Action Mechanism of Metformin and Its Application in Hematological Malignancy Treatments: A Review
Abstract
1. Introduction
2. Metformin Mechanism of Action
2.1. Metformin Anti-Hyperglycemic Effects Mechanism
2.1.1. Systemic Actions of Metformin
2.1.2. Cellular Actions of Metformin
AMPK-Dependent Mechanisms
AMPK-Independent Mechanisms
2.2. Metformin Anti-Cancer Mechanism
2.2.1. Activation of LKB1-AMPK-mTOR Signaling Pathway
- Regulation of epigenetic modifying enzyme activity [97]: AMPK directly and indirectly regulates histone acetylation and alters gene expression patterns via the epigenetic regulation of various chromatin functions [98,99]. Activated AMPK phosphorylates a variety of substrates including histone acetyltransferases (HATs), histone deacetylases (HDACs) and deoxyribonucleic acid (DNA) methyltransferases (DNMTs), often leading to their inhibition [98,99,100];
- Activation of p53: Activated P53 promotes apoptosis, cycle arrest, autophagy and the inhibition of the phosphoinositide 3-kinase (PI3K)–protein kinase B (AKT) signaling pathway [101,102]. The effect of P53 in tumor apoptosis was also associated with the reduced expression of transcription factor specificity protein 1 (Sp1), Sp3, Sp4 and oncogenes (B-cell lymphoma-2 (BCL-2), mTOR, vascular endothelial growth factor (VEGF) and MYC, etc.) [103,104];
- Activation or inhibition of the expression of other cancer-related signaling pathways:
- 3.1
- Activation of the AMPK-forkhead box O1/3 (FOXO1/3) signaling pathway [105,106,107]: FOXO is a proliferation-associated transcription factor, and the activation of FOXO3a has been shown to be required for the pro-apoptotic and chemotherapeutic effects of metformin in a variety of tumor models [108,109];
- 3.2
- Suppression of Hippo pathway expression [110,111]: In some (cancer) models, metformin has been shown to repress the activity or expression of yes-associated protein (YAP)/tea domain transcription factor (TAZ), which are two central effectors of the Hippo pathway that are mediated by AMPK [112,113,114,115,116,117];
- 3.3
- Inhibition of the receptor tyrosine kinase (TK) pathway: this includes epidermal growth factor receptor (EGFR) signaling, further targeting downstream effectors AKT, mTOR, extracellular regulated protein kinases (ERK), etc. [118];
- 3.4
- Inhibition of the PI3K/AKT pathway: in vitro and in vivo experiments showed that the PI3K/AKT signaling pathway inhibited by metformin stimulated the expression of phosphatase and tensin homolog (PTEN) and inositol triphosphate 3 (IP3) [119];
- 3.5
- Affecting natural killer (NK) cell signaling pathways: the anti-cancer properties of specific immune regulation, the inhibition of cell proliferation and the induction of cell cycle arrest are exerted via the inhibition of tumor cell metastasis, endothelial cell proliferation and the alteration of NK cells’ ligand expression on the surface of tumor cells [120,121,122,123];
- 3.6
- Inhibition of cancer stem cells (CSCs): CSCs are cancer cells with unlimited renewal capacity. A number of studies have shown that metformin inhibits the biological activity of CSCs in a variety of tumors, including gastric, endometrial and ovarian cancers [126]. Subsequently, metformin activates additional signaling pathways for targeting CSCs via the activation of AMPK, which include PI3K–AKT–mTOR, insulin-insulin growth factor1 (IGF1), MAPK, sonic hedgehog (Shh), Wnt, TGFB, Notch, Hippo and NFkB pathways [126];
- Mitigation of hypoxia and other tumor responses caused by hypoxia: Metformin leads to slow tumor growth by decreasing the expression of hypoxia-inducible factor-1α (HIF-1α), which in turn decreases the expression of HIF1-targeted genes [129,130,131,132]. The inhibition of HIF-1α simultaneously suppresses the immunosuppressive activity of myeloid-derived suppressor cells (MDSCs) and improves T-cell immunity in the tumor microenvironment (TME) [133,134,135]. These immune responses are ultimately also involved in the inhibition of angiogenesis [136];
- Inhibition of adipogenesis: The cancer cells themselves require more nutrients and energy; thus, the rate of adipogenesis is higher [137]. AMPK inhibits lipogenesis by targeting the activity or expression of many lipogenic enzymes. On the one hand, AMPK phosphorylates and inhibits 3-hydroxy-3-methylglutaryl CoA reductase (HMGCR), which catalyzes the rate-limiting step in cholesterol synthesis. On the other hand, AMPK phosphorylates and inactivates acetyl CoA carboxylase (ACC), the main enzyme involved in the biosynthesis of fatty acid and HMGCR, resulting in the inhibition of cholesterol biosynthesis [138]. In addition, AMPK also phosphorylates sterol regulatory element binding protein-1c (SREBP-1c) at Ser-372, which restricts its cleavage and nuclear translocation. This process results in the downregulation of SREBP-1c target genes, including those encoding ACC1 and fatty acid synthase (FASN), and leads to reduced lipogenesis [139];
- Activation of ataxia telangiectasia-mutated gene (ATM), which leads to the activation of DNA damage repair pathways and the inhibition of tumor growth [140].
2.2.2. Activation of AMPK-Independent Signaling Pathways
2.2.3. Altered Energy Metabolism of Tumors
2.2.4. Improved Immune and Inflammatory Response
2.2.5. Reduced Tumor Vascular Metastasis and Invasion
3. Therapeutic Application of Metformin in Hematological Malignancies
3.1. Leukemia
3.1.1. Acute Myeloid Leukemia (AML)
3.1.2. Chronic Myeloid Leukemia (CML)
3.1.3. Acute Lymphocytic Leukemia (ALL)
3.1.4. Chronic Lymphocytic Leukemia (CLL)
3.2. Multiple Myeloma (MM)
3.3. Lymphoma
3.4. Ongoing Studies
4. Adverse Effects of Metformin
5. Limitations of Metformin in Cancer Treatment
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, J.; Bell, C.; Campbell, V.; DeBacker, J.; Tamberg, E.; Lee, C.; Mehta, S. Noninvasive Ventilation in Patients With Hematologic Malignancy. J. Intensive Care Med. 2017, 885066617690725. [Google Scholar]
- Al-Dorzi, H.M.; Al Orainni, H.; Al Eid, F.; Tlayjeh, H.; Itani, A.; Al Hejazi, A.; Arabi, Y.M. Characteristics and predictors of mortality of patients with hematologic malignancies requiring invasive mechanical ventilation. Ann. Thorac. Med. 2017, 12, 259–265. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.; Li, Q.; Xu, A.; Hu, Y.; Sun, C. Ferroptosis in hematological malignancies and its potential network with abnormal tumor metabolism. Biomed. Pharmacother. 2022, 148, 112747. [Google Scholar] [CrossRef] [PubMed]
- Eppert, K.; Takenaka, K.; Lechman, E.R.; Waldron, L.; Nilsson, B.; van Galen, P.; Metzeler, K.H.; Poeppl, A.; Ling, V.; Beyene, J.; et al. Stem cell gene expression programs influence clinical outcome in human leukemia. Nat. Med. 2011, 17, 1086–1093. [Google Scholar] [CrossRef]
- Westin, J.; Sehn, L.H. CAR T cells as a second-line therapy for large B-cell lymphoma: A paradigm shift? Blood 2022, 139, 2737–2746. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Zhen, C.; Liu, J.; Shang, P. β-Phenethyl Isothiocyanate Induces Cell Death in Human Osteosarcoma through Altering Iron Metabolism, Disturbing the Redox Balance, and Activating the MAPK Signaling Pathway. Oxid. Med. Cell. Longev. 2020, 2020, 5021983. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Model List of Essential Medicines. 22nd List. 2021. Available online: https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2021.02 (accessed on 2 November 2022).
- DeFronzo, R.A.; Goodman, A.M.; Group, M.M.S. Efficacy of metformin in patients with non-insulin-dependent diabetes mellitus. N. Engl. J. Med. 1995, 333, 541–549. [Google Scholar] [CrossRef]
- Maruthur, N.M.; Tseng, E.; Hutfless, S.; Wilson, L.M.; Suarez-Cuervo, C.; Berger, Z.; Chu, Y.; Iyoha, E.; Segal, J.B.; Bolen, S. Diabetes Medications as Monotherapy or Metformin-Based Combination Therapy for Type 2 Diabetes: A Systematic Review and Meta-analysis. Ann. Intern. Med. 2016, 164, 740–751. [Google Scholar] [CrossRef]
- Evans, J.M.; Donnelly, L.A.; Emslie-Smith, A.M.; Alessi, D.R.; Morris, A.D. Metformin and reduced risk of cancer in diabetic patients. BMJ 2005, 330, 1304–1305. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Li, S. The prognostic value of metformin for cancer patients with concurrent diabetes: A systematic review and meta-analysis. Diabetes Obes. Metab. 2014, 16, 707–710. [Google Scholar] [CrossRef]
- Zhang, P.; Li, H.; Tan, X.; Chen, L.; Wang, S. Association of metformin use with cancer incidence and mortality: A meta-analysis. Cancer Epidemiol. 2013, 37, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Noto, H.; Goto, A.; Tsujimoto, T.; Noda, M. Cancer risk in diabetic patients treated with metformin: A systematic review and meta-analysis. PLoS ONE 2012, 7, e33411. [Google Scholar] [CrossRef]
- Franciosi, M.; Lucisano, G.; Lapice, E.; Strippoli, G.F.; Pellegrini, F.; Nicolucci, A. Metformin therapy and risk of cancer in patients with type 2 diabetes: Systematic review. PLoS ONE 2013, 8, e71583. [Google Scholar] [CrossRef] [PubMed]
- Gandini, S.; Puntoni, M.; Heckman-Stoddard, B.M.; Dunn, B.K.; Ford, L.; DeCensi, A.; Szabo, E. Metformin and cancer risk and mortality: A systematic review and meta-analysis taking into account biases and confounders. Cancer Prev. Res. 2014, 7, 867–885. [Google Scholar] [CrossRef]
- Yin, M.; Zhou, J.; Gorak, E.J.; Quddus, F. Metformin is associated with survival benefit in cancer patients with concurrent type 2 diabetes: A systematic review and meta-analysis. Oncologist 2013, 18, 1248–1255. [Google Scholar] [CrossRef] [PubMed]
- Deng, D.; Yang, Y.; Tang, X.; Skrip, L.; Qiu, J.; Wang, Y.; Zhang, F. Association between metformin therapy and incidence, recurrence and mortality of prostate cancer: Evidence from a meta-analysis. Diabetes Metab. Res. Rev. 2015, 31, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Raval, A.D.; Thakker, D.; Vyas, A.; Salkini, M.; Madhavan, S.; Sambamoorthi, U. Impact of metformin on clinical outcomes among men with prostate cancer: A systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2015, 18, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Zhang, Y.; Gong, Y.I.; He, J.; Chen, X. Metformin and lung cancer risk of patients with type 2 diabetes mellitus: A meta-analysis. Biomed. Rep. 2015, 3, 235–241. [Google Scholar] [CrossRef]
- Sakoda, L.C.; Ferrara, A.; Achacoso, N.S.; Peng, T.; Ehrlich, S.F.; Quesenberry, C.P., Jr.; Habel, L.A. Metformin use and lung cancer risk in patients with diabetes. Cancer Prev. Res. 2015, 8, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.J.; Gallagher, E.J.; Sigel, K.; Mhango, G.; Galsky, M.D.; Smith, C.B.; LeRoith, D.; Wisnivesky, J.P. Survival of patients with stage IV lung cancer with diabetes treated with metformin. Am. J. Respir. Crit. Care Med. 2015, 191, 448–454. [Google Scholar] [CrossRef]
- Sandulache, V.C.; Hamblin, J.S.; Skinner, H.D.; Kubik, M.W.; Myers, J.N.; Zevallos, J.P. Association between metformin use and improved survival in patients with laryngeal squamous cell carcinoma. Head Neck 2014, 36, 1039–1043. [Google Scholar] [CrossRef] [PubMed]
- Yen, Y.C.; Lin, C.; Lin, S.W.; Lin, Y.S.; Weng, S.F. Effect of metformin on the incidence of head and neck cancer in diabetics. Head Neck 2015, 37, 1268–1273. [Google Scholar] [CrossRef] [PubMed]
- Col, N.F.; Ochs, L.; Springmann, V.; Aragaki, A.K.; Chlebowski, R.T. Metformin and breast cancer risk: A meta-analysis and critical literature review. Breast Cancer Res. Treat. 2012, 135, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Chen, K.; Jia, X.; Tian, Y.; Dai, Y.; Li, D.; Xie, J.; Tao, M.; Mao, Y. Metformin Use Is Associated With Better Survival of Breast Cancer Patients With Diabetes: A Meta-Analysis. Oncologist 2015, 20, 1236–1244. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lai, S.T.; Xie, L.; Zhao, J.D.; Ma, N.Y.; Zhu, J.; Ren, Z.G.; Jiang, G.L. Metformin is associated with reduced risk of pancreatic cancer in patients with type 2 diabetes mellitus: A systematic review and meta-analysis. Diabetes Res. Clin. Pract. 2014, 106, 19–26. [Google Scholar] [CrossRef]
- Fransgaard, T.; Thygesen, L.C.; Gögenur, I. Metformin Increases Overall Survival in Patients with Diabetes Undergoing Surgery for Colorectal Cancer. Ann. Surg. Oncol. 2016, 23, 1569–1575. [Google Scholar] [CrossRef]
- Mei, Z.B.; Zhang, Z.J.; Liu, C.Y.; Liu, Y.; Cui, A.; Liang, Z.L.; Wang, G.H.; Cui, L. Survival benefits of metformin for colorectal cancer patients with diabetes: A systematic review and meta-analysis. PLoS ONE 2014, 9, e91818. [Google Scholar] [CrossRef] [PubMed]
- Nevadunsky, N.S.; Van Arsdale, A.; Strickler, H.D.; Moadel, A.; Kaur, G.; Frimer, M.; Conroy, E.; Goldberg, G.L.; Einstein, M.H. Metformin use and endometrial cancer survival. Gynecol. Oncol. 2014, 132, 236–240. [Google Scholar] [CrossRef]
- Dilokthornsakul, P.; Chaiyakunapruk, N.; Termrungruanglert, W.; Pratoomsoot, C.; Saokaew, S.; Sruamsiri, R. The effects of metformin on ovarian cancer: A systematic review. Int. J. Gynecol. Cancer 2013, 23, 1544–1551. [Google Scholar] [CrossRef]
- Romero, I.L.; McCormick, A.; McEwen, K.A.; Park, S.; Karrison, T.; Yamada, S.D.; Pannain, S.; Lengyel, E. Relationship of type II diabetes and metformin use to ovarian cancer progression, survival, and chemosensitivity. Obstet. Gynecol. 2012, 119, 61–67. [Google Scholar] [CrossRef]
- Kumar, S.; Meuter, A.; Thapa, P.; Langstraat, C.; Giri, S.; Chien, J.; Rattan, R.; Cliby, W.; Shridhar, V. Metformin intake is associated with better survival in ovarian cancer: A case-control study. Cancer 2013, 119, 555–562. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Zheng, Z.J.; Shi, R.; Su, Q.; Jiang, Q.; Kip, K.E. Metformin for liver cancer prevention in patients with type 2 diabetes: A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2012, 97, 2347–2353. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.J.; Zheng, Y.X.; Zhou, P.C.; Xiao, Y.N.; Tan, H.Z. Metformin use improves survival of diabetic liver cancer patients: Systematic review and meta-analysis. Oncotarget 2016, 7, 66202–66211. [Google Scholar] [CrossRef]
