Beyond the Cardiovascular Effects of Glucagon-like Peptide-1 Receptor Agonists: Body Slimming and Plaque Stabilization. Are New Statins Born?
Abstract
:1. Introduction
2. GLP-1 Receptors and Their Agonists
3. GLP-1RAs and Reduction in Cardiovascular Risk
4. Metabolic Effects of GLP-1RA Administration
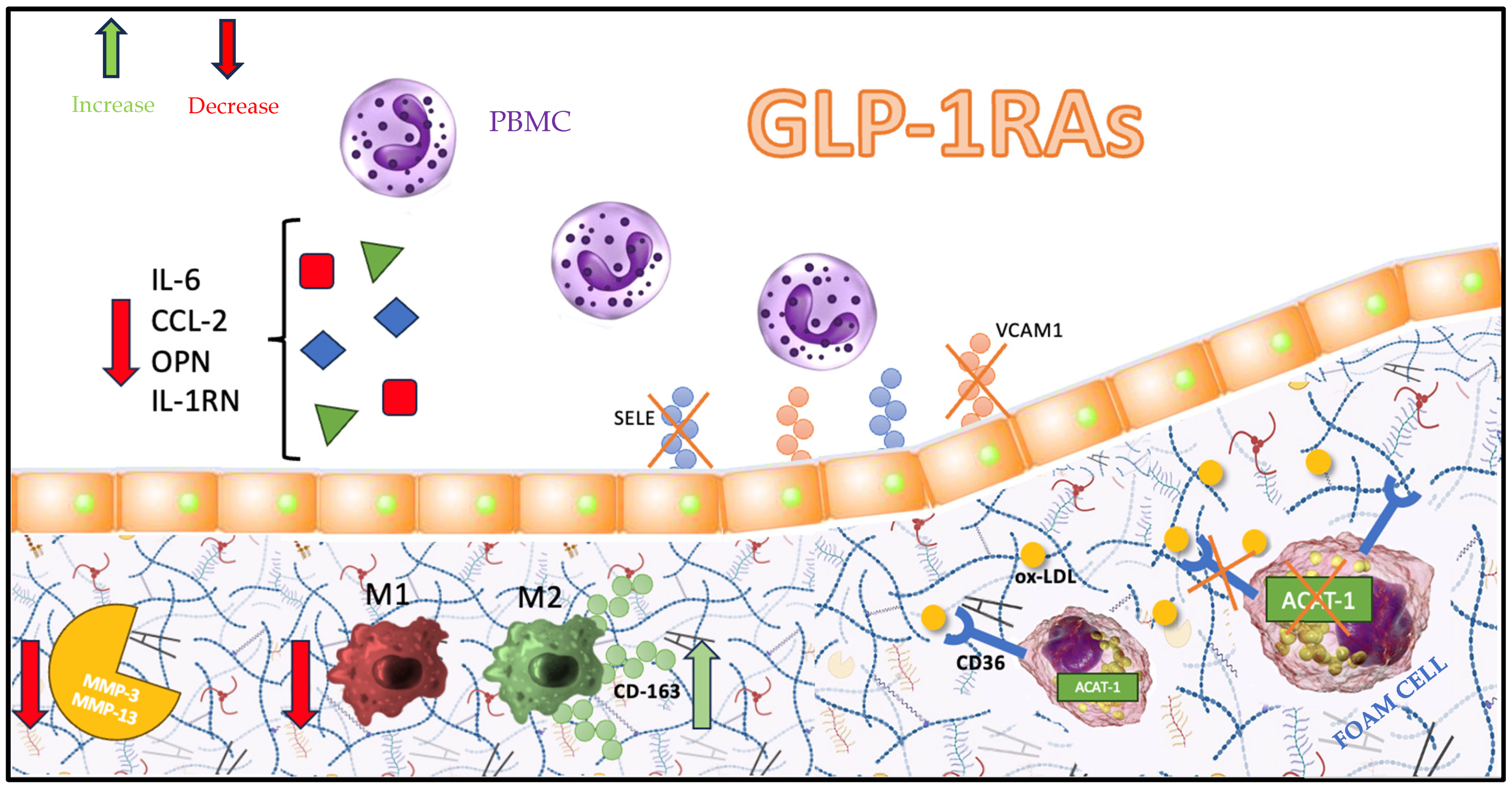
5. Atherosclerotic Plaque Pathways Targeted by GLP-1RAs
6. Clinical Implications
7. Conclusions and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Classics in arteriosclerosis research: On experimental cholesterin steatosis and its significance in the origin of some pathological processes by N. Anitschkow and S. Chalatow, translated by Mary Z. Pelias, 1913. Arterioscler. Dallas Tex 1983, 3, 178–182.
