Abstract
Thiazole carboxamide derivatives were synthesized in this investigation, with a subsequent examination of their impact on GluA2 AMPA receptors. The synthesized compounds, namely MMH-1-5, were subjected to characterization using high-resolution mass spectrometry (HRMS), proton nuclear magnetic resonance (1H-NMR), and carbon-13 nuclear magnetic resonance (13C-NMR). The present work thoroughly investigates the impact of five thiazole derivatives on GluA2 AMPA receptors. This investigation examined their effects on both whole-cell currents and receptor kinetics. In addition, the cytotoxicity of the samples was assessed using the MTS test. The compound MMH-5 had the highest effect level, resulting in a notable drop in current amplitude by a factor of six. Similarly, MMH-4 and MMH-3 also caused major reductions in the current amplitude. The compounds mentioned above also influenced the rates of deactivation and desensitization. MMH-5 and MMH-4 exhibited an increase in deactivation, while MMH-5 showed reduced desensitization. Our research findings highlight the efficacy of MMH-5 as a negative allosteric modulator of GluA2 AMPA receptors, exerting substantial effects on both the magnitude and time course of receptor activity. Significantly, the compound MMH-2 demonstrated noteworthy cytotoxic effects, as evidenced by cell viability rates dropping below 6.79% for all cancer cell lines and 17.52% for the normal cell line (LX-2). Of particular interest is the pronounced cytotoxicity observed in MMH-5, suggesting its potential as a safe neuroprotective agent targeting the AMPA receptor, as indicated by cell viability percentages exceeding 85.44% across all cancer and normal cell lines. Docking simulations were performed to determine possible modes of interaction between MMH5 and the GluA2-AMPA receptor (PDB:7RZ5). The abovementioned facts and the well-documented effects of further thiazole derivatives provide a strong foundation for future research endeavors to enhance tailored treatments for neurological disorders that rely heavily on GluA2 signaling. The present study elucidates the intricate association between thiazole derivatives and GluA2 receptors, providing valuable perspectives on the prospects of enhanced and specific therapeutic interventions for diverse neurological conditions.
1. Introduction
Ionotropic glutamate receptors are the linchpins of fast excitatory synaptic transmission in the central nervous system (CNS). Among the subclasses of these receptors, the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors hold a particularly critical role [1,2]. These tetrameric proteins are complex assemblies, with each receptor comprising a combination of four subunits—GluA1 to GluA4 [3]. In the typical hippocampus, the predominant configuration of synaptic AMPA receptors consists of heteromeric complexes formed by GluA1 and GluA2 subunits, with a significantly smaller proportion containing GluA2 and GluA3 subunits [4]. Each subunit contributes specialized characteristics to the receptor’s overall function, which include ion selectivity, conductance properties, and drug sensitivities [5].
The GluA2 subunit distinguishes itself in several key aspects: it modifies the AMPA receptor’s ion channel to be impermeable to calcium, thereby shaping synaptic strength and influencing long-term potentiation and depression. Positive allosteric modulators can enhance GluA2 currents through two distinct mechanisms. For instance, substances like aniracetam primarily work to stabilize the closed clamshell conformation when agonists are bound, preventing deactivation. On the other hand, modulators like cyclothiazide mainly function by stabilizing the receptor, thereby slowing down the process of desensitization [6,7]. By not engaging in direct competition with endogenous neurotransmitters, the negative allosteric regulation of the AMPA receptor enables precise control over the frequency and amplitude of excitatory neural activity. This control remains unaffected by variations in neurotransmitter concentrations or the polarization state of the synaptic membrane [8]. The application of negative allosteric modulation on AMPA receptors proves beneficial in managing neurological disorders associated with excessive glutamatergic activity. This approach involves the use of compounds such as 2,3-benzodiazepines, quinazolinones, and pyridines [9,10,11]. Notably, benzodiazepines are frequently employed in the management of psychiatric disorders, making them a widely recognized option in this context [12].
Furthermore, the GluA2 subunit is critical in receptor trafficking, which has downstream effects on synaptic plasticity and network function [13,14]. The physiological and pathological significance of the GluA2 subunit is vast. Dysfunctions or alterations in GluA2 expression have been linked to various neurological conditions, including but not limited to Alzheimer’s disease, epilepsy, and ischemic stroke [15,16]. Additionally, the unique kinetic properties of AMPA receptors that contain GluA2 subunits warrant particular attention. Specifically, these subunits influence the receptor’s desensitization and deactivation kinetics. Desensitization is the process through which receptors lose sensitivity due to continuous agonist binding, while deactivation is characterized by the cessation of ionic current upon the removal of the agonist [17,18,19]. The dynamics of these processes can markedly influence the strength and duration of synaptic signaling. A thorough comprehension of the kinetic properties of GluA2-containing AMPA receptors is vital for understanding the intricacies of synaptic behavior and neural networks and is crucial for the rational design of therapeutic interventions aimed at modulating these receptors [20].
Building on the physiological and pathological significance of GluA2-containing AMPA receptors, exploring compounds that can modulate their behavior is essential. Our study focuses on novel thiazole carboxamide derivatives; these compounds bear a close relationship to existing pharmacologically active agents as they share the thiazole ring found in thiazole derivatives such as vitamin B1 (thiamine); dasatinib, an anticancer drug [21]; and Riluzole (Figure 1), known for its neuroprotective effects in conditions like amyotrophic lateral sclerosis [22,23]. In a separate study, a series of thiazolamide derivatives were synthesized and assessed for their potential as mGluR1 antagonists. Notably, St.1 (Figure 1) exhibited strong activity against mGluR1, yet faced challenges related to inadequate metabolic stability and limited water solubility [24]. Buettelmann and colleagues revealed a collection of thiazole-4-carboxamide derivatives in a patent application, positioning them as mGluR5a receptor antagonists. These derivatives underwent various substitutions on the thiazole carboxamide core to identify potent mGluR5a receptor antagonists. Among the compounds in this series, St.2 (Figure 1) emerged as the most active, demonstrating a favorable mGluR5a binding affinity with a Ki value of 18 nM [25]. Previously, our research team synthesized a heterocyclic-based curcumin compound featuring methoxy substitutions on the phenyl ring. These compounds exhibited substantial activity on GluA2 receptor subtypes, notably exemplified by St.3 (Figure 1) [15].
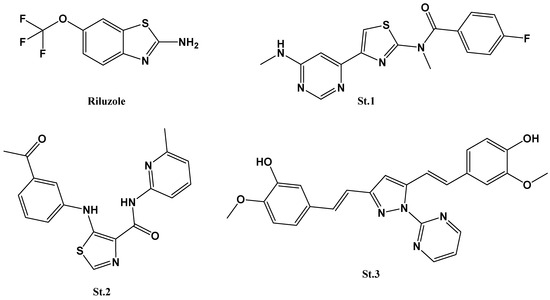
Figure 1.