- Bailey, C.J. Metformin: Historical overview. Diabetologia 2017, 60, 1566–1576. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, C. Studies in the metabolism changes induced by administration of guanidine bases: I. Influence of injected guanidine hydrochloride upon blood sugar content. J. Biol. Chem. 1918, 33, 253–265. [Google Scholar] [CrossRef]
- Werner, E.A.; Bell, J. CCXIV.—The preparation of methylguanidine, and of ββ-dimethylguanidine by the interaction of dicyanodiamide, and methylammonium and dimethylammonium chlorides respectively. J. Chem. Soc. Trans. 1922, 121, 1790–1794. [Google Scholar] [CrossRef]
- Alušík, Š.; Paluch, Z. Metformin: The past, presence, and future. Minerva Med. 2015, 106, 233–238. [Google Scholar] [PubMed]
- King, P.; Peacock, I.; Donnelly, R. The UK prospective diabetes study (UKPDS): Clinical and therapeutic implications for type 2 diabetes. Br. J. Clin. Pharmacol. 1999, 48, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Rojas, L.B.; Gomes, M.B. Metformin: An old but still the best treatment for type 2 diabetes. Diabetol. Metab. Syndr. 2013, 5, 6. [Google Scholar] [CrossRef]
- Agius, L.; Ford, B.E.; Chachra, S.S. The Metformin Mechanism on Gluconeogenesis and AMPK Activation: The Metabolite Perspective. Int. J. Mol. Sci. 2020, 21, 3240. [Google Scholar] [CrossRef]
- Jones, V.C.; Dietze, E.C.; Jovanovic-Talisman, T.; McCune, J.S.; Seewaldt, V.L. Metformin and Chemoprevention: Potential for Heart-Healthy Targeting of Biologically Aggressive Breast Cancer. Front. Public Health 2020, 8, 509714. [Google Scholar] [CrossRef] [PubMed]
- Nasri, H.; Rafieian-Kopaei, M. Metformin: Current knowledge. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2014, 19, 658. [Google Scholar]
- Sliwinska, A.; Drzewoski, J. Molecular action of metformin in hepatocytes: An updated insight. Curr. Diabetes Rev. 2015, 11, 175–181. [Google Scholar] [CrossRef]
- Ruderman, N.B.; Carling, D.; Prentki, M.; Cacicedo, J.M. AMPK, insulin resistance, and the metabolic syndrome. J. Clin. Investig. 2013, 123, 2764–2772. [Google Scholar] [CrossRef] [PubMed]
- Natali, A.; Ferrannini, E. Effects of metformin and thiazolidinediones on suppression of hepatic glucose production and stimulation of glucose uptake in type 2 diabetes: A systematic review. Diabetologia 2006, 49, 434–441. [Google Scholar] [CrossRef]
- Petersen, M.C.; Vatner, D.F.; Shulman, G.I. Regulation of hepatic glucose metabolism in health and disease. Nat. Rev. Endocrinol. 2017, 13, 572–587. [Google Scholar] [CrossRef]
- Stumvoll, M.; Nurjhan, N.; Perriello, G.; Dailey, G.; Gerich, J.E. Metabolic effects of metformin in non-insulin-dependent diabetes mellitus. N. Engl. J. Med. 1995, 333, 550–554. [Google Scholar] [CrossRef]
- Holman, R.R.; Paul, S.K.; Bethel, M.A.; Matthews, D.R.; Neil, H.A.W. 10-year follow-up of intensive glucose control in type 2 diabetes. N. Engl. J. Med. 2008, 359, 1577–1589. [Google Scholar] [CrossRef]
- Hong, J.; Zhang, Y.; Lai, S.; Lv, A.; Su, Q.; Dong, Y.; Zhou, Z.; Tang, W.; Zhao, J.; Cui, L.; et al. Effects of metformin versus glipizide on cardiovascular outcomes in patients with type 2 diabetes and coronary artery disease. Diabetes Care 2013, 36, 1304–1311. [Google Scholar] [CrossRef]
- Zhao, M.; Li, X.W.; Chen, Z.; Hao, F.; Tao, S.X.; Yu, H.Y.; Cheng, R.; Liu, H. Neuro-Protective Role of Metformin in Patients with Acute Stroke and Type 2 Diabetes Mellitus via AMPK/Mammalian Target of Rapamycin (mTOR) Signaling Pathway and Oxidative Stress. Med. Sci. Monit. 2019, 25, 2186–2194. [Google Scholar] [CrossRef]
- Zhou, J.; Massey, S.; Story, D.; Li, L. Metformin: An Old Drug with New Applications. Int. J. Mol. Sci. 2018, 19, 2863. [Google Scholar] [CrossRef] [PubMed]
- Adekola, K.U.; Dalva Aydemir, S.; Ma, S.; Zhou, Z.; Rosen, S.T.; Shanmugam, M. Investigating and targeting chronic lymphocytic leukemia metabolism with the human immunodeficiency virus protease inhibitor ritonavir and metformin. Leuk. Lymphoma 2015, 56, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Shin, N.R.; Lee, J.C.; Lee, H.Y.; Kim, M.S.; Whon, T.W.; Lee, M.S.; Bae, J.W. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut 2014, 63, 727–735. [Google Scholar] [CrossRef] [PubMed]
- McCreight, L.J.; Bailey, C.J.; Pearson, E.R. Metformin and the gastrointestinal tract. Diabetologia 2016, 59, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Minamii, T.; Nogami, M.; Ogawa, W. Mechanisms of metformin action: In and out of the gut. J. Diabetes Investig. 2018, 9, 701–703. [Google Scholar] [CrossRef] [PubMed]
- Maida, A.; Lamont, B.; Cao, X.; Drucker, D. Metformin regulates the incretin receptor axis via a pathway dependent on peroxisome proliferator-activated receptor-α in mice. Diabetologia 2011, 54, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Coll, A.P.; Chen, M.; Taskar, P.; Rimmington, D.; Patel, S.; Tadross, J.A.; Cimino, I.; Yang, M.; Welsh, P.; Virtue, S. GDF15 mediates the effects of metformin on body weight and energy balance. Nature 2020, 578, 444–448. [Google Scholar] [CrossRef]
- Zhou, G.; Myers, R.; Li, Y.; Chen, Y.; Shen, X.; Fenyk-Melody, J.; Wu, M.; Ventre, J.; Doebber, T.; Fujii, N. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Investig. 2001, 108, 1167–1174. [Google Scholar] [CrossRef]
- Andrzejewski, S.; Siegel, P.M.; St-Pierre, J. Metabolic Profiles Associated With Metformin Efficacy in Cancer. Front. Endocrinol. 2018, 9, 372. [Google Scholar] [CrossRef]
- El-Mir, M.-Y.; Nogueira, V.; Fontaine, E.; Avéret, N.; Rigoulet, M.; Leverve, X. Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J. Biol. Chem. 2000, 275, 223–228. [Google Scholar] [CrossRef]
- Vander Heiden, M.G. Targeting cancer metabolism: A therapeutic window opens. Nat. Rev. Drug Discov. 2011, 10, 671–684. [Google Scholar] [CrossRef] [PubMed]
- Shaw, R.J.; Lamia, K.A.; Vasquez, D.; Koo, S.-H.; Bardeesy, N.; DePinho, R.A.; Montminy, M.; Cantley, L.C. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science 2005, 310, 1642–1646. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Tian, X.; Zhang, B.; Li, M.; Wang, Y.; Yang, C.; Wu, J.; Wei, X.; Qu, Q.; Yu, Y.; et al. Low-dose metformin targets the lysosomal AMPK pathway through PEN2. Nature 2022, 603, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.S.; Li, M.; Ma, T.; Zong, Y.; Cui, J.; Feng, J.W.; Wu, Y.Q.; Lin, S.Y.; Lin, S.C. Metformin Activates AMPK through the Lysosomal Pathway. Cell Metab. 2016, 24, 521–522. [Google Scholar] [CrossRef] [PubMed]
- Ben Sahra, I.; Regazzetti, C.; Robert, G.; Laurent, K.; Le Marchand-Brustel, Y.; Auberger, P.; Tanti, J.F.; Giorgetti-Peraldi, S.; Bost, F. Metformin, independent of AMPK, induces mTOR inhibition and cell-cycle arrest through REDD1. Cancer Res. 2011, 71, 4366–4372. [Google Scholar] [CrossRef]
- Foretz, M.; Hébrard, S.; Leclerc, J.; Zarrinpashneh, E.; Soty, M.; Mithieux, G.; Sakamoto, K.; Andreelli, F.; Viollet, B. Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. J. Clin. Investig. 2010, 120, 2355–2369. [Google Scholar] [CrossRef]
- Inoki, K.; Kim, J.; Guan, K.-L. AMPK and mTOR in cellular energy homeostasis and drug targets. Annu. Rev. Pharmacol. Toxicol. 2012, 52, 381–400. [Google Scholar] [CrossRef]
- Xu, J.; Ji, J.; Yan, X.-H. Cross-talk between AMPK and mTOR in regulating energy balance. Crit. Rev. Food Sci. Nutr. 2012, 52, 373–381. [Google Scholar] [CrossRef]
- Zachariah Tom, R.; Garcia-Roves, P.M.; Sjögren, R.J.; Jiang, L.Q.; Holmström, M.H.; Deshmukh, A.S.; Vieira, E.; Chibalin, A.V.; Björnholm, M.; Zierath, J.R. Effects of AMPK activation on insulin sensitivity and metabolism in leptin-deficient ob/ob mice. Diabetes 2014, 63, 1560–1571. [Google Scholar] [CrossRef]
- O’neill, H.M. AMPK and exercise: Glucose uptake and insulin sensitivity. Diabetes Metab. J. 2013, 37, 1–21. [Google Scholar] [CrossRef]
- Fullerton, M.D.; Galic, S.; Marcinko, K.; Sikkema, S.; Pulinilkunnil, T.; Chen, Z.-P.; O’neill, H.M.; Ford, R.J.; Palanivel, R.; O’brien, M. Single phosphorylation sites in Acc1 and Acc2 regulate lipid homeostasis and the insulin-sensitizing effects of metformin. Nat. Med. 2013, 19, 1649–1654. [Google Scholar] [CrossRef]
- Ford, R.J.; Fullerton, M.D.; Pinkosky, S.L.; Day, E.A.; Scott, J.W.; Oakhill, J.S.; Bujak, A.L.; Smith, B.K.; Crane, J.D.; Blümer, R.M.; et al. Metformin and salicylate synergistically activate liver AMPK, inhibit lipogenesis and improve insulin sensitivity. Biochem. J. 2015, 468, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Boudaba, N.; Marion, A.; Huet, C.; Pierre, R.; Viollet, B.; Foretz, M. AMPK Re-Activation Suppresses Hepatic Steatosis but its Downregulation Does Not Promote Fatty Liver Development. EBioMedicine 2018, 28, 194–209. [Google Scholar] [CrossRef] [PubMed]
- Viollet, B.; Guigas, B.; Garcia, N.S.; Leclerc, J.; Foretz, M.; Andreelli, F. Cellular and molecular mechanisms of metformin: An overview. Clin. Sci. 2012, 122, 253–270. [Google Scholar] [CrossRef] [PubMed]
- Foretz, M.; Guigas, B.; Bertrand, L.; Pollak, M.; Viollet, B. Metformin: From mechanisms of action to therapies. Cell Metab. 2014, 20, 953–966. [Google Scholar] [CrossRef]
- Drahota, Z.; Palenickova, E.; Endlicher, R.; Milerova, M.; Brejchova, J.; Vosahlikova, M.; Svoboda, P.; Kazdova, L.; Kalous, M.; Cervinkova, Z.; et al. Biguanides inhibit complex I, II and IV of rat liver mitochondria and modify their functional properties. Physiol. Res. 2014, 63, 1–11. [Google Scholar] [CrossRef]
- Miller, R.A.; Chu, Q.; Xie, J.; Foretz, M.; Viollet, B.; Birnbaum, M.J. Biguanides suppress hepatic glucagon signalling by decreasing production of cyclic AMP. Nature 2013, 494, 256–260. [Google Scholar] [CrossRef]
- Ouyang, J.; Parakhia, R.A.; Ochs, R.S. Metformin activates AMP kinase through inhibition of AMP deaminase. J. Biol. Chem. 2011, 286, 1–11. [Google Scholar] [CrossRef]
- Madiraju, A.K.; Erion, D.M.; Rahimi, Y.; Zhang, X.M.; Braddock, D.T.; Albright, R.A.; Prigaro, B.J.; Wood, J.L.; Bhanot, S.; MacDonald, M.J.; et al. Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature 2014, 510, 542–546. [Google Scholar] [CrossRef]
- Marini, C.; Salani, B.; Massollo, M.; Amaro, A.; Esposito, A.I.; Orengo, A.M.; Capitanio, S.; Emionite, L.; Riondato, M.; Bottoni, G.; et al. Direct inhibition of hexokinase activity by metformin at least partially impairs glucose metabolism and tumor growth in experimental breast cancer. Cell Cycle 2013, 12, 3490–3499. [Google Scholar] [CrossRef]
- Salani, B.; Marini, C.; Rio, A.D.; Ravera, S.; Massollo, M.; Orengo, A.M.; Amaro, A.; Passalacqua, M.; Maffioli, S.; Pfeffer, U. Metformin impairs glucose consumption and survival in Calu-1 cells by direct inhibition of hexokinase-II. Sci. Rep. 2013, 3, 2070. [Google Scholar] [CrossRef] [PubMed]
- Picone, P.; Vilasi, S.; Librizzi, F.; Contardi, M.; Nuzzo, D.; Caruana, L.; Baldassano, S.; Amato, A.; Mulè, F.; San Biagio, P.L.; et al. Biological and biophysics aspects of metformin-induced effects: Cortex mitochondrial dysfunction and promotion of toxic amyloid pre-fibrillar aggregates. Aging 2016, 8, 1718–1734. [Google Scholar] [CrossRef]
- Shoshan-Barmatz, V.; Anand, U.; Nahon-Crystal, E.; Di Carlo, M.; Shteinfer-Kuzmine, A. Adverse Effects of Metformin From Diabetes to COVID-19, Cancer, Neurodegenerative Diseases, and Aging: Is VDAC1 a Common Target? Front. Physiol. 2021, 12, 730048. [Google Scholar] [CrossRef] [PubMed]
- LaMoia, T.E.; Butrico, G.M.; Kalpage, H.A.; Goedeke, L.; Hubbard, B.T.; Vatner, D.F.; Gaspar, R.C.; Zhang, X.-M.; Cline, G.W.; Nakahara, K. Metformin, phenformin, and galegine inhibit complex IV activity and reduce glycerol-derived gluconeogenesis. Proc. Natl. Acad. Sci. USA 2022, 119, e2122287119. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Q.; Chen, G. Combined therapeutic effect and molecular mechanisms of metformin and cisplatin in human lung cancer xenografts in nude mice. J. Cancer Res. Ther. 2015, 11, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.B.; Kim, Y.; Kim, D.; Cho, E.Y.; Han, J.; Kim, H.K.; Shim, Y.M.; Kim, D.H. Metformin and tenovin-6 synergistically induces apoptosis through LKB1-independent SIRT1 down-regulation in non-small cell lung cancer cells. J. Cell. Mol. Med. 2019, 23, 2872–2889. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, X.; Li, T.; Yi, E.; Li, Y. Metformin synergistic pemetrexed suppresses non-small-cell lung cancer cell proliferation and invasion in vitro. Cancer Med. 2017, 6, 1965–1975. [Google Scholar] [CrossRef]
- Saraei, P.; Asadi, I.; Kakar, M.A.; Moradi-Kor, N. The beneficial effects of metformin on cancer prevention and therapy: A comprehensive review of recent advances. Cancer Manag. Res. 2019, 11, 3295. [Google Scholar] [CrossRef]
- Mallik, R.; Chowdhury, T.A. Metformin in cancer. Diabetes Res. Clin. Pr. 2018, 143, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Heckman-Stoddard, B.M.; Gandini, S.; Puntoni, M.; Dunn, B.K.; DeCensi, A.; Szabo, E. Repurposing old drugs to chemoprevention: The case of metformin. Semin. Oncol. 2016, 43, 123–133. [Google Scholar] [CrossRef]
- Mishra, A.K.; Dingli, D. Metformin inhibits IL-6 signaling by decreasing IL-6R expression on multiple myeloma cells. Leukemia 2019, 33, 2695–2709. [Google Scholar] [CrossRef] [PubMed]
- Sekino, N.; Kano, M.; Matsumoto, Y.; Sakata, H.; Akutsu, Y.; Hanari, N.; Murakami, K.; Toyozumi, T.; Takahashi, M.; Otsuka, R.; et al. Antitumor effects of metformin are a result of inhibiting nuclear factor kappa B nuclear translocation in esophageal squamous cell carcinoma. Cancer Sci. 2018, 109, 1066–1074. [Google Scholar] [CrossRef]
