Circulating miRNA 122-5p Expression Predicts Mortality and Cardiovascular Events in Chronic Hemodialysis Patients: A Multicentric, Pilot, Prospective Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants’ Selection Criteria
2.2. Clinical and Laboratory Assessment
2.3. miRNA 122-5p Extraction and Quantification of Relative Expression
2.4. Prospective Follow-Up and Study Endpoint
2.5. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
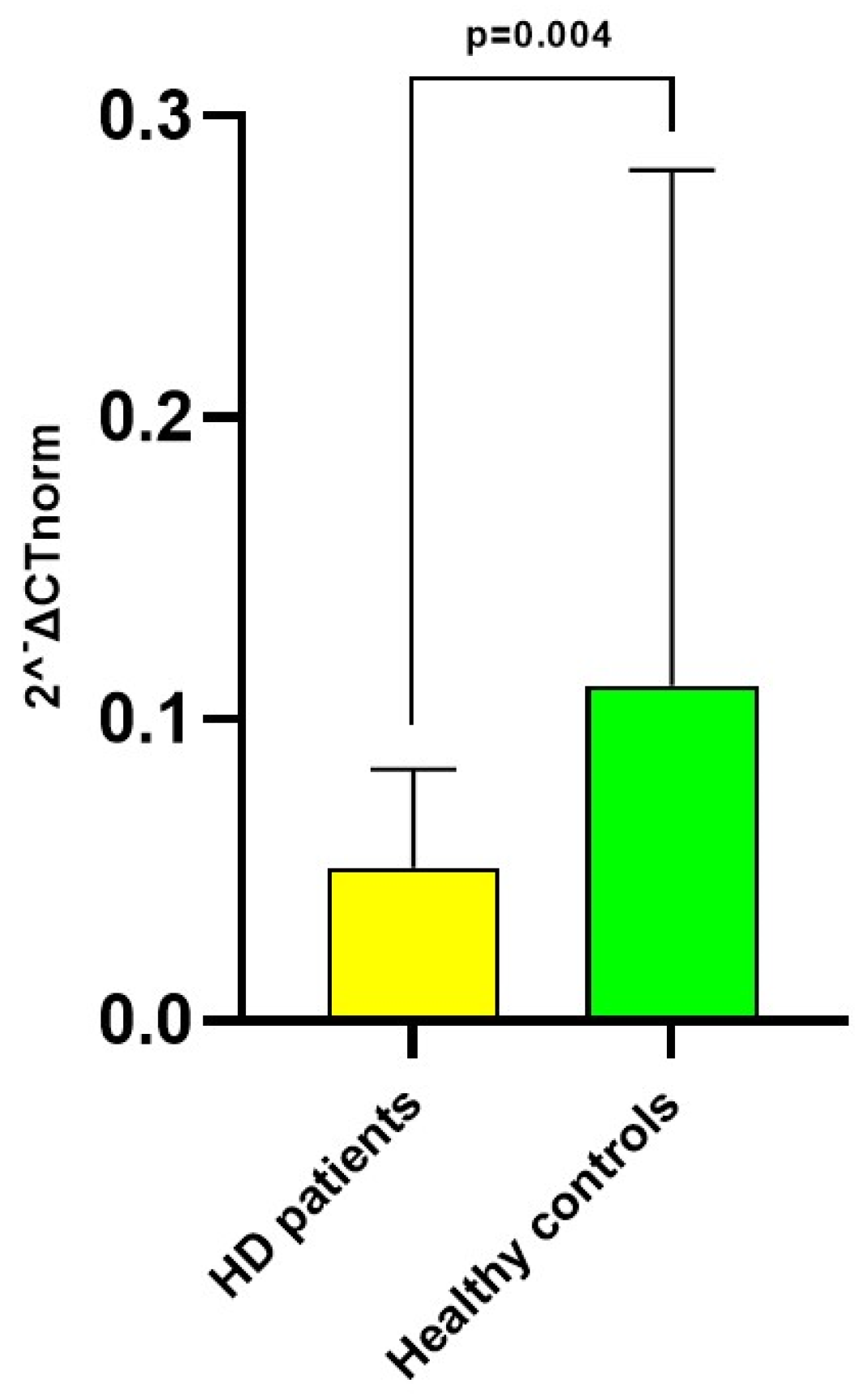
3.2. miRNA 122-5p Expression in Hemodialysis Patients
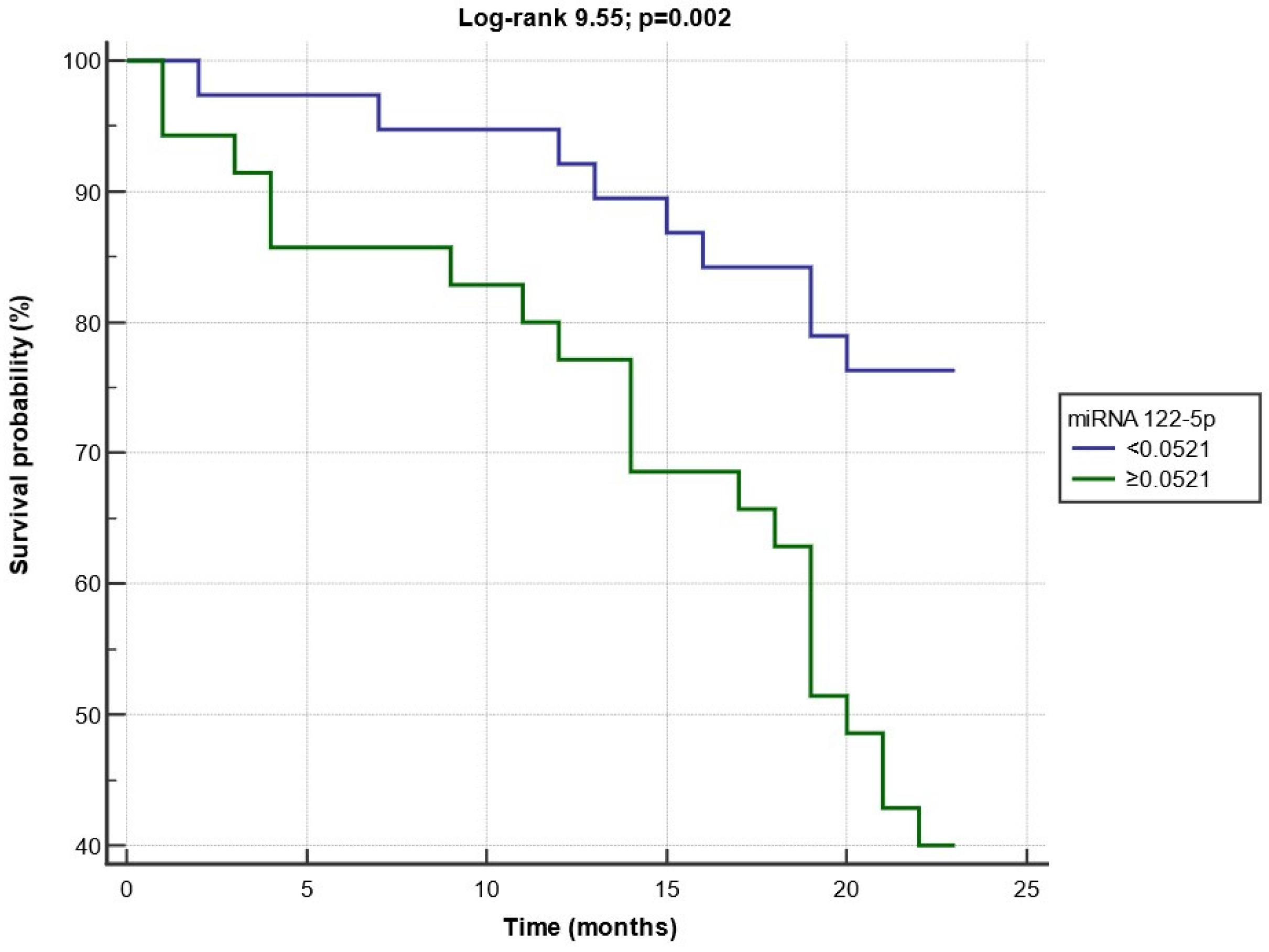
3.3. Composite Endpoint during the Follow-Up Period
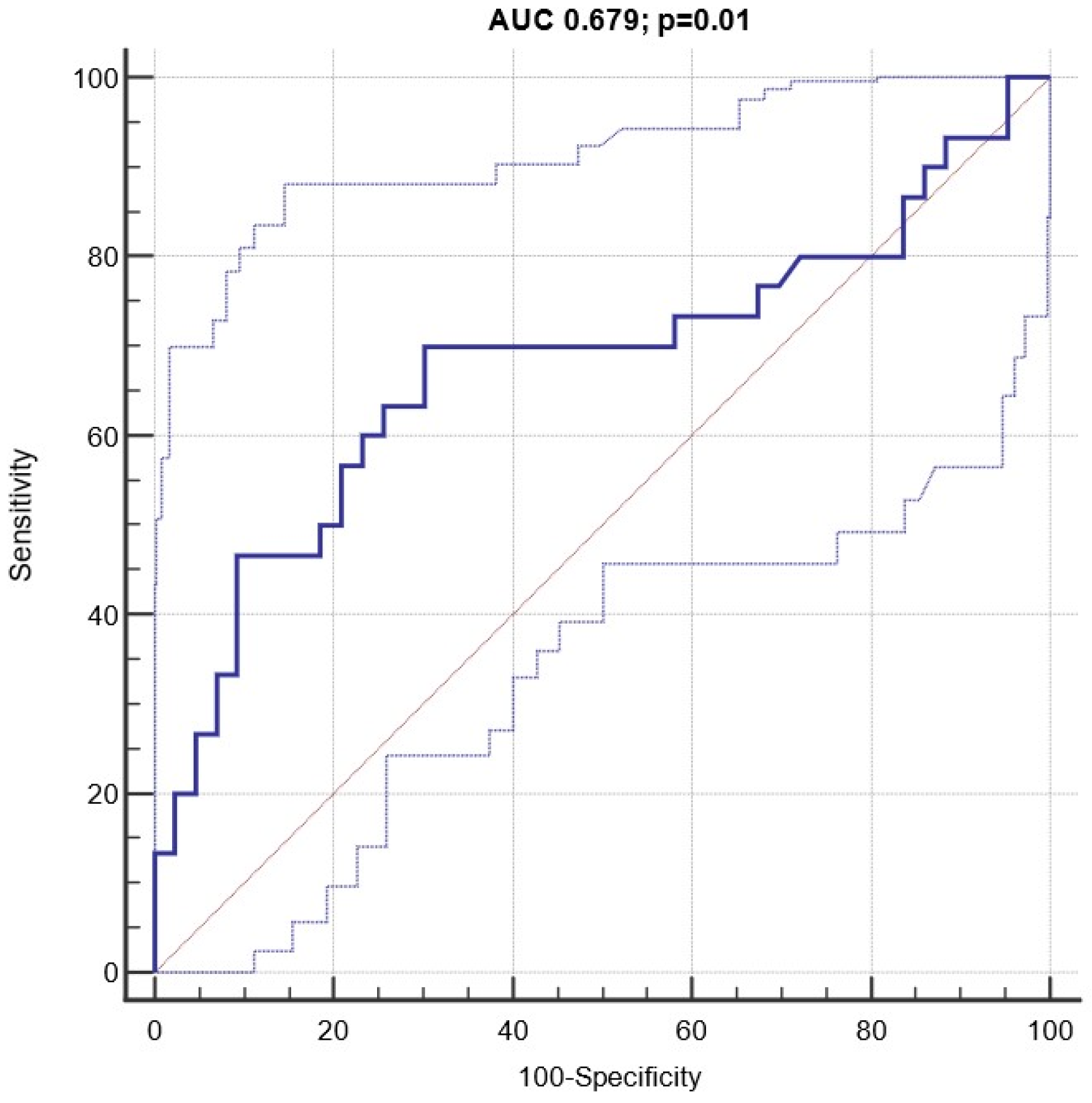
3.4. Diagnostic and Prognostic Capacity of Circulating miRNA 122-5p Expression
3.5. Cox Regression Analyses for the Composite Endpoint
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jankowski, J.; Floege, J.; Fliser, D.; Bohm, M.; Marx, N. Cardiovascular Disease in Chronic Kidney Disease: Pathophysiological Insights and Therapeutic Options. Circulation 2021, 143, 1157–1172. [Google Scholar] [CrossRef] [PubMed]
- Thompson, S.; James, M.; Wiebe, N.; Hemmelgarn, B.; Manns, B.; Klarenbach, S.; Tonelli, M. Cause of Death in Patients with Reduced Kidney Function. J. Am. Soc. Nephrol. 2015, 26, 2504–2511. [Google Scholar] [CrossRef] [PubMed]
- Zoccali, C. Traditional and emerging cardiovascular and renal risk factors: An epidemiologic perspective. Kidney Int. 2006, 70, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-Y.; Xi, Y.; Zhu, W.-N.; Zeng, C.; Zhang, Z.-Q.; Guo, Z.-C.; Hao, D.-L.; Liu, G.; Feng, L.; Chen, H.-Z.; et al. Positive regulation of hepatic miR-122 expression by HNF4α. J. Hepatol. 2011, 55, 602–611. [Google Scholar] [CrossRef]
- Esau, C.; Davis, S.; Murray, S.F.; Yu, X.X.; Pandey, S.K.; Pear, M.; Watts, L.; Booten, S.L.; Graham, M.; McKay, R.; et al. miR-122 regulation of lipid metabolism revealed by in vivo antisense targeting. Cell Metab. 2006, 3, 87–98. [Google Scholar] [CrossRef]
