Repurposing an Antioxidant to Kill Mycobacterium tuberculosis by Targeting the 50S Subunit of the Ribosome
Abstract
1. Introduction
2. Materials and Methods
2.1. Drugs and Reagents
2.2. M. tuberculosis Strains and Cell Culture
2.3. MIC Determination
2.4. Cytotoxicity Determination
2.5. Kill Curve Assay
2.6. Drug Synergy according to the Checkerboard Method
2.7. Intracellular Antibacterial Activity Assay
2.8. Protective Effect of SKQ-1 on Galleria mellonella In Vivo
2.9. Mutation Frequency and Acquisition of Resistant Mutants
2.10. The Coupled Transcription/Translation Assay
2.11. Statistical Analysis
3. Results
3.1. Activity of SKQ-1 against Mtb
3.2. Kill Curve Dynamics
3.3. Activity of SKQ-1 against Intracellular Mtb
3.4. The Antibacterial Synergy of SKQ-1
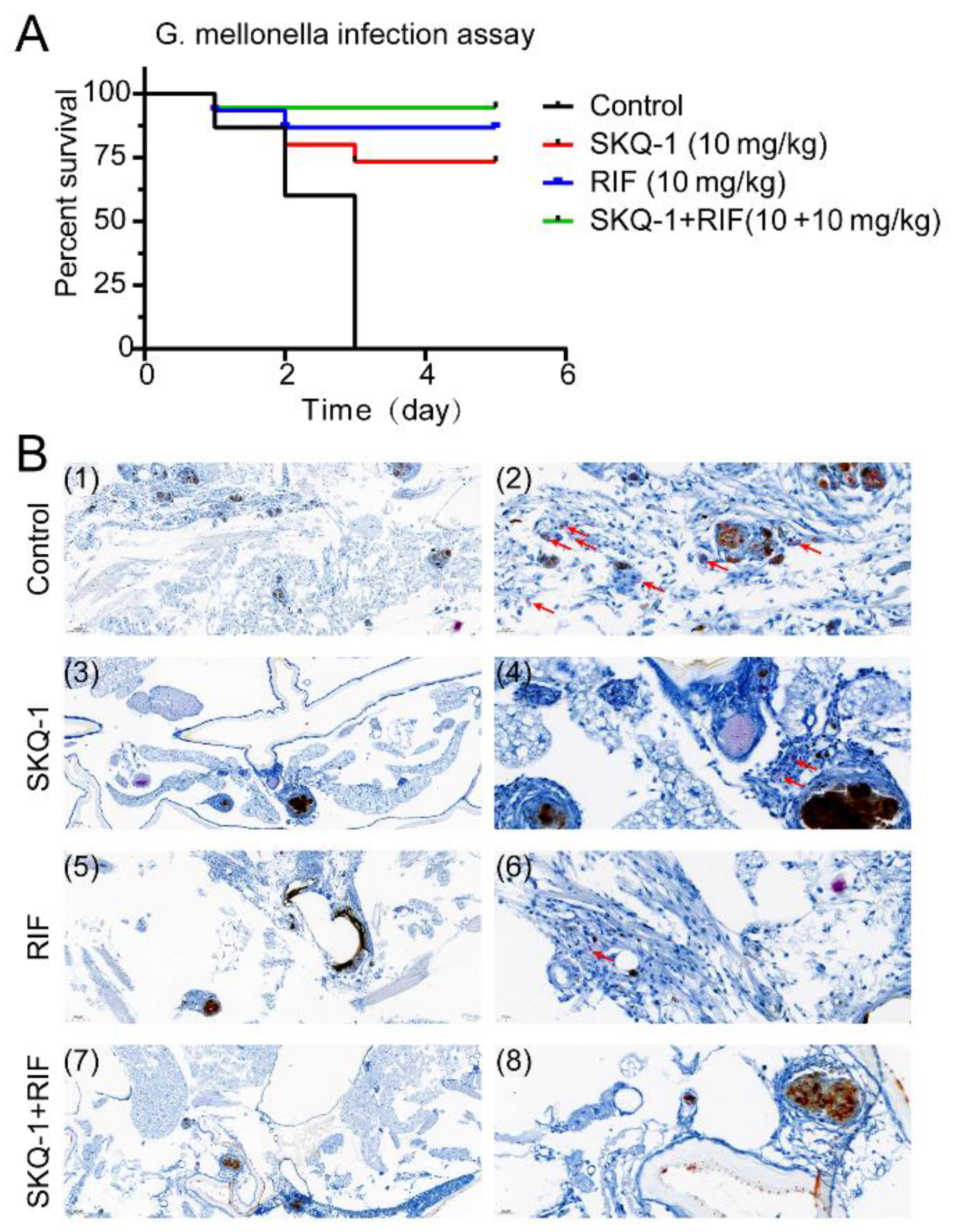
3.5. SKQ-1 in Combination with RIF Protects G. mellonella from Mtb
3.6. SKQ-1 Targets Ribosomes in Mtb
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bagcchi, S. WHO’s global tuberculosis report 2022. Lancet Microbe 2023, 4, e20. [Google Scholar] [CrossRef] [PubMed]
- Wolff, K.A.; Nguyen, L. Strategies for potentiation of ethionamide and folate antagonists against Mycobacterium tuberculosis. Expert. Rev. Anti. Infect. Ther. 2012, 10, 971–981. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zumla, A.I.; Gillespie, S.H.; Hoelscher, M.; Philips, P.P.; Cole, S.T.; Abubakar, I.; McHugh, T.D.; Schito, M.; Maeurer, M.; Nunn, A.J. New antituberculosis drugs, regimens, and adjunct therapies: Needs, advances, and future prospects. Lancet Infect. Dis. 2014, 14, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Zitko, J.; Doležal, M. Old Drugs and New Targets as an Outlook for the Treatment of Tuberculosis. Curr. Med. Chem. 2018, 25, 5142–5167. [Google Scholar] [CrossRef] [PubMed]
- An, Q.; Li, C.; Chen, Y.; Deng, Y.; Yang, T.; Luo, Y. Repurposed drug candidates for antituberculosis therapy. Eur. J. Med. Chem. 2020, 192, 112175. [Google Scholar] [CrossRef]
- Zumla, A.; Nahid, P.; Cole, S.T. Advances in the development of new tuberculosis drugs and treatment regimens. Nat. Rev. Drug Discov. 2013, 12, 388–404. [Google Scholar] [CrossRef]