- Menon, S.; Dibble, C.C.; Talbott, G.; Hoxhaj, G.; Valvezan, A.J.; Takahashi, H.; Cantley, L.C.; Manning, B.D. Spatial control of the TSC complex integrates insulin and nutrient regulation of mTORC1 at the lysosome. Cell 2014, 156, 771–785. [Google Scholar] [CrossRef] [PubMed]
- Bar-Peled, L.; Schweitzer, L.D.; Zoncu, R.; Sabatini, D.M. Ragulator is a GEF for the rag GTPases that signal amino acid levels to mTORC1. Cell 2012, 150, 1196–1208. [Google Scholar] [CrossRef]
- Bar-Peled, L.; Chantranupong, L.; Cherniack, A.D.; Chen, W.W.; Ottina, K.A.; Grabiner, B.C.; Spear, E.D.; Carter, S.L.; Meyerson, M.; Sabatini, D.M. A Tumor suppressor complex with GAP activity for the Rag GTPases that signal amino acid sufficiency to mTORC1. Science 2013, 340, 1100–1106. [Google Scholar] [CrossRef] [PubMed]
- Bridgeman, S.C.; Ellison, G.C.; Melton, P.E.; Newsholme, P.; Mamotte, C.D.S. Epigenetic effects of metformin: From molecular mechanisms to clinical implications. Diabetes Obes. Metab. 2018, 20, 1553–1562. [Google Scholar] [CrossRef]
- Salminen, A.; Kauppinen, A.; Kaarniranta, K. AMPK/Snf1 signaling regulates histone acetylation: Impact on gene expression and epigenetic functions. Cell. Signal. 2016, 28, 887–895. [Google Scholar] [CrossRef]
- Vancura, A.; Vancurova, I. Metformin induces protein acetylation in cancer cells. Oncotarget 2017, 8, 39939–39940. [Google Scholar] [CrossRef]
- Hu, T.; Chung, Y.M.; Guan, M.; Ma, M.; Ma, J.; Berek, J.S.; Hu, M.C. Reprogramming ovarian and breast cancer cells into non-cancerous cells by low-dose metformin or SN-38 through FOXO3 activation. Sci. Rep. 2014, 4, 5810. [Google Scholar] [CrossRef]
- Pernicova, I.; Korbonits, M. Metformin—Mode of action and clinical implications for diabetes and cancer. Nat. Rev. Endocrinol. 2014, 10, 143–156. [Google Scholar] [CrossRef]
- Yu, X.; Mao, W.; Zhai, Y.; Tong, C.; Liu, M.; Ma, L.; Yu, X.; Li, S. Anti-tumor activity of metformin: From metabolic and epigenetic perspectives. Oncotarget 2017, 8, 5619. [Google Scholar] [CrossRef] [PubMed]
- Safe, S.; Nair, V.; Karki, K. Metformin-induced anticancer activities: Recent insights. Biol. Chem. 2018, 399, 321–335. [Google Scholar] [CrossRef] [PubMed]
- Nair, V.; Sreevalsan, S.; Basha, R.; Abdelrahim, M.; Abudayyeh, A.; Rodrigues Hoffman, A.; Safe, S. Mechanism of metformin-dependent inhibition of mammalian target of rapamycin (mTOR) and Ras activity in pancreatic cancer: Role of specificity protein (Sp) transcription factors. J. Biol. Chem. 2014, 289, 27692–27701. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Hong, L.; Luo, C.; Li, Z.; Zhu, Y.; Huang, T.; Zhang, Y.; Yuan, H.; Hu, Y.; Wen, T.; et al. Metformin inhibits estrogen-dependent endometrial cancer cell growth by activating the AMPK-FOXO1 signal pathway. Cancer Sci. 2016, 107, 1806–1817. [Google Scholar] [CrossRef] [PubMed]
- de Queiroz, E.A.; Akamine, E.H.; de Carvalho, M.H.; Sampaio, S.C.; Fortes, Z.B. Metformin reduces the Walker-256 tumor development in obese-MSG rats via AMPK and FOXO3a. Life Sci. 2015, 121, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Tao, C.; Huang, X.; He, H.; Shi, H.; Zhang, Q.; Wu, H. Metformin induces apoptosis of human hepatocellular carcinoma HepG2 cells by activating an AMPK/p53/miR-23a/FOXA1 pathway. OncoTargets Ther. 2016, 9, 2845–2853. [Google Scholar]
- Celestini, V.; Tezil, T.; Russo, L.; Fasano, C.; Sanese, P.; Forte, G.; Peserico, A.; Lepore Signorile, M.; Longo, G.; De Rasmo, D. Uncoupling FoxO3A mitochondrial and nuclear functions in cancer cells undergoing metabolic stress and chemotherapy. Cell Death Dis. 2018, 9, 231. [Google Scholar] [CrossRef]
- Grossi, V.; Fasano, C.; Celestini, V.; Lepore Signorile, M.; Sanese, P.; Simone, C. Chasing the Foxo3: Insights into its new mitochondrial lair in colorectal cancer landscape. Cancers 2019, 11, 414. [Google Scholar] [CrossRef]
- Piccolo, S.; Dupont, S.; Cordenonsi, M. The biology of YAP/TAZ: Hippo signaling and beyond. Physiol. Rev. 2014, 94, 1287–1312. [Google Scholar] [CrossRef]
- Yu, F.X.; Zhao, B.; Guan, K.L. Hippo Pathway in Organ Size Control, Tissue Homeostasis, and Cancer. Cell 2015, 163, 811–828. [Google Scholar] [CrossRef]
- Hajimoradi Javarsiani, M.; Sajedianfard, J.; Haghjooy Javanmard, S. The effects of metformin on the hippo pathway in the proliferation of melanoma cancer cells: A preclinical study. Arch. Physiol. Biochem. 2022, 128, 1150–1155. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, J.; Chen, H.; Wang, R.; Li, P.; Miao, Y.; Liu, P. Metformin suppresses proliferation and invasion of drug-resistant breast cancer cells by activation of the Hippo pathway. J. Cell. Mol. Med. 2020, 24, 5786–5796. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.; Guo, J.; Wu, Y.; Chen, W.; Du, J.; Yang, L.; Wang, X.; Gong, K.; Dai, J.; Miao, S.; et al. Metformin-repressed miR-381-YAP-snail axis activity disrupts NSCLC growth and metastasis. J. Exp. Clin. Cancer Res. 2020, 39, 6. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zheng, Q.; Li, Y.; Wang, G.; Gao, S.; Zhang, X.; Yan, X.; Zhang, X.; Xie, J.; Wang, Y.; et al. Metformin targets a YAP1-TEAD4 complex via AMPKα to regulate CCNE1/2 in bladder cancer cells. J. Exp. Clin. Cancer Res. 2019, 38, 376. [Google Scholar] [CrossRef]
- Yuan, X.; Wei, W.; Bao, Q.; Chen, H.; Jin, P.; Jiang, W. Metformin inhibits glioma cells stemness and epithelial-mesenchymal transition via regulating YAP activity. Biomed. Pharm. 2018, 102, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Tang, B.; Wang, C.; Sun, D.; Zhang, R.; Luo, N.; Han, Z.; Liang, R.; Gao, Z.; Wang, L. Metformin mediates resensitivity to 5-fluorouracil in hepatocellular carcinoma via the suppression of YAP. Oncotarget 2016, 7, 46230–46241. [Google Scholar] [CrossRef]
- Zhang, H.-H.; Guo, X.-L. Combinational strategies of metformin and chemotherapy in cancers. Cancer Chemother. Pharmacol. 2016, 78, 13–26. [Google Scholar] [CrossRef]
- Roncolato, F.; Lindemann, K.; Willson, M.L.; Martyn, J.; Mileshkin, L. PI3K/AKT/mTOR inhibitors for advanced or recurrent endometrial cancer. Cochrane Database Syst. Rev. 2019, 10, Cd012160. [Google Scholar] [CrossRef]
- Liu, Y.L.; Yu, J.M.; Song, X.R.; Wang, X.W.; Xing, L.G.; Gao, B.B. Regulation of the chemokine receptor CXCR4 and metastasis by hypoxia-inducible factor in non small cell lung cancer cell lines. Cancer Biol. Ther. 2006, 5, 1320–1326. [Google Scholar] [CrossRef]
- Helbig, G.; Christopherson, K.W., 2nd; Bhat-Nakshatri, P.; Kumar, S.; Kishimoto, H.; Miller, K.D.; Broxmeyer, H.E.; Nakshatri, H. NF-kappaB promotes breast cancer cell migration and metastasis by inducing the expression of the chemokine receptor CXCR4. J. Biol. Chem. 2003, 278, 21631–21638. [Google Scholar] [CrossRef]
- Dallaglio, K.; Bruno, A.; Cantelmo, A.R.; Esposito, A.I.; Ruggiero, L.; Orecchioni, S.; Calleri, A.; Bertolini, F.; Pfeffer, U.; Noonan, D.M. Paradoxic effects of metformin on endothelial cells and angiogenesis. Carcinogenesis 2014, 35, 1055–1066. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Liu, C.; He, Z.; Cai, Y.; Chen, J. Metformin inhibits cervical cancer cell proliferation by modulating PI3K/Akt-induced major histocompatibility complex class I-related chain A gene expression. J. Exp. Clin. Cancer Res. 2020, 39, 127. [Google Scholar] [CrossRef] [PubMed]
- Nelson, L.E.; Valentine, R.J.; Cacicedo, J.M.; Gauthier, M.-S.; Ido, Y.; Ruderman, N.B. A novel inverse relationship between metformin-triggered AMPK-SIRT1 signaling and p53 protein abundance in high glucose-exposed HepG2 cells. Am. J. Physiol.-Cell Physiol. 2012, 303, C4–C13. [Google Scholar] [CrossRef] [PubMed]
- Kauppinen, A.; Suuronen, T.; Ojala, J.; Kaarniranta, K.; Salminen, A. Antagonistic crosstalk between NF-κB and SIRT1 in the regulation of inflammation and metabolic disorders. Cell. Signal. 2013, 25, 1939–1948. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.-L.; Liu, L. Effect of metformin on stem cells: Molecular mechanism and clinical prospect. World J. Stem Cells 2020, 12, 1455. [Google Scholar] [CrossRef] [PubMed]
- Pierotti, M.; Berrino, F.; Gariboldi, M.; Melani, C.; Mogavero, A.; Negri, T.; Pasanisi, P.; Pilotti, S. Targeting metabolism for cancer treatment and prevention: Metformin, an old drug with multi-faceted effects. Oncogene 2013, 32, 1475–1487. [Google Scholar] [CrossRef] [PubMed]
- Pollak, M. Insulin and insulin-like growth factor signalling in neoplasia. Nat. Rev. Cancer 2008, 8, 915–928. [Google Scholar] [CrossRef]
- Li, L.; Wang, L.; Li, J.; Fan, Z.; Yang, L.; Zhang, Z.; Zhang, C.; Yue, D.; Qin, G.; Zhang, T. Metformin-induced reduction of CD39 and CD73 blocks myeloid-derived suppressor cell activity in patients with ovarian cancer. Cancer Res. 2018, 78, 1779–1791. [Google Scholar] [CrossRef]
- Wheaton, W.W.; Weinberg, S.E.; Hamanaka, R.B.; Soberanes, S.; Sullivan, L.B.; Anso, E.; Glasauer, A.; Dufour, E.; Mutlu, G.M.; Budigner, G.S. Metformin inhibits mitochondrial complex I of cancer cells to reduce tumorigenesis. elife 2014, 3, e02242. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, J.; Yi, G.; Deng, M.; Liu, H.; Liang, M.; Shi, B.; Fu, X.; Chen, Y.; Chen, L.; et al. Metformin suppresses hypoxia-induced stabilization of HIF-1α through reprogramming of oxygen metabolism in hepatocellular carcinoma. Oncotarget 2016, 7, 873–884. [Google Scholar] [CrossRef]
- Ye, J.; Chen, K.; Qi, L.; Li, R.; Tang, H.; Zhou, C.; Zhai, W. Metformin suppresses hypoxia-induced migration via the HIF-1α/VEGF pathway in gallbladder cancer in vitro and in vivo. Oncol. Rep. 2018, 40, 3501–3510. [Google Scholar] [CrossRef] [PubMed]
- Baumann, T.; Dunkel, A.; Schmid, C.; Schmitt, S.; Hiltensperger, M.; Lohr, K.; Laketa, V.; Donakonda, S.; Ahting, U.; Lorenz-Depiereux, B. Regulatory myeloid cells paralyze T cells through cell–cell transfer of the metabolite methylglyoxal. Nat. Immunol. 2020, 21, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Uehara, T.; Eikawa, S.; Nishida, M.; Kunisada, Y.; Yoshida, A.; Fujiwara, T.; Kunisada, T.; Ozaki, T.; Udono, H. Metformin induces CD11b+-cell-mediated growth inhibition of an osteosarcoma: Implications for metabolic reprogramming of myeloid cells and anti-tumor effects. Int. Immunol. 2019, 31, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Yin, K.; Tang, X.; Tian, J.; Zhang, Y.; Ma, J.; Xu, H.; Xu, Q.; Wang, S. Metformin inhibits the function of granulocytic myeloid-derived suppressor cells in tumor-bearing mice. Biomed. Pharmacother. 2019, 120, 109458. [Google Scholar] [CrossRef]
- Subarsky, P.; Hill, R.P. The hypoxic tumour microenvironment and metastatic progression. Clin. Exp. Metastasis 2003, 20, 237–250. [Google Scholar] [CrossRef]
- Mounier, C.; Bouraoui, L.; Rassart, E. Lipogenesis in cancer progression. Int. J. Oncol. 2014, 45, 485–492. [Google Scholar] [CrossRef]
- Ha, J.; Daniel, S.; Broyles, S.S.; Kim, K.-H. Critical phosphorylation sites for acetyl-CoA carboxylase activity. J. Biol. Chem. 1994, 269, 22162–22168. [Google Scholar] [CrossRef]
- Li, Y.; Xu, S.; Mihaylova, M.M.; Zheng, B.; Hou, X.; Jiang, B.; Park, O.; Luo, Z.; Lefai, E.; Shyy, J.Y.; et al. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 2011, 13, 376–388. [Google Scholar] [CrossRef]
- Tomic, T.; Botton, T.; Cerezo, M.; Robert, G.; Luciano, F.; Puissant, A.; Gounon, P.; Allegra, M.; Bertolotto, C.; Bereder, J.M.; et al. Metformin inhibits melanoma development through autophagy and apoptosis mechanisms. Cell Death Dis. 2011, 2, e199. [Google Scholar] [CrossRef]
- Jiang, S.; Lu, Q. A new contribution for an old drug: Prospect of metformin in colorectal oncotherapy. J. Cancer Res. Ther. 2021, 17, 1608–1617. [Google Scholar] [CrossRef]
- Kalender, A.; Selvaraj, A.; Kim, S.Y.; Gulati, P.; Brûlé, S.; Viollet, B.; Kemp, B.E.; Bardeesy, N.; Dennis, P.; Schlager, J.J. Metformin, independent of AMPK, inhibits mTORC1 in a rag GTPase-dependent manner. Cell Metab. 2010, 11, 390–401. [Google Scholar] [CrossRef]
- Feng, Y.; Ke, C.; Tang, Q.; Dong, H.; Zheng, X.; Lin, W.; Ke, J.; Huang, J.; Yeung, S.C.; Zhang, H. Metformin promotes autophagy and apoptosis in esophageal squamous cell carcinoma by downregulating Stat3 signaling. Cell Death Dis. 2014, 5, e1088. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Furuno, A.; Sakurai, J.; Sakamoto, A.; Park, H.R.; Shin-Ya, K.; Tsuruo, T.; Tomida, A. Chemical genomics identifies the unfolded protein response as a target for selective cancer cell killing during glucose deprivation. Cancer Res. 2009, 69, 4225–4234. [Google Scholar] [CrossRef] [PubMed]
- Melnik, S.; Dvornikov, D.; Müller-Decker, K.; Depner, S.; Stannek, P.; Meister, M.; Warth, A.; Thomas, M.; Muley, T.; Risch, A.; et al. Cancer cell specific inhibition of Wnt/β-catenin signaling by forced intracellular acidification. Cell Discov. 2018, 4, 37. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.H.; Song, I.H.; Sung, E.G.; Lee, T.J.; Kim, J.Y. Metformin-induced apoptosis facilitates degradation of the cellular caspase 8 (FLICE)-like inhibitory protein through a caspase-dependent pathway in human renal cell carcinoma A498 cells. Oncol. Lett. 2018, 16, 2030–2038. [Google Scholar] [CrossRef]
- Zhao, Y.; Luo, Q.; Mo, J.; Li, J.; Ye, D.; Ao, Z.; Chen, L.; Liu, J. Metformin in combination with JS-K inhibits growth of renal cell carcinoma cells via reactive oxygen species activation and inducing DNA breaks. J. Cancer 2020, 11, 3701–3712. [Google Scholar] [CrossRef] [PubMed]
- Vial, G.; Detaille, D.; Guigas, B. Role of mitochondria in the mechanism (s) of action of metformin. Front. Endocrinol. 2019, 10, 294. [Google Scholar] [CrossRef] [PubMed]
- Saladini, S.; Aventaggiato, M.; Barreca, F.; Morgante, E.; Sansone, L.; Russo, M.A.; Tafani, M. Metformin Impairs Glutamine Metabolism and Autophagy in Tumour Cells. Cells 2019, 8, 49. [Google Scholar] [CrossRef] [PubMed]
