Metformin in Differentiated Thyroid Cancer: Molecular Pathways and Its Clinical Implications
Abstract
:1. Introduction
2. Molecular Insights of Metformin in Thyroid Cancer
2.1. Proposed Direct Antitumor Mechanisms Related to Metformin in DTC
2.2. Proposed Indirect Antitumor Mechanisms Related to Metformin in DTC
3. Clinical Evidence of Metformin Use in Thyroid Cancer
3.1. Thyroid Cancer Risk Reduction
3.2. Metformin and TSH Suppression
3.3. Role of Metformin as Co-Adjuvant Therapy in Radioactive Iodine Therapy in DTC
3.4. Role of Metformin in Thyroid Cancer Metastases
3.5. Role of Metformin in Thyroid Nodules
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mallik, R.; Chowdhury, T.A. Metformin in cancer. Diabetes Res. Clin. Pract. 2018, 143, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Vancura, A.; Bu, P.; Bhagwat, M.; Zeng, J.; Vancurova, I. Metformin as an Anticancer Agent. Trends Pharmacol. Sci. 2018, 39, 867–878. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Goswami, S.; Giacomini, K.M.; Altman, R.B.; Klein, T.E. Metformin pathways: Pharmacokinetics and pharmacodynamics. Pharm. Genom. 2012, 22, 820–827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, J.M.M.; Donnelly, L.A.; Emslie-Smith, A.M.; Alessi, D.R.; Morris, A.D. Metformin and reduced risk of cancer in diabetic patients. Br. Med. J. 2005, 330, 1304–1305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malek, M.; Aghili, R.; Emami, Z.; Khamseh, M.E. Risk of Cancer in Diabetes: The Effect of Metformin. ISRN Endocrinol. 2013, 2013, 636927. [Google Scholar] [CrossRef]
- Zingales, V.; Distefano, A.; Raffaele, M.; Zanghì, A.; Barbagallo, I.; Vanella, L. Metformin: A Bridge between Diabetes and Prostate Cancer. Front. Oncol. 2017, 7, 243. [Google Scholar] [CrossRef]
- Zhou, X.-L.; Xue, W.-H.; Ding, X.-F.; Li, L.-F.; Dou, M.-M.; Zhang, W.-J.; Lv, Z.; Fan, Z.-R.; Zhao, J.; Wang, L.-X. Association between metformin and the risk of gastric cancer in patients with type 2 diabetes mellitus: A meta-analysis of cohort studies. Oncotarget 2017, 8, 55622–55631. [Google Scholar] [CrossRef] [Green Version]
- Campbell, J.M.; Bellman, S.M.; Stephenson, M.D.; Lisy, K. Metformin reduces all-cause mortality and diseases of ageing independent of its effect on diabetes control: A systematic review and meta-analysis. Ageing Res. Rev. 2017, 40, 31–44. [Google Scholar] [CrossRef]
- Thakur, S.; Daley, B.; Klubo-Gwiezdzinska, J. The role of an anti-diabetic drug metformin in the treatment of endocrine tumors. J. Mol. Endocrinol. 2019, 63, R17–R35. [Google Scholar] [CrossRef]
- He, X.; Wu, D.; Hu, C.; Xu, T.; Liu, Y.; Liu, C.; Tang, W. Role of Metformin in the Treatment of Patients with Thyroid Nodules and Insulin Resistance: A Systematic Review and Meta-Analysis. Thyroid 2019, 29, 359–367. [Google Scholar] [CrossRef]
- World Health Organization. Cancer Today–IARC. 2022. Available online: https://gco.iarc.fr (accessed on 14 February 2022).