- Gold, B.; Pingle, M.; Brickner, S.J.; Shah, N.; Roberts, J.; Rundell, M.; Bracken, W.C.; Warrier, T.; Somersan, S.; Venugopal, A.; et al. Nonsteroidal anti-inflammatory drug sensitizes Mycobacterium tuberculosis to endogenous and exogenous antimicrobials. Proc. Natl. Acad. Sci. USA 2012, 109, 16004–16011. [Google Scholar] [CrossRef]
- Byrne, S.T.; Denkin, S.M.; Zhang, Y. Aspirin and ibuprofen enhance pyrazinamide treatment of murine tuberculosis. J. Antimicrob. Chemother. 2007, 59, 313–316. [Google Scholar] [CrossRef]
- Maitra, A.; Bates, S.; Shaik, M.; Evangelopoulos, D.; Abubakar, I.; McHugh, T.D.; Lipman, M.; Bhakta, S. Repurposing drugs for treatment of tuberculosis: A role for non-steroidal anti-inflammatory drugs. Br. Med. Bull. 2016, 118, 138–148. [Google Scholar] [CrossRef]
- Vashisht, R.; Brahmachari, S.K. Metformin as a potential combination therapy with existing front-line antibiotics for Tuberculosis. J. Transl. Med. 2015, 13, 83. [Google Scholar] [CrossRef]
- Oglesby, W.; Kara, A.M.; Granados, H.; Cervantes, J.L. Metformin in tuberculosis: Beyond control of hyperglycemia. Infection 2019, 47, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Antonenko, Y.N.; Roginsky, V.A.; Pashkovskaya, A.A.; Rokitskaya, T.I.; Kotova, E.A.; Zaspa, A.A.; Chernyak, B.V.; Skulachev, V.P. Protective effects of mitochondria-targeted antioxidant SkQ in aqueous and lipid membrane environments. J. Membr. Biol. 2008, 222, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Shabalina, I.G.; Vyssokikh, M.Y.; Gibanova, N.; Csikasz, R.I.; Edgar, D.; Hallden-Waldemarson, A.; Rozhdestvenskaya, Z.; Bakeeva, L.E.; Vays, V.B.; Pustovidko, A.V.; et al. Improved health-span and lifespan in mtDNA mutator mice treated with the mitochondrially targeted antioxidant SkQ1. Aging 2017, 9, 315–339. [Google Scholar] [CrossRef] [PubMed]
- Novikova, Y.P.; Gancharova, O.S.; Eichler, O.V.; Philippov, P.P.; Grigoryan, E.N. Preventive and therapeutic effects of SkQ1-containing Visomitin eye drops against light-induced retinal degeneration. Biochemistry 2014, 79, 1101–1110. [Google Scholar] [CrossRef] [PubMed]