- Gofman, J.W.; Lindgren, F.T.; Elliott, H. Ultracentrifugal studies of lipoproteins of human serum. J. Biol. Chem. 1949, 179, 973–979. [Google Scholar] [CrossRef]
- Gofman, J.W.; Lindgren, F.; Elliott, H.; Mantz, W.; Hewitt, J.; Strisower, B.; Herring, V.; Lyon, T.P. The role of lipids and lipoproteins in atherosclerosis. Science 1950, 111, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Gofman, J.W.; Glazier, F.; Tamplin, A.; Strisower, B.; De Lalla, O. Lipoproteins, coronary heart disease, and atherosclerosis. Physiol. Rev. 1954, 34, 589–607. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, J.L.; Brown, M.S. Familial hypercholesterolemia: Identification of a defect in the regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity associated with overproduction of cholesterol. Proc. Natl. Acad. Sci. USA 1973, 70, 2804–2808. [Google Scholar] [CrossRef]
- Shah, P.K.; Lecis, D. Inflammation in atherosclerotic cardiovascular disease. F1000Research 2019, 8, F1000. [Google Scholar] [CrossRef]
- Ross, R. Atherosclerosis—An Inflammatory Disease. N. Engl. J. Med. 1999, 340, 115–126. [Google Scholar] [CrossRef]
- Libby, P. Inflammation during the life cycle of the atherosclerotic plaque. Cardiovasc. Res. 2021, 117, 2525–2536. [Google Scholar] [CrossRef] [PubMed]
- Boren, J.; Chapman, M.J.; Krauss, R.M.; Packard, C.J.; Bentzon, J.F.; Binder, C.J.; Daemen, M.J.; Demer, L.L.; Hegele, R.A.; Nicholls, S.J.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: Pathophysiological, genetic, and therapeutic insights: A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2020, 41, 2313–2330. [Google Scholar] [CrossRef] [PubMed]
- Hurt-Camejo, E.; Camejo, G.; Rosengren, B.; Lopez, F.; Ahlström, C.; Fager, G.; Bondjers, G. Effect of arterial proteoglycans and glycosaminoglycans on low density lipoprotein oxidation and its uptake by human macrophages and arterial smooth muscle cells. Arterioscler. Thromb. J. Vasc. Biol. 1992, 12, 569–583. [Google Scholar] [CrossRef] [PubMed]
- Kattoor, A.J.; Goel, A.; Mehta, J.L. LOX-1: Regulation, Signaling and Its Role in Atherosclerosis. Antioxidants 2019, 8, 218. [Google Scholar] [CrossRef]
- Hofmann, A.; Brunssen, C.; Morawietz, H. Contribution of lectin-like oxidized low-density lipoprotein receptor-1 and LOX-1 modulating compounds to vascular diseases. Vasc. Pharmacol. 2017, 107, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Aikawa, M. Mechanisms of plaque stabilization with statins. Am. J. Cardiol. 2003, 91, 4B–8B. [Google Scholar] [CrossRef] [PubMed]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef] [PubMed]
- Silverman, M.G.; Ference, B.A.; Im, K.; Wiviott, S.D.; Giugliano, R.P.; Grundy, S.M.; Braunwald, E.; Sabatine, M.S. Association Between Lowering LDL-C and Cardiovascular Risk Reduction Among Different Therapeutic Interventions: A Systematic Review and Meta-analysis. JAMA 2016, 316, 1289–1297. [Google Scholar] [CrossRef] [PubMed]
