Preventive Effects of Collagen-Derived Dipeptide Prolyl-Hydroxyproline against Dexamethasone-Induced Muscle Atrophy in Mouse C2C12 Skeletal Myotubes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and Treatment
2.2. Immunofluorescence Analysis
2.3. RNA Preparation and Quantitative RT-PCR (qPCR)
2.4. Western Blot Assay
2.5. Statistical Analysis
3. Results
3.1. Pro-Hyp Suppressed DEX-Induced C2C12 Myotube Atrophy
3.2. Pro-Hyp Ameliorates Muscle-Atrophy-Associated Genes in DEX-Induced Myotube Atrophy
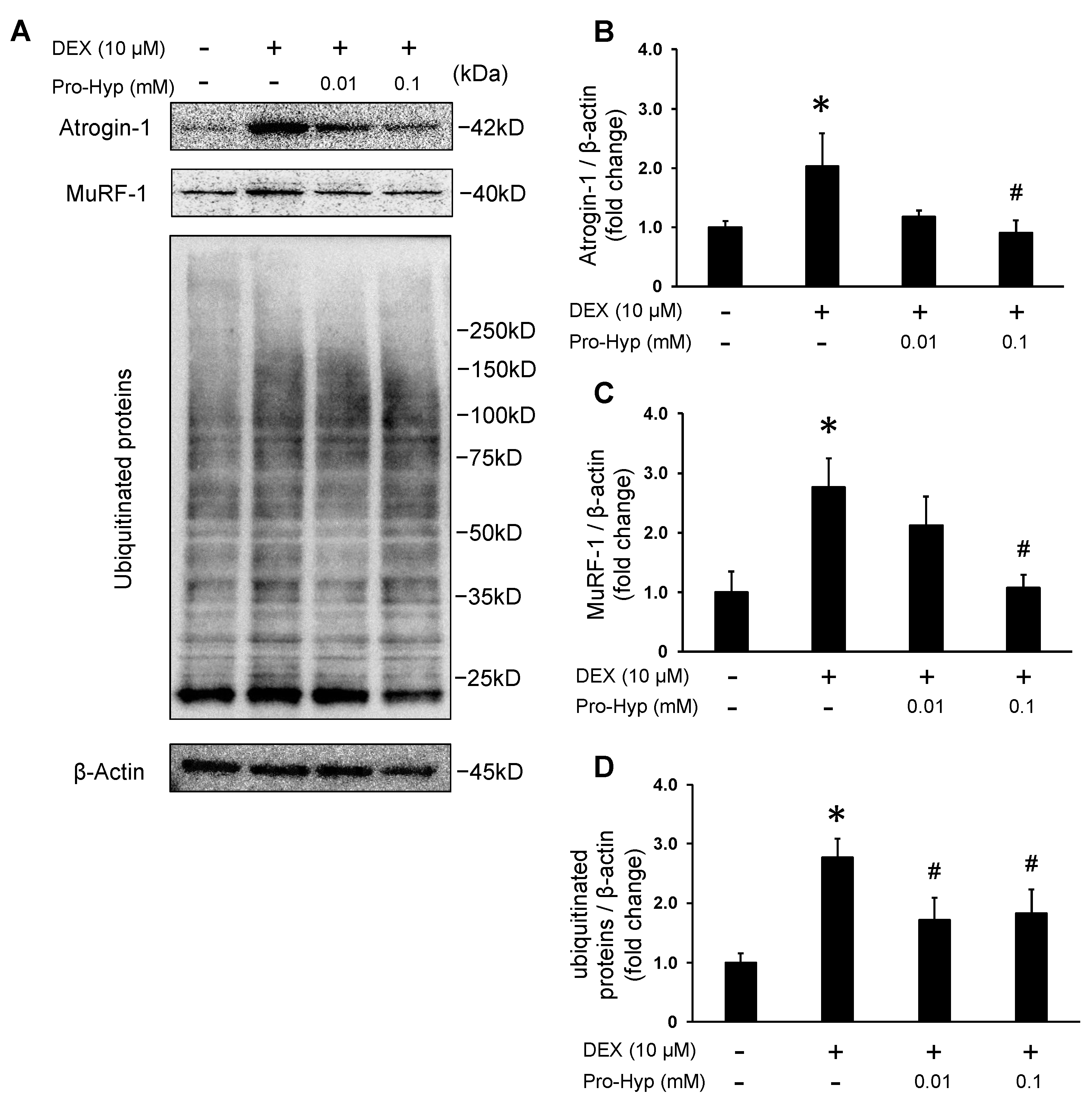
3.3. Pro-Hyp Attenuates Protein Levels of Muscle-Atrophy-Associated Ubiquitin Ligases and Ubiquitinated Proteins in DEX-Induced Myotube Atrophy
3.4. Pro-Hyp Prevented DEX-Induced Muscle Atrophy through Akt/mTOR/Foxo3a Signaling
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fanzani, A.; Conraads, V.M.; Penna, F.; Martinet, W. Molecular and Cellular Mechanisms of Skeletal Muscle Atrophy: An Update. J. Cachexia Sarcopenia Muscle 2012, 3, 163–179. [Google Scholar] [CrossRef]
- Mancini, D.M.; Walter, G.; Reichek, N.; Lenkinski, R.; McCully, K.K.; Mullen, J.L.; Wilson, J.R. Contribution of Skeletal Muscle Atrophy to Exercise Intolerance and Altered Muscle Metabolism in Heart Failure. Circulation 1992, 85, 1364–1373. [Google Scholar] [CrossRef] [PubMed]
- Dubashynskaya, N.V.; Bokatyi, A.N.; Skorik, Y.A. Dexamethasone Conjugates: Synthetic Approaches and Medical Prospects. Biomedicines 2021, 9, 341. [Google Scholar] [CrossRef]
- Schakman, O.; Kalista, S.; Barbé, C.; Loumaye, A.; Thissen, J.P. Glucocorticoid-Induced Skeletal Muscle Atrophy. Int. J. Biochem. Cell Biol. 2013, 45, 2163–2172. [Google Scholar] [CrossRef]
- Bodine, S.C.; Baehr, L.M. Skeletal Muscle Atrophy and the E3 Ubiquitin Ligases MuRF1 and MAFbx/Atrogin-1. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E469–E484. [Google Scholar] [CrossRef] [PubMed]
- Stitt, T.N.; Drujan, D.; Clarke, B.A.; Panaro, F.; Timofeyva, Y.; Kline, W.O.; Gonzalez, M.; Yancopoulos, G.D.; Glass, D.J. The IGF-1/PI3K/Akt Pathway Prevents Expression of Muscle Atrophy-Induced Ubiquitin Ligases by Inhibiting FOXO Transcription Factors. Mol. Cell 2004, 14, 395–403. [Google Scholar] [CrossRef]
- Kim, J.; Park, M.Y.; Kim, H.K.; Park, Y.; Whang, K.-Y. Cortisone and Dexamethasone Inhibit Myogenesis by Modulating the AKT/MTOR Signaling Pathway in C2C12. Biosci. Biotechnol. Biochem. 2016, 80, 2093–2099. [Google Scholar] [CrossRef]