- Brzheskiy, V.V.; Efimova, E.L.; Vorontsova, T.N.; Alekseev, V.N.; Gusarevich, O.G.; Shaidurova, K.N.; Ryabtseva, A.A.; Andryukhina, O.M.; Kamenskikh, T.G.; Sumarokova, E.S.; et al. Results of a Multicenter, Randomized, Double-Masked, Placebo-Controlled Clinical Study of the Efficacy and Safety of Visomitin Eye Drops in Patients with Dry Eye Syndrome. Adv. Ther. 2015, 32, 1263–1279. [Google Scholar] [CrossRef]
- Qu, M.; Wan, L.; Dong, M.; Wang, Y.; Xie, L.; Zhou, Q. Hyperglycemia-induced severe mitochondrial bioenergetic deficit of lacrimal gland contributes to the early onset of dry eye in diabetic mice. Free Radic. Biol. Med. 2021, 166, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Chistyakov, D.V.; Baksheeva, V.E.; Tiulina, V.V.; Goriainov, S.V.; Azbukina, N.V.; Gancharova, O.S.; Arifulin, E.A.; Komarov, S.V.; Chistyakov, V.V.; Tikhomirova, N.K.; et al. Mechanisms and Treatment of Light-Induced Retinal Degeneration-Associated Inflammation: Insights from Biochemical Profiling of the Aqueous Humor. Int. J. Mol. Sci. 2020, 21, 704. [Google Scholar] [CrossRef]
- Wang, G.; Dong, W.; Lu, H.; Lu, W.; Feng, J.; Wang, X.; Chen, H.; Liu, M.; Tan, C. Enniatin A1, A Natural Compound with Bactericidal Activity against Mycobacterium tuberculosis In Vitro. Molecules 2019, 25, 38. [Google Scholar] [CrossRef]
- Odds, F.C. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 2003, 52, 1. [Google Scholar] [CrossRef]
- Dong, W.; Wang, G.; Feng, J.; Li, P.; Wang, R.; Lu, H.; Lu, W.; Wang, C.; Wang, X.; Chen, H.; et al. MiR-25 blunts autophagy and promotes the survival of Mycobacterium tuberculosis by regulating NPC1. iScience 2022, 25, 104279. [Google Scholar] [CrossRef]
- Asai, M.; Li, Y.; Spiropoulos, J.; Cooley, W.; Everest, D.; Robertson, B.D.; Langford, P.R.; Newton, S.M. A novel biosafety level 2 compliant tuberculosis infection model using a DeltaleuDDeltapanCD double auxotroph of Mycobacterium tuberculosis H37Rv and Galleria mellonella. Virulence 2020, 11, 811–824. [Google Scholar] [CrossRef] [PubMed]