Structures of thiazole and methoxyphenyl containing compounds with protentional AMPA receptor activities.
Importantly, some structurally similar thiazole derivatives, including Riluzole, have already been studied for their interactions with AMPA receptors [25,26]. Beyond their influence on AMPA receptors, these compounds exhibit additional pharmacological properties such as anti-inflammatory, anticancer, and neuroprotective effects [27,28]. This multidimensional therapeutic portfolio hints at a broad potential application in neurological disorders. However, the direction in which we steer the spotlight is toward understanding the kinetic subtleties of GluA2-containing AMPA receptors—focusing on how agonists and antagonists influence their molecular performance. These findings pave the way for comprehensive efforts regarding the development of negative allosteric modulators and anchoring the explorations in the structure–activity relationship (SAR), to dissect the precise mechanisms by which thiazole derivatives alter AMPA receptor behavior and in silico techniques to investigate possible binding interactions between the most potent compound and the AMPA-GluA2 target accordingly. Moreover, the cytotoxic activities of the thiazole derivatives were evaluated on various cell lines. This exploration aimed to identify more effective therapies for neurological conditions in which GluA2 signaling plays a pivotal role.
2. Materials and Methods
2.1. Materials
The chemicals used in this study were obtained from well-regarded suppliers, such as Chemicals Company, Sigma-Aldrich, and Alfa Aesar. The following substances were used in their original state, eliminating the need for additional purification: 2-(3,4-dimethoxyphenyl)-4-methylthiazole-5-carboxylic acid (Sigma-Aldrich, Burlington, MA, USA; catalog # BOG00154); 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC) (Alfa Aesar; Karlsruhe, Germany; catalog # A10807); 4-(dimethylamino)pyridine (DMAP) (Sigma-Aldrich; Gillingham, UK; catalog # 39405-50G); 3,4,5-trimethoxyaniline (Sigma Aldrich, Burlington, MA, USA; catalog # T68209-10G), silica gel (Sigma-Aldrich; Shanghai, China; catalog # S74874); 4-tert-butylaniline (Sigma Aldrich; Germany; catalog # 209864); 3,4-dimethoxyaniline (Sigma-Aldrich; Taufkirchen, Germany; catalog # A83008); 3,5-dimethoxyaniline (Sigma-Aldrich; Taufkirchen, Germany; catalog # D130001); and 4-methylthioaniline (Sigma-Aldrich; Burlington, MA, USA; catalog # L04950). The experimental requirements were DMEM media (Sigma-Aldrich; Burlington, MA, USA; catalog # D0822), L-glutamine solution (Sigma-Aldrich; Burlington, MA, USA; catalog #D0822), and dinitrosalicylic acid (DNSA) (Sigma-Aldrich; Burlington, MA, USA; catalog #128848).
2.2. Instrumentation
The NMR spectra of the synthesized thiazole derivatives were scrutinized using a Bruker DPX-500 High-Performance Digital FT-NMR Spectrometer (Billerica, MA, USA) in DMSO-d6 solutions at the Faculty of Science, University of Jordan, Jordan. The 1H-NMR spectra were obtained at a 500 MHz frequency, and the 13C-NMR spectra were acquired at 125 MHz. Chemical shift values (δ) were measured in parts per million (ppm), with coupling constants specified in Hertz (Hz). High-resolution mass spectra (HRMS) data were generated using a water LCT Premier XE Mass Spectrometer (Waters Corporation, Milford, MA, USA) employing the positive polarity mode’s electrospray ionization (ESI) technique. This research occurred at the Pharmacy Faculty of Gazi University in Ankara, Turkey.
2.3. General Procedure for the Synthesis of Thiazole Carboxamide (MMH1-5)
Following Scheme 1, in a clean, round-bottom flask, 2-(3,4-dimethoxyphenyl)-4-methylthiazole-5-carboxylic acid (300 mg, 1.074 mmol) was dissolved in 15 mL of dichloromethane (DCM). Subsequently, dimethylaminopyridine (DMAP) (45 mg, 0.361 mmol) was introduced, and the mixture was stirred under an argon atmosphere to prevent oxidation. After 5 to 10 min, a coupling reagent, 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDCI, 305.91 mg, 1.5639 mmol), was added and stirred under argon. Then, following 30 min, the aniline derivative was incorporated, and the reaction blend was stirred with a mechanical stirrer for 48 h. Thin-layer chromatography (TLC) papers were treated with ninhydrin to identify the presence of aniline, while another set of papers was treated with bromocresol to confirm the presence of acid, ensuring the purity of the product. Subsequently, the reaction mixture was subjected to an extraction process with diluted hydrochloric acid in a separatory funnel. The lower layer was collected in a conical flask. Anhydrous sodium sulfate was introduced as a drying agent, and the mixture was filtered through filter paper. Silica gel (approximately 3–4 spatulas) was then added to the filtrate, and the rotary vacuum evaporator was employed to remove the reaction mixture, taking into account the boiling point of DCM at 39.6 °C. This process resulted in a flask containing silica loaded with the product. The product-loaded silica was further purified through silica gel column chromatography using a solvent system of DCM and ethyl acetate in a 2:1 ratio. The elution was collected in tubes, and the solvent evaporated [29,30].
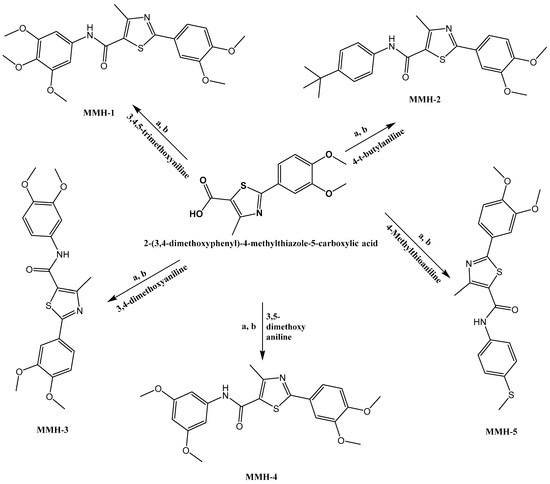
Scheme 1.
Upon stirring 2-(3,4-dimethoxyphenyl)-4-methylthiazole-5-carboxylic acid starting materials along with each aniline derivative within a 15 mL volume of DCM, the subsequent steps were executed as follows: (a) the introduction of DMAP and (b) the addition of EDC. Both steps were carried out under an inert gas environment.
