Investigating the Stirred Tank Bioreactor Co-Cultures of the Secondary Metabolite Producers Streptomyces noursei and Penicillium rubens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains
2.2. Cultivation Medium
2.3. Cultivation Conditions
2.4. Co-Culture Initiation Methods
- PRSN1: simultaneous inoculation of S. noursei and P. rubens;
- PRSN2: S. noursei inoculation delayed by 24 h relative to P. rubens;
- PRSN3: S. noursei inoculation delayed by 48 h relative to P. rubens.
2.5. Analytical Procedures
3. Results
3.1. Production of Secondary Metabolites
3.2. Changes in Dissolved Oxygen Levels
3.3. Consumption of Lactose and Glucose
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Demain, A.L.; Fang, A. The natural functions of secondary metabolites. In Advances in Biochemical Engineering/Biotechnology; Springer: Berlin/Heidelberg, Germany, 2000; Volume 69, pp. 1–39. [Google Scholar]
- Hegemann, J.D.; Birkelbach, J.; Walesch, S.; Müller, R. Current developments in antibiotic discovery. EMBO Rep. 2023, 24, e56184. [Google Scholar] [CrossRef]
- Walesch, S.; Birkelbach, J.; Jézéquel, G.; Haeckl, J.; Hegemann, J.D.; Hesterkamp, T.; Hirsch, A.K.H.; Hammann, P.; Müller, R. Fighting antibiotic resistance—Strategies and (pre)clinical developments to find new antibacterials. EMBO Rep. 2023, 24, e56033. [Google Scholar] [CrossRef]
- Keller, N.P. Fungal secondary metabolism: Regulation, function and drug discovery. Nat. Rev. Microbiol. 2019, 17, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Rendon, D.; Passari, A.K.; Ruiz-Villafán, B.; Rodríguez-Sanoja, R.; Sánchez, S.; Demain, A.L. Impact of novel microbial secondary metabolites on the pharma industry. Appl. Microbiol. Biotechnol. 2022, 106, 1855–1878. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Orhan, I.E.; Banach, M.; Rollinger, J.M.; Barreca, D.; Weckwerth, W.; Bauer, R.; Bayer, E.A.; et al. