curr. Opin. Immunol. 1996, 8, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Banumathy, G.; Singh, V.; Pavithra, S.R.; Tatu, U. Heat Shock Protein 90 Function Is Essential for Plasmodium Falciparum Growth in Human Erythrocytes. J. Biol. Chem. 2003, 278, 18336–18345. [Google Scholar] [CrossRef] [PubMed]
- Angel, S.O.; Figueras, M.J.; Alomar, M.L.; Echeverria, P.C.; Deng, B. Toxoplasma Gondii Hsp90: Potential Roles in Essential Cellular Processes of the Parasite. Parasitology 2014, 141, 1138–1147. [Google Scholar] [CrossRef]
- Echeverria, P.C.; Matrajt, M.; Harb, O.S.; Zappia, M.P.; Costas, M.A.; Roos, D.S.; Dubremetz, J.F.; Angel, S.O. Toxoplasma Gondii Hsp90 Is a Potential Drug Target Whose Expression and Subcellular Localization Are Developmentally Regulated. J. Mol. Biol. 2005, 350, 723–734. [Google Scholar] [CrossRef]
- Corigliano, M.G.; Sander, V.A.; Sánchez López, E.F.; Ramos Duarte, V.A.; Mendoza Morales, L.F.; Angel, S.O.; Clemente, M. Heat Shock Proteins 90 KDa: Immunomodulators and Adjuvants in Vaccine Design Against Infectious Diseases. Front. Bioeng. Biotechnol. 2021, 8, 622186. [Google Scholar] [CrossRef]
- Gerges, N.Z.; Tran, I.C.; Backos, D.S.; Harrell, J.M.; Chinkers, M.; Pratt, W.B.; Esteban, J.A. Independent Functions of Hsp90 in Neurotransmitter Release and in the Continuous Synaptic Cycling of AMPA Receptors. J. Neurosci. 2004, 24, 4758–4766. [Google Scholar] [CrossRef]
- Karney-Grobe, S.; Russo, A.; Frey, E.; Milbrandt, J.; DiAntonio, A. HSP90 Is a Chaperone for DLK and Is Required for Axon Injury Signaling. Proc. Natl. Acad. Sci. USA 2018, 115, E9899–E9908. [Google Scholar] [CrossRef]
- Hajdú, G.; Gecse, E.; Taisz, I.; Móra, I.; Sőti, C. Toxic Stress-Specific Cytoprotective Responses Regulate Learned Behavioral Decisions in C. Elegans. BMC Biol. 2021, 19, 26. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Oosten-Hawle, P. Organismal Roles of Hsp90. Biomolecules 2023, 13, 251. https://doi.org/10.3390/biom13020251
van Oosten-Hawle P. Organismal Roles of Hsp90. Biomolecules. 2023; 13(2):251. https://doi.org/10.3390/biom13020251
Chicago/Turabian Stylevan Oosten-Hawle, Patricija. 2023. "Organismal Roles of Hsp90" Biomolecules 13, no. 2: 251. https://doi.org/10.3390/biom13020251
APA Stylevan Oosten-Hawle, P. (2023). Organismal Roles of Hsp90. Biomolecules, 13(2), 251. https://doi.org/10.3390/biom13020251