2-(3,4-Dimethoxyphenyl)-4-methyl-N-(3,4,5-trimethoxyphenyl)thiazole-5-carboxamide (MMH1)
For the compound 2-(3,4-dimethoxyphenyl)-4-methyl-N-(3,4,5-trimethoxyphenyl)thiazole-5-carboxamide (MMH1), its 1H NMR (500 MHz, DMSO) is δ 10.08 (s, 1H, NH-amide), 7.54 (d, J = 8 Hz, 1H, Ar-H), 7.50 (s, 1H, Ar-H), 7.14 (s, 2H, Ar-H), 7.10 (d, J = 8.5 Hz, 1H, Ar-H), 3.87, 3.85 (s, 6H, -O-CH3), 3.77 (s, 6H, -O-CH3), 3.65 (s, 3H, -O-CH3), and 2.65 (s, 3H, thiazole-CH3), and the 13C NMR (DMSO) is δ 166.80 (C=O), 160.18, 156.44, 153.10, 151.74, 149.55, 135.29, 134.38, 125.62, 125.29, 120.13, 112.48, 109.34, 98.54, 60.58 (-O-CH3), 56.22 (-O-CH3), 56.17 (-O-CH3), 56.07 (-O-CH3), and 17.68 (thiazole-CH3). HRMS (m/z): [M + H]+ is calculated for C22H24N2O6S as 445.1430, which is found to be 445.1436.
N-(4-(Tert-butyl)phenyl)-2-(3,4-dimethoxyphenyl)-4-methylthiazole-5-carboxamide (MMH2)
For the compound N-(4-(tert-butyl)phenyl)-2-(3,4-dimethoxyphenyl)-4-methylthiazole-5-carboxamide (MMH2), its 1H NMR (500 MHz, DMSO) is δ 10.12 (s, 1H, NH-amide), 7.61 (d, J = 10.5 Hz, 2H, Ar-H), 7.55 (d, J = 10.5 Hz, 1H, Ar-H), 7.50 (s, 1H, Ar-H), 7.38 (d, J = 10.5 Hz, 2H, Ar-H), 7.11 (s, 1H, Ar-H), 3.87 (s, 3H, -O-CH3), 3.85 (s, 3H, -O-CH3), 2.64 (s, 3H, thiazole-CH3), and 1.29 (s, 9H, t-butyl), and the 13C NMR (DMSO) is δ 166.80 (C=O), 160.22, 160.21, 155.98, 155.97, 151.73, 149.57, 146.84, 136.56, 125.77, 120.64, 120.15, 112.52, 109.40, 56.18 (-O-CH3), 56.09 (-O-CH3), 34.55 (C-(CH3)3), 31.65 (C-(CH3)3), and 17.60 (thiazole-CH3). HRMS (m/z): [M + H]+ is calculated for C23H26N2O3S as 411.1750, which is found to be 411.1743.
N,2-bis(3,4-dimethoxyphenyl)-4-methylthiazole-5-carboxamide (MMH3)
For the compound N,2-bis(3,4-dimethoxyphenyl)-4-methylthiazole-5-carboxamide (MMH3), its 1H NMR (500 MHz, DMSO) is δ 10.03 (s, 1H, NH-amide), 7.55 (dd, J = 10.5, 2 Hz, 1H, Ar-H), 7.50 (d, J = 2.5 Hz, 1H, Ar-H), 7.39 (d, J = 3 Hz, 1H, Ar-H), 7.25 (dd, J = 8, 3 Hz, 1H, Ar-H), 7.11 (d, J = 10.5 Hz, 1H, Ar-H), 6.94 (d, J = 10.5 Hz, 1H, Ar-H), 3.87, 3.85 (s, 6H, -O-CH3), 3.75 (s, 6H, -O-CH3), and 2.65 (s, 3H, thiazole-CH3), and the 13C NMR (DMSO) is δ166.67 (C=O), 160.00, 156.06, 151.70, 149.55, 148.92, 145.86, 132.64, 125.67, 125.55, 120.11, 112.88, 112.49, 112.34, 109.33, 105.97, 56.18 (-O-CH3), 56.07 (-O-CH3), 55.88 (-O-CH3), and 17.63 (thiazole-CH3). HRMS (m/z): [M + H]+ is calculated for C21H22N2O5S as 415.1340, which is found to be 415.1348.
2-(3,4-Dimethoxyphenyl)-N-(3,5-dimethoxyphenyl)-4-methylthiazole-5-carboxamide (MMH4)
For the compound 2-(3,4-dimethoxyphenyl)-N-(3,5-dimethoxyphenyl)-4-methylthiazole-5-carboxamide (MMH4), its 1H NMR (500 MHz, DMSO) is δ 10.12 (s, 1H, NH-amide), 7.55 (d, J = 10.5 Hz, 1H, Ar-H), 7.50 (s, 1H, Ar-H), 7.11 (d, J = 10.5 Hz, 1H, Ar-H), 7.97 (d, J = 3 Hz, 2H, Ar-H), 6.29 (s, 1H, Ar-H), 3.87, 3.85 (s, 6H, -O-CH3), 3.75 (s, 6H, -O-CH3), and 2.92 (s, 3H, thiazole-CH3), and the 13C NMR (DMSO) is δ: 166.92 (C=O), 160.87, 160.38, 156.38, 151.76, 149.55, 146.90, 143.90, 140.83, 125.61, 125.41, 120.16, 112.50, 109.36, 98.97, 96.46, 56.18 (-O-CH3), 56.08 (-O-CH3), 55.62 (-O-CH3), and 17.65 (thiazole-CH3). HRMS (m/z): [M + H]+ is calculated for C21H22N2O5S as 415.1340, which is found to be 415.1334.
2-(3,4-Dimethoxyphenyl)-4-methyl-N-(4-(methylthio)phenyl)thiazole-5-carboxamide (MMH5)
For the compound 2-(3,4-dimethoxyphenyl)-4-methyl-N-(4-(methylthio)phenyl)thiazole-5-carboxamide (MMH5), its 1H NMR (500 MHz, DMSO) is δ 10.19 (s, 1H, NH-amide), 7.66 (d, J = 9 Hz, 2H, Ar-H), 7.55 (d, J = 10.5 Hz, 1H, Ar-H), 7.50 (s, 1H, Ar-H), 7.28 (d, J = 8.5 Hz, 2H, Ar-H), 7.11 (d, J = 10.5 Hz, 1H, Ar-H), 3.87 (s, 3H, -O-CH3), 3.84 (s, 3H, -O-CH3), 2.64 (s, 3H, thiazole-CH3), and 2.47 (s, 3H, -S-CH3), and the 13C NMR (DMSO) is δ166.89 (C=O), 160.24, 156.25, 151.74, 149.54, 136.55, 133.26, 127.62, 127.30, 125.62, 121.47, 120.15, 112.48, 109.34, 56.18 (-O-CH3), 56.08 (-O-CH3), 17.64 (thiazole-CH3), and 15.85 (-S-CH3). HRMS (m/z): [M + H]+ is calculated for C20H20N2O3S2 as 401.1010, which is found to be 401.1008.
