Abstract
Multiple myeloma (MM) is a dyscrasia of plasma cells (PCs) characterized by abnormal immunoglobulin (Ig) production. The disease remains incurable due to a multitude of mutations and structural abnormalities in MM cells, coupled with a favorable microenvironment and immune suppression that eventually contribute to the development of drug resistance. The bone marrow microenvironment (BMME) is composed of a cellular component comprising stromal cells, endothelial cells, osteoclasts, osteoblasts, and immune cells, and a non-cellular component made of the extracellular matrix (ECM) and the liquid milieu, which contains cytokines, growth factors, and chemokines. The bone marrow stromal cells (BMSCs) are involved in the adhesion of MM cells, promote the growth, proliferation, invasion, and drug resistance of MM cells, and are also crucial in angiogenesis and the formation of lytic bone lesions. Classical immunophenotyping in combination with advanced immune profiling using single-cell sequencing technologies has enabled immune cell-specific gene expression analysis in MM to further elucidate the roles of specific immune cell fractions from peripheral blood and bone marrow (BM) in myelomagenesis and progression, immune evasion and exhaustion mechanisms, and development of drug resistance and relapse. The review describes the role of BMME components in MM development and ongoing clinical trials using immunotherapeutic approaches.
1. Introduction
B-cell development and maturation is a tightly regulated process. Throughout adult life, the bone marrow (BM) serves as the primary hotspot for a finite number of pluripotent hematopoietic stem cells (HSCs) to sequentially give rise to all the blood cell types through a process called hematopoiesis (Figure 1).
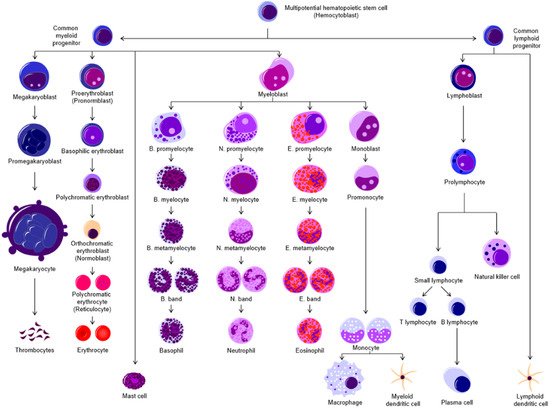
Figure 1.
Hematopoiesis is the process by which all the blood cell types are produced through differentiation from increasingly lineage-committed precursor cells in the adult BM. Broadly, the cells can be classified as myeloid or lymphoid in origin. The myeloid lineage gives rise to thrombocytes (platelets), erythrocytes, granulocytes (mast cells, basophils, neutrophils, eosinophils), macrophages, and myeloid-derived dendritic cells (DCs). The lymphoid lineage produces T- and B-lymphocytes, natural killer (NK) cells, and lymphoid DCs.
Immature plasma cells (PCs) originate from the lymphoid progenitor lineage [1,2], express a surface B-cell immunoglobulin receptor (BCR), and move to secondary lymphoid organs [3,4]. Here, the B-cells start to mature while remaining non-proliferative and transcriptionally dormant but primed for antigen recognition through the toll-like receptors (TLR) or the BCR [3,5]. B-cell activation can then occur in a T-cell-independent or dependent manner to generate acute or long-term immunity. The latter process involves complex activation by cytokines and affinity maturation in the germinal center of the lymph nodes. During activation and differentiation, B-cells are equipped to express a host of antibodies through the physiological process of class-switch recombination and somatic hypermutation [3,5,6]. However, these processes are highly error-prone and can cause the accumulation of genomic changes such as chromosomal translocations and aneuploidies [3,5]. Such translocations can place proto-oncogenes under the control of powerful enhancers in the Ig gene locus which can create aberrant PCs that can establish multiple myeloma (MM) precursor conditions such as monoclonal gammopathy of uncertain significance (MGUS) and smoldering MM (SMM) [5,7,8,9].
In MM, a clonal subset of these transformed post-germinal-center B-cells undergoes malignant proliferation in the favorable microenvironment of the BM (Figure 2) to produce abnormal levels of Igs that are secreted into the serum and urine and termed as Bence-Jones proteins [9,10]. Monoclonal antibody accumulation in the BM can cause anemia, hypercalcemia and lytic bone lesions with accompanying bone pain and fractures, and impaired kidney function leading to renal failure [6,8,9]. These bone- and organ-damaging events are characteristic features of MM, often abbreviated as “CRAB” which stands for hyperCalcemia, Renal involvement, Anemia, and Bone lesions [8,9].
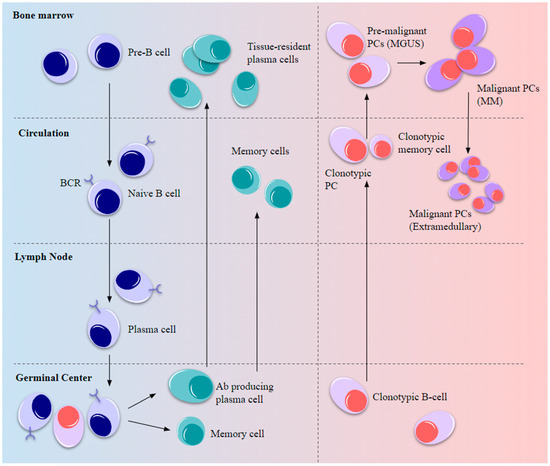
Figure 2.
Normal B-cell development and myelomagenesis. Upon BCR activation by antigenic stimulus, B-cell precursors migrate from the BM to peripheral lymph nodes to elicit a short-term immune response and the remaining cells differentiate into long-lived circulating plasma and memory cells, eventually becoming resident cells in the tissue niches. Some of the post-germinal-center B-cells carrying oncogenic mutations enter the circulation as plasmablasts and memory B-cells that migrate to the BM as pre-malignant cells to establish MGUS. Further advantageous oncogenic mutations and the favorable BMME drive malignancy and MM development after which the cells become niche-independent and re-enter the circulation, resulting in extramedullary plasmacytoma.
Epidemiology, Diagnosis, and Cytogenetics in Multiple Myeloma
As of 2020, 176,404 new cases of MM were reported globally, accounting for close to 1% of all newly diagnosed cancer cases and 1.2% of all cancer-related deaths [11]. MM is often labelled as a neoplasm of ageing, with the age at diagnosis often being ≥ 65 years [9,12,13]. Very rarely, MM has been reported in individuals younger than 30 years of age at frequencies of 0.02% to 0.3% of all diagnosed cases [14]. In 1979, the Durie-Salmon staging system was developed for evaluating MM based on the presence of anemia and bone disease [15,16]. The International Myeloma Working Group defines the International and Revised International Staging systems (ISS and R-ISS) for MM based on either clinical and laboratory parameters only (ISS) or in combination with cytogenetic abnormalities (R-ISS) [17,18]. Chromosomal aneuploidy is a complex yet distinguishing feature in MM, with the disease often presenting as hyperdiploid or non-hyperdiploid, involving translocations with the IgH locus [19,20]. Fluorescent in situ hybridization (FISH) is most commonly employed to detect cytogenetic abnormalities in MM [19,20,21]. Hyperdiploidy is observed in nearly half of MM cases and is associated with a good prognosis [5,22,23]. Primary reciprocal translocations specific to B-cell chromosomal rearrangements, observed in nearly half the MGUS and MM cases, are early events in myeloma pathogenesis and are considered high-risk (HR) abnormalities as they bring oncogenes, mainly cyclins, fibroblast growth factor receptor (FGFR) 3, multiple myeloma SET domain (MMSET), and musculoaponeurotic fibrosarcoma (c-MAF), under the control of powerful IgH enhancers [5,22,24,25,26]. Secondary genetic alterations indicate disease progression, generally do not involve the IgH locus, and may or may not be clonal. Further, these changes are seen in a non-hyperdiploid background with reduced dependence on the BMME and often indicate a poor prognosis (Figure 3) [5,25]. Detailed classification and staging based on clinical and cytogenetic parameters are listed in Table 1.
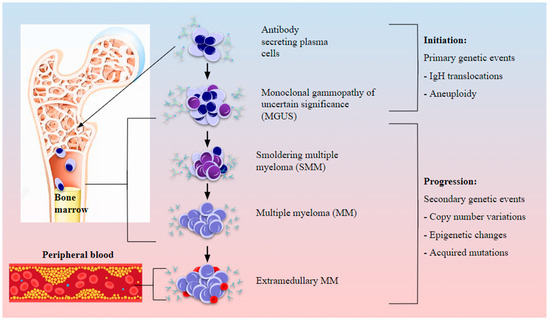
Figure 3.
Cytogenetic abnormalities and involvement of the BMME in myeloma initiation and progression. Abnormal PCs in the BM harbor chromosomal abnormalities such as translocations and aneuploidy which could establish the precursor condition of monoclonal gammopathy of uncertain significance (MGUS). BM involvement and modification of the immune landscape by myeloma cells sets off myeloma genesis, first as smoldering MM (SMM), and with the accumulation of secondary genetic abnormalities such as CNVs and epigenetic changes as well as oncogenic driver mutations, active MM is established which eventually progresses to aggressive extramedullary plasmacytoma upon bone lysis and egress from the marrow to peripheral blood.

Table 1.
Staging of myeloma based on myeloma-defining events, cytogenetic abnormalities, and prognosis [27,28,29,30,31,32,33,34,35,36,37,38]. * CRAB criteria defined by the International Myeloma Working Group (IMWG) refer to organ involvement based on hyperCalcemia (serum calcium > 10.5 mg/dL), Renal impairment (serum creatinine > 2 mg/dL), Anemia (hemoglobin < 10 g/dL), and Bone lesions (osteolysis or osteoporosis on any skeletal examination). LR = Low Risk, SR = Standard Risk, IR = Intermediate Risk, HR = High Risk, IS = Improved Survival, AP = Adverse Prognosis for progression-free/overall survival.
2. Marrow, Microenvironment, and Multiple Myeloma
The BMME comprises an extracellular matrix (ECM), blood vessels, cells from the hematopoietic lineage, supporting cells required for the regulation of hematopoiesis, and cells from the osteo-lineage [39,40,41]. Normal hematopoiesis occurs in two distinct niches in the BM: the central niche which is in the core of the BM and the endosteal niche which is close to the surface of the bone and the terminal epiphysis containing the trabecular marrow (Figure 4) [40,42].
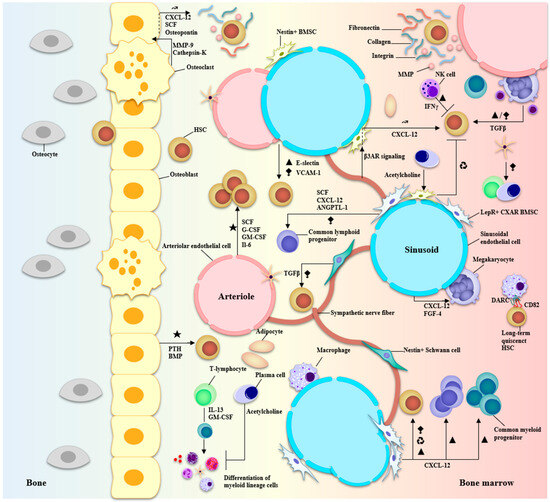
Figure 4.
Anatomy of the BMME and niche interactions of BM-resident cells during hematopoiesis. Two major niches can be described in the bone marrow: vascular and endosteal. The vascular niche is at the core of the bone marrow comprising both arteriolar and sinusoidal vessels and is associated with LepR+ or nestin+ BMSCs and CXAR cells, macrophages, megakaryocytes, sympathetic nerve fibers, Schwann cells, endothelial cells, and adipocytes. The endosteal niche is closer to terminal bone and comprises osteolineage cells—osteoblasts and osteoclasts—involved in bone formation and recycling. Secreted growth factors, cytokines, CAMs, and ECM molecules participate in an intricate signaling network to regulate HSC quiescence and differentiation/proliferation programs. The major factors involved are CXCL-12, TGF-β, G-CSF, GM-CSF, MMPs, collagens, and integrins. Particularly, the Schwann cells and stromal cells associated with the vasculature help in the regulation of HSC migration and differentiation while cells at the endosteal surface promote HSC quiescence and maintenance of their self-renewal capacity. ★ Activation of hematopoiesis. ▲ proliferation. ⧪ retention in the BM ↝ mobilization ♻ recycling.
Hematopoiesis starts in the embryonic stage, primarily to produce red blood cells from transitory, non-renewing erythroid progenitor cells that carry oxygen to tissues during growth and development. This is the “primitive” phase of hematopoiesis [43,44]. As embryonic development progresses, the primitive wave allows definitive hematopoiesis to begin in various fetal niches, giving rise to erythroid-myeloid progenitor cells [43,44]. HSCs progressively move into the fetal liver and then the BM, which is the primary site for adult hematopoiesis [1,44]. The HSCs generate increasingly lineage-committed progenitor cells that give rise to all the blood cells (Figure 1). The first line of cells are the common lymphoid progenitors (CLP) and the common myeloid progenitors (CMP) [45]. Subsequent differentiation of CLP gives rise to B and T lymphocytes, which are integral for antigen-specific adaptive immunity, and to natural killer (NK) [46] cells, which are part of the innate immune system. CMPs differentiate into erythroid/platelet progenitors which produce the red blood cells (erythrocytes) and platelets (thrombocytes), and granulocyte-macrophage progenitors which produce granulocytes (neutrophils, eosinophils, basophils, and mast cells), dendritic cells (DCs), and monocytes, which differentiate into macrophages [45,47].
Disruptions to genome integrity, transcription efficiency, and cell-specific protein expression capacity affect several cellular processes including hematopoiesis [48]. Such events can drive the malignant transformation of BM cells and alter the environment in a manner disruptive to normal cells but favorable for their own survival and proliferation [49]. A double-edged hypothesis is suggested by several studies whereby both the microenvironment and the hematopoietic cells themselves are suggested to initiate or promote malignancy [42,50,51,52]. Cells of the hematological lineage and the BMME can co-evolve to promote tumorigenesis, cancer progression, and the development of drug resistance. In MM, the BMME exhibits distinct, stage-specific profiles (Figure 5).
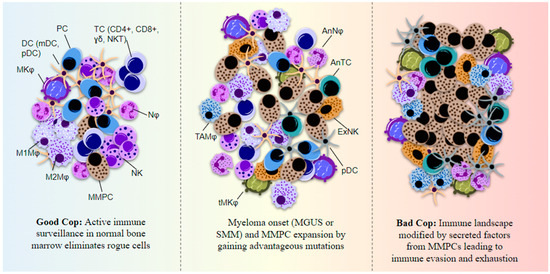
Figure 5.
Immune cell function in myeloma BM is altered by secreted factors from the MMPCs and the cells lose their normal immune surveillance capacity. An immunosuppressive state is then established, helping MMPCs evade immune system detection. The immune cells show features of exhaustion and anergy. Myeloma cells primarily use secreted IL-6 and TGF-β to disrupt normal immune system activation and express cell adhesion molecules (CAMs) such as PSGL-1, ICAM-1, and VLA-4 which helps in attachment to various immune cells as well as the BMSCs or ECM. PD-L1 is another surface-expressed ligand on MMPCs that can bind NK- and T-cell programmed cell death protein (PD)-1 to suppress their cytotoxic functions and cause immune exhaustion. MMPCs also express CXCL-12 which results in macrophage polarization to M2 or the tumor-associated macrophage phenotype. MMPC-derived cytokines including IL-6 and IL-10, along with secreted M-CSF and VEGF, affect DC maturation and proliferation in the BM. PC = plasma cell, TC = T-cell, NKT = natural killer-like T-cell, Nφ = neutrophil, NK = natural killer cell, MMPC = multiple myeloma plasma cell, Mφ = macrophage, MKφ = megakaryocyte, TAMφ = tumor-associated macrophage, tMKφ = transformed megakaryocyte, DC = dendritic cell, mDC = monocytic DC, pDC = plasmacytoid DC, ExNK = exhausted NK, AnNφ = anergic Nφ, AnTC = anergic TC.
2.1. ECM Components
The ECM proteins in the BMME are fibronectin, collagens, laminin, tenascin, thrombospondin, and elastin. The ECM also contains other glycoproteins, cell adhesion molecules (CAMs), proteases, and growth factors required for tissue scaffolding and homeostasis [53,54,55,56,57]. Glycoproteins and collagens help in the adhesion and expansion of HSCs and support the development, proliferation, and maturation of B lymphocytes and erythroid lineage cells. Fibronectin provides anchorage for hematopoietic progenitor cells and plays a crucial role in myelopoiesis and thrombopoiesis [46,58,59]. Proteoglycans, particularly heparan sulphate, are important for differentiation, lineage specification, and homing during hematopoiesis [60,61,62,63]. CAMs and integrins are essential for homing and regulating the survival and proliferation of HSCs [56,64,65]. Serine proteases such as CD26, neutrophil serine proteases, and matrix metalloproteases (MMPs), especially MMP-9, are required for the mobilization of HSCs during hematopoiesis by cleavage of interactions with specific adhesion molecules such as integrins, vascular CAMs (VCAMs), and fibronectin, and also in association with growth factors like granulocyte colony-stimulating factor (G-CSF) and granulocyte-monocyte colony-stimulating growth factor (GM-CSF) [66,67].
Transcriptomic analysis of MM cells showed enrichment for genes associated with the ECM [68]. Dysregulation of fibronectin, collagens, and lysyl oxidase (LOX) leads to BM fibrosis and is a poor prognostic factor and indicator of disease progression in hematological malignancies, particularly MM [68,69,70,71]. Fibronectin binding by MM cells triggers the activation of nuclear factor (NF)-κB signaling and the development of cell adhesion-mediated drug resistance (CAMDR) [23,69]. Collagen expression varies with disease stage, with the BM of MM patients having high plasmacytosis showing lower levels of collagen I but increased collagen IV compared to MGUS and normal BM [69]. As the disease progresses, collagens I, II, and III are re-expressed, indicating supportive roles in aggressive disease [68]. MMP-2 and MMP-9 are known to be overexpressed in MM and cause lytic bone lesions by degrading collagens I and IV [72,73]. Drug-resistant phenotypes were observed with MM patient BM cells cultured on fibronectin or BM stromal cells (BMSCs), alongside increased expression of CAMs in those with post-chemotherapeutic drug resistance. Further, tumor necrosis factor (TNF)-mediated apoptosis resistance was also observed from the cells cultured with BMSCs but not fibronectin [69]. Serglycin is an intracellular proteoglycan exclusively found in immature myeloid cells in the BM and is found to be upregulated in MMPCs where it could potentially create a pro-inflammatory environment and alter bone development [74]. Increased levels of laminin and laminin receptors mediate progression from MGUS to MM and promote migration [75].
2.2. Vascular Compartment
The vascular niche of the BM consists of a network of blood vessels arising from BM-resident endothelial cells (BMECs) and perivascular stromal cells, which play critical roles in cellular trafficking, hematopoiesis, and osteogenesis [76,77,78,79,80]. Hematopoiesis in the BM also depends on blood vessel permeability [77].