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Ossai, J.; Khatabi, B.; Nybo, S.E.; Kharel, M.K. Renewed interests in the discovery of bioactive actinomycete metabolites driven by emerging technologies. J. Appl. Microbiol. 2022, 132, 59–77. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Park, T.J.; Cao, H. Advances in mining and expressing microbial biosynthetic gene clusters. Crit. Rev. Microbiol. 2022, 49, 18–37. [Google Scholar] [CrossRef] [PubMed]
- Brakhage, A.A. Regulation of fungal secondary metabolism. Nat. Rev. Microbiol. 2013, 11, 21–32. [Google Scholar] [CrossRef]
- Selegato, D.M.; Castro-Gamboa, I. Enhancing chemical and biological diversity by co-cultivation. Front. Microbiol. 2023, 14, 67. [Google Scholar] [CrossRef]
- Knowles, S.L.; Raja, H.A.; Roberts, C.D.; Oberlies, N.H. Fungal–fungal co-culture: A primer for generating chemical diversity. Nat. Prod. Rep. 2022, 39, 1557–1573. [Google Scholar] [CrossRef]
- Bertrand, S.; Bohni, N.; Schnee, S.; Schumpp, O.; Gindro, K.; Wolfender, J.L. Metabolite induction via microorganism co-culture: A potential way to enhance chemical diversity for drug discovery. Biotechnol. Adv. 2014, 32, 1180–1204. [Google Scholar] [CrossRef] [PubMed]
- Arora, D.; Gupta, P.; Jaglan, S.; Roullier, C.; Grovel, O.; Bertrand, S. Expanding the chemical diversity through microorganisms co-culture: Current status and outlook. Biotechnol. Adv. 2020, 40, 107521. [Google Scholar] [CrossRef] [PubMed]
- Ravikrishnan, A.; Blank, L.M.; Srivastava, S.; Raman, K. Investigating metabolic interactions in a microbial co-culture through integrated modelling and experiments. Comput. Struct. Biotechnol. J. 2020, 18, 1249–1258. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, A.R.; Segrè, D. A multidimensional perspective on microbial interactions. FEMS Microbiol. Lett. 2019, 366, 125. [Google Scholar] [CrossRef]
- Goers, L.; Freemont, P.; Polizzi, K.M. Co-culture systems and technologies: Taking synthetic biology to the next level. J. R. Soc. Interface 2014, 11, 20140065. [Google Scholar] [CrossRef]