- Shen, P.; Reineke, L.C.; Knutsen, E.; Chen, M.; Pichler, M.; Ling, H.; Calin, G.A. Metformin blocks MYC protein synthesis in colorectal cancer via mTOR-4EBP-eIF4E and MNK1-eIF4G-eIF4E signaling. Mol. Oncol. 2018, 12, 1856–1870. [Google Scholar] [CrossRef]
- Lei, Y.; Yi, Y.; Liu, Y.; Liu, X.; Keller, E.T.; Qian, C.N.; Zhang, J.; Lu, Y. Metformin targets multiple signaling pathways in cancer. Chin. J. Cancer 2017, 36, 17. [Google Scholar] [CrossRef]
- Blandino, G.; Valerio, M.; Cioce, M.; Mori, F.; Casadei, L.; Pulito, C.; Sacconi, A.; Biagioni, F.; Cortese, G.; Galanti, S.; et al. Metformin elicits anticancer effects through the sequential modulation of DICER and c-MYC. Nat. Commun. 2012, 3, 865. [Google Scholar] [CrossRef] [PubMed]
- Pulito, C.; Mori, F.; Sacconi, A.; Goeman, F.; Ferraiuolo, M.; Pasanisi, P.; Campagnoli, C.; Berrino, F.; Fanciulli, M.; Ford, R.J.; et al. Metformin-induced ablation of microRNA 21-5p releases Sestrin-1 and CAB39L antitumoral activities. Cell Discov. 2017, 3, 17022. [Google Scholar] [CrossRef] [PubMed]
- Cabello, P.; Pineda, B.; Tormo, E.; Lluch, A.; Eroles, P. The Antitumor Effect of Metformin Is Mediated by miR-26a in Breast Cancer. Int. J. Mol. Sci. 2016, 17, 1298. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Iwama, H.; Yamashita, T.; Kobayashi, K.; Fujihara, S.; Fujimori, T.; Kamada, H.; Kobara, H.; Masaki, T. The anti-diabetic drug metformin inhibits pancreatic cancer cell proliferation in vitro and in vivo: Study of the microRNAs associated with the antitumor effect of metformin. Oncol. Rep. 2016, 35, 1582–1592. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, L.; Zhang, G.; Wang, Y.; Chen, H.; Kong, R.; Pan, S.; Sun, B. Crucial microRNAs and genes in metformin’s anti-pancreatic cancer effect explored by microRNA-mRNA integrated analysis. Investig. New Drugs 2018, 36, 20–27. [Google Scholar] [CrossRef]
- Zhang, J.; Shen, C.; Wang, L.; Ma, Q.; Xia, P.; Qi, M.; Yang, M.; Han, B. Metformin inhibits epithelial-mesenchymal transition in prostate cancer cells: Involvement of the tumor suppressor miR30a and its target gene SOX4. Biochem. Biophys. Res. Commun. 2014, 452, 746–752. [Google Scholar] [CrossRef]
- Kalogirou, C.; Schäfer, D.; Krebs, M.; Kurz, F.; Schneider, A.; Riedmiller, H.; Kneitz, B.; Vergho, D. Metformin-Derived Growth Inhibition in Renal Cell Carcinoma Depends on miR-21-Mediated PTEN Expression. Urol. Int. 2016, 96, 106–115. [Google Scholar] [CrossRef]
- Fujimori, T.; Kato, K.; Fujihara, S.; Iwama, H.; Yamashita, T.; Kobayashi, K.; Kamada, H.; Morishita, A.; Kobara, H.; Mori, H.; et al. Antitumor effect of metformin on cholangiocarcinoma: In vitro and in vivo studies. Oncol. Rep. 2015, 34, 2987–2996. [Google Scholar] [CrossRef]
- Jiang, X.; Ma, N.; Wang, D.; Li, F.; He, R.; Li, D.; Zhao, R.; Zhou, Q.; Wang, Y.; Zhang, F.; et al. Metformin inhibits tumor growth by regulating multiple miRNAs in human cholangiocarcinoma. Oncotarget 2015, 6, 3178–3194. [Google Scholar] [CrossRef]
- Chiyo, T.; Kato, K.; Iwama, H.; Fujihara, S.; Fujita, K.; Tadokoro, T.; Ohura, K.; Samukawa, E.; Yamana, Y.; Kobayashi, N.; et al. Therapeutic potential of the antidiabetic drug metformin in small bowel adenocarcinoma. Int. J. Oncol. 2017, 50, 2145–2153. [Google Scholar] [CrossRef]
- Wang, F.; Xu, J.; Liu, H.; Liu, Z.; Xia, F. Metformin induces apoptosis by microRNA-26a-mediated downregulation of myeloid cell leukaemia-1 in human oral cancer cells. Mol. Med. Rep. 2016, 13, 4671–4676. [Google Scholar] [CrossRef]
- Najafi, M.; Cheki, M.; Rezapoor, S.; Geraily, G.; Motevaseli, E.; Carnovale, C.; Clementi, E.; Shirazi, A. Metformin: Prevention of genomic instability and cancer: A review. Mutat. Res. Genet. Toxicol. Environ. Mutagen 2018, 827, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.G.; Hien, T.T.; Han, E.H.; Hwang, Y.P.; Choi, J.H.; Kang, K.W.; Kwon, K.I.; Kim, B.H.; Kim, S.K.; Song, G.Y.; et al. Metformin inhibits P-glycoprotein expression via the NF-κB pathway and CRE transcriptional activity through AMPK activation. Br. J. Pharm. 2011, 162, 1096–1108. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Warburg, O. Uber den stoffwechsel der karzinomezellen. Biochem Z 1924, 152, 309–344. [Google Scholar]
- Zu, X.L.; Guppy, M. Cancer metabolism: Facts, fantasy, and fiction. Biochem. Biophys. Res. Commun. 2004, 313, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Loubiere, C.; Clavel, S.; Gilleron, J.; Harisseh, R.; Fauconnier, J.; Ben-Sahra, I.; Kaminski, L.; Laurent, K.; Herkenne, S.; Lacas-Gervais, S.; et al. The energy disruptor metformin targets mitochondrial integrity via modification of calcium flux in cancer cells. Sci. Rep. 2017, 7, 5040. [Google Scholar] [CrossRef] [PubMed]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Di Magno, L.; Di Pastena, F.; Bordone, R.; Coni, S.; Canettieri, G. The Mechanism of Action of Biguanides: New Answers to a Complex Question. Cancers 2022, 14, 3220. [Google Scholar]
- Huang, N.L.; Chiang, S.H.; Hsueh, C.H.; Liang, Y.J.; Chen, Y.J.; Lai, L.P. Metformin inhibits TNF-alpha-induced IkappaB kinase phosphorylation, IkappaB-alpha degradation and IL-6 production in endothelial cells through PI3K-dependent AMPK phosphorylation. Int. J. Cardiol. 2009, 134, 169–175. [Google Scholar] [CrossRef]
- Kelly, B.; Tannahill, G.M.; Murphy, M.P.; O’Neill, L.A. Metformin Inhibits the Production of Reactive Oxygen Species from NADH:Ubiquinone Oxidoreductase to Limit Induction of Interleukin-1β (IL-1β) and Boosts Interleukin-10 (IL-10) in Lipopolysaccharide (LPS)-activated Macrophages. J. Biol. Chem. 2015, 290, 20348–20359. [Google Scholar] [CrossRef] [PubMed]
- Pearce, E.L.; Walsh, M.C.; Cejas, P.J.; Harms, G.M.; Shen, H.; Wang, L.S.; Jones, R.G.; Choi, Y. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature 2009, 460, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Eikawa, S.; Nishida, M.; Mizukami, S.; Yamazaki, C.; Nakayama, E.; Udono, H. Immune-mediated antitumor effect by type 2 diabetes drug, metformin. Proc. Natl. Acad. Sci. USA 2015, 112, 1809–1814. [Google Scholar] [CrossRef] [PubMed]
- Nishino, M.; Ramaiya, N.H.; Hatabu, H.; Hodi, F.S. Monitoring immune-checkpoint blockade: Response evaluation and biomarker development. Nat. Rev. Clin. Oncol. 2017, 14, 655–668. [Google Scholar] [CrossRef]
- Chen, Y.C.; Li, H.; Wang, J. Mechanisms of metformin inhibiting cancer invasion and migration. Am. J. Transl. Res. 2020, 12, 4885–4901. [Google Scholar]
- Hsu, S.K.; Cheng, K.C.; Mgbeahuruike, M.O.; Lin, Y.H.; Wu, C.Y.; Wang, H.D.; Yen, C.H.; Chiu, C.C.; Sheu, S.J. New Insight into the Effects of Metformin on Diabetic Retinopathy, Aging and Cancer: Nonapoptotic Cell Death, Immunosuppression, and Effects beyond the AMPK Pathway. Int. J. Mol. Sci. 2021, 22, 9453. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, Y.H.; Park, E.H.; Lee, S.J.; Kim, H.; Kim, A.; Lee, S.B.; Shim, S.; Jang, H.; Myung, J.K.; et al. Effects of metformin and phenformin on apoptosis and epithelial-mesenchymal transition in chemoresistant rectal cancer. Cancer Sci. 2019, 110, 2834–2845. [Google Scholar] [CrossRef]
- Huang, D.; He, X.; Zou, J.; Guo, P.; Jiang, S.; Lv, N.; Alekseyev, Y.; Luo, L.; Luo, Z. Negative regulation of Bmi-1 by AMPK and implication in cancer progression. Oncotarget 2016, 7, 6188–6200. [Google Scholar] [CrossRef]
- Kang, S.; Kim, B.R.; Kang, M.H.; Kim, D.Y.; Lee, D.H.; Oh, S.C.; Min, B.W.; Um, J.W. Anti-metastatic effect of metformin via repression of interleukin 6-induced epithelial-mesenchymal transition in human colon cancer cells. PLoS ONE 2018, 13, e0205449. [Google Scholar] [CrossRef]
- Huang, H. Matrix Metalloproteinase-9 (MMP-9) as a Cancer Biomarker and MMP-9 Biosensors: Recent Advances. Sensors 2018, 18, 3249. [Google Scholar] [CrossRef]
- Teng, Y.; Ross, J.L.; Cowell, J.K. The involvement of JAK-STAT3 in cell motility, invasion, and metastasis. Jakstat 2014, 3, e28086. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, H.A.; Iliopoulos, D.; Struhl, K. Metformin inhibits the inflammatory response associated with cellular transformation and cancer stem cell growth. Proc. Natl. Acad. Sci. USA 2013, 110, 972–977. [Google Scholar] [CrossRef] [PubMed]
- Jun, K.H.; Lee, J.E.; Kim, S.H.; Jung, J.H.; Choi, H.J.; Kim, Y.I.; Chin, H.M.; Yang, S.H. Clinicopathological significance of N-cadherin and VEGF in advanced gastric cancer brain metastasis and the effects of metformin in preclinical models. Oncol. Rep. 2015, 34, 2047–2053. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.C.; Li, G.Y.; Li, P.P.; Sun, X.; Li, W.M.; Li, Y.; Lu, S.Y.; Liu, P.J. Suppression of hypoxia-induced excessive angiogenesis by metformin via elevating tumor blood perfusion. Oncotarget 2017, 8, 73892–73904. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.C.; Li, G.Y.; Wang, B.; Han, S.X.; Sun, X.; Jiang, Y.N.; Shen, Y.W.; Zhou, C.; Feng, J.; Lu, S.Y.; et al. Metformin inhibits metastatic breast cancer progression and improves chemosensitivity by inducing vessel normalization via PDGF-B downregulation. J. Exp. Clin. Cancer Res. 2019, 38, 235. [Google Scholar] [CrossRef] [PubMed]
- Quesada, A.E.; Nguyen, N.D.; Rios, A.; Brown, R.E. Morphoproteomics identifies constitutive activation of the mTORC2/Akt and NF-κB pathways and expressions of IGF-1R, Sirt1, COX-2, and FASN in peripheral T-cell lymphomas: Pathogenetic implications and therapeutic options. Int. J. Clin. Exp. Pathol. 2014, 7, 8732–8739. [Google Scholar] [PubMed]
- Koo, Y.X.; Tan, D.S.; Tan, I.B.; Tai, D.W.; Ha, T.; Ong, W.S.; Quek, R.; Tao, M.; Lim, S.T. Effect of concomitant statin, metformin, or aspirin on rituximab treatment for diffuse large B-cell lymphoma. Leuk. Lymphoma 2011, 52, 1509–1516. [Google Scholar]
- Chang, S.H.; Luo, S.; O’Brian, K.K.; Thomas, T.S.; Colditz, G.A.; Carlsson, N.P.; Carson, K.R. Association between metformin use and progression of monoclonal gammopathy of undetermined significance to multiple myeloma in US veterans with diabetes mellitus: A population-based retrospective cohort study. Lancet Haematol. 2015, 2, e30–e36. [Google Scholar] [CrossRef]
- Boursi, B.; Mamtani, R.; Yang, Y.X.; Weiss, B.M. Impact of metformin on the progression of MGUS to multiple myeloma. Leuk. Lymphoma 2017, 58, 1265–1267. [Google Scholar] [CrossRef]
- Zi, F.M.; He, J.S.; Li, Y.; Wu, C.; Yang, L.; Yang, Y.; Wang, L.J.; He, D.H.; Zhao, Y.; Wu, W.J.; et al. Metformin displays anti-myeloma activity and synergistic effect with dexamethasone in in vitro and in vivo xenograft models. Cancer Lett. 2015, 356 Pt B, 443–453. [Google Scholar] [CrossRef]
- Wu, W.; Merriman, K.; Nabaah, A.; Seval, N.; Seval, D.; Lin, H.; Wang, M.; Qazilbash, M.H.; Baladandayuthapani, V.; Berry, D.; et al. The association of diabetes and anti-diabetic medications with clinical outcomes in multiple myeloma. Br. J. Cancer 2014, 111, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.Y.; Xiao, D.; Wang, L.; Dong, L.H.; Yan, Z.X.; Shen, Z.X.; Chen, S.J.; Chen, Y.; Zhao, W.L. Therapeutic metformin/AMPK activation blocked lymphoma cell growth via inhibition of mTOR pathway and induction of autophagy. Cell Death Dis. 2012, 3, e275. [Google Scholar] [CrossRef] [PubMed]
- Jagannathan, S.; Abdel-Malek, M.A.; Malek, E.; Vad, N.; Latif, T.; Anderson, K.C.; Driscoll, J.J. Pharmacologic screens reveal metformin that suppresses GRP78-dependent autophagy to enhance the anti-myeloma effect of bortezomib. Leukemia 2015, 29, 2184–2191. [Google Scholar] [CrossRef] [PubMed]
- You, R.; Wang, B.; Chen, P.; Zheng, X.; Hou, D.; Wang, X.; Zhang, B.; Chen, L.; Li, D.; Lin, X.; et al. Metformin sensitizes AML cells to chemotherapy through blocking mitochondrial transfer from stromal cells to AML cells. Cancer Lett. 2022, 532, 215582. [Google Scholar] [CrossRef]
- Zhou, F.J.; Zeng, C.X.; Kuang, W.; Cheng, C.; Liu, H.C.; Yan, X.Y.; Chen, X.P.; Zhou, G.; Cao, S. Metformin exerts a synergistic effect with venetoclax by downregulating Mcl-1 protein in acute myeloid leukemia. J. Cancer 2021, 12, 6727–6739. [Google Scholar] [CrossRef]
- Gámez, B.; Morris, E.V.; Olechnowicz, S.W.Z.; Webb, S.; Edwards, J.R.; Sowman, A.; Turner, C.J.; Edwards, C.M. The antidiabetic drug metformin acts on the bone microenvironment to promote myeloma cell adhesion to preosteoblasts and increase myeloma tumour burden in vivo. Transl. Oncol. 2022, 15, 101301. [Google Scholar] [CrossRef]
- Arnason, T.G.; MacDonald-Dickinson, V.; Gaunt, M.C.; Davies, G.F.; Lobanova, L.; Trost, B.; Gillespie, Z.E.; Waldner, M.; Baldwin, P.; Borrowman, D.; et al. Activation of the Anaphase Promoting Complex Reverses Multiple Drug Resistant Cancer in a Canine Model of Multiple Drug Resistant Lymphoma. Cancers 2022, 14, 4215. [Google Scholar] [CrossRef]
- Yuan, F.; Cheng, C.; Xiao, F.; Liu, H.; Cao, S.; Zhou, G. Inhibition of mTORC1/P70S6K pathway by Metformin synergistically sensitizes Acute Myeloid Leukemia to Ara-C. Life Sci. 2020, 243, 117276. [Google Scholar] [CrossRef]
- Singh, A.R.; Gu, J.J.; Zhang, Q.; Torka, P.; Sundaram, S.; Mavis, C.; Hernandez-Ilizaliturri, F.J. Metformin sensitizes therapeutic agents and improves outcome in pre-clinical and clinical diffuse large B-cell lymphoma. Cancer Metab. 2020, 8, 10. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, W.; Yan, Z.; Zhao, W.; Mi, J.; Li, J.; Yan, H. Metformin induces autophagy and G0/G1 phase cell cycle arrest in myeloma by targeting the AMPK/mTORC1 and mTORC2 pathways. J. Exp. Clin. Cancer Res. 2018, 37, 63. [Google Scholar] [CrossRef] [PubMed]
