Isolation, Characterization, and Biocompatibility of Bisporitin, a Ribotoxin-like Protein from White Button Mushroom (Agaricus bisporus)
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Mushroom Samples for Bisporitin Purification
2.3. Protein Purification
2.4. Analytical Procedures
2.5. Protein Synthesis Inhibition In Vitro
2.6. Enzymatic Assays
2.6.1. Ribonuclease Activity on Yeast High-Molecular-Weight RNA
2.6.2. Ribonucleolytic Activity on Rabbit Ribosomes
2.6.3. Nicking Endonuclease Activity on Supercoiled pUC18 DNA
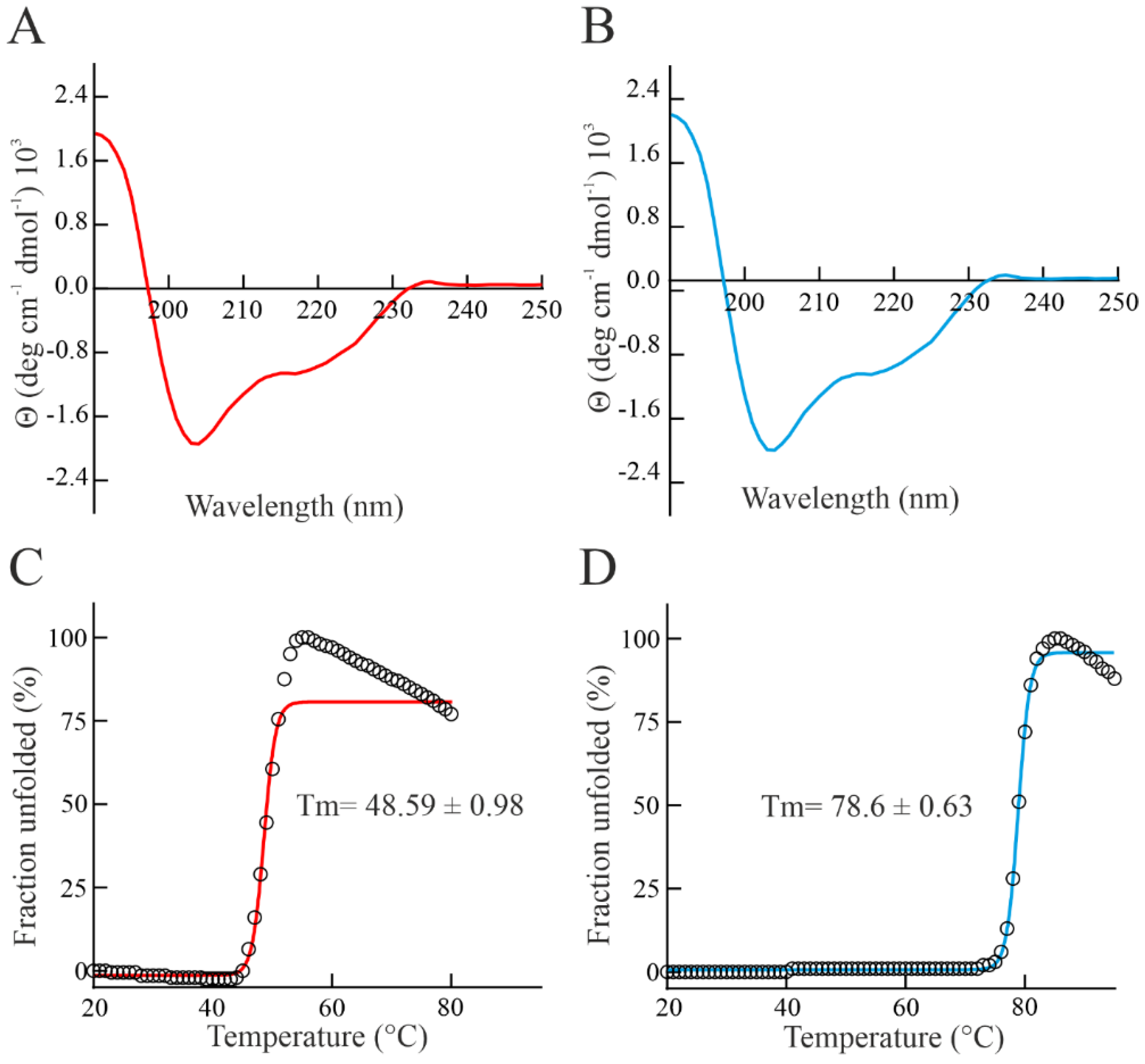
2.7. Circular Dichroism and Thermal Stability Determination
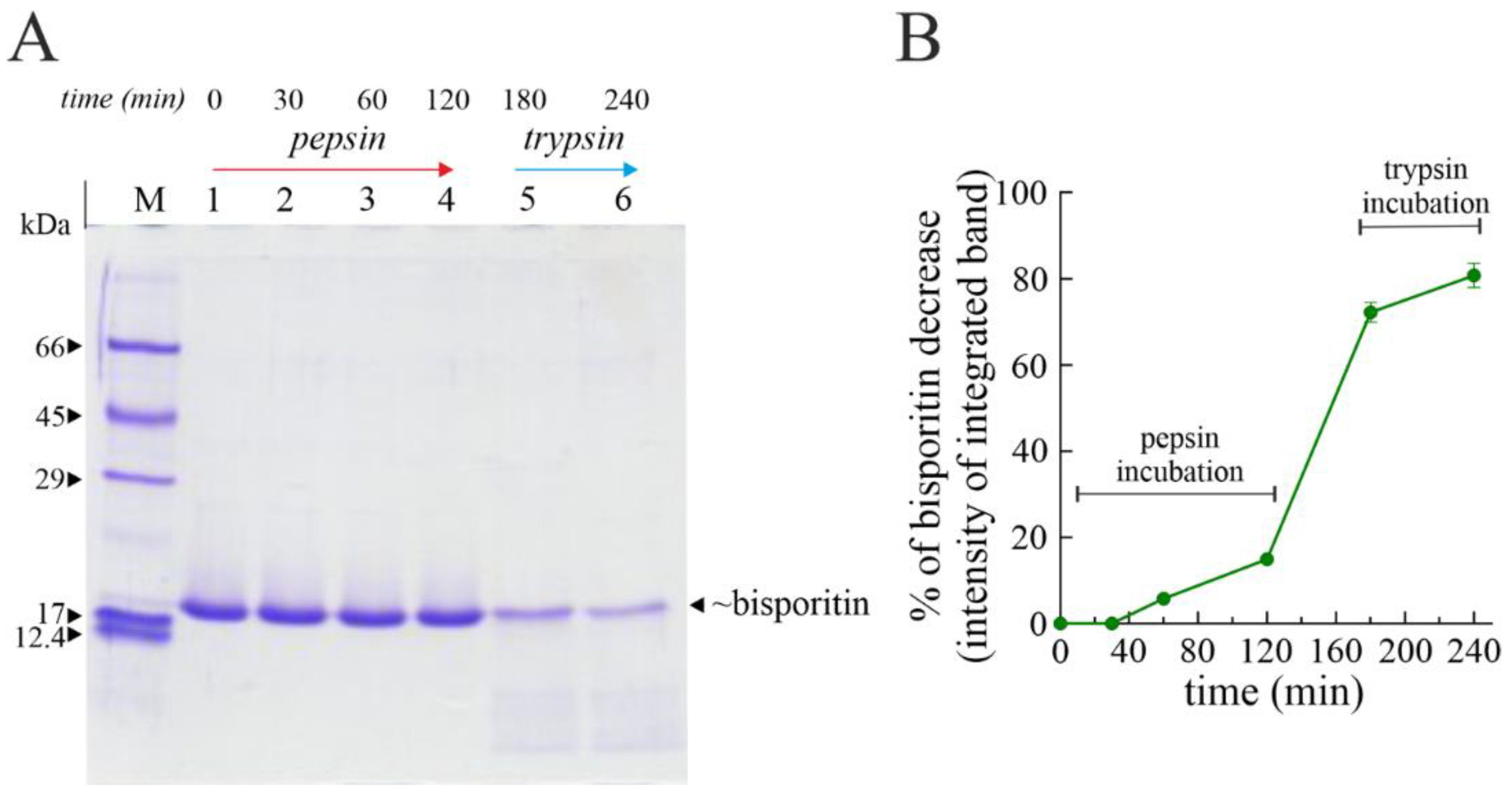
2.8. In Vitro Digestibility
2.9. Cell Cultures
2.10. Statistical Analysis
3. Results and Discussion
3.1. Purification of Bisporitin
3.2. Enzymatic Properties of Bisporitin
3.3. Spectroscopic Properties and Thermostability of Bisporitin
3.4. Digestibility of Bisporitin In Vitro
3.5. Susceptibility of Human Cell Lines to Bisporitin
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Imbach, E.J. Pilzflora des Kantons Luzern und der angrenzen Innerschweiz. Mitt. Nat. Ges. Luzern 1946, 15, 5–85. (In German) [Google Scholar]
- Royse, D.J.; Baars, J.; Tan, Q. Current Overview of Mushroom Production in the World. In Edible and Medicinal Mushrooms; Diego, Z.D., Pardo-Giménez, A., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2017; pp. 5–13. [Google Scholar]
- Kerrigan, R.W. Global genetic resources for Agaricus breeding and cultivation. Can. J. Bot. 1995, 73, 973–979. [Google Scholar] [CrossRef]
- Bhushan, A.; Kulshreshtha, M. The Medicinal Mushroom Agaricus bisporus: Review of Phytopharmacology and Potential Role in the Treatment of Various Diseases. J. Nat. Sci. Med. 2018, 1, 4–9. [Google Scholar]
- Usman, M.; Murtaza, G.; Ditta, A. Nutritional, Medicinal, and Cosmetic Value of Bioactive Compounds in Button Mushroom (Agaricus bisporus): A Review. Appl. Sci. 2021, 11, 5943. [Google Scholar] [CrossRef]
- El Sebaaly, Z.; Hammoud, M.; Sassine, Y.N. History, Health Benefits, Market, and Production Status of Button Mushroom. In Mushrooms: Agaricus bisporus; Sassine, Y.N., Ed.; CABI: Boston, MA, USA, 2021; pp. 1–65. [Google Scholar]