- Rosero-Chasoy, G.; Rodríguez-Jasso, R.M.; Aguilar, C.N.; Buitrón, G.; Chairez, I.; Ruiz, H.A. Microbial co-culturing strategies for the production high value compounds, a reliable framework towards sustainable biorefinery implementation—An overview. Bioresour. Technol. 2021, 321, 124458. [Google Scholar] [CrossRef]
- Kapoore, R.V.; Padmaperuma, G.; Maneein, S.; Vaidyanathan, S. Co-culturing microbial consortia: Approaches for applications in biomanufacturing and bioprocessing. Crit. Rev. Biotechnol. 2021, 42, 46–72. [Google Scholar] [CrossRef]
- Boruta, T.; Marczyk, A.; Rychta, K.; Przydacz, K.; Bizukojc, M. Confrontation between Penicillium rubens and Aspergillus terreus: Investigating the production of fungal secondary metabolites in submerged co-cultures. J. Biosci. Bioeng. 2020, 130, 503–513. [Google Scholar] [CrossRef]
- Luti, K.J.K.; Mavituna, F. Elicitation of Streptomyces coelicolor with E. coli in a bioreactor enhances undecylprodigiosin production. Biochem. Eng. J. 2011, 53, 281–285. [Google Scholar] [CrossRef]
- Boruta, T.; Ścigaczewska, A.; Bizukojć, M. “Microbial Wars” in a Stirred Tank Bioreactor: Investigating the Co-Cultures of Streptomyces rimosus and Aspergillus terreus, Filamentous Microorganisms Equipped with a Rich Arsenal of Secondary Metabolites. Front. Bioeng. Biotechnol. 2021, 9, 713639. [Google Scholar] [CrossRef]
- Boruta, T.; Ścigaczewska, A.; Bizukojć, M. Production of secondary metabolites in stirred tank bioreactor co-cultures of Streptomyces noursei and Aspergillus terreus. Front. Bioeng. Biotechnol. 2022, 10, 1011220. [Google Scholar] [CrossRef] [PubMed]
- Boruta, T.; Ścigaczewska, A.; Ruda, A.; Bizukojć, M. Effects of the Coculture Initiation Method on the Production of Secondary Metabolites in Bioreactor Cocultures of Penicillium rubens and Streptomyces rimosus. Molecules 2023, 28, 6044. [Google Scholar] [CrossRef]