2.2.1. Vasculature-Associated Cells
Maintenance of adult HSCs is mediated through surface expression of CAMs like C-X-C motif chemokine ligand (CXCL)-12, nestin, and melanoma CAM (MCAM) (CD146), expressed differentially by BMECs and BMSCs [77,78,80,81,82,83]. Cytokines and CAMs secreted by different subsets of BMECs are important for the regulation of homing and the differentiation of HSCs. E-selectin helps in the homing of HSCs to the BM and proliferation, while VCAM-1 is essential for HSC retention [76,79,84]. Platelet endothelial CAM-1 (PECAM-1) (CD31)-expressing endothelial cells are found in the BM across all developmental stages and are also expressed on the surface of both myeloid and pre-B-lymphoid hematopoietic progenitor cell subsets [85]. In mice, CD31+ microvascular endothelial cells have been shown to rescue short- and long-term multilineage hematopoiesis following lethal irradiation of the BM [86]. Endothelial cell-secreted cytokines have both pro- and anti-hematopoietic activity. Stem cell factor (SCF), GM-CSF, interleukin (IL)-6, IL-1α, IL-11, and G-CSF are reported to stimulate hematopoiesis while thymosin-β4 is an inhibitor [87,88]. BMEC-secreted chemokines such as CXCL-12 and fibroblast growth factor (FGF)-4 are important for the localization of megakaryocyte progenitors to the vascular niche for the production, maturation, and release of platelets through VCAM-1 and VLA-4 signaling [89,90]. Granulopoiesis and lymphopoiesis are supported by BMSCs [80,91]. Leptin receptor (LepR)+ BMSCs, including skeletal stem cells, are rare during early development but are more abundant in adulthood and produce high levels of SCF, CXCL-12, and angiopoietin-like 1 (ANGPTL-1), which are required for HSC maintenance [80,92]. A subset of lymphoid progenitors is found in the periarteriolar niche and depend on secreted SCF from LepR + osteolectin+ BMSCs for their maintenance in this niche. Another subset of LepR+ BMSCs also secretes IL-7 for early lymphoid progenitors. However, the exact niche occupied by them is uncertain [80,92].
BMECs are “activated” in MM conditions, contributing to angiogenesis, immune escape, ECM adhesion, migration, proliferation, and entry of myeloma cells into circulation [93]. Secretion of cytokines and growth factors like vascular endothelial growth factor (VEGF), IL-6, and insulin-like growth factor (IGF)-1 by BMECs support MM cell growth in the BMME [94]. CAMs such as homing CAM (HCAM), very late-activation antigen (VLA)-4, intracellular CAM (ICAM), and neural CAM (NCAM), produced by MMPCs and their regulatory partners like integrins, cadherins, and IgCAMs, are critical for progression from precursor conditions to active myeloma [95,96,97]. Although CAMs appear to be required for PC homing to the BM, expression of NCAM and lymphocyte function-associated antigen (LFA)-3 was notably higher in MMPCs while selectin-1 and VLA-2 were absent [95]. CXCL-12 produced by BMSCs is important for neovascularization and ECM adhesion of MM cells, invasion, and chemoresistance [98,99]. VEGF is important in BMEC differentiation and crucial for MMPC migration, with increased levels detected in aggressive MM plasma compared to earlier stages and correlated to higher levels of circulating endothelial progenitors, indicating a role in BMEC recruitment for neovascularization [100,101,102]. VEGF receptor 2 (VEGFR2) has been targeted in a murine MM model and shown to inhibit angiogenesis and tumor growth [103]. E-selectin is essential for homing and maintaining MMPCs in the supportive BMME and evading bortezomib cytotoxicity, with higher levels observed in advanced-stage and drug-refractory MM [95]. MMPCs attach to BMSC fibronectin and collagen via ligands such as VLA-4 and syndecan-1, respectively, and attachment with BMECs is facilitated by CD44 interaction with endothelial hyaluronic acid [95].
2.2.2. The Blood Vessel Niches
Two distinct types of blood vessels can be identified in the BM: arterial (CD31 high/CD45−/Sca-1 high/Nestin+) and sinusoidal (CD31+/CD45−/Sca-1 low/Nestin−) [77]. Highly quiescent HSCs are found close to the endosteal surface and away from regions with rapid blood flow [104]. Nestin+ non-myelinating Schwann cells have been found in the arteriolar niche, where they help to maintain hibernating HSCs through activation of the transforming growth factor (TGF)-β pathway [76,77,105]. Further, Nestin+ cells in arterial blood vessels co-localize with HSCs and serve as a site for leukocyte trafficking and homing to the BM [76,77,81]. Knockout studies have shown that multipotent Nestin, CXCL-12, SCF, and LepR+ stromal cells, which localize near the sinusoidal and arteriolar endothelium, are essential for supporting hematopoietic cell maintenance and differentiation [106,107,108]. Sinusoidal megakaryocytes appear to have dual roles in regulating HSCs: maintenance of HSC quiescence via CXCL-4 and TGF-β [109,110] while supporting proliferation of HSCs via FGF-1 [109,111].
In hematological malignancies, tumor cells have been shown to compete with HSCs for niche occupancy in the early stages and eventually evolve niche independence with progression [112,113]. Like normal PCs, MMPCs also employ CXCR-4-CXCL-12 signaling to migrate via sinusoidal vessels to the BM [114]. P-selectin glycoprotein ligand 1 (PSGL1) expressed on MMPCs interacts with endothelial P-selectin and facilitates the “rolling” of MM cells in BM microvasculature [114]. It has been hypothesized that reduced CXCR-4 expression on MMPCs, which is also shown to occur with bortezomib treatment, promotes their egress from the BM to peripheral blood to establish extramedullary disease by detaching MMPCs from BMSCs [115].
2.3. Osteolineage Cells
Mesenchymal stem cells produce the osteolineage cells—chondrocytes, adipocytes, and osteoblasts—which have important roles in hematopoiesis [105,116,117,118]. One of the major complications in MM is lytic bone disease which occurs due to imbalances in osteolineage cells, with hyperactive osteoclasts and decreased osteoblast activity.
2.3.1. Osteoblasts
Endosteal osteoblasts are known to be an HSC niche [116,118,119]. Osteoblasts promote adult HSC proliferation and maturation via parathyroid hormone (PTH) and bone morphogenic protein (BMP) signaling [120,121,122]. Osteoblast-secreted cathepsin-X degrades CXCL-12, which is required for HSC engraftment, homing, maintenance, and mobilization in the BM niches [123]. In mice, depletion of osteoblasts caused niche migration of HSCs from the BM to the spleen and liver and showed impairment in B lymphopoiesis [124,125]. However, emerging evidence indicates that mature N-cadherin-expressing osteoblasts are not essential for maintaining adult hematopoiesis in the BM [126,127,128]. CD146+ stromal osteoprogenitor cells produce angiopoietin-1 (ANGPT-1), which is important for maintaining the vascular HSC niche [129].
MM cells residing in the osteoblastic niche are protected from apoptosis, have more oncogenic potential, and inhibit osteoblastogenesis [130]. Stimulation of osteoblasts with PTH-1 or bortezomib has been shown to block cell growth in MM model systems [131,132]. Osteoblasts secrete decorin-A to induce cell cycle arrest and apoptosis of myeloma cells and recruit immune cells to the BM for potential anti-tumor activity. In contrast, inhibition of osteoblast growth by myeloma cells is achieved through DKK-1 signaling [133]. Cell-to-cell contact between VLA-4 on myeloma cells and VCAM-1 on osteoblast progenitors suppresses Runt-related transcription factor 2 (RUNX2)/core binding factor-α1 (CBFA1)-mediated transcription and inhibits osteoblast formation [134,135].
2.3.2. Osteoclasts
Osteoclasts are bone-chewing cells that secrete enzymes such as MMP-9 and cathepsin K, which degrade CXCL-12, SCF, and osteopontin on the osteoblast surface and promote HSC mobilization [116,136]. Osteoclasts play an indirect role in B-lymphocyte development by mobilizing their progenitors to BM niches via expression of CXCL-12 and IL-7 in stromal cells [137]. Another indirect mechanism of hematopoietic regulation by osteoclasts is by modulating osteoblast differentiation [138]. It has been suggested that osteoclasts form an HSC niche, based on observations from a mouse model where mice depleted in osteoclasts developed osteopetrosis that caused extramedullary hematopoiesis [138,139,140].
It is well known that osteolytic activity is abnormally increased in MM leading to lytic bone disease, fractures, and release of growth factors outside the bone, thereby promoting aggressive disease [133,141,142]. VLA-4–VCAM-1 contacts are essential not only in the suppression of osteoblastogenesis but also for increased osteoclast activity [134]. MM cells cause upregulation of receptor activator of NF-κB (RANKL) and downregulation of osteoprotegerin (OPG) secretion by BM-MSCs (via VLA-4 interactions) and osteoblasts, leading to osteoclast differentiation and hyperactivity [143,144]. Other cytokines and factors overexpressed in the MM BM such as macrophage inflammatory protein (MIP)-1α and IL-3 promote osteoclastogenesis and inhibit osteoblast formation in a stage-dependent manner [141]. Proteasome inhibitors (PIs) are also helpful in targeting osteoclasts via inhibition of nuclear factor (NF)-κB and P38/MAPK signaling and pro-inflammatory cytokines such as MIP-1α, IL-6, TNF-α, and IL-1β [145,146].
2.3.3. CXCL-12-Abundant Reticular (CXAR) Cells
Recently, it has been demonstrated that mesenchymal CXAR cells—progenitors of the adipo-osteogenic lineage of cells—are essential for releasing HSCs from quiescence as well as for maintaining them in an undifferentiated state. CXAR cells also play a role in HSC self-renewal and proliferation of lymphoid and myeloid progenitors [147,148,149]. Most perivascular HSCs are in direct contact with CXAR cells and Nestin+ stromal cells in this niche [150]. Further, HSPCs and lymphoid progenitors in the BM are found in close association with CXAR cells in the BM [148,151]. Studies have demonstrated the importance of CXAR-cell chemokine signaling for HSC maintenance in the BM. Greenbaum et al. have shown that depletion of CXCL-12 from CXAR cells causes mobilization of HSPCs from the BM and B-lymphoid progenitor loss [152]. In mice, treatment with LPS increased CCL-2 (macrophage chemoattractant protein (MCP)-1) expression in CXAR cells and other BM MSCs required for monocyte egress into the bloodstream [153].
CXCL-12 is important for MM cell evasion of apoptosis and survival by upregulating survivin, B-cell lymphoma (BCL)-2, and ATP-binding cassette (ABCC)-1, a multidrug resistance transporter [154]. Further, CXCL-12 promotes homing of CXCR-4-expressing MM cells to CXCL-12+ stromal niches in the BM and retention through strong adhesion of α4β1 integrin on MMPCs to VCAM-1 in the BM microvasculature [114,155].
2.3.4. Adipocytes
In the adult BM, adipocytes are reported to negatively regulate hematopoiesis, although emerging evidence indicates their contribution to hematopoiesis through lineage-specific differentiation [156,157,158,159]. One factor attributed to lesser hematopoietic support by BM adipocytes is the reduced production of growth factors such as G-CSF and GM-CSF [156,160]. Characterization with electron microscopy revealed that mouse BM adipocytes are innervated by sympathetic nerves and found close to endothelial cells in the sinusoidal vasculature. This study also identified BM adipocytes interacting with developing myeloid/granulocyte lineage cells and erythroblast islands [161]. Differential numbers of CD45+ HSCs have been reported in mice with adipocyte-rich tail BM having two- to three-fold lower numbers compared to adipocyte-poor thoracic BM [156]. Human BM adipocytes were shown to support the complete differentiation of CD34+ HSPCs into myeloid and B- and NK-lymphoid lineages but did not help maintain immature HSC quiescence in a co-culture model [162].
BM adipocytes in MM show altered expression profiles with decreased adiponectin and increased inflammatory cytokines, adipokines, and free fatty acids, which induce lipolysis of adipocytes and support MM cell proliferation and metastasis [163]. These altered BM adipocytes in MM also promote aggressive bone disease [164]. Low serum adiponectin levels have been reported in MGUS and MM patients, indicating a myeloma-suppressive role [164,165]. Adipocyte-derived MCP-1 and CXCL-12α are important for the recruitment of MMPCs to the BM where BMME cell-secreted growth factors like IL-6, TNF-α, IGF-1, and hepatocyte growth factor (HGF) promote their retention, proliferation, and survival by evading apoptosis and inducing chemoresistance [166]. In a mouse model, direct cell-cell adhesion with adipocytes increased DNA synthesis and prevented apoptosis of MM cells [167]. The association of increased leptin levels has been noted with increasing MM stage and could potentially have a role in the progression from MGUS to active MM through OPG/RANKL signaling [24,165]. These data suggest that BM adipocytes support myeloma progression.
2.4. Supporting Cells
Supporting cells are also involved in the direct or indirect regulation of hematopoiesis. Neurons of the sympathetic nervous system (SNS) influence osteoblast activity and the osteocyte network and mediate hematopoietic progenitor cell mobility through G-CSF [168]. Further, sympathetic nerve fibers use β3 adrenergic receptor signaling to regulate CXCL-12 expression in Nes+ mesenchymal and stromal progenitor cells, which is required for HSC mobilization from the BM [81,169]. Adrenergic signaling mediated by the interaction of CCR7 and CXCR-4 with the β2 adrenergic receptor and concerted chemokine expression is also important for leukocyte release from the lymph nodes as well as their homing to the BM through endothelial adhesion molecule signaling [169,170,171].
The SNS is a well-known driver of malignancy by modulating the BMME in acute lymphoblastic leukemia (ALL) [172]. However, reduced adrenergic signaling leading to sympathetic neuropathy supports myeloid malignancies, as shown in mice studies [173,174]. In fact, β-adrenergic receptor blockers have been used with some success in MM patients to reduce overall mortality and in MM cell lines to prevent proliferation and survival [175,176].
2.5. Hematopoietic Progeny
Lineage-specific cells such as megakaryocytes, macrophages, neutrophils, and T lymphocytes arising from the differentiation of HSCs contribute to the regulation of hematopoiesis by manipulating HSC quiescence and mobilization across niches. Further, these cells themselves influence and are influenced by malignant PCs during the development and progression of myeloma (Figure 3).
2.5.1. Megakaryocytes
Megakaryocytes promote HSC proliferation and quiescence through TGF-β1 and CXCL-4 signaling and, under stress, secrete FGF and IGF-1 to activate HSC proliferation [109,110,177]. Further, it has been shown that megakaryocytes are closely associated with platelet- and myeloid-biased HSCs [177,178]. The myeloid-biased HSCs express megakaryocyte-like transcripts such as transcription factor GATA1 (GATA-binding protein 1), the TPO (thrombopoietin) receptor Mpl, and the platelet and endothelial aggregation factor VWF (von Willebrand Factor), as well as the megakaryocyte-specific integrin CD41 [179]. It has also been shown that the depletion of megakaryocytes leads to decreased thrombopoietin (TPO) expression, resulting in an overall reduction in HSC numbers in the BM [180]. Further, megakaryocyte ablation has also been shown to reprogram the associated VWF+ myeloid-biased HSCs towards more lineage-balanced HSCs [178]. Recently, a feedback mechanism has also been proposed whereby microparticles released by megakaryocytes can elicit differentiation of HSCs and HSPCs into functional megakaryocytes [181].
The specific role of megakaryocytes in myelomagenesis still needs to be studied. However, it has been reported that cytokines such as TPO, IL-6, and soluble P-selectin, which participate in myeloma progression, are also required for thrombopoiesis and megakaryocytopoiesis [182]. In a study by Lemancewicz et al., newly diagnosed MM (NDMM) patients had significantly increased levels of cytokines, including TPO and soluble P-selectin, compared to controls. Further, stage-specific differences in cytokine expression and megakaryocyte numbers were observed [182]. In mouse cell lines, megakaryocytes were shown to be necessary for MMPC growth in the BM, and this could be through IL-6 and APRIL [183,184]. IL-1β is produced by myeloid and megakaryocytic cells and myeloma cells and could induce osteoclast activity, mediate homing and adhesion of MMPCs in the BM, and consequently induce pro-inflammatory IL-6 signaling [185].
2.5.2. Macrophages
Resident macrophages in the BM contribute to HSPC retention and use G-CSF for their egress into circulation [169,186,187]. It has been reported that CD234 (Duffy antigen receptor for chemokines (DARC))-expressing macrophages mediate long-term HSC quiescence by binding CD82/KAI1 and downstream TGF-β1/Smad3 signaling [188]. Resident macrophages indirectly regulate HSC maintenance through interactions with osteoblasts and Nestin+ MSCs [189]. Through depletion experiments, CD169+ VCAM-1+ macrophages were shown to be essential for erythropoiesis and HSPC and progenitor retention in the BM [190]. Macrophages expressing α-smooth muscle actin also indirectly regulate hematopoiesis via prostaglandin E2, which enhances CXCL-12 production by Nestin+ cells [191,192].
In MGUS and MM patient BM, macrophages were closely associated with MMPCs, with high levels of CD68+ macrophages, a indicator of aggressive disease and poor prognosis [193,194,195,196]. Interaction of MM cells with BMSCs via CXCL-12 results in the recruitment of monocytes from peripheral blood, specifically macrophages, and polarizes them towards M2 or a tumor-supportive phenotype and prevents chemotherapy-mediated MM cell apoptosis [197,198]. Macrophages are important for MMPC proliferation, migration, homing to BM, survival, development of drug resistance, immunosuppression, and angiogenesis [199,200,201,202,203,204,205]. M2 macrophages, also known as tumor-associated macrophages (TAM), produce IL-6 and other cytokines (IL-10, IL-12, VEGF, IL-1β, IGF-1) important for MMPC proliferation [200,206,207]. MMPCs expressing PSGL1 and ICAM-1 can polarize macrophages towards the M2 or TAM phenotype in co-culture models by enhancing CD206 expression and also induce drug resistance through the Src, ERK, and c-MYC pathways [205]. MMPCs secrete pro-angiogenic VEGF and FGF-2 and convert macrophages to an endothelial cell-like phenotype to produce abnormal vasculature in a “vascular mimicry” process [208]. IL-10 and TGF-β secreted by TAMs, along with IL-32 secreted by MM cells, are thought to play a role in immune suppression by reducing T-cell surveillance through reduced MHC-II activation and decreased production of IFN-γ, IL-2, and TNF-α [197,198,209,210].
2.5.3. Neutrophils
During acute infection or inflammation, neutrophils promote emergency myelopoiesis by reactive oxygen species (ROS) production through HSPC proliferation. Post-transplantation and upon sinusoidal injury, a subset of Gr1+CD115− neutrophils secrete TNFα to drive sinusoidal regeneration and endothelial cell proliferation, which are niche components vital for normal hematopoiesis [211,212]. CD62L-low CXCR-4-high neutrophils are identified as “aged” and undergo circadian clearance from the BM, consequently causing a size reduction of the hematopoietic niche [213]. Interestingly, neutrophils in the intestine also have a paracrine effect on granulopoiesis in the BM by suppressing IL23-mediated signaling [214].
Neutrophils are implicated in immune evasion and chemoresistance development in MM [215,216,217,218]. The gene expression profile of MM patient peripheral blood neutrophils was found to be different from that of healthy normal neutrophil gene expression, particularly in FC-γ-R-mediated phagocytosis, endocytosis, leukocyte transendothelial migration, chemokine signaling, and TLR pathways, with little evidence for their role in T-cell suppression [219]. However, some genes such as CSK, GSA, MEGF, PGM1, and PROK2 were found to be upregulated in high-density neutrophils from the BM of MM patients and were associated with progression from MGUS to MM, and mature neutrophils alone were shown to impact MM patient survival [114].
2.5.4. T Cells
All sub-populations of T cells including CD4+ T-helper cells, CD4+ regulatory T cells, CD8+ T cells, γδ-T cells, and NK-T cells produce cytokines important for regulating hematopoiesis [220,221]. Th1 cells help maintain the homeostasis of hematopoietic progenitor cells (HPC). Reduced Th1 activity coupled with low Stat4 expression and increased Th2 cells results in reduced HPC numbers and proliferation in the BM, while increased Th1 activity with reduced Stat6 expression is favorable for HPC proliferation [220,222,223]. In mice, CD4+ Th cells were shown to be important for the development of myeloid lineage cells from their progenitors [223]. Activated Th1 cells are important for the differentiation of macrophages through secreted IL-3, GM-CSF, and M-CSF. However, secretion of IFNɣ and TNFɑ have the contrasting effect of suppressing hematopoiesis. Th2 cells also produce IL-3 and GM-CSF similar to Th1 cells while producing lineage-specific cytokines such as IL-4, IL-5, IL-9, and IL-13, which influence hematopoiesis [220]. IL-5 produced by T cells is required for eosiniophilopoiesis both during primitive hematopoiesis and during allergen sensitization at later life stages [224,225]. Another important T-cell subset, Th-17 cells, produces IL-17, which is important for neutrophil homeostasis by controlling tissue migration and neutrophil apoptosis [226].
