Combination Drug Therapy for the Management of Chronic Neuropathic Pain
Abstract
:1. Introduction
2. Pathophysiological Mechanisms of Chronic NP
3. Pharmacological Treatment of Chronic NP: Drug Classes/Monotherapy
4. Pharmacological Treatment of Chronic NP: Combination Therapy
4.1. General Considerations
4.2. Preclinical and Clinical Data on Combination Therapy
4.2.1. NSAIDs and Opioids (Including Tapentadol/Tramadol)
4.2.2. NSAIDs and Gabapentinoids/Antiepileptics
4.2.3. Gabapentinoids and Opioids (Including Tapentadol/Tramadol)
4.2.4. Gabapentinoids and Antidepressants
4.2.5. Additional Combination Therapies
4.3. Unmet Medical Needs
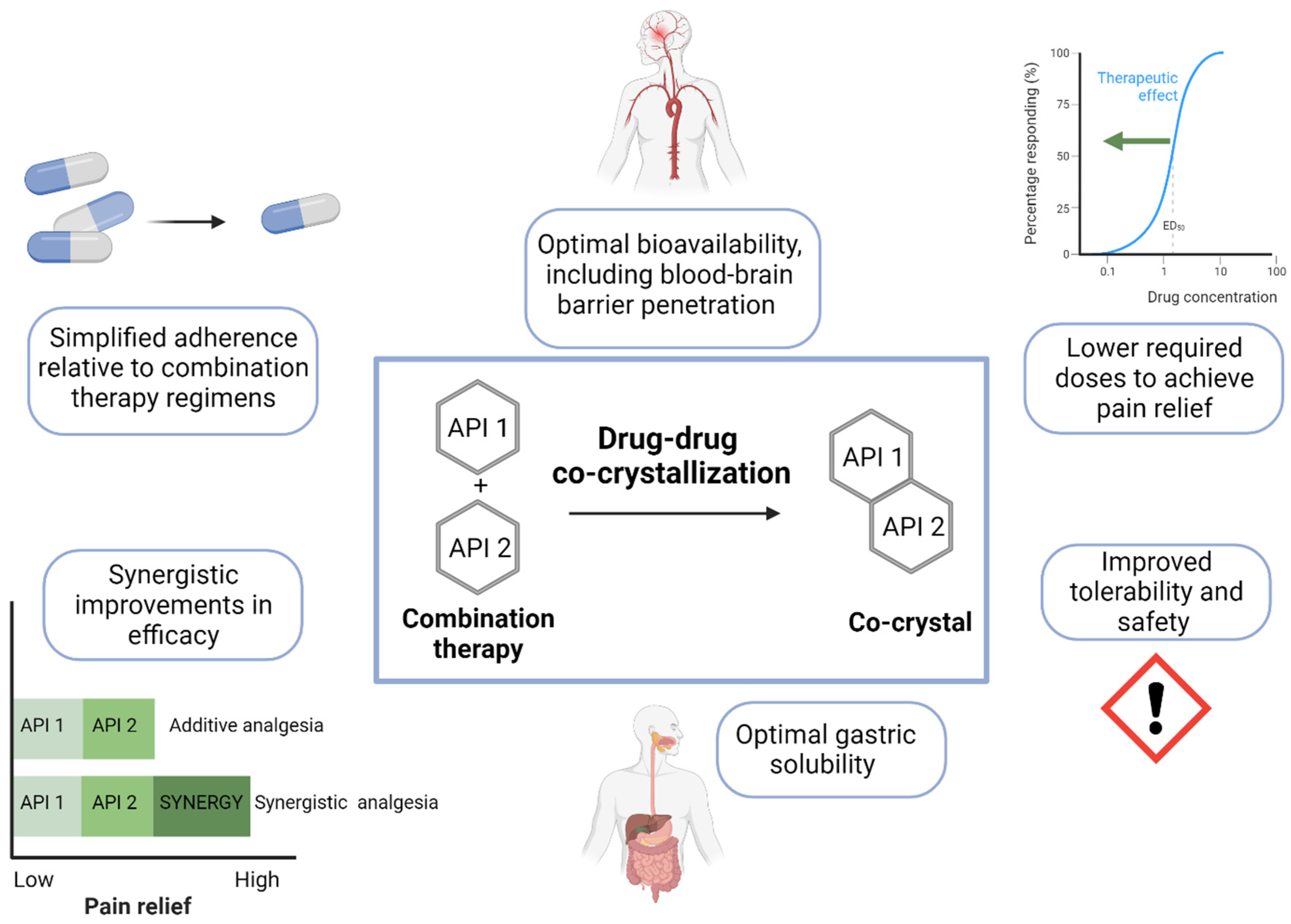
5. Pharmacological Treatment of Chronic NP: Co-Crystallization
| Drug Combinations | Doses | Pre-Clinical Study | Clinical Study | Key Findings: Analgesia | Key Findings: Physicochemical Properties | Reference |
|---|---|---|---|---|---|---|
| Tramadol hydrochloride + Celecoxib co-crystal | Rat: 0.625–320 mg/kg (i.p. or p.o.) | Postoperative pain model in rats | Moderate to severe acute pain following Bunionectomy + Osteotomy (NCT03108482) or abdominal hysterectomy (NCT3062644) | Rat: Tramadol hydrochloride and celecoxib co-crystal exhibited synergistic analgesic efficacy greater than monotherapy or the theoretical additive effects of the single agents; effects of the co-crystal were comparable to strong opioids, with improved tolerability; reduced ulcerogenic activity | Faster intrinsic dissolution rate of celecoxib and slower intrinsic dissolution rate of tramadol | [146,149,151,152] |
| Human: 200 mg twice daily (NCT03108482) | Human (NCT03108482): superior analgesic efficacy of combination twice daily compared with tramadol 50 mg four times daily or celecoxib 100 mg twice daily | NCT03108482 | ||||
| 100 mg twice daily (NCT3062644) | Human (NCT3062644): non-inferior analgesic efficacy to tramadol 100 mg four times daily, improved risk/benefit ratio vs. tramadol alone with lower cumulative opioid exposure | NCT03062644; EudraCT: 2016-000593-38 | ||||
| Ketoprofen lysine salt (KLS) + Gabapentin (GABA) co-crystal | Rat: 67.5 mg/kg, 2 cps (acute treatment) | Carrageenan paw edema model in rat | NA | KLS and gabapentin had supra-additive effects in reducing mechanical allodynia and thermal hyperalgesia in NP rats; co-crystal showed lower gastric mucosal damage relative to single compounds | Increased gastric solubility of KLS, consistent with a supersaturation profile | [155] |
| Rat: 11.60 mg/kg, 1 cps (repeated treatment for 7 days) | CCI in rat |
6. Conclusions and Future Directions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Finnerup, N.B.; Kuner, R.; Jensen, T.S. Neuropathic Pain: From Mechanisms to Treatment. Physiol. Rev. 2021, 101, 259–301. [Google Scholar] [CrossRef] [PubMed]
- Vos, T.; Flaxman, A.D.; Naghavi, M.; Lozano, R.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; Aboyans, V.; et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2163–2196. [Google Scholar] [CrossRef] [PubMed]
- Scholz, J.; Finnerup, N.B.; Attal, N.; Aziz, Q.; Baron, R.; Bennett, M.I.; Benoliel, R.; Cohen, M.; Cruccu, G.; Davis, K.D.; et al. The IASP classification of chronic pain for ICD-11: Chronic neuropathic pain. Pain 2019, 160, 53–59. [Google Scholar] [CrossRef] [PubMed]
- van Hecke, O.; Austin, S.K.; Khan, R.A.; Smith, B.H.; Torrance, N. Neuropathic pain in the general population: A systematic review of epidemiological studies. Pain 2014, 155, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Berger, A.; Dukes, E.M.; Oster, G. Clinical characteristics and economic costs of patients with painful neuropathic disorders. J. Pain 2004, 5, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Derry, S.; Bell, R.F.; Straube, S.; Wiffen, P.J.; Aldington, D.; Moore, R.A. Pregabalin for neuropathic pain in adults. Cochrane Database Syst. Rev. 2019, 1, CD007076. [Google Scholar] [CrossRef]
- Finnerup, N.B.; Attal, N.; Haroutounian, S.; McNicol, E.; Baron, R.; Dworkin, R.H.; Gilron, I.; Haanpaa, M.; Hansson, P.; Jensen, T.S.; et al. Pharmacotherapy for neuropathic pain in adults: A systematic review and meta-analysis. Lancet Neurol. 2015, 14, 162–173. [Google Scholar] [CrossRef]
- Gilron, I.; Dickenson, A.H. Emerging drugs for neuropathic pain. Expert. Opin. Emerg. Drugs 2014, 19, 329–341. [Google Scholar] [CrossRef]
- Sultan, A.; Gaskell, H.; Derry, S.; Moore, R.A. Duloxetine for painful diabetic neuropathy and fibromyalgia pain: Systematic review of randomised trials. BMC Neurol. 2008, 8, 29. [Google Scholar] [CrossRef]
- IASP. International Association for the Study of Pain (IASP) Taxonomy. Available online: https://www.iasp-pain.org/resources/terminology/?navItemNumber=576 (accessed on 1 November 2023).