- Houbraken, J.; Frisvad, J.C.; Samson, R.A. Fleming’s penicillin producing strain is not Penicillium chrysogenum but P. rubens. IMA Fungus 2011, 2, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Fjærvik, E.; Zotchev, S.B. Biosynthesis of the polyene macrolide antibiotic nystatin in Streptomyces noursei. Appl. Microbiol. Biotechnol. 2005, 67, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Vaněk, Z.; Půža, M.; Cudlín, J.; Vondráček, M.; Rickards, R.W. Metabolites of Streptomyces noursei. X. Biogenesis of cycloheximide. Folia Microbiol. 1969, 14, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Pohl, C.; Polli, F.; Schütze, T.; Viggiano, A.; Mózsik, L.; Jung, S.; de Vries, M.; Bovenberg, R.A.L.; Meyer, V.; Driessen, A.J.M. A Penicillium rubens platform strain for secondary metabolite production. Sci. Rep. 2020, 10, 7630. [Google Scholar] [CrossRef] [PubMed]
- Van Santen, J.A.; Poynton, E.F.; Iskakova, D.; Mcmann, E.; Alsup, T.A.; Clark, T.N.; Fergusson, C.H.; Fewer, D.P.; Hughes, A.H.; Mccadden, C.A.; et al. The Natural Products Atlas 2.0: A database of microbially-derived natural products. Nucleic Acids Res. 2022, 50, D1317–D1323. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Zhuang, Y.; Kong, F.; Zhang, C.; Zhu, W. Phenolic Polyketides from the Co-Cultivation of Marine-Derived Penicillium sp. WC-29-5 and Streptomyces fradiae 007. Mar. Drugs 2014, 12, 2079–2088. [Google Scholar] [CrossRef]
- Krespach, M.K.C.; Stroe, M.C.; Netzker, T.; Rosin, M.; Zehner, L.M.; Komor, A.J.; Beilmann, J.M.; Krüger, T.; Scherlach, K.; Kniemeyer, O.; et al. Streptomyces polyketides mediate bacteria–fungi interactions across soil environments. Nat. Microbiol. 2023, 8, 1348–1361. [Google Scholar] [CrossRef]
- Boruta, T.; Ścigaczewska, A. Enhanced oxytetracycline production by Streptomyces rimosus in submerged co-cultures with Streptomyces noursei. Molecules 2021, 26, 6036. [Google Scholar] [CrossRef]

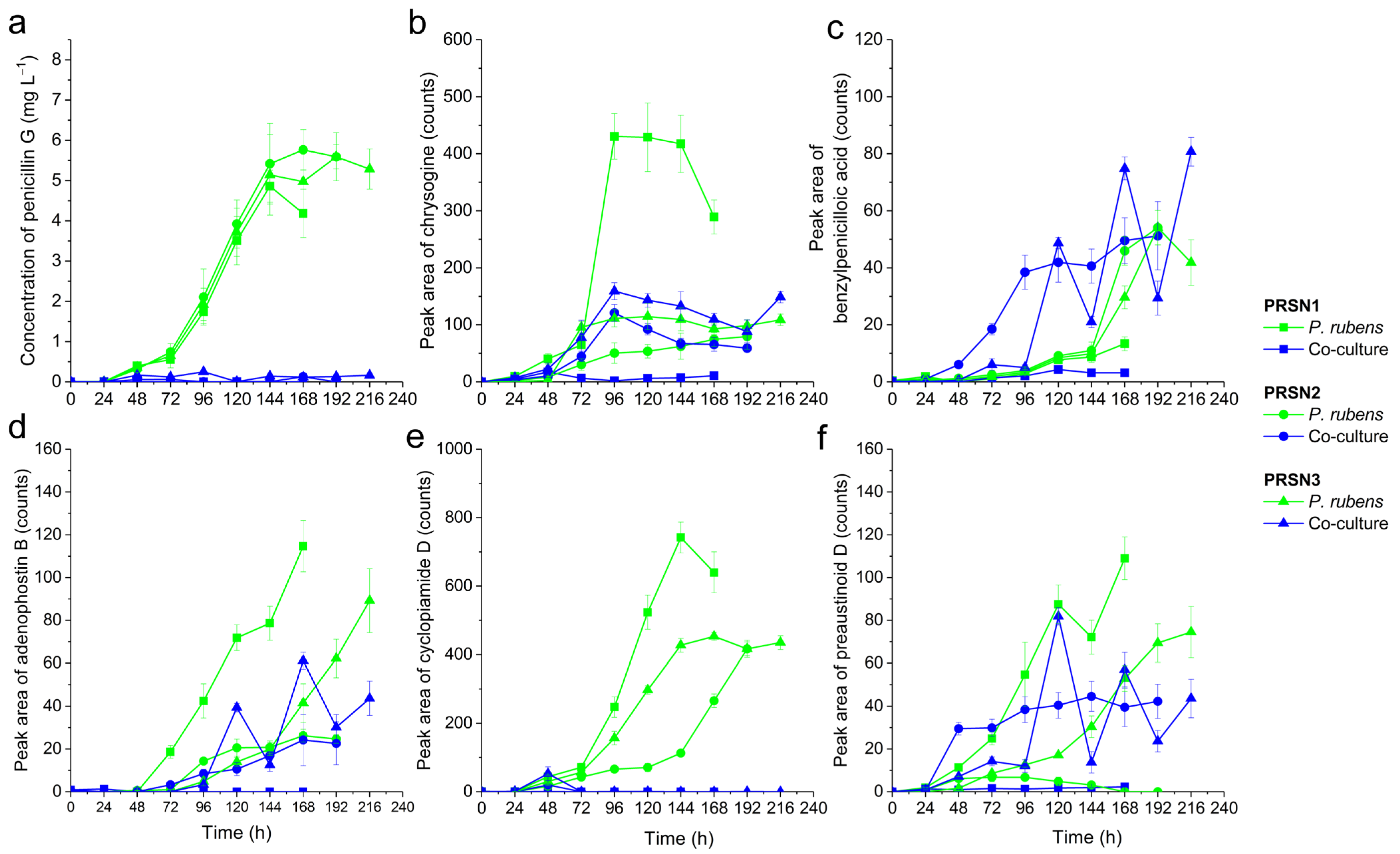

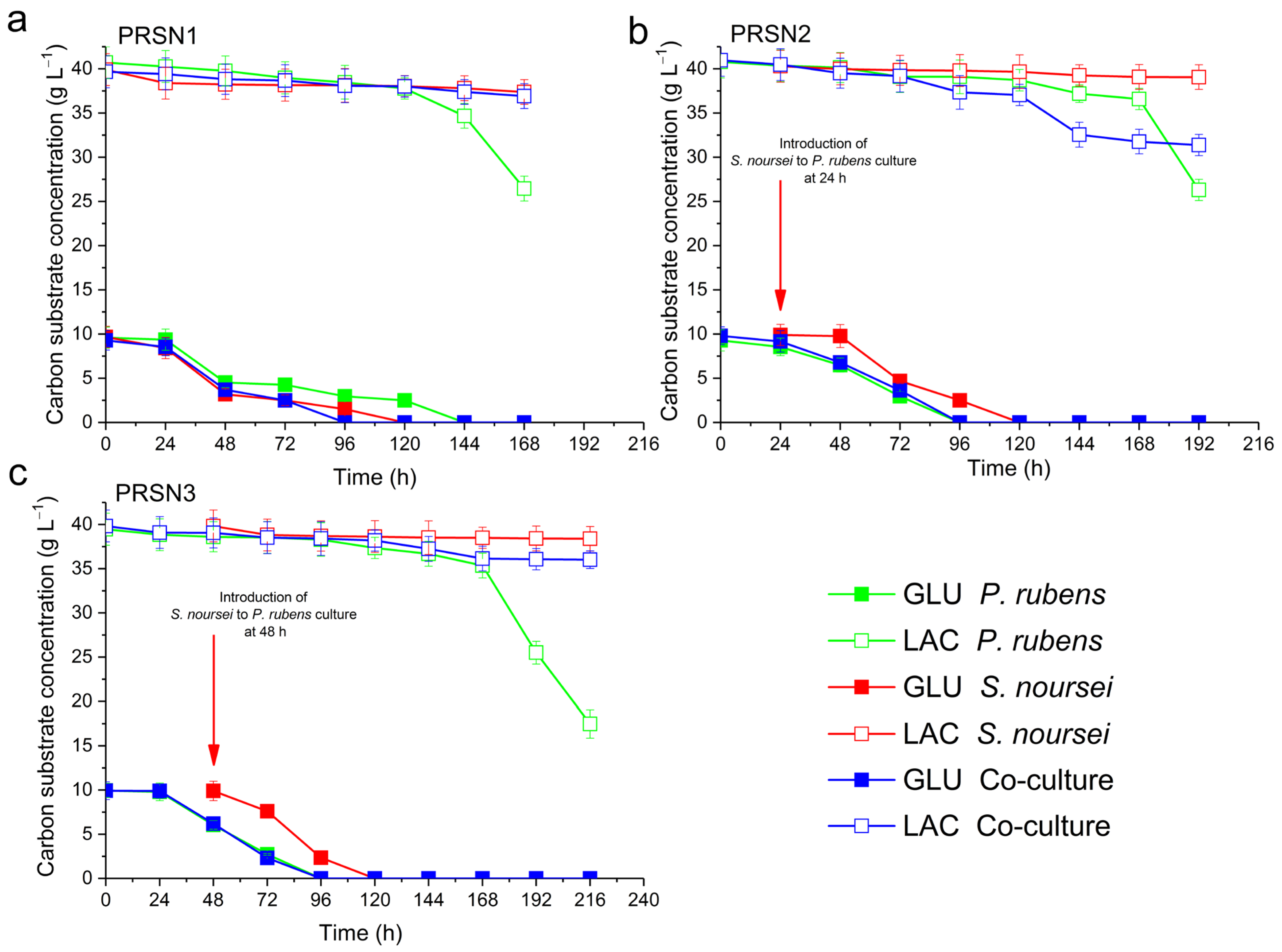
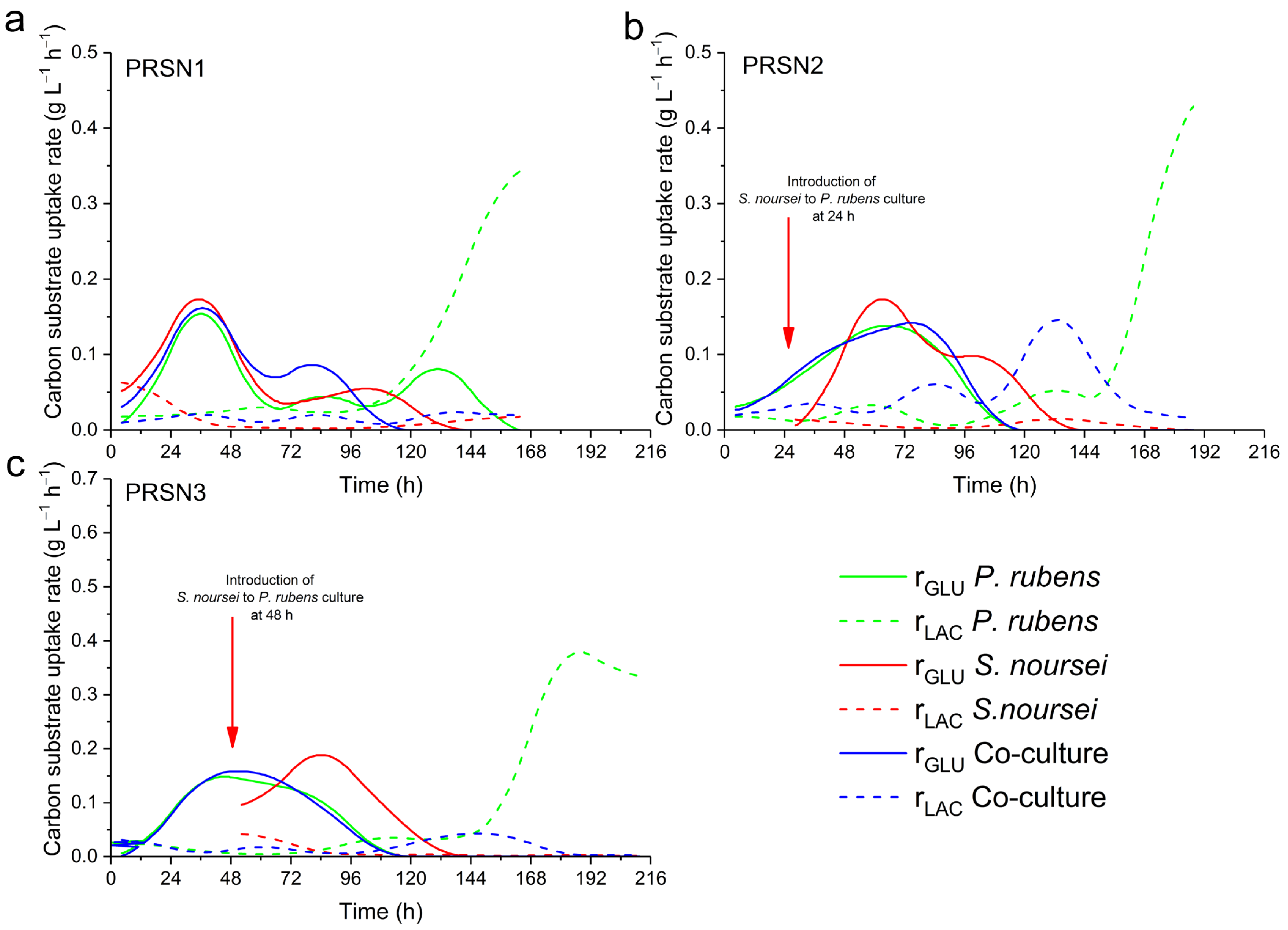