2.4. HEK293T Cell Patch Clamp Recordings
The QIAGEN Plasmid Mini Kit was used for DNA preparation to extract high-copy plasmid DNA (up to 20 µg). The GluA2 subunit, specifically, the flip isoform obtained from S. F. Heinemann at the Salk Institute, was sub-cloned into the pRK vector for expression in Human Embryonic Kidney cells 293 (HEK293T) obtained from Sigma, Germany. HEK293T cells were chosen for their ease of cultivation and transfection efficiency. Transfection involved GluA2 and GFP-expressing constructs, and we used either jetPRIME or Lipofectamine 2000 as transfection reagents. The HEK293T cells were cultured in DMEM with 10% FBS, streptomycin, and sodium pyruvate. The cells were incubated at 37 °C with 5% CO2 and subcultured regularly. After 36–48 h post-transfection, highly fluorescent cells were selected for recordings, as explained in our previous work [31].
Electrophysiological recordings were carried out using a patch clamp setup equipped with a rapid perfusion system. Recordings were performed at 22 °C with a membrane potential of −60 mV, amplified using an integrated patch amplifier, and digitized. Patch pipettes had a resistance range of 2–4 MΩ and were filled with a specific solution. The extracellular medium composition was standardized. A sample size of six viable cells was used to calculate the mean inhibition. The solution exchange time was approximately 500 milliseconds; this has been detailed further in previous works [32,33].
Data were analyzed using Igor Pro7 software. Receptor desensitization and deactivation rates were determined by fitting the current decay using a double exponential model. Weighted mean time constants (τw) were calculated. Statistical analyses were performed using ANOVA to assess differences among chemical compounds, with significance levels indicated.
2.5. Molecular Docking Studies
In this research, an integrated single-click docking method was employed to perform molecular docking of the most active compound (MMH5). The compound’s chemical structure was sketched and then docked with the 7RZ5 protein structure (PDB code: 7Rz5), which was downloaded from the RCSB repository server. Subsequently, the protein structures were analyzed with PyMOL v2.5. The native ligand, non-protein atoms, and crystallographic waters were removed. Polar hydrogen atoms were added to the previous structure, and sidechains were protonated, assuming a physiological pH of 7.4 using an H++ server. Using the 1-click docking free server (https://mcule.com/apps/1-click-docking accessed on 18 October 2023), the binding site as well as the binding site dimensions were identified according to the literature. Additionally, the interaction profile was analyzed between the compound MMH5 and the receptor using the Protein–Ligand Interaction Profiler (PLIP) service [34,35].
2.6. Cell Culture and Cytotoxicity Assay (MTS)
All of the thiazole derivatives’ cytotoxicity was investigated using cultured cell lines, including HepG2, MCF-7, HeLa, CaCo-2, cancer cell lines, and the normal hepatic cell line LX-2, which were grown separately in RPMI-1640 media (Sigma, Norwich, UK). The media were supplemented with 1% L-glutamine (Sigma, London, UK), 1% penicillin/streptomycin antibiotics (BI, New Delhi, India), and 10% fetal bovine serum. These cells were cultured under humid conditions with 5% CO2 at 37 °C. In 96-well plates, cells were seeded at a density of 2.6 × 104 cells per well. After 48 h, cancer cells were exposed to thiazole derivatives (MMH1-5) at one concentration (50 µM) for 24 h. Cell viability was assessed using the CellTiter 96® Aqueous One Solution Cell Proliferation (MTS) Assay from Promega Corporation, Madison, WI, USA, following the manufacturer’s instructions. Following the treatment, 20 µL of MTS solution was added to each well containing 100 µL of medium, and the plates with thiazole derivatives were incubated at 37 °C for 2 h. Absorbance was measured at 490 nm [36,37].
3. Results and Discussion
3.1. Chemistry
Following the procedure outlined in Scheme 1, a sequence of thiazole derivatives, namely MMH1-5, were synthesized. The synthesis initiation involved dissolving 2-(3,4-dimethoxyphenyl)-4-methylthiazole-5-carboxylic acid in dichloromethane (DCM). Subsequently, a blend of dimethylaminopyridine (DMAP) and EDCI was introduced, and the components were thoroughly mixed within an inert gas environment. The corresponding aniline derivative was added after a 30 min incubation period in each experiment. The resulting reaction was allowed to stir for 48 h. Following this reaction, an extraction process was conducted by utilizing a 32% hydrochloric acid (HCl) solution, followed by treatment with anhydrous sodium sulfate. The HRMS values for all the synthesized compounds confirmed their respective molecular weights. The 1H-NMR spectra verified the synthesis of these compounds, with each compound displaying a single peak for the N-H amide proton around 10.05 ppm. A signal peak representing nine protons was also evident for the compound featuring a t-butyl substituent at 1.29 ppm. The 13C-NMR spectrum exhibited a distinctive signal for the carbonyl group at around 166 ppm, for methoxy groups at around 56, and a signal was observed for all compounds at 17 ppm, which represents the methyl group of the thiazole ring substituted methyl.
3.2. Thiazole Derivatives’ Effects on GluA2 Currents and Kinetics: Unveiling Desensitization and Deactivation Dynamics
The effects of thiazole carboxamide derivatives were investigated on AMPA-R homomeric subunit GluA2 whole-cell currents, as well as their impact on deactivation (τw deact) and desensitization rates (τw des). The detailed results are presented in Tables S1–S5. Figure 2a illustrates the alterations in GluA2 whole-cell currents, following exposure to the thiazole derivatives. The A/AI ratio was calculated to quantify these changes, representing the current amplitudes before and after thiazole derivative exposure (Figure 2b). Compound MMH-5 demonstrated the most substantial effect, causing a remarkable 6-fold decrease in current amplitude. Following closely, MMH-4 induced a 5-fold reduction, while MMH-3 resulted in a 4-fold decrease. MMH-2 and MMH-1 exhibited comparatively milder effects, reducing GluA2 activity by nearly 3-fold.
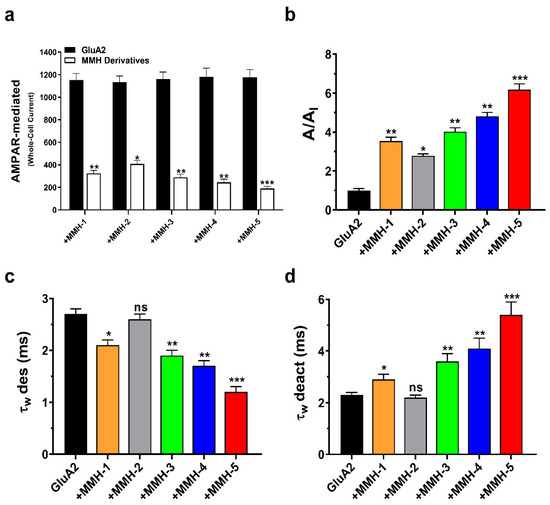
Figure 2.