- Treede, R.D.; Rief, W.; Barke, A.; Aziz, Q.; Bennett, M.I.; Benoliel, R.; Cohen, M.; Evers, S.; Finnerup, N.B.; First, M.B.; et al. Chronic pain as a symptom or a disease: The IASP Classification of Chronic Pain for the International Classification of Diseases (ICD-11). Pain 2019, 160, 19–27. [Google Scholar] [CrossRef]
- Fitzcharles, M.A.; Cohen, S.P.; Clauw, D.J.; Littlejohn, G.; Usui, C.; Hauser, W. Nociplastic pain: Towards an understanding of prevalent pain conditions. Lancet 2021, 397, 2098–2110. [Google Scholar] [CrossRef]
- Treede, R.D.; Jensen, T.S.; Campbell, J.N.; Cruccu, G.; Dostrovsky, J.O.; Griffin, J.W.; Hansson, P.; Hughes, R.; Nurmikko, T.; Serra, J. Neuropathic pain: Redefinition and a grading system for clinical and research purposes. Neurology 2008, 70, 1630–1635. [Google Scholar] [CrossRef]
- Sommer, C.; Geber, C.; Young, P.; Forst, R.; Birklein, F.; Schoser, B. Polyneuropathies. Dtsch. Arztebl. Int. 2018, 115, 83–90. [Google Scholar] [CrossRef]
- Walling, A.D.; Dickson, G. Guillain-Barre syndrome. Am. Fam. Physician 2013, 87, 191–197. [Google Scholar]
- Patil, A.; Goldust, M.; Wollina, U. Herpes zoster: A Review of Clinical Manifestations and Management. Viruses 2022, 14, 192. [Google Scholar] [CrossRef]
- Cortes-Altamirano, J.L.; Reyes-Long, S.; Bandala, C.; Morraz-Varela, A.; Bonilla-Jaime, H.; Alfaro-Rodriguez, A. Neuropathic Pain in Parkinson’s Disease. Neurol. India 2022, 70, 1879–1886. [Google Scholar] [CrossRef]
- Chio, A.; Mora, G.; Lauria, G. Pain in amyotrophic lateral sclerosis. Lancet Neurol. 2017, 16, 144–157. [Google Scholar] [CrossRef]
- Szok, D.; Tajti, J.; Nyari, A.; Vecsei, L. Therapeutic Approaches for Peripheral and Central Neuropathic Pain. Behav. Neurol. 2019, 2019, 8685954. [Google Scholar] [CrossRef]
- Kremer, M.; Salvat, E.; Muller, A.; Yalcin, I.; Barrot, M. Antidepressants and gabapentinoids in neuropathic pain: Mechanistic insights. Neuroscience 2016, 338, 183–206. [Google Scholar] [CrossRef] [PubMed]
- Dworkin, R.H.; O’Connor, A.B.; Backonja, M.; Farrar, J.T.; Finnerup, N.B.; Jensen, T.S.; Kalso, E.A.; Loeser, J.D.; Miaskowski, C.; Nurmikko, T.J.; et al. Pharmacologic management of neuropathic pain: Evidence-based recommendations. Pain 2007, 132, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Sindrup, S.H.; Otto, M.; Finnerup, N.B.; Jensen, T.S. Antidepressants in the treatment of neuropathic pain. Basic Clin. Pharmacol. Toxicol. 2005, 96, 399–409. [Google Scholar] [CrossRef]
- Patel, R.; Dickenson, A.H. Mechanisms of the gabapentinoids and alpha 2 delta-1 calcium channel subunit in neuropathic pain. Pharmacol. Res. Perspect. 2016, 4, e00205. [Google Scholar] [CrossRef]
- Bannister, K.; Qu, C.; Navratilova, E.; Oyarzo, J.; Xie, J.Y.; King, T.; Dickenson, A.H.; Porreca, F. Multiple sites and actions of gabapentin-induced relief of ongoing experimental neuropathic pain. Pain 2017, 158, 2386–2395. [Google Scholar] [CrossRef]
- Maneuf, Y.P.; Hughes, J.; McKnight, A.T. Gabapentin inhibits the substance P-facilitated K+)-evoked release of [3H]glutamate from rat caudial trigeminal nucleus slices. Pain 2001, 93, 191–196. [Google Scholar] [CrossRef]
- Ahn, S.H.; Park, H.W.; Lee, B.S.; Moon, H.W.; Jang, S.H.; Sakong, J.; Bae, J.H. Gabapentin effect on neuropathic pain compared among patients with spinal cord injury and different durations of symptoms. Spine 2003, 28, 341–346, discussion 346–347. [Google Scholar] [CrossRef]
- Arendt-Nielsen, L.; Curatolo, M.; Drewes, A. Human experimental pain models in drug development: Translational pain research. Curr. Opin. Investig. Drugs 2007, 8, 41–53. [Google Scholar]
- Moore, R.A.; Wiffen, P.J.; Derry, S.; McQuay, H.J. Gabapentin for chronic neuropathic pain and fibromyalgia in adults. Cochrane Database Syst. Rev. 2011, 4, CD007938. [Google Scholar] [CrossRef]
- Deng, Y.; Luo, L.; Hu, Y.; Fang, K.; Liu, J. Clinical practice guidelines for the management of neuropathic pain: A systematic review. BMC Anesthesiol. 2016, 16, 12. [Google Scholar] [CrossRef] [PubMed]
- Beakley, B.D.; Kaye, A.M.; Kaye, A.D. Tramadol, Pharmacology, Side Effects, and Serotonin Syndrome: A Review. Pain Physician 2015, 18, 395–400. [Google Scholar] [PubMed]
- Minami, K.; Ogata, J.; Uezono, Y. What is the main mechanism of tramadol? Naunyn Schmiedebergs Arch. Pharmacol. 2015, 388, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Bohnert, A.S.; Ilgen, M.A.; Trafton, J.A.; Kerns, R.D.; Eisenberg, A.; Ganoczy, D.; Blow, F.C. Trends and regional variation in opioid overdose mortality among Veterans Health Administration patients, fiscal year 2001 to 2009. Clin. J. Pain 2014, 30, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Fischer, B.; Jones, W.; Urbanoski, K.; Skinner, R.; Rehm, J. Correlations between prescription opioid analgesic dispensing levels and related mortality and morbidity in Ontario, Canada, 2005–2011. Drug Alcohol Rev. 2014, 33, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Fields, H. State-dependent opioid control of pain. Nat. Rev. Neurosci. 2004, 5, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Brunton, S. Approach to assessment and diagnosis of chronic pain. J. Fam. Pract. 2004, 53, S3–S10. [Google Scholar] [PubMed]
- Maione, S.; Radanova, L.; De Gregorio, D.; Luongo, L.; De Petrocellis, L.; Di Marzo, V.; Imming, P. Effects of metabolites of the analgesic agent dipyrone (metamizol) on rostral ventromedial medulla cell activity in mice. Eur. J. Pharmacol. 2015, 748, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Bjorkman, R.L.; Hedner, T.; Hallman, K.M.; Henning, M.; Hedner, J. Localization of the central antinociceptive effects of diclofenac in the rat. Brain Res. 1992, 590, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Pini, L.A.; Vitale, G.; Sandrini, M. Serotonin and opiate involvement in the antinociceptive effect of acetylsalicylic acid. Pharmacology 1997, 54, 84–91. [Google Scholar] [CrossRef]
- Pernia-Andrade, A.J.; Tortorici, V.; Vanegas, H. Induction of opioid tolerance by lysine-acetylsalicylate in rats. Pain 2004, 111, 191–200. [Google Scholar] [CrossRef]
- Vanegas, H.; Vazquez, E.; Tortorici, V. NSAIDs, Opioids, Cannabinoids and the Control of Pain by the Central Nervous System. Pharmaceuticals 2010, 3, 1335–1347. [Google Scholar] [CrossRef]
- Borer, J.S.; Simon, L.S. Cardiovascular and gastrointestinal effects of COX-2 inhibitors and NSAIDs: Achieving a balance. Arthritis Res. Ther. 2005, 7 (Suppl. S4), S14–S22. [Google Scholar] [CrossRef]
- Zhou, T.J.; Tang, J.; White, P.F. Propacetamol versus ketorolac for treatment of acute postoperative pain after total hip or knee replacement. Anesth. Analg. 2001, 92, 1569–1575. [Google Scholar] [CrossRef] [PubMed]
- Diblasio, C.J.; Snyder, M.E.; Kattan, M.W.; Russo, P. Ketorolac: Safe and effective analgesia for the management of renal cortical tumors with partial nephrectomy. J. Urol. 2004, 171, 1062–1065. [Google Scholar] [CrossRef] [PubMed]
- Varrassi, G.; Panella, L.; Piroli, A.; Marinangeli, F.; Varrassi, S.; Wolman, I.; Niv, D. The effects of perioperative ketorolac infusion on postoperative pain and endocrine-metabolic response. Anesth. Analg. 1994, 78, 514–519. [Google Scholar] [CrossRef]