- Miyaaki, H.; Ichikawa, T.; Kamo, Y.; Taura, N.; Honda, T.; Shibata, H.; Milazzo, M.; Fornari, F.; Gramantieri, L.; Bolondi, L.; et al. Significance of serum and hepatic microRNA-122 levels in patients with non-alcoholic fatty liver disease. Liver Int. 2014, 34, e302–e307. [Google Scholar] [CrossRef]
- Kia, R.; Kelly, L.; Sison-Young, R.L.C.; Zhang, F.; Pridgeon, C.S.; Heslop, J.A.; Metcalfe, P.; Kitteringham, N.R.; Baxter, M.; Harrison, S.; et al. MicroRNA-122: A novel hepatocyte-enriched in vitro marker of drug-induced cellular toxicity. Toxicol. Sci. 2014, 144, 173–185. [Google Scholar] [CrossRef]
- Novák, J.; Olejníčková, V.; Tkáčová, N.; Santulli, G. Mechanistic Role of MicroRNAs in Coupling Lipid Metabolism and Atherosclerosis. Adv. Exp. Med. Biol. 2015, 887, 79–100. [Google Scholar] [CrossRef]
- Huang, X.; Huang, F.; Yang, D.; Dong, F.; Shi, X.; Wang, H.; Zhou, X.; Wang, S.; Dai, S. Expression of microRNA-122 con-tributes to apoptosis in H9C2 myocytes. J. Cell Mol. Med. 2012, 16, 2637–2646. [Google Scholar] [CrossRef]
- D’agostino, M.; Mauro, D.; Zicarelli, M.; Carullo, N.; Greco, M.; Andreucci, M.; Coppolino, G.; Bolignano, D. miRNAs in Uremic Cardiomyopathy: A Comprehensive Review. Int. J. Mol. Sci. 2023, 24, 5425. [Google Scholar] [CrossRef]
- Pofi, R.; Giannetta, E.; Galea, N.; Francone, M.; Campolo, F.; Barbagallo, F.; Gianfrilli, D.; Venneri, M.A.; Filardi, T.; Cristini, C.; et al. Diabetic Cardiomiopathy Progression is Triggered by miR122-5p and Involves Extracellular Matrix: A 5-Year Prospective Study. JACC Cardiovasc. Imaging 2021, 14, 1130–1142. [Google Scholar] [CrossRef] [PubMed]
- Cortez-Dias, N.; Costa, M.C.; Carrilho-Ferreira, P.; Silva, D.; Jorge, C.; Calisto, C.; Pessoa, T.; Martins, S.R.; de Sousa, J.C.; da Silva, P.C.; et al. Circulating miR-122-5p/miR-133b Ratio Is a Specific Early Prognostic Biomarker in Acute Myocardial Infarction. Circ. J. 2016, 80, 2183–2191. [Google Scholar] [CrossRef] [PubMed]