| Retention Time (min) | Experimental (m/z), ESI− | Suggested Formula, [M−H]− | Suggested Metabolite | Theoretical (m/z) of Suggested Metabolite, ESI− | Δ(m/z) = Experimental (m/z)—Theoretical (m/z) | Confidence Level of Metabolite Identification | Producer |
|---|---|---|---|---|---|---|---|
| 6.5 | 333.0928 | C16H17N2O4S | Penicillin G | 333.0909 | +0.0019 | Identified | P. rubens |
| 5.7 | 351.0989 | C16H19N2O5S | Benzylpenicilloic acid | 351.1015 | −0.0026 | Putatively annotated | P. rubens |
| 4.7 | 189.0756 | C10H9N2O2 | Chrysogine | 189.0665 | +0.0091 | Putatively annotated | P. rubens |
| 8.3 | 491.2644 | C27H39O8 | Preaustinoid D | 491.2645 | −0.0001 | Putatively annotated | P. rubens |
| 6.5 | 335.1042 | C19H15N2O4 | Cyclopiamide D | 335.1031 | +0.0011 | Putatively annotated | P. rubens |
| 4.9 | 710.0464 | C18H27N5O19P3 | Adenophostin B | 710.0513 | −0.0049 | Putatively annotated | P. rubens |
| 5.9 | 924.4999 | C47H74O17N | Nystatin A1 | 924.4957 | +0.0042 | Identified | S. noursei |
| 4.9 | 601.3576 | C27H49O9N6 | Desferrioxamine E | 601.3561 | +0.0015 | Putatively annotated | S. noursei |
| 4.8 | 585.3549 | C27H49O8N6 | Deshydroxynocardamine | 585.3612 | −0.0063 | Putatively annotated | S. noursei |
| 5.3 | 280.1520 | C15H22O4N | Cycloheximide | 280.1549 | −0.0029 | Putatively annotated | S. noursei |
| 5.9 | 280.1520 | C15H22O4N | Secocycloheximide | 280.1549 | −0.0029 | Putatively annotated | S. noursei |
| 5.8 | 280.1520 | C15H22O4N | A75943 | 280.1549 | −0.0029 | Putatively annotated | S. noursei |
| 5.3 | 292.1167 | C15H18ON | Phenatic acid A | 291.1185 | −0.0018 | Putatively annotated | S. noursei |
| 6.2 | 292.1167 | C15H18ON | Obscurolide C2 | 291.1185 | −0.0018 | Putatively annotated | S. noursei |
| 6.0 | 276.1201 | C15H18O4N1 | Obscurolide B3 | 276.1236 | −0.0053 | Putatively annotated | S. noursei |
| 6.3 | 274.1042 | C15H16O4N | 3-(2-Hydroxy-3,5-dimethyl-phenacyl)glutarimide (actiphenol) | 274.1079 | −0.0037 | Putatively annotated | S. noursei |
