Chronic Exposure to Low-Molecular-Weight Polycyclic Aromatic Hydrocarbons Promotes Lipid Accumulation and Metabolic Inflammation
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. RNA Extraction and RT-PCR Analysis
2.3. Enzyme-Linked Immunosorbent Assays (ELISA)
2.4. Staining and Visualization of Neutral Lipids
2.5. Statistical Analysis
3. Results
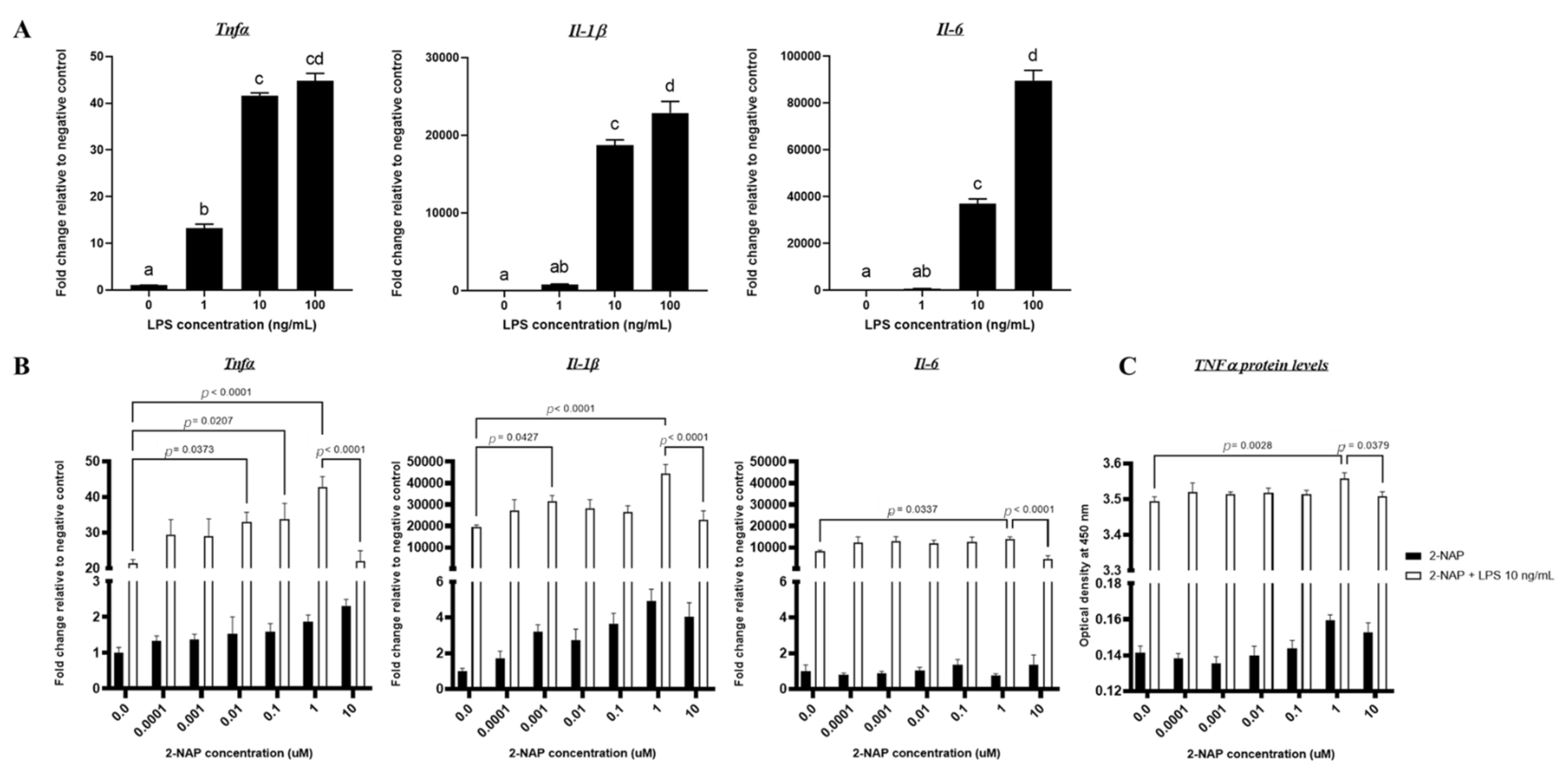
3.1. In Vitro Effects of Varying LPS Concentrations on RAW264.7 Macrophages
3.2. Acute 2-NAP Treatment Enhances Inflammatory Responses in RAW264.7 Macrophages
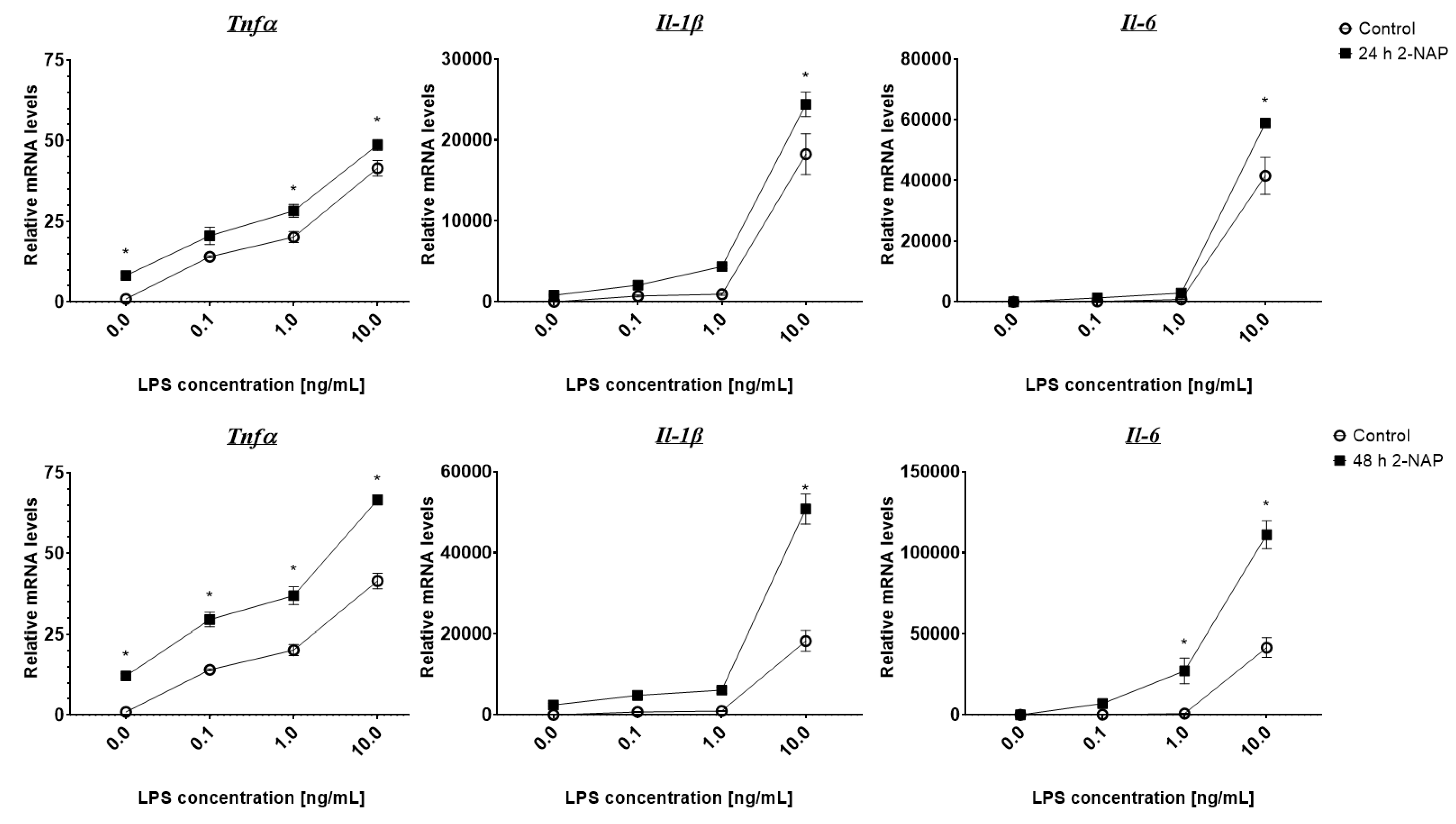
3.3. Chronic 2-Naphthol Exposure Increases the Expression of Inflammatory Genes in Macrophages
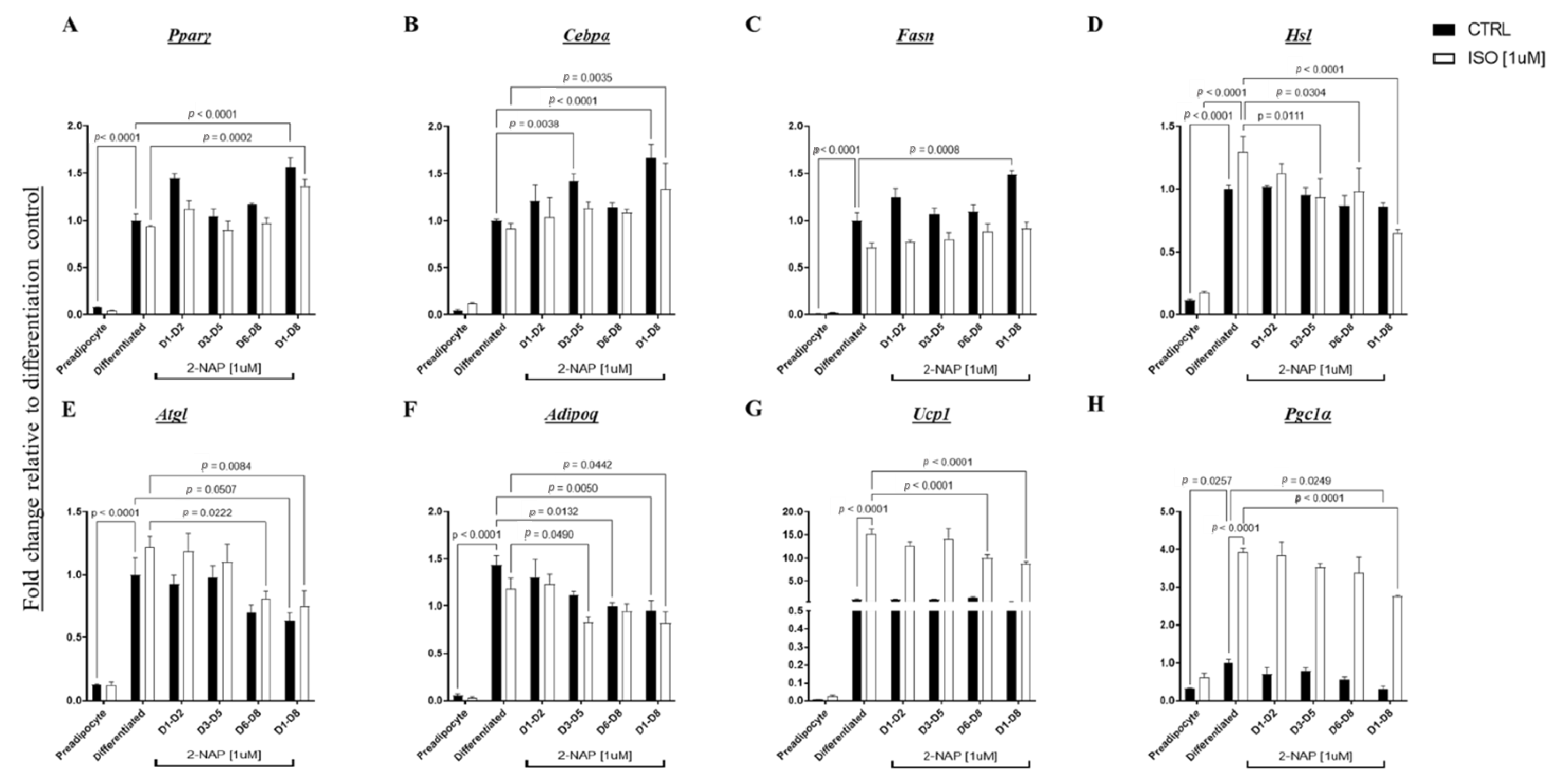
3.4. Chronic 2-Naphthol Treatment Up-Regulates An Adipogenic and Lipogenic Program Gene Expression in 3T3-L1 Cells
3.5. Qualitative Analysis Shows An Increase in Lipid Accumulation in 2-NAP Treated 3T3-L1 Adipocytes
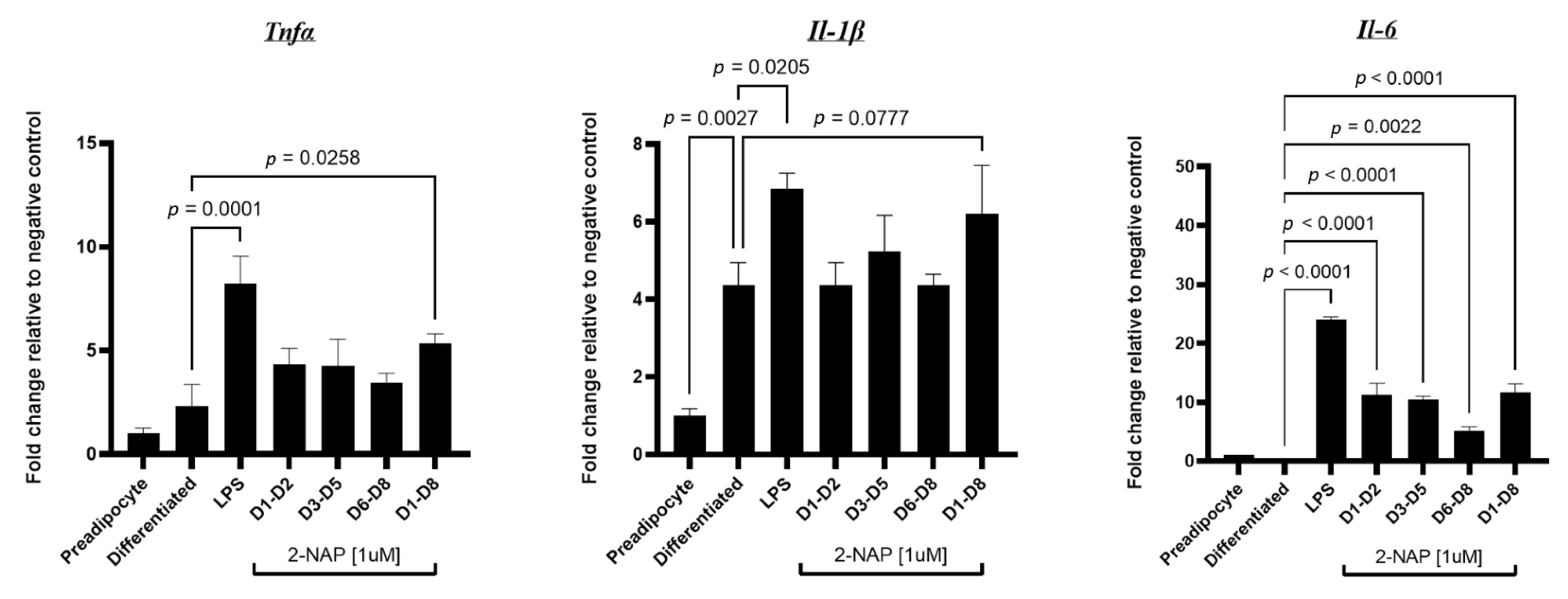
3.6. Chronic 2-NAP Exposure Increases the Expression of Inflammatory Genes in 3T3-L1 Cells
3.7. Brown Adipocytes Exposed to 2-NAP during Differentiation Displayed A Decrease in Thermogenesis and An Increase in Adipogenic Gene Expression
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, C.; Ying, Z.; Harkema, J.; Sun, Q.; Rajagopalan, S. Epidemiological and Experimental Links between Air Pollution and Type 2 Diabetes. Toxicol. Pathol. 2013, 41, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Grover, P.L.; Sims, P.; Huberman, E.; Marquardt, H.; Kuroki, T.; Heidelberger, C. In vitro transformation of rodent cells by K-region derivatives of polycyclic hydrocarbons. Proc. Natl. Acad. Sci. 1971, 68, 1098–1101. [Google Scholar] [CrossRef] [PubMed]