- Li, M.; Maso, L.D.; Vaccarella, S. Global trends in thyroid cancer incidence and the impact of overdiagnosis. Lancet Diabetes Endocrinol. 2020, 8, 468–470. [Google Scholar] [CrossRef]
- Seib, C.D.; Sosa, J.A. Evolving Understanding of the Epidemiology of Thyroid Cancer. Endocrinol. Metab. Clin. N. Am. 2018, 48, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, Q.; Yang, Y.; Yao, J.; Liao, L.; Dong, J. High prevalence of thyroid carcinoma in patients with insulin resistance: A meta-analysis of case-control studies. Aging 2021, 13, 22232–22241. [Google Scholar] [CrossRef] [PubMed]
- Kheder, S.; Sisley, K.; Hadad, S.; Balasubramanian, S. Effects of prolonged exposure to low dose metformin in thyroid cancer cell lines. J. Cancer 2017, 8, 1053–1061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Xu, S.; Renko, K.; Derwahl, M. Metformin Inhibits Growth of Thyroid Carcinoma Cells, Suppresses Self-Renewal of Derived Cancer Stem Cells, and Potentiates the Effect of Chemotherapeutic Agents. J. Clin. Endocrinol. Metab. 2012, 97, E510–E520. [Google Scholar] [CrossRef] [Green Version]
- Cho, S.W.; Yi, K.H.; Han, S.K.; Sun, H.J.; Kim, Y.A.; Oh, B.-C.; Park, Y.J.; Park, D.J. Therapeutic potential of metformin in papillary thyroid cancer in vitro and in vivo. Mol. Cell. Endocrinol. 2014, 393, 24–29. [Google Scholar] [CrossRef]
- Hanly, E.K.; Bednarczyk, R.B.; Tuli, N.Y.; Moscatello, A.L.; Halicka, H.D.; Li, J.; Geliebter, J.; Darzynkiewicz, Z.; Tiwari, R.K. mTOR inhibitors sensitize thyroid cancer cells to cytotoxic effect of vemurafenib. Oncotarget 2015, 6, 39702–39713. [Google Scholar] [CrossRef] [Green Version]
- Choi, Y.K.; Park, K.-G. Metabolic roles of AMPK and metformin in cancer cells. Mol. Cells 2013, 36, 279–287. [Google Scholar] [CrossRef] [Green Version]
- Ben Sahra, I.; Tanti, J.F.; Bost, F. The combination of metformin and 2 deoxyglucose inhibits autophagy and induces AMPK-dependent apoptosis in prostate cancer cells. Autophagy 2010, 6, 670–671. [Google Scholar] [CrossRef]
- Pierotti, M.A.; Berrino, F.; Gariboldi, M.; Melani, C.; Mogavero, A.; Negri, T.; Pasanisi, P.; Pilotti, S. Targeting metabolism for cancer treatment and prevention: Metformin, an old drug with multi-faceted effects. Oncogene 2012, 32, 1475–1487. [Google Scholar] [CrossRef] [PubMed]
- Hadad, S.; Iwamoto, T.; Jordan, L.; Purdie, C.; Bray, S.; Baker, L.; Jellema, G.; Deharo, S.; Hardie, D.G.; Pusztai, L.; et al. Evidence for biological effects of metformin in operable breast cancer: A pre-operative, window-of-opportunity, randomized trial. Breast Cancer Res. Treat. 2011, 128, 783–794. [Google Scholar] [CrossRef] [PubMed]
- Kourelis, T.V.; Siegel, R.D. Metformin and cancer: New applications for an old drug. Med. Oncol. 2011, 29, 1314–1327. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.J. Insulin-Like Growth Factor Pathway and the Thyroid. Front. Endocrinol. 2021, 12, 502. [Google Scholar] [CrossRef]