- Ricard-Blum, S. The Collagen Family. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978. [Google Scholar] [CrossRef] [PubMed]
- Hulmes, D.J. The Collagen Superfamily—Diverse Structures and Assemblies. Essays Biochem. 1992, 27, 49–67. [Google Scholar] [PubMed]
- Ottani, V.; Raspanti, M.; Ruggeri, A. Collagen Structure and Functional Implications. Micron 2001, 32, 251–260. [Google Scholar] [CrossRef]
- Husek, P.; Svagera, Z.; Vsianský, F.; Franeková, J.; Simek, P. Prolyl-Hydroxyproline Dipeptide in Non-Hydrolyzed Morning Urine and Its Value in Postmenopausal Osteoporosis. Clin. Chem. Lab. Med. 2008, 46, 1391–1397. [Google Scholar] [CrossRef]
- Kusubata, M.; Koyama, Y.-I.; Tometsuka, C.; Shigemura, Y.; Sato, K. Detection of Endogenous and Food-Derived Collagen Dipeptide Prolylhydroxyproline (Pro-Hyp) in Allergic Contact Dermatitis-Affected Mouse Ear. Biosci. Biotechnol. Biochem. 2015, 79, 1356–1361. [Google Scholar] [CrossRef] [PubMed]
- Brodsky, B.; Ramshaw, J.A. The Collagen Triple-Helix Structure. Matrix Biol. 1997, 15, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Iwai, K.; Hasegawa, T.; Taguchi, Y.; Morimatsu, F.; Sato, K.; Nakamura, Y.; Higashi, A.; Kido, Y.; Nakabo, Y.; Ohtsuki, K. Identification of Food-Derived Collagen Peptides in Human Blood after Oral Ingestion of Gelatin Hydrolysates. J. Agric. Food Chem. 2005, 53, 6531–6536. [Google Scholar] [CrossRef] [PubMed]
- Watanabe-Kamiyama, M.; Shimizu, M.; Kamiyama, S.; Taguchi, Y.; Sone, H.; Morimatsu, F.; Shirakawa, H.; Furukawa, Y.; Komai, M. Absorption and Effectiveness of Orally Administered Low Molecular Weight Collagen Hydrolysate in Rats. J. Agric. Food Chem. 2010, 58, 835–841. [Google Scholar] [CrossRef] [PubMed]
- König, D.; Oesser, S.; Scharla, S.; Zdzieblik, D.; Gollhofer, A. Specific Collagen Peptides Improve Bone Mineral Density and Bone Markers in Postmenopausal Women—A Randomized Controlled Study. Nutrients 2018, 10, 97. [Google Scholar] [CrossRef]
- Bello, A.E.; Oesser, S. Collagen Hydrolysate for the Treatment of Osteoarthritis and Other Joint Disorders: A Review of the Literature. Curr. Med. Res. Opin. 2006, 22, 2221–2232. [Google Scholar] [CrossRef] [PubMed]