Modulation of GluA2 subunit-driven whole-cell current and kinetics by MMH compounds. (a) Whole-cell current amplitude (in picoamperes) illustrating the impact of glutamate alone (black) on the GluA2 subunit and the effect of five MMH compounds (at a concentration of 16 µM) (white). (b) Calculated A/AI ratio, where A represents the current with glutamate alone, and AI is the inhibitory current observed after applying the MMH compounds. (c) Effect of the compounds on the desensitization rate (τw des). MMH-1 (orange), MMH-2 (gray), MMH-3 (green), MMH-4 (blue), and MMH-5 (red) are shown. (d) Effect of the compounds on the deactivation rate (τw deact). MMH-1 (orange), MMH-2 (gray), MMH-3 (green), MMH-4 (blue), and MMH-5 (red) are displayed. The experiments were conducted under controlled conditions, including a pH of 7.4, a temperature of 22 °C, and a voltage of −60 mV. Data are presented as the mean ± standard error of the mean (SEM), with n = 6 representing the number of patch cells tested. Statistical significance of one-way ANOVA results: (*) p < 0.05, (**) p < 0.01, (***) p < 0.001; ns, not significant.
Shifting our focus toward the kinetics of the GluA2 subunit, interesting dynamics were observed for the thiazole derivatives. Specifically, MMH-5 and MMH-4 led to a notable increase in the deactivation rate, approximately 3-fold (Figure 2d). In contrast, MMH-3 had a milder effect, elevating the deactivation rate by 1.5-fold. Regarding desensitization rates (Figure 2c), MMH-5 demonstrated a significant reduction, reducing the desensitization rate approximately by half. MMH-4 and MMH-3 also exhibited a reduction in desensitization, though to a smaller degree, each resulting in approximately a 1-fold decrease. These findings highlight the distinct effects of the thiazole derivatives on both GluA2 whole-cell currents and the kinetics of the GluA2 subunit, shedding light on the potential modulatory roles of these compounds.
Compounds with heterocycles have garnered substantial attention in recent years due to their potential therapeutic applications. These interactions can lead to anticonvulsant properties, with carbonyl groups, heteroatoms, and aromatic or heterocyclic rings playing pivotal roles in these pharmacophore groups. In our previous research on 2-oxo-3H-benzoxazole derivatives, these compounds exhibited essential pharmacophores that interact with amino acids in the binding site of AMPA receptor subunits, resulting in receptor inhibition and potential anticonvulsant effects [38]. Previous research has identified critical residues surrounding the binding pocket, including the pre-M1, M3, and M4 helices, as well as the S2-M4 linker. These residues are expected to play a crucial role in compound binding, as evidenced by interactions with known antagonists like DNQX and ZK200775. Our analysis suggests that these residues are also pivotal in interacting with our thiazole derivatives, which exhibit AMPA receptor antagonistic effects akin to DNQX and ZK200775. This suggests that thiazole compounds may function as AMPA receptor antagonists, likely employing a binding mechanism similar to the aforementioned antagonists [39,40].
The AMPA receptor-affecting compounds encompass the primary pharmacophore groups, including carbonyl, hetero atoms, and halogenated aromatic or heterocyclic rings. These groups are capable of facilitating various binding interactions with AMPA receptor amino acids, such as hydrogen, hydrophobic, and π–π interactions involving amino acids like ASN791, PHE623, PRO520, and LEU620 [41,42]. The carbonyl functional group and/or hetero atoms, like oxygen, can establish hydrogen bonds, while aromatic or heterocyclic rings contribute to hydrophobic interactions [41]. These pharmacophores are fundamental for substantial binding interactions with various AMPA receptor subtypes. Each compound in our study incorporates a thiazole moiety, facilitating π–π or hydrogen bonding interactions, along with a carbonyl amide group capable of establishing hydrogen bonding interactions.
The investigation into compounds that can modulate the behavior of GluA2-containing AMPA receptors is of paramount importance, given the physiological and pathological implications of these receptors. The study at hand delves into a novel class of compounds known as thiazole carboxamide derivatives. These compounds exhibit structural similarities to well-established pharmacologically active agents, featuring the shared thiazole ring like Riluzole, renowned for its neuroprotective properties in conditions such as amyotrophic lateral sclerosis [22,23]. Similar to our compounds, the thiazolamide derivatives were synthesized to explore their potential as mGluR1 antagonists. Notably, they exhibited substantial activity against mGluR1—although it faced challenges related to metabolic stability and water solubility [24]—as well as thiazole-4-carboxamide derivatives, as mGluR5a receptor antagonists were displaying a favorable mGluR5a binding affinity [25]. Previous research from our team involved the synthesis of heterocyclic-based curcumin compounds featuring methoxy substitutions on the phenyl ring, with notable activity on GluA2 receptor subtypes [11].
3.3. Molecular Docking and SAR
The compound with the greatest efficacy, MMH-5, was chosen for molecular docking studies to predict its potential binding interactions with the AMPA receptor. Based on the findings from Figure 3 and Table 1, it was observed that approximately nine binding interactions occurred between MMH-5 and specific amino acids, including GLN-293, ASP-473, PHE-438, VAL-395, and LEU-742. These interactions were characterized by ideal distances, all within 3.0 Å or less. Notably, a robust hydrogen bond was formed between GLN-293 and the H-amide group at a distance of just 2.7 Å. Detailed information on the other binding interactions and their respective distances can be found in Table 1 and Figure 3.
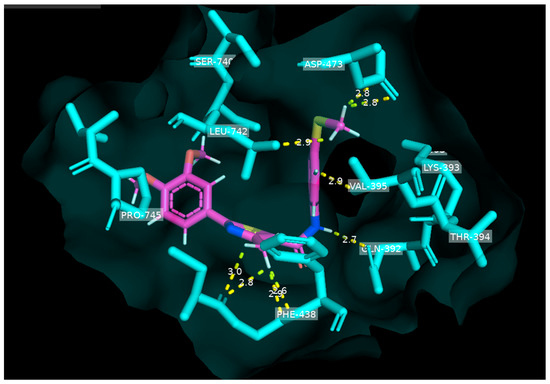
Figure 3.
Computational molecular docking of MMH-5 compound within the binding site of GluA2-AMPA receptor (PDB ID: 7RZ5): analyzing hydrogen bonds, bond distances, and binding site surface interactions.

Table 1.
Detailed interactions and binding scores of MMH-5 compound within the binding pocket of GluA2-AMPA (PDB ID: 7RZ5) target protein.