- Ference, B.A.; Yoo, W.; Alesh, I.; Mahajan, N.; Mirowska, K.K.; Mewada, A.; Kahn, J.; Afonso, L.; Williams, K.A.; Flack, J.M.; et al. Effect of long-term exposure to lower low-density lipoprotein cholesterol beginning early in life on the risk of coronary heart disease: A Mendelian randomization analysis. J. Am. Coll. Cardiol. 2012, 60, 2631–2639. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, S.; Bosch, J.; Dagenais, G.; Zhu, J.; Xavier, D.; Liu, L.; Pais, P.; López-Jaramillo, P.; Leiter, L.A.; Dans, A.; et al. Cholesterol Lowering in Intermediate-Risk Persons without Cardiovascular Disease. N. Engl. J. Med. 2016, 374, 2021–2031. [Google Scholar] [CrossRef]
- Ridker, P.M.; Danielson, E.; Fonseca, F.A.; Genest, J.; Gotto, A.M., Jr.; Kastelein, J.J.; Koenig, W.; Libby, P.; Lorenzatti, A.J.; MacFadyen, J.G.; et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N. Engl. J. Med. 2008, 359, 2195–2207. [Google Scholar] [CrossRef]
- Bierman, E.L. George Lyman Duff Memorial Lecture. Atherogenesis in diabetes. Arterioscler. Thromb. J. Vasc. Biol. 1992, 12, 647–656. [Google Scholar] [CrossRef]
- Pyörälä, K.; Laakso, M.; Uusitupa, M. Diabetes and atherosclerosis: An epidemiologic view. Diabetes Metab. Rev. 1987, 3, 463–524. [Google Scholar] [CrossRef]
- Prandi, F.R.; Evangelista, I.; Sergi, D.; Palazzuoli, A.; Romeo, F. Mechanisms of cardiac dysfunction in diabetic cardiomyopathy: Molecular abnormalities and phenotypical variants. Heart Fail. Rev. 2023, 28, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Prandi, F.R.; Lecis, D.; Illuminato, F.; Milite, M.; Celotto, R.; Lerakis, S.; Romeo, F.; Barillà, F. Epigenetic Modifications and Non-Coding RNA in Diabetes-Mellitus-Induced Coronary Artery Disease: Pathophysiological Link and New Therapeutic Frontiers. Int. J. Mol. Sci. 2022, 23, 4589. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.; Akindehin, S.E.; Orsso, C.E.; Waldner, R.C.; DiMarchi, R.D.; Müller, T.D.; Haqq, A.M. Recent Advances in Incretin-Based Pharmacotherapies for the Treatment of Obesity and Diabetes. Front. Endocrinol. 2022, 13, 838410. [Google Scholar] [CrossRef]
- Longo, S.; Rizza, S.; Federici, M. Microbiota-gut-brain axis: Relationships among the vagus nerve, gut microbiota, obesity, and diabetes. Acta Diabetol. 2023, 60, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Mangmool, S.; Parichatikanond, W. Multifaceted Roles of GLP-1 and Its Analogs: A Review on Molecular Mechanisms with a Cardiotherapeutic Perspective. Pharmaceuticals 2023, 16, 836. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M. Incretin therapies: Highlighting common features and differences in the modes of action of glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Diabetes Obes. Metab. 2016, 18, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Lutz, T.A.; Osto, E. Glucagon-like peptide-1, glucagon-like peptide-2, and lipid metabolism. Curr. Opin. Lipidol. 2016, 27, 257–263. [Google Scholar] [CrossRef]
- Dandona, P.; Ghanim, H.; Chaudhuri, A. Incretins: B eyond type 2 diabetes. Diabetes Obes. Metab. 2018, 20, 59–67. [Google Scholar] [CrossRef]
- Owens, D.R.; Monnier, L.; Hanefeld, M. A review of glucagon-like peptide-1 receptor agonists and their effects on lowering postprandial plasma glucose and cardiovascular outcomes in the treatment of type 2 diabetes mellitus. Diabetes Obes. Metab. 2017, 19, 1645–1654. [Google Scholar] [CrossRef]
- Gumieniczek, A.; Berecka-Rycerz, A. Metabolism and Chemical Degradation of New Antidiabetic Drugs: A Review of Analytical Approaches for Analysis of Glutides and Gliflozins. Biomedicines 2023, 11, 2127. [Google Scholar] [CrossRef]