- Lavoie, E.J.; Tulley, L.; Bedenko, V.; Hoffmann, D. Mutagenicity of methylated fluorenes and benzofluorenes. Mutat. Res. Lett. 1981, 91, 167–176. [Google Scholar] [CrossRef]
- Nisbet, I.C.T.; LaGoy, P.K. Toxic equivalency factors (TEFs) for polycyclic aromatic hydrocarbons (PAHs). Regul. Toxicol. Pharmacol. 1992, 16, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Lu, H.; Chen, S.; Amagai, T. Pollution level, phase distribution and source analysis of polycyclic aromatic hydrocarbons in residential air in Hangzhou, China. J. Hazard. Mater. 2009, 162, 1165–1170. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.O.; Goldstone, M.E.; Kirk, P.W.W.; Lester, J.N.; Perry, R. Phase distribution and particle size dependency of polycyclic aromatic hydrocarbons in the urban atmosphere. Chemosphere 1991, 22, 503–520. [Google Scholar] [CrossRef]
- Cautreels, W.; Van Cauwenberghe, K. Experiments on the distribution of organic pollutants between airborne particulate matter and the corresponding gas phase. Atmospheric Environ. 1978, 12, 1133–1141. [Google Scholar] [CrossRef]
- Mumtaz, M.; George, J. Toxicological profile for polycyclic aromatic hydrocarbons; Agency for Toxic Substances and Disease Registry (ATSDR): Atlanta, GA, USA, 1995; pp. 1–458.
- El-Masri, H.; Mumtaz, M.; Todd, D.G.; McClure, P.; Marable, B.; Singh, M. Toxicological Profile for Naphthalene, 1-Methylnaphthalene, and 2- Methylnaphthalene (Update); Agency for Toxic Substances and Disease Registry (ATSDR): Atlanta, GA, USA, 2005; pp. 1–291.