- Rincón, L.M.; Rodríguez-Serrano, M.; Conde, E.; Lanza, V.F.; Sanmartín, M.; González-Portilla, P.; Paz-García, M.; Del Rey, J.M.; Menacho, M.; Bermejo, M.-L.G.; et al. Serum microRNAs are key predictors of long-term heart failure and cardiovascular death after myocardial infarction. ESC Heart Fail. 2022, 9, 3367–3379. [Google Scholar] [CrossRef]
- Rivoli, L.; Vliegenthart, A.D.B.; de Potter, C.M.J.; van Bragt, J.J.M.H.; Tzoumas, N.; Gallacher, P.; Farrah, T.E.; Dhaun, N.; Dear, J.W. The effect of renal dysfunction and haemodialysis on circulating liver specific miR-122. Br. J. Clin. Pharmacol. 2016, 83, 584–592. [Google Scholar] [CrossRef]
- Bolignano, D.; Greco, M.; Presta, P.; Duni, A.; Vita, C.; Pappas, E.; Mirabelli, M.; Lakkas, L.; Naka, K.K.; Brunetti, A.; et al. A small circulating miR-NAs signature predicts mortality and adverse cardiovascular outcomes in chronic hemodialysis patients. Clin. Kidney J. 2023, 16, 868–878. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 1, 1–39.e14. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Neal, C.S.; Michael, M.Z.; Pimlott, L.K.; Yong, T.Y.; Li, J.Y.; Gleadle, J.M. Circulating microRNA ex-pression is reduced in chronic kidney disease. Nephrol. Dial. Transplant. 2011, 26, 3794–3802. [Google Scholar] [CrossRef]
- Emilian, C.; Goretti, E.; Prospert, F.; Pouthier, D.; Duhoux, P.; Gilson, G.; Devaux, Y.; Wagner, D.R. MicroRNAs in patients on chronic hemodialysis (MINOS study). Clin. J. Am. Soc. Nephrol. 2012, 7, 619–623. [Google Scholar] [CrossRef]
- Yasuda, K.; Okuda, K.; Endo, N.; Ishiwatari, Y.; Ikeda, R.; Hayashi, H.; Yokozeki, K.; Kobayashi, S.; Irie, Y. Hypoaminotransferasemia in patients undergoing long-term hemodialysis: Clinical and biochemical appraisal. Gastroenterology 1995, 109, 1295–1300. [Google Scholar] [CrossRef]