T-cells are known to be altered in numbers and function in MM. There is a reduction in the overall CD4+ and CD8+ T-cell population, skewed Th1/Th2 ratio, and T-cell anergy [227,228]. Nearly half of the T-cell population described in MM are of the CD3 + CD8 + CD57+ effector-memory subtype and lack surface TCR expression [228,229]. Flow cytometric analysis revealed increased expression of programmed cell death protein (PD)-1, cytotoxic T lymphocyte-associated protein (CTLA)-4, CD160, and 2B4 (CD244/signaling lymphocytic activation molecule (SLAM) F4)) in the BM T-cells of MM patients, indicative of an immunosuppressive microenvironment favorable for immune evasion by MM cells [230]. MMPCs express the ligand PDL-1 for the T-cell PD-1 receptor, and this interaction results in immune suppression due to impaired T-cell proliferation and cytokine secretion with T-cell exhaustion, promoting relapse of MM in a mouse model [227,231]. This also correlates with clinical studies showing increased PD-1+ T-cells in the BM of relapsed/refractory (RR)MM compared to NDMM or MM-under-remission patients [227]. MM cells also secrete TGF-β, which reduces IL-2-mediated T-cell proliferation [231,232]. By “trogocytosis”, T-cells acquire MM cell surface-derived proteins upon contact and establish a novel subset of regulatory T-cells, often without functional significance, but potentially serving as prognostic markers or therapeutic targets [233]. Other MM-specific T-cells are CD57+ CD8+ terminal effector T-cells detected from both BM and peripheral blood, but their function is unclear [234]. Th17 cells have also been reported from both the peripheral blood and BM of MM patients. Whereas IL-17 promotes tumor growth, Th17 cells are correlated with long-term survival in MM patients [228]. A small subset of γδ-T cells was also identified with adaptive and innate immune responses against myeloma [235].
2.5.5. B Cells
BM B lymphocytes also play a role in regulating normal hematopoiesis. Patel and Pietras have demonstrated that acetylcholine secreted by B cells during both homeostasis and inflammation acts on Chrna7+ mesenchymal stromal cells and, in turn, promotes expression of CXCL-12 and ANGPT1 in the HSC niche, leading to arrest of HSC cell cycling and myeloid lineage differentiation [236]. CD138+ BMPCs are crucial for myelopoiesis in the murine BMME. A subset of “myeloid-like B cells” promotes emergency myelopoiesis during systemic infection, primarily through IL-10 [237,238]. In mice, a subset of macrophages has also been observed to originate from pre/pro-B-cells expressing non-rearranged BCR genes and co-expressing both myeloid and lymphoid lineage markers [239].
MM is a B-cell malignancy, and MMPCs would significantly influence and be influenced by the BMME. MMPCs establish crosstalk with almost all the BM’s cell types, ECM, and vasculature to harness them for their proliferation and survival [8]. MMPCs are late-stage B-cells expressing the characteristic markers CD138 (syndecan-1), CD38, and monoclonal Ig light-chain, but lacking the CD19 marker for pre-differentiated B-cells and other B-cell lymphomas. In addition, they do not frequently express other B-cell antigens such as CD20, CD22, CD24, and CD45 [240]. MMPC VLA-4 binds to VCAM-1 on BMSCs while MMPC CD138 and VLA-4 bind to the BM-ECM collagen-1 and fibronectin, respectively, and in turn help in BM adhesion of MMPCs and inducing IL-6 transcription [241,242]. The major cytokines secreted by MMPCs and involved in myeloma cell proliferation, survival, drug resistance, migration, angiogenesis, and osteolysis are IL-6, TNF-α, VEGF, and IGF-1 [243]. Older studies have reported circulating pre-B cells and clonotypic and possibly non-malignant PCs from the peripheral blood of MM patients. However, the latter could be indicative of MGUS [244,245,246]. MMPCs have been shown to bind soluble IFN-γ1, supporting MMPC proliferation and dexamethasone resistance [247]. Clonogenic populations of PC progenitors have also been identified from the BM of MM patients, some of which are CD19+, confer drug resistance, and contribute to MRD as potential stem cells for MM recurrence [248,249].
2.5.6. NK Cells
NK cells appear to have contradictory roles in regulating hematopoiesis as they produce both growth factors and inhibitory cytokines [250,251]. In vitro cell culture and murine in vivo studies have shown that IL-2-activated NK cells promote hematopoiesis, particularly affecting megakaryocyte and granulocyte lineages in the absence of other cytokines or growth factors. Conversely, IL-2-activated NK cells have been shown to have an inhibitory effect on hematopoiesis in the presence of cytokines and other normal growth conditions, partly via IFN-γ signaling [250].
Conflicting reports exist regarding NK cell numbers in MM, but it is known that NK-cell-mediated cytotoxicity is reduced and worsens with the advancement of MM [252,253,254,255,256]. Cytokines such as TGF-β, IL-10, IL-6, and prostaglandin-E2 (PGE2) secreted by MM cells promote MM cell proliferation and suppress NK cell activity through downstream signaling via STAT-3, which inhibits IFN-γ-mediated antibody-dependent cellular cytotoxicity (ADCC) and reduces the NK cell response to inflammatory cytokines such as IL-12 and IL-15 [256]. Further, the monoclonal Igs secreted by myeloma cells affected NK cells, as shown in a study where the use of monoclonal IgA on NK cells resulted in a reduction of cytolytic granules and the creation of vacuoles [257]. In an MM mouse model, the BM effector Killer Cell Lectin-Like Receptor (KLR)G1-NK cell subpopulation was substantially reduced with myeloma progression due to impaired chemokine receptor-ligand ratios with downregulation of CXCR-3 and CXCR-12 and increased CXCL-9 and CXCL-10 [258]. NK cells from MM patients show a reduction in activating receptors NKG2D (aka killer cell lectin-like receptor K1/KLRK1), DNAX accessory molecule (DNAM)-1, and CD161, and an increase in inhibitory CD158a (inhibitory Killer Ig-like Receptor/KIR). NKT cells that express both NK- and T-cell receptors also show impaired cytotoxicity [114]. MM cells also express the PD-L1 ligand that can bind PD-1 on T and NK cells and suppress DNAM-1 on these cells to prevent activation of immune responses [256].
2.5.7. Dendritic Cells (DCs)
BM-resident DCs retain recirculated mature B- and T-memory cells [259,260]. In mice, perivascular DCs formed clusters that were embedded with recirculating B- and T-cells, and selective ablation of these DCs resulted in the elimination of mature B-cells from this niche [259]. Monocytes, including DCs, secrete G-CSF, which is important for HSPC mobilization [261]. Bone-marrow-resident DCs found close to venous sinusoids and arterioles use CXCR-2 signaling to regulate endothelial function and are important for the mobilization of hematopoietic stem and progenitor cells (HSPC) [262]. It is also reported that classical DCs expressing CD8α (murine) or X-C motif chemokine receptor 1 (XCR1) and C-type lectin domain containing 9 (CLEC9) (human) induce erythropoiesis during stress [263].
DC dysfunction is well-studied in MM, with plasmacytoid DCs (pDC) particularly supporting myeloma cell proliferation, survival, and immune evasion [264,265,266,267]. DCs from MM patients have been shown to lack surface markers such as CD80 and CD86, and have a higher prevalence of immature and inactive DC types [268,269]. MMPC-derived IL-6, IL-10, M-CSF, and VEGF inhibit DC maturation, development, and antigen presentation capacity by driving DC progenitors toward non-specific or inactive monocytic lineages [269]. DCs in MM can promote myeloma cell proliferation and produce IL-17 to expand the BM Th-17 cell population, which then plays a role in osteoclastogenesis and bone lysis [270]. Thus, DC dysfunction significantly drives MM’s immune evasion and drug resistance.
2.6. Clonal Hematopoiesis and Development of Multiple Myeloma
A subset of HSCs in the BM acquires advantageous somatic mutations over time and undergo selective expansion in a process termed “clonal hematopoiesis” (CH) [271,272,273]. When some of these mutations are oncogenic, it is termed clonal hematopoiesis of indeterminate potential (CHIP), which leads to establishing precursor conditions like MGUS and malignant transformation to active MM [271,272]. CHIP is rarely detected in the younger population but occurs at a frequency of up to 10% in those over 70 years [273]. Frequently mutated genes in CH are the epigenetic regulators Tet Methylcytosine Dioxygenase 2 (TET2), DNA methyltransferase (DNMT3A), and additional sex combs like 1 (ASXL1) [273,274]. CHIP-associated variants are indicators of high-risk disease with poor prognosis and response to treatment in MM, particularly in those undergoing transplant [275,276]. Identification of CHIP is based on the presence of at least 2% higher variant allele frequency (VAF) in genes associated with hematological malignancies as determined from sequencing data with no indications of hematopoietic dysplasia or abnormal blast counts [273,277].
3. Immune Profiling Using Single-Cell Transcriptome Sequencing in Multiple Myeloma
In hematological malignancies, immune system dysfunction is well-established, and a deeper understanding of the immune landscape can inform precision diagnostics, drug response prediction, and targeted treatment. Recent technological advancement has seen an influx of several non- and minimally invasive approaches for individualized immune profiling from limited samples to obtain gene/protein expression information from bulk immune cells and single cells [278]. In this section, we attempt to review the application of one of the most promising technologies in this area—single-cell transcriptome sequencing (SCTS)—in immune profiling of hematological malignancies, focusing on MM. Table 2 provides a detailed list of recent single-cell sequencing studies in MM focusing on the immune microenvironment.

Table 2.
Recent single-cell sequencing studies in MM exploring the role of immune cells and the BMME in myelomagenesis, progression, and drug response. NA = not available.
SCTS is an emerging technology used to resolve gene expression differences between cell populations of a heterogeneous tumor sample, and it has been employed in hematological malignancies to understand aberrant immune cell frequency and function. Many SCTS studies have been carried out for MM in recent years, focusing on the BM tumor microenvironment, the immune landscape and immune evasion, and the development of drug resistance and relapse.
Single-cell sequencing of around 75,000 cells from 14 MM patients by Liu et al. provided insights into the malleability of the tumor microenvironment and its role in tumor heterogeneity and MM progression, as demonstrated by co-clustering patient immune and PCs with similar genetic backgrounds [282]. The authors also traced the journey of PCs from SMM to active myeloma and relapse using somatic alteration, cell type-specific marker expression, and gene expression data in relation to the tumor microenvironment. Unlike the malignant cells, the clustering of non-malignant cells was independent of tumor stage or origin. However, patient-specific differences in expression were observed, further underscoring the involvement of the tumor microenvironment’s interaction with the genetic background [282]. BM cells from different stages of MM progression and healthy donors were used for SCTS, revealing that NK cell abundance increases early in the disease along with altered chemokine receptor expression. In this study, the authors found that granzyme K+ cytotoxic T-memory cells were lost as early as SMM and could play a critical role in MM immunosurveillance in mouse models. Additionally, major histocompatibility complex (MHC)-class II dysregulation in CD14+ monocytes leads to T-cell suppression in vitro [281]. A compromised tumor microenvironment was also demonstrated in a study combining SCTS with validation using bulk RNA sequencing (RNAseq), flow cytometry, and functional experiments [285]. Results showed enrichment of exhausted NK cells and CD8+ T cells, macrophage reprogramming to a mixed M1-M2 phenotype, and two TAM clusters present only in the MM stage with increased M2 scores. Interaction analysis showed enrichment for two ligand–receptor pairs between macrophages and malignant PCs involving phagocytosis suppression and the macrophage inhibitory factor, which alters macrophage phenotype, and these alterations correlated with disease progression and adverse outcomes. In a multi-omics study that included SCTS as part of the MMRF immune atlas pilot project, 18 MM patient BM samples were used to compare immune cell profiles using scRNA-seq, cytometry by time-of-flight (CyTOF), and cellular indexing of transcriptomes and epitopes by sequencing (CITE-seq). The techniques exhibited differences in identifying T-cell, macrophage, and monocyte numbers. Overall, ISS stage 3 patients had decreased CD4+ T/CD8+ T-cell ratios, and ras-related C3 botulinum toxin substrate 2 (RAC2) and proteasome 20S subunit beta 9 (PSMB9) expression was upregulated in NK cells of progressive versus non-progressive MM [283]. SCTS of 10 MM individuals pre and post two cycles of a bortezomib-cyclophosphamide-dexamethasone (VCD) regimen revealed increased immune-reactive and stress-associated pathways while unfolded-protein response (UPR) and metabolic-related programs were reduced. Low immune-reactive gene expression indicated poor survival and non-responsiveness to drugs, likely by reduced MHC-class I-mediated antigen presentation capacity (APC) and immune surveillance, and upregulation of immune escape genes. The authors also found a connection between the tumor-intrinsic immune reactive program and the immunosuppressive microenvironment arising from immune cell exhaustion and checkpoint molecule expression in T-cells, NK cells, and monocytes [284]. Another study focusing specifically on SCTS of DCs and monocytes from 10 MM patients found five distinct clusters for each cell type [286]. Using trajectory analysis, one subset—monocyte-derived DCs (mono-DCs)—was shown to be generated from intermediate monocytes. Compared to healthy controls, conventional DC2 (cDC2), mono-DC, and intermediate monocytes of MM patients exhibited impaired APC. Additionally, the regulatory function of interferon regulatory factor 1 (IRF1) was shown to be decreased in the cDC2, mono-DC, and intermediate monocytes of MM patients.
4. Immunotherapy in Multiple Myeloma
Immunotherapy has come a long way from the Nobel Prize-winning first documented use of allogeneic bone marrow hematopoietic cell transplantation in 1968 by Edward Donnall Thomas for leukemia treatment [287]. The primary immunotherapies for hematological cancers are checkpoint inhibitors, vaccines, cell-based antibodies, and oncolytic viruses [288]. Here, we will review the antibody- and cell-based methods. In cancer, immunotherapy refers to either activating or rescuing an anti-tumor immunogenic response or suppressing an undesirable immune dysfunction state to destroy existing cancer cells or control the progression. This is often achieved by identifying and targeting specific proteins usually expressed only by malignant cells using antibody- or cell-based methods. Monoclonal or bispecific antibodies are designed against the malignant cell proteins to destroy cancer cells while leaving healthy cells untouched. Similarly, healthy immune cells (mainly T- or NK-cells) derived from the patient are engineered in the laboratory to express specific antibodies or peptides against cancer cell-specific proteins and termed chimeric antigen receptor (CAR) cells, which can home onto cancer cells and kill them via cell-mediated cytotoxic responses. The patient’s immune function can also be rescued using specific recombinant cytokines and growth factors. Immunotherapy can be used as a monotherapy or in combination with a cytotoxic/chemotherapeutic drug to eliminate cancer cells while minimizing off-target effects.
Bispecific T-cell engagers (BiTEs) have two specific variable region chains—one for the immune cell receptor and one for the cancer-specific target antigen—to improve specificity and reduce off-target action.
MM’s standard-of-care treatment has remained relatively the same since autologous stem cell transplantation (ASCT) was developed in the 1980s and, later, PIs in the 2000s (Figure 6). MM remains an incurable disease with high relapse and drug resistance rates [289,290,291,292,293]. Cell- and antibody-based immunotherapy are emerging as invaluable tools in MM treatment, with clinical trials showing great promise. B-cell maturation antigen (BCMA) and SLAMF7 are the two novel targets in MM immunotherapy. BCMA is an important member of the TNFR superfamily expressed by all malignant MMPCs and required for survival, proliferation, and myeloma progression. At the same time, SLAMF7 is thought to play a role In BM stromal interactions with MMPCs to promote survival and is also highly expressed by all stages of MMPCs [294,295]. The landmark advancement in MM therapy was the development and approval of the monoclonal antibodies elotuzumab (anti-SLAMF7) and daratumumab (anti-CD38) in 2015 for both monotherapy and combination forms, particularly in the case of RRMM [296,297,298,299,300,301]. The first anti-BCMA CAR-T cells were made by lentiviral vector-mediated transfection in 2013 using a single-chain variable fragment from mouse anti-BCMA antibody combined with hinge and transmembrane regions of human CD8α, CD3ζ T-cell activation domain, and a costimulatory molecule (CD28), and the first clinical trial took place in 2016 to show potent cytotoxicity in refractory MM [302,303]. Since then, a wide array of immunotherapies have been developed, including chimeric antigen receptor-T (CAR-T) cells, bispecific antibodies, antibody-drug conjugates, and immune checkpoint inhibitors [262,289,290]. A combination of the PI3K inhibitor with BCMA was used to develop the Bb2121 CAR T-cell therapy, designed to improve the memory phenotype for RRMM. In clinical trials, this showed minimal residual disease (MRD) negativity and median progression-free survival (PFS) of 17.7 months [304]. LCAR-B38M, a dual epitope anti-BCMA-targeting CAR-T therapy, underwent a Phase 1 clinical trial and showed a median duration of response (DOR) of 16 months, median PFS of 15 months, and median PFS for patients achieving CR of 24 months [305]. The CARAMBA Phase 1/2A clinical trial employs CAR-T cells targeting SLAMF7, utilizing a novel virus-free transient mRNA expression system to minimize the incidence of cytokine release syndrome (CRS) in the first European virus-free CAR-T clinical trial [306]. CAR-NK cell therapy has also been developed against SLAMF7, which was shown to eliminate human MM cells in a mouse xenograft model and is currently under phase 1 clinical trial evaluation (NCT03710421) [307]. A majority of MMPCs express CD56—required for MM cell peripheral blood egress and setup of extramedullary disease—which is also an emerging CAR cell therapy target showing positive results in a mouse model and currently in clinical trials (NCT03473496, NCT03271632) [308,309]. Another exciting target is CD19, which is rarely expressed by MMPCs but could potentially be expressed by “stem” clones in some patients. It has also been tested in two clinical trials with no conclusive results [308]. Other important targets in CAR cell therapy are CD138, κ-light chain, G-protein coupled receptor (GPRC) 5D, NKG2D, and New York Esophageal Squamous Cell Carcinoma (NY-ESO)-1, also being investigated in several clinical trials [308]. Table 3 lists successful ongoing and completed clinical trials for immunotherapy in MM at various stages of the disease. An anti-BCMA antibody, erlanatamab, is currently in a Phase 1 dose escalation clinical trial (NCT03269136) for RRMM post-PI or anti-CD38 treatment over an 8.1 month follow-up, with 67% of participants showing Grade 1 or 2 CRS, 31% complete remission (CR), 64% overall response rate (ORR), and 91% probability of event-free status at 6 months [310]. Several other anti-BCMA BiTEs and BsAbs are in various stages of clinical trials, and more targets are being explored, including CD138, CD38, CD19, SLAMF7, Fc receptor-like 5 (FcRL5), CS1-NKG2D, GPRC5D, and NY-ESO-1 [311].
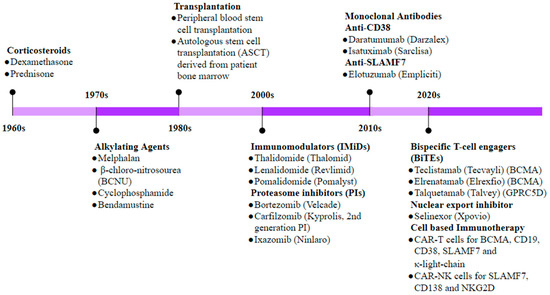
Figure 6.