- Scinicariello, F.; Buser, M.C. Urinary Polycyclic Aromatic Hydrocarbons and Childhood Obesity: NHANES (2001–2006). El-Masri 2014, 122, 299–303. [Google Scholar] [CrossRef]
- Yan, Z.; Zhang, H.; Maher, C.; Arteaga-Solis, E.; Champagne, F.A.; Wu, L.; McDonald, J.D.; Yan, B.; Schwartz, G.J.; Miller, R.L. Prenatal Polycyclic Aromatic Hydrocarbon, Adiposity, Peroxisome Proliferator-Activated Receptor (PPAR) γ Methylation in Offspring, Grand-Offspring Mice. PLoS ONE 2014, 9, e110706. [Google Scholar] [CrossRef]
- Pei, X.-H.; Nakanishi, Y.; Inoue, H.; Takayama, K.; Bai, F.; Hara, N. Polycyclic aromatic hydrocarbons induce IL-8 expression through nuclear factor kB activation in A549 cell line. Cytokine 2002, 19, 236–241. [Google Scholar] [CrossRef]
- Vandebriel, R.J.; Meredith, C.; Scott, M.P.; Roholl, P.J.M.; Van Loveren, H. Effects of in Vivo Exposure to Bis(tri-n-butyltin)oxide, Hexachlorobenzene, and Benzo(a)pyrene on Cytokine (Receptor) mRNA Levels in Cultured Rat Splenocytes and on IL-2 Receptor Protein Levels 1 Effects of in Vivo Exposure to Bis(tri-n-butyltin)oxide, He. Toxicol. Appl. Pharmacol. 1998, 148, 126–136. [Google Scholar] [CrossRef]
- Ndiaye, M.; Le Ferrec, E.; Lagadic-Gossmann, D.; Bastien Corre, S.; Gilot, D.; Rie Lecureur, V.; Monteiro, P.; Rauch, C.; Galibert, M.-D.; Fardel, O. Aryl Hydrocarbon Receptor-and Calcium-dependent Induction of the Chemokine CCL1 by the Environmental Contaminant Benzo[a]pyrene. J. Biol. Chem. 2006, 281, 19906–19915. [Google Scholar] [CrossRef] [PubMed]