- Muszyńska, B.; Kała, K.; Rojowski, J.; Grzywacz, A.; Opoka, W. Composition and Biological Properties of Agaricus bisporus Fruiting Bodies-a Review. Pol. J. Food Nutr. Sci. 2017, 67, 173–181. [Google Scholar] [CrossRef]
- Ramos, M.; Burgos, N.; Barnard, A.; Evans, G.; Preece, J.; Graz, M.; Ruthes, A.C.; Jiménez-Quero, A.; Martínez-Abad, A.; Vilaplana, F.; et al. Agaricus bisporus and its by-products as a source of valuable extracts and bioactive compounds. Food Chem. 2019, 292, 176–187. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Ho, K.-J.; Hsieh, Y.-J.; Wang, L.-T.; Mau, J.-L. Contents of lovastatin, γ-aminobutyric acid and ergothioneine in mushroom fruiting bodies and mycelia. LWT 2012, 47, 274–278. [Google Scholar] [CrossRef]
- Ng, T.B. Peptides and proteins from fungi. Peptides 2004, 25, 1055–1073. [Google Scholar] [CrossRef]
- Ismaya, W.T.; Tjandrawinata, R.R.; Rachmawati, H. Lectins from the Edible Mushroom Agaricus bisporus and Their Therapeutic Potentials. Molecules 2020, 25, 2368. [Google Scholar] [CrossRef]
- Wang, H.X.; Ng, T.B. Examination of lectins, polysaccharopeptide, polysaccharide, alkaloid, coumarin and trypsin inhibitors for inhibitory activity against human immunodeficiency virus reverse transcriptase and glycohydrolases. Planta Med. 2001, 67, 669–672. [Google Scholar] [CrossRef]
- Othman, A.M.; Elsayed, M.A.; Elshafei, A.M.; Hassan, M.M. Purification and biochemical characterization of two isolated laccase isoforms from Agaricus bisporus CU13 and their potency in dye decolorization. Int. J. Biol. Macromol. 2018, 113, 1142–1148. [Google Scholar] [CrossRef]
- Wannet, W.J.; Wassenaar, R.W.; Jorissen, H.J.; van der Drift, C.; Op den Camp, H.J. Purification and characterization of an acid phosphatase from the commercial mushroom Agaricus bisporus. Antonie Leeuwenhoek 2000, 77, 215–222. [Google Scholar] [CrossRef]
- Hu, Y.; Zhu, M.; Tian, G.; Zhao, L.; Wang, H.; Ng, T.B. Isolation of a protease-resistant and pH-stable α-galactosidase displaying hydrolytic efficacy toward raffinose family oligosaccharides from the button mushroom Agaricus bisporus. Int. J. Biol. Macromol. 2017, 104, 576–583. [Google Scholar] [CrossRef]
- Bonnen, A.M.; Anton, L.H.; Orth, A.B. Lignin-Degrading Enzymes of the Commercial Button Mushroom, Agaricus bisporus. Appl. Environ. Microbiol. 1994, 60, 960–965. [Google Scholar] [CrossRef]