- Dalva-Aydemir, S.; Bajpai, R.; Martinez, M.; Adekola, K.U.; Kandela, I.; Wei, C.; Singhal, S.; Koblinski, J.E.; Raje, N.S.; Rosen, S.T.; et al. Targeting the metabolic plasticity of multiple myeloma with FDA-approved ritonavir and metformin. Clin. Cancer Res. 2015, 21, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Rosilio, C.; Lounnas, N.; Nebout, M.; Imbert, V.; Hagenbeek, T.; Spits, H.; Asnafi, V.; Pontier-Bres, R.; Reverso, J.; Michiels, J.F.; et al. The metabolic perturbators metformin, phenformin and AICAR interfere with the growth and survival of murine PTEN-deficient T cell lymphomas and human T-ALL/T-LL cancer cells. Cancer Lett. 2013, 336, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Glamoclija, U.; Mahmutovic, L.; Bilajac, E.; Soljic, V.; Vukojevic, K.; Suljagic, M. Metformin and Thymoquinone Synergistically Inhibit Proliferation of Imatinib-Resistant Human Leukemic Cells. Front. Pharm. 2022, 13, 867133. [Google Scholar] [CrossRef]
- Liu, L.; Patnana, P.K.; Xie, X.; Frank, D.; Nimmagadda, S.C.; Rosemann, A.; Liebmann, M.; Klotz, L.; Opalka, B.; Khandanpour, C. High Metabolic Dependence on Oxidative Phosphorylation Drives Sensitivity to Metformin Treatment in MLL/AF9 Acute Myeloid Leukemia. Cancers 2022, 14, 486. [Google Scholar] [CrossRef] [PubMed]
- Saber, M.M.; Al-Mahallawi, A.M.; Stork, B. Metformin dampens cisplatin cytotoxicity on leukemia cells after incorporation into cubosomal nanoformulation. Biomed. Pharm. 2021, 143, 112140. [Google Scholar] [CrossRef] [PubMed]
- Valiulienė, G.; Vitkevičienė, A.; Skliutė, G.; Borutinskaitė, V.; Navakauskienė, R. Pharmaceutical Drug Metformin and MCL1 Inhibitor S63845 Exhibit Anticancer Activity in Myeloid Leukemia Cells via Redox Remodeling. Molecules 2021, 26, 2303. [Google Scholar] [CrossRef] [PubMed]
- Na, Y.J.; Yu, E.S.; Kim, D.S.; Lee, D.H.; Oh, S.C.; Choi, C.W. Metformin enhances the cytotoxic effect of nilotinib and overcomes nilotinib resistance in chronic myeloid leukemia cells. Korean J. Intern. Med. 2021, 36 (Suppl. S1), S196–S206. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Itoh, M.; Tohda, S. Metformin suppresses the growth of leukemia cells partly through downregulation of AXL receptor tyrosine kinase. Leuk. Res. 2020, 94, 106383. [Google Scholar] [CrossRef]
- Zhou, X.; Kuang, Y.; Liang, S.; Wang, L. Metformin inhibits cell proliferation in SKM-1 cells via AMPK-mediated cell cycle arrest. J. Pharm. Sci. 2019, 141, 146–152. [Google Scholar] [CrossRef]
- Vitkevičienė, A.; Janulis, V.; Žučenka, A.; Borutinskaitė, V.; Kaupinis, A.; Valius, M.; Griškevičius, L.; Navakauskienė, R. Oxidative phosphorylation inhibition induces anticancerous changes in therapy-resistant-acute myeloid leukemia patient cells. Mol. Carcinog. 2019, 58, 2008–2016. [Google Scholar] [CrossRef]
- Chen, H.L.; Ma, P.; Chen, Y.L.; Sun, L.; Xing, Y.; Wang, F.; Wang, F.; Cao, W.J.; Huang, Y.M.; Zhang, R.H. Effect of Metformin on Proliferation Capacity, Apoptosis and Glycolysis in K562 Cells. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2019, 27, 1387–1394. [Google Scholar]
- Renner, K.; Seilbeck, A.; Kauer, N.; Ugele, I.; Siska, P.J.; Brummer, C.; Bruss, C.; Decking, S.M.; Fante, M.; Schmidt, A.; et al. Combined Metabolic Targeting With Metformin and the NSAIDs Diflunisal and Diclofenac Induces Apoptosis in Acute Myeloid Leukemia Cells. Front. Pharm. 2018, 9, 1258. [Google Scholar] [CrossRef] [PubMed]
- Asik, A.; Kayabasi, C.; Ozmen Yelken, B.; Yılmaz Susluer, S.; Dogan Sigva, Z.O.; Balcı Okcanoglu, T.; Saydam, G.; Biray Avci, C.; Gunduz, C. Antileukemic effect of paclitaxel in combination with metformin in HL-60 cell line. Gene 2018, 647, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Ravera, S.; Cossu, V.; Tappino, B.; Nicchia, E.; Dufour, C.; Cavani, S.; Sciutto, A.; Bolognesi, C.; Columbaro, M.; Degan, P.; et al. Concentration-dependent metabolic effects of metformin in healthy and Fanconi anemia lymphoblast cells. J. Cell. Physiol. 2018, 233, 1736–1751. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Kong, P.; Wang, J.; Xu, Y.; Gao, C.; Guo, G. Effects of metformin on proliferation and apoptosis of human megakaryoblastic Dami and MEG-01 cells. J. Pharm. Sci. 2017, 135, 14–21. [Google Scholar] [CrossRef]
- Yi, Y.; Gao, L.; Wu, M.; Ao, J.; Zhang, C.; Wang, X.; Lin, M.; Bergholz, J.; Zhang, Y.; Xiao, Z.J. Metformin Sensitizes Leukemia Cells to Vincristine via Activation of AMP-activated Protein Kinase. J. Cancer 2017, 8, 2636–2642. [Google Scholar] [CrossRef]
- Sabnis, H.S.; Bradley, H.L.; Tripathi, S.; Yu, W.M.; Tse, W.; Qu, C.K.; Bunting, K.D. Synergistic cell death in FLT3-ITD positive acute myeloid leukemia by combined treatment with metformin and 6-benzylthioinosine. Leuk. Res. 2016, 50, 132–140. [Google Scholar] [CrossRef]
- Velez, J.; Pan, R.; Lee, J.T.; Enciso, L.; Suarez, M.; Duque, J.E.; Jaramillo, D.; Lopez, C.; Morales, L.; Bornmann, W.; et al. Biguanides sensitize leukemia cells to ABT-737-induced apoptosis by inhibiting mitochondrial electron transport. Oncotarget 2016, 7, 51435–51449. [Google Scholar] [CrossRef]
- Voltan, R.; Rimondi, E.; Melloni, E.; Gilli, P.; Bertolasi, V.; Casciano, F.; Rigolin, G.M.; Zauli, G.; Secchiero, P. Metformin combined with sodium dichloroacetate promotes B leukemic cell death by suppressing anti-apoptotic protein Mcl-1. Oncotarget 2016, 7, 18965–18977. [Google Scholar] [CrossRef]
- Wang, F.; Liu, Z.; Zeng, J.; Zhu, H.; Li, J.; Cheng, X.; Jiang, T.; Zhang, L.; Zhang, C.; Chen, T.; et al. Metformin synergistically sensitizes FLT3-ITD-positive acute myeloid leukemia to sorafenib by promoting mTOR-mediated apoptosis and autophagy. Leuk. Res. 2015, 39, 1421–1427. [Google Scholar] [CrossRef]
- Shi, R.; Lin, J.; Gong, Y.; Yan, T.; Shi, F.; Yang, X.; Liu, X.; Naren, D. The antileukemia effect of metformin in the Philadelphia chromosome-positive leukemia cell line and patient primary leukemia cell. Anti-Cancer Drugs 2015, 26, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Lirio, A.; Pérez-Yarza, G.; Fernández-Suárez, M.R.; Alonso-Tejerina, E.; Boyano, M.D.; Asumendi, A. Metformin Induces Cell Cycle Arrest and Apoptosis in Drug-Resistant Leukemia Cells. Leuk. Res. Treat. 2015, 2015, 516460. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Liu, L.L.; Mao, X.; Zhang, D.H. Effects of metformin on FOXM1 expression and on the biological behavior of acute leukemia cell lines. Mol. Med. Rep. 2014, 10, 3193–3198. [Google Scholar] [CrossRef] [PubMed]
- Leclerc, G.M.; Leclerc, G.J.; Kuznetsov, J.N.; DeSalvo, J.; Barredo, J.C. Metformin induces apoptosis through AMPK-dependent inhibition of UPR signaling in ALL lymphoblasts. PLoS ONE 2013, 8, e74420. [Google Scholar] [CrossRef]
- Huai, L.; Wang, C.; Zhang, C.; Li, Q.; Chen, Y.; Jia, Y.; Li, Y.; Xing, H.; Tian, Z.; Rao, Q.; et al. Metformin induces differentiation in acute promyelocytic leukemia by activating the MEK/ERK signaling pathway. Biochem. Biophys. Res. Commun. 2012, 422, 398–404. [Google Scholar] [CrossRef]
- Liu, M.K.; Cheng, L.L.; Yi, H.M.; He, Y.; Li, X.; Fu, D.; Dai, Y.T.; Fang, H.; Cheng, S.; Xu, P.P.; et al. Enhanced lipid metabolism confers the immunosuppressive tumor microenvironment in CD5-positive non-MYC/BCL2 double expressor lymphoma. Front. Oncol. 2022, 12, 885011. [Google Scholar] [CrossRef]
- Bagaloni, I.; Visani, A.; Biagiotti, S.; Ruzzo, A.; Navari, M.; Etebari, M.; Mundo, L.; Granai, M.; Lazzi, S.; Isidori, A.; et al. Metabolic Switch and Cytotoxic Effect of Metformin on Burkitt Lymphoma. Front. Oncol. 2021, 11, 661102. [Google Scholar] [CrossRef]
- Cristofoletti, C.; Bresin, A.; Picozza, M.; Picchio, M.C.; Monzo, F.; Helmer Citterich, M.; Passarelli, F.; Frezzolini, A.; Scala, E.; Monopoli, A.; et al. Blood and skin-derived Sezary cells: Differences in proliferation-index, activation of PI3K/AKT/mTORC1 pathway and its prognostic relevance. Leukemia 2019, 33, 1231–1242. [Google Scholar] [CrossRef]
- Chukkapalli, V.; Gordon, L.I.; Venugopal, P.; Borgia, J.A.; Karmali, R. Metabolic changes associated with metformin potentiates Bcl-2 inhibitor, Venetoclax, and CDK9 inhibitor, BAY1143572 and reduces viability of lymphoma cells. Oncotarget 2018, 9, 21166–21181. [Google Scholar] [CrossRef]
- Granato, M.; Gilardini Montani, M.S.; Romeo, M.A.; Santarelli, R.; Gonnella, R.; D’Orazi, G.; Faggioni, A.; Cirone, M. Metformin triggers apoptosis in PEL cells and alters bortezomib-induced Unfolded Protein Response increasing its cytotoxicity and inhibiting KSHV lytic cycle activation. Cell. Signal. 2017, 40, 239–247. [Google Scholar] [CrossRef]
- Schlesser, C.; Meul, T.; Stathopoulos, G.; Meiners, S. Metformin Induces Resistance of Cancer Cells to the Proteasome Inhibitor Bortezomib. Biomolecules 2022, 12, 756. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Chen, S.; Xiang, W.; Xiao, M.; Xiao, H. The mechanism of treatment of multiple myeloma with metformin by way of metabolism. Arch. Med. Sci. 2021, 17, 1056–1063. [Google Scholar] [CrossRef]
- Kocemba-Pilarczyk, K.A.; Trojan, S.; Ostrowska, B.; Lasota, M.; Dudzik, P.; Kusior, D.; Kot, M. Influence of metformin on HIF-1 pathway in multiple myeloma. Pharm. Rep. 2020, 72, 1407–1417. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhao, Y.; Zhang, E.; Yan, H.; Lv, N.; Cai, Z. The synergistic effect of PFK15 with metformin exerts anti-myeloma activity via PFKFB3. Biochem. Biophys. Res. Commun. 2019, 515, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.Y.; Wang, C.; Liu, Y.F.; Wang, W.Q.; Hao, Q.Q.; Ma, J.; Sun, L.; Sun, H. Metformin Induces Apoptosis of Human Multiple Myeloma Cell U266 through the Mitochondrial Apoptotic Pathway. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2018, 26, 489–492. [Google Scholar] [PubMed]
- Zhao, Y.; Zhang, E.; Lv, N.; Ma, L.; Yao, S.; Yan, M.; Zi, F.; Deng, G.; Liu, X.; He, J.; et al. Metformin and FTY720 Synergistically Induce Apoptosis in Multiple Myeloma Cells. Cell. Physiol. Biochem. 2018, 48, 785–800. [Google Scholar] [CrossRef]
- Lu, B.; Gui, S.Y.; Zhou, H.H.; Liu, Z.L. Effect of Metformin on Proliferation of Multiple Myeloma Cells. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2017, 25, 1097–1100. [Google Scholar]
- Short, N.J.; Rytting, M.E.; Cortes, J.E. Acute myeloid leukaemia. Lancet 2018, 392, 593–606. [Google Scholar] [CrossRef]
- Green, A.S.; Chapuis, N.; Maciel, T.T.; Willems, L.; Lambert, M.; Arnoult, C.; Boyer, O.; Bardet, V.; Park, S.; Foretz, M.; et al. The LKB1/AMPK signaling pathway has tumor suppressor activity in acute myeloid leukemia through the repression of mTOR-dependent oncogenic mRNA translation. Blood 2010, 116, 4262–4273. [Google Scholar] [CrossRef]
- Martelli, A.M.; Chiarini, F.; Evangelisti, C.; Ognibene, A.; Bressanin, D.; Billi, A.M.; Manzoli, L.; Cappellini, A.; McCubrey, J.A. Targeting the liver kinase B1/AMP-activated protein kinase pathway as a therapeutic strategy for hematological malignancies. Expert. Opin. Ther. Targets 2012, 16, 729–742. [Google Scholar] [CrossRef] [PubMed]
- Rosilio, C.; Ben-Sahra, I.; Bost, F.; Peyron, J.F. Metformin: A metabolic disruptor and anti-diabetic drug to target human leukemia. Cancer Lett. 2014, 346, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Huai, L.; Wang, C.C.; Zhang, C.P.; Li, Q.H.; Chen, Y.R.; Jia, Y.J.; Wang, M.; Wang, J.X. Effect of metformin on acute promyelocytic leukemia cell line NB4 and its mechanism. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2012, 20, 1322–1326. [Google Scholar] [PubMed]
- Ceacareanu, A.C.; Nimako, G.K.; Wintrob, Z.A. Missing the benefit of metformin in acute myeloid leukemia: A problem of contrast? J. Res. Pharm. Pract. 2017, 6, 145. [Google Scholar] [CrossRef]
- Groffen, J.; Heisterkamp, N.; Stam, K. Oncogene activation by chromosomal translocation in chronic myelocytic leukemia. Cold Spring Harb. Symp. Quant. Biol. 1986, 51 Pt 2, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Deininger, M.W.; Goldman, J.M.; Melo, J.V. The molecular biology of chronic myeloid leukemia. BLOOD-NEW YORK 2000, 96, 3343–3356. [Google Scholar]
- Vakana, E.; Altman, J.K.; Glaser, H.; Donato, N.J.; Platanias, L.C. Antileukemic effects of AMPK activators on BCR-ABL-expressing cells. Blood 2011, 118, 6399–6402. [Google Scholar] [CrossRef] [PubMed]
- Pokorny, R.; Stenehjem, D.D.; Gilreath, J.A. Impact of metformin on tyrosine kinase inhibitor response in chronic myeloid leukemia. J. Oncol. Pharm. Pr. 2022, 28, 916–923. [Google Scholar] [CrossRef] [PubMed]
- Paganin, M.; Ferrando, A. Molecular pathogenesis and targeted therapies for NOTCH1-induced T-cell acute lymphoblastic leukemia. Blood Rev. 2011, 25, 83–90. [Google Scholar] [CrossRef]
- Aifantis, I.; Raetz, E.; Buonamici, S. Molecular pathogenesis of T-cell leukaemia and lymphoma. Nat. Rev. Immunol. 2008, 8, 380–390. [Google Scholar] [CrossRef]
- Grimaldi, C.; Chiarini, F.; Tabellini, G.; Ricci, F.; Tazzari, P.L.; Battistelli, M.; Falcieri, E.; Bortul, R.; Melchionda, F.; Iacobucci, I.; et al. AMP-dependent kinase/mammalian target of rapamycin complex 1 signaling in T-cell acute lymphoblastic leukemia: Therapeutic implications. Leukemia 2012, 26, 91–100. [Google Scholar] [CrossRef]
- Pan, J.; Chen, C.; Jin, Y.; Fuentes-Mattei, E.; Velazquez-Tores, G.; Benito, J.M.; Konopleva, M.; Andreeff, M.; Lee, M.H.; Yeung, S.C. Differential impact of structurally different anti-diabetic drugs on proliferation and chemosensitivity of acute lymphoblastic leukemia cells. Cell Cycle 2012, 11, 2314–2326. [Google Scholar] [CrossRef] [PubMed]