- Liberato, I.R.; Lopes, E.P.; Cavalcante, M.A.; Pinto, T.C.; Moura, I.F.; Loureiro Junior, L. Liver enzymes in patients with chronic kidney disease undergoing peritoneal dialysis and hemodialysis. Clinics 2012, 67, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Benfari, G.; Miller, W.L.; Antoine, C.; Rossi, A.; Lin, G.; Oh, J.K.; Roger, V.L.; Thapa, P.; Enriquez-Sarano, M. Diastolic Determinants of Excess Mortality in Heart Failure with Reduced Ejection Fraction. JACC Heart Fail. 2019, 7, 808–817. [Google Scholar] [CrossRef]
- Donal, E.; Lund, L.H.; Oger, E.; Hage, C.; Persson, H.; Reynaud, A.; Ennezat, P.; Bauer, F.; Drouet, E.; Linde, C.; et al. New echocardiographic predictors of clinical outcome in patients presenting with heart failure and a preserved left ventricular ejection fraction: A subanalysis of the Ka (Karolinska) Ren (Rennes) Study. Eur. J. Heart Fail. 2015, 17, 680–688. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Zhang, Z.; Dong, Z.; Liu, X.; Liu, Y.; Li, X.; Xu, Y.; Guo, Y.; Wang, N.; Zhang, M.; et al. MicroRNA-122-5p Aggravates Angioten-sin II-Mediated Myocardial Fibrosis and Dysfunction in Hypertensive Rats by Regulating the Elabela/Apelin-APJ and ACE2-GDF15-Porimin Signaling. J. Cardiovasc. Transl. Res. 2022, 15, 535–547. [Google Scholar] [CrossRef]
- Cengiz, M.; Karatas, O.F.; Koparir, E.; Yavuzer, S.; Ali, C.; Yavuzer, H.; Kirat, E.; Karter, Y.; Ozen, M. Differential expression of hypertension-associated microRNAs in the plasma of patients with white coat hyper-tension. Medicine 2015, 94, e693. [Google Scholar] [CrossRef]
- Ghafouri-Fard, S.; Shirvani-Farsani, Z.; Hussen, B.M.; Taheri, M.; Samsami, M. The key roles of non-coding RNAs in the pathophysiology of hypertension. Eur. J. Pharmacol. 2022, 931, 175220. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Peng, X.; Du, G.; Zhang, Z.; Zhai, Y.; Xiong, X.; Luo, X. MicroRNA-122-5p Inhibition Improves Inflammation and Oxidative Stress Damage in Dietary-Induced Non-alcoholic Fatty Liver Disease Through Targeting FOXO3. Front Physiol. 2022, 13, 803445. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, C.; Zhang, C.; Wang, Y.; Zhai, K.; Tong, Z. MicroRNA-122-5p regulates coagulation and inflammation through MASP1 and HO-1 genes. Infect. Genet. Evol. 2022, 100, 105268. [Google Scholar] [CrossRef]