- Lecureur, V.; Le Ferrec, E.; N'Diaye, M.; Le Vee, M.; Gardyn, C.; Gilot, D.; Fardel, O. ERK-dependent induction of TNFα expression by the environmental contaminant benzo(a)pyrene in primary human macrophages. FEBS Lett. 2005, 579, 1904–1910. [Google Scholar] [CrossRef] [PubMed]
- Myers, M.J.; Schook, L.B.; Bick, P.H. Mechanisms of Benzo(A)Pyrene-lnduced Modulation of Antigen Presentation. J. Pharmacol. Exp. Ther. 1987, 242, 399–404. [Google Scholar]
- Lyte, M.; Bick, P.H. Modulation of interleukin-1 production by macrophages following benzo(a)pyrene exposure. Int. J. Immunopharmacol. 1986, 8, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Qiu, B.; Simon, C.M. BODIPY 493/503 Staining of Neutral Lipid Droplets for Microscopy and Quantification by Flow Cytometry. Bio Protoc. 2016, 6, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Baris, E.; Efe, H.; Gumustekin, M.; Arici, A.; Tosun, M. Varenicline Prevents LPS-Induced Inflammatory Response via Nicotinic Acetylcholine Receptors in RAW 264.7 Macrophages. Front. Mol. Biosci. 2021, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Bajaj, M. Normal Adipose Tissue Biology: Adipocytokines and Inflammation. Pathobiol. Hum. Dis. A Dyn. Encycl. Dis. Mech. 2014, 488–497. [Google Scholar] [CrossRef]
- Diggs, D.L.; Huderson, A.C.; Harris, K.L.; Myers, J.N.; Banks, L.D.; Rekhadevi, P.V.; Niaz, M.S.; Ramesh, A. Polycyclic Aromatic Hydrocarbons and digestive tract cancers-a perspective. J. Environ. Sci. Heal. Part C. 2011, 29, 324–357. [Google Scholar] [CrossRef]
- Marris, C.R.; Kompella, S.N.; Miller, M.R.; Incardona, J.P.; Brette, F.; Hancox, J.C.; Sørhus, E.; Shiels, H.A. Polyaromatic hydrocarbons in pollution: A heart-breaking matter. J. Physiol. 2020, 598, 227–247. [Google Scholar] [CrossRef]