- Bailey, C.J.; Flatt, P.R.; Conlon, J.M. An update on peptide-based therapies for type 2 diabetes and obesity. Peptides 2023, 161, 170939. [Google Scholar] [CrossRef]
- Belli, M.; Bellia, A.; Sergi, D.; Barone, L.; Lauro, D.; Barillà, F. Glucose variability: A new risk factor for cardiovascular disease. Acta Diabetol. 2023, 60, 1291–1299. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, M.A.; Claggett, B.; Diaz, R.; Dickstein, K.; Gerstein, H.C.; Kober, L.V.; Lawson, F.C.; Ping, L.; Wei, X.; Lewis, E.F.; et al. Lixisenatide in Patients with Type 2 Diabetes and Acute Coronary Syndrome. N. Engl. J. Med. 2015, 373, 2247–2257. [Google Scholar] [CrossRef] [PubMed]
- Marso, S.P.; Daniels, G.H.; Brown-Frandsen, K.; Kristensen, P.; Mann, J.F.; Nauck, M.A.; Nissen, S.E.; Pocock, S.; Poulter, N.R.; Ravn, L.S.; et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Husain, M.; Bain, S.C.; Jeppesen, O.K.; Lingvay, I.; Sørrig, R.; Treppendahl, M.B.; Vilsbøll, T. Semaglutide (SUSTAIN and PIONEER) reduces cardiovascular events in type 2 diabetes across varying cardiovascular risk. Diabetes Obes. Metab. 2020, 22, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Marso, S.P.; Holst, A.G.; Vilsbøll, T. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2017, 376, 891–892. [Google Scholar] [CrossRef]
- Nauck, M.A.; Quast, D.R.; Wefers, J.; Meier, J.J. GLP-1 receptor agonists in the treatment of type 2 diabetes-state-of-the-art. Mol. Metab. 2021, 46, 101102. [Google Scholar] [CrossRef]
- Holman, R.R.; Bethel, M.A.; Mentz, R.J.; Thompson, V.P.; Lokhnygina, Y.; Buse, J.B.; Chan, J.C.; Choi, J.; Gustavson, S.M.; Iqbal, N.; et al. Effects of Once-Weekly Exenatide on Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 1228–1239. [Google Scholar] [CrossRef]
- Gerstein, H.C.; Sattar, N.; Rosenstock, J.; Ramasundarahettige, C.; Pratley, R.; Lopes, R.D.; Lam, C.S.P.; Khurmi, N.S.; Heenan, L.; Del Prato, S.; et al. Cardiovascular and Renal Outcomes with Efpeglenatide in Type 2 Diabetes. N. Engl. J. Med. 2021, 385, 896–907. [Google Scholar] [CrossRef]
- Kristensen, S.L.; Rørth, R.; Jhund, P.S.; Docherty, K.F.; Sattar, N.; Preiss, D.; Køber, L.; Petrie, M.C.; McMurray, J.J.V. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: A systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019, 7, 776–785. [Google Scholar] [CrossRef]
- Giugliano, D.; Scappaticcio, L.; Longo, M.; Caruso, P.; Maiorino, M.I.; Bellastella, G.; Ceriello, A.; Chiodini, P.; Esposito, K. GLP-1 receptor agonists and cardiorenal outcomes in type 2 diabetes: An updated meta-analysis of eight CVOTs. Cardiovasc. Diabetol. 2021, 20, 189. [Google Scholar] [CrossRef] [PubMed]
- Ferdinand, K.C.; White, W.B.; Calhoun, D.A.; Lonn, E.M.; Sager, P.T.; Brunelle, R.; Jiang, H.H.; Threlkeld, R.J.; Robertson, K.E.; Geiger, M.J. Effects of the once-weekly glucagon-like peptide-1 receptor agonist dulaglutide on ambulatory blood pressure and heart rate in patients with type 2 diabetes mellitus. Hypertension 2014, 64, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Lauder, L.; Mahfoud, F.; Azizi, M.; Bhatt, D.L.; Ewen, S.; Kario, K.; Parati, G.; Rossignol, P.; Schlaich, M.P.; Teo, K.K.; et al. Hypertension management in patients with cardiovascular comorbidities. Eur. Heart J. 2023, 44, 2066–2077. [Google Scholar] [CrossRef] [PubMed]