Timeline of therapeutic developments in MM. Historically, corticosteroids were the first drugs to be used in MM treatment, and in the late 1970s, BM stem cell transplantation became one of the most promising treatments in eligible patients. Discovery of the proteasome inhibitor (PI) bortezomib was a significant advancement in MM treatment and remains the standard first-line therapy in most MM regimens today. The last decade has witnessed significant research into targeting the dysregulated immune landscape as well as myeloma cell-specific markers through development of antibody- and cell-based immunotherapeutic approaches.

Table 3.
Ongoing immunotherapy clinical trials for MM {adapted from the NCBI Clinical Trials Registry. Source: [312]}.
5. Conclusions
This review emphasizes the intricate, dynamic network of cellular crosstalk between MMPCs and their BMME. All the components of the normal BM, including the ECM, blood vessels, osteo-adipo lineage cells, supporting cells, and the hematopoietic progeny, are eventually transformed by the myeloma cells to abet their destructive march through the bone and eventually into the bloodstream. These cells not only inhibit MM cell apoptosis, whether autonomous or drug-induced, but also contribute to the development of osteolysis. Therefore, it is imperative to consider these cellular interactions in the pursuit of innovative MM treatment approaches. A deeper understanding of the interactions among healthy cells within the BMME will provide valuable insights into how MM disrupts this milieu. Ongoing research efforts are focused on the development of novel MM treatment targets. In recent years, the introduction of agents targeting MM cells and their interactions with the BMME, such as proteasome inhibitors (e.g., bortezomib and carfilzomib) and immunomodulatory agents (e.g., thalidomide, lenalidomide, and pomalidomide), has significantly reduced mortality in MM patients. However, further elucidation of the molecular mechanisms governing MM-bone interactions and the MM niche is necessary to identify novel approaches that can positively impact bone disease and MM tumor progression.
SCTS studies in MM have greatly enhanced our understanding of the tumor microenvironment and the interplay of immune cells, MMPCs, and the BME. The studies reviewed here represent only the beginning of the ever-growing breadth of single-cell sequencing analysis. To truly impact clinical outcomes through genomic medicine, it is imperative to elucidate MM complexity through extensive large-cohort analyses to predict treatment response and improve overall prognosis. Multi-omics investigations, which integrate genetic sequencing with phenotypic assessments, promise to provide a comprehensive overview of dysregulation within various cell types and compartments throughout the disease continuum, spanning from MGUS and SMM to full-blown MM. Some limitations faced during single-cell analysis include the need for pure populations of cells for studies dealing exclusively with profiling PCs, and this becomes difficult in early stages such as MGUS or SMM, where the PC burden would be low and distinguishing malignant and non-malignant PCs becomes challenging as there would be some overlap between surface markers. Ultimately, this wealth of data will facilitate the molecular profiling of tumors across all disease stages, offering a dual clinical benefit for patients: precision medicine and precision diagnosis. While precision medicine has been strongly emphasized, aimed at tailoring therapies to individual MM patients based on molecular profiles, precision diagnostic strategies are also in development. These strategies target asymptomatic individuals with MGUS and SMM, offering early interventions to impede progression to MM. Harnessing the genomic insights accumulated over the past decade will lead to innovative approaches that can potentially enhance outcomes for MM patients.
Although great strides have been made in cancer immunotherapy, the burden of off-target effects, graft versus host disease, and cytokine release syndrome remains a significant cause of mortality post-treatment. MM remains an incurable disease with a complex pathophysiology. The interplay between MMPCs and the bone marrow (BM) microenvironment plays a pivotal role in MM’s pathogenesis and progression. Current treatment standards and most active anti-MM drugs target both MM cells and the BM microenvironment. By directing efforts toward these diseased cells and their microenvironment, novel treatment strategies may be identified to improve outcomes for individuals grappling with this incurable blood cancer.
Author Contributions
N.S.S. and B.C. conceptualized the article, performed the literature search, wrote the initial draft of the manuscript, performed the critical review, and prepared the final version of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
Financial support was provided by The Department of Science and Technology Fund for Improvement of S&T Infrastructure in Higher Educational Institutions (DST-FIST) (SR/FST/LS-II/2019/615) and The Departments Information Technology, Biotechnology and Science and Technology, Government of Karnataka, India.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are grateful for the financial support provided by The Department of Science and Technology Fund for Improvement of S&T Infrastructure in Higher Educational Institutions (DST-FIST) (SR/FST/LS-II/2019/615) and The Departments Information Technology, Biotechnology and Science and Technology, Government of Karnataka, India.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rieger, M.A.; Schroeder, T. Hematopoiesis. Cold Spring Harb. Perspect. Biol. 2012, 4, a008250. [Google Scholar] [CrossRef] [PubMed]
- Elsaid, R.; Soares-Da-Silva, F.; Peixoto, M.; Amiri, D.; Mackowski, N.; Pereira, P.; Bandeira, A.; Cumano, A. Hematopoiesis: A Layered Organization Across Chordate Species. Front. Cell Dev. Biol. 2020, 8, 606642. [Google Scholar] [CrossRef] [PubMed]
- González, D.; van der Burg, M.; García-Sanz, R.; Fenton, J.A.; Langerak, A.W.; González, M.; van Dongen, J.J.M.; Miguel, J.F.S.; Morgan, G.J. Immunoglobulin gene rearrangements and the pathogenesis of multiple myeloma. Blood 2007, 110, 3112–3121. [Google Scholar] [CrossRef] [PubMed]
- Godin, I.; Cumano, A. Hematopoietic Stem Cell Development; Springer Science & Business Media: Berlin, Germany, 2010; 178p. [Google Scholar]
- Barwick, B.G.; Gupta, V.A.; Vertino, P.M.; Boise, L.H. Cell of Origin and Genetic Alterations in the Pathogenesis of Multiple Myeloma. Front. Immunol. 2019, 10, 1121. [Google Scholar] [CrossRef]
- Cowan, A.J.; Green, D.J.; Kwok, M.; Lee, S.; Coffey, D.G.; Holmberg, L.A.; Tuazon, S.; Gopal, A.K.; Libby, E.N. Diagnosis and Management of Multiple Myeloma: A Review. JAMA 2022, 327, 464–477. [Google Scholar] [CrossRef] [PubMed]
- Rustad, E.H.; Yellapantula, V.; Leongamornlert, D.; Bolli, N.; Ledergor, G.; Nadeu, F.; Angelopoulos, N.; Dawson, K.J.; Mitchell, T.J.; Osborne, R.J.; et al. Timing the initiation of multiple myeloma. Nat. Commun. 2020, 11, 1917. [Google Scholar] [CrossRef] [PubMed]
- Morgan, G.J.; Walker, B.A.; Davies, F.E. The genetic architecture of multiple myeloma. Nat. Rev. Cancer 2012, 12, 335–348. [Google Scholar] [CrossRef]
- Padala, S.A.; Barsouk, A.; Barsouk, A.; Rawla, P.; Vakiti, A.; Kolhe, R.; Kota, V.; Ajebo, G.H. Epidemiology, Staging, and Management of Multiple Myeloma. Med. Sci. 2021, 9, 3. [Google Scholar] [CrossRef]
- Röllig, C.; Knop, S.; Bornhäuser, M. Multiple myeloma. Lancet 2015, 385, 2197–2208. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Zhou, L.; Yu, Q.; Wei, G.; Wang, L.; Huang, Y.; Hu, K.; Hu, Y.; Huang, H. Measuring the global, regional, and national burden of multiple myeloma from 1990 to 2019. BMC Cancer 2021, 21, 606. [Google Scholar] [CrossRef]
- Turesson, I.; Bjorkholm, M.; Blimark, C.H.; Kristinsson, S.; Velez, R.; Landgren, O. Rapidly changing myeloma epidemiology in the general population: Increased incidence, older patients, and longer survival. Eur. J. Haematol. 2018, 101, 237–244. [Google Scholar] [CrossRef]
- Kazandjian, D. Multiple myeloma epidemiology and survival: A unique malignancy. Semin. Oncol. 2016, 43, 676–681. [Google Scholar] [CrossRef] [PubMed]
- International Myeloma Foundation. Durie-Salmon Staging System. Available online: https://www.myeloma.org/ (accessed on 8 October 2022).
- Filonzi, G.; Mancuso, K.; Zamagni, E.; Nanni, C.; Spinnato, P.; Cavo, M.; Fanti, S.; Salizzoni, E.; Bazzocchi, A. A Comparison of Different Staging Systems for Multiple Myeloma: Can the MRI Pattern Play a Prognostic Role? AJR Am. J. Roentgenol. 2017, 209, 152–158. [Google Scholar] [CrossRef]
- International Myeloma Foundation. International Staging System (ISS) and Revised ISS (R-ISS). Available online: https://www.myeloma.org/ (accessed on 8 October 2022).
- International Myeloma Foundation. International Myeloma Working Group (IMWG) criteria for the diagnosis of multiple myeloma. Available online: https://www.myeloma.org/ (accessed on 8 October 2022).
- Sawyer, J.R. The prognostic significance of cytogenetics and molecular profiling in multiple myeloma. Cancer Genet. 2011, 204, 3–12. [Google Scholar] [CrossRef]
- Liebisch, P.; Viardot, A.; Baßermann, N.; Wendl, C.; Roth, K.; Goldschmidt, H.; Einsele, H.; Straka, C.; Stilgenbauer, S.; Döhner, H.; et al. Value of comparative genomic hybridization and fluorescence in situ hybridization for molecular diagnostics in multiple myeloma. Br. J. Haematol. 2003, 122, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Tassone, P.; Tagliaferri, P.; Rossi, M.; Gaspari, M.; Terracciano, R.; Venuta, S. Genetics and molecular profiling of multiple myeloma: Novel tools for clinical management? Eur. J. Cancer 2006, 42, 1530–1538. [Google Scholar] [CrossRef] [PubMed]
- Bergsagel, P.L.; Kuehl, W.M. Molecular Pathogenesis and a Consequent Classification of Multiple Myeloma. J. Clin. Oncol. 2005, 23, 6333–6338. [Google Scholar] [CrossRef]
- Hideshima, T.; Mitsiades, C.; Tonon, G.; Richardson, P.G.; Anderson, K.C. Understanding multiple myeloma pathogenesis in the bone marrow to identify new therapeutic targets. Nat. Rev. Cancer 2007, 7, 585–598. [Google Scholar] [CrossRef]
- Fairfield, H.; Falank, C.; Avery, L.; Reagan, M.R. Multiple myeloma in the marrow: Pathogenesis and treatments. Ann. N. Y. Acad. Sci. 2016, 1364, 32–51. [Google Scholar] [CrossRef] [PubMed]
- Kuehl, W.M.; Bergsagel, P.L. Multiple myeloma: Evolving genetic events and host interactions. Nat. Rev. Cancer 2002, 2, 175–187. [Google Scholar] [CrossRef]
- Bergsagel, P.L.; Kuehl, W.M. Chromosome translocations in multiple myeloma. Oncogene 2001, 20, 5611–5622. [Google Scholar] [CrossRef]
- Rajan, A.M.; Rajkumar, S.V. Interpretation of cytogenetic results in multiple myeloma for clinical practice. Blood Cancer J. 2015, 5, e365. [Google Scholar] [CrossRef] [PubMed]
- Hillengass, J.; Moehler, T.; Hundemer, M. Monoclonal gammopathy and smoldering multiple myeloma: Diagnosis, staging, prognosis, management. Recent Results Cancer Res. 2011, 183, 113–131. [Google Scholar] [PubMed]
- Korde, N.; Kristinsson, S.Y.; Landgren, O. Monoclonal gammopathy of undetermined significance (MGUS) and smoldering multiple myeloma (SMM): Novel biological insights and development of early treatment strategies. Blood 2011, 117, 5573–5581. [Google Scholar] [CrossRef] [PubMed]
- Seong, C.; Delasalle, K.; Hayes, K.; Weber, D.; Dimopoulos, M.; Swantkowski, J.; Huh, Y.; Glassman, A.; Champlin, R.; Alexanian, R. Prognostic value of cytogenetics in multiple myeloma. Br. J. Haematol. 1998, 101, 189–194. [Google Scholar] [CrossRef]
- Kumar, S.; Fonseca, R.; Ketterling, R.P.; Dispenzieri, A.; Lacy, M.Q.; Gertz, M.A.; Hayman, S.R.; Buadi, F.K.; Dingli, D.; Knudson, R.A.; et al. Trisomies in multiple myeloma: Impact on survival in patients with high-risk cytogenetics. Blood 2012, 119, 2100–2105. [Google Scholar] [CrossRef]
- Sonneveld, P.; Avet-Loiseau, H.; Lonial, S.; Usmani, S.; Siegel, D.; Anderson, K.C.; Chng, W.-J.; Moreau, P.; Attal, M.; Kyle, R.A.; et al. Treatment of multiple myeloma with high-risk cytogenetics: A consensus of the International Myeloma Working Group. Blood 2016, 127, 2955–2962. [Google Scholar] [CrossRef]
- Billecke, L.; Penas, E.M.M.; May, A.M.; Engelhardt, M.; Nagler, A.; Leiba, M.; Schiby, G.; Kröger, N.; Zustin, J.; Marx, A.; et al. Cytogenetics of extramedullary manifestations in multiple myeloma. Br. J. Haematol. 2013, 161, 87–94. [Google Scholar] [CrossRef]
- Besse, L.; Sedlarikova, L.; Greslikova, H.; Kupska, R.; Almasi, M.; Penka, M.; Jelinek, T.; Pour, L.; Adam, Z.; Kuglik, P.; et al. Cytogenetics in multiple myeloma patients progressing into extramedullary disease. Eur. J. Haematol. 2016, 97, 93–100. [Google Scholar] [CrossRef]
- Harrison, S.J.; Perrot, A.; Alegre, A.; Simpson, D.; Wang, M.C.; Spencer, A.; Delimpasi, S.; Hulin, C.; Sunami, K.; Facon, T.; et al. Subgroup analysis of ICARIA-MM study in relapsed/refractory multiple myeloma patients with high-risk cytogenetics. Br. J. Haematol. 2021, 194, 120–131. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Kastritis, E.; Christoulas, D.; Migkou, M.; Gavriatopoulou, M.; Gkotzamanidou, M.; Iakovaki, M.; Matsouka, C.; Mparmparoussi, D.; Roussou, M.; et al. Treatment of patients with relapsed/refractory multiple myeloma with lenalidomide and dexamethasone with or without bortezomib: Prospective evaluation of the impact of cytogenetic abnormalities and of previous therapies. Leukemia 2010, 24, 1769–1778. [Google Scholar] [CrossRef] [PubMed]
- Bhutani, M.; Foureau, D.M.; Atrash, S.; Voorhees, P.M.; Usmani, S.Z. Extramedullary multiple myeloma. Leukemia 2019, 34, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Lonial, S.; Mitsiades, C.S.; Richardson, P.G. Treatment Options for Relapsed and Refractory Multiple Myeloma. Clin. Cancer Res. 2011, 17, 1264–1277. [Google Scholar] [CrossRef] [PubMed]
- Shafat, M.S.; Gnaneswaran, B.; Bowles, K.M.; Rushworth, S.A. The bone marrow microenvironment—Home of the leukemic blasts. Blood Rev. 2017, 31, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Morrison, S.J.; Scadden, D.T. The bone marrow niche for haematopoietic stem cells. Nature 2014, 505, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Yu, V.W.; Scadden, D.T. Heterogeneity of the bone marrow niche. Curr. Opin. Hematol. 2016, 23, 331–338. [Google Scholar] [CrossRef]
- Méndez-Ferrer, S.; Bonnet, D.; Steensma, D.P.; Hasserjian, R.P.; Ghobrial, I.M.; Gribben, J.G.; Andreeff, M.; Krause, D.S. Bone marrow niches in haematological malignancies. Nat. Rev. Cancer 2020, 20, 285–298. [Google Scholar] [CrossRef]
- Orkin, S.H.; Zon, L.I. Hematopoiesis: An Evolving Paradigm for Stem Cell Biology. Cell 2008, 132, 631–644. [Google Scholar] [CrossRef]
- Jagannathan-Bogdan, M.; Zon, L.I. Hematopoiesis. Development 2013, 140, 2463–2467. [Google Scholar] [CrossRef]
- Pucella, J.N.; Upadhaya, S.; Reizis, B. The Source and Dynamics of Adult Hematopoiesis: Insights from Lineage Tracing. Annu. Rev. Cell Dev. Biol. 2020, 36, 529–550. [Google Scholar] [CrossRef] [PubMed]
- Yokota, T.; Oritani, K.; Mitsui, H.; Aoyama, K.; Ishikawa, J.; Sugahara, H.; Matsumura, I.; Tsai, S.; Tomiyama, Y.; Kanakura, Y.; et al. Growth-supporting activities of fibronectin on hematopoietic stem/progenitor cells in vitro and in vivo: Structural requirement for fibronectin activities of CS1 and cell-binding domains. Blood 1998, 91, 3263–3272. [Google Scholar] [CrossRef]
- Smith, C. Hematopoietic Stem Cells and Hematopoiesis. Cancer Control. 2003, 10, 9–16. [Google Scholar] [CrossRef]
- Waterstrat, A.; Van Zant, G. Effects of aging on hematopoietic stem and progenitor cells. Curr. Opin. Immunol. 2009, 21, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Fröbel, J.; Landspersky, T.; Percin, G.; Schreck, C.; Rahmig, S.; Ori, A.; Nowak, D.; Essers, M.; Waskow, C.; Oostendorp, R.A.J. The Hematopoietic Bone Marrow Niche Ecosystem. Front. Cell Dev. Biol. 2021, 9, 705410. [Google Scholar] [CrossRef] [PubMed]