- Eggo, M.C.; Bachrach, L.K.; Burrow, G.N. Interaction of TSH, Insulin and Insulin-like Growth Factors in Regulating Thyroid Growth and Function. Growth Factors 1990, 2, 99–109. [Google Scholar] [CrossRef]
- Iglesias-Osma, M.C.; Blanco, E.J.; Carretero-Hernandez, M.; Catalano-Iniesta, L.; Sanchez-Robledo, V.; Garcia-Barrado, M.J.; Vicente-Garcia, T.; Burks, D.J.; Carretero, J. The influence of the lack of insulin receptor substrate 2 (IRS2) on the thyroid gland. Sci. Rep. 2019, 9, 5673. [Google Scholar] [CrossRef] [Green Version]
- Morale, M.G.; Tamura, R.E.; Rubio, I.G.S. Metformin and Cancer Hallmarks: Molecular Mechanisms in Thyroid, Prostate and Head and Neck Cancer Models. Biomolecules 2022, 12, 357. [Google Scholar] [CrossRef]
- Thakur, S.; Daley, B.; Gaskins, K.; Vasko, V.V.; Boufraqech, M.; Patel, D.; Sourbier, C.; Reece, J.M.; Cheng, S.-Y.; Kebebew, E.; et al. Metformin Targets Mitochondrial Glycerophosphate Dehydrogenase to Control Rate of Oxidative Phosphorylation and Growth of Thyroid Cancer In Vitro and In Vivo. Clin. Cancer Res. 2018, 24, 4030–4043. [Google Scholar] [CrossRef] [Green Version]
- Rotondi, M.; Coperchini, F.; Chiovato, L. CXCL8 in thyroid disease: From basic notions to potential applications in clinical practice. Cytokine Growth Factor Rev. 2013, 24, 539–546. [Google Scholar] [CrossRef]
- Bauerle, K.T.; Schweppe, R.E.; Haugen, B.R. Inhibition of nuclear factor-kappa B differentially affects thyroid cancer cell growth, apoptosis, and invasion. Mol. Cancer 2010, 9, 117. [Google Scholar] [CrossRef] [Green Version]
- Fang, W.; Ye, L.; Shen, L.; Cai, J.; Huang, F.; Wei, Q.; Fei, X.; Chen, X.; Guan, H.; Wang, W.; et al. Tumor-associated macrophages promote the metastatic potential of thyroid papillary cancer by releasing CXCL8. Carcinogenesis 2014, 35, 1780–1787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rotondi, M.; Coperchini, F.; Pignatti, P.; Magri, F.; Chiovato, L. Metformin Reverts the Secretion of CXCL8 Induced by TNF-α in Primary Cultures of Human Thyroid Cells: An Additional Indirect Anti-Tumor Effect of the Drug. J. Clin. Endocrinol. Metab. 2015, 100, E427–E432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bikas, A.; Jensen, K.; Patel, A.; Costello, J., Jr.; McDaniel, D.; Klubo-Gwiezdzinska, J.; Larin, O.; Hoperia, V.; Burman, K.D.; Boyle, L.; et al. Glucose-deprivation increases thyroid cancer cells sensitivity to metformin. Endocr.-Relat. Cancer 2015, 22, 919–932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, C.; Wei, W.; Qiu, Z.-L.; Song, H.-J.; Zhang, X.-Y.; Sun, Z.-K.; Luo, Q.-Y. Metformin reduces glycometabolism of papillary thyroid carcinoma in vitro and in vivo. J. Mol. Endocrinol. 2017, 58, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Xu, S.; Chen, G.; Derwahl, M.; Liu, C. Metformin and thyroid disease. J. Endocrinol. 2017, 233, R43–R51. [Google Scholar] [CrossRef]
