Establishment of a Cell Line Stably Expressing the Growth Hormone Secretagogue Receptor to Identify Crocin as a Ghrelin Agonist
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Teaghrelin Isolation
2.3. Cell Culture and Lentiviral Transduction
2.4. Polymerase Chain Reaction (PCR)
2.5. PrestoBlue Assay for Cell Viability
2.6. Immunoblotting Analysis
2.7. Immunofluorescence Staining
2.8. Silence of GHSR1a by RNA Interference
2.9. Homology Modeling and Docking
2.10. Statistical Analysis
3. Results
3.1. Generation of Human HEK293T Cells Expressing GHSR1a
3.2. Effects of Ghrelin, Teaghrelin, and SP-Analog on Cell Viability of GHSR1a-mCherry HEK293T and A549 Cells
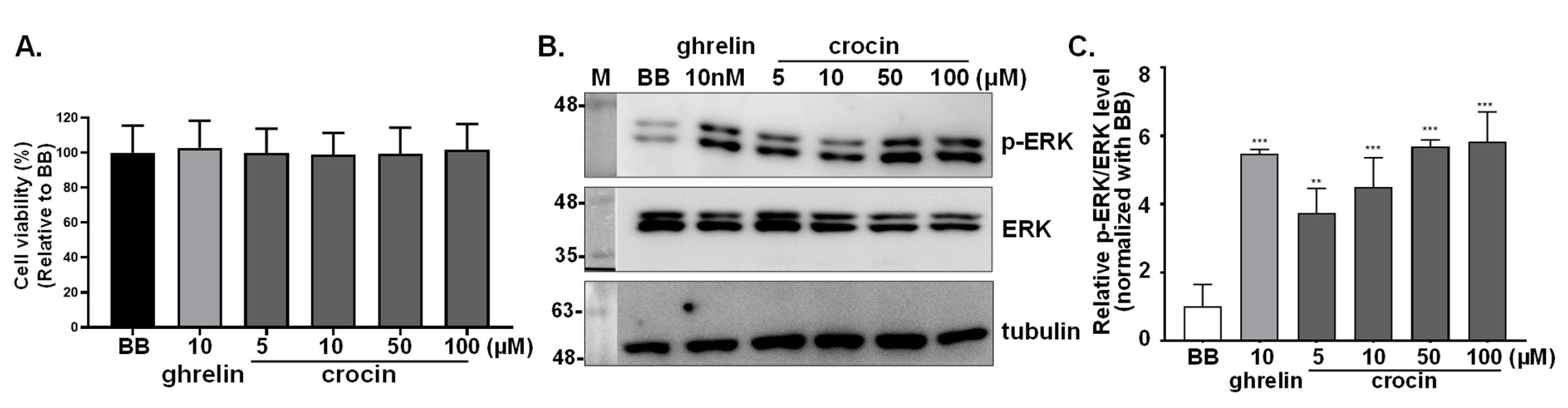
3.3. Activation of Intracellular ERK1/2 Signaling in GHSR1a-mCherry HEK293T Cells
3.4. Activation of Intracellular ERK1/2 Signaling in A549 Cells
3.5. Modeling of Crocin Docking to the GHSR1a Receptor
3.6. Detection of Crocin as a Ghrelin Agonist
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kojima, M.; Hosoda, H.; Date, Y.; Nakazato, M.; Matsuo, H.; Kangawa, K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 1999, 402, 656–660. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, P.; Zheng, H.; Smith, R.G. Ghrelin stimulation of growth hormone release and appetite is mediated through the growth hormone secretagogue receptor. Proc. Natl. Acad. Sci. USA 2004, 101, 4679–4684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Guo, S.; Zhuang, Y.; Yun, Y.; Xu, P.; He, X.; Guo, J.; Yin, W.; Xu, H.E.; Xie, X.; et al. Molecular recognition of an acyl-peptide hormone and activation of ghrelin receptor. Nat. Commun. 2021, 12, 5064. [Google Scholar] [CrossRef] [PubMed]
- Heppner, K.M.; Chaudhary, N.; Muller, T.D.; Kirchner, H.; Habegger, K.M.; Ottaway, N.; Smiley, D.L.; Dimarchi, R.; Hofmann, S.M.; Woods, S.C.; et al. Acylation type determines ghrelin’s effects on energy homeostasis in rodents. Endocrinology 2012, 153, 4687–4695. [Google Scholar] [CrossRef]
- Chuang, J.C.; Perello, M.; Sakata, I.; Osborne-Lawrence, S.; Savitt, J.M.; Lutter, M.; Zigman, J.M. Ghrelin mediates stress-induced food-reward behavior in mice. J. Clin. Investig. 2011, 121, 2684–2692. [Google Scholar] [CrossRef]
- Diano, S.; Farr, S.A.; Benoit, S.C.; McNay, E.C.; da Silva, I.; Horvath, B.; Gaskin, F.S.; Nonaka, N.; Jaeger, L.B.; Banks, W.A.; et al. Ghrelin controls hippocampal spine synapse density and memory performance. Nat. Neurosci. 2006, 9, 381–388. [Google Scholar] [CrossRef]
- Akamizu, T.; Takaya, K.; Irako, T.; Hosoda, H.; Teramukai, S.; Matsuyama, A.; Tada, H.; Miura, K.; Shimizu, A.; Fukushima, M.; et al. Pharmacokinetics, safety, and endocrine and appetite effects of ghrelin administration in young healthy subjects. Eur. J. Endocrinol. 2004, 150, 447–455. [Google Scholar] [CrossRef] [Green Version]
- Giorgioni, G.; Del Bello, F.; Quaglia, W.; Botticelli, L.; Cifani, C.; Micioni Di Bonaventura, E.; Micioni Di Bonaventura, M.V.; Piergentili, A. Advances in the Development of Nonpeptide Small Molecules Targeting Ghrelin Receptor. J. Med. Chem. 2022, 65, 3098–3118. [Google Scholar] [CrossRef]