- Mavrakis, K.J.; Wolfe, A.L.; Oricchio, E.; Palomero, T.; de Keersmaecker, K.; McJunkin, K.; Zuber, J.; James, T.; Khan, A.A.; Leslie, C.S.; et al. Genome-wide RNA-mediated interference screen identifies miR-19 targets in Notch-induced T-cell acute lymphoblastic leukaemia. Nat. Cell. Biol. 2010, 12, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Mayr, M.; Liem, D.; Zhang, J.; Li, X.; Avliyakulov, N.K.; Yang, J.I.; Young, G.; Vondriska, T.M.; Ladroue, C.; Madhu, B.; et al. Proteomic and metabolomic analysis of cardioprotection: Interplay between protein kinase C epsilon and delta in regulating glucose metabolism of murine hearts. J. Mol. Cell. Cardiol. 2009, 46, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Niture, S.K.; Gnatt, A.; Jaiswal, A.K. Retraction: Oncogene PKCε controls INrf2-Nrf2 interaction in normal and cancer cells through phosphorylation of INrf2. J. Cell Sci. 2017, 130, 815. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.M.; Gao, W.W.; Chan, C.P.; Siu, Y.T.; Wong, C.M.; Kok, K.H.; Ching, Y.P.; Takemori, H.; Jin, D.Y. LKB1 tumor suppressor and salt-inducible kinases negatively regulate human T-cell leukemia virus type 1 transcription. Retrovirology 2013, 10, 40. [Google Scholar] [CrossRef] [PubMed]
- Huntly, B.J.; Gilliland, D.G. Leukaemia stem cells and the evolution of cancer-stem-cell research. Nat. Rev. Cancer 2005, 5, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Iliopoulos, D.; Hirsch, H.A.; Struhl, K. Metformin decreases the dose of chemotherapy for prolonging tumor remission in mouse xenografts involving multiple cancer cell types. Cancer Res. 2011, 71, 3196–3201. [Google Scholar] [CrossRef]
- Ramos-Peñafiel, C.; Olarte-Carrillo, I.; Cerón-Maldonado, R.; Rozen-Fuller, E.; Kassack-Ipiña, J.J.; Meléndez-Mier, G.; Collazo-Jaloma, J.; Martínez-Tovar, A. Effect of metformin on the survival of patients with ALL who express high levels of the ABCB1 drug resistance gene. J. Transl. Med. 2018, 16, 245. [Google Scholar] [CrossRef] [PubMed]
- Trucco, M.; Barredo, J.C.; Goldberg, J.; Leclerc, G.M.; Hale, G.A.; Gill, J.; Setty, B.; Smith, T.; Lush, R.; Lee, J.K.; et al. A phase I window, dose escalating and safety trial of metformin in combination with induction chemotherapy in relapsed refractory acute lymphoblastic leukemia: Metformin with induction chemotherapy of vincristine, dexamethasone, PEG-asparaginase, and doxorubicin. Pediatr. Blood Cancer 2018, 65, e27224. [Google Scholar]
- Vu, K.; Busaidy, N.; Cabanillas, M.E.; Konopleva, M.; Faderl, S.; Thomas, D.A.; O’Brien, S.; Broglio, K.; Ensor, J.; Escalante, C.; et al. A randomized controlled trial of an intensive insulin regimen in patients with hyperglycemic acute lymphoblastic leukemia. Clin. Lymphoma Myeloma Leuk. 2012, 12, 355–362. [Google Scholar] [CrossRef]
- Ramos-Peñafiel, C.O.; Martínez-Murillo, C.; Santoyo-Sánchez, A.; Jiménez-Ponce, F.; Rozen-Fuller, E.; Collazo-Jaloma, J.; Olarte-Carrillo, I.; Martínez-Tovar, A. Effect of metformin addition to an acute lymphoblastic leukemia chemotherapy treatment. Rev. Médica Inst. Mex. Seguro Soc. 2014, 52, 270–275. [Google Scholar]
- Bostrom, B.; Uppal, P.; Chu, J.; Messinger, Y.; Gandrud, L.; McEvoy, R. Safety and efficacy of metformin for therapy-induced hyperglycemia in children with acute lymphoblastic leukemia. J. Pediatr. Hematol. Oncol. 2013, 35, 504–508. [Google Scholar] [CrossRef] [PubMed]
- Bruno, S.; Ledda, B.; Tenca, C.; Ravera, S.; Orengo, A.M.; Mazzarello, A.N.; Pesenti, E.; Casciaro, S.; Racchi, O.; Ghiotto, F.; et al. Metformin inhibits cell cycle progression of B-cell chronic lymphocytic leukemia cells. Oncotarget 2015, 6, 22624–22640. [Google Scholar] [CrossRef] [PubMed]
- Herishanu, Y.; Pérez-Galán, P.; Liu, D.; Biancotto, A.; Pittaluga, S.; Vire, B.; Gibellini, F.; Njuguna, N.; Lee, E.; Stennett, L.; et al. The lymph node microenvironment promotes B-cell receptor signaling, NF-kappaB activation, and tumor proliferation in chronic lymphocytic leukemia. Blood 2011, 117, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Yaktapour, N.; Übelhart, R.; Schüler, J.; Aumann, K.; Dierks, C.; Burger, M.; Pfeifer, D.; Jumaa, H.; Veelken, H.; Brummer, T.; et al. Insulin-like growth factor-1 receptor (IGF1R) as a novel target in chronic lymphocytic leukemia. Blood 2013, 122, 1621–1633. [Google Scholar] [CrossRef]
- Hofbauer, S.W.; Piñón, J.D.; Brachtl, G.; Haginger, L.; Wang, W.; Jöhrer, K.; Tinhofer, I.; Hartmann, T.N.; Greil, R. Modifying akt signaling in B-cell chronic lymphocytic leukemia cells. Cancer Res. 2010, 70, 7336–7344. [Google Scholar] [CrossRef]
- Zhuang, J.; Hawkins, S.F.; Glenn, M.A.; Lin, K.; Johnson, G.G.; Carter, A.; Cawley, J.C.; Pettitt, A.R. Akt is activated in chronic lymphocytic leukemia cells and delivers a pro-survival signal: The therapeutic potential of Akt inhibition. Haematologica 2010, 95, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Hazan-Halevy, I.; Harris, D.M.; Li, P.; Ferrajoli, A.; Faderl, S.; Keating, M.J.; Estrov, Z. STAT-3 activates NF-kappaB in chronic lymphocytic leukemia cells. Mol. Cancer Res. 2011, 9, 507–515. [Google Scholar] [CrossRef]
- Kesanakurti, D.; Chetty, C.; Rajasekhar Maddirela, D.; Gujrati, M.; Rao, J.S. Essential role of cooperative NF-κB and Stat3 recruitment to ICAM-1 intronic consensus elements in the regulation of radiation-induced invasion and migration in glioma. Oncogene 2013, 32, 5144–5155. [Google Scholar] [CrossRef]
- Del Gaizo Moore, V.; Brown, J.R.; Certo, M.; Love, T.M.; Novina, C.D.; Letai, A. Chronic lymphocytic leukemia requires BCL2 to sequester prodeath BIM, explaining sensitivity to BCL2 antagonist ABT-737. J. Clin. Investig. 2007, 117, 112–121. [Google Scholar] [CrossRef]
- van Delft, M.F.; Wei, A.H.; Mason, K.D.; Vandenberg, C.J.; Chen, L.; Czabotar, P.E.; Willis, S.N.; Scott, C.L.; Day, C.L.; Cory, S.; et al. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell 2006, 10, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, S.V. Evolving diagnostic criteria for multiple myeloma. Hematol. Am. Soc. Hematol. Educ. Program 2015, 2015, 272–278. [Google Scholar] [CrossRef] [PubMed]
- White-Al Habeeb, N.M.; Garcia, J.; Fleshner, N.; Bapat, B. Metformin Elicits Antitumor Effects and Downregulates the Histone Methyltransferase Multiple Myeloma SET Domain (MMSET) in Prostate Cancer Cells. Prostate 2016, 76, 1507–1518. [Google Scholar] [CrossRef] [PubMed]
- Birmann, B.M.; Neuhouser, M.L.; Rosner, B.; Albanes, D.; Buring, J.E.; Giles, G.G.; Lan, Q.; Lee, I.M.; Purdue, M.P.; Rothman, N.; et al. Prediagnosis biomarkers of insulin-like growth factor-1, insulin, and interleukin-6 dysregulation and multiple myeloma risk in the Multiple Myeloma Cohort Consortium. Blood 2012, 120, 4929–4937. [Google Scholar] [CrossRef] [PubMed]
- Verdura, S.; Cuyàs, E.; Martin-Castillo, B.; Menendez, J.A. Metformin as an archetype immuno-metabolic adjuvant for cancer immunotherapy. Oncoimmunology 2019, 8, e1633235. [Google Scholar] [CrossRef] [PubMed]
- Azab, A.K.; Hu, J.; Quang, P.; Azab, F.; Pitsillides, C.; Awwad, R.; Thompson, B.; Maiso, P.; Sun, J.D.; Hart, C.P.; et al. Hypoxia promotes dissemination of multiple myeloma through acquisition of epithelial to mesenchymal transition-like features. Blood 2012, 119, 5782–5794. [Google Scholar] [CrossRef]
- Kocemba, K.A.; van Andel, H.; de Haan-Kramer, A.; Mahtouk, K.; Versteeg, R.; Kersten, M.J.; Spaargaren, M.; Pals, S.T. The hypoxia target adrenomedullin is aberrantly expressed in multiple myeloma and promotes angiogenesis. Leukemia 2013, 27, 1729–1737. [Google Scholar] [CrossRef]
- Colla, S.; Storti, P.; Donofrio, G.; Todoerti, K.; Bolzoni, M.; Lazzaretti, M.; Abeltino, M.; Ippolito, L.; Neri, A.; Ribatti, D.; et al. Low bone marrow oxygen tension and hypoxia-inducible factor-1α overexpression characterize patients with multiple myeloma: Role on the transcriptional and proangiogenic profiles of CD138(+) cells. Leukemia 2010, 24, 1967–1970. [Google Scholar] [CrossRef] [PubMed]
- Storti, P.; Bolzoni, M.; Donofrio, G.; Airoldi, I.; Guasco, D.; Toscani, D.; Martella, E.; Lazzaretti, M.; Mancini, C.; Agnelli, L.; et al. Hypoxia-inducible factor (HIF)-1α suppression in myeloma cells blocks tumoral growth in vivo inhibiting angiogenesis and bone destruction. Leukemia 2013, 27, 1697–1706. [Google Scholar] [CrossRef] [PubMed]
- Tyszka-Czochara, M.; Bukowska-Strakova, K.; Kocemba-Pilarczyk, K.A.; Majka, M. Caffeic Acid Targets AMPK Signaling and Regulates Tricarboxylic Acid Cycle Anaplerosis while Metformin Downregulates HIF-1α-Induced Glycolytic Enzymes in Human Cervical Squamous Cell Carcinoma Lines. Nutrients 2018, 10, 841. [Google Scholar] [CrossRef]
- Guimarães, T.A.; Farias, L.C.; Santos, E.S.; de Carvalho Fraga, C.A.; Orsini, L.A.; de Freitas Teles, L.; Feltenberger, J.D.; de Jesus, S.F.; de Souza, M.G.; Santos, S.H.; et al. Metformin increases PDH and suppresses HIF-1α under hypoxic conditions and induces cell death in oral squamous cell carcinoma. Oncotarget 2016, 7, 55057–55068. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Li, L.; Hu, J.; Li, G.; Zhang, Y.; Dai, X.; De, Z.; Xu, F. Metformin Inhibits Multiple Myeloma Serum-induced Endothelial Cell Thrombosis by Down-Regulating miR-532. Ann. Vasc. Surg. 2022, 85, 347–357.e2. [Google Scholar] [CrossRef] [PubMed]
- Ragbourne, S.C.; Maghsoodi, N.; Streetly, M.; Crook, M.A. The Association between Metabolic Syndrome and Multiple Myeloma. Acta Haematol. 2021, 144, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Boursi, B.; Weiss, B.M.; Haynes, K.; Mamtani, R.; Yang, Y.X. Reappraisal of risk factors for monoclonal gammopathy of undetermined significance. Am. J. Hematol. 2016, 91, 581–584. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Naserian, M.; Ramazani, E.; Iranshahi, M.; Tayarani-Najaran, Z. The Role of SAPK/JNK Pathway in the Synergistic Effects of Metformin and Dacarbazine on Apoptosis in Raji and Ramos Lymphoma Cells. Curr. Mol. Pharm. 2018, 11, 336–342. [Google Scholar] [CrossRef]
- Anderson, J.R.; Armitage, J.O.; Weisenburger, D.D. Epidemiology of the non-Hodgkin’s lymphomas: Distributions of the major subtypes differ by geographic locations. Non-Hodgkin’s Lymphoma Classification Project. Ann. Oncol. 1998, 9, 717–720. [Google Scholar] [CrossRef]
- Wright, G.W.; Huang, D.W.; Phelan, J.D.; Coulibaly, Z.A.; Roulland, S.; Young, R.M.; Wang, J.Q.; Schmitz, R.; Morin, R.D.; Tang, J.; et al. A Probabilistic Classification Tool for Genetic Subtypes of Diffuse Large B Cell Lymphoma with Therapeutic Implications. Cancer Cell 2020, 37, 551–568.e14. [Google Scholar] [CrossRef]
- Schmitz, R.; Wright, G.W.; Huang, D.W.; Johnson, C.A.; Phelan, J.D.; Wang, J.Q.; Roulland, S.; Kasbekar, M.; Young, R.M.; Shaffer, A.L.; et al. Genetics and Pathogenesis of Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2018, 378, 1396–1407. [Google Scholar] [CrossRef]
- Chapuy, B.; Stewart, C.; Dunford, A.J.; Kim, J.; Kamburov, A.; Redd, R.A.; Lawrence, M.S.; Roemer, M.G.M.; Li, A.J.; Ziepert, M.; et al. Molecular subtypes of diffuse large B cell lymphoma are associated with distinct pathogenic mechanisms and outcomes. Nat. Med. 2018, 24, 679–690. [Google Scholar] [CrossRef]
- Jiang, X.N.; Zhang, Y.; Wang, W.G.; Sheng, D.; Zhou, X.Y.; Li, X.Q. Alteration of Cholesterol Metabolism by Metformin Is Associated With Improved Outcome in Type II Diabetic Patients With Diffuse Large B-Cell Lymphoma. Front. Oncol. 2021, 11, 608238. [Google Scholar] [CrossRef]
- Casulo, C.; Friedberg, J.W. Burkitt lymphoma- a rare but challenging lymphoma. Best Pr. Res. Clin. Haematol. 2018, 31, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Maurer, M.J.; Larson, M.C.; Allmer, C.; Feldman, A.L.; Bennani, N.N.; Thompson, C.A.; Porrata, L.F.; Habermann, T.M.; Witzig, T.E.; et al. Impact of metformin use on the outcomes of newly diagnosed diffuse large B-cell lymphoma and follicular lymphoma. Br. J. Haematol. 2019, 186, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Zhang, G.; Righolt, C.; Johnston, J.B.; Banerji, V.; Gibson, S.B.; Mahmud, S.M. Metformin Is Not Associated with Incidence Risk of Non-Hodgkin Lymphomas among Diabetic Patients. Cancer Epidemiol. Biomark. Prev. 2018, 27, 610–612. [Google Scholar] [CrossRef] [PubMed]
- Smyth, L.; Blunt, D.N.; Gatov, E.; Nagamuthu, C.; Croxford, R.; Mozessohn, L.; Cheung, M.C. Statin and cyclooxygenase-2 inhibitors improve survival in newly diagnosed diffuse large B-cell lymphoma: A large population-based study of 4913 subjects. Br. J. Haematol. 2020, 191, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.H. Metformin is associated with a lower risk of non-Hodgkin lymphoma in patients with type 2 diabetes. Diabetes Metab. 2019, 45, 458–464. [Google Scholar] [CrossRef]
- Wynn, A.; Vacheron, A.; Zuber, J.; Solomon, S.S. Metformin Associated With Increased Survival in Type 2 Diabetes Patients With Pancreatic Cancer and Lymphoma. Am. J. Med. Sci. 2019, 358, 200–203. [Google Scholar] [CrossRef]
- Singh, A.; Gu, J.; Yanamadala, V.; Czuczman, M.S.; Hernandez-Ilizaliturri, F.J. Metformin lowers the mitochondrial potential of lymphoma cells and its use during front-line rituximab-based chemo-immunotherapy improves the clinical outcome of diffuse Large B-cell lymphoma. Blood 2013, 122, 1825. [Google Scholar] [CrossRef]
- Fan, X.; Zhao, B.; Yang, B.; Zhao, Y.; Ye, J.; Lu, Y.; Wang, C.; Xiong, H.; Chen, S.; Janin, A. Metformin prolonged the survival of diffuse large B-cell lymphoma and grade 3b follicular lymphoma patients responding to first-line treatment with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone: A prospective phase II clinical trial. Transl. Cancer Res. 2018, 2018, 4. [Google Scholar]
- Bolen, S.; Feldman, L.; Vassy, J.; Wilson, L.; Yeh, H.C.; Marinopoulos, S.; Wiley, C.; Selvin, E.; Wilson, R.; Bass, E.B.; et al. Systematic review: Comparative effectiveness and safety of oral medications for type 2 diabetes mellitus. Ann. Intern. Med. 2007, 147, 386–399. [Google Scholar] [CrossRef]
- Dawed, A.Y.; Zhou, K.; van Leeuwen, N.; Mahajan, A.; Robertson, N.; Koivula, R.; Elders, P.J.M.; Rauh, S.P.; Jones, A.G.; Holl, R.W.; et al. Variation in the Plasma Membrane Monoamine Transporter (PMAT) (Encoded by SLC29A4) and Organic Cation Transporter 1 (OCT1) (Encoded by SLC22A1) and Gastrointestinal Intolerance to Metformin in Type 2 Diabetes: An IMI DIRECT Study. Diabetes Care 2019, 42, 1027–1033. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wu, Z.; Zhao, P. The effects of metformin in the treatment of osteoarthritis: Current perspectives. Front. Pharm. 2022, 13, 952560. [Google Scholar] [CrossRef] [PubMed]