- Landi, N.; Pacifico, S.; Ragucci, S.; Iglesias, R.; Piccolella, S.; Amici, A.; Di Giuseppe, A.M.A.; Di Maro, A. Purification, characterization and cytotoxicity assessment of Ageritin: The first ribotoxin from the basidiomycete mushroom Agrocybe aegerita. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 1113–1121. [Google Scholar] [CrossRef]
- Landi, N.; Ragucci, S.; Culurciello, R.; Russo, R.; Valletta, M.; Pedone, P.V.; Pizzo, E.; Di Maro, A. Ribotoxin-like proteins from Boletus edulis: Structural properties, cytotoxicity and in vitro digestibility. Food Chem. 2021, 359, 129931. [Google Scholar] [CrossRef] [PubMed]
- Landi, N.; Grundner, M.; Ragucci, S.; Pavšič, M.; Mravinec, M.; Pedone, P.V.; Sepčić, K.; Di Maro, A. Characterization and cytotoxic activity of ribotoxin-like proteins from the edible mushroom Pleurotus eryngii. Food Chem. 2022, 396, 133655. [Google Scholar] [CrossRef] [PubMed]
- Landi, N.; Ragucci, S.; Russo, R.; Valletta, M.; Pizzo, E.; Ferreras, J.M.; Di Maro, A. The ribotoxin-like protein Ostreatin from Pleurotus ostreatus fruiting bodies: Confirmation of a novel ribonuclease family expressed in basidiomycetes. Int. J. Biol. Macromol. 2020, 161, 1329–1336. [Google Scholar] [CrossRef] [PubMed]
- Olombrada, M.; Lázaro-Gorines, R.; López-Rodríguez, J.C.; Martínez-Del-Pozo, Á.; Oñaderra, M.; Maestro-López, M.; Lacadena, J.; Gavilanes, J.G.; García-Ortega, L. Fungal Ribotoxins: A Review of Potential Biotechnological Applications. Toxins 2017, 9, 71. [Google Scholar] [CrossRef]
- Landi, N.; Ragucci, S.; Russo, R.; Pedone, P.V.; Chambery, A.; Di Maro, A. Structural insights into nucleotide and protein sequence of Ageritin: A novel prototype of fungal ribotoxin. J. Biochem. 2019, 165, 415–422. [Google Scholar] [CrossRef]
- Ragucci, S.; Landi, N.; Russo, R.; Valletta, M.; Pedone, P.V.; Chambery, A.; Di Maro, A. Ageritin from Pioppino Mushroom: The Prototype of Ribotoxin-Like Proteins, a Novel Family of Specific Ribonucleases in Edible Mushrooms. Toxins 2021, 13, 263. [Google Scholar] [CrossRef] [PubMed]
- Landi, N.; Hussain, H.Z.F.; Pedone, P.V.; Ragucci, S.; Di Maro, A. Ribotoxic Proteins, Known as Inhibitors of Protein Synthesis, from Mushrooms and Other Fungi According to Endo’s Fragment Detection. Toxins 2022, 14, 403. [Google Scholar] [CrossRef] [PubMed]