- Naito, T.; Uchino, J.; Kojima, T.; Matano, Y.; Minato, K.; Tanaka, K.; Mizukami, T.; Atagi, S.; Higashiguchi, T.; Muro, K.; et al. A multicenter, open-label, single-arm study of anamorelin (ONO-7643) in patients with cancer cachexia and low body mass index. Cancer 2022, 128, 2025–2035. [Google Scholar] [CrossRef]
- Ishida, J.; Saitoh, M.; Ebner, N.; Springer, J.; Anker, S.D.; Haehling, S. Growth hormone secretagogues: History, mechanism of action, and clinical development. JCSM Rapid Commun. 2020, 3, 25–37. [Google Scholar] [CrossRef]
- Sigalos, J.T.; Pastuszak, A.W. The Safety and Efficacy of Growth Hormone Secretagogues. Sex. Med. Rev. 2018, 6, 45–53. [Google Scholar] [CrossRef]
- Sadakane, C.; Muto, S.; Nakagawa, K.; Ohnishi, S.; Saegusa, Y.; Nahata, M.; Hattori, T.; Asaka, M.; Takeda, H. 10-Gingerol, a component of rikkunshito, improves cisplatin-induced anorexia by inhibiting acylated ghrelin degradation. Biochem. Biophys. Res. Commun. 2011, 412, 506–511. [Google Scholar] [CrossRef]
- Kang, M.; Yoshimatsu, H.; Oohara, A.; Kurokawa, M.; Ogawa, R.; Sakata, T. Ginsenoside Rg1 modulates ingestive behavior and thermal response induced by interleukin-1β in rats. Physiol. Behav. 1995, 57, 393–396. [Google Scholar] [CrossRef]
- Fujimoto, K.; Sakata, T.; Ishimaru, T.; Etou, H.; Ookuma, K.; Kurokawa, M.; Machidori, H. Attenuation of anorexia induced by heat or surgery during sustained administration of ginsenoside Rg1 into rat third ventricle. Psychopharmacology 1989, 99, 257–260. [Google Scholar] [CrossRef]
- Fujitsuka, N.; Asakawa, A.; Uezono, Y.; Minami, K.; Yamaguchi, T.; Niijima, A.; Yada, T.; Maejima, Y.; Sedbazar, U.; Sakai, T. Potentiation of ghrelin signaling attenuates cancer anorexia–cachexia and prolongs survival. Transl. Psychiatry 2011, 1, e23. [Google Scholar] [CrossRef] [Green Version]
- Jang, Y.; Kim, T.-K.; Shim, W.-S. Naringin exhibits in vivo prokinetic activity via activation of ghrelin receptor in gastrointestinal motility dysfunction rats. Pharmacology 2013, 92, 191–197. [Google Scholar] [CrossRef]
- Jang, Y.; Kim, S.-W.; Oh, J.; Hong, G.-S.; Seo, E.-K.; Oh, U.; Shim, W.-S. Ghrelin receptor is activated by naringin and naringenin, constituents of a prokinetic agent Poncirus fructus. J. Ethnopharmacol. 2013, 148, 459–465. [Google Scholar] [CrossRef]
- Tahaghoghi-Hajghorbani, S.; Ebrahimzadeh, M.; Rafiei, A.; Golpour, M.; Hosseini-Khah, Z.; Akhtari, J. Improvement of chemotherapy through reducing of cachexia by using Citrus unshiu peel extract. J. Ethnopharmacol. 2019, 242, 111929. [Google Scholar] [CrossRef]
- Yang, C.S.; Chen, T.; Ho, C.-T. Redox and Other Biological Activities of Tea Catechins That May Affect Health: Mechanisms and Unresolved Issues. J. Agric. Food Chem. 2022, 70, 7887–7899. [Google Scholar] [CrossRef]
- Dou, Q.P. Tea in Health and Disease. Nutrients 2019, 11, 929. [Google Scholar] [CrossRef]