- Krause, D.S.; Scadden, D.T. A hostel for the hostile: The bone marrow niche in hematologic neoplasms. Haematologica 2015, 100, 1376–1387. [Google Scholar] [CrossRef]
- Kumar, R.; Godavarthy, P.S.; Krause, D.S. The bone marrow microenvironment in health and disease at a glance. J. Cell Sci. 2018, 131, jcs201707. [Google Scholar] [CrossRef] [PubMed]
- Duarte, D.; Hawkins, E.D.; Celso, C.L. The interplay of leukemia cells and the bone marrow microenvironment. Blood 2018, 131, 1507–1511. [Google Scholar] [CrossRef] [PubMed]
- Zanetti, C.; Krause, D.S. “Caught in the net”: The extracellular matrix of the bone marrow in normal hematopoiesis and leukemia. Exp. Hematol. 2020, 89, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Lee-Thedieck, C.; Schertl, P.; Klein, G. The extracellular matrix of hematopoietic stem cell niches. Adv. Drug Deliv. Rev. 2021, 181, 114069. [Google Scholar] [CrossRef] [PubMed]
- Klein, G. The extracellular matrix of the hematopoietic microenvironment. Experientia 1995, 51, 914–926. [Google Scholar] [CrossRef]
- Horton, P.D.; Dumbali, S.; Wenzel, P.L. Mechanoregulation in Hematopoiesis and Hematologic Disorders. Curr. Stem Cell Rep. 2020, 6, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Zhong, H. Roles of the bone marrow niche in hematopoiesis, leukemogenesis, and chemotherapy resistance in acute myeloid leukemia. Hematology 2018, 23, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Wirth, F.; Lubosch, A.; Hamelmann, S.; Nakchbandi, I.A. Fibronectin and Its Receptors in Hematopoiesis. Cells 2020, 9, 2717. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, R.; Riordan, M.A.; Wenc, K.; Kreczko, S.; Zhou, M.; Dainiak, N. Dual role of fibronectin in hematopoietic differentiation. Blood 1989, 73, 111–116. [Google Scholar] [CrossRef]
- Papy-Garcia, D.; Albanese, P. Heparan sulfate proteoglycans as key regulators of the mesenchymal niche of hematopoietic stem cells. Glycoconj. J. 2017, 34, 377–391. [Google Scholar] [CrossRef] [PubMed]
- Netelenbos, T.; Born, J.v.D.; Kessler, F.L.; Zweegman, S.; Merle, P.A.; van Oostveen, J.W.; Zwaginga, J.J.; Huijgens, P.C.; Dräger, A.M. Proteoglycans on bone marrow endothelial cells bind and present SDF-1 towards hematopoietic progenitor cells. Leukemia 2003, 17, 175–184. [Google Scholar] [CrossRef]
- Keating, A.; Gordon, M.Y. Hierarchical organization of hematopoietic microenvironments: Role of proteoglycans. Leukemia 1988, 2, 766–769. [Google Scholar]
- Kharchenko, M.F.; Rybakova, L.P.; Golenko, O.D.; Kornilova, N.V.; Zakharov, I.M. The role of glycosaminoglycans and proteoglycans in hemopoiesis and the physiological functions of the blood cells. Fiziol. Zhurnal Im. IM 1996, 82, 18–25. [Google Scholar]
- Grenier, J.M.P.; Testut, C.; Fauriat, C.; Mancini, S.J.C.; Aurrand-Lions, M. Adhesion Molecules Involved in Stem Cell Niche Retention During Normal Haematopoiesis and in Acute Myeloid Leukaemia. Front. Immunol. 2021, 12, 756231. [Google Scholar] [CrossRef]
- Cambi, A.; van Helden, S.F.G.; Figdor, C.G. Roles for Integrins and Associated Proteins in the Haematopoietic System. In Madame Curie Bioscience Database; Landes Bioscience: Austin, TX, USA, 2013. [Google Scholar]
- Velders, G.A.; Fibbe, W.E. Involvement of proteases in cytokine-induced hematopoietic stem cell mobilization. Ann. N. Y. Acad. Sci. 2005, 1044, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Nervi, B.; Link, D.C.; DiPersio, J.F. Cytokines and hematopoietic stem cell mobilization. J. Cell. Biochem. 2006, 99, 690–705. [Google Scholar] [CrossRef]
- Glavey, S.V.; Naba, A.; Manier, S.; Clauser, K.; Tahri, S.; Park, J.; Reagan, M.R.; Moschetta, M.; Mishima, Y.; Gambella, M.; et al. Proteomic characterization of human multiple myeloma bone marrow extracellular matrix. Leukemia 2017, 31, 2426–2434. [Google Scholar] [CrossRef] [PubMed]
- Tancred, T.M.; Belch, A.R.; Reiman, T.; Pilarski, L.M.; Kirshner, J. Altered Expression of Fibronectin and Collagens I and IV in Multiple Myeloma and Monoclonal Gammopathy of Undetermined Significance. J. Histochem. Cytochem. 2008, 57, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Tadmor, T.; Bejar, J.; Attias, D.; Mischenko, E.; Sabo, E.; Neufeld, G.; Vadasz, Z. The expression of lysyl-oxidase gene family members in myeloproliferative neoplasms. Am. J. Hematol. 2013, 88, 355–358. [Google Scholar] [CrossRef]
- Lucero, H.A.; Kagan, H.M. Lysyl oxidase: An oxidative enzyme and effector of cell function. Cell. Mol. Life Sci. 2006, 63, 2304–2316. [Google Scholar] [CrossRef] [PubMed]
- Vacca, A.; Ribatti, D.; Presta, M.; Minischetti, M.; Iurlaro, M.; Ria, R.; Albini, A.; Bussolino, F.; Dammacco, F. Bone marrow neovascularization, plasma cell angiogenic potential, and matrix metalloproteinase-2 secretion parallel progression of human multiple myeloma. Blood 1999, 93, 3064–3073. [Google Scholar] [CrossRef]
- Barillé, S.; Akhoundi, C.; Collette, M.; Mellerin, M.P.; Rapp, M.J.; Harousseau, J.L.; Bataille, R.; Amiot, M. Metalloproteinases in Multiple Myeloma: Production of Matrix Metalloproteinase-9 (MMP-9), Activation of proMMP-2, and Induction of MMP-1 by Myeloma Cells. Blood 1997, 90, 1649–1655. [Google Scholar] [CrossRef]
- Korpetinou, A.; Skandalis, S.S.; Labropoulou, V.T.; Smirlaki, G.; Noulas, A.; Karamanos, N.K.; Theocharis, A.D. Serglycin: At the Crossroad of Inflammation and Malignancy. Front. Oncol. 2014, 3, 327. [Google Scholar] [CrossRef]
- Sidhu, I.; Barwe, S.P.; Gopalakrishnapillai, A. The extracellular matrix: A key player in the pathogenesis of hematologic malignancies. Blood Rev. 2020, 48, 100787. [Google Scholar] [CrossRef]
- Itkin, T.; Gur-Cohen, S.; Spencer, J.A.; Schajnovitz, A.; Ramasamy, S.K.; Kusumbe, A.P.; Ledergor, G.; Jung, Y.; Milo, I.; Poulos, M.G.; et al. Distinct bone marrow blood vessels differentially regulate haematopoiesis. Nature 2016, 532, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, S.K.; Kusumbe, A.P.; Itkin, T.; Gur-Cohen, S.; Lapidot, T.; Adams, R.H. Regulation of Hematopoiesis and Osteogenesis by Blood Vessel–Derived Signals. Annu. Rev. Cell Dev. Biol. 2016, 32, 649–675. [Google Scholar] [CrossRef] [PubMed]
- Corselli, M.; Chin, C.J.; Parekh, C.; Sahaghian, A.; Wang, W.; Ge, S.; Evseenko, D.; Wang, X.; Montelatici, E.; Lazzari, L.; et al. Perivascular support of human hematopoietic stem/progenitor cells. Blood 2013, 121, 2891–2901. [Google Scholar] [CrossRef]
- Perlin, J.R.; Sporrij, A.; Zon, L.I. Blood on the tracks: Hematopoietic stem cell-endothelial cell interactions in homing and engraftment. J. Mol. Med. 2017, 95, 809–819. [Google Scholar] [CrossRef] [PubMed]
- Comazzetto, S.; Shen, B.; Morrison, S.J. Niches that regulate stem cells and hematopoiesis in adult bone marrow. Dev. Cell 2021, 56, 1848–1860. [Google Scholar] [CrossRef]
- Méndez-Ferrer, S.; Michurina, T.V.; Ferraro, F.; Mazloom, A.R.; MacArthur, B.D.; Lira, S.A.; Scadden, D.T.; Ma’ayan, A.; Enikolopov, G.N.; Frenette, P.S. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 2010, 466, 829. [Google Scholar] [CrossRef] [PubMed]
- Harkness, L.; Zaher, W.; Ditzel, N.; Isa, A.; Kassem, M. CD146/MCAM defines functionality of human bone marrow stromal stem cell populations. Stem Cell Res Ther. 2016, 7, 4. [Google Scholar] [CrossRef]
- Kunisaki, Y.; Bruns, I.; Scheiermann, C.; Ahmed, J.; Pinho, S.; Zhang, D.; Mizoguchi, T.; Wei, Q.; Lucas, D.; Ito, K.; et al. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature 2013, 502, 637–643. [Google Scholar] [CrossRef]
- Ulyanova, T.; Scott, L.M.; Priestley, G.V.; Jiang, Y.; Nakamoto, B.; Koni, P.A.; Papayannopoulou, T. VCAM-1 expression in adult hematopoietic and nonhematopoietic cells is controlled by tissue-inductive signals and reflects their developmental origin. Blood 2005, 106, 86–94. [Google Scholar] [CrossRef]
- Baumann, C.I.; Bailey, A.S.; Li, W.; Ferkowicz, M.J.; Yoder, M.C.; Fleming, W.H. PECAM-1 is expressed on hematopoietic stem cells throughout ontogeny and identifies a population of erythroid progenitors. Blood 2004, 104, 1010–1016. [Google Scholar] [CrossRef]
- Li, B.; Bailey, A.S.; Jiang, S.; Liu, B.; Goldman, D.C.; Fleming, W.H. Endothelial cells mediate the regeneration of hematopoietic stem cells. Stem Cell Res. 2010, 4, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Li, W.M.; Huang, W.Q.; Huang, Y.H.; Jiang, D.Z.; Wang, Q.R. Positive and negative hematopoietic cytokines produced by bone marrow endothelial cells. Cytokine 2000, 12, 1017–1023. [Google Scholar] [CrossRef] [PubMed]
- Davis, T.; Black, A.; Lee, K. Soluble factor(s) alone produced by primary porcine microvascular endothelial cells support the proliferation and differentiation of human CD34+ hematopoietic progenitor cells with a high replating potential. Transpl. Proc. 1997, 29, 2003–2004. [Google Scholar] [CrossRef]
- Avecilla, S.T.; Hattori, K.; Heissig, B.; Tejada, R.; Liao, F.; Shido, K.; Jin, D.K.; Dias, S.; Zhang, F.; Hartman, T.E.; et al. Chemokine-mediated interaction of hematopoietic progenitors with the bone marrow vascular niche is required for thrombopoiesis. Nat. Med. 2003, 10, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Kopp, H.-G.; Avecilla, S.T.; Hooper, A.T.; Shmelkov, S.V.; Ramos, C.A.; Zhang, F.; Rafii, S. Tie2 activation contributes to hemangiogenic regeneration after myelosuppression. Blood 2005, 106, 505–513. [Google Scholar] [CrossRef]
- Dexter, T.M.; Allen, T.D.; Lajtha, L.G. Conditions controlling the proliferation of haemopoietic stem cells in vitro. J. Cell. Physiol. 1977, 91, 335–344. [Google Scholar] [CrossRef]
- Cordeiro Gomes, A.; Hara, T.; Lim, V.Y.; Herndler-Brandstetter, D.; Nevius, E.; Sugiyama, T.; Tani-Ichi, S.; Schlenner, S.; Richie, E.; Rodewald, H.-R.; et al. Hematopoietic Stem Cell Niches Produce Lineage-Instructive Signals to Control Multipotent Progenitor Differentiation. Immunity 2016, 45, 1219–1231. [Google Scholar] [CrossRef]
- Ria, R.; Vacca, A. Bone Marrow Stromal Cells-Induced Drug Resistance in Multiple Myeloma. Int. J. Mol. Sci. 2020, 21, 613. [Google Scholar] [CrossRef]
- Hideshima, T.; Anderson, K.C. Signaling Pathway Mediating Myeloma Cell Growth and Survival. Cancers 2021, 13, 216. [Google Scholar] [CrossRef]
- Bou Zerdan, M.; Nasr, L.; Kassab, J.; Saba, L.; Ghossein, M.; Yaghi, M.; Dominguez, B.; Chaulagain, C.P. Adhesion molecules in multiple myeloma oncogenesis and targeted therapy. Int. J. Hematol. Oncol. 2022, 11, IJH39. [Google Scholar] [CrossRef]
- Cook, G.; Dumbar, M.; Franklin, I.M. The role of adhesion molecules in multiple myeloma. Acta Haematol. 1997, 97, 81–89. [Google Scholar] [CrossRef]
- Vacca, A.; Di Loreto, M.; Ribatti, D.; Di Stefano, R.; Gadaleta-Caldarola, G.; Iodice, G.; Caloro, D.; Dammacco, F. Bone marrow of patients with active multiple myeloma: Angiogenesis and plasma cell adhesion molecules LFA-1, VLA-4, LAM-1, and CD44. Am. J. Hematol. 1995, 50, 9–14. [Google Scholar] [CrossRef]
- Alsayed, Y.; Ngo, H.; Runnels, J.; Leleu, X.; Singha, U.K.; Pitsillides, C.M.; Spencer, J.A.; Kimlinger, T.; Ghobrial, J.M.; Jia, X.Y.; et al. Mechanisms of regulation of CXCR4/SDF-1 (CXCL12)-dependent migration and homing in multiple myeloma. Blood 2007, 109, 2708–2717. [Google Scholar] [CrossRef]
- Meng, W.; Xue, S.; Chen, Y. The role of CXCL12 in tumor microenvironment. Gene 2018, 641, 105–110. [Google Scholar] [CrossRef]
- Tenreiro, M.M.; Correia, M.L.; Brito, M.A. Endothelial progenitor cells in multiple myeloma neovascularization: A brick to the wall. Angiogenesis 2017, 20, 443–462. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Witzig, T.E.; Timm, M.; Haug, J.; Wellik, L.; Fonseca, R.; Greipp, P.R.; Rajkumar, S.V. Expression of VEGF and its receptors by myeloma cells. Leukemia 2003, 17, 2025–2031. [Google Scholar] [CrossRef] [PubMed]
- Valković, T.; Babarović, E.; Lučin, K.; Štifter, S.; Aralica, M.; Pećanić, S.; Seili-Bekafigo, I.; Duletić-Načinović, A.; Nemet, D.; Jonjić, N. Plasma levels of osteopontin and vascular endothelial growth factor in association with clinical features and parameters of tumor burden in patients with multiple myeloma. Biomed. Res. Int. 2014, 2014, 513170. [Google Scholar] [CrossRef] [PubMed]
- Baek, Y.-Y.; Lee, D.-K.; Kim, J.; Kim, J.-H.; Park, W.; Kim, T.; Han, S.; Jeoung, D.; You, J.C.; Lee, H.; et al. Arg-Leu-Tyr-Glu tetrapeptide inhibits tumor progression by suppressing angiogenesis and vascular permeability via VEGF receptor-2 antagonism. Oncotarget 2016, 8, 11763–11777. [Google Scholar] [CrossRef][Green Version]
- Lévesque, J.-P.; Helwani, F.M.; Winkler, I.G. The endosteal ‘osteoblastic’ niche and its role in hematopoietic stem cell homing and mobilization. Leukemia 2010, 24, 1979–1992. [Google Scholar] [CrossRef]
- Calvi, L.M.; Link, D.C. The hematopoietic stem cell niche in homeostasis and disease. Blood 2015, 126, 2443–2451. [Google Scholar] [CrossRef]
- Zhou, B.O.; Yue, R.; Murphy, M.M.; Peyer, J.G.; Morrison, S.J. Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell 2014, 15, 154–168. [Google Scholar] [CrossRef]
- Ding, L.; Saunders, T.L.; Enikolopov, G.; Morrison, S.J. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature 2012, 481, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Wolock, S.L.; Krishnan, I.; Tenen, D.E.; Matkins, V.; Camacho, V.; Patel, S.; Agarwal, P.; Bhatia, R.; Tenen, D.G.; Klein, A.M.; et al. Mapping Distinct Bone Marrow Niche Populations and Their Differentiation Paths. Cell Rep. 2019, 28, 302–311.e5. [Google Scholar] [CrossRef]
- Zhao, M.; Perry, J.M.; Marshall, H.; Venkatraman, A.; Qian, P.; He, X.C.; Ahamed, J.; Li, L. Megakaryocytes maintain homeostatic quiescence and promote post-injury regeneration of hematopoietic stem cells. Nat. Med. 2014, 20, 1321–1326. [Google Scholar] [CrossRef] [PubMed]
- Bruns, I.; Lucas, D.; Pinho, S.; Ahmed, J.; Lambert, M.P.; Kunisaki, Y.; Scheiermann, C.; Schiff, L.; Poncz, M.; Bergman, A.; et al. Megakaryocytes regulate hematopoietic stem cell quiescence through CXCL4 secretion. Nat. Med. 2014, 20, 1315–1320. [Google Scholar] [CrossRef]
- Olson, T.S.; Caselli, A.; Otsuru, S.; Hofmann, T.J.; Williams, R.; Paolucci, P.; Dominici, M.; Horwitz, E.M. Megakaryocytes promote murine osteoblastic HSC niche expansion and stem cell engraftment after radioablative conditioning. Blood 2013, 121, 5238–5249. [Google Scholar] [CrossRef] [PubMed]
- Akinduro, O.; Weber, T.S.; Ang, H.; Haltalli, M.L.R.; Ruivo, N.; Duarte, D.; Rashidi, N.M.; Hawkins, E.D.; Duffy, K.R.; Celso, C.L. Proliferation dynamics of acute myeloid leukaemia and haematopoietic progenitors competing for bone marrow space. Nat. Commun. 2018, 9, 519. [Google Scholar] [CrossRef] [PubMed]
- Boyd, A.L.; Campbell, C.J.; Hopkins, C.I.; Fiebig-Comyn, A.; Russell, J.; Ulemek, J.; Foley, R.; Leber, B.; Xenocostas, A.; Collins, T.J.; et al. Niche displacement of human leukemic stem cells uniquely allows their competitive replacement with healthy HSPCs. J. Exp. Med. 2014, 211, 1925–1935. [Google Scholar] [CrossRef]
- García-Ortiz, A.; Rodríguez-García, Y.; Encinas, J.; Maroto-Martín, E.; Castellano, E.; Teixidó, J.; Martínez-López, J. The Role of Tumor Microenvironment in Multiple Myeloma Development and Progression. Cancers 2021, 13, 217. [Google Scholar] [CrossRef]
- Stessman, H.A.F.; Mansoor, A.; Zhan, F.; Janz, S.; Linden, M.A.; Baughn, L.B.; Van Ness, B. Reduced CXCR4 expression is associated with extramedullary disease in a mouse model of myeloma and predicts poor survival in multiple myeloma patients treated with bortezomib. Leukemia 2013, 27, 2075–2077. [Google Scholar] [CrossRef]
- Asada, N.; Katayama, Y. Regulation of hematopoiesis in endosteal microenvironments. Int. J. Hematol. 2014, 99, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Calvi, L.M. Osteolineage cells and regulation of the hematopoietic stem cell. Best Pract. Res. Clin. Haematol. 2013, 26, 249–252. [Google Scholar] [CrossRef]