- Zdzieblik, D.; Oesser, S.; Gollhofer, A.; König, D. Improvement of Activity-Related Knee Joint Discomfort Following Supplementation of Specific Collagen Peptides. Appl. Physiol. Nutr. Metab. 2017, 42, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Shigemura, Y.; Iwai, K.; Morimatsu, F.; Iwamoto, T.; Mori, T.; Oda, C.; Taira, T.; Park, E.Y.; Nakamura, Y.; Sato, K. Effect of Prolyl-Hydroxyproline (Pro-Hyp), a Food-Derived Collagen Peptide in Human Blood, on Growth of Fibroblasts from Mouse Skin. J. Agric. Food Chem. 2009, 57, 444–449. [Google Scholar] [CrossRef]
- Kimira, Y.; Ogura, K.; Taniuchi, Y.; Kataoka, A.; Inoue, N.; Sugihara, F.; Nakatani, S.; Shimizu, J.; Wada, M.; Mano, H. Collagen-Derived Dipeptide Prolyl-Hydroxyproline Promotes Differentiation of MC3T3-E1 Osteoblastic Cells. Biochem. Biophys. Res. Commun. 2014, 453, 498–501. [Google Scholar] [CrossRef]
- Ide, K.; Takahashi, S.; Sakai, K.; Taga, Y.; Ueno, T.; Dickens, D.; Jenkins, R.; Falciani, F.; Sasaki, T.; Ooi, K.; et al. The Dipeptide Prolyl-Hydroxyproline Promotes Cellular Homeostasis and Lamellipodia-Driven Motility via Active Β1-Integrin in Adult Tendon Cells. J. Biol. Chem. 2021, 297, 100819. [Google Scholar] [CrossRef] [PubMed]
- Nakatani, S.; Mano, H.; Sampei, C.; Shimizu, J.; Wada, M. Chondroprotective Effect of the Bioactive Peptide Prolyl-Hydroxyproline in Mouse Articular Cartilage in Vitro and in Vivo. Osteoarthr. Cartil. 2009, 17, 1620–1627. [Google Scholar] [CrossRef]
- Kimira, Y.; Sato, T.; Sakamoto, M.; Osawa, Y.; Mano, H. Collagen-Derived Dipeptide Pro-Hyp Enhanced ATDC5 Chondrocyte Differentiation under Hypoxic Conditions. Molecules 2023, 28, 4664. [Google Scholar] [CrossRef]
- Kitakaze, T.; Sakamoto, T.; Kitano, T.; Inoue, N.; Sugihara, F.; Harada, N.; Yamaji, R. The Collagen Derived Dipeptide Hydroxyprolyl-Glycine Promotes C2C12 Myoblast Differentiation and Myotube Hypertrophy. Biochem. Biophys. Res. Commun. 2016, 478, 1292–1297. [Google Scholar] [CrossRef] [PubMed]
- Jendricke, P.; Centner, C.; Zdzieblik, D.; Gollhofer, A.; König, D. Specific Collagen Peptides in Combination with Resistance Training Improve Body Composition and Regional Muscle Strength in Premenopausal Women: A Randomized Controlled Trial. Nutrients 2019, 11, 892. [Google Scholar] [CrossRef]
- Zdzieblik, D.; Jendricke, P.; Oesser, S.; Gollhofer, A.; König, D. The Influence of Specific Bioactive Collagen Peptides on Body Composition and Muscle Strength in Middle-Aged, Untrained Men: A Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2021, 18, 4837. [Google Scholar] [CrossRef]