- Wang, Y.; Chang, W.; Zhang, Y.; Zhang, L.; Ding, H.; Qi, H.; Xue, S.; Yu, H.; Hu, L.; Liu, D.; et al. Circulating miR-22-5p and miR-122-5p are promising novel biomarkers for diagnosis of acute myocardial infarction. J. Cell. Physiol. 2019, 234, 4778–4786. [Google Scholar] [CrossRef]
- Willeit, P.; Skroblin, P.; Kiechl, S.; Fernandez-Hernando, C.; Mayr, M. Liver microRNAs: Potential mediators and biomarkers for metabolic and cardiovascular disease? Eur. Heart J. 2016, 37, 3260–3266. [Google Scholar] [CrossRef]
- Ravel, V.; Streja, E.; Molnar, M.Z.; Rezakhani, S.; Soohoo, M.; Kovesdy, C.P.; Kalantar-Zadeh, K.; Moradi, H. Association of aspartate aminotransferase with mortality in hemodialysis patients. Nephrol. Dial. Transplant. 2015, 31, 814–822. [Google Scholar] [CrossRef] [PubMed]

| All HD Patients n = 74 | Endpoint—No n = 44 | Endpoint—Yes n = 30 | p | |
|---|---|---|---|---|
| Age (years) | 68.6 ± 12.3 | 66.6 ± 11.9 | 72.5 ± 12.5 | 0.04 |
| Males n (%) | 56 (75.7) | 33 (75) | 23 (76.7) | 0.90 |
| Dry weight (kg) | 71 ± 15.3 | 72.1 ± 16.8 | 71.7 ± 13.2 | 0.92 |
| BMI (kg/m2) | 25.4 ± 4.9 | 26.2 ± 5.2 | 25.1 ± 3.8 | 0.76 |
| Waist–hip ratio (cm) | 0.94 ± 0.12 | 0.92 ± 0.23 | 0.95 ± 0.19 | 0.69 |
| Type of dialysis | ||||
| - Hemodialysis n (%) | 41 (55.4) | 20 (45.4) | 21 (70) | 0.64 |
| - Hemodiafiltration n (%) | 33 (44.6) | 24 (54.5) | 9 (30) | 0.06 |
| Kt/V | 1.44 ± 0.27 | 1.46 ± 0.27 | 1.40 ± 0.28 | 0.29 |
| History of any CV disease | 36 (48.6) | 12 (27.3) | 18 (60) | 0.08 |
| Dialysis vintage (mo.) | 35.5 [17–68.2] | 35.5 [21.2–66.7] | 40 [15.5–71.2] | 0.76 |
| Diabetes n (%) | 20 (27) | 11 (25) | 9 (30) | 0.79 |
| Glycemia (mg/dL) | 113 ± 49.4 | 125 ± 42 | 140.5 ± 58.2 | 0.18 |
| Hemoglobin (g/dL) | 10.9 ± 1.1 | 11.1 ± 1.15 | 10.7 ± 1.1 | 0.11 |
| SBP (mmHg) | 139.6 ± 23.8 | 141.4 ± 19.4 | 136.9 ± 29.3 | 0.42 |