- Acharya, M.; Dunning, J. Does the use of non-steroidal anti-inflammatory drugs after cardiac surgery increase the risk of renal failure? Interact. Cardiovasc. Thorac. Surg. 2010, 11, 461–467. [Google Scholar] [CrossRef]
- Elia, N.; Lysakowski, C.; Tramer, M.R. Does multimodal analgesia with acetaminophen, nonsteroidal antiinflammatory drugs, or selective cyclooxygenase-2 inhibitors and patient-controlled analgesia morphine offer advantages over morphine alone? Meta-analyses of randomized trials. Anesthesiology 2005, 103, 1296–1304. [Google Scholar] [CrossRef] [PubMed]
- McGettigan, P.; Henry, D. Cardiovascular risk and inhibition of cyclooxygenase: A systematic review of the observational studies of selective and nonselective inhibitors of cyclooxygenase 2. JAMA 2006, 296, 1633–1644. [Google Scholar] [CrossRef] [PubMed]
- Nussmeier, N.A.; Whelton, A.A.; Brown, M.T.; Joshi, G.P.; Langford, R.M.; Singla, N.K.; Boye, M.E.; Verburg, K.M. Safety and efficacy of the cyclooxygenase-2 inhibitors parecoxib and valdecoxib after noncardiac surgery. Anesthesiology 2006, 104, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.J.; Campen, D.; Hui, R.; Spence, M.; Cheetham, C.; Levy, G.; Shoor, S.; Ray, W.A. Risk of acute myocardial infarction and sudden cardiac death in patients treated with cyclo-oxygenase 2 selective and non-selective non-steroidal anti-inflammatory drugs: Nested case-control study. Lancet 2005, 365, 475–481. [Google Scholar] [CrossRef]
- Zhang, J.; Ding, E.L.; Song, Y. Adverse effects of cyclooxygenase 2 inhibitors on renal and arrhythmia events: Meta-analysis of randomized trials. JAMA 2006, 296, 1619–1632. [Google Scholar] [CrossRef]
- Fries, J.F.; Miller, S.R.; Spitz, P.W.; Williams, C.A.; Hubert, H.B.; Bloch, D.A. Toward an epidemiology of gastropathy associated with nonsteroidal antiinflammatory drug use. Gastroenterology 1989, 96, 647–655. [Google Scholar] [CrossRef]
- Baron, R.; Forster, M.; Binder, A. Subgrouping of patients with neuropathic pain according to pain-related sensory abnormalities: A first step to a stratified treatment approach. Lancet Neurol. 2012, 11, 999–1005. [Google Scholar] [CrossRef]
- Chaparro, L.E.; Wiffen, P.J.; Moore, R.A.; Gilron, I. Combination pharmacotherapy for the treatment of neuropathic pain in adults. Cochrane Database Syst. Rev. 2012, 2012, CD008943. [Google Scholar] [CrossRef]
- Varrassi, G.; Alon, E.; Bagnasco, M.; Lanata, L.; Mayoral-Rojals, V.; Paladini, A.; Pergolizzi, J.V.; Perrot, S.; Scarpignato, C.; Tolle, T. Towards an Effective and Safe Treatment of Inflammatory Pain: A Delphi-Guided Expert Consensus. Adv. Ther. 2019, 36, 2618–2637. [Google Scholar] [CrossRef]
- Alcantara Montero, A.; Balsalobre Gongora, S.; Narganes Pineda, D.M.; Blanco Polanco, B. Multimodal analgesia and pharmacological synergy in pain management. Semergen 2020, 46, 284–285. [Google Scholar] [CrossRef]
- Virani, A.; Mailis, A.; Shapiro, L.E.; Shear, N.H. Drug interactions in human neuropathic pain pharmacotherapy. Pain 1997, 73, 3–13. [Google Scholar] [CrossRef]
- Torres, N.B.; Altafini, C. Drug combinatorics and side effect estimation on the signed human drug-target network. BMC Syst. Biol. 2016, 10, 74. [Google Scholar] [CrossRef]
- Eisenberg, E.; Suzan, E. Drug combinations in the treatment of neuropathic pain. Curr. Pain Headache Rep. 2014, 18, 463. [Google Scholar] [CrossRef]
- Gilron, I.; Jensen, T.S.; Dickenson, A.H. Combination pharmacotherapy for management of chronic pain: From bench to bedside. Lancet Neurol. 2013, 12, 1084–1095. [Google Scholar] [CrossRef]
- Mao, J.; Gold, M.S.; Backonja, M.M. Combination drug therapy for chronic pain: A call for more clinical studies. J. Pain 2011, 12, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Qaseem, A.; Wilt, T.J.; McLean, R.M.; Forciea, M.A.; Clinical Guidelines Committee of the American College of Physicians. Noninvasive Treatments for Acute, Subacute, and Chronic Low Back Pain: A Clinical Practice Guideline From the American College of Physicians. Ann. Intern. Med. 2017, 166, 514–530. [Google Scholar] [CrossRef] [PubMed]
- Berger, A.; Sadosky, A.; Dukes, E.; Edelsberg, J.; Oster, G. Clinical characteristics and patterns of healthcare utilization in patients with painful neuropathic disorders in UK general practice: A retrospective cohort study. BMC Neurol. 2012, 12, 8. [Google Scholar] [CrossRef]
- Harrisson, S.A.; Ogollah, R.; Dunn, K.M.; Foster, N.E.; Konstantinou, K. Prevalence, Characteristics, and Clinical Course of Neuropathic Pain in Primary Care Patients Consulting with Low Back-related Leg Pain. Clin. J. Pain 2020, 36, 813–824. [Google Scholar] [CrossRef]
- Gore, M.; Dukes, E.; Rowbotham, D.J.; Tai, K.S.; Leslie, D. Clinical characteristics and pain management among patients with painful peripheral neuropathic disorders in general practice settings. Eur. J. Pain 2007, 11, 652–664. [Google Scholar] [CrossRef]
- Verma, V.; Singh, N.; Singh Jaggi, A. Pregabalin in neuropathic pain: Evidences and possible mechanisms. Curr. Neuropharmacol. 2014, 12, 44–56. [Google Scholar] [CrossRef]
- Hahn, J.; Jo, Y.; Yoo, S.H.; Shin, J.; Yu, Y.M.; Ah, Y.M. Risk of major adverse events associated with gabapentinoid and opioid combination therapy: A systematic review and meta-analysis. Front. Pharmacol. 2022, 13, 1009950. [Google Scholar] [CrossRef]
- Gatti, A.; Sabato, A.F.; Occhioni, R.; Colini Baldeschi, G.; Reale, C. Controlled-release oxycodone and pregabalin in the treatment of neuropathic pain: Results of a multicenter Italian study. Eur. Neurol. 2009, 61, 129–137. [Google Scholar] [CrossRef]
- Lytle, E.; Claus, C.; Yoon, E.; Tong, D.; Soo, T. The Impact of Intraoperative Local Ketorolac on Opioid Use in the Management of Postoperative Pain in Thoracolumbar Spinal Fusions: A Retrospective Cohort Study. Int. J. Spine Surg. 2020, 14, 294–299. [Google Scholar] [CrossRef]
- Adams, A.J.; Buczek, M.J.; Flynn, J.M.; Shah, A.S. Perioperative Ketorolac for Supracondylar Humerus Fracture in Children Decreases Postoperative Pain, Opioid Usage, Hospitalization Cost, and Length-of-Stay. J. Pediatr. Orthop. 2019, 39, e447–e451. [Google Scholar] [CrossRef]
- White, P.F.; Raeder, J.; Kehlet, H. Ketorolac: Its role as part of a multimodal analgesic regimen. Anesth. Analg. 2012, 114, 250–254. [Google Scholar] [CrossRef]
- De Oliveira, G.S., Jr.; Agarwal, D.; Benzon, H.T. Perioperative single dose ketorolac to prevent postoperative pain: A meta-analysis of randomized trials. Anesth. Analg. 2012, 114, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Fornasari, D.; Allegri, M.; Gerboni, S.; Fanelli, G. A “novel” association to treat pain: Tramadol/dexketoprofen. The first drug of a “new pharmacological class”. Acta Biomed. 2017, 88, 17–24. [Google Scholar] [CrossRef]
- Bennett, R.M.; Kamin, M.; Karim, R.; Rosenthal, N. Tramadol and acetaminophen combination tablets in the treatment of fibromyalgia pain: A double-blind, randomized, placebo-controlled study. Am. J. Med. 2003, 114, 537–545. [Google Scholar] [CrossRef]