It is noteworthy that the fundamental pharmacophore groups previously discussed concerning compounds affecting the AMPA receptor and SAR studies, such as carbonyl, hetero atoms, and heterocyclic rings, play a pivotal role in facilitating a diverse spectrum of binding interactions with AMPA receptor amino acids. In the case of the compound MMH5, all of these crucial features were present. The amide group formed a hydrogen bond with GLN-392; the heterocyclic (thiazole) engaged in four binding interactions with PHE-438; the phenyl-amide ring presented a more important binding interaction than the second phenyl ring; and the presence of a methylthio group was optimal for establishing three binding interactions with ASP-473 and LEU-742 amino acids. It became evident that the inclusion of larger substituents such as trimethoxy groups or tert-butyl, as seen in MMH1 and MMH2, respectively, did not effectively facilitate binding interactions. In fact, they introduced a restrictive effect that resulted in diminished activity with GluA2 amino acids and the absence of three binding interactions. On the contrary, the presence of smaller substituents like methylthio proved to be highly effective in promoting binding interactions with ASP-473 and LEU-742, leading to robust binding interactions and the potent activity of compound MMH5.
The intricacies of thiazole compounds’ effects on AMPA receptors featuring GluA2 subunits provide an intriguing focus for neuroscience and pharmacology. Our data elucidate a compelling correlation between the molecular architecture of thiazole derivatives and their consequential modulation of receptor kinetics. Specifically, MMH1, MMH3, and MMH4, each with their unique configurations of 3,4-dimethoxyphenyl and additional substituents like 3,4,5-trimethoxyphenyl, demonstrated substantial decreases in amplitude along with significant changes in deactivation and desensitization times (t deact and t des) when compared to glutamate alone. These structural nuances, particularly the presence and positioning of methoxy groups on the phenyl rings, may enhance the electron-donating abilities of these molecules. This likely influences their interaction affinity and kinetics with the GluA2 subunits, echoing the behaviors of known AMPA receptor modulators like talampanel and GYKI 53655. The unique kinetic behavior of MMH5, characterized by a 4-(methylthio)phenyl moiety, underscores the impact of even slight structural alterations on receptor modulation. It was clear that these thiazole carboxamide derivatives have structural similarities to established pharmacologically active agents. This unique approach presents the opportunity to explore these compounds as potential modulators of GluA2-containing AMPA receptors, a focus that aligns with the growing understanding of the physiological and pathological significance of these receptors. The study’s emphasis on the pharmacophore groups shared by these compounds and their potential interactions with AMPA receptor amino acids adds an innovative layer to the research. Furthermore, the data delve into the critical residues involved in compound binding, shedding light on the compounds’ possible mechanisms of action. The findings extend to the modulation of receptor kinetics, highlighting how subtle structural alterations can significantly impact receptor behavior. This holistic approach not only enhances our understanding of AMPA receptor modulation, but also opens doors to the development of novel therapeutics in the fields of neurobiology and drug discovery.
3.4. Cytotoxicity Results
Cytotoxicity assessments were conducted on the thiazole derivatives to ensure their safe use at a concentration approximating the effective dose of 15 µM on the GluA2 receptor. Figure 4 presents the cell viability percentages at a 50 µM concentration for various cancer cell lines and a normal cell line (LX-2). Notably, compound MMH2 (N-(4-(tert-butyl)phenyl)-2-(3,4-dimethoxyphenyl)-4-methylthiazole-5-carboxamide) displayed remarkable cytotoxicity, with cell viability values falling below 6.79% for all cancer cell lines and at 17.52% for the normal cell line (LX-2) at 50 µM concentration, in comparison with positive-control anticancer drug 5-fluorouracil cell viability values falling below 22.99% for all cancer cell lines and at 10.17% for the normal cell line (LX-2) at the same concentration. In contrast, the cell viability percentages for the other compounds exceeded 80% for nearly all compounds across a wide range of cell lines. These values were compared to the positive control, the anticancer drug 5-fluorouracil. It is particularly noteworthy to highlight the cytotoxicity of MMH5, as it stood out as the most potent compound targeting the GluA2 receptor subtype, with cell viability percentages surpassing 85.44% across all cancer and normal cell lines, affirming its safety for use as a neuroprotective agent on the AMPA receptor. The intriguing anticancer activities observed for compound MMH2 against all cancer cell lines can be attributed to the presence of the tert-butyl substituent on the phenyl ring. This structural feature enhances the compound’s lipophilicity, facilitating its easy cell membrane penetration and interaction with intracellular targets.
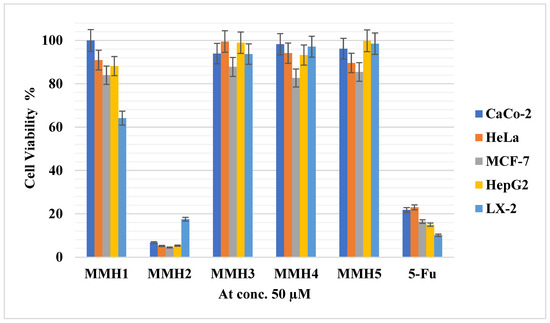
Figure 4.
Cell viability values at 50 µM on various cell lines of the thiazole derivatives in comparison with positive control.
4. Conclusions
In summary, our research investigates the impact of thiazole carboxamide derivatives on GluA2 AMPA receptors. These experiments have provided valuable insights into the complex interplay between these chemicals and receptor kinetics, enhancing our understanding of their potential as regulators of AMPA receptor activity. MMH5 is notable for its strong negative allosteric modulation effects, which substantially impact both the amplitude and kinetics of the receptor. This underscores the potential therapeutic relevance of MMH5. Furthermore, our extensive evaluations of cytotoxicity have provided evidence supporting the safety of these compounds at concentrations proximate to the efficacious dosage of the GluA2 receptor. However, it is worth noting that compound MMH2 exhibits substantial cytotoxic properties on cancer cell lines, thereby demonstrating its potential as an anticancer agent. This effect is attributed to the distinctive structural characteristic of a tert-butyl substituent on the phenyl ring, which enhances its ability to penetrate cellular membranes and interact with intracellular targets. As mentioned earlier, the results provide a robust basis for future investigations and research initiatives to develop customized therapeutics for neurological disorders in which GluA2 signaling plays a crucial role. The observed effects of these thiazole derivatives and the encouraging cytotoxicity outcomes provide intriguing opportunities for enhancing tailored therapies and therapeutic approaches in neurology and pharmacology. The significant regulatory impacts of MMH5 highlight the possibility of focused interventions in many neurological illnesses, signaling the advent of a new era in tailored pharmacology. The conclusion of this research underscores the potential for future work in the fields of neurology and pharmacology. This includes in-depth investigations into the mechanistic details of thiazole carboxamide derivatives’ interactions with GluA2 AMPA receptors and the synthesis of novel compounds for enhanced pharmacological effects. Moreover, the transition to preclinical studies and clinical trials is essential for evaluating the compounds’ safety and efficacy as potential therapeutic agents. Additionally, the broad applications of these compounds in treating neurological disorders and their potential for customized pharmacological interventions mark a promising path for future research and therapeutic development.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biom13121694/s1, Figure S1: NMR Spectrum of MMH1; Figure S2: NMR Spectrum of MMH2; Figure S3: NMR Spectrum of MMH3; Figure S4: NMR Spectrum of MMH4; Figure S5: NMR Spectrum of MMH5; Table S1: Whole-cell recordings for compound MMH1; Table S2: Whole-cell recordings for compound MMH2; Table S3: Whole-cell recordings for compound MMH3; Table S4: Whole-cell recordings for compound MMH4; Table S5: Whole-cell recordings for compound MMH5.