| DBP (mmHg) | 71.4 ± 12.2 | 73.7 ± 10.2 | 58 ± 14.2 | 0.04 |
| Pulse pressure (mmHg) | 68.1 ± 26.8 | 57.5 ± 23.2 | 76.8 ± 25.6 | 0.03 |
| Serum creatinine (mg/dL) | 8.2 [6.9–9.2] | 8.9 [7.5–9.9] | 8.60 [7–10.64] | 0.11 |
| Urea (mg/dL) | 133.1 ± 34 | 132.2 ± 32 | 134.9 ± 32.8 | 0.58 |
| Sodium (mg/dL) | 136.3 ± 15.2 | 135.6 ± 19.6 | 137.4 ± 3.1 | 0.60 |
| Potassium (mg/dL) | 4.97 ± 0.68 | 5.01 ± 0.65 | 4.91 ± 0.73 | 0.52 |
| Phosphate (mg/dL) | 4.7 ± 1.29 | 4.75 ± 1.24 | 4.65 ± 1.38 | 0.74 |
| Calcium (mg/dL) | 9.23 ± 0.64 | 9.30 ± 0.69 | 9.12 ± 0.57 | 0.26 |
| iPTH (pg/mL) | 251.9 [145.9–386] | 207 [129–255] | 239.8 [124.5–358.9] | 0.59 |
| Uric acid (mg/dL) | 5.8 ± 1.1 | 5.8 ± 1.04 | 5.82 ± 1.15 | 0.92 |
| Albumin (g/dL) | 3.97 ± 0.24 | 3.99 ± 0.19 | 3.94 ± 0.31 | 0.41 |
| CK-MB (UI/L) | 21 ± 8.1 | 18.8 ± 6.6 | 24.2 ± 9.2 | 0.02 |
| hs-cTnI (pg/mL) | 21.4 [11.6–61.7] | 19.3 [7.7–22.2] | 23.1 [20.6–53.6] | 0.04 |
| ALP (U/L) | 79 [62.7–88.5] | 79 [62–87] | 85.5 [67.5–92] | 0.23 |
| Total cholesterol (mg/dL) | 147.5 ± 45.1 | 141.8 ± 28.9 | 155.8 ± 61.3 | 0.19 |
| HDL (mg/dL) | 41.6 ± 10.1 | 40.9 ± 9.3 | 42.5 ± 11.3 | 0.50 |
| LDL (mg/dL) | 83.6 ± 33 | 77.9 ± 25.8 | 91.9 ± 30.5 | 0.05 |
| Triglycerides (mg/dL) | 126 [85.5–194.5] | 127 [99–173] | 140 [90.5–265.7] | 0.61 |
| AST (U/L) | 12.3 ± 4.4 | 11.8 ± 3.8 | 13 ± 5.2 | 0.57 |
| ALT (U/L) | 11.9 ± 5 | 11.3 ± 4.4 | 12.8 ± 5.9 | 0.66 |
| ESR (mm/h) | 29 [20–44.7] | 33 [25–47] | 29 [25.2–45.2] | 0.36 |
| C-reactive protein (mg/L) | 0.81 [0.23–3.23] | 0.30 [0.12–0.52] | 0.61 [0.11–0.93] | 0.23 |
| Fibrinogen (mg/dL) | 421.6 ± 100.9 | 414.8 ± 60.2 | 431.6 ± 54 | 0.58 |
| RBC (n × 103) | 3.70 ± 0.73 | 3.71 ± 0.50 | 3.69 ± 0.99 | 0.88 |
| WBC (n × 103) | 7.01 ± 3.30 | 7.17 ± 2.9 | 6.77 ± 1.94 | 0.60 |
| PLT (n × 103) | 219.2 ± 72.1 | 216.7 ± 75.6 | 222.8 ± 67.6 | 0.72 |
| Serum iron (mg/dL) | 70.9 ± 25.2 | 72.2 ± 25.9 | 69 ± 24.2 | 0.59 |
| TSAT (%) | 28 [20.2–34.3] | 28.2 [22.1–32.1] | 28.9 [23.1–34.5] | 0.88 |