- Cantini, F.; Bellandi, F.; Niccoli, L.; Di Munno, O. Fluoxetin combined with cyclobenzaprine in the treatment of fibromyalgia. Minerva Med. 1994, 85, 97–100. [Google Scholar]
- Calandre, E.P.; Morillas-Arques, P.; Molina-Barea, R.; Rodriguez-Lopez, C.M.; Rico-Villademoros, F. Trazodone plus pregabalin combination in the treatment of fibromyalgia: A two-phase, 24-week, open-label uncontrolled study. BMC Musculoskelet. Disord. 2011, 12, 95. [Google Scholar] [CrossRef]
- Goldenberg, D.; Mayskiy, M.; Mossey, C.; Ruthazer, R.; Schmid, C. A randomized, double-blind crossover trial of fluoxetine and amitriptyline in the treatment of fibromyalgia. Arthritis Rheum. 1996, 39, 1852–1859. [Google Scholar] [CrossRef]
- Simpson, D.A. Gabapentin and venlafaxine for the treatment of painful diabetic neuropathy. J. Clin. Neuromuscul. Dis. 2001, 3, 53–62. [Google Scholar] [CrossRef]
- Hanna, M.; O’Brien, C.; Wilson, M.C. Prolonged-release oxycodone enhances the effects of existing gabapentin therapy in painful diabetic neuropathy patients. Eur. J. Pain. 2008, 12, 804–813. [Google Scholar] [CrossRef]
- Romano, C.L.; Romano, D.; Bonora, C.; Mineo, G. Pregabalin, celecoxib, and their combination for treatment of chronic low-back pain. J. Orthop. Traumatol. 2009, 10, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Rowbotham, M.; Harden, N.; Stacey, B.; Bernstein, P.; Magnus-Miller, L. Gabapentin for the treatment of postherpetic neuralgia: A randomized controlled trial. JAMA 1998, 280, 1837–1842. [Google Scholar] [CrossRef] [PubMed]
- Arai, Y.C.; Matsubara, T.; Shimo, K.; Suetomi, K.; Nishihara, M.; Ushida, T.; Kobayashi, K.; Suzuki, C.; Kinoshita, A.; Kondo, M.; et al. Low-dose gabapentin as useful adjuvant to opioids for neuropathic cancer pain when combined with low-dose imipramine. J. Anesth. 2010, 24, 407–410. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, T.; Hasegawa, K.; Shintani, D.; Yano, Y.; Sato, S.; Yabuno, A.; Kurosaki, A.; Yoshida, H.; Fujiwara, K. Combination Therapy of Pregabalin with Tramadol for Treatment of Peripheral Neuropathy in Patients with Gynecological Cancer Receiving Taxane Containing Chemotherapy. Gan Kagaku Ryoho 2017, 44, 227–231. [Google Scholar]
- Afonso, A.S.; Carnaval, T.; Cés, S.V. Combination Therapy for Neuropathic Pain: A Review of Recent Evidence. J. Clin. Med. 2021, 10, 3533. [Google Scholar] [CrossRef]
- Zin, C.S.; Nissen, L.M.; O’Callaghan, J.P.; Duffull, S.B.; Smith, M.T.; Moore, B.J. A randomized, controlled trial of oxycodone versus placebo in patients with postherpetic neuralgia and painful diabetic neuropathy treated with pregabalin. J. Pain 2010, 11, 462–471. [Google Scholar] [CrossRef]
- Boyce-Rustay, J.M.; Honore, P.; Jarvis, M.F. Animal models of acute and chronic inflammatory and nociceptive pain. Methods Mol. Biol. 2010, 617, 41–55. [Google Scholar] [CrossRef]
- Fehrenbacher, J.C.; Vasko, M.R.; Duarte, D.B. Models of inflammation: Carrageenan- or complete Freund’s Adjuvant (CFA)-induced edema and hypersensitivity in the rat. Curr. Protoc. Pharmacol. 2012, 56, 5.4. [Google Scholar] [CrossRef]
- Brennan, T.J.; Vandermeulen, E.P.; Gebhart, G.F. Characterization of a rat model of incisional pain. Pain 1996, 64, 493–502. [Google Scholar] [CrossRef]
- Ren, K.; Dubner, R. Inflammatory Models of Pain and Hyperalgesia. ILAR J. 1999, 40, 111–118. [Google Scholar] [CrossRef]
- Tappe-Theodor, A.; Negus, S.S.; Martin, T.J. Editorial: Preclinical Animal Models and Measures of Pain: Improving Predictive Validity for Analgesic Drug Development. Front. Pain Res. 2022, 3, 867786. [Google Scholar] [CrossRef]
- Romero-Alejo, E.; Puig, M.M.; Romero, A. Antihyperalgesic effects of dexketoprofen and tramadol in a model of postoperative pain in mice—Effects on glial cell activation. J. Pharm. Pharmacol. 2016, 68, 1041–1050. [Google Scholar] [CrossRef]
- McQuay, H.J.; Moore, R.A.; Berta, A.; Gainutdinovs, O.; Fulesdi, B.; Porvaneckas, N.; Petronis, S.; Mitkovic, M.; Bucsi, L.; Samson, L.; et al. Randomized clinical trial of dexketoprofen/tramadol 25 mg/75 mg in moderate-to-severe pain after total hip arthroplasty. Br. J. Anaesth. 2016, 116, 269–276. [Google Scholar] [CrossRef]
- Moore, R.A.; McQuay, H.J.; Tomaszewski, J.; Raba, G.; Tutunaru, D.; Lietuviete, N.; Galad, J.; Hagymasy, L.; Melka, D.; Kotarski, J.; et al. Dexketoprofen/tramadol 25 mg/75 mg: Randomised double-blind trial in moderate-to-severe acute pain after abdominal hysterectomy. BMC Anesthesiol. 2016, 16, 9. [Google Scholar] [CrossRef]
- Moore, R.A.; Gay-Escoda, C.; Figueiredo, R.; Toth-Bagi, Z.; Dietrich, T.; Milleri, S.; Torres-Lagares, D.; Hill, C.M.; Garcia-Garcia, A.; Coulthard, P.; et al. Dexketoprofen/tramadol: Randomised double-blind trial and confirmation of empirical theory of combination analgesics in acute pain. J. Headache Pain 2015, 16, 541. [Google Scholar] [CrossRef]
- Miranda, H.F.; Puig, M.M.; Romero, M.A.; Prieto, J.C. Effects of tramadol and dexketoprofen on analgesia and gastrointestinal transit in mice. Fundam. Clin. Pharmacol. 2009, 23, 81–88. [Google Scholar] [CrossRef]
- Miranda, H.F.; Romero, M.A.; Puig, M.M. Antinociceptive and anti-exudative synergism between dexketoprofen and tramadol in a model of inflammatory pain in mice. Fundam. Clin. Pharmacol. 2012, 26, 373–382. [Google Scholar] [CrossRef]
- Lin, W.Y.; Cheng, Y.T.; Huang, Y.H.; Lin, F.S.; Sun, W.Z.; Yen, C.T. Synergistic symptom-specific effects of ketorolac-tramadol and ketorolac-pregabalin in a rat model of peripheral neuropathy. J. Chin. Med. Assoc. 2019, 82, 457–463. [Google Scholar] [CrossRef]
- Hurley, R.W.; Chatterjea, D.; Rose Feng, M.; Taylor, C.P.; Hammond, D.L. Gabapentin and pregabalin can interact synergistically with naproxen to produce antihyperalgesia. Anesthesiology 2002, 97, 1263–1273. [Google Scholar] [CrossRef]
- Yoon, M.H.; Yaksh, T.L. Evaluation of interaction between gabapentin and ibuprofen on the formalin test in rats. Anesthesiology 1999, 91, 1006–1013. [Google Scholar] [CrossRef]
- Stepanovic-Petrovic, R.M.; Tomic, M.A.; Vuckovic, S.M.; Poznanovic, G.; Ugresic, N.D.; Prostran, M.S.; Boskovic, B. Pharmacological interaction between oxcarbazepine and two COX inhibitors in a rat model of inflammatory hyperalgesia. Pharmacol. Biochem. Behav. 2011, 97, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Narai, Y.; Imamachi, N.; Saito, Y. Gabapentin augments the antihyperalgesic effects of diclofenac sodium through spinal action in a rat postoperative pain model. Anesth. Analg. 2012, 115, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Picazo, A.; Castaneda-Hernandez, G.; Ortiz, M.I. Examination of the interaction between peripheral diclofenac and gabapentin on the 5% formalin test in rats. Life Sci. 2006, 79, 2283–2287. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Juarez, J.V.; Jaramillo-Morales, O.A.; Corona-Ramos, J.N.; Medina-Lopez, J.R.; Lopez-Munoz, F.J. Antinociceptive Interactions between Meloxicam and Gabapentin in Neuropathic Pain Depend on the Ratio used in Combination in Rats. Drug Dev. Res. 2016, 77, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Nozawa, K.; Karasawa, Y.; Shidahara, Y.; Ushida, T. Efficacy of Combination Therapy with Pregabalin in Neuropathic Pain: A Preclinical Study in the Rat L5 Spinal Nerve Ligation Model. J. Pain Res. 2022, 15, 3469–3478. [Google Scholar] [CrossRef] [PubMed]