- Kilanowicz, A.; Czerski, B.; Sapota, A. The disposition and metabolism of naphthalene in rats. Int. J. Occup. Med. Environ. Health 1999, 12, 209–219. [Google Scholar]
- Berry, D.C.; Stenesen, D.; Zeve, D.; Graff, J.M. The developmental origins of adipose tissue. Development 2013, 140, 3939–3949. [Google Scholar] [CrossRef] [PubMed]
- Hu, E.; Liang, P.; Spiegelman, B.M. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J. Biol. Chem. 1996, 271, 10697–10703. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, T.; Kamon, J.; Waki, H.; Terauchi, Y.; Kubota, N.; Hara, K.; Mori, Y.; Ide, T.; Murakami, K.; Tsuboyama-Kasaoka, N.; et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat. Medi-Cine Vol. 2001, 7, 941–946. [Google Scholar] [CrossRef] [PubMed]
- Arita, Y.; Kihara, S.; Ouchi, N.; Takahashi, M.; Maeda, K.; Miyagawa, J.I.; Hotta, K.; Shimomura, I.; Nakamura, T.; Miyaoka, K.; et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem. Biophys. Res. Commun. 1999, 257, 79–83. [Google Scholar] [CrossRef]
- Wang, Z.V.; Scherer, P.E. Adiponectin, the past two decades. J. Mol. Cell Biol. 2016, 8, 93–100. [Google Scholar] [CrossRef]
- Stern, J.H.; Rutkowski, J.M.; Scherer, P.E. Adiponectin, Leptin, and Fatty Acids in the Maintenance of Metabolic Homeostasis through Adipose Tissue Crosstalk. Cell Metab. 2016, 23, 770–784. [Google Scholar] [CrossRef]
- Kajimura, S.; Seale, P.; Spiegelman, B.M. Transcriptional Control of Brown Fat Development. Cell Metab. 2010, 11, 257–262. [Google Scholar] [CrossRef]
- Wang, W.; Seale, P. Control of brown and beige fat development. Nat. Rev. Mol. Cell Biol. 2016, 17, 691–702. [Google Scholar] [CrossRef]