- Yaribeygi, H.; Farrokhi, F.R.; Abdalla, M.A.; Sathyapalan, T.; Banach, M.; Jamialahmadi, T.; Sehebkar, A. The Effects of Glucagon-Like Peptide-1 Receptor Agonists and Dipeptydilpeptidase-4 Inhibitors on Blood Pressure and Cardiovascular Complications in Diabetes. J. Diabetes Res. 2021, 2021, 6518221. [Google Scholar] [CrossRef] [PubMed]
- Skov, J.; Pedersen, M.; Holst, J.J.; Madsen, B.; Goetze, J.P.; Rittig, S.; Jonassen, T.; Frøkiaer, J.; Dejgaard, A.; Christiansen, J.S. Glucagon-like peptide-1 (GLP-1): Effect on kidney hemodynamics and renin-angiotensin-aldosterone system in healthy men. J. Clin. Endocrinol. Metab. 2013, 98, E664–E671. [Google Scholar] [CrossRef]
- Le, Y.; Zheng, Z.; Xue, J.; Cheng, M.; Guan, M.; Xue, Y. Effects of exendin-4 on the intrarenal renin-angiotensin system and interstitial fibrosis in unilateral ureteral obstruction mice: Exendin-4 and unilateral ureteral obstruction. J. Renin-Angiotensin-Aldosterone Syst. JRAAS 2016, 17, 1470320316677918. [Google Scholar] [CrossRef]
- Martins, F.L.; Bailey, M.A.; Girardi, A.C.C. Endogenous Activation of Glucagon-Like Peptide-1 Receptor Contributes to Blood Pressure Control: Role of Proximal Tubule Na+/H+ Exchanger Isoform 3, Renal Angiotensin II, and Insulin Sensitivity. Hypertension 2020, 76, 839–848. [Google Scholar] [CrossRef]
- Waldrop, G.; Zhong, J.; Peters, M.; Goud, A.; Chen, Y.H.; Davis, S.N.; Mukherjee, B.; Rajagopalan, S. Incretin-based therapy in type 2 diabetes: An evidence based systematic review and meta-analysis. J. Diabetes Complications 2018, 32, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Petit, J.-M.; Cercueil, J.P.; Loffroy, R.; Denimal, D.; Bouillet, B.; Fourmont, C.; Chevallier, O.; Duvillard, L.; Vergès, B. Effect of liraglutide therapy on liver fat content in patients with inadequately controlled type 2 diabetes. The Lira-NAFLD study. J. Clin. Endocrinol. Metab. 2016, 102, 407–415. [Google Scholar] [CrossRef]
- Sun, F.; Wu, S.; Wang, J.; Guo, S.; Chai, S.; Yang, Z.; Li, L.; Zhang, Y.; Ji, L.; Zhan, S. Effect of glucagon-like peptide-1 receptor agonists on lipid profiles among type 2 diabetes: A systematic review and network meta-analysis. Clin. Ther. 2015, 37, 225–241.e8. [Google Scholar] [CrossRef]
- Peradze, N.; Farr, O.M.; Perakakis, N.; Lázaro, I.; Sala-Vila, A.; Mantzoros, C.S. Short-term treatment with high dose liraglutide improves lipid and lipoprotein profile and changes hormonal mediators of lipid metabolism in obese patients with no overt type 2 diabetes mellitus: A randomized, placebo-controlled, cross-over, double-blind clinical trial. Cardiovasc. Diabetol. 2019, 18, 141. [Google Scholar] [CrossRef] [PubMed]
- Sposito, A.C.; Berwanger, O.; De Carvalho, L.S.F.; Saraiva, J.F.K. GLP-1RAs in type 2 diabetes: Mechanisms that underlie cardiovascular effects and overview of cardiovascular outcome data. Cardiovasc. Diabetol. 2018, 17, 157. [Google Scholar] [CrossRef] [PubMed]
- Akopian, D.; Medh, J.D. Genetics and molecular biology: Macrophage ACAT depletion-mechanisms of atherogenesis. Curr. Opin. Lipidol. 2006, 17, 85–88. [Google Scholar] [CrossRef]