- Le, P.M.; Andreeff, M.; Battula, V.L. Osteogenic niche in the regulation of normal hematopoiesis and leukemogenesis. Haematologica 2018, 103, 1945–1955. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Yin, T.; Wiegraebe, W.; He, X.C.; Miller, D.; Stark, D.; Perko, K.; Alexander, R.; Schwartz, J.; Grindley, J.C.; et al. Detection of functional haematopoietic stem cell niche using real-time imaging. Nature 2008, 457, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Calvi, L.M.; Adams, G.B.; Weibrecht, K.W.; Weber, J.M.; Olson, D.P.; Knight, M.C.; Martin, R.P.; Schipani, E.; Divieti, P.; Bringhurst, F.R.; et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature 2003, 425, 841–846. [Google Scholar] [CrossRef]
- Zhang, J.; Niu, C.; Ye, L.; Huang, H.; He, X.; Tong, W.-G.; Ross, J.; Haug, J.; Johnson, T.; Feng, J.Q.; et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature 2003, 425, 836–841. [Google Scholar] [CrossRef] [PubMed]
- Taichman, R.S.; Emerson, S.G. The Role of Osteoblasts in the Hematopoietic Microenvironment. Stem Cells 1998, 16, 7–15. [Google Scholar] [CrossRef]
- Staudt, N.D.; Aicher, W.K.; Kalbacher, H.; Stevanovic, S.; Carmona, A.K.; Bogyo, M.; Klein, G. Cathepsin X is secreted by human osteoblasts, digests CXCL-12 and impairs adhesion of hematopoietic stem and progenitor cells to osteoblasts. Haematologica 2010, 95, 1452–1460. [Google Scholar] [CrossRef]
- Visnjic, D.; Kalajzic, Z.; Rowe, D.W.; Katavic, V.; Lorenzo, J.; Aguila, H.L. Hematopoiesis is severely altered in mice with an induced osteoblast deficiency. Blood 2004, 103, 3258–3264. [Google Scholar] [CrossRef]
- Zhu, J.; Garrett, R.; Jung, Y.; Zhang, Y.; Kim, N.; Wang, J.; Joe, G.J.; Hexner, E.; Choi, Y.; Taichman, R.S.; et al. Osteoblasts support B-lymphocyte commitment and differentiation from hematopoietic stem cells. Blood 2007, 109, 3706–3712. [Google Scholar] [CrossRef] [PubMed]
- Kiel, M.J.; Radice, G.L.; Morrison, S.J. Lack of Evidence that Hematopoietic Stem Cells Depend on N-Cadherin-Mediated Adhesion to Osteoblasts for Their Maintenance. Cell Stem Cell 2007, 1, 204–217. [Google Scholar] [CrossRef] [PubMed]
- Kiel, M.J.; Acar, M.; Radice, G.L.; Morrison, S.J. Hematopoietic Stem Cells Do Not Depend on N-Cadherin to Regulate Their Maintenance. Cell Stem Cell 2009, 4, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Greenbaum, A.; Link, D. N-Cadherin in Osteolineage Cells Is Not Required for Maintenance of Hematopoietic Stem Cells. Blood 2011, 118, 2390. [Google Scholar] [CrossRef]
- Sacchetti, B.; Funari, A.; Michienzi, S.; Di Cesare, S.; Piersanti, S.; Saggio, I.; Tagliafico, E.; Ferrari, S.; Robey, P.G.; Riminucci, M.; et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell 2007, 131, 324–336. [Google Scholar] [CrossRef] [PubMed]
- Toscani, D.; Bolzoni, M.; Accardi, F.; Aversa, F.; Giuliani, N. The osteoblastic niche in the context of multiple myeloma. Ann. N. Y. Acad. Sci. 2015, 1335, 45–62. [Google Scholar] [CrossRef] [PubMed]
- Pennisi, A.; Ling, W.; Li, X.; Khan, S.; Wang, Y.; Barlogie, B.; Shaughnessy, J.D.; Yaccoby, S. Consequences of Daily Administered Parathyroid Hormone on Myeloma Growth, Bone Disease, and Molecular Profiling of Whole Myelomatous Bone. PLoS ONE 2010, 5, e15233. [Google Scholar] [CrossRef]
- Giuliani, N.; Morandi, F.; Tagliaferri, S.; Lazzaretti, M.; Bonomini, S.; Crugnola, M.; Mancini, C.; Martella, E.; Ferrari, L.; Tabilio, A.; et al. The proteasome inhibitor bortezomib affects osteoblast differentiation in vitro and in vivo in multiple myeloma patients. Blood 2007, 110, 334–338. [Google Scholar] [CrossRef]
- Reagan, M.R.; Liaw, L.; Rosen, C.J.; Ghobrial, I.M. Dynamic interplay between bone and multiple myeloma: Emerging roles of the osteoblast. Bone 2015, 75, 161–169. [Google Scholar] [CrossRef]
- Giuliani, N.; Rizzoli, V.; Roodman, G.D. Multiple myeloma bone disease: Pathophysiology of osteoblast inhibition. Blood 2006, 108, 3992–3996. [Google Scholar] [CrossRef]
- Giuliani, N.; Colla, S.; Morandi, F.; Lazzaretti, M.; Sala, R.; Bonomini, S.; Grano, M.; Colucci, S.; Svaldi, M.; Rizzoli, V.; et al. Myeloma cells block RUNX2/CBFA1 activity in human bone marrow osteoblast progenitors and inhibit osteoblast formation and differentiation. Blood 2005, 106, 2472–2483. [Google Scholar] [CrossRef]
- Miyamoto, T. Role of osteoclasts in regulating hematopoietic stem and progenitor cells. World J. Orthop. 2013, 4, 198–206. [Google Scholar] [CrossRef]
- Mansour, A.; Anginot, A.; Mancini, S.J.C.; Schiff, C.; Carle, G.F.; Wakkach, A.; Blin-Wakkach, C. Osteoclast activity modulates B-cell development in the bone marrow. Cell Res. 2011, 21, 1102–1115. [Google Scholar] [CrossRef]
- Mansour, A.; Abou-Ezzi, G.; Sitnicka, E.; Jacobsen, S.E.W.; Wakkach, A.; Blin-Wakkach, C. Osteoclasts promote the formation of hematopoietic stem cell niches in the bone marrow. J. Exp. Med. 2012, 209, 537–549. [Google Scholar] [CrossRef]
- Tagaya, H.; Kunisada, T.; Yamazaki, H.; Yamane, T.; Tokuhisa, T.; Wagner, E.F.; Sudo, T.; Shultz, L.D.; Hayashi, S.I. Intramedullary and extramedullary B lymphopoiesis in osteopetrotic mice. Blood 2000, 95, 3363–3370. [Google Scholar] [CrossRef]
- Zeytin, I.C.; Alkan, B.; Ozdemir, C.; Cetinkaya, D.; Okur, F.V. Modeling osteoclast defect and altered hematopoietic stem cell niche in osteopetrosis with patient-derived iPSCs. Res. Sq. 2021, 1. [Google Scholar] [CrossRef]
- Mansour, A.; Wakkach, A.; Blin-Wakkach, C. Emerging Roles of Osteoclasts in the Modulation of Bone Microenvironment and Immune Suppression in Multiple Myeloma. Front. Immunol. 2017, 8, 954. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, N.; Colla, S.; Rizzoli, V. New insight in the mechanism of osteoclast activation and formation in multiple myeloma: Focus on the receptor activator of NF-kappaB ligand (RANKL). Exp. Hematol. 2004, 32, 685–691. [Google Scholar] [CrossRef]
- Sezer, O.; Heider, U.; Zavrski, I.; Kühne, C.A.; Hofbauer, L.C.; Chiarle, R.; Gong, J.Z.; Guasparri, I.; Pesci, A.; Cai, J.; et al. RANK ligand and osteoprotegerin in myeloma bone disease. Blood 2003, 101, 2094–2098. [Google Scholar] [CrossRef] [PubMed]
- Pearse, R.N.; Sordillo, E.M.; Yaccoby, S.; Wong, B.R.; Liau, D.F.; Colman, N.; Michaeli, J.; Epstein, J.; Choi, Y. Multiple myeloma disrupts the TRANCE/ osteoprotegerin cytokine axis to trigger bone destruction and promote tumor progression. Proc. Natl. Acad. Sci. USA 2001, 98, 11581–11586. [Google Scholar] [CrossRef]
- Breitkreutz, I.; Raab, M.S.; Vallet, S.; Hideshima, T.; Raje, N.; Mitsiades, C.; Chauhan, D.; Okawa, Y.; Munshi, N.C.; Richardson, P.G.; et al. Lenalidomide inhibits osteoclastogenesis, survival factors and bone-remodeling markers in multiple myeloma. Leukemia 2008, 22, 1925–1932. [Google Scholar] [CrossRef] [PubMed]
- Hongming, H.; Jian, H. Bortezomib inhibits maturation and function of osteoclasts from PBMCs of patients with multiple myeloma by downregulating TRAF6. Leuk. Res. 2009, 33, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Omatsu, Y.; Sugiyama, T.; Kohara, H.; Kondoh, G.; Fujii, N.; Kohno, K.; Nagasawa, T. The essential functions of adipo-osteogenic progenitors as the hematopoietic stem and progenitor cell niche. Immunity 2010, 33, 387–399. [Google Scholar] [CrossRef]
- Sugiyama, T.; Kohara, H.; Noda, M.; Nagasawa, T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity 2006, 25, 977–988. [Google Scholar] [CrossRef]
- Schreck, C.; Bock, F.; Grziwok, S.; Oostendorp, R.A.; Istvánffy, R. Regulation of hematopoiesis by activators and inhibitors of Wnt signaling from the niche. Ann. N. Y. Acad. Sci. 2014, 1310, 32–43. [Google Scholar] [CrossRef]
- Anthony, B.A.; Link, D.C. Regulation of hematopoietic stem cells by bone marrow stromal cells. Trends Immunol. 2014, 35, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Tokoyoda, K.; Egawa, T.; Sugiyama, T.; Choi, B.-I.; Nagasawa, T. Cellular Niches Controlling B Lymphocyte Behavior within Bone Marrow during Development. Immunity 2004, 20, 707–718. [Google Scholar] [CrossRef]
- Greenbaum, A.; Hsu, Y.-M.S.; Day, R.B.; Schuettpelz, L.G.; Christopher, M.J.; Borgerding, J.N.; Nagasawa, T.; Link, D.C. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature 2013, 495, 227–230. [Google Scholar] [CrossRef]
- Shi, C.; Jia, T.; Mendez-Ferrer, S.; Hohl, T.M.; Serbina, N.V.; Lipuma, L.; Leiner, I.; Li, M.O.; Frenette, P.S.; Pamer, E.G. Bone marrow mesenchymal stem and progenitor cells induce monocyte emigration in response to circulating toll-like receptor ligands. Immunity. Immunity 2011, 34, 590–601. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, J.; Lei, W.; Wang, H.; Ni, Y.; Liu, Y.; Yan, H.L.; Tian, Y.; Wang, Z.; Yang, Z.; et al. CXCL12-CXCR4/CXCR7 Axis in Cancer: From Mechanisms to Clinical Applications. Int. J. Biol. Sci. 2023, 19, 3341–3359. [Google Scholar] [CrossRef] [PubMed]
- Domanska, U.M.; Kruizinga, R.C.; Nagengast, W.B.; Timmer-Bosscha, H.; Huls, G.; de Vries, E.G.E.; Walenkamp, A.M.E. A review on CXCR4/CXCL12 axis in oncology: No place to hide. Eur. J. Cancer 2013, 49, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Naveiras, O.; Nardi, V.; Wenzel, P.L.; Hauschka, P.V.; Fahey, F.; Daley, G.Q. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature 2009, 460, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Tratwal, J.; Rojas-Sutterlin, S.; Bataclan, C.; Blum, S.; Naveiras, O. Bone marrow adiposity and the hematopoietic niche: A historical perspective of reciprocity, heterogeneity, and lineage commitment. Best Pract. Res. Clin. Endocrinol. Metab. 2021, 35, 101564. [Google Scholar] [CrossRef] [PubMed]
- Cuminetti, V.; Arranz, L. Bone Marrow Adipocytes: The Enigmatic Components of the Hematopoietic Stem Cell Niche. J. Clin. Med. 2019, 8, 707. [Google Scholar] [CrossRef]
- Zinngrebe, J.; Debatin, K.M.; Fischer-Posovszky, P. Adipocytes in hematopoiesis and acute leukemia: Friends, enemies, or innocent bystanders? Leukemia 2020, 34, 2305–2316. [Google Scholar] [CrossRef] [PubMed]
- Corre, J.; Barreau, C.; Cousin, B.; Chavoin, J.; Caton, D.; Fournial, G.; Penicaud, L.; Casteilla, L.; Laharrague, P. Human subcutaneous adipose cells support complete differentiation but not self-renewal of hematopoietic progenitors. J. Cell. Physiol. 2006, 208, 282–288. [Google Scholar] [CrossRef]
- Robles, H.; Park, S.; Joens, M.S.; Fitzpatrick, J.A.; Craft, C.S.; Scheller, E.L. Characterization of the bone marrow adipocyte niche with three-dimensional electron microscopy. Bone 2018, 118, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Corre, J.; Planat-Benard, V.; Corberand, J.X.; Pénicaud, L.; Casteilla, L.; Laharrague, P. Human bone marrow adipocytes support complete myeloid and lymphoid differentiation from human CD34+ cells. Br. J. Haematol. 2004, 127, 344–347. [Google Scholar] [CrossRef]
- Morris, E.V.; Edwards, C.M. Bone Marrow Adipose Tissue: A New Player in Cancer Metastasis to Bone. Front. Endocrinol. 2016, 7, 90. [Google Scholar] [CrossRef]
- Jafari, A.; Fairfield, H.; Andersen, T.L.; Reagan, M.R. Myeloma-bone marrow adipocyte axis in tumour survival and treatment response. Br. J. Cancer 2021, 125, 775–777. [Google Scholar] [CrossRef]
- Morris, E.V.; Edwards, C.M. Adipokines, adiposity, and bone marrow adipocytes: Dangerous accomplices in multiple myeloma. J. Cell. Physiol. 2018, 233, 9159–9166. [Google Scholar] [CrossRef]
- Morris, E.V.; Edwards, C.M. Bone marrow adiposity and multiple myeloma. Bone 2019, 118, 42–46. [Google Scholar] [CrossRef]
- Caers, J.; Deleu, S.; Belaid, Z.; De Raeve, H.; Van Valckenborgh, E.; De Bruyne, E.; DeFresne, M.-P.; Van Riet, I.; Van Camp, B.; Vanderkerken, K. Neighboring adipocytes participate in the bone marrow microenvironment of multiple myeloma cells. Leukemia 2007, 21, 1580–1584. [Google Scholar] [CrossRef] [PubMed]
- Katayama, Y.; Battista, M.; Kao, W.M.; Hidalgo, A.; Peired, A.J.; Thomas, S.A.; Frenette, S. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell 2006, 124, 407–421. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Frenette, P.S. Niches for Hematopoietic Stem Cells and Their Progeny. Immunity 2018, 48, 632–648. [Google Scholar] [CrossRef]
- Scheiermann, C.; Kunisaki, Y.; Lucas, D.; Chow, A.; Jang, J.E.; Zhang, D.; Hashimoto, D.; Merad, M.; Frenette, P.S. Adrenergic nerves govern circadian leukocyte recruitment to tissues. Immunity 2012, 37, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Ikuta, K.; Scheiermann, C. Circadian Control of Immunity. Front. Immunol. 2020, 11, 618843. [Google Scholar] [CrossRef]
- Hanns, P.; Paczulla, A.M.; Medinger, M.; Konantz, M.; Lengerke, C. Stress and catecholamines modulate the bone marrow microenvironment to promote tumorigenesis. Cell Stress Chaperones 2019, 3, 221–235. [Google Scholar] [CrossRef]
- Arranz, L.; Sánchez-Aguilera, A.; Martín-Pérez, D.; Isern, J.; Langa, X.; Tzankov, A.; Lundberg, P.; Muntión, S.; Tzeng, Y.-S.; Lai, D.; et al. Neuropathy of haematopoietic stem cell niche is essential for myeloproliferative neoplasms. Nature 2014, 512, 78–81. [Google Scholar] [CrossRef]
- Hanoun, M.; Zhang, D.; Mizoguchi, T.; Pinho, S.; Pierce, H.; Kunisaki, Y.; Lacombe, J.; Armstrong, S.A.; Dührsen, U.; Frenette, P.S. Acute Myelogenous Leukemia-Induced Sympathetic Neuropathy Promotes Malignancy in an Altered Hematopoietic Stem Cell Niche. Cell Stem Cell 2014, 15, 365–375. [Google Scholar] [CrossRef]
- Hwa, Y.L.; Shi, Q.; Kumar, S.K.; Lacy, M.Q.; Gertz, M.A.; Kapoor, P.; Buadi, F.K.; Leung, N.; Dingli, D.; Go, R.S.; et al. Beta-blockers improve survival outcomes in patients with multiple myeloma: A retrospective evaluation. Am. J. Hematol. 2017, 92, 50–55. [Google Scholar] [CrossRef]
- Kozanoglu, I.; Yandim, M.K.; Cincin, Z.B.; Ozdogu, H.; Cakmakoglu, B.; Baran, Y. New indication for therapeutic potential of an old well-known drug (propranolol) for multiple myeloma. J. Cancer Res. Clin. Oncol. 2012, 139, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Zhan, H.; Kaushansky, K. Megakaryocytes as the Regulator of the Hematopoietic Vascular Niche. Front. Oncol. 2022, 12, 912060. [Google Scholar] [CrossRef]
- Pinho, S.; Marchand, T.; Yang, E.; Wei, Q.; Nerlov, C.; Frenette, P.S. Lineage-Biased Hematopoietic Stem Cells Are Regulated by Distinct Niches. Dev. Cell 2018, 44, 634–641.e4. [Google Scholar] [CrossRef]
- Noetzli, L.J.; French, S.L.; Machlus, K.R. New Insights Into the Differentiation of Megakaryocytes From Hematopoietic Progenitors. Arter. Thromb. Vasc. Biol. 2019, 39, 1288–1300. [Google Scholar] [CrossRef] [PubMed]
- Nakamura-Ishizu, A.; Takubo, K.; Fujioka, M.; Suda, T. Megakaryocytes are essential for HSC quiescence through the production of thrombopoietin. Biochem. Biophys. Res. Commun. 2014, 454, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Kao, C.Y.; Papoutsakis, E.T. How do megakaryocytic microparticles target and deliver cargo to alter the fate of hematopoietic stem cells? J. Control. Release 2017, 247, 1–18. [Google Scholar] [CrossRef]
- Lemancewicz, D.; Bolkun, L.; Mantur, M.; Semeniuk, J.; Kloczko, J.; Dzieciol, J. Bone marrow megakaryocytes, soluble P-selectin and thrombopoietic cytokines in multiple myeloma patients. Platelets 2014, 25, 181–187. [Google Scholar] [CrossRef]
- Wong, D.; Winter, O.; Hartig, C.; Siebels, S.; Szyska, M.; Tiburzy, B.; Meng, L.; Kulkarni, U.; Fähnrich, A.; Bommert, K.; et al. Eosinophils and megakaryocytes support the early growth of murine MOPC315 myeloma cells in their bone marrow niches. PLoS ONE 2014, 9, e109018. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Garcia-Gerique, L.; Bonner, E.E.; Mastio, J.; Rosenwasser, M.; Cruz, Z.; Lawler, M.; Bernabei, L.; Muthumani, K.; Liu, Q.; et al. S100A8/S100A9 Promote Progression of Multiple Myeloma via Expansion of Megakaryocytes. Cancer Res Commun. 2023, 3, 420–430. [Google Scholar] [CrossRef]
- Costes, V.; Portier, M.; Lu, Z.-Y.; Rossi, J.-F.; Bataille, R.; Klein, B. Interleukin-1 in multiple myeloma: Producer cells and their role in the control of IL-6 production. Br. J. Haematol. 1998, 103, 1152–1160. [Google Scholar] [CrossRef] [PubMed]
- Winkler, I.G.; Sims, N.A.; Pettit, A.R.; Barbier, V.; Nowlan, B.; Helwani, F.; Poulton, I.J.; Van Rooijen, N.; Alexander, K.; Raggatt, L.J.; et al. Bone marrow macrophages maintain hematopoietic stem cell (HSC) niches and their depletion mobilizes HSCs. Blood 2010, 116, 4815–4828. [Google Scholar] [CrossRef]