- Chen, T.-Y.; Wang, M.M.; Hsieh, S.-K.; Hsieh, M.-H.; Chen, W.-Y.; Tzen, J.T.C. Pancreatic lipase inhibition of strictinin isolated from Pu’er tea (Cammelia sinensis) and its anti-obesity effects in C57BL6 mice. J. Funct. Foods 2018, 48, 1–8. [Google Scholar] [CrossRef]
- Lo, Y.-H.; Chen, Y.-J.; Chang, C.-I.; Lin, Y.-W.; Chen, C.-Y.; Lee, M.-R.; Lee, V.S.Y.; Tzen, J.T.C. Teaghrelins, Unique Acylated Flavonoid Tetraglycosides in Chin-Shin Oolong Tea, Are Putative Oral Agonists of the Ghrelin Receptor. J. Agric. Food Chem. 2014, 62, 5085–5091. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Wu, C.J.; Lin, Y.C.; Wu, R.H.; Chen, W.Y.; Kuo, P.C.; Tzen, J.T.C. Identification of two teaghrelins in Shy-jih-chuen oolong tea. J. Food Biochem. 2019, 43, e12810. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, S.-K.; Lin, H.-Y.; Chen, C.-J.; Jhuo, C.-F.; Liao, K.-Y.; Chen, W.-Y.; Tzen, J.T.C. Promotion of myotube differentiation and attenuation of muscle atrophy in murine C2C12 myoblast cells treated with teaghrelin. Chem. Biol. Interact. 2020, 315, 108893. [Google Scholar] [CrossRef] [PubMed]
- Jhuo, C.-F.; Hsieh, S.-K.; Chen, C.-J.; Chen, W.-Y.; Tzen, J.T.C. Teaghrelin Protects SH-SY5Y Cells against MPP+-Induced Neurotoxicity through Activation of AMPK/SIRT1/PGC-1α and ERK1/2 Pathways. Nutrients 2020, 12, 3665. [Google Scholar] [CrossRef]
- Lo, Y.-H.; Chen, Y.-J.; Chung, T.-Y.; Lin, N.-H.; Chen, W.-Y.; Chen, C.-Y.; Lee, M.-R.; Chou, C.-C.; Tzen, J.T.C. Emoghrelin, a unique emodin derivative in Heshouwu, stimulates growth hormone secretion via activation of the ghrelin receptor. J. Ethnopharmacol. 2015, 159, 1–8. [Google Scholar] [CrossRef]
- Hsieh, S.-K.; Chung, T.-Y.; Li, Y.-C.; Lo, Y.-H.; Lin, N.-H.; Kuo, P.-C.; Chen, W.-Y.; Tzen, J.T.C. Ginkgoghrelins, unique acylated flavonoid diglycosides in Folium Ginkgo, stimulate growth hormone secretion via activation of the ghrelin receptor. J. Ethnopharmacol. 2016, 193, 237–247. [Google Scholar] [CrossRef]
- Wu, C.-J.; Chien, M.-Y.; Lin, N.-H.; Lin, Y.-C.; Chen, W.-Y.; Chen, C.-H.; Tzen, J.T.C. Echinacoside Isolated from Cistanche tubulosa Putatively Stimulates Growth Hormone Secretion via Activation of the Ghrelin Receptor. Molecules 2019, 24, 720. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.C.; Wu, C.J.; Kuo, P.C.; Chen, W.Y.; Tzen, J.T.C. Quercetin 3-O-malonylglucoside in the leaves of mulberry (Morus alba) is a functional analog of ghrelin. J. Food Biochem. 2020, 44, e13379. [Google Scholar] [CrossRef]
- Wu, R.; Xiao, D.; Shan, X.; Dong, Y.; Tao, W.W. Rapid and prolonged antidepressant-like effect of Ccrocin is associated with GHSR-mediated hippocampal plasticity-related proteins in mice exposed to prenatal stress. ACS Chem. Neurosci. 2020, 11, 1159–1170. [Google Scholar] [CrossRef]
- Brooks, B.R.; Bruccoleri, R.E.; Olafson, B.D.; States, D.J.; Swaminathan, S.; Karplus, M. CHARMM—A program for macromolecular energy, minimization, and dynamics calculations. J. Comput. Chem. 1983, 4, 187–217. [Google Scholar] [CrossRef]