- Cannon, B.; Nedergaard, J. The biochemistry of an inefficient tissue: Brown adipose tissue. Essays in Biochem. 1985, 20, 110–164. [Google Scholar]
- Cannon, B.; Nedergaard, J. Nonshivering thermogenesis and its adequate measurement in metabolic studies. J. Exp. Biol. 2011, 214, 242–253. [Google Scholar] [CrossRef]
- Cannon, B.; Nedergaard, J.A.N. Brown Adipose Tissue: Function and Physiological Significance. Physiol. Rev. 2004, 277–359. [Google Scholar] [CrossRef] [PubMed]
- Cinti, S. The adipose organ. Prostaglandins Leukot. Essent. Fat. Acids 2005, 73, 9–15. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Olefsky, J.M.; Glass, C.K. Macrophages, Inflammation, and Insulin Resistance. Annu. Rev. Physiol. 2010, 72, 219–246. [Google Scholar] [CrossRef] [PubMed]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Barnes, G.T.; Yang, Q.; Tan, G.; Yang, D.; Chou, C.J.; Sole, J.; Nichols, A.; Ross, J.S.; Tartaglia, L.A.; et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Investig. 2003, 112, 1821–1830. [Google Scholar] [CrossRef]
- Crewe, C.; An, Y.A.; Scherer, P.E. The ominous triad of adipose tissue dysfunction: Inflammation, fibrosis, and impaired angiogenesis. J. Clin. Investig. 2017, 127, 74. [Google Scholar] [CrossRef]
- Sun, K.; Kusminski, C.M.; Scherer, P.E. Adipose tissue remodeling and obesity. J. Clin. Investig. 2011, 121, 2094–2101. [Google Scholar] [CrossRef]
- Fu, H.; Liu, X.; Li, W.; Zu, Y.; Zhou, F.; Shou, Q.; Ding, Z. PM2.5 Exposure Induces Inflammatory Response in Macrophages via the TLR4/COX-2/NF-κB Pathway. Inflammation 2020, 43, 1948–1958. [Google Scholar] [CrossRef]