- Lévesque, J.-P.; Summers, K.M.; Millard, S.M.; Bisht, K.; Winkler, I.G.; Pettit, A.R. Role of macrophages and phagocytes in orchestrating normal and pathologic hematopoietic niches. Exp. Hematol. 2021, 100, 12–31.e1. [Google Scholar] [CrossRef] [PubMed]
- Hur, J.; Choi, J.I.; Lee, H.; Nham, P.; Kim, T.W.; Chae, C.W.; Yun, J.Y.; Kang, J.A.; Kang, J.; Lee, S.E.; et al. CD82/KAI1 Maintains the Dormancy of Long-Term Hematopoietic Stem Cells through Interaction with DARC-Expressing Macrophages. Cell Stem Cell 2016, 18, 508–521. [Google Scholar] [CrossRef]
- Man, Y.; Yao, X.; Yang, T.; Wang, Y. Hematopoietic Stem Cell Niche During Homeostasis, Malignancy, and Bone Marrow Transplantation. Front. Cell Dev. Biol. 2021, 9, 621214. [Google Scholar] [CrossRef] [PubMed]
- Chow, A.; Huggins, M.; Ahmed, J.; Hashimoto, D.; Lucas, D.; Kunisaki, Y.; Pinho, S.; Leboeuf, M.; Noizat, C.; van Rooijen, N.; et al. CD169+ macrophages provide a niche promoting erythropoiesis under homeostasis and stress. Nat. Med. 2013, 19, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Ludin, A.; Itkin, T.; Gur-Cohen, S.; Mildner, A.; Shezen, E.; Golan, K.; Kollet, O.; Kalinkovich, A.; Porat, Z.; D’Uva, G.; et al. Monocytes-macrophages that express α-smooth muscle actin preserve primitive hematopoietic cells in the bone marrow. Nat. Immunol. 2012, 13, 1072–1082. [Google Scholar] [CrossRef] [PubMed]
- Casanova-Acebes, M.; A-González, N.; Weiss, L.A.; Hidalgo, A. Innate immune cells as homeostatic regulators of the hematopoietic niche. Int. J. Hematol. 2014, 99, 685–694. [Google Scholar] [CrossRef]
- Suyanı, E.; Sucak, G.T.; Akyürek, N.; Şahin, S.; Baysal, N.A.; Yağcı, M.; Haznedar, R. Tumor-associated macrophages as a prognostic parameter in multiple myeloma. Ann. Hematol. 2013, 92, 669–677. [Google Scholar] [CrossRef]
- Chen, X.; Chen, J.; Zhang, W.; Sun, R.; Liu, T.; Zheng, Y.; Wu, Y. Prognostic value of diametrically polarized tumor-associated macrophages in multiple myeloma. Oncotarget 2017, 8, 112685–112696. [Google Scholar] [CrossRef]
- Wang, H.; Hu, W.-M.; Xia, Z.-J.; Liang, Y.; Lu, Y.; Lin, S.-X.; Tang, H. High numbers of CD163+ tumor-associated macrophages correlate with poor prognosis in multiple myeloma patients receiving bortezomib-based regimens. J. Cancer 2019, 10, 3239–3245. [Google Scholar] [CrossRef]
- Panchabhai, S.; Kelemen, K.; Ahmann, G.; Sebastian, S.; Mantei, J.; Fonseca, R. Tumor-associated macrophages and extracellular matrix metalloproteinase inducer in prognosis of multiple myeloma. Leukemia 2015, 30, 951–954. [Google Scholar] [CrossRef] [PubMed]
- Beider, K.; Bitner, H.; Leiba, M.; Gutwein, O.; Koren-Michowitz, M.; Ostrovsky, O.; Abraham, M.; Wald, H.; Galun, E.; Peled, A.; et al. Multiple myeloma cells recruit tumor-supportive macrophages through the CXCR4/CXCL12 axis and promote their polarization toward the M2 phenotype. Oncotarget. 2014, 5, 11283–11296. [Google Scholar] [CrossRef]
- Sun, J.; Park, C.; Guenthner, N.; Gurley, S.; Zhang, L.; Lubben, B.; Adebayo, O.; Bash, H.; Chen, Y.; Maksimos, M.; et al. Tumor-associated macrophages in multiple myeloma: Advances in biology and therapy. J. Immunother. Cancer 2022, 10, e003975. [Google Scholar] [CrossRef] [PubMed]
- Opperman, K.S.; Vandyke, K.; Psaltis, P.J.; Noll, J.E.; Zannettino, A.C.W. Macrophages in multiple myeloma: Key roles and therapeutic strategies. Cancer Metastasis Rev. 2021, 40, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Alexandrakis, M.G.; Goulidaki, N.; Pappa, C.A.; Boula, A.; Psarakis, F.; Neonakis, I.; Tsirakis, G. Interleukin-10 Induces Both Plasma Cell Proliferation and Angiogenesis in Multiple Myeloma. Pathol. Oncol. Res. 2015, 21, 929–934. [Google Scholar] [CrossRef]
- Berardi, S.; Ria, R.; Reale, A.; De Luisi, A.; Catacchio, I.; Moschetta, M.; Vacca, A. Multiple myeloma macrophages: Pivotal players in the tumor microenvironment. J. Oncol. 2013, 2013, 183602. [Google Scholar] [CrossRef]
- Sun, M.; Xiao, Q.; Wang, X.; Yang, C.; Chen, C.; Tian, X.; Wang, S.; Li, H.; Qiu, S.; Shu, J.; et al. Tumor-associated macrophages modulate angiogenesis and tumor growth in a xenograft mouse model of multiple myeloma. Leuk. Res. 2021, 110, 106709. [Google Scholar] [CrossRef]
- Cencini, E.; Sicuranza, A.; Ciofini, S.; Fabbri, A.; Bocchia, M.; Gozzetti, A. Tumor-Associated Macrophages in Multiple Myeloma: Key Role in Disease Biology and Potential Therapeutic Implications. Curr. Oncol. 2023, 30, 6111–6133. [Google Scholar] [CrossRef]
- Liu, Y.; Yan, H.; Gu, H.; Zhang, E.; He, J.; Cao, W.; Qu, J.; Xu, R.; Cao, L.; He, D.; et al. Myeloma-derived IL-32γ induced PD-L1 expression in macrophages facilitates immune escape via the PFKFB3-JAK1 axis. OncoImmunology 2022, 11, 2057837. [Google Scholar] [CrossRef]
- Cencini, E.; Fabbri, A.; Sicuranza, A.; Gozzetti, A.; Bocchia, M. The Role of Tumor-Associated Macrophages in Hematologic Malignancies. Cancers 2021, 13, 3597. [Google Scholar] [CrossRef]
- Kim, J.; Denu, R.A.; Dollar, B.A.; Escalante, L.E.; Kuether, J.P.; Callander, N.S.; Asimakopoulos, F.; Hematti, P. Macrophages and mesenchymal stromal cells support survival and proliferation of multiple myeloma cells. Br. J. Haematol. 2012, 158, 336–346. [Google Scholar] [CrossRef]
- Lentzsch, S.; Gries, M.; Janz, M.; Bargou, R.; Dö, B.; Mapara, M.Y. Macrophage inflammatory protein 1-alpha (MIP-1α) triggers migration and signaling cascades mediating survival and proliferation in multiple myeloma (MM) cells. Blood 2003, 101, 3568–3573. [Google Scholar] [CrossRef]
- Vacca, A.; Ribatti, D. Angiogenesis and vasculogenesis in multiple myeloma: Role of inflammatory cells. Recent Results Cancer Res. 2011, 183, 87–95. [Google Scholar] [PubMed]
- Smith, L.K.; Boukhaled, G.M.; Condotta, S.A.; Mazouz, S.; Guthmiller, J.J.; Vijay, R.; Butler, N.S.; Bruneau, J.; Shoukry, N.H.; Krawczyk, C.M.; et al. Interleukin-10 Directly Inhibits CD8+ T Cell Function by Enhancing N-Glycan Branching to Decrease Antigen Sensitivity. Immunity 2018, 48, 299–312.e5. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Dong, M.; Liu, X.; Shen, Q.; He, D.; Huang, X.; Zhang, E.; Lin, X.; Chen, Q.; Guo, X.; et al. Multiple myeloma cell-derived IL-32γ increases the immunosuppressive function of macrophages by promoting indoleamine 2,3-dioxygenase (IDO) expression. Cancer Lett. 2019, 446, 38–48. [Google Scholar] [CrossRef]
- Cossío, I.; Lucas, D.; Hidalgo, A. Neutrophils as regulators of the hematopoietic niche. Blood 2019, 133, 2140–2148. [Google Scholar] [CrossRef]
- Bowers, E.; Slaughter, A.; Frenette, P.S.; Kuick, R.; Pello, O.M.; Lucas, D. Granulocyte-derived TNFα promotes vascular and hematopoietic regeneration in the bone marrow. Nat. Med. 2018, 24, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Casanova-Acebes, M.; Pitaval, C.; Weiss, L.A.; Nombela-Arrieta, C.; Chèvre, R.; A-González, N.; Kunisaki, Y.; Zhang, D.; van Rooijen, N.; Silberstein, L.E.; et al. Rhythmic modulation of the hematopoietic niche through neutrophil clearance. Cell 2013, 153, 1025–1035. [Google Scholar] [CrossRef]
- Stark, M.A.; Huo, Y.; Burcin, T.L.; Morris, M.A.; Olson, T.S.; Ley, K. Phagocytosis of Apoptotic Neutrophils Regulates Granulopoiesis via IL-23 and IL-17. Immunity 2005, 22, 285–294. [Google Scholar] [CrossRef]
- Ramachandran, I.R.; Condamine, T.; Lin, C.; Herlihy, S.E.; Garfall, A.; Vogl, D.T.; Gabrilovich, D.I.; Nefedova, Y. Bone marrow PMN-MDSCs and neutrophils are functionally similar in protection of multiple myeloma from chemotherapy. Cancer Lett. 2016, 371, 117–124. [Google Scholar] [CrossRef]
- Romano, A.; Parrinello, N.L.; Simeon, V.; Puglisi, F.; La Cava, P.; Bellofiore, C.; Giallongo, C.; Camiolo, G.; D’auria, F.; Grieco, V.; et al. High-density neutrophils in MGUS and multiple myeloma are dysfunctional and immune-suppressive due to increased STAT3 downstream signaling. Sci. Rep. 2020, 10, 1983. [Google Scholar] [CrossRef] [PubMed]
- Petersson, J.; Askman, S.; Pettersson, Å.; Wichert, S.; Hellmark, T.; Johansson, C.M.; Hansson, M. Bone Marrow Neutrophils of Multiple Myeloma Patients Exhibit Myeloid-Derived Suppressor Cell Activity. J. Immunol. Res. 2021, 2021, 6344344. [Google Scholar] [CrossRef]
- Botta, C.; Mendicino, F.; Martino, E.A.; Vigna, E.; Ronchetti, D.; Correale, P.; Morabito, F.; Neri, A.; Gentile, M. Mechanisms of Immune Evasion in Multiple Myeloma: Open Questions and Therapeutic Opportunities. Cancers 2021, 13, 3213. [Google Scholar] [CrossRef]
- Ho, M.; Xiao, A.; Yi, D.; Zanwar, S.; Bianchi, G. Treating Multiple Myeloma in the Context of the Bone Marrow Microenvironment. Curr. Oncol. 2022, 29, 8975–9005. [Google Scholar] [CrossRef] [PubMed]
- Alexander, L.D.; Dent, A.L.; Kaplan, M.H. T cell regulation of hematopoiesis. Front. Biosci. 2008, 13, 6229–6236. [Google Scholar] [CrossRef]
- Riether, C. Regulation of hematopoietic and leukemia stem cells by regulatory T cells. Front. Immunol. 2022, 13, 1049301. [Google Scholar] [CrossRef]
- Bonomo, A.; Monteiro, A.C.; Gonçalves-Silva, T.; Cordeiro-Spinetti, E.; Galvani, R.G.; Balduino, A. A T Cell View of the Bone Marrow. Front. Immunol. 2016, 7, 184. [Google Scholar] [CrossRef]
- Monteiro, J.P.; Benjamin, A.; Costa, E.S.; Barcinski, M.A.; Bonomo, A. Normal hematopoiesis is maintained by activated bone marrow CD4+ T cells. Blood 2005, 105, 1484–1491. [Google Scholar] [CrossRef] [PubMed]
- Gharaee-Kermani, M.; McGarry, B.; Lukacs, N.; Huffnagle, G.; Egan, R.W.; Phan, S.H. The role of IL-5 in bleomycin-induced pulmonary fibrosis. J. Leukoc. Biol. 1998, 64, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Pelaia, C.; Paoletti, G.; Puggioni, F.; Racca, F.; Pelaia, G.; Canonica, G.W.; Heffler, E. Interleukin-5 in the Pathophysiology of Severe Asthma. Front. Physiol. 2019, 10, 1514. [Google Scholar] [CrossRef]
- Zenobia, C.; Hajishengallis, G. Basic biology and role of interleukin-17 in immunity and inflammation. Periodontology 2000 2015, 69, 142–159. [Google Scholar] [CrossRef] [PubMed]
- Lopes, R.; Caetano, J.; Ferreira, B.; Barahona, F.; Carneiro, E.A.; João, C. The Immune Microenvironment in Multiple Myeloma: Friend or Foe? Cancers 2021, 13, 625. [Google Scholar] [CrossRef] [PubMed]
- Bryant, C.; Suen, H.; Brown, R.; Yang, S.; Favaloro, J.; Aklilu, E.; Gibson, J.; Ho, P.J.; Iland, H.; Fromm, P.; et al. Long-term survival in multiple myeloma is associated with a distinct immunological profile, which includes proliferative cytotoxic T-cell clones and a favourable Treg/Th17 balance. Blood Cancer J. 2013, 3, e148. [Google Scholar] [CrossRef]
- Brown, R.; Yuen, B.P.E.; Gibson, J.; Joshua, D. The Expression of T Cell Related Costimulatory Molecules in Multiple Myeloma. Leuk. Lymphoma 1998, 31, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Zelle-Rieser, C.; Thangavadivel, S.; Biedermann, R.; Brunner, A.; Stoitzner, P.; Willenbacher, E.; Greil, R.; Jöhrer, K. T cells in multiple myeloma display features of exhaustion and senescence at the tumor site. J. Hematol. Oncol. 2016, 9, 116. [Google Scholar] [CrossRef]
- Lagreca, I.; Riva, G.; Nasillo, V.; Barozzi, P.; Castelli, I.; Basso, S.; Bettelli, F.; Giusti, D.; Cuoghi, A.; Bresciani, P.; et al. The Role of T Cell Immunity in Monoclonal Gammopathy and Multiple Myeloma: From Immunopathogenesis to Novel Therapeutic Approaches. Int. J. Mol. Sci. 2022, 23, 5242. [Google Scholar] [CrossRef] [PubMed]
- Cook, G.; Campbell, J.D.M.; Carr, C.E.; Boyd, K.S.; Franklin, I.M. Transforming growth factor β from multiple myeloma cells inhibits proliferation and IL-2 responsiveness in T lymphocytes. J. Leukoc. Biol. 1999, 66, 981–988. [Google Scholar] [CrossRef]
- Brown, R.; Suen, H.; Favaloro, J.; Yang, S.; Ho, P.J.; Gibson, J.; Joshua, D. Trogocytosis generates acquired regulatory T cells adding further complexity to the dysfunctional immune response in multiple myeloma. OncoImmunology 2012, 1, 1658–1660. [Google Scholar] [CrossRef][Green Version]
- Joshua, D.E.; Vuckovic, S.; Favaloro, J.; Lau, K.H.A.; Yang, S.; Bryant, C.E.; Gibson, J.; Ho, P.J. Treg and Oligoclonal Expansion of Terminal Effector CD8+ T Cell as Key Players in Multiple Myeloma. Front. Immunol. 2021, 12, 620596. [Google Scholar] [CrossRef]
- Bonneville, M.; O’Brien, R.L.; Born, W.K. Gammadelta T cell effector functions: A blend of innate programming and acquired plasticity. Nat. Rev. Immunol. 2010, 10, 467–478. [Google Scholar] [CrossRef]
- Patel, S.B.; Pietras, E.M. B cells regulate hematopoietic stem cells via cholinergic signaling. Nat. Immunol. 2022, 23, 476–478. [Google Scholar] [CrossRef] [PubMed]
- Kanayama, M.; Izumi, Y.; Akiyama, M.; Hayashi, T.; Atarashi, K.; Roers, A.; Sato, T.; Ohteki, T. Myeloid-like B cells boost emergency myelopoiesis through IL-10 production during infection. J. Exp. Med. 2023, 220, e20221221. [Google Scholar] [CrossRef]
- Meng, L.; Almeida, L.N.; Clauder, A.-K.; Lindemann, T.; Luther, J.; Link, C.; Hofmann, K.; Kulkarni, U.; Wong, D.M.; David, J.-P.; et al. Bone Marrow Plasma Cells Modulate Local Myeloid-Lineage Differentiation via IL-10. Front. Immunol. 2019, 10, 1183. [Google Scholar] [CrossRef]
- Audzevich, T.; Bashford-Rogers, R.; Mabbott, N.A.; Frampton, D.; Freeman, T.C.; Potocnik, A.; Kellam, P.; Gilroy, D.W. Pre/pro-B cells generate macrophage populations during homeostasis and inflammation. Proc. Natl. Acad. Sci. USA 2017, 114, E3954–E3963. [Google Scholar] [CrossRef]
- Mahindra, A.; Hideshima, T.; Anderson, K.C. Multiple myeloma: Biology of the disease. Blood Rev. 2010, 24 (Suppl. 1), S5–S11. [Google Scholar] [CrossRef]
- Uchiyama, H.; Barut, B.A.; Mohrbacher, A.F.; Chauhan, D.; Anderson, K.C. Adhesion of human myeloma-derived cell lines to bone marrow stromal cells stimulates interleukin-6 secretion. Blood 1993, 82, 3712–3720. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, D.; Uchiyama, H.; Akbarali, Y.; Urashima, M.; Yamamoto, K.; Libermann, T.; Anderson, K. Multiple myeloma cell adhesion-induced interleukin-6 expression in bone marrow stromal cells involves activation of NF-kappa B. Blood 1996, 87, 1104–1112. [Google Scholar] [CrossRef]
- Shapiro-Shelef, M.; Calame, K. Plasma cell differentiation and multiple myeloma. Curr. Opin. Immunol. 2004, 16, 226–234. [Google Scholar] [CrossRef]
- Pilarski, L.M.; Mant, M.J.; Ruether, B.A. Pre-B cells in peripheral blood of multiple myeloma patients. Blood 1985, 66, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Pilarski, L.M.; Masellis-Smith, A.; Szczepek, A.; Mant, M.J.; Belch, A.R. Circulating Clonotypic B Cells in the Biology of Multiple Myeloma: Speculations on the Origin of Myeloma. Leuk. Lymphoma 1996, 22, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Santonocito, A.M.; Consoli, U.; Bagnato, S.; Milone, G.; Palumbo, G.A.; Di Raimondo, F.; Stagno, F.; Guglielmo, P.; Giustolisi, R. Flow cytometric detection of aneuploid CD38(++) plasmacells and CD19(+) B-lymphocytes in bone marrow, peripheral blood and PBSC harvest in multiple myeloma patients. Leuk. Res. 2004, 28, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Novak, A.J.; Grote, D.M.; Ziesmer, S.C.; Rajkumar, V.; Doyle, S.E.; Ansell, S.M. A role for IFN-λ1 in multiple myeloma B cell growth. Leukemia 2008, 22, 2240–2246. [Google Scholar] [CrossRef] [PubMed]
- Boucher, K.; Parquet, N.; Widen, R.; Shain, K.; Baz, R.; Alsina, M.; Koomen, J.; Anasetti, C.; Dalton, W.; Perez, L.E. Stemness of B-cell Progenitors in Multiple Myeloma Bone Marrow. Clin. Cancer Res. 2012, 18, 6155–6168. [Google Scholar] [CrossRef] [PubMed]
- Sahota, S.; Hamblin, T.; Oscier, D.G.; Stevenson, F. Assessment of the role of clonogenic B lymphocytes in the pathogenesis of multiple myeloma. Leukemia 1994, 8, 1285–1289. [Google Scholar] [PubMed]
- Murphy, W.J.; Keller, J.R.; Harrison, C.L.; Young, H.A.; Longo, D.L. Interleukin-2-activated natural killer cells can support hematopoiesis in vitro and promote marrow engraftment in vivo. Blood 1992, 80, 670–677. [Google Scholar] [CrossRef] [PubMed]
- Murphy, W.J.; Longo, D.L. NK Cells in the Regulation of Hematopoiesis. Methods 1996, 9, 344–351. [Google Scholar] [CrossRef]