- Ragucci, S.; Pacifico, S.; Ruocco, M.R.; Crescente, G.; Nasso, R.; Simonetti, M.; Masullo, M.; Piccolella, S.; Pedone, P.V.; Landi, N.; et al. Ageritin from poplar mushrooms: Scale-up purification and cytotoxicity towards undifferentiated and differentiated SH-SY5Y cells. Food Funct. 2019, 10, 6342–6350. [Google Scholar] [CrossRef] [PubMed]
- Citores, L.; Ragucci, S.; Ferreras, J.M.; Di Maro, A.; Iglesias, R. Ageritin, a Ribotoxin from Poplar Mushroom (Agrocybe aegerita) with Defensive and Antiproliferative Activities. ACS Chem. Biol. 2019, 14, 1319–1327. [Google Scholar] [CrossRef]
- Rotondo, R.; Ragucci, S.; Castaldo, S.; Landi, N.; Oliva, M.A.; Pedone, P.V.; Di Maro, A.; Arcella, A. Ageritin-The Ribotoxin-like Protein from Poplar Mushroom (Cyclocybe aegerita) Sensitizes Primary Glioblastoma Cells to Conventional Temozolomide Chemotherapy. Molecules 2022, 27, 2385. [Google Scholar] [CrossRef]
- Khirehgesh, M.R.; Sharifi, J.; Safari, F.; Akbari, B. Immunotoxins and nanobody-based immunotoxins: Review and update. J. Drug Target. 2021, 29, 848–862. [Google Scholar] [CrossRef]
- Di Maro, A.; Chambery, A.; Daniele, A.; Casoria, P.; Parente, A. Isolation and characterization of heterotepalins, type 1 ribosome-inactivating proteins from Phytolacca heterotepala leaves. Phytochemistry 2007, 68, 767–776. [Google Scholar] [CrossRef]
- Langer, M.; Rothe, M.; Eck, J.; Möckel, B.; Zinke, H. A nonradioactive assay for ribosome-inactivating proteins. Anal. Biochem. 1996, 243, 150–153. [Google Scholar] [CrossRef]
- Greiner-Stoeffele, T.; Grunow, M.; Hahn, U. A general ribonuclease assay using methylene blue. Anal. Biochem. 1996, 240, 24–28. [Google Scholar] [CrossRef]
- Iglesias, R.; Citores, L.; Ragucci, S.; Russo, R.; Di Maro, A.; Ferreras, J.M. Biological and antipathogenic activities of ribosome-inactivating proteins from Phytolacca dioica L. Biochim. Biophys. Acta Gen. Subj. 2016, 1860, 1256–1264. [Google Scholar] [CrossRef]