Funding
This research received no external funding.
Institutional Review Board Statement
The study did not require ethical approval for studies not involving humans or animals.
Informed Consent Statement
Not applicable.
Data Availability Statement
This published article and its Supplementary Materials include all data generated or analyzed during this study.
Acknowledgments
The author thanks An-Najah National University and Gazi University for cooperating in facilitating this research. Additionally, the author expresses gratitude to Mohammad Qneibi for his valuable support in the analysis and execution of the GluA2 subunit receptor tests conducted in the research laboratory/Department of Biomedical Sciences, Faculty of Medicine and Health Sciences, An-Najah National University. The author would also like to thank Mohammed T. Qaoud for providing the opportunity and the assistance to perform his molecular modeling research at Faculty of Pharmacy, Cyprus International University, Nicosia, Cyprus.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Stroebel, D.; Paoletti, P. Architecture and function of NMDA receptors: An evolutionary perspective. J. Physiol. 2021, 599, 2615–2638. [Google Scholar] [CrossRef] [PubMed]
- Narisawa-Saito, M.; Silva, A.J.; Yamaguchi, T.; Hayashi, T.; Yamamoto, T.; Nawa, H. Growth factor-mediated Fyn signaling regulates α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor expression in rodent neocortical neurons. Proc. Natl. Acad. Sci. USA 1999, 96, 2461–2466. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chen, S.; Swensen, A.C.; Qian, W.-J.; Gouaux, E. Architecture and subunit arrangement of native AMPA receptors elucidated by cryo-EM. Science 2019, 364, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Greger, I.H.; Watson, J.F.; Cull-Candy, S.G. Structural and functional architecture of AMPA-type glutamate receptors and their auxiliary proteins. Neuron 2017, 94, 713–730. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.; Vissel, B. The essential role of AMPA receptor GluR2 subunit RNA editing in the normal and diseased brain. Front. Mol. Neurosci. 2012, 5, 34. [Google Scholar] [CrossRef]
- Ptak, C.P.; Ahmed, A.H.; Oswald, R.E. Probing the allosteric modulator binding site of GluR2 with thiazide derivatives. Biochemistry 2009, 48, 8594–8602. [Google Scholar] [CrossRef]
- Krintel, C.; Frydenvang, K.; Olsen, L.; Kristensen, M.T.; de Barrios, O.; Naur, P.; Francotte, P.; Pirotte, B.; Gajhede, M.; Kastrup, J.S. Thermodynamics and structural analysis of positive allosteric modulation of the ionotropic glutamate receptor GluA2. Biochem. J. 2012, 441, 173–178. [Google Scholar] [CrossRef]
- Bigge, C.F.; Nikam, S.S. AMPA receptor agonists, antagonists and modulators: Their potential for clinical utility. Expert Opin. Ther. Pat. 1997, 7, 1099–1114. [Google Scholar] [CrossRef]
- Di Bonaventura, C.; Labate, A.; Maschio, M.; Meletti, S.; Russo, E. AMPA receptors and perampanel behind selected epilepsies: Current evidence and future perspectives. Expert Opin. Pharmacother. 2017, 18, 1751–1764. [Google Scholar] [CrossRef]
- Lazzaro, J.; Paternain, A.V.; Lerma, J.; Chenard, B.; Ewing, F.; Huang, J.; Welch, W.; Ganong, A.; Menniti, F.S. Functional characterization of CP-465,022, a selective, noncompetitive AMPA receptor antagonist. Neuropharmacology 2002, 42, 143–153. [Google Scholar] [CrossRef]
- Zwart, R.; Sher, E.; Ping, X.; Jin, X.; Sims, J.; Chappell, A.; Gleason, S.; Hahn, P.; Gardinier, K.; Gernert, D. Perampanel, an antagonist of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors, for the treatment of epilepsy: Studies in human epileptic brain and nonepileptic brain and in rodent models. J. Pharmacol. Exp. Ther. 2014, 351, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Hattab, S.; Kittana, N.; Qasarweh, L.; Tayem, Y. Prevalence and factors associated with polypharmacy among patients treated for psychiatric disorders in Palestine. Palest. Med. Pharm. J. 2023, 8, 1. [Google Scholar] [CrossRef]
- Cull-Candy, S.G.; Farrant, M. Ca2+-permeable AMPA receptors and their auxiliary subunits in synaptic plasticity and disease. J. Physiol. 2021, 599, 2655–2671. [Google Scholar] [CrossRef]
- Wu, Q.-L.; Gao, Y.; Li, J.-T.; Ma, W.-Y.; Chen, N.-H. The role of AMPARs composition and trafficking in synaptic plasticity and diseases. Cell. Mol. Neurobiol. 2022, 42, 2489–2504. [Google Scholar] [CrossRef]
- Qneibi, M.; Hamed, O.; Jaradat, N.; Hawash, M.; Al-Kerm, R.; Al-Kerm, R.; Sobuh, S.; Tarazi, S. The AMPA receptor biophysical gating properties and binding site: Focus on novel curcumin-based diazepines as non-competitive antagonists. Bioorg. Chem. 2021, 116, 105406. [Google Scholar] [CrossRef]
- Hosaka, T.; Yamashita, T.; Tamaoka, A.; Kwak, S. Extracellular RNAs as biomarkers of sporadic amyotrophic lateral sclerosis and other neurodegenerative diseases. Int. J. Mol. Sci. 2019, 20, 3148. [Google Scholar] [CrossRef]
- Balannik, V.; Menniti, F.S.; Paternain, A.V.; Lerma, J.; Stern-Bach, Y. Molecular mechanism of AMPA receptor noncompetitive antagonism. Neuron 2005, 48, 279–288. [Google Scholar] [CrossRef]
- Zhang, W.; Cho, Y.; Lolis, E.; Howe, J.R. Structural and single-channel results indicate that the rates of ligand binding domain closing and opening directly impact AMPA receptor gating. J. Neurosci. 2008, 28, 932–943. [Google Scholar] [CrossRef]
- Qneibi, M.; Hawash, M.; Jaradat, N.; Bdir, S. Affecting AMPA receptor biophysical gating properties with negative allosteric modulators. Mol. Neurobiol. 2022, 59, 5264–5275. [Google Scholar] [CrossRef]