- Xiao, X.; Bi, M.; Jiao, Q.; Chen, X.; Du, X.; Jiang, H. A new understanding of GHSR1a—Independent of ghrelin activation. Ageing Res. Rev. 2020, 64, 101187. [Google Scholar] [CrossRef]
- Ramirez, V.T.; Van Oeffelen, W.E.; Torres-Fuentes, C.; Chruścicka, B.; Druelle, C.; Golubeva, A.V.; Van De Wouw, M.; Dinan, T.G.; Cryan, J.F.; Schellekens, H. Differential functional selectivity and downstream signaling bias of ghrelin receptor antagonists and inverse agonists. FASEB J. 2019, 33, 518–531. [Google Scholar] [CrossRef]
- Zhu, J.; Yao, J.; Huang, R.; Wang, Y.; Jia, M.; Huang, Y. Ghrelin promotes human non-small cell lung cancer A549 cell proliferation through PI3K/Akt/mTOR/P70S6K and ERK signaling pathways. Biochem. Biophys. Res. Commun. 2018, 498, 616–620. [Google Scholar] [CrossRef]
- Chruścicka, B.; Cowan, C.S.; Fitzsimons, S.E.W.; Borroto-Escuela, D.O.; Druelle, C.M.; Stamou, P.; Bergmann, C.A.; Dinan, T.G.; Slattery, D.A.; Fuxe, K. Molecular, biochemical and behavioural evidence for a novel oxytocin receptor and serotonin 2C receptor heterocomplex. Neuropharmacology 2021, 183, 108394. [Google Scholar] [CrossRef]
- Zhang, H.; Sturchler, E.; Zhu, J.; Nieto, A.; Cistrone, P.A.; Xie, J.; He, L.; Yea, K.; Jones, T.; Turn, R. Autocrine selection of a GLP-1R G-protein biased agonist with potent antidiabetic effects. Nat. Commun. 2015, 6, 8918. [Google Scholar] [CrossRef] [Green Version]
- Cordisco Gonzalez, S.; Mustafaá, E.R.n.; Rodriguez, S.S.; Perello, M.; Raingo, J. Dopamine receptor Type 2 and ghrelin receptor coexpression alters CaV2. 2 modulation by G protein signaling cascades. ACS Chem. Neurosci. 2019, 11, 3–13. [Google Scholar] [CrossRef]
- Bouzo-Lorenzo, M.; Santo-Zas, I.; Lodeiro, M.; Nogueiras, R.; Casanueva, F.F.; Castro, M.; Pazos, Y.; Tobin, A.B.; Butcher, A.J.; Camiña, J.P. Distinct phosphorylation sites on the ghrelin receptor, GHSR1a, establish a code that determines the functions of ss-arrestins. Sci. Rep. 2016, 6, 22495. [Google Scholar] [CrossRef]
- M’Kadmi, C.; Leyris, J.-P.; Onfroy, L.; Galés, C.; Saulière, A.; Gagne, D.; Damian, M.; Mary, S.; Maingot, M.; Denoyelle, S. Agonism, antagonism, and inverse agonism bias at the ghrelin receptor signaling. J. Biol. Chem. 2015, 290, 27021–27039. [Google Scholar] [CrossRef] [Green Version]
- Schellekens, H.; van Oeffelen, W.E.; Dinan, T.G.; Cryan, J.F. Promiscuous dimerization of the growth hormone secretagogue receptor (GHS-R1a) attenuates ghrelin-mediated signaling. J. Biol. Chem. 2013, 288, 181–191. [Google Scholar] [CrossRef]
- Liang, Q.-H.; Jiang, Y.; Zhu, X.; Cui, R.-R.; Liu, G.-Y.; Liu, Y.; Wu, S.-S.; Liao, X.-B.; Xie, H.; Zhou, H.-D. Ghrelin attenuates the osteoblastic differentiation of vascular smooth muscle cells through the ERK pathway. PLoS ONE 2012, 7, e33126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khatib, M.N.; Shankar, A.; Kirubakaran, R.; Agho, K.; Simkhada, P.; Gaidhane, S.; Saxena, D.; Gode, D.; Gaidhane, A.; Zahiruddin, S.Q. Effect of ghrelin on mortality and cardiovascular outcomes in experimental rat and mice models of heart failure: A systematic review and meta-analysis. PLoS ONE 2015, 10, e0126697. [Google Scholar] [CrossRef] [PubMed]