- Vigersky, R.A.; Filmore-Nassar, A.; Glass, A.R. Thyrotropin Suppression by Metformin. J. Clin. Endocrinol. Metab. 2006, 91, 225–227. [Google Scholar] [CrossRef] [PubMed]
- Alevizaki, M. Metformin and the thyroid: Some questions still remain. Clin. Endocrinol. 2013, 78, 503–504. [Google Scholar] [CrossRef]
- Krysiak, R.; Okopień, B. Thyrotropin-lowering effect of metformin in a patient with resistance to thyroid hormone. Clin. Endocrinol. 2011, 75, 404–406. [Google Scholar] [CrossRef]
- Cannarella, R.; Condorelli, R.A.; Barbagallo, F.; Aversa, A.; Calogero, A.E.; La Vignera, S. TSH lowering effects of metformin: A possible mechanism of action. J. Endocrinol. Investig. 2020, 44, 1547–1550. [Google Scholar] [CrossRef]
- Lupoli, R.; Di Minno, A.; Tortora, A.; Ambrosino, P.; Lupoli, G.A.; Di Minno, M.N.D. Effects of Treatment With Metformin on TSH Levels: A Meta-analysis of Literature Studies. J. Clin. Endocrinol. Metab. 2014, 99, E143–E148. [Google Scholar] [CrossRef] [Green Version]
- Tseng, C.-H. Metformin Reduces Thyroid Cancer Risk in Taiwanese Patients with Type 2 Diabetes. PLoS ONE 2014, 9, e109852. [Google Scholar] [CrossRef] [PubMed]
- Ben Sahra, I.; Laurent, K.; Loubat, A.; Giorgetti-Peraldi, S.; Colosetti, P.; Auberger, P.; Bost, F. The antidiabetic drug metformin exerts an antitumoral effect in vitro and in vivo through a decrease of cyclin D1 level. Oncogene 2008, 27, 3576–3586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frid, A.; Sterner, G.N.; Londahl, M.; Wiklander, C.; Cato, A.; Vinge, E.; Andersson, A. Novel assay of metformin levels in patients with type 2 diabetes and varying levels of renal function: Clinical recommendations. Diabetes Care 2010, 33, 1291–1293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanzhi, C.; Cui, H.; Kang, L.; Zhang, X.; Jin, Z.; Lu, L.; Fan, Z. Metformin inhibits thyroid cancer cell growth, migration, and EMT through the mTOR pathway. Tumor Biol. 2015, 36, 6295–6304. [Google Scholar] [CrossRef]
- Cho, Y.Y.; Kang, M.J.; Kim, S.K.; Jung, J.H.; Hahm, J.R.; Kim, T.H.; Nam, J.Y.; Lee, B.-W.; Lee, Y.-H.; Chung, J.H.; et al. Protective Effect of Metformin Against Thyroid Cancer Development: A Population-Based Study in Korea. Thyroid 2018, 28, 864–870. [Google Scholar] [CrossRef]
- Klubo-Gwiezdzinska, J.; Burman, K.D.; Van Nostrand, D.; Mete, M.; Jacqueline, J.; Wartofsky, L. Potential Use of Recombinant Human Thyrotropin in the Treatment of Distant Metastases in Patients with Differentiated Thyroid Cancer. Endocr. Pract. 2013, 19, 139–148. [Google Scholar] [CrossRef] [Green Version]
- Jang, E.K.; Kim, W.G.; Kwon, H.; Choi, Y.M.; Jeon, M.J.; Kim, T.Y.; Shong, Y.K.; Kim, E.Y. Metformin Is Associated with a Favorable Outcome in Diabetic Patients with Cervical Lymph Node Metastasis of Differentiated Thyroid Cancer. Eur. Thyroid J. 2015, 4, 181–188. [Google Scholar] [CrossRef] [Green Version]