- Roth, R.H.; Zhang, Y.; Huganir, R.L. Dynamic imaging of AMPA receptor trafficking in vitro and in vivo. Curr. Opin. Neurobiol. 2017, 45, 51–58. [Google Scholar] [CrossRef]
- Pkn, S.; Sahoo, J.; Paidesetty, S.K.; Mohanta, G.P. Thiazoles as potent anticancer agents: A review. Indian Drugs 2016, 53, 5–11. [Google Scholar]
- Bryson, H.M.; Fulton, B.; Benfield, P. Riluzole: A review of its pharmacodynamic and pharmacokinetic properties and therapeutic potential in amyotrophic lateral sclerosis. Drugs 1996, 52, 549–563. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Calabrese, V.; Giordano, J. Demonstrated hormetic mechanisms putatively subserve riluzole-induced effects in neuroprotection against amyotrophic lateral sclerosis (ALS): Implications for research and clinical practice. Ageing Res. Rev. 2021, 67, 101273. [Google Scholar] [CrossRef]
- Satoh, A.; Nagatomi, Y.; Hirata, Y.; Ito, S.; Suzuki, G.; Kimura, T.; Maehara, S.; Hikichi, H.; Satow, A.; Hata, M. Discovery and in vitro and in vivo profiles of 4-fluoro-N-[4-[6-(isopropylamino) pyrimidin-4-yl]-1, 3-thiazol-2-yl]-N-methylbenzamide as novel class of an orally active metabotropic glutamate receptor 1 (mGluR1) antagonist. Bioorg. Med. Chem. Lett. 2009, 19, 5464–5468. [Google Scholar] [CrossRef]
- Mishra, C.B.; Kumari, S.; Tiwari, M. Thiazole: A promising heterocycle for the development of potent CNS active agents. Eur. J. Med. Chem. 2015, 92, 1–34. [Google Scholar] [CrossRef]
- Kretschmer, B.D.; Kratzer, U.; Schmidt, W.J. Riluzole, a glutamate release inhibitor, and motor behavior. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1998, 358, 181–190. [Google Scholar] [CrossRef]
- Agarwal, S.; Kalal, P.; Gandhi, D.; Prajapat, P. Thiazole containing Heterocycles with CNS activity. Curr. Drug Discov. Technol. 2018, 15, 178–195. [Google Scholar] [CrossRef]
- Singh, A.; Malhotra, D.; Singh, K.; Chadha, R.; Bedi, P.M.S. Thiazole derivatives in medicinal chemistry: Recent advancements in synthetic strategies, structure activity relationship and pharmacological outcomes. J. Mol. Struct. 2022, 1266, 133479. [Google Scholar] [CrossRef]
- Iwaszkiewicz-Grzes, D.; Cholewinski, G.; Kot-Wasik, A.; Trzonkowski, P.; Dzierzbicka, K. Synthesis and biological activity of mycophenolic acid-amino acid derivatives. Eur. J. Med. Chem. 2013, 69, 863–871. [Google Scholar] [CrossRef]
- Kim, I.H.; Lee, I.H.; Nishiwaki, H.; Hammock, B.D.; Nishi, K. Structure–activity relationships of substituted oxyoxalamides as inhibitors of the human soluble epoxide hydrolase. Bioorg. Med. Chem. 2014, 22, 1163–1175. [Google Scholar] [CrossRef]
- Qneibi, M.; Nassar, S.; Bdir, S.; Hidmi, A. α-Lipoic Acid Derivatives as Allosteric Modulators for Targeting AMPA-Type Glutamate Receptors’ Gating Modules. Cells 2022, 11, 3608. [Google Scholar] [CrossRef]
- Jaradat, N.; Hawash, M.; Qneibi, M.; Shtayeh, T.; Sobuh, S.; Arar, M.; Bdir, S. The effect of novel negative allosteric 2,3-benzodiazepine on glutamate AMPA receptor and their cytotoxicity. J. Mol. Struct. 2022, 1261, 132936. [Google Scholar] [CrossRef]
- Jaradat, N.; Qneibi, M.; Hawash, M.; Al-Maharik, N.; Qadi, M.; Abualhasan, M.N.; Ayesh, O.; Bsharat, J.; Khadir, M.; Morshed, R. Assessing Artemisia arborescens essential oil compositions, antimicrobial, cytotoxic, anti-inflammatory, and neuroprotective effects gathered from two geographic locations in Palestine. Ind. Crops Prod. 2022, 176, 114360. [Google Scholar] [CrossRef]
- Adasme, M.F.; Linnemann, K.L.; Bolz, S.N.; Kaiser, F.; Salentin, S.; Haupt, V.J.; Schroeder, M. PLIP 2021: Expanding the scope of the protein–ligand interaction profiler to DNA and RNA. Nucleic Acids Res. 2021, 49, W530–W534. [Google Scholar] [CrossRef]
- Schrödinger, Inc. The PyMOL Molecular Graphics System, version 1.8; Schrödinger, Inc.: New York, NY, USA, 2015. [Google Scholar]
- Jaradat, N.; Al-Maharik, N. Fingerprinting, Antimicrobial, Antioxidant, Anticancer, Cyclooxygenase and Metabolic Enzymes Inhibitory Characteristic Evaluations of Stachys viticina Boiss. Essential Oil. Molecules 2019, 24, 3880. [Google Scholar] [CrossRef]
- Özcan, G.; Akman, G.; Khalilia, W. Induction of Apoptosis by Hypoxia in C-4 I Human Cervical Cancer Cells. Palest. Med. Pharm. J. 2020, 7, 2. [Google Scholar]
- Qneibi, M.; Hawash, M.; Bdir, S.; Nacak Baytas, S. Targeting the kinetics mechanism of AMPA receptor inhibition by 2-oxo-3H-benzoxazole derivatives. Bioorg. Chem. 2022, 129, 106163. [Google Scholar] [CrossRef]
- Szymańska, E.; Nielsen, B.; Johansen, T.N.; Cuñado Moral, A.M.; Pickering, D.S.; Szczepańska, K.; Mickowska, A.; Kieć-Kononowicz, K. Pharmacological characterization and binding modes of novel racemic and optically active phenylalanine-based antagonists of AMPA receptors. Eur. J. Med. Chem. 2017, 138, 874–883. [Google Scholar] [CrossRef]
- Armstrong, N.; Gouaux, E. Mechanisms for Activation and Antagonism of an AMPA-Sensitive Glutamate Receptor: Crystal Structures of the GluR2 Ligand Binding Core. Neuron 2000, 28, 165–181. [Google Scholar] [CrossRef]
- El-Helby, A.-G.A.; Ayyad, R.R.; El-Adl, K.; Elwan, A. Quinoxalin-2 (1H)-one derived AMPA-receptor antagonists: Design, synthesis, molecular docking and anticonvulsant activity. Med. Chem. Res. 2017, 26, 2967–2984. [Google Scholar] [CrossRef]
- Qneibi, M.; Jaradat, N.; Hawash, M.; Olgac, A.; Emwas, N. Ortho versus Meta Chlorophenyl-2, 3-Benzodiazepine Analogues: Synthesis, Molecular Modeling, and Biological Activity as AMPAR Antagonists. ACS Omega 2020, 5, 3588–3595. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).