- Keskinbora, K.; Pekel, A.F.; Aydinli, I. Gabapentin and an opioid combination versus opioid alone for the management of neuropathic cancer pain: A randomized open trial. J. Pain. Symptom Manag. 2007, 34, 183–189. [Google Scholar] [CrossRef] [PubMed]
- De la O-Arciniega, M.; Diaz-Reval, M.I.; Cortes-Arroyo, A.R.; Dominguez-Ramirez, A.M.; Lopez-Munoz, F.J. Anti-nociceptive synergism of morphine and gabapentin in neuropathic pain induced by chronic constriction injury. Pharmacol. Biochem. Behav. 2009, 92, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, H.; Shen, C.; Luo, J. Morphine and pregabalin in the treatment of neuropathic pain. Exp. Ther. Med. 2017, 13, 1393–1397. [Google Scholar] [CrossRef] [PubMed]
- Gilron, I.; Bailey, J.M.; Tu, D.; Holden, R.R.; Jackson, A.C.; Houlden, R.L. Nortriptyline and gabapentin, alone and in combination for neuropathic pain: A double-blind, randomised controlled crossover trial. Lancet 2009, 374, 1252–1261. [Google Scholar] [CrossRef] [PubMed]
- Tesfaye, S.; Sloan, G.; Petrie, J.; White, D.; Bradburn, M.; Julious, S.; Rajbhandari, S.; Sharma, S.; Rayman, G.; Gouni, R.; et al. Comparison of amitriptyline supplemented with pregabalin, pregabalin supplemented with amitriptyline, and duloxetine supplemented with pregabalin for the treatment of diabetic peripheral neuropathic pain (OPTION-DM): A multicentre, double-blind, randomised crossover trial. Lancet 2022, 400, 680–690. [Google Scholar] [CrossRef] [PubMed]
- Tesfaye, S.; Wilhelm, S.; Lledo, A.; Schacht, A.; Tolle, T.; Bouhassira, D.; Cruccu, G.; Skljarevski, V.; Freynhagen, R. Duloxetine and pregabalin: High-dose monotherapy or their combination? The “COMBO-DN study”—A multinational, randomized, double-blind, parallel-group study in patients with diabetic peripheral neuropathic pain. Pain 2013, 154, 2616–2625. [Google Scholar] [CrossRef]
- Chakrabarty, S.; Biswas, S.; Maiti, T.; Das, A.; Mandal, A.; Banerjee, P. Pregabalin and Amitriptyline as Monotherapy or as Low-Dose Combination in Patients of Neuropathic Pain: A Randomized, Controlled Trial to Evaluate Efficacy and Safety in an Eastern India Teaching Hospital. Ann. Indian. Acad. Neurol. 2019, 22, 437–441. [Google Scholar] [CrossRef]
- Pecikoza, U.B.; Tomic, M.A.; Micov, A.M.; Stepanovic-Petrovic, R.M. Metformin Synergizes with Conventional and Adjuvant Analgesic Drugs to Reduce Inflammatory Hyperalgesia in Rats. Anesth. Analg. 2017, 124, 1317–1329. [Google Scholar] [CrossRef]
- Ortiz, M.I. Synergistic interaction between diclofenac and pyrilamine on nociception, inflammation, and gastric damage in rats. Can. J. Physiol. Pharmacol. 2017, 95, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, M.I.; Carino-Cortes, R.; Ponce-Monter, H.A.; Gonzalez-Garcia, M.P.; Castaneda-Hernandez, G.; Salinas-Caballero, M. Synergistic Interaction of Matricaria Chamomilla Extract with Diclofenac and Indomethacin on Carrageenan-Induced Paw Inflammation in Rats. Drug Dev. Res. 2017, 78, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, M.I.; Carino-Cortes, R.; Ponce-Monter, H.A.; Castaneda-Hernandez, G.; Chavez-Pina, A.E. Pharmacological interaction of alpha-bisabolol and diclofenac on nociception, inflammation, and gastric integrity in rats. Drug Dev. Res. 2018, 79, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Lara, C.A.; Ortiz, M.I.; Rodriguez-Ramos, F.; Chavez-Pina, A.E. Synergistic interaction between docosahexaenoic acid and diclofenac on inflammation, nociception, and gastric security models in rats. Drug Dev. Res. 2018, 79, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Dudhgaonkar, S.P.; Tandan, S.K.; Kumar, D.; Naik, A.K.; Raviprakash, V. Ameliorative effect of combined administration of inducible nitric oxide synthase inhibitor with cyclooxygenase-2 inhibitors in neuropathic pain in rats. Eur. J. Pain 2007, 11, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Varela, L.F.; Herrera, J.E.; Caram-Salas, N.L.; Rocha-Gonzalez, H.I.; Granados-Soto, V. Isobolographic analyses of the gabapentin-metamizol combination after local peripheral, intrathecal and oral administration in the rat. Pharmacology 2007, 79, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Tomic, M.A.; Vuckovic, S.M.; Stepanovic-Petrovic, R.M.; Ugresic, N.D.; Prostran, M.S.; Boskovic, B. Synergistic interactions between paracetamol and oxcarbazepine in somatic and visceral pain models in rodents. Anesth. Analg. 2010, 110, 1198–1205. [Google Scholar] [CrossRef] [PubMed]
- Wallin, J.; Cui, J.G.; Yakhnitsa, V.; Schechtmann, G.; Meyerson, B.A.; Linderoth, B. Gabapentin and pregabalin suppress tactile allodynia and potentiate spinal cord stimulation in a model of neuropathy. Eur. J. Pain 2002, 6, 261–272. [Google Scholar] [CrossRef]
- Guindon, J.; Beaulieu, P. Antihyperalgesic effects of local injections of anandamide, ibuprofen, rofecoxib and their combinations in a model of neuropathic pain. Neuropharmacology 2006, 50, 814–823. [Google Scholar] [CrossRef]
- Guindon, J.; De Lean, A.; Beaulieu, P. Local interactions between anandamide, an endocannabinoid, and ibuprofen, a nonsteroidal anti-inflammatory drug, in acute and inflammatory pain. Pain 2006, 121, 85–93. [Google Scholar] [CrossRef]
- Ulugol, A.; Ozyigit, F.; Yesilyurt, O.; Dogrul, A. The additive antinociceptive interaction between WIN 55,212-2, a cannabinoid agonist, and ketorolac. Anesth. Analg. 2006, 102, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Reval, M.I.; Cardenas, Y.; Huerta, M.; Trujillo, X.; Sanchez-Pastor, E.A.; Gonzalez-Trujano, M.E.; Virgen-Ortiz, A.; Perez-Hernandez, M.G. Activation of Peripheral Cannabinoid Receptors Synergizes the Effect of Systemic Ibuprofen in a Pain Model in Rat. Pharmaceuticals 2022, 15, 910. [Google Scholar] [CrossRef]
- Kazantzis, N.P.; Casey, S.L.; Seow, P.W.; Mitchell, V.A.; Vaughan, C.W. Opioid and cannabinoid synergy in a mouse neuropathic pain model. Br. J. Pharmacol. 2016, 173, 2521–2531. [Google Scholar] [CrossRef] [PubMed]
- Quinonez-Bastidas, G.N.; Osuna-Martinez, U.; Reda-Licea, A.L.; Lopez-Ortiz, M.; Regla, I.; Navarrete, A. Synergistic action between a synthetic cannabinoid compound and tramadol in neuropathic pain rats. Acta Pharm. 2022, 72, 509–527. [Google Scholar] [CrossRef] [PubMed]
- Grenald, S.A.; Young, M.A.; Wang, Y.; Ossipov, M.H.; Ibrahim, M.M.; Largent-Milnes, T.M.; Vanderah, T.W. Synergistic attenuation of chronic pain using mu opioid and cannabinoid receptor 2 agonists. Neuropharmacology 2017, 116, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.A.; Jesus, C.H.A.; Martins, L.L.; Fukuyama, A.H.; Gasparin, A.T.; Crippa, J.A.; Zuardi, A.W.; Hallak, J.E.C.; Genaro, K.; de Castro Junior, C.J.; et al. Pharmacological Interaction between Cannabidiol and Tramadol on Experimental Diabetic Neuropathic Pain: An Isobolographic Analysis. Cannabis Cannabinoid Res. 2023. ahead of print. [Google Scholar] [CrossRef]
- Siddall, P.J.; Molloy, A.R.; Walker, S.; Mather, L.E.; Rutkowski, S.B.; Cousins, M.J. The efficacy of intrathecal morphine and clonidine in the treatment of pain after spinal cord injury. Anesth. Analg. 2000, 91, 1493–1498. [Google Scholar] [CrossRef]