| 6.3 | 290.1028 | C15H16O5N | 3-[2-[2-Hydroxy- 3-methylphenyl-5-(hydroxymethyl)]- 2-oxoethyl]glutarimide | 290.1028 | 0.0000 | Putatively annotated | S. noursei |
| 4.8 | 310.1324 | C15H20O6N | Streptoglutarimide F | 310.1291 | +0.0033 | Putatively annotated | S. noursei |
| 7.2 | 257.1300 | C15H17O2N2 | Albonoursin | 257.1290 | +0.0010 | Putatively annotated | S. noursei |
| 4.3 | 238.1678 | C11H20O1N5 | Argvalin | 238.1668 | −0.0010 | Putatively annotated | S. noursei |
| 7.2 | 163.0753 | C10H11O2 | 2-Phenylethyl acetate | 163.0759 | −0.0006 | Putatively annotated | S. noursei |
| 6.5 | 293.1290 | C18H17O2N2 | Endophenazine A | 293.1290 | 0.0000 | Putatively annotated | S. noursei |
| 4.7 | 263.1064 | C13H15N2O4 | Spinoxazine A | 263.1032 | +0.0032 | Putatively annotated | S. noursei |
| 4.9 | 264.0859 | C11H14N5OS | 2-Methylthio-cis-zeatin | 264.0919 | −0.0060 | Putatively annotated | S. noursei |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boruta, T.; Ścigaczewska, A.; Bizukojć, M. Investigating the Stirred Tank Bioreactor Co-Cultures of the Secondary Metabolite Producers Streptomyces noursei and Penicillium rubens. Biomolecules 2023, 13, 1748. https://doi.org/10.3390/biom13121748
Boruta T, Ścigaczewska A, Bizukojć M. Investigating the Stirred Tank Bioreactor Co-Cultures of the Secondary Metabolite Producers Streptomyces noursei and Penicillium rubens. Biomolecules. 2023; 13(12):1748. https://doi.org/10.3390/biom13121748
Chicago/Turabian StyleBoruta, Tomasz, Anna Ścigaczewska, and Marcin Bizukojć. 2023. "Investigating the Stirred Tank Bioreactor Co-Cultures of the Secondary Metabolite Producers Streptomyces noursei and Penicillium rubens" Biomolecules 13, no. 12: 1748. https://doi.org/10.3390/biom13121748
APA StyleBoruta, T., Ścigaczewska, A., & Bizukojć, M. (2023). Investigating the Stirred Tank Bioreactor Co-Cultures of the Secondary Metabolite Producers Streptomyces noursei and Penicillium rubens. Biomolecules, 13(12), 1748. https://doi.org/10.3390/biom13121748