- Zaidi, S.A.H.; Lemtalsi, T.; Xu, Z.; Santana, I.; Sandow, P.; Labazi, L.; Caldwell, R.W.; Caldwell, R.B.; Rojas, M.A. Role of acyl-coenzyme A: Cholesterol transferase 1 (ACAT1) in retinal neovascularization. J. Neuroinflammation 2023, 20, 14. [Google Scholar] [CrossRef]
- Freeman, N.E.; Rusinol, A.E.; Linton, M.; Hachey, D.L.; Fazio, S.; Sinensky, M.S.; Thewke, D. Acyl-coenzyme A: Cholesterol acyltransferase promotes oxidized LDL/oxysterol-induced apoptosis in macrophages. J. Lipid Res. 2005, 46, 1933–1943. [Google Scholar] [CrossRef] [PubMed]
- Kharbanda, R.K.; Wallace, S.; Walton, B.; Donald, A.; Cross, J.M.; Deanfield, J. Systemic Acyl-CoA: Cholesterol acyltransferase inhibition reduces inflammation and improves vascular function in hypercholesterolemia. Circulation 2005, 111, 804–807. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, Y.; Sato, K.; Watanabe, T.; Nohtomi, K.; Terasaki, M.; Nagashima, M.; Hirano, T. A glucagon-like peptide-1 analog liraglutide suppresses macrophage foam cell formation and atherosclerosis. Peptides 2014, 54, 19–26. [Google Scholar] [CrossRef]
- Nagashima, M.; Watanabe, T.; Terasaki, M.; Tomoyasu, M.; Nohtomi, K.; Kim-Kaneyama, J.; Miyazaki, A.; Hirano, T. Native incretins prevent the development of atherosclerotic lesions in apolipoprotein E knockout mice. Diabetologia 2011, 54, 2649–2659. [Google Scholar] [CrossRef]
- Silverstein, R.L.; Li, W.; Park, Y.M.; Rahaman, S.O. Mechanisms of cell signaling by the scavenger receptor CD36: Implications in atherosclerosis and thrombosis. Trans. Am. Clin. Climatol. Assoc. 2010, 121, 206–220. [Google Scholar] [PubMed]
- Rakipovski, G.; Rolin, B.; Nøhr, J.; Klewe, I.; Frederiksen, K.S.; Augustin, R.; Hecksher-Sørensen, J.; Ingvorsen, C.; Polex-Wolf, J.; Knudsen, L.B. The GLP-1 Analogs Liraglutide and Semaglutide Reduce Atherosclerosis in ApoE−/− and LDLr−/− Mice by a Mechanism That Includes Inflammatory Pathways. JACC Basic Transl. Sci. 2018, 3, 844–857. [Google Scholar] [CrossRef]
- Zwakenberg, S.R.; van der Schouw, Y.T.; Schalkwijk, C.G.; Spijkerman, A.M.W.; Beulens, J.W.J. Bone markers and cardiovascular risk in type 2 diabetes patients. Cardiovasc. Diabetol. 2018, 17, 45. [Google Scholar] [CrossRef] [PubMed]
- Giachelli, C.M.; Lombardi, D.; Johnson, R.J.; Murry, C.E.; Almeida, M. Evidence for a role of osteopontin in macrophage infiltration in response to pathological stimuli in vivo. Am. J. Pathol. 1998, 152, 353–358. [Google Scholar]
- Bruen, R.; Curley, S.; Kajani, S.; Crean, D.; O’Reilly, M.E.; Lucitt, M.B.; Godson, C.G.; McGillicuddy, F.C.; Belton, O. Liraglutide dictates macrophage phenotype in apolipoprotein E null mice during early atherosclerosis. Cardiovasc. Diabetol. 2017, 16, 143. [Google Scholar] [CrossRef] [PubMed]