| Ferritin (mcg/L) | 197.9 [103–318] | 178.3 [92–251] | 258.1 [129.7–341.8] | 0.32 |
| miRNA 122-5p (2−ΔCT) | 0.051 [0.019–0.083] | 0.034 [0.019–0.061] | 0.071 [0.022–0.106] | 0.01 |
| All HD Patients n = 74 | Endpoint—No n = 44 | Endpoint—Yes n = 30 | p | |
|---|---|---|---|---|
| LAVi (mL/m2) | 28.3 [21.9–42.8] | 26.3 [18.3–42.9] | 29.2 [23.6–41.9] | 0.05 |
| LAD (cm) | 3.7 [3.3–4.1] | 3.8 [3.4–4.5] | 3.7 [3.2–3.9] | 0.37 |
| LVEDVi (mL/m2) | 51.3 ± 21.9 | 50.2 ± 20 | 53.1 ± 25.2 | 0.17 |
| LVMi (g/m2) | 131.1 ± 36.8 | 128.8 ± 33.2 | 134.8 ± 41.3 | 0.05 |
| Ejection fraction (%) | 57.5 ± 9.1 | 58.9 ± 7.6 | 55.3 ± 10.9 | 0.11 |
| Vmax (m/s) | 2.5 [2.2–2.9] | 2.44 [2–2.78] | 2.49 [1.92–2.89] | 0.23 |
| TAPSE (mm) | 20 ± 4.8 | 21 [18–25] | 21 [19–26] | 0.97 |
| E/e’ | 13.2 ± 4.2 | 10.5 ± 3.6 | 16.2 ± 4.8 | 0.01 |
| Fractional shortening (%) | 37 ± 8.7 | 37.5 ± 7.9 | 36.2 ± 10.2 | 0.60 |
| RAVi (mL/m2) | 29.5 [14–25.3] | 18.1 [11.8–24.3] | 20.3 [15.9–24.9] | 0.23 |
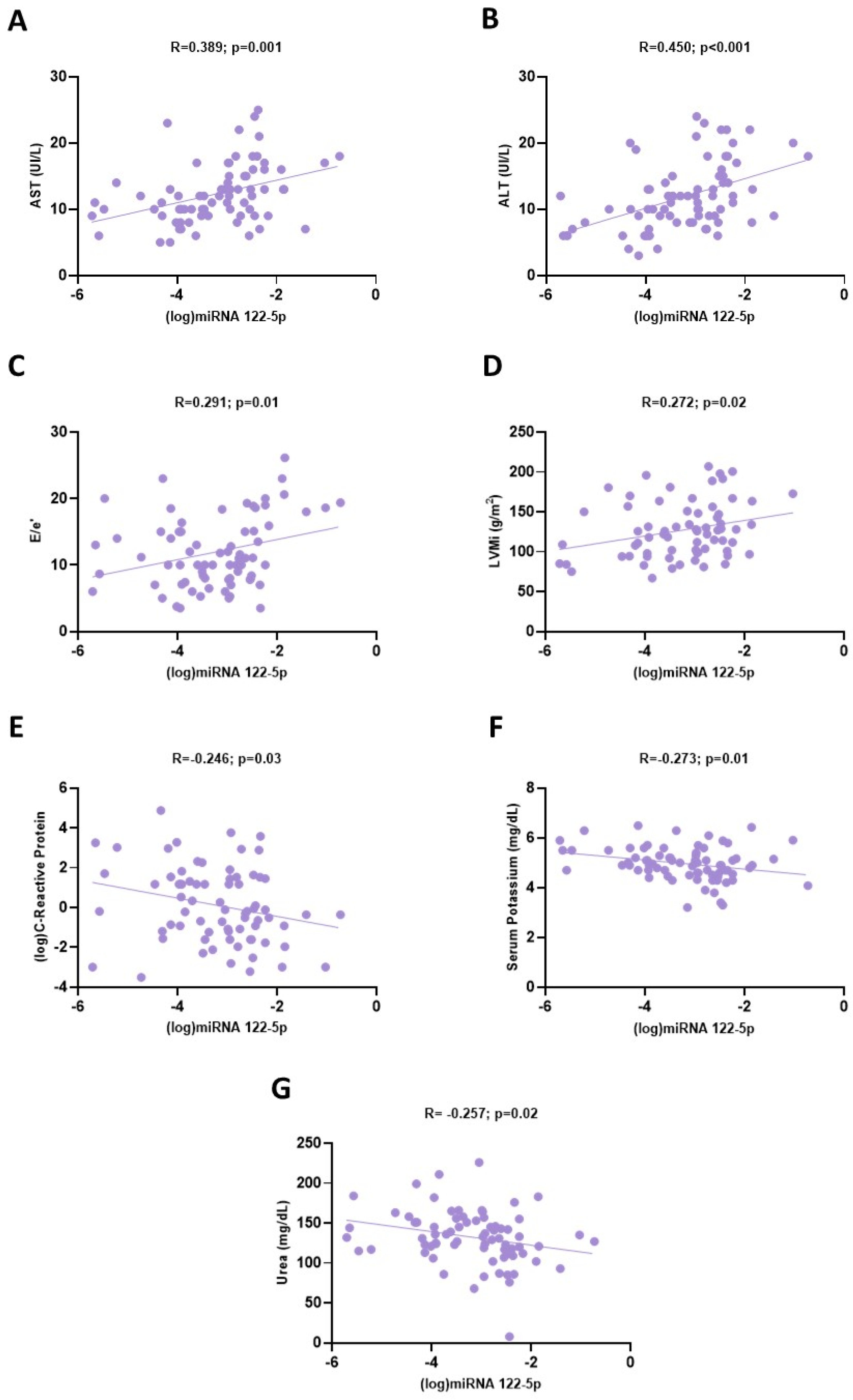
| Univariate R/Rho | p | Multivariate R2 (p) | Multivariate β | p | |
|---|---|---|---|---|---|
| Variable | 0.39 (<0.001) | ||||
| ALT | 0.450 | <0.001 | 0.333 | 0.02 | |
| E/e’ | 0.291 | 0.01 | 0.265 | 0.02 | |
| (log)C-reactive protein | −0.246 | 0.03 | −0.219 | 0.04 | |
| LVMi | 0.272 | 0.02 | 0.168 | 0.12 | |
| Serum potassium | −0.273 | 0.01 | 0.127 | 0.25 | |
| AST | 0.389 | 0.001 | 0.089 | 0.55 | |
| Urea | −0.257 | 0.02 | −0.047 | 0.67 |
| Units of Increase | HR | 95% CI | χ2 | p | |
|---|---|---|---|---|---|
| Univariate Analysis | |||||
| Pulse pressure | 1 mmHg | 1.026 | 1.008–1.044 | 8.003 | 0.005 |
| E/e’ | 1U | 1.155 | 1.081–1.234 | 18.343 | <0.001 |
| CK-MB | 1 UI/L | 1.062 | 1.014–1.112 | 6.618 | 0.01 |
| miRNA 122-5p | 2−ΔCT | 3.235 | 1.343–4.805 | 4.651 | 0.03 |
| Age | years | 1.036 | 0.994–1.081 | 2.754 | 0.09 |
| LAVi | mL/m2 | 1.016 | 0.983–1.049 | 1.137 | 0.34 |
| LVMi | g/m2 | 1.011 | 0.996–1.025 | 2.232 | 0.14 |