- Amr, Y.M. Multi-day low dose ketamine infusion as adjuvant to oral gabapentin in spinal cord injury related chronic pain: A prospective, randomized, double blind trial. Pain Physician 2010, 13, 245–249. [Google Scholar] [CrossRef]
- Velazquez, K.T.; Mohammad, H.; Sweitzer, S.M. Protein kinase C in pain: Involvement of multiple isoforms. Pharmacol. Res. 2007, 55, 578–589. [Google Scholar] [CrossRef]
- Vellani, V.; Giacomoni, C. Gabapentin Inhibits Protein Kinase C Epsilon Translocation in Cultured Sensory Neurons with Additive Effects When Coapplied with Paracetamol (Acetaminophen). Sci. World J. 2017, 2017, 3595903. [Google Scholar] [CrossRef]
- Tesfaye, S.; Vileikyte, L.; Rayman, G.; Sindrup, S.H.; Perkins, B.A.; Baconja, M.; Vinik, A.I.; Boulton, A.J.; The Toronto Expert Panel on Diabetic Neuropathy. Painful diabetic peripheral neuropathy: Consensus recommendations on diagnosis, assessment and management. Diabetes Metab. Res. Rev. 2011, 27, 629–638. [Google Scholar] [CrossRef]
- Lotsch, J.; Weyer-Menkhoff, I.; Tegeder, I. Current evidence of cannabinoid-based analgesia obtained in preclinical and human experimental settings. Eur. J. Pain 2018, 22, 471–484. [Google Scholar] [CrossRef] [PubMed]
- Parri, N.; Silvagni, D.; Chiarugi, A.; Cortis, E.; D’Avino, A.; Lanari, M.; Marchisio, P.G.; Vezzoli, C.; Zampogna, S.; Staiano, A. Paracetamol and ibuprofen combination for the management of acute mild-to-moderate pain in children: Expert consensus using the Nominal Group Technique (NGT). Ital. J. Pediatr. 2023, 49, 36. [Google Scholar] [CrossRef] [PubMed]
- Abushanab, D.; Al-Badriyeh, D. Efficacy and Safety of Ibuprofen Plus Paracetamol in a Fixed-Dose Combination for Acute Postoperative Pain in Adults: Meta-Analysis and a Trial Sequential Analysis. CNS Drugs 2021, 35, 105–120. [Google Scholar] [CrossRef] [PubMed]
- Batlle, L.; Mattie, R.; Irwin, R. A Medication Combination for the Treatment of Central Poststroke Pain via the Adjuvant Use of Prednisone with Gabapentin: A Case Report. PM R. 2016, 8, 278–281. [Google Scholar] [CrossRef] [PubMed]
- Playne, R.; Anderson, B.J.; Frampton, C.; Stanescu, I.; Atkinson, H.C. Analgesic effectiveness, pharmacokinetics, and safety of a paracetamol/ibuprofen fixed-dose combination in children undergoing adenotonsillectomy: A randomized, single-blind, parallel group trial. Paediatr. Anaesth. 2018, 28, 1087–1095. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Du, S.; Zhang, R.; Jia, X.; Yang, T.; Zhang, X. Drug-drug cocrystals: Opportunities and challenges. Asian J. Pharm. Sci. 2021, 16, 307–317. [Google Scholar] [CrossRef]
- Emami, S.; Siahi-Shadbad, M.; Adibkia, K.; Barzegar-Jalali, M. Recent advances in improving oral drug bioavailability by cocrystals. Bioimpacts 2018, 8, 305–320. [Google Scholar] [CrossRef]
- Modani, S.; Gunnam, A.; Yadav, B.; Nangia, A.K.; Shastri, N.R. Generation and Evaluation of Pharmacologically Relevant Drug–Drug Cocrystal for Gout Therapy. Cryst. Growth Des. 2020, 20, 3577–3583. [Google Scholar] [CrossRef]
- Cheney, M.L.; Weyna, D.R.; Shan, N.; Hanna, M.; Wojtas, L.; Zaworotko, M.J. Coformer selection in pharmaceutical cocrystal development: A case study of a meloxicam aspirin cocrystal that exhibits enhanced solubility and pharmacokinetics. J. Pharm. Sci. 2011, 100, 2172–2181. [Google Scholar] [CrossRef]
- Buschmann, H.H.; Carandell, L.S.; Buchholz, J.B.; Bertan, J.C.C.; Artero, J.R. Co-Crystals of Duloxetine and Naproxen. EP2291345B1, 13 March 2013. [Google Scholar]
- Nugrahani, I.; Utami, D.; Nugraha, Y.P.; Uekusa, H.; Hasianna, R.; Darusman, A.A. Cocrystal construction between the ethyl ester with parent drug of diclofenac: Structural, stability, and anti-inflammatory study. Heliyon 2019, 5, e02946. [Google Scholar] [CrossRef] [PubMed]
- Machado, S.M.T.; Castro, R.A.E.; Maria, T.M.R.; Canotilho, J.; Eusebio, M.E.S. Levetiracetam + nonsteroidal anti-inflammatory drug binary systems: A contribution to the development of new solid dosage forms. Int. J. Pharm. 2017, 533, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Almansa, C.; Frampton, C.S.; Vela, J.M.; Whitelock, S.; Plata-Salaman, C.R. Co-crystals as a new approach to multimodal analgesia and the treatment of pain. J. Pain Res. 2019, 12, 2679–2689. [Google Scholar] [CrossRef] [PubMed]
- Almansa, C.; Merce, R.; Tesson, N.; Farran, J.; Tomas, J.; Plata-Salaman, C.R. Co-crystal of Tramadol Hydrochloride–Celecoxib (ctc): A Novel API−API Co-crystal for the Treatment of Pain. Cryst. Growth Des. 2017, 17, 1884–1892. [Google Scholar] [CrossRef]
- Wadman, M. The pain game. Nature 2007, 448, 400–401. [Google Scholar] [CrossRef] [PubMed]
- Nissen, S.E.; Yeomans, N.D.; Solomon, D.H.; Luscher, T.F.; Libby, P.; Husni, M.E.; Graham, D.Y.; Borer, J.S.; Wisniewski, L.M.; Wolski, K.E.; et al. Cardiovascular Safety of Celecoxib, Naproxen, or Ibuprofen for Arthritis. N. Engl. J. Med. 2016, 375, 2519–2529. [Google Scholar] [CrossRef] [PubMed]
- Merlos, M.; Portillo-Salido, E.; Brenchat, A.; Aubel, B.; Buxens, J.; Fisas, A.; Codony, X.; Romero, L.; Zamanillo, D.; Vela, J.M. Administration of a co-crystal of tramadol and celecoxib in a 1:1 molecular ratio produces synergistic antinociceptive effects in a postoperative pain model in rats. Eur. J. Pharmacol. 2018, 833, 370–378. [Google Scholar] [CrossRef]
- Videla, S.; Lahjou, M.; Vaque, A.; Sust, M.; Encabo, M.; Soler, L.; Sans, A.; Sicard, E.; Gascon, N.; Encina, G.; et al. Single-dose pharmacokinetics of co-crystal of tramadol-celecoxib: Results of a four-way randomized open-label phase I clinical trial in healthy subjects. Br. J. Clin. Pharmacol. 2017, 83, 2718–2728. [Google Scholar] [CrossRef]
- Viscusi, E.R.; de Leon-Casasola, O.; Cebrecos, J.; Jacobs, A.; Morte, A.; Ortiz, E.; Sust, M.; Vaque, A.; Gottlieb, I.; Daniels, S.; et al. Celecoxib-tramadol co-crystal in patients with moderate-to-severe pain following bunionectomy with osteotomy: A phase 3, randomized, double-blind, factorial, active- and placebo-controlled trial. Pain Pract. 2023, 23, 8–22. [Google Scholar] [CrossRef]
- Langford, R.; Morte, A.; Sust, M.; Cebrecos, J.; Vaque, A.; Ortiz, E.; Fettiplace, J.; Adeyemi, S.; Raba, G.; But-Husaim, L.; et al. Efficacy and safety of co-crystal of tramadol-celecoxib (CTC) in acute moderate-to-severe pain after abdominal hysterectomy: A randomized, double-blind, phase 3 trial (STARDOM2). Eur. J. Pain 2022, 26, 2083–2096. [Google Scholar] [CrossRef]
- Lopez-Cedrun, J.; Videla, S.; Burgueno, M.; Juarez, I.; Aboul-Hosn, S.; Martin-Granizo, R.; Grau, J.; Puche, M.; Gil-Diez, J.L.; Hueto, J.A.; et al. Co-crystal of Tramadol-Celecoxib in Patients with Moderate to Severe Acute Post-surgical Oral Pain: A Dose-Finding, Randomised, Double-Blind, Placebo- and Active-Controlled, Multicentre, Phase II Trial. Drugs R&D 2018, 18, 137–148. [Google Scholar] [CrossRef]
- Esteve Pharmaceuticals. Seglentis: Prescribing Information 2021. Available online: https://www.kowapharma.com/documents/SEGLENTIS_Prescribing_Information.pdf (accessed on 1 November 2023).