- Bruen, R.; Curley, S.; Kajani, S.; Lynch, G.; O’Reilly, M.E.; Dillon, E.T.; Brennan, E.P.; Barry, M.; Sheehan, S.; McGillicuddy, F.C.; et al. Liraglutide Attenuates Preestablished Atherosclerosis in Apolipoprotein E-Deficient Mice via Regulation of Immune Cell Phenotypes and Proinflammatory Mediators. J. Pharmacol. Exp. Ther. 2019, 370, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Chen, L.; Li, D.; Xu, H.; Chen, J.; Min, X.; He, M.; Wu, T.; Zhong, J.; Yang, H.; et al. Effect of GLP-1/GLP-1R on the Polarization of Macrophages in the Occurrence and Development of Atherosclerosis. Med. Inflamm. 2021, 2021, 5568159. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Hu, Y.; He, S.; Ye, Q.; Lv, Z.; Liu, J.; Chen, X. Dulaglutide inhibits high glucose- induced endothelial dysfunction and NLRP3 inflammasome activation. Arch. Biochem. Biophys. 2019, 671, 203–209. [Google Scholar] [CrossRef]
- Andreadi, A.; Muscoli, S.; Tajmir, R.; Meloni, M.; Muscoli, C.; Ilari, S.; Mollace, V.; Della Morte, D.; Bellia, A.; Di Daniele, N.; et al. Recent Pharmacological Options in Type 2 Diabetes and Synergic Mechanism in Cardiovascular Disease. Int. J. Mol. Sci. 2023, 24, 1646. [Google Scholar] [CrossRef]
- Amaro, A.; Sugimoto, D.; Wharton, S. Efficacy and safety of semaglutide for weight management: Evidence from the STEP program. Postgrad. Med. 2022, 134, 5–17. [Google Scholar] [CrossRef]
- Wilding, J.P.H.; Batterham, R.L.; Calanna, S.; Davies, M.; Van Gaal, L.F.; Lingvay, I.; McGowan, B.M.; Rosenstock, J.; Tran, M.T.D.; Wadden, T.A.; et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N. Engl. J. Med. 2021, 384, 989–1002. [Google Scholar] [CrossRef]
- Liao, C.; Liang, X.; Zhang, X.; Li, Y. The effects of GLP-1 receptor agonists on visceral fat and liver ectopic fat in an adult population with or without diabetes and nonalcoholic fatty liver disease: A systematic review and meta-analysis. PLoS ONE 2023, 18, e0289616. [Google Scholar] [CrossRef]
- Lingvay, I.; Brown-Frandsen, K.; Colhoun, H.M.; Deanfield, J.; Emerson, S.S.; Esbjerg, S.; Hardt-Lindberg, S.; Hovingh, G.K.; Kahn, S.E.; Kushner, R.F.; et al. Semaglutide for cardiovascular event reduction in people with overweight or obesity: SELECT study baseline characteristics. Obesity 2023, 31, 111–122. [Google Scholar] [CrossRef]
- Perry, T.A.; Greig, N.H. A new Alzheimer’s disease interventive strategy: GLP-1. Curr. Drug Targets 2004, 5, 565–571. [Google Scholar] [CrossRef]
- During, M.J.; Cao, L.; Zuzga, D.S.; Francis, J.S.; Fitzsimons, H.L.; Jiao, X.; Bland, R.J.; Klugmann, M.; Banks, W.A.; Drucker, D.J.; et al. Glucagon-like peptide-1 receptor is involved in learning and neuroprotection. Nat. Med. 2003, 9, 1173–1179. [Google Scholar] [CrossRef] [PubMed]
- Bassil, F.; Fernagut, P.-O.; Bezard, E.; Meissner, W.G. Insulin, IGF-1 and GLP-1 signaling in neurodegenerative disorders: Targets for disease modification? Prog. Neurobiol. 2014, 118, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Monney, M.; Jornayvaz, F.R.; Gariani, K. GLP-1 receptor agonists effect on cognitive function in patients with and without type 2 diabetes. Diabetes Metab. 2023, 49, 101470. [Google Scholar] [CrossRef] [PubMed]
- Jantrapirom, S.; Nimlamool, W.; Chattipakorn, N.; Chattipakorn, S.; Temviriyanukul, P.; Inthachat, W.; Govitrapong, P.; Potikanond, S. Liraglutide Suppresses Tau Hyperphosphorylation, Amyloid Beta Accumulation through Regulating Neuronal Insulin Signaling and BACE-1 Activity. Int. J. Mol. Sci. 2020, 21, 1725. [Google Scholar] [CrossRef]