- Tella, S.H.; Kommalapati, A.; Esquivel, M.A.; Correa, R. Potential Role of Metabolic Intervention in the Management of Advanced Differentiated Thyroid Cancer. Front. Oncol. 2017, 7, 160. [Google Scholar] [CrossRef] [Green Version]
- Bikas, A.; Van Nostrand, D.; Jensen, K.E.; Desale, S.; Mete, M.; Patel, A.; Wartofsky, L.; Vasko, V.V.; Burman, K.D. Metformin Attenuates131I-Induced Decrease in Peripheral Blood Cells in Patients with Differentiated Thyroid Cancer. Thyroid 2016, 26, 280–286. [Google Scholar] [CrossRef]
- Sui, M.; Yu, Y.; Zhang, H.; Di, H.; Liu, C.; Fan, Y. Efficacy of Metformin for Benign Thyroid Nodules in Subjects With Insulin Resistance: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2018, 9, 494. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.; Yan, T.; Wang, G.; Chen, Y.; Zhu, Y.; Jiang, Z.; Yang, M.; Li, C.; Li, Z.; Yu, P.; et al. Correlation between Insulin Resistance and Thyroid Nodule in Type 2 Diabetes Mellitus. Int. J. Endocrinol. 2017, 2017, 1617458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anil, C.; Kut, A.; Atesagaoglu, B.; Nar, A.; Tutuncu, N.B.; Gursoy, A. Metformin Decreases Thyroid Volume and Nodule Size in Subjects with Insulin Resistance: A Preliminary Study. Med. Princ. Pract. 2016, 25, 233–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
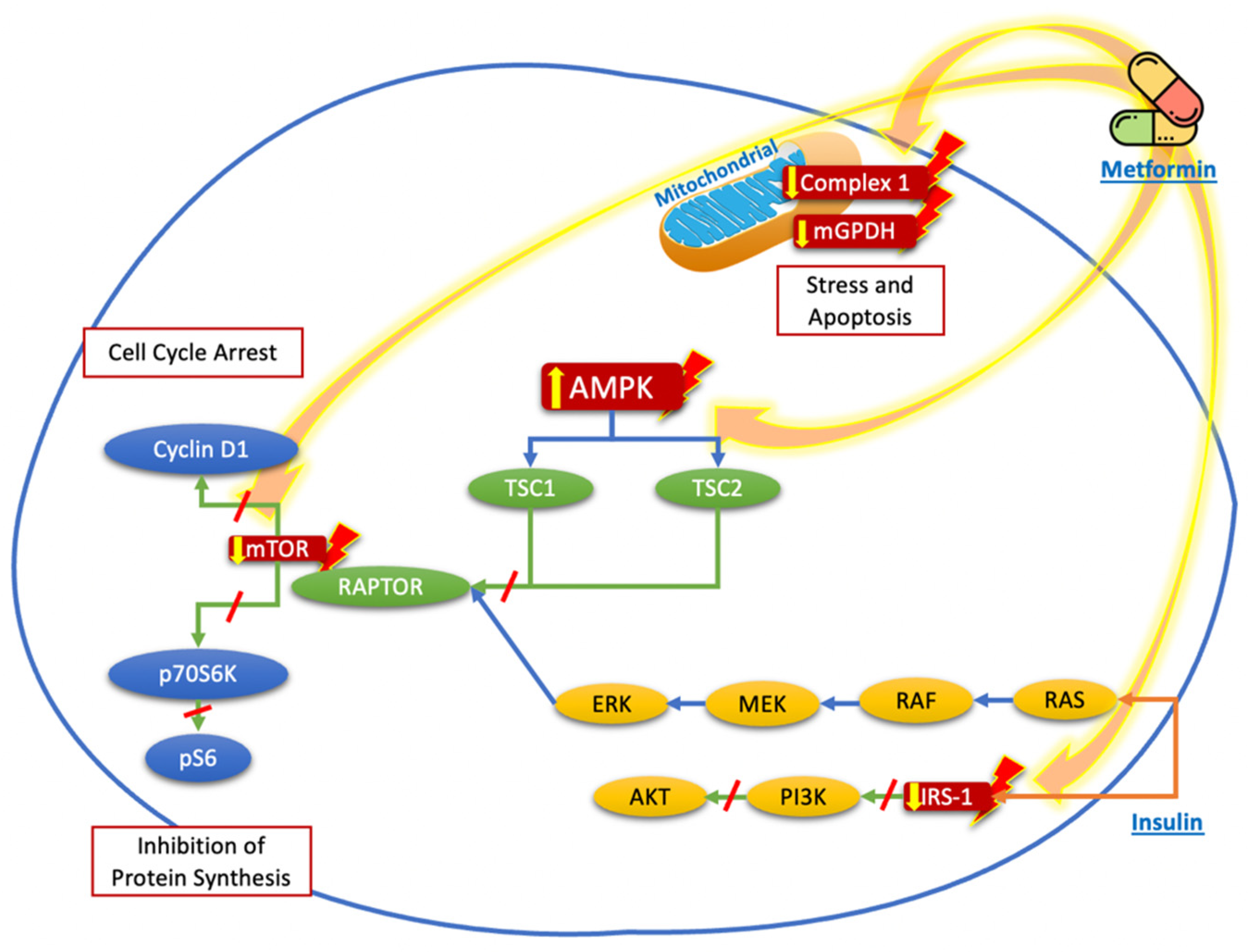
| References | Pathways/Target | Effect |
|---|---|---|
| Mitochondrial action | ||
| Thakur et al. [29] | Inhibition of mitochondrial complex 1 and mitochondrial glycerophosphate dehydrogenase (mGPDH) | Reduction in oxidative phosphorylation, decreased energy production, cell stress, and tumor cell apoptosis |
| Mechanisms involved in the inflammatory state | ||
| Rotondi et al. [33] Bauerle [31] | Inhibits interleukin 8 (CXCL8) stimulated by tumor necrosis factor alpha (TNF-α) which decrease nuclear factor κB (NF-κB) | Reduced growth and progression of thyroid cancer |
| AMPK-dependent | ||
| Chen et al. [17] Cho et al. [18] Hanly et al. [19] | Activation of AMPK triggers tuberous sclerosis complex 2 (TSC2), which inhibits the mTOR signaling pathway and hampers the activation of ribosomal protein S6 kinase beta-1 (p70S6K/pS6) and reduction in cyclin D1 | Inhibition of protein synthesis and cell cycle arrest |
| Pierotti et al. [22] | AMPK activation phosphorylates an inhibitory serine residue in the insulin receptor substrate-1 (IRS-1), leading to downregulation of insulin-like growth factor 1 receptor (IGF-1R), which decreases the signaling of phosphatidylinositol 3-kinase/protein kinase pathway (PI3K/AKT), leading to the reduction in mTOR pathway activation | Inhibition of protein synthesis and cell cycle arrest |
| AMPK-independent | ||
| Hadad et al. [23] | Tumor necrosis factor receptor 1 (TNFR1) and G1/S checkpoint regulation | Reduction in cancer cell growth |