- Wang, X.; Gao, W.; Zhang, J.; Zhang, H.; Li, J.; He, X.; Ma, H. Subunit, amino acid composition and in vitro digestibility of protein isolates from Chinese kabuli and desi chickpea (Cicer arietinum L.) cultivars. Food Res. Int. 2010, 43, 567–572. [Google Scholar] [CrossRef]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of Cell Viability by the MTT Assay. Cold Spring Harb. Protoc. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, A.; García-Ortega, L.; Ragucci, S.; Russo, R.; Landi, N.; Berisio, R.; Di Maro, A. Structural and enzymatic properties of Ageritin, a novel metal-dependent ribotoxin-like protein with antitumor activity. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 2888–2894. [Google Scholar] [CrossRef]
- Zhou, H.; Tan, Y.; McClements, D.J. Applications of the INFOGEST In Vitro Digestion Model to Foods: A Review. Annu. Rev. Food Sci. Technol. 2022, 14, 1. [Google Scholar] [CrossRef] [PubMed]
- Landi, N.; Ruocco, M.R.; Ragucci, S.; Aliotta, F.; Nasso, R.; Pedone, P.V.; Di Maro, A. Quinoa as source of type 1 ribosome inactivating proteins: A novel knowledge for a revision of its consumption. Food Chem. 2021, 342, 128337. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ragucci, S.; Hussain, H.Z.F.; Bosso, A.; Landi, N.; Clemente, A.; Pedone, P.V.; Pizzo, E.; Di Maro, A. Isolation, Characterization, and Biocompatibility of Bisporitin, a Ribotoxin-like Protein from White Button Mushroom (Agaricus bisporus). Biomolecules 2023, 13, 237. https://doi.org/10.3390/biom13020237
Ragucci S, Hussain HZF, Bosso A, Landi N, Clemente A, Pedone PV, Pizzo E, Di Maro A. Isolation, Characterization, and Biocompatibility of Bisporitin, a Ribotoxin-like Protein from White Button Mushroom (Agaricus bisporus). Biomolecules. 2023; 13(2):237. https://doi.org/10.3390/biom13020237
Chicago/Turabian StyleRagucci, Sara, Hafiza Zumra Fatima Hussain, Andrea Bosso, Nicola Landi, Angela Clemente, Paolo Vincenzo Pedone, Elio Pizzo, and Antimo Di Maro. 2023. "Isolation, Characterization, and Biocompatibility of Bisporitin, a Ribotoxin-like Protein from White Button Mushroom (Agaricus bisporus)" Biomolecules 13, no. 2: 237. https://doi.org/10.3390/biom13020237
APA StyleRagucci, S., Hussain, H. Z. F., Bosso, A., Landi, N., Clemente, A., Pedone, P. V., Pizzo, E., & Di Maro, A. (2023). Isolation, Characterization, and Biocompatibility of Bisporitin, a Ribotoxin-like Protein from White Button Mushroom (Agaricus bisporus). Biomolecules, 13(2), 237. https://doi.org/10.3390/biom13020237