- Arnold, S.E.; Arvanitakis, Z.; Macauley-Rambach, S.L.; Koenig, A.M.; Wang, H.Y.; Ahima, R.S.; Craft, S.; Gandy, S.; Buettner, C.; Stoeckel, L.E.; et al. Brain insulin resistance in type 2 diabetes and Alzheimer disease: Concepts and conundrums. Nat. Rev. Neurol. 2018, 14, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Laurindo, L.F.; Barbalho, S.M.; Guiguer, E.L.; da Silva Soares de Souza, M.; de Souza, G.A.; Fidalgo, T.M.; Araújo, A.C.; de Souza Gonzaga, H.F.; de Bortoli Teixeira, D.; de Oliveira Silva Ullmann, T.; et al. GLP-1a: Going beyond Traditional Use. Int. J. Mol. Sci. 2022, 23, 739. [Google Scholar] [CrossRef]
- Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group, Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N. Engl. J. Med. 1998, 339, 1349–1357. [CrossRef]
| Trial (Duration) | GLP-1RA | Primary Endpoint | Results |
|---|---|---|---|
| ELIXA (5 years) | Lixisenatide | CV death, MI, stroke, or hospitalization for UA | No benefit |
| LEADER (1.5 years) | Liraglutide | First occurrence of death from CV causes, nonfatal MI, or nonfatal stroke | Significant decrease |
| SUSTAIN-6 (2.1 years) | Injectable semaglutide | First occurrence of CV death, nonfatal MI, or nonfatal stroke | Significant decrease |
| PIONEER 6 (15.9 months) | Oral semaglutide | First occurrence of death from CV causes, nonfatal MI, or nonfatal stroke | No benefit |
| EXSCEL (7 years) | Exenatide | First occurrence of death from CV causes, nonfatal MI, or nonfatal stroke | No benefit |
| HARMONY (2.4 years) | Albiglutide | CV death, nonfatal MI, or stroke | Significant decrease |
| REWIND (8.4 years) | Dulaglutide | First occurrence nonfatal MI, nonfatal stroke, or death from CV causes | No benefit |
| AMPLITUDE-O (3 years) | Efpeglenatide | First MACE: composite of nonfatal MI, nonfatal stroke, or death from CV or undetermined causes | Significant decrease |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lecis, D.; Prandi, F.R.; Barone, L.; Belli, M.; Sergi, D.; Longo, S.; Muscoli, S.; Romeo, F.; Federici, M.; Lerakis, S.; et al. Beyond the Cardiovascular Effects of Glucagon-like Peptide-1 Receptor Agonists: Body Slimming and Plaque Stabilization. Are New Statins Born? Biomolecules 2023, 13, 1695. https://doi.org/10.3390/biom13121695
Lecis D, Prandi FR, Barone L, Belli M, Sergi D, Longo S, Muscoli S, Romeo F, Federici M, Lerakis S, et al. Beyond the Cardiovascular Effects of Glucagon-like Peptide-1 Receptor Agonists: Body Slimming and Plaque Stabilization. Are New Statins Born? Biomolecules. 2023; 13(12):1695. https://doi.org/10.3390/biom13121695
Chicago/Turabian StyleLecis, Dalgisio, Francesca Romana Prandi, Lucy Barone, Martina Belli, Domenico Sergi, Susanna Longo, Saverio Muscoli, Francesco Romeo, Massimo Federici, Stamatios Lerakis, and et al. 2023. "Beyond the Cardiovascular Effects of Glucagon-like Peptide-1 Receptor Agonists: Body Slimming and Plaque Stabilization. Are New Statins Born?" Biomolecules 13, no. 12: 1695. https://doi.org/10.3390/biom13121695
APA StyleLecis, D., Prandi, F. R., Barone, L., Belli, M., Sergi, D., Longo, S., Muscoli, S., Romeo, F., Federici, M., Lerakis, S., & Barillà, F. (2023). Beyond the Cardiovascular Effects of Glucagon-like Peptide-1 Receptor Agonists: Body Slimming and Plaque Stabilization. Are New Statins Born? Biomolecules, 13(12), 1695. https://doi.org/10.3390/biom13121695