- Asai, M.; Li, Y.; Khara, J.S.; Robertson, B.D.; Langford, P.R.; Newton, S.M. Galleria mellonella: An Infection Model for Screening Compounds against the Mycobacterium tuberculosis Complex. Front. Microbiol. 2019, 10, 2630. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.; Kumar, P.; Parashar, V.; Vilcheze, C.; Veyron-Churlet, R.; Freundlich, J.S.; Barnes, S.W.; Walker, J.R.; Szymonifka, M.J.; Marchiano, E.; et al. Antituberculosis thiophenes define a requirement for Pks13 in mycolic acid biosynthesis. Nat. Chem. Biol. 2013, 9, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Lemieux, M.R.; Siricilla, S.; Mitachi, K.; Eslamimehr, S.; Wang, Y.; Yang, D.; Pressly, J.D.; Kong, Y.; Park, F.; Franzblau, S.G.; et al. An antimycobacterial pleuromutilin analogue effective against dormant bacilli. Bioorganic. Med. Chem. 2018, 26, 4787–4796. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, S.; Takhar, P.; Baldeviano, C.; Glover, W.; Zhang, Y. Isoniazid induces its own resistance in nonreplicating Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2007, 51, 2100–2104. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, M.; Sala, C.; Dhar, N.; Vocat, A.; Sambandamurthy, V.K.; Sharma, S.; Marriner, G.; Balasubramanian, V.; Cole, S.T. In vitro and in vivo activities of three oxazolidinones against nonreplicating Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2014, 58, 3217–3223. [Google Scholar] [CrossRef] [PubMed]
- Leach, K.L.; Brickner, S.J.; Noe, M.C.; Miller, P.F. Linezolid, the first oxazolidinone antibacterial agent. Ann. N. Y. Acad. Sci. 2011, 1222, 49–54. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, H.; Han, Y.; Huo, L.; Cao, Y.; Xu, X.; Ai, L. Rapid and simultaneous determination of ten anti-tuberculosis drugs in human plasma by UPLC-MS/MS with applications in therapeutic drug monitoring. J. Chromatogr. B 2020, 1152, 122246. [Google Scholar] [CrossRef]