- Aramini, A.; Bianchini, G.; Lillini, S.; Tomassetti, M.; Pacchiarotti, N.; Canestrari, D.; Cocchiaro, P.; Novelli, R.; Dragani, M.C.; Palmerio, F.; et al. Ketoprofen, lysine and gabapentin co-crystal magnifies synergistic efficacy and tolerability of the constituent drugs: Pre-clinical evidences towards an innovative therapeutic approach for neuroinflammatory pain. Biomed. Pharmacother. 2023, 163, 114845. [Google Scholar] [CrossRef] [PubMed]
- Van Den Abeele, J.; Brouwers, J.; Mattheus, R.; Tack, J.; Augustijns, P. Gastrointestinal Behavior of Weakly Acidic BCS Class II Drugs in Man—Case Study of Diclofenac Potassium. J. Pharm. Sci. 2016, 105, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Kavanagh, O.N.; Croker, D.M.; Walker, G.M.; Zaworotko, M.J. Pharmaceutical cocrystals: From serendipity to design to application. Drug Discov. Today 2019, 24, 796–804. [Google Scholar] [CrossRef]
- Wallace, J.L. Prostaglandins, NSAIDs, and gastric mucosal protection: Why doesn’t the stomach digest itself? Physiol. Rev. 2008, 88, 1547–1565. [Google Scholar] [CrossRef]
- De Palma, C.; Di Paola, R.; Perrotta, C.; Mazzon, E.; Cattaneo, D.; Trabucchi, E.; Cuzzocrea, S.; Clementi, E. Ibuprofen-arginine generates nitric oxide and has enhanced anti-inflammatory effects. Pharmacol. Res. 2009, 60, 221–228. [Google Scholar] [CrossRef]
| Drug Combinations | Dose (Human Studies Only) | Pre-Clinical Study | Clinical Study | Key Findings | Reference |
|---|---|---|---|---|---|
| NSAID and Opioids (Including Tapentadol and Tramadol) | |||||
| Dexketoprofen + Tramadol | Dexketoprofen, 25 mg Tramadol, 75 mg | Postoperative pain model (plantar incision) in mouse (von Frey test) | Moderate or severe pain following third molar extraction, total hip arthroplasty, or abdominal hysterectomy | Mouse: Additive anti-hyperalgesic effect of combination; inhibition of microglia activation in the spinal cord | [90] |
| Human: Combination therapy was superior to either agent as monotherapy; greater peak pain relief of combination over monotherapy, particularly at times >6 h; adverse events were unremarkable | [91,92,93] | ||||
| Dexketoprofen + Tramadol | Acetic acid writhing test, tail-flick test, and formalin test in mouse | NA | Synergistic analgesia, increased risk for constipation | [94] | |
| Dexketoprofen + Tramadol | Musculoskeletal pain and complete Freund’s adjuvant inflammatory pain models in mouse (hot plate test, acetone test, and spontaneous pain behaviors) | NA | Synergistic analgesia | [95] | |
| Ketorolac + Tramadol | SNI in rat (von Frey test, acetone test, spontaneous pain behaviors) | NA | Synergistic analgesia with subeffective doses of tramadol + ketorolac | [96] | |
| NSAIDs and gabapentinoids/antiepileptics | |||||
| Pregabalin + Ketorolac | SNI in rat (von Frey test, acetone test, spontaneous pain behaviors) | NA | Synergistic analgesia with subeffective doses pregabalin + ketorolac | [96] | |
| Gabapentin + Naproxen Pregabalin + Naproxen | Carrageenan paw edema model in rat (radiant heat paw-withdrawal test) | NA | Synergistic anti-hyperalgesia with 50:1, 10:1, and 1:1 combinations for assessments of thermal hyperalgesia; only additive effects for paw edema and for 1:50 for thermal hyperalgesia | [97] | |
| Gabapentin + Ibuprofen | Formalin test in rat | NA | Additive analgesia | [98] | |
| Ibuprofen + Oxcarbazepine | Carrageenan paw edema model in rat (modified paw pressure test) | NA | Synergistic analgesia | [99] | |
| Gabapentin + Diclofenac | Postoperative pain model (hindpaw incision) in rat (von Frey test) | NA | Synergistic analgesia with subeffective doses of gabapentin + diclofenac 1 | [100] | |
| Gabapentin + Diclofenac | Formalin test in rat | NA | Synergistic analgesia | [101] | |
| Gabapentin + Meloxicam | Chronic constriction injury (sciatic nerve) in rat (von Frey test and acetone test) | NA | Additive anti-hyperalgesia in the von Frey test, synergistic anti-allodynia with the acetone test only at one specific dose tested (10 mg/kg gabapentin + 1 mg/kg meloxicam) | [102] | |
| Pregabalin + Celecoxib | Spinal nerve ligation model in rat (von Frey test) | NA | Additive anti-allodynic effect | [103] | |
| Gabapentinoids and opioids (including tapentadol and tramadol) | |||||
| Pregabalin + Oxycodone | Doses titrated to achieve optimal efficacy and tolerability (mean doses at end of study: 141.5 mg + 35.8 mg) (oral) | NA | Moderate to severe neuropathic pain (including failed back surgery syndrome, stenosis medullary spinal canal, post-herpetic neuralgia, painful diabetic neuropathy) | Both combination therapy and oxycodone monotherapy alleviated neuropathic pain; combination therapy was superior to pregabalin monotherapy and allowed a reduction of the dose for both oxycodone (22%) and pregabalin (51%) | [67] |
| Pregabalin + Oxycodone | Pregabalin titrated to achieve optimal efficacy and tolerability (mean pregabalin dose: 227.6 mg/day); oxycodone: 10 mg/day (oral) | NA | Post-herpetic neuralgia or painful diabetic neuropathy | No added benefit of combination therapy | [84] |
| Gabapentin + Oxycodone | Doses titrated to achieve optimal efficacy and tolerability (Gabapentin: 48% of patients: <1200 mg 36% of patients: 12–1800 mg 16% of patients: >1800 mg + Oxycodone: Up to 80 mg daily) (oral) | NA | DPNP | Oxycodone + gabapentin significantly improved pain relief vs. gabapentin alone. Oxycodone + gabapentin co-administration was associated with less escape medication use and fewer nights of disturbed sleep | [78] |
| Pregabalin + Tramadol | L5 spinal nerve ligation model in rat (von Frey test) | NA | Synergistic anti-allodynic effect of combinations as compared to single compounds | [103] | |
| Gabapentin + Morphine, tramadol, or fentanyl | Doses titrated to achieve optimal efficacy and tolerability (mean doses at end of study: Gabapentin: 1287.1 mg/day Morphine: 90 mg/day Tramadol: 400 mg/day Fentanyl: 68.1 µg/48 h) (oral) | NA | Nonresponsive neuropathic cancer pain | Stronger reduction in burning and shooting pain after 4 and 13 days and a stronger reduction in allodynia at 4 days with combination therapy relative to opioids alone. Combination therapy group also had significantly fewer side effects | [104] |
| Gabapentin + Morphine | CCI in rat (von Frey and acetone tests) | NA | Synergistic anti-allodynic effects, synergistic anti-hyperalgesia effects; anti-allodynic effect of combination therapy persisted longer than morphine alone 120 m | [105] | |
| Pregabalin + Morphine | Doses titrated to achieve optimal efficacy and tolerability (mean doses at end of study: 142.5 mg + 41.8 mg daily) (oral) | NA | Moderate to severe chronic neuropathic pain (including failed back surgery syndrome, stenosis medullary spinal canal, post-herpetic neuralgia, painful diabetic neuropathy) | Combination therapy was more effective in reducing pain intensity at 3 months than pregabalin monotherapy. Furthermore, while reducing the average dosage, combination therapy improved quality of life compared with the patients in the other two groups | [106] |
| Gabapentinoids and antidepressants | |||||
| Pregabalin + Duloxetine Pregabalin + Venlafaxine | Spinal nerve ligation model in rat (von Frey test) | NA | Additive anti-allodynic effect of pregabalin + duloxetine, potentially antagonistic effects with pregabalin + venlafaxine | [103] | |
| Gabapentin + Venlafaxine | Doses titrated to achieve optimal efficacy and tolerability (dose range: Gabapentin: 300–3600 mg daily Venlafaxine: 37.5–150 mg daily) (oral) | NA | Painful diabetic neuropathy | Significant improvement in pain reduction, mood, and quality of life observed with combination therapy relative to gabapentin alone | [77] |