| LDL | mg/dL | 1.013 | 0.999–1.028 | 1.904 | 0.11 |
| hs-CTNi | pg/mL | 1.006 | 0.997–1.015 | 1.301 | 0.22 |
| Multivariate Analysis | |||||
| E/e’ | 1U | 1.085 | 1.028–1.146 | 8.736 | 0.003 |
| miRNA 30a-5p | 2−ΔCT (×103) | 1.115 | 1.009–1.232 | 4.576 | 0.03 |
| CK-MB | 1 UI/L | 1.007 | 0.997–1.018 | 2.008 | 0.15 |
| Pulse pressure | 1 mmHg | 1.013 | 0.988–1.038 | 1.084 | 0.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duni, A.; Greco, M.; Presta, P.; Arena, R.; Pappas, E.; Lakkas, L.; Naka, K.K.; Brunetti, A.; Foti, D.P.; Andreucci, M.; et al. Circulating miRNA 122-5p Expression Predicts Mortality and Cardiovascular Events in Chronic Hemodialysis Patients: A Multicentric, Pilot, Prospective Study. Biomolecules 2023, 13, 1663. https://doi.org/10.3390/biom13111663
Duni A, Greco M, Presta P, Arena R, Pappas E, Lakkas L, Naka KK, Brunetti A, Foti DP, Andreucci M, et al. Circulating miRNA 122-5p Expression Predicts Mortality and Cardiovascular Events in Chronic Hemodialysis Patients: A Multicentric, Pilot, Prospective Study. Biomolecules. 2023; 13(11):1663. https://doi.org/10.3390/biom13111663
Chicago/Turabian StyleDuni, Anila, Marta Greco, Pierangela Presta, Roberta Arena, Ethymios Pappas, Lampros Lakkas, Katerina K. Naka, Antonio Brunetti, Daniela Patrizia Foti, Michele Andreucci, and et al. 2023. "Circulating miRNA 122-5p Expression Predicts Mortality and Cardiovascular Events in Chronic Hemodialysis Patients: A Multicentric, Pilot, Prospective Study" Biomolecules 13, no. 11: 1663. https://doi.org/10.3390/biom13111663
APA StyleDuni, A., Greco, M., Presta, P., Arena, R., Pappas, E., Lakkas, L., Naka, K. K., Brunetti, A., Foti, D. P., Andreucci, M., Coppolino, G., Dounousi, E., & Bolignano, D. (2023). Circulating miRNA 122-5p Expression Predicts Mortality and Cardiovascular Events in Chronic Hemodialysis Patients: A Multicentric, Pilot, Prospective Study. Biomolecules, 13(11), 1663. https://doi.org/10.3390/biom13111663