- Luo, M.; Zhou, W.; Patel, H.; Srivastava, A.P.; Symersky, J.; Bonar, M.M.; Faraldo-Gómez, J.D.; Liao, M.; Mueller, D.M. Bedaquiline inhibits the yeast and human mitochondrial ATP synthases. Commun. Biol. 2020, 3, 452. [Google Scholar] [CrossRef]
- Zhao, Y.; Fox, T.; Manning, K.; Stewart, A.; Tiffin, N.; Khomo, N.; Leslie, J.; Boulle, A.; Mudaly, V.; Kock, Y.; et al. Improved Treatment Outcomes with Bedaquiline When Substituted for Second-line Injectable Agents in Multidrug-resistant Tuberculosis: A Retrospective Cohort Study. Clin. Infect. Dis. 2019, 68, 1522–1529. [Google Scholar] [CrossRef]
- Zhang, X.; Falagas, M.E.; Vardakas, K.Z.; Wang, R.; Qin, R.; Wang, J.; Liu, Y. Systematic review and meta-analysis of the efficacy and safety of therapy with linezolid containing regimens in the treatment of multidrug-resistant and extensively drug-resistant tuberculosis. J. Thorac. Dis. 2015, 7, 603–615. [Google Scholar] [CrossRef] [PubMed]
- Hashemian, S.M.; Farhadi, T.; Ganjparvar, M. Linezolid: A review of its properties, function, and use in critical care. Drug Des. Dev. Ther. 2018, 12, 1759–1767. [Google Scholar] [CrossRef] [PubMed]
- Campbell, E.A.; Korzheva, N.; Mustaev, A.; Murakami, K.; Nair, S.; Goldfarb, A.; Darst, S.A. Structural mechanism for rifampicin inhibition of bacterial rna polymerase. Cell 2001, 104, 901–912. [Google Scholar] [CrossRef] [PubMed]
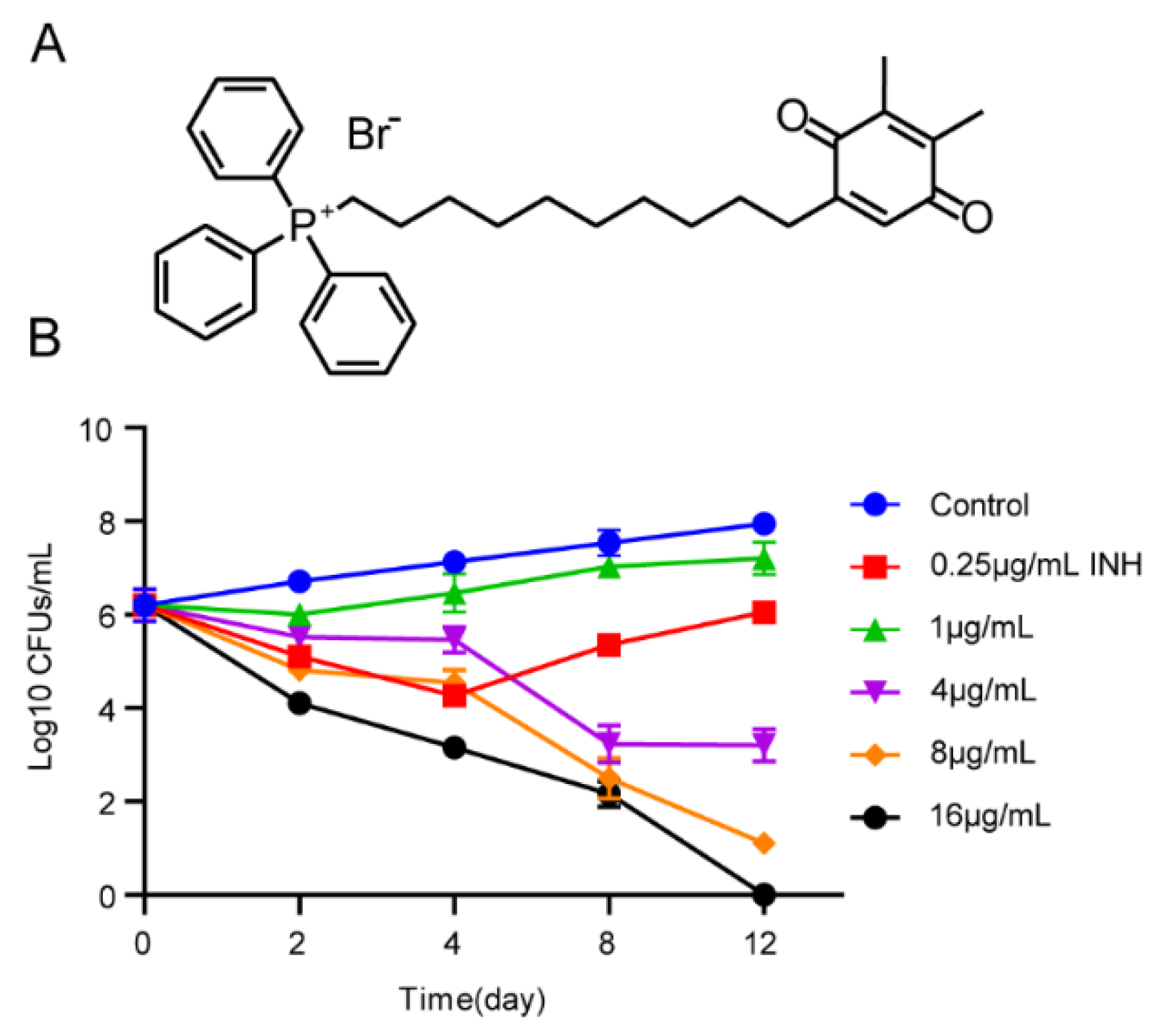


| Strains | MIC (μg/mL) |
|---|---|
| H37Rv (Susceptible) | 1 |
| M. bovis (Susceptible) | 2 |
| H37Ra (Susceptible) | 1 |
| BCG (Susceptible) | 2 |
| CR1 (RIF-R) | 2 |
| CR2 (RIF-R) | 2 |
| CR3 (INH-R) | 2 |
| CR4 (INH-R) | 1 |
| CR5 (EMB-R) | 1 |
| CR6 (MDR) | 4 |
| Drug | MIC (μg/mL) | FIC | FICI | Remarks | |
|---|---|---|---|---|---|
| Combination | Alone | Combination | |||
| SKQ-1 | 1 | 0.0625 | 0.0625 | 0.1875 | synergy |
| RIF | 0.0156 | 0.002 | 0.125 | ||
| SKQ-1 | 1 | 0.0312 | 0.0312 | 0.0624 | synergy |
| INH | 0.0625 | 0.002 | 0.0312 | ||
| SKQ-1 | 1 | 0.004 | 0.004 | 1.004 | additive |
| EMB | 1 | 1 | 1 | ||
| SKQ-1 | 1 | 0.0156 | 0.0156 | 1.0156 | additive |
| LNZ | 0.5 | 0.5 | 1 | ||
| SKQ-1 | 1 | 0.0156 | 0.0156 | 0.5156 | additive |