| Gabapentin + Nortriptyline | Doses titrated to achieve optimal efficacy and tolerability | NA | Diabetic neuropathy or postherpetic neuralgia | Stronger pain reduction observed with combination therapy relative to either drug as monotherapy | [107] |
| Pregabalin + Amitriptyline Pregabalin + Duloxetine | Doses titrated to achieve optimal efficacy and tolerability (mean doses at pain assessment: Amitriptyline + pregabalin: 56 mg + 347 mg Pregabalin + Amitriptyline: 397 mg + 52 mg Duloxetine + pregabalin: 76 mg + 405 mg) (oral) | NA | Moderate–severe DPNP | Combination therapy and monotherapy had similar analgesic effects, but combination therapy had a stronger analgesic effect in those unresponsive to monotherapy | [108] |
| Pregabalin + Duloxetine | 300 + 60 mg daily (oral) | NA | Patients with moderate-severe DPNP who were unresponsive to monotherapy | There was no significant benefit observed with combination therapy relative to high-dose monotherapy of either drug on the Brief Pain Inventory Modified Short Form average pain score. The combination was safe and well tolerated | [109] |
| Pregabalin + Amitriptyline | 75 + 10 mg daily (oral) | NA | NP | Combination therapy with low-dose pregabalin and amitriptyline was equally effective but more tolerable compared to higher dosage monotherapy with either drug in reducing NP symptom inventory score | [110]. |
| Additional combinations | |||||
| Metformin + Ibuprofen Metformin + Aspirin Metformin + Tramadol Metformin + Pregabalin | Carrageenan paw edema model in rat (von Frey test) | NA | Synergistic analgesia with a ~5-fold reduction of doses of both drugs required for pain relief in all tested combinations | [111] | |
| Diclofenac + Pyrilamine | Formalin model in rat Carrageenan paw edema model in rat | NA | Synergistic anti-inflammatory and analgesic effects; Level of gastric damage was reduced with the combination therapy compared with diclofenac alone | [112] | |
| Diclofenac + Matricaria chamomilla extract (MCE) Indomethacin + Matricaria chamomilla extract (MCE) | Carrageenan paw edema model in rat | NA | Synergistic anti-inflammatory effect (reduced paw inflammation); reduced levels of gastric damage caused by NSAID-MCE combinations compared with monotherapies | [113] | |
| Diclofenac + α-bisabolol | Carrageenan paw edema model and formalin test in rat | NA | Synergistic analgesia and ant-inflammatory efficacy (reduced paw inflammation); reduced levels of gastric damage observed with combinations relative to monotherapies | [114] | |
| Diclofenac + Docosahexaenoic acid (DHA) | Formalin model in rat Carrageenan paw edema model in rat | NA | Synergistic analgesia and ant-inflammatory efficacy (reduced paw inflammation); reduced levels of gastric damage observed with combinations relative to monotherapies | [115] | |
| Rofecoxib + Aminoguanidine hydrochloride Meloxicam + Aminoguanidine hydrochloride | CCI model of neuropathic pain in rat (pressure test, hot plate test, cold stimuli paw withdrawal test) | NA | Improved analgesia outcomes (i.e., higher withdrawal thresholds than monotherapies) | [116] | |
| Gabapentin + Metamizole | Formalin model in rat | NA | Synergistic analgesia (systemic administration resulted in the highest synergism) 1 | [117] | |
| Paracetamol + Oxcarbazepine | Carrageenan paw edema model in rat and acetic acid-induced writhing test in mouse | NA | Synergistic anti-hyperalgesia | [118] | |
| Gabapentin + Pregabalin | Partial sciatic nerve injury (von Frey test) | NA | Subeffective doses of combination potentiated the effects of spinal cord stimulation in neuropathic rats on tactile allodynia and neuronal spinal hyperexcitability 1 | [119] | |
| Ibuprofen + Anandamide | Formalin model in rat Partial sciatic nerve ligation in rat (von Frey test and noxious heat paw withdrawal) | NA | Synergistic analgesia in the formalin model; improved anti-hyperalgesic and anti-allodynic effects in the partial sciatic nerve ligation model (no conclusions regarding additive or synergistic effects) | [120,121] | |
| Rofecoxib + Anandamide | Partial sciatic nerve ligation in rat (von Frey test and noxious heat paw withdrawal) | NA | Improved anti-hyperalgesic and anti-allodynic effects in the partial sciatic nerve ligation model (no conclusions regarding additive or synergistic effects) | [120] | |
| Ketorolac + WIN 55,212-2 (synthetic cannabinoid) | Acetic acid-induced writhing test and tail-flick test in rat | NA | Additive analgesia in the acetic acid induced writhing test, no effect or added benefit of ketorolac in addition to WIN 55,212-2 in the thermal tail-flick test | [122] | |
| Ibuprofen + WIN 55,212-2 (synthetic cannabinoid) | Formalin model in rat | NA | Synergistic analgesia | [123] | |
| Morphine + WIN 55,212-2 (synthetic cannabinoid) | CCI (von Frey test and cold allodynia) | NA | Synergistic analgesia (no synergistic effect observed on motor coordination) | [124] | |
| PhAR-DBH-Me + Tramadol | Spinal nerve ligation, cisplatin-induced NP (von Frey test) | NA | Synergistic analgesia in rats exposed to spinal nerve ligation but not in rats exposed to cisplatin-induced NP | [125] | |
| JWH015 (CB2 agonist) + Morphine | Post-operative pain (plantar incision), SNI, formalin model (von Frey test and thermal withdrawal) | NA | Synergistic efficacy observed in inflammatory, post-operative, and SNI pain; gastrointestinal impairment and conditioned place preference associated with morphine was reduced with combination JWH015 | [126] | |
| Cannabidiol + Tramadol | STZ-induced diabetic neuropathy (von Frey test) | NA | Additive, but not synergistic, analgesia | [127] | |
| Morphine + Clonidine | Doses titrated to achieve optimal efficacy and tolerability (rescue analgesia with paracetamol or dextromoramide was permitted) (i.t.) 1 | NA | Patients with neuropathic pain after spinal cord injury unresponsive to other treatments | Synergistic efficacy was observed in that morphine + clonidine was associated with stronger pain relief than either as monotherapy or placebo | [128] |
| Gabapentin + Ketamine | 300 mg gabapentin 3× daily (oral) + 80 mg ketamine (i.v.) | NA | Patients with neuropathic pain after spinal cord injury | Gabapentin + ketamine produced greater pain relief than did gabapentin alone, but pain returned two weeks after cessation of the ketamine infusion | [129] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boccella, S.; De Filippis, L.; Giorgio, C.; Brandolini, L.; Jones, M.; Novelli, R.; Amorizzo, E.; Leoni, M.L.G.; Terranova, G.; Maione, S.; et al. Combination Drug Therapy for the Management of Chronic Neuropathic Pain. Biomolecules 2023, 13, 1802. https://doi.org/10.3390/biom13121802
Boccella S, De Filippis L, Giorgio C, Brandolini L, Jones M, Novelli R, Amorizzo E, Leoni MLG, Terranova G, Maione S, et al. Combination Drug Therapy for the Management of Chronic Neuropathic Pain. Biomolecules. 2023; 13(12):1802. https://doi.org/10.3390/biom13121802
Chicago/Turabian StyleBoccella, Serena, Lidia De Filippis, Cristina Giorgio, Laura Brandolini, Meghan Jones, Rubina Novelli, Ezio Amorizzo, Matteo Luigi Giuseppe Leoni, Gaetano Terranova, Sabatino Maione, and et al. 2023. "Combination Drug Therapy for the Management of Chronic Neuropathic Pain" Biomolecules 13, no. 12: 1802. https://doi.org/10.3390/biom13121802
APA StyleBoccella, S., De Filippis, L., Giorgio, C., Brandolini, L., Jones, M., Novelli, R., Amorizzo, E., Leoni, M. L. G., Terranova, G., Maione, S., Luongo, L., Leone, M., Allegretti, M., Minnella, E. M., & Aramini, A. (2023). Combination Drug Therapy for the Management of Chronic Neuropathic Pain. Biomolecules, 13(12), 1802. https://doi.org/10.3390/biom13121802