- Marshall, S.M. 60 years of metformin use: A glance at the past and a look to the future. Diabetologia 2017, 60, 1561–1565. [Google Scholar] [CrossRef] [PubMed]
- Aroda, V.R.; Edelstein, S.L.; Goldberg, R.B.; Knowler, W.C.; Marcovina, S.M.; Orchard, T.J.; Bray, G.A.; Schade, D.S.; Temprosa, M.G.; White, N.H.; et al. Long-term Metformin Use and Vitamin B12 Deficiency in the Diabetes Prevention Program Outcomes Study. J. Clin. Endocrinol. Metab. 2016, 101, 1754–1761. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Ahn, C.W.; Fang, S.; Lee, H.S.; Park, J.S. Association between metformin dose and vitamin B12 deficiency in patients with type 2 diabetes. Medicine 2019, 98, e17918. [Google Scholar] [CrossRef]
- Ting, R.Z.; Szeto, C.C.; Chan, M.H.; Ma, K.K.; Chow, K.M. Risk factors of vitamin B(12) deficiency in patients receiving metformin. Arch. Intern. Med. 2006, 166, 1975–1979. [Google Scholar] [CrossRef]
- Bauman, W.A.; Shaw, S.; Jayatilleke, E.; Spungen, A.M.; Herbert, V. Increased intake of calcium reverses vitamin B12 malabsorption induced by metformin. Diabetes Care 2000, 23, 1227–1231. [Google Scholar] [CrossRef]
- Wang, Y.W.; He, S.J.; Feng, X.; Cheng, J.; Luo, Y.T.; Tian, L.; Huang, Q. Metformin: A review of its potential indications. Drug Des. Devel. Ther. 2017, 11, 2421–2429. [Google Scholar] [CrossRef]
- Boucaud-Maitre, D.; Ropers, J.; Porokhov, B.; Altman, J.J.; Bouhanick, B.; Doucet, J.; Girardin, E.; Kaloustian, E.; Lassmann Vague, V.; Emmerich, J. Lactic acidosis: Relationship between metformin levels, lactate concentration and mortality. Diabet. Med. 2016, 33, 1536–1543. [Google Scholar] [CrossRef]
- Al-Abri, S.A.; Hayashi, S.; Thoren, K.L.; Olson, K.R. Metformin overdose-induced hypoglycemia in the absence of other antidiabetic drugs. Clin. Toxicol. 2013, 51, 444–447. [Google Scholar] [CrossRef]
- Salpeter, S.R.; Greyber, E.; Pasternak, G.A.; Salpeter, E.E. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus. Cochrane Database Syst. Rev. 2010, 2010, Cd002967. [Google Scholar]
- Donnan, K.; Segar, L. SGLT2 inhibitors and metformin: Dual antihyperglycemic therapy and the risk of metabolic acidosis in type 2 diabetes. Eur. J. Pharm. 2019, 846, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, E.; Sargeant, J.A.; Zaccardi, F.; Khunti, K.; Webb, D.R.; Davies, M.J. Where Does Metformin Stand in Modern Day Management of Type 2 Diabetes? Pharmaceuticals 2020, 13, 427. [Google Scholar] [CrossRef] [PubMed]
- Adler, A.I.; Shaw, E.J.; Stokes, T.; Ruiz, F. Newer agents for blood glucose control in type 2 diabetes: Summary of NICE guidance. BMJ 2009, 338, b1668. [Google Scholar] [CrossRef]
- Tanner, C.; Wang, G.; Liu, N.; Andrikopoulos, S.; Zajac, J.D.; Ekinci, E.I. Metformin: Time to review its role and safety in chronic kidney disease. Med. J. Aust. 2019, 211, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Wensink, M.J.; Lu, Y.; Tian, L.; Shaw, G.M.; Rizzi, S.; Jensen, T.K.; Mathiesen, E.R.; Skakkebæk, N.E.; Lindahl-Jacobsen, R.; Eisenberg, M.L. Preconception Antidiabetic Drugs in Men and Birth Defects in Offspring: A Nationwide Cohort Study. Ann. Intern. Med. 2022, 175, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Alhowail, A. Potential mechanisms of metformin-induced memory impairment. Eur. Rev. Med. Pharm. Sci. 2021, 25, 4757–4761. [Google Scholar]
- Wang, G.S.; Hoyte, C. Review of Biguanide (Metformin) Toxicity. J. Intensive Care Med. 2019, 34, 863–876. [Google Scholar] [CrossRef] [PubMed]
- Update on metformin safety in pregnancy. Drug Ther. Bull. 2022, 60, 99. [CrossRef] [PubMed]
- Notaro, A.L.G.; Neto, F.T.L. The use of metformin in women with polycystic ovary syndrome: An updated review. J Assist Reprod. Genet. 2022, 39, 573–579. [Google Scholar] [CrossRef]
- Bailey, C.J.; Gwilt, M. Diabetes, Metformin and the Clinical Course of Covid-19: Outcomes, Mechanisms and Suggestions on the Therapeutic Use of Metformin. Front. Pharm. 2022, 13, 784459. [Google Scholar] [CrossRef] [PubMed]
- Syngelaki, A.; Nicolaides, K.H.; Balani, J.; Hyer, S.; Akolekar, R.; Kotecha, R.; Pastides, A.; Shehata, H. Metformin versus Placebo in Obese Pregnant Women without Diabetes Mellitus. N. Engl. J. Med. 2016, 374, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Ferro, A.; Goyal, S.; Kim, S.; Wu, H.; Taunk, N.K.; Schiff, D.; Pirlamarla, A.; Haffty, B.G. Evaluation of Diabetic Patients with Breast Cancer Treated with Metformin during Adjuvant Radiotherapy. Int. J. Breast Cancer 2013, 2013, 659723. [Google Scholar] [CrossRef]
- Mortezaee, K.; Shabeeb, D.; Musa, A.E.; Najafi, M.; Farhood, B. Metformin as a Radiation Modifier; Implications to Normal Tissue Protection and Tumor Sensitization. Curr. Clin. Pharm. 2019, 14, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.L.; Kolozsvary, A.; Isrow, D.M.; Al Feghali, K.; Lapanowski, K.; Jenrow, K.A.; Kim, J.H. A Novel Mechanism of High Dose Radiation Sensitization by Metformin. Front. Oncol. 2019, 9, 247. [Google Scholar] [CrossRef] [PubMed]
- Suissa, S.; Azoulay, L. Metformin and cancer: Mounting evidence against an association. Diabetes Care 2014, 37, 1786–1788. [Google Scholar] [CrossRef]
- Mamtani, R.; Pfanzelter, N.; Haynes, K.; Finkelman, B.S.; Wang, X.; Keefe, S.M.; Haas, N.B.; Vaughn, D.J.; Lewis, J.D. Incidence of bladder cancer in patients with type 2 diabetes treated with metformin or sulfonylureas. Diabetes Care 2014, 37, 1910–1917. [Google Scholar] [CrossRef] [PubMed]
- Pollak, M.N. Investigating metformin for cancer prevention and treatment: The end of the beginning. Cancer Discov. 2012, 2, 778–790. [Google Scholar] [CrossRef]
- Samuel, S.M.; Varghese, E.; Varghese, S.; Büsselberg, D. Challenges and perspectives in the treatment of diabetes associated breast cancer. Cancer Treat. Rev. 2018, 70, 98–111. [Google Scholar] [CrossRef]
- Hatoum, D.; McGowan, E.M. Recent advances in the use of metformin: Can treating diabetes prevent breast cancer? Biomed. Res. Int 2015, 2015, 548436. [Google Scholar] [CrossRef]
- Samuel, S.M.; Varghese, E.; Kubatka, P.; Triggle, C.R.; Büsselberg, D. Metformin: The Answer to Cancer in a Flower? Current Knowledge and Future Prospects of Metformin as an Anti-Cancer Agent in Breast Cancer. Biomolecules 2019, 9, 846. [Google Scholar] [CrossRef] [PubMed]
- Scherbakov, A.M.; Sorokin, D.V.; Tatarskiy, V.V., Jr.; Prokhorov, N.S.; Semina, S.E.; Berstein, L.M.; Krasil’nikov, M.A. The phenomenon of acquired resistance to metformin in breast cancer cells: The interaction of growth pathways and estrogen receptor signaling. IUBMB Life 2016, 68, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Farmer, R.E.; Ford, D.; Forbes, H.J.; Chaturvedi, N.; Kaplan, R.; Smeeth, L.; Bhaskaran, K. Metformin and cancer in type 2 diabetes: A systematic review and comprehensive bias evaluation. Int. J. Epidemiol. 2017, 46, 728–744. [Google Scholar] [CrossRef] [PubMed]
- Elgendy, M.; Cirò, M.; Hosseini, A.; Weiszmann, J.; Mazzarella, L.; Ferrari, E.; Cazzoli, R.; Curigliano, G.; DeCensi, A.; Bonanni, B.; et al. Combination of Hypoglycemia and Metformin Impairs Tumor Metabolic Plasticity and Growth by Modulating the PP2A-GSK3β-MCL-1 Axis. Cancer Cell 2019, 35, 798–815.e15. [Google Scholar] [CrossRef]
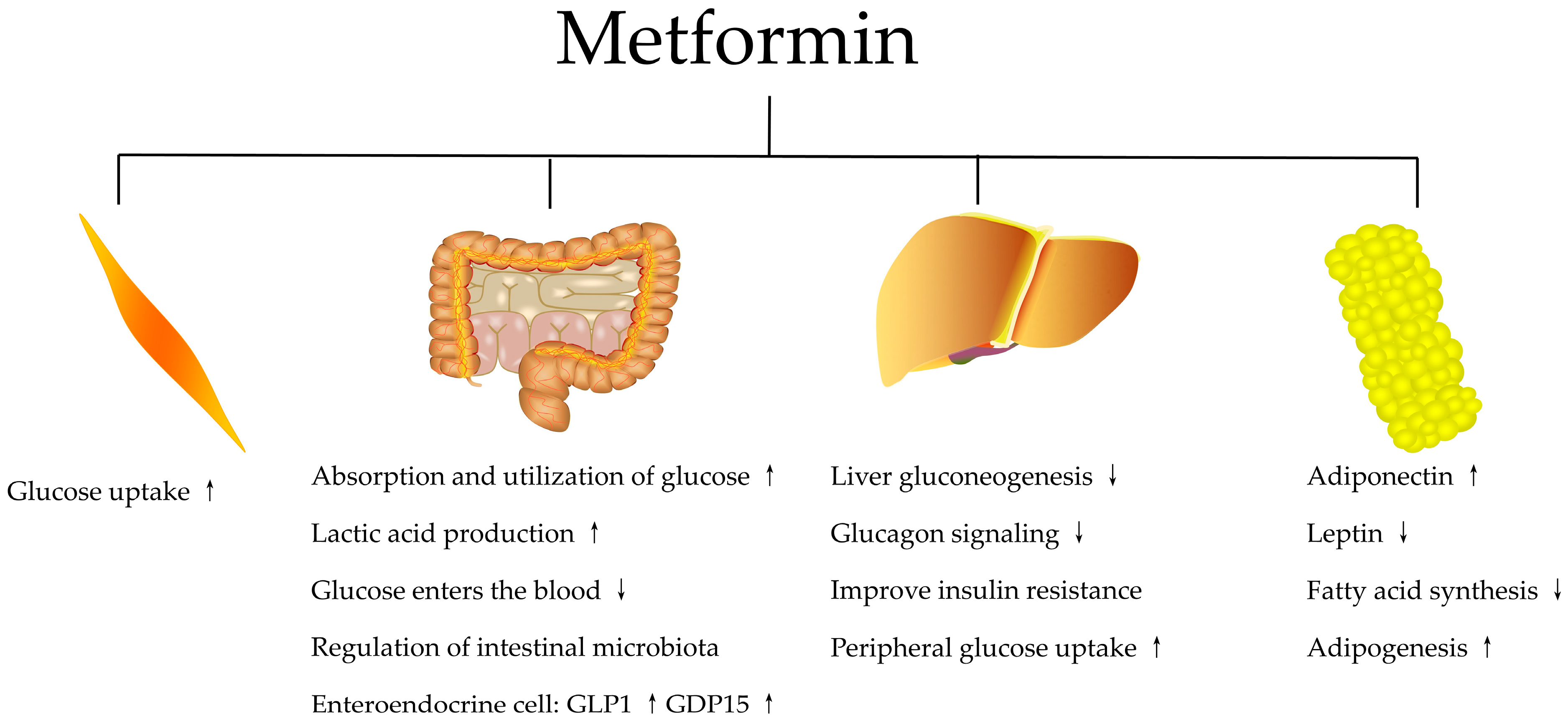

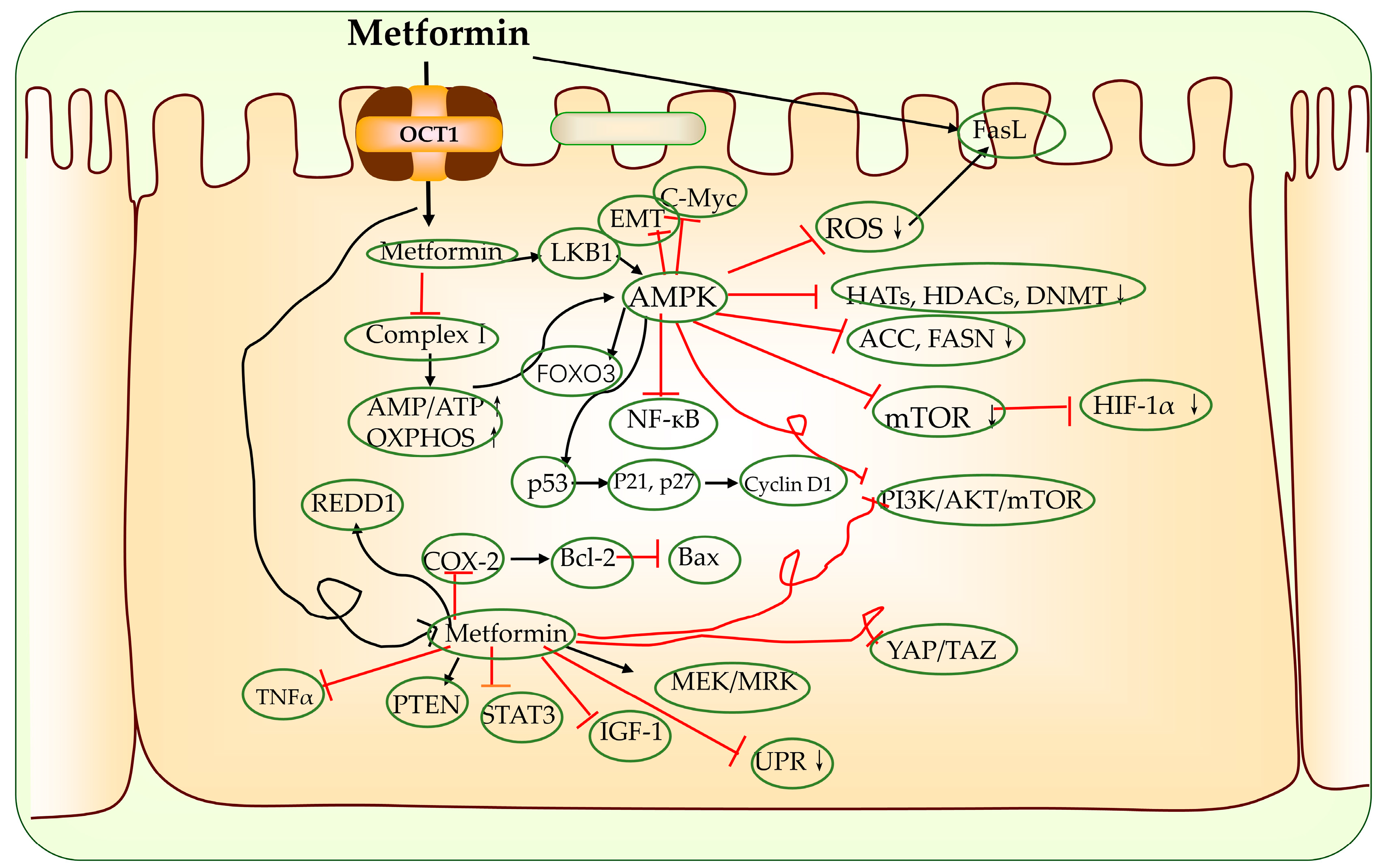
| Research Subjects | Models | Metformin Dosage | Year | Joint Effect of Other Drugs | Effects | Ref. |
|---|---|---|---|---|---|---|
| NCG mice | Acute Myelocytic Leukemia | 40 mg/kg/day i.v., 4 days | 2022 | Ara-C | Metformin potentiated the anti-tumor efficacy of Ara-C in vivo in an NCG immunodeficient mouse xenograft model by inhibiting the mitochondrial transfer and OXPHOS activity in the engrafted human AML cells. | [195] |
| Female nude mice (aged 5 weeks; average weight, 16 g) | Acute Myelocytic Leukemia | 200 mg/kg i.p., 14 days | 2022 | Venetoclax | Metformin downregulated the expression of anti-apoptotic proteins Mcl-1 and Bcl-xl by inhibiting protein production, and shows a synergistic anti-tumor effect with ABT-199 in acute myeloid leukemia. | [196] |
| Female C57BL/KaLwRij mice (aged 5, 6 weeks) | Multiple Myeloma | 2.5 mg/mL p.o., 4 weeks | 2022 | - | Metformin increased OPN expression in preosteoblasts, increasing myeloma cell adherence. | [197] |
| MDR canines were male/neutered with recurrent B-cell lymphoma. | Lymphoma | 250 mg OD or 500 mg BID p.o., 0–184 days | 2022 | CHOP or Doxorubicin | Metformin reduced MDR protein markers in all canines in the study. | [198] |
| BALC female nude mice (SPF level) | Acute Myelocytic Leukemia | 125 mg/kg i.p., 21 days | 2020 | Ara-C | The synergistic anti-tumor effect of Ara-C/metformin in AML was via inhibiting the mTORC1/P70S6K pathway. | [199] |
| SCID mice (aged 6–8 weeks) | Burkitt’s Lymphoma | 2 μg/mL p.o., 3 months | 2020 | Rituximab | Metformin in combination with rituximab showed improved survival compared with rituximab monotherapy. | [200] |
| NOD/SCID mice (aged 5 weeks) | Multiple Myeloma | 250 mg/kg/day p.o., 21 days | 2018 | - | Metformin inhibited the proliferation of myeloma cells by inducing autophagy and cell-cycle arrest. The molecular mechanism involved the dual repression of mTORC1 and mTORC2 pathways via AMPK activation. | [201] |
| mice | Multiple Myeloma | 600 μg/mL i.v., 18 or 27 days | 2015 | Bortezomib | Metformin suppressed GRP78, and supported the pharmacologic repositioning of metformin to enhance the anti-myeloma benefit of bortezomib. | [194] |
| NOD/SCID CB17-strain 394 (white) mice (aged 5, 6 weeks) | Multiple Myeloma | 125 mg/kg i.p., 1 week | 2015 | Ritonavir | Ritonavir and metformin effectively suppressed AKT and mTORC1 phosphorylation and pro-survival BCL-2 family member MCL-1 expression in multiple myeloma cell lines. | [202] |
| male CB17/SCID mice (aged 4 weeks) | Multiple Myeloma | 200 mg/kg/day i.p., 21 days | 2015 | Dexamethasone | Metformin inhibited multiple myeloma cell proliferation via the IGF-1R/PI3K/AKT/mTOR signaling pathway. | [191] |
| Pten-deficient mice (tPTEN−/−) | Lymphoma | 2 mg/mouse/day i.p., 18 days | 2013 | - | Metformin strongly decreased the growth of luciferase-expressing tPTEN−/− cells xenografted in nude mice. | [203] |
| nude mice (aged 5–6 weeks) | Lymphoma | 4 mg/kg/day (i.p.) or 3 mg/kg/day (p.o.) for 21 days | 2012 | Doxorubicin or Temsirolimus | Metformin induced AMPK activation, mTOR inhibition and remarkably blocked tumor growth in murine lymphoma xenografts. | [193] |
| Research Subjects | Models | Metformin Dosage | Year | Joint Effect of Other Drugs | Effects | Ref. |
|---|---|---|---|---|---|---|
| U937 and HL-60 cells | AML | 5 mM, 24 h | 2022 | Ara-C | Metformin inhibited mitochondrial transfer and significantly enhanced the chemosensitivity of AML cells co-cultured with BMSCs. | [195] |
| LAMA-84s, LAMA-84r and K562 cells | CML | 2.5–80 mM, 48 h | 2022 | thymoquinone | Metformin and thymoquinone monotherapies possessed a significant anti-leukemic effect that was more pronounced when combinatorial therapies are applied. | [204] |
| K562, K562TRBSR, MOLM13, THP1, HEL, HL60, HL60TRBSR, OCI/AML3 cells, KG1 cells and HEK293T. | AML | 5 mM, 72 h | 2022 | - | AML cells with an MLL/AF9 genotype have a high dependency on OXPHOS and could be therapeutically targeted by metformin. | [205] |
| 697 cells | ALL | 0–15 mM, 48 h | 2022 | cisplatin | Metformin and cisplatin exerted a cytotoxic effect on 697 cells. When both drugs were combined they demonstrated antagonistic effects. | [206] |
| KG-1, Kasumi-1 and THP-1 cells | AML | 0–2 mM, 48 h | 2022 | venetoclax | Metformin downregulated the expression of anti-apoptotic proteins Mcl-1 and Bcl-xl by inhibiting protein production, and showed a synergistic anti-tumor effect with ABT-199 in acute myeloid leukemia. | [196] |
| NB4, KG1 and KG1A cell lines | Leukemia | 10 mM, 24 h or 72 h | 2021 | MCL1 Inhibitor S63845 | A combined treatment with metformin and S63845 had a stronger inhibitory effect on AML cell oxidative phosphorylation and glycolysis rate and, consequently, on cellular ATP levels. The apoptosis induced by treatment was related to the change of ROS level. | [207] |
| K562 and KU812 cells | CML | 0–10 mM, 48 h | 2021 | nilotinib | Metformin was effective in decreasing phosphorylated JNK levels, resulting in the restoration of nilotinib sensitivity. | [208] |
| OCI/AML2, K562, OCI/AML3, and THP-1 cells | Leukemia | 0–10 mM, 24–72 h | 2020 | TP-0903 | Metformin inhibited phosphorylation-dependent activation of TAM RTKs, which regulate molecular pathways associated with leukemia. | [209] |
| HL-60 and THP-1 cells | AML | 0–12 mM, 24–72 h | 2020 | Ara-C | The synergistic anti-tumor effect of Ara-C and metformin in AML was achieved via inhibition of the mTORC1/P70S6K pathway. | [199] |
| SKM-1 cells | AML-MDS | 0–20 mM, 24–72 h | 2019 | - | Metformin inhibited proliferation of SKM-1 cells, potentially via an AMPK-mediated cell cycle arrest. | [210] |
| chemoresistant AML patients | AML | 10 mM, 24 h | 2019 | Cytarabine, Venetoclax | Metformin decreased therapy-resistant-AML cell oxidative phosphorylation in vitro, while co-treatment with cytarabine and venetoclax slightly increased the effect. | [211] |
| K562 cells | CML | 0–30 mM, 48 h | 2019 | - | Metformin can inhibit the growth and proliferation of K562 cells and promote the apoptosis of K562 cells by inhibiting glycolysis energy metabolism. The PI3K/Akt/mTOR signaling pathway may be one of the molecular mechanisms of metformin on k562 cells. | [212] |