| BDQ | 0.0625 | 0.0312 | 0.5 | ||
| Type | Location | Reference | After | Gene | Annotation |
|---|---|---|---|---|---|
| SNP | 1476234 | G | A | rrl | 23S ribosomal RNA |
| SNP | 1476260 | A | G | rrl | 23S ribosomal RNA |
| SNP | 1476268 | A | T | rrl | 23S ribosomal RNA |
| SNP | 1476332 | G | C | rrl | 23S ribosomal RNA |
| SNP | 1476353 | G | T | rrl | 23S ribosomal RNA |
| SNP | 1476358 | T | C | rrl | 23S ribosomal RNA |
| SNP | 1476408 | G | A | rrl | 23S ribosomal RNA |
| SNP | 1476466 | C | T | rrl | 23S ribosomal RNA |
| SNP | 1476481 | T | C | rrl | 23S ribosomal RNA |
| SNP | 1476506 | T | C | rrl | 23S ribosomal RNA |
| SNP | 1476530 | C | T | rrl | 23S ribosomal RNA |
| SNP | 1476547 | C | T | rrl | 23S ribosomal RNA |
| SNP | 1476567 | C | T | rrl | 23S ribosomal RNA |
| SNP | 1476584 | C | T | rrl | 23S ribosomal RNA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, W.; Wang, G.; Bai, Y.; Li, Y.; Zhao, L.; Lu, W.; Wang, C.; Zhang, Z.; Lu, H.; Wang, X.; et al. Repurposing an Antioxidant to Kill Mycobacterium tuberculosis by Targeting the 50S Subunit of the Ribosome. Biomolecules 2023, 13, 1793. https://doi.org/10.3390/biom13121793
Dong W, Wang G, Bai Y, Li Y, Zhao L, Lu W, Wang C, Zhang Z, Lu H, Wang X, et al. Repurposing an Antioxidant to Kill Mycobacterium tuberculosis by Targeting the 50S Subunit of the Ribosome. Biomolecules. 2023; 13(12):1793. https://doi.org/10.3390/biom13121793
Chicago/Turabian StyleDong, Wenqi, Gaoyan Wang, Yajuan Bai, Yuxin Li, Liying Zhao, Wenjia Lu, Chenchen Wang, Zhaoran Zhang, Hao Lu, Xiangru Wang, and et al. 2023. "Repurposing an Antioxidant to Kill Mycobacterium tuberculosis by Targeting the 50S Subunit of the Ribosome" Biomolecules 13, no. 12: 1793. https://doi.org/10.3390/biom13121793
APA StyleDong, W., Wang, G., Bai, Y., Li, Y., Zhao, L., Lu, W., Wang, C., Zhang, Z., Lu, H., Wang, X., Chen, H., & Tan, C. (2023). Repurposing an Antioxidant to Kill Mycobacterium tuberculosis by Targeting the 50S Subunit of the Ribosome. Biomolecules, 13(12), 1793. https://doi.org/10.3390/biom13121793