- Jurisic, V.; Srdic, T.; Konjevic, G.; Markovic, O.; Colovic, M. Clinical stage-depending decrease of NK cell activity in multiple myeloma patients. Med. Oncol. 2007, 24, 312–317. [Google Scholar] [CrossRef]
- Frassanito, M.A.; Silvestris, F.; Cafforio, P.; Silvestris, N.; Dammacco, F. IgG M-components in active myeloma patients induce a down-regulation of natural killer cell activity. Int. J. Clin. Lab. Res. 1997, 27, 48–54. [Google Scholar] [CrossRef]
- García-Sanz, R.; González, M.; Orfão, A.; Moro, M.J.; Hernández, J.M.; Borrego, D.; Carnero, M.; Casanova, F.; Bárez, A.; Jiménez, R.; et al. Analysis of natural killer-associated antigens in peripheral blood and bone marrow of multiple myeloma patients and prognostic implications. Br. J. Haematol. 1996, 93, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Schütt, P.; Brandhorst, D.; Stellberg, W.; Poser, M.; Ebeling, P.; Müller, S.; Buttkereit, U.; Opalka, B.; Lindemann, M.; Grosse-Wilde, H.; et al. Immune parameters in multiple myeloma patients: Influence of treatment and correlation with opportunistic infections. Leuk. Lymphoma 2006, 47, 1570–1582. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, J.; Benson, D.M., Jr. The role of natural killer cells in immunity against multiple myeloma. Leuk. Lymphoma 2012, 53, 1666–1676. [Google Scholar] [CrossRef]
- Moţa, G.; Galatiuc, C.; Popescu, I.; Hirt, M.; Cialâcu, V.; Sulică, A. IgA monoclonal and polyclonal proteins as regulatory factors of the NK cytotoxic activity. Rom. J. Virol. 2001, 50, 17–31. [Google Scholar]
- Ponzetta, A.; Benigni, G.; Antonangeli, F.; Sciumè, G.; Sanseviero, E.; Zingoni, A.; Ricciardi, M.R.; Petrucci, M.T.; Santoni, A.; Bernardini, G. Multiple Myeloma Impairs Bone Marrow Localization of Effector Natural Killer Cells by Altering the Chemokine Microenvironment. Cancer Res. 2015, 75, 4766–4777. [Google Scholar] [CrossRef] [PubMed]
- Sapoznikov, A.; Pewzner-Jung, Y.; Kalchenko, V.; Krauthgamer, R.; Shachar, I.; Jung, S. Perivascular clusters of dendritic cells provide critical survival signals to B cells in bone marrow niches. Nat. Immunol. 2008, 9, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Cavanagh, L.L.; Bonasio, R.; Mazo, I.B.; Halin, C.; Cheng, G.; van der Velden, A.W.M.; Cariappa, A.; Chase, C.; Russell, P.; Starnbach, M.N.; et al. Activation of bone marrow-resident memory T cells by circulating, antigen-bearing dendritic cells. Nat. Immunol. 2005, 6, 1029. [Google Scholar] [CrossRef]
- Christopher, M.J.; Rao, M.; Liu, F.; Woloszynek, J.R.; Link, D.C. Expression of the G-CSF receptor in monocytic cells is sufficient to mediate hematopoietic progenitor mobilization by G-CSF in mice. J. Exp. Med. 2011, 208, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Melchers, F. Checkpoints that control B cell development. J. Clin. Investig. 2015, 125, 2203–2210. [Google Scholar] [CrossRef]
- Kim, T.S.; Hanak, M.; Trampont, P.C.; Braciale, T.J. Stress-associated erythropoiesis initiation is regulated by type 1 conventional dendritic cells. J. Clin. Investig. 2015, 125, 3965–3980. [Google Scholar] [CrossRef]
- Chauhan, D.; Singh, A.V.; Brahmandam, M.; Carrasco, R.; Bandi, M.; Hideshima, T.; Bianchi, G.; Podar, K.; Tai, Y.-T.; Mitsiades, C.; et al. Functional interaction of plasmacytoid dendritic cells with multiple myeloma cells: A therapeutic target. Cancer Cell 2009, 16, 309–323. [Google Scholar] [CrossRef] [PubMed]
- Kukreja, A.; Hutchinson, A.; Dhodapkar, K.; Mazumder, A.; Vesole, D.; Angitapalli, R.; Jagannath, S.; Dhodapkar, M.V. Enhancement of clonogenicity of human multiple myeloma by dendritic cells. J. Exp. Med. 2006, 203, 1859–1865. [Google Scholar] [CrossRef]
- Brimnes, M.K.; Svane, I.M.; Johnsen, H.E. Impaired functionality and phenotypic profile of dendritic cells from patients with multiple myeloma. Clin. Exp. Immunol. 2006, 144, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.; Das, D.S.; Song, Y.; Richardson, P.; Munshi, N.C.; Chauhan, D.; Anderson, K.C. Targeting PD1–PDL1 immune checkpoint in plasmacytoid dendritic cell interactions with T cells, natural killer cells and multiple myeloma cells. Leukemia 2015, 29, 1441–1444. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.D.; Pope, B.; Murray, A.; Esdale, W.; Sze, D.M.; Gibson, J.; Ho, P.J.; Hart, D.; Joshua, D. Dendritic cells from patients with myeloma are numerically normal but functionally defective as they fail to up-regulate CD80 (B7-1) expression after huCD40LT stimulation because of inhibition by transforming growth factor-beta1 and interleukin-10. Blood 2001, 98, 2992–2998. [Google Scholar] [CrossRef]
- Ratta, M.; Fagnoni, F.; Curti, A.; Vescovini, R.; Sansoni, P.; Oliviero, B.; Fogli, M.; Ferri, E.; Della Cuna, G.R.; Tura, S.; et al. Dendritic cells are functionally defective in multiple myeloma: The role of interleukin-6. Blood 2002, 100, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Tucci, M.; Stucci, S.; Strippoli, S.; Dammacco, F.; Silvestris, F. Dendritic cells and malignant plasma cells: An alliance in multiple myeloma tumor progression? Oncologist 2011, 16, 1040–1048. [Google Scholar] [CrossRef] [PubMed]
- Meier, J.; Jensen, J.L.; Dittus, C.; Coombs, C.C.; Rubinstein, S. Game of clones: Diverse implications for clonal hematopoiesis in lymphoma and multiple myeloma. Blood Rev. 2022, 56, 100986. [Google Scholar] [CrossRef]
- Testa, S.; Kumar, J.; Goodell, A.J.; Zehnder, J.L.; Alexander, K.M.; Sidana, S.; Arai, S.; Witteles, R.M.; Liedtke, M. Prevalence, mutational spectrum and clinical implications of clonal hematopoiesis of indeterminate potential in plasma cell dyscrasias. Semin. Oncol. 2022, 49, 465–475. [Google Scholar] [CrossRef]
- DeStefano, C.B.; Gibson, S.J.; Sperling, A.S.; Richardson, P.G.; Ghobrial, I.; Mo, C.C. The emerging importance and evolving understanding of clonal hematopoiesis in multiple myeloma. Semin. Oncol. 2022, 49, 19–26. [Google Scholar] [CrossRef]
- Bou Zerdan, M.; Nasr, L.; Saba, L.; Meouchy, P.; Safi, N.; Allam, S.; Bhandari, J.; Chaulagain, C.P. A Synopsis Clonal Hematopoiesis of Indeterminate Potential in Hematology. Cancers 2022, 14, 3663. [Google Scholar] [CrossRef] [PubMed]
- Gelli, E.; Laudisi, A.; Martinuzzi, C.; Soncini, D.; Becherini, P.; Guolo, F.; Conticello, C.; Derudas, D.; Di Raimondo, F.; Cagnetta, A.; et al. Clonal Hematopoiesis: Exploiting Molecular Landscape of Multiple Myeloma Patients for Choosing the Most Appropriate Therapeutic Strategy. Blood 2022, 140 (Suppl. 1), 7064–7065. [Google Scholar] [CrossRef]
- Mouhieddine, T.H.; Sperling, A.S.; Redd, R.; Park, J.; Leventhal, M.; Gibson, C.J.; Manier, S.; Nassar, A.H.; Capelletti, M.; Huynh, D.; et al. Clonal hematopoiesis is associated with adverse outcomes in multiple myeloma patients undergoing transplant. Nat. Commun. 2020, 11, 2996. [Google Scholar] [CrossRef] [PubMed]
- Heuser, M.; Thol, F.; Ganser, A. Clonal Hematopoiesis of Indeterminate Potential. Dtsch. Ärzteblatt Int. 2016, 113, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Lyons, Y.A.; Wu, S.Y.; Overwijk, W.W.; Baggerly, K.A.; Sood, A.K. Immune cell profiling in cancer: Molecular approaches to cell-specific identification. Npj Precis. Oncol. 2017, 1, 26. [Google Scholar] [CrossRef]
- Ledergor, G.; Weiner, A.; Zada, M.; Wang, S.-Y.; Cohen, Y.C.; Gatt, M.E.; Snir, N.; Magen, H.; Koren-Michowitz, M.; Herzog-Tzarfati, K.; et al. Single cell dissection of plasma cell heterogeneity in symptomatic and asymptomatic myeloma. Nat. Med. 2018, 24, 1867–1876. [Google Scholar] [CrossRef]
- Khoo, W.H.; Ledergor, G.; Weiner, A.; Roden, D.L.; Terry, R.L.; McDonald, M.M.; Chai, R.C.; De Veirman, K.; Owen, K.L.; Opperman, K.S.; et al. A niche-dependent myeloid transcriptome signature defines dormant myeloma cells. Blood 2019, 134, 30–43. [Google Scholar] [CrossRef]
- Zavidij, O.; Haradhvala, N.J.; Mouhieddine, T.H.; Sklavenitis-Pistofidis, R.; Cai, S.; Reidy, M.; Rahmat, M.; Flaifel, A.; Ferland, B.; Su, N.K.; et al. Single-cell RNA sequencing reveals compromised immune microenvironment in precursor stages of multiple myeloma. Nat. Cancer 2020, 1, 493–506. [Google Scholar] [CrossRef]
- Liu, R.; Gao, Q.; Foltz, S.M.; Fowles, J.S.; Yao, L.; Wang, J.T.; Cao, S.; Sun, H.; Wendl, M.C.; Sethuraman, S.; et al. Co-evolution of tumor and immune cells during progression of multiple myeloma. Nat. Commun. 2021, 12, 282. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Jayasinghe, R.G.; Lee, B.H.; Bhasin, S.S.; Pilcher, W.; Doxie, D.B.; Gonzalez-Kozlova, E.; Dasari, S.; Fiala, M.A.; Pita-Juarez, Y.; et al. Comprehensive Characterization of the Multiple Myeloma Immune Microenvironment Using Integrated scRNA-seq, CyTOF, and CITE-seq Analysis. Cancer Res. Commun. 2022, 2, 1255–1265. [Google Scholar] [CrossRef]
- Chen, M.; Wan, Y.; Li, X.; Xiang, J.; Chen, X.; Jiang, J.; Han, X.; Zhong, L.; Xiao, F.; Liu, J.; et al. Dynamic single-cell RNA-seq analysis reveals distinct tumor program associated with microenvironmental remodeling and drug sensitivity in multiple myeloma. Cell Biosci. 2023, 13, 19. [Google Scholar] [CrossRef]
- Li, J.; Yang, Y.; Wang, W.; Xu, J.; Sun, Y.; Jiang, J.; Tan, H.; Ren, L.; Wang, Y.; Ren, Y.; et al. Single-cell atlas of the immune microenvironment reveals macrophage reprogramming and the potential dual macrophage-targeted strategy in multiple myeloma. Br. J. Haematol. 2023, 201, 917–934. [Google Scholar] [CrossRef]
- Jiang, J.; Xiang, J.; Chen, M.; Wan, Y.; Zhong, L.; Han, X.; Chen, X.; Wang, J.; Xiao, F.; Liu, J.; et al. Distinct mechanisms of dysfunctional antigen-presenting DCs and monocytes by single-cell sequencing in multiple myeloma. Cancer Sci. 2023, 114, 2750–2760. [Google Scholar] [CrossRef] [PubMed]
- Donnall Thomas, E. A history of haemopoietic cell transplantation. Br. J. Haematol. 1999, 105, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Lanier, O.L.; Pérez-Herrero, E.; Andrea, A.P.D.; Bahrami, K.; Lee, E.; Ward, D.M.; Ayala-Suárez, N.; Rodríguez-Méndez, S.M.; Peppas, N.A. Immunotherapy approaches for hematological cancers. iScience 2022, 25, 105326. [Google Scholar] [CrossRef] [PubMed]
- Holthof, L.C.; Mutis, T. Challenges for Immunotherapy in Multiple Myeloma: Bone Marrow Microenvironment-Mediated Immune Suppression and Immune Resistance. Cancers 2020, 12, 988. [Google Scholar] [CrossRef]
- A Shah, U.; Mailankody, S. Emerging immunotherapies in multiple myeloma. BMJ 2020, 370, m3176. [Google Scholar] [CrossRef]
- Ntanasis-Stathopoulos, I.; Gavriatopoulou, M.; Kastritis, E.; Terpos, E.; Dimopoulos, M.A. Multiple myeloma: Role of autologous transplantation. Cancer Treat. Rev. 2020, 82, 101929. [Google Scholar] [CrossRef]
- Mateos, M.-V.; Ludwig, H.; Bazarbachi, A.; Beksac, M.; Bladé, J.; Boccadoro, M.; Cavo, M.; Delforge, M.; Dimopoulos, M.A.; Facon, T.; et al. Insights on Multiple Myeloma Treatment Strategies. HemaSphere 2019, 3, e163. [Google Scholar] [CrossRef]
- Gandolfi, S.; Laubach, J.P.; Hideshima, T.; Chauhan, D.; Anderson, K.C.; Richardson, P.G. The proteasome and proteasome inhibitors in multiple myeloma. Cancer Metastasis Rev. 2017, 36, 561–584. [Google Scholar] [CrossRef]
- Palma, B.D.; Marchica, V.; Catarozzo, M.T.; Giuliani, N.; Accardi, F. Monoclonal and Bispecific Anti-BCMA Antibodies in Multiple Myeloma. J. Clin. Med. 2020, 9, 3022. [Google Scholar] [CrossRef]
- Gogishvili, T.; Danhof, S.; Prommersberger, S.; Rydzek, J.; Schreder, M.; Brede, C.; Einsele, H.; Hudecek, M. SLAMF7-CAR T cells eliminate myeloma and confer selective fratricide of SLAMF7+ normal lymphocytes. Blood 2017, 130, 2838–2847. [Google Scholar] [CrossRef]
- Nishida, H.; Yamada, T. Monoclonal Antibody Therapies in Multiple Myeloma: A Challenge to Develop Novel Targets. J. Oncol. 2019, 2019, 6084012. [Google Scholar] [CrossRef] [PubMed]
- Bapatla, A.; Kaul, A.; Dhalla, P.S.; Armenta-Quiroga, A.S.; Khalid, R.; Garcia, J.; Khan, S. Role of Daratumumab in Combination With Standard Therapies in Patients With Relapsed and Refractory Multiple Myeloma. Cureus 2021, 13, e15440. [Google Scholar] [CrossRef] [PubMed]
- Trudel, S.; Moreau, P.; Touzeau, C. Update on elotuzumab for the treatment of relapsed/refractory multiple myeloma: Patients’ selection and perspective. OncoTargets Ther. 2019, 12, 5813–5822. [Google Scholar] [CrossRef] [PubMed]
- Abodunrin, F.O.; Tauseef, A.; Silberstein, P. Role of Daratumumab in Relapsed and Refractory Multiple Myeloma Patients: Meta-Analysis and Literature Review. Blood 2021, 138, 4734. [Google Scholar] [CrossRef]
- Bruzzese, A.; Martino, E.A.; Vigna, E.; Iaccino, E.; Mendicino, F.; Lucia, E.; Olivito, V.; Filippelli, G.; Neri, A.; Morabito, F.; et al. Elotuzumab in multiple myeloma. Expert Opin. Biol. Ther. 2022, 23, 7–10. [Google Scholar] [CrossRef]
- Grosicki, S.; Bednarczyk, M.; Barchnicka, A.; Grosicka, O. Elotuzumab in the treatment of relapsed and refractory multiple myeloma. Futur. Oncol. 2021, 17, 1581–1591. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, R.O.; Evbuomwan, M.O.; Pittaluga, S.; Rose, J.J.; Raffeld, M.; Yang, S.; Gress, R.E.; Hakim, F.T.; Kochenderfer, J.N. B-cell maturation antigen is a promising target for adoptive T-cell therapy of multiple myeloma. Clin. Cancer Res. 2013, 19, 2048–2060. [Google Scholar] [CrossRef]
- Ali, S.A.; Shi, V.; Maric, I.; Wang, M.; Stroncek, D.F.; Rose, J.J.; Brudno, J.N.; Stetler-Stevenson, M.; Feldman, S.A.; Hansen, B.G.; et al. T cells expressing an anti–B-cell maturation antigen chimeric antigen receptor cause remissions of multiple myeloma. Blood 2016, 128, 1688–1700. [Google Scholar] [CrossRef]
- Raje, N.S.; Berdeja, J.G.; Lin, Y.; Munshi, N.C.; Siegel, D.S.D.; Liedtke, M.; Jagannath, S.; Madduri, D.; Rosenblatt, J.; Maus, M.V.; et al. bb2121 anti-BCMA CAR T-cell therapy in patients with relapsed/refractory multiple myeloma: Updated results from a multicenter phase I study. J. Clin. Oncol. 2018, 36 (Suppl. 15), 8007. [Google Scholar] [CrossRef]
- Zhao, W.H.; Liu, J.; Wang, B.-Y.; Chen, Y.-X.; Cao, X.-M.; Yang, Y.; Zhang, Y.-L.; Wang, F.-X.; Zhang, P.-Y.; Lei, B.; et al. A phase 1, open-label study of LCAR-B38M, a chimeric antigen receptor T cell therapy directed against B cell maturation antigen, in patients with relapsed or refractory multiple myeloma. J. Hematol. Oncol. 2018, 11, 141. [Google Scholar] [CrossRef]
- Prommersberger, S.; Reiser, M.; Beckmann, J.; Danhof, S.; Amberger, M.; Quade-Lyssy, P.; Einsele, H.; Hudecek, M.; Bonig, H.; Ivics, Z. CARAMBA: A first-in-human clinical trial with SLAMF7 CAR-T cells prepared by virus-free Sleeping Beauty gene transfer to treat multiple myeloma. Gene Ther. 2021, 28, 560–571. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.; Deng, Y.; Benson, D.M.; He, S.; Hughes, T.; Zhang, J.; Peng, Y.; Mao, H.; Yi, L.; Ghoshal, K.; et al. CS1-specific chimeric antigen receptor (CAR)-engineered natural killer cells enhance in vitro and in vivo antitumor activity against human multiple myeloma. Leukemia 2013, 28, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Pan, B.; Huang, H.; Xu, K. Non-BCMA targeted CAR-T cell therapies for multiple myeloma. ImmunoMedicine 2021, 1, e1030. [Google Scholar] [CrossRef]
- Gagelmann, N.; Riecken, K.; Wolschke, C.; Berger, C.; Ayuk, F.A.; Fehse, B.; Kröger, N. Development of CAR-T cell therapies for multiple myeloma. Leukemia 2020, 34, 2317–2332. [Google Scholar] [CrossRef]
- Bahlis, N.J.; Costello, C.L.; Raje, N.S.; Levy, M.Y.; Dholaria, B.; Solh, M.; Tomasson, M.H.; Damore, M.A.; Jiang, S.; Basu, C.; et al. Elranatamab in relapsed or refractory multiple myeloma: The MagnetisMM-1 phase 1 trial. Nat. Med. 2023, 29, 2023. [Google Scholar] [CrossRef]
- Caraccio, C.; Krishna, S.; Phillips, D.J.; Schürch, C.M. Bispecific Antibodies for Multiple Myeloma: A Review of Targets, Drugs, Clinical Trials, and Future Directions. Front. Immunol. 2020, 11, 501. [Google Scholar] [CrossRef]
- CTG Labs—NCBI. Available online: https://clinicaltrials.gov/search?cond=multiple%20myeloma&viewType=Table&limit=50&aggFilters=phase:1%202%203%204,results:with,status:com%20act%20not%20rec&intr=Immunotherapy (accessed on 23 September 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).