| U937 and THP-1 cells | AML | 0–10 mM, 24 h or 48 h | 2018 | Diflunisal, Diclofenac | Low concentrations of metformin and the two NSAIDs diclofenac and diflunisal exerted a synergistic inhibitory effect on AML proliferation and induced apoptosis, most likely by blocking tumor-cell metabolism. | [213] |
| HL-60 cells | APL | 0–1 μM, 24 h, 48 h, and 72 h | 2018 | Paclitaxel | The combination of paclitaxel and metformin triggered differentiation and apoptosis according to gene expression changes. | [214] |
| HL60 cells | APL | 15 μM, 150 μM, and 1.5 mM, 4 h | 2018 | - | Low concentrations (15 and 150 µM) increased both oxidative phosphorylation and the oxidative stress response, acting on the AMPK/Sirt1 pathway, while high concentration (1.5 mM) inhibited the respiratory chain and altered cell morphology, becoming toxic to the cells. | [215] |
| Dami and MEG-01 cells | AMKL | 4 mM, 0–72 h | 2017 | - | Metformin inhibited the proliferation and induced the apoptosis of human megakaryoblastic cell lines. | [216] |
| K562 and A301 cells | CML ALL | 0–25 mM, 24 h | 2017 | Vincristine | AMPK activation was critical to metformin’s effects on vincristine-induced apoptosis. | [217] |
| MV4-11, MOLM-14, OCI-AML3, Nomo-1, THP-1 and HL-60 cells | AML | 10 mM, 24 h or 48 h | 2016 | 6-Benzylthioinosine | The combination of 6-BT with metformin resulted in significant cytotoxicity (60–70%) in monocytic AML cell lines and was associated with the inhibition of FLT3-ITD activated STAT5 and reduced c-Myc and GLUT-1 expression. | [218] |
| OCI-AML3, REH, NALM-6-6, and KBM5 cells | AML ALL CML | 0–10 mM, 0–16 h | 2016 | ABT-737 | Inhibition of mitochondrial metabolism by metformin or phenformin was associated with increased leukemia cell susceptibility to induction of intrinsic apoptosis. | [219] |
| EHEB, JVM-2 and MEC-2 cells | Leukemic | 0.1–10 mM, 0–48 h | 2016 | Sodium Dichloroacetate | The combination of metformin and DCA increased the cytotoxicity in the B-type leukemia cell line and the cell culture derived from B-CLL patients, which was the result of cell inhibition and apoptosis promotion. | [220] |
| MV4-11(FLT3-ITD positive) and THP-1(FLT3-ITD negative) cells | AML | 0.2–16 mM, 0–72 h | 2015 | Sorafenib | In the presence of metformin, the anti-cancer potential of sorafenib, accompanied by increased LC3 levels, was found to be synergistically enhanced with the remarkably reduced protein expression of the mTOR/p70S6K/4EBP1 pathway, while not appreciably altering the cell cycle. | [221] |
| SUP-B15, K562 and K562R cells | ALL CML | 0–50 mM, 0–72 h | 2015 | - | Metformin mediated anti-leukemia effects by proapoptosis and inhibition of mTORC1 signaling, potentiating the anti-cancer efficacy of imatinib in Ph+ ALL cells. Metformin-induced autophagy was associated with the activation of the ERK pathway. | [222] |
| 10E1-CEM cells | ALL | 10 mM, 0–72 h | 2015 | - | Metformin induced cell-cycle arrest and apoptosis in drug-resistant leukemia cells. | [223] |
| Kassumi, NB-4, THP-1, ML-2, K562, Jurkat, Raji and HUT-78 cells | AML ALL | 0–8 μM, 0–48 h | 2014 | - | Metformin may be involved in the downregulation of FOXM1. Metformin promoted the apoptosis of ML-2 cells, induced cell-cycle arrest at the G0/G1 and G2/M phases, and inhibited proliferation. | [224] |
| CCRF-CEM, Jurkat, REH and NALM6 cells | ALL | 0–10 mM, 48 h | 2013 | - | Metformin induced ALL cell death by triggering ER and proteotoxic stress and simultaneously down-regulating the physiologic UPR response responsible for effectively buffering proteotoxic stress. | [225] |
| Kasumi-1, SKNO-1, HL-60, KG-1a and NB4 cells | AML APL | 0–5 mM, 0–72 h | 2012 | ATRA | The synergism between metformin and ATRA triggered the maturation pathway in APL cells. | [226] |
| Research Subjects | Models | Metformin Dosage | Year | Joint Effect of Other Drugs | Effects | Ref. |
|---|---|---|---|---|---|---|
| OCI-LY-10, DB and THP-1 cells | Lymphoma | 200 μM, 72 h | 2022 | - | Metformin could target altered lipid metabolism and decrease M2 macrophages in DLBCL, especially in CD5+ non-DE lymphoma. | [227] |
| DAUDI cells | Burkitt‘s lymphoma | 10 mM, 0–72 h | 2021 | - | Metformin could induce cell death in BL cells by stressing cellular metabolism via the induction of GLUT1, PKM2, and LDHA. | [228] |
| Raji, U2392 and RL cell lines | Lymphoma | 4, 8, or 16 mM, 0–72 h | 2020 | Rituximab | Metformin caused cell-cycle arrest in the G1 phase. Metformin induced apoptosis, ROS production, and increased mitochondrial membrane permeability. Metformin exhibited additive/synergistic effects when combined with traditional chemotherapy or rituximab in vitro. | [200] |
| Hut78, H9 and HH cells | Lymphoma | 1 or 10 mM, 2 h | 2019 | - | Metformin inhibited mTORC1 signaling and migration of SS cells induced by SDF-1. | [229] |
| Daudi, SUDHL-4 and Jeko-1 cells | Lymphoma | 0–10 mM, 48 h or 7 days | 2018 | Venetoclax, and BAY-1143572 | Metformin inhibited oxidative phosphorylation in lymphoma cells. Metformin increased caspase 3/7 activity in venetoclax and BAY-1143572-treated lymphoma cells. Metformin showed potentiation with venetoclax and BAY-1143572 in a cell-type-dependent manner. | [230] |
| BC3 and BCBL1 cells | Primary exudative lymphoma | 15, 20 and 30 mM, 24 h | 2017 | Bortezomib | The cytotoxic effect of metformin was correlated with intracellular reactive oxygen species reduction, activation of AMPK and the inhibition of pro-survival pathways such as mTOR and STAT3. Metformin altered UPR activated by bortezomib, leading to a reduced expression of BiP, upregulation of CHOP and downregulation of Bcl-2. | [231] |
| Jurkat, Sil-ALL, SupT1 and Ke37 cells | Lymphoma | 0–10 mM, 0–48 h | 2013 | 2-deoxyglucose | Metformin synergized with 2DG to impair tumour cell survival. | [203] |
| SU-DHL-4, Nalmawa, DB, SU-DHL-5, Daudi, Jurkat, 6T-CEM, Kappas, H9 and HUT78 cells | Lymphoma | 0–40 mM, 0–72 h | 2012 | - | Metformin-induced AMPK activation was associated with the inhibition of mTOR signaling without involving AKT. | [193] |
| Research Subjects | Models | Metformin Dosage | Year | Joint Effect of Other Drugs | Effects | Ref. |
|---|---|---|---|---|---|---|
| Jeko-1 cells | MCL | 10 mM, 0–72 h | 2022 | Bortezomib | Metformin treatment induced the resistance of cancer cells to the proteasome inhibitor Bortezomib by impairing the activity and assembly of the 26S proteasome complexes. | [232] |
| 5TGM1, MM.1S cells | MM | 0–10 mM, 48 h | 2022 | - | Metformin increased OPN expression in preosteoblasts, increasing myeloma cell adherence. | [197] |
| RPMI-8226 cells | MM | 0–40 mM, 48 h | 2020 | Melphalan | Metformin could promote DNA damage induced by melphalan and decreased the concentration of ATP in the process of repairing DNA damage to hinder the anti-apoptotic process of tumor cells. | [233] |
| L363 and RPMI-8226 cells | MM | 0–10 mM, 0–72 h | 2020 | - | Metformin inhibited HIF-1 signaling in MM cells, and the effect of metformin was mainly oxygen dependent. Metformin triggered the growth arrest without inducing apoptosis in either normoxic or hypoxic conditions. | [234] |
| I-8266, U266- B1, MM.1S, OPM1, OPM2, ANBL6, OCI-MY5, JJN3, KP6, DP6, KAS-6/1, KMS12PE, K562 and NALM6 cells | MM CML ALL | 0–80 mM, 0–72 h | 2019 | - | Metformin specifically decreased IL-6R expression which is mediated via AMPK, mTOR, and miR34a. | [92] |
| RPMI8226, ARP-1 and OPM2 cells | MM | 0–40 mM, 24 h | 2019 | PFK15 | Metformin was found to inhibit PFKFB3 protein expression. PFK15 also demonstrated a synergistic effect with metformin to eliminate MM cells. | [235] |
| U266 cells | MM | 5–50 mM, 0–72 h | 2018 | - | Metformin could inhibit cell proliferation and induced U266 cell apoptosis via the mitochondrial apoptotic pathway. | [236] |
| RPMI-8226 and U266 cells | MM | 0–80 mM, 0–72 h | 2018 | - | Metformin inhibited the proliferation of myeloma cells by inducing autophagy and cell-cycle arrest. The molecular mechanism involved the dual repression of mTORC1 and mTORC2 pathways via AMPK activation. | [201] |
| U266, RPMI8226, LP-1 and NCI-H929 cells | MM | 20 mM, 24 h | 2018 | FTY720 | Exposure to metformin in combination with FTY720 potently induced apoptosis in MM cells in a ROS-dependent manner. | [237] |
| RPMI-8226 and U266 cells | MM | 0–80 mM, 0–72 h | 2017 | - | Metformin can inhibit the proliferation and induce apoptosis of RPMI8226 and U266 cell lines, which may be related to downregulation of the STAT3 signal transduction pathway. | [238] |
| RPMI-8226 cells | MM | 500 μM, 24 h | 2015 | Bortezomib | Metformin inhibited GRP78 to enhance the anti-myeloma effect of bortezomib. | [194] |
| KMS11, L363, and JJN3 cells | MM | 0–5 mM, 0–72 h | 2015 | Ritonavir | Ritonavir and metformin effectively suppressed AKT and mTORC1 phosphorylation and prosurvival BCL-2 family member MCL-1 expression in multiple myeloma cell lines. | [202] |
| RPMI8226, MM.1S, MM.1R, and U266 cells | MM | 0–80 mM, 0–72 h | 2015 | Dexamethasone | Metformin inhibitedMM cell proliferation via the IGF-1R/PI3K/AKT/mTOR signaling pathway. | [191] |
| NCT Number | Official Title | Actual Enrollment | Status | Type of Disease | Combination Treatment | Phase |
|---|---|---|---|---|---|---|
| 03118128 | Effect of the Addition of Metformin Hydrochloride on the Prognosis of Patients With B-cell Precursor (Ph+ Negative) Acute Lymphoblastic Leukemia with High Expression of ABCB1 Gene | 102 | Completed | ALL | Conventional Chemotherapy | NA |
| 01750567 | A Phase II Pilot Study of Metformin Therapy in Patients with Relapsed Chronic Lymphocytic Leukemia and Un-treated CLL Patients with Genomic Deletion 11q | 40 | Recruiting | Relapsed CLL | None | Phase 2 |
| 05326984 | Effect of Metformin on ABCB1 and AMPK Expression in Adolescents with Newly Diagnosed Acute Lymphoblastic Leukemia | 20 | Recruiting | ALL | Conventional Chemotherapy | NA |
| 04741945 | STOP-LEUKEMIA: Repurposing Metformin as a Leukemia-preventive Drug in CCUS and LR-MDS | 24 | Recruiting | Preleukemia, MDS, Cytopenia | None | Phase 2 |
| 01324180 | A Phase I Window, Dose Escalating and Safety Trial of Metformin in Combination with Induction Chemotherapy in Relapsed Refractory Acute Lymphoblastic Leukemia: Metformin with Induction Chemotherapy of Vincristine, Dexamethasone, Doxorubicin, and PEG-asparaginase (VPLD) | 14 | Completed | ALL | VLPD | Phase 1 |
| 02948283 | A Pilot Feasibility Study of Metformin/Ritonavir Combination Treatment in Patients with Relapsed/Refractory Multiple Myeloma or Chronic Lymphocytic Leukemia | 3 | Completed | R/R MM, R/R CLL | Ritonavir | Phase 1 |
| 01486043 | Metformin as an Adjunctive Therapy for Transient Hyperglycemia in Patients with Acute Lymphoblastic Leukemia During Induction Chemotherapy | 4 | Terminated | ALL | Insulin | NA |
| 01849276 | A Phase I Study of Metformin and Cytarabine for the Treatment of Relapsed/Refractory Acute Myeloid Leukemia | 2 | Terminated | R/R AML | Cytarabine | Phase 1 |
| 00659568 | A Phase I Study of Temsirolimus in Combination with Metformin in Advanced Solid Tumours | 28 | Completed | Lymphoma | Temsirolimus | Phase 1 |
| 02967276 | Phase II Trial, Open Label, Clinical Activity of Metformin in Combination with High-dose of Dexamethasone (HDdexa) in Patients with Relapsed/Refractory Multiple Myeloma | 28 | Unknown | MM | High doses of dexamethasone | Phase 2 |
| 04850846 | A Randomized Placebo-Controlled Phase 2 Study of Metformin for the Prevention of Progression of Monoclonal Gammopathy of Undetermined Significance and Smoldering Multiple Myeloma | 80 | Recruiting | MGUS, SMM | None | Phase 2 |
| 03829020 | An Open-Label Phase 1 Study of Metformin and Nelfinavir in Combination with Bortezomib in Patients with Relapsed and/or Refractory Multiple Myeloma | 9 | Active, not recruiting | R/R PCM | Bortezomib, Nelfinavir Mesylate | Phase 1 |
| 03200015 | Effect of Metformin in Combination with R-CHOP for the First Line Treatment of Patients with Diffuse Large B-cell Lymphoma | 15 | Unknown | DLBCL | RCHOP | Phase 2 |
| 02815397 | A Phase II Study Evaluating the Efficacy and Safety of Metformin in Combination with Standard Induction Therapy (DA-EPOCH-R) for Previously Untreated C-myc+ Diffuse Large B-Cell Lymphoma | 2 | Terminated | DLBCL | DA-EPOCHR | Phase 2 |
| 03600363 | A Prospective Randomized Controlled Phase II Clinical Trial of Metformin in the Maintenance Therapy of High Risk Diffuse Large B Lymphoma/Stage III Follicular Lymphoma Patients with Complete Remission | 250 | Unknown | DLBCL, Stage III FL | RCHOP | Phase 2 |
| 02531308 | A Phase ll Study Evaluating the Efficacy and Safety of Metformin in Combination with Standard Induction Therapy (RM-CHOP) for Previously Untreated Aggressive Diffuse Large B-cell Lymphoma | 5 | Terminated | DLBCL | RCHOP | Phase 2 |
| 02145559 | A Pharmacodynamic Study of Sirolimus and Metformin in Patients with Advanced Solid Tumors | 24 | Completed | Lymphoma | Sirolimus | Phase 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Zhou, F.; Guan, J.; Zhou, L.; Chen, B. Action Mechanism of Metformin and Its Application in Hematological Malignancy Treatments: A Review. Biomolecules 2023, 13, 250. https://doi.org/10.3390/biom13020250
Zhang Y, Zhou F, Guan J, Zhou L, Chen B. Action Mechanism of Metformin and Its Application in Hematological Malignancy Treatments: A Review. Biomolecules. 2023; 13(2):250. https://doi.org/10.3390/biom13020250
Chicago/Turabian StyleZhang, Yi, Fang Zhou, Jiaheng Guan, Lukun Zhou, and Baoan Chen. 2023. "Action Mechanism of Metformin and Its Application in Hematological Malignancy Treatments: A Review" Biomolecules 13, no. 2: 250. https://doi.org/10.3390/biom13020250
APA StyleZhang, Y., Zhou, F., Guan, J., Zhou, L., & Chen, B. (2023). Action Mechanism of Metformin and Its Application in Hematological Malignancy Treatments: A Review. Biomolecules, 13(2), 250. https://doi.org/10.3390/biom13020250