- Woll, P.J.; Rozengurt, E. [D-Arg1, D-Phe5, D-Trp7, 9, Leu11] substance P, a potent bombesin antagonist in murine Swiss 3T3 cells, inhibits the growth of human small cell lung cancer cells in vitro. Proc. Natl. Acad. Sci. USA 1988, 85, 1859–1863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guha, S.; Eibl, G.; Kisfalvi, K.; Fan, R.S.; Burdick, M.; Reber, H.; Hines, O.J.; Strieter, R.; Rozengurt, E. Broad-spectrum G protein–coupled receptor antagonist, [D-Arg1, D-Trp5, 7, 9, Leu11] SP: A dual inhibitor of growth and angiogenesis in pancreatic cancer. Cancer Res. 2005, 65, 2738–2745. [Google Scholar] [CrossRef] [Green Version]
- Camina, J. Cell biology of the ghrelin receptor. J. Neuroendocrinol. 2006, 18, 65–76. [Google Scholar] [CrossRef]
- Whiteside, E.J.; Seim, I.; Pauli, J.P.; O’Keeffe, A.J.; Thomas, P.B.; Carter, S.L.; Walpole, C.M.; Fung, J.N.; Josh, P.; Herington, A.C. Identification of a long non-coding RNA gene, growth hormone secretagogue receptor opposite strand, which stimulates cell migration in non-small cell lung cancer cell lines. Int. J. Oncol. 2013, 43, 566–574. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Chen, Y.F.; Li, F.; Zhang, H.Y. Fructus Gardenia (Gardenia jasminoides Ellis) phytochemistry, pharmacology of cardiovascular, and safety with the perspective of new drugs development. J. Asian Nat. Prod. Res. 2013, 15, 94–110. [Google Scholar] [CrossRef]
- Song, Y.N.; Wang, Y.; Zheng, Y.H.; Liu, T.L.; Zhang, C. Crocins: A comprehensive review of structural characteristics, pharmacokinetics and therapeutic effects. Fitoterapia 2021, 153, 104969. [Google Scholar] [CrossRef]





| Ligand | Total Energy (kJ mol−1) | Van der Waals’ Force (kJ mol−1) | H Bond (kJ mol−1) |
|---|---|---|---|
| teaghrelin | −157.97 | −17.87 | 16.63 |
| crocin | −146.43 | −15.65 | 15.97 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.-H.; Tseng, C.-Y.; Hsu, W.-L.; Tzen, J.T.C. Establishment of a Cell Line Stably Expressing the Growth Hormone Secretagogue Receptor to Identify Crocin as a Ghrelin Agonist. Biomolecules 2022, 12, 1813. https://doi.org/10.3390/biom12121813
Wang C-H, Tseng C-Y, Hsu W-L, Tzen JTC. Establishment of a Cell Line Stably Expressing the Growth Hormone Secretagogue Receptor to Identify Crocin as a Ghrelin Agonist. Biomolecules. 2022; 12(12):1813. https://doi.org/10.3390/biom12121813
Chicago/Turabian StyleWang, Chia-Hao, Ching-Yu Tseng, Wei-Li Hsu, and Jason T. C. Tzen. 2022. "Establishment of a Cell Line Stably Expressing the Growth Hormone Secretagogue Receptor to Identify Crocin as a Ghrelin Agonist" Biomolecules 12, no. 12: 1813. https://doi.org/10.3390/biom12121813
APA StyleWang, C.-H., Tseng, C.-Y., Hsu, W.-L., & Tzen, J. T. C. (2022). Establishment of a Cell Line Stably Expressing the Growth Hormone Secretagogue Receptor to Identify Crocin as a Ghrelin Agonist. Biomolecules, 12(12), 1813. https://doi.org/10.3390/biom12121813