| Kourelis et al. [24] | Inhibition of unfolded protein response (UPR) | Apoptosis, prevents angiogenesis, and induces toxicity on cancer stem cells |
| Reference | Study Design | Objective | Patients Characteristics | Metformin Dose | Duration of Treatment or Follow Up | Conclusions |
|---|---|---|---|---|---|---|
| Tseng et al. [42] | Clinical observational trial | To investigate the association between metformin use and thyroid cancer risk. | 795,321 metformin users and 619,402 non-metformin users, Taiwanese patients with T2D. | Cumulative dose of 263,000 mg. | 9 months | Metformin decreased thyroid cancer risk by 32%. |
| Cho et al. [46] | Retrospective cohort study | To investigate the association between metformin and thyroid cancer development. | Korean population: 128,453 metformin users and 128,453 non-users. | Mean cumulative dose of 868,169 (±563,221) mg. | 1633 (±915) days | Metformin reduced risk cancer by 31%. |
| Klubo-Gwiezdzinska et al. [47] | Single-center observational study | Whether the efficacy of conventional treatment of DTC is affected by therapy with metformin in patients with diabetes. | Patients with diabetes treated (n = 34) or not (n = 21) with metformin and control patients without diabetes (185). | 500–2000 mg/day. | 4.4 (±3) years | Age, locoregional metastases, distant metastases, and lack of treatment with metformin were associated with increased risk for shortened progression-free survival. Metformin-treated individuals had smaller tumor size and better remission rates. |
| Jang et al. [48] | Retrospective study | To evaluate the clinical outcome of patients with diabetes and DTC according to metformin treatment. | 60 patients with diabetes and 201 control patients with DTC after total thyroidectomy. | Mean dose of 979 mg. | 7.4 (±4.8) years | Metformin treatment was associated with longer disease-free survival. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Sáenz, M.; Lobaton-Ginsberg, M.; Ferreira-Hermosillo, A. Metformin in Differentiated Thyroid Cancer: Molecular Pathways and Its Clinical Implications. Biomolecules 2022, 12, 574. https://doi.org/10.3390/biom12040574
García-Sáenz M, Lobaton-Ginsberg M, Ferreira-Hermosillo A. Metformin in Differentiated Thyroid Cancer: Molecular Pathways and Its Clinical Implications. Biomolecules. 2022; 12(4):574. https://doi.org/10.3390/biom12040574
Chicago/Turabian StyleGarcía-Sáenz, Manuel, Miry Lobaton-Ginsberg, and Aldo Ferreira-Hermosillo. 2022. "Metformin in Differentiated Thyroid Cancer: Molecular Pathways and Its Clinical Implications" Biomolecules 12, no. 4: 574. https://doi.org/10.3390/biom12040574
APA StyleGarcía-Sáenz, M., Lobaton-Ginsberg, M., & Ferreira-Hermosillo, A. (2022). Metformin in Differentiated Thyroid Cancer: Molecular Pathways and Its Clinical Implications. Biomolecules, 12(4), 574. https://doi.org/10.3390/biom12040574