- Zheng, Z.; Zhang, X.; Wang, J.; Dandekar, A.; Kim, H.; Qiu, Y.; Xu, X.; Cui, Y.; Wang, A.; Chen, L.C.; et al. Exposure to fine airborne particulate matters induces hepatic fibrosis in murine models. J. Hepatol. 2015, 63, 1397–1404. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Xu, X.; Zhang, X.; Wang, A.; Zhang, C.; Hüttemann, M.; Grossman, L.I.; Chen, L.C.; Rajagopalan, S.; Sun, Q.; et al. Exposure to ambient particulate matter induces a NASH-like phenotype and impairs hepatic glucose metabolism in an animal model. J. Hepatol. 2013, 58, 148–154. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bright, A.; Li, F.; Movahed, M.; Shi, H.; Xue, B. Chronic Exposure to Low-Molecular-Weight Polycyclic Aromatic Hydrocarbons Promotes Lipid Accumulation and Metabolic Inflammation. Biomolecules 2023, 13, 196. https://doi.org/10.3390/biom13020196
Bright A, Li F, Movahed M, Shi H, Xue B. Chronic Exposure to Low-Molecular-Weight Polycyclic Aromatic Hydrocarbons Promotes Lipid Accumulation and Metabolic Inflammation. Biomolecules. 2023; 13(2):196. https://doi.org/10.3390/biom13020196
Chicago/Turabian StyleBright, Asia, Fenfen Li, Miranda Movahed, Hang Shi, and Bingzhong Xue. 2023. "Chronic Exposure to Low-Molecular-Weight Polycyclic Aromatic Hydrocarbons Promotes Lipid Accumulation and Metabolic Inflammation" Biomolecules 13, no. 2: 196. https://doi.org/10.3390/biom13020196
APA StyleBright, A., Li, F., Movahed, M., Shi, H., & Xue, B. (2023). Chronic Exposure to Low-Molecular-Weight Polycyclic Aromatic Hydrocarbons Promotes Lipid Accumulation and Metabolic Inflammation. Biomolecules, 13(2), 196. https://doi.org/10.3390/biom13020196