- Zdzieblik, D.; Oesser, S.; Baumstark, M.W.; Gollhofer, A.; König, D. Collagen Peptide Supplementation in Combination with Resistance Training Improves Body Composition and Increases Muscle Strength in Elderly Sarcopenic Men: A Randomised Controlled Trial. Br. J. Nutr. 2015, 114, 1237–1245. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Park, S.H.; Kim, D.S.; Choi, W.; Jang, J.; Rahmawati, L.; Jang, W.Y.; Lim, H.K.; Hwang, J.Y.; Gu, G.R.; et al. The Preventive Effect of Specific Collagen Peptides against Dexamethasone-Induced Muscle Atrophy in Mice. Molecules 2023, 28, 1950. [Google Scholar] [CrossRef]
- Kim, J.E.; Kwon, E.Y.; Han, Y. A Collagen Hydrolysate Containing Tripeptides Ameliorates Sarcopenia in Middle-Aged Mice. Molecules 2022, 27, 9. [Google Scholar] [CrossRef]
- Kimira, Y.; Odaira, H.; Nomura, K.; Taniuchi, Y.; Inoue, N.; Nakatani, S.; Shimizu, J.; Wada, M.; Mano, H. Collagen-Derived Dipeptide Prolyl-Hydroxyproline Promotes Osteogenic Differentiation through Foxg1. Cell. Mol. Biol. Lett. 2017, 22, 27. [Google Scholar] [CrossRef]
- Chen, L.; Chen, L.; Wan, L.; Huo, Y.; Huang, J.; Li, J.; Lu, J.; Xin, B.; Yang, Q.; Guo, C. Matrine Improves Skeletal Muscle Atrophy by Inhibiting E3 Ubiquitin Ligases and Activating the Akt/MTOR/FoxO3α Signaling Pathway in C2C12 Myotubes and Mice. Oncol. Rep. 2019, 42, 479–494. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.S.; Kong, I.D. Irisin Prevents Dexamethasone-Induced Atrophy in C2C12 Myotubes. Pflug. Arch. 2020, 472, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Blau, H.M.; Pavlath, G.K.; Hardeman, E.C.; Chiu, C.P.; Silberstein, L.; Webster, S.G.; Miller, S.C.; Webster, C. Plasticity of the Differentiated State. Science 1985, 230, 758–766. [Google Scholar] [CrossRef]
- Gomes, M.D.; Lecker, S.H.; Jagoe, R.T.; Navon, A.; Goldberg, A.L. Atrogin-1, a Muscle-Specific F-Box Protein Highly Expressed during Muscle Atrophy. Proc. Natl. Acad. Sci. USA 2001, 98, 14440–14445. [Google Scholar] [CrossRef] [PubMed]
- Lagirand-Cantaloube, J.; Offner, N.; Csibi, A.; Leibovitch, M.P.; Batonnet-Pichon, S.; Tintignac, L.A.; Segura, C.T.; Leibovitch, S.A. The Initiation Factor EIF3-f Is a Major Target for Atrogin1/MAFbx Function in Skeletal Muscle Atrophy. EMBO J. 2008, 27, 1266–1276. [Google Scholar] [CrossRef]
- Clarke, B.A.; Drujan, D.; Willis, M.S.; Murphy, L.O.; Corpina, R.A.; Burova, E.; Rakhilin, S.V.; Stitt, T.N.; Patterson, C.; Latres, E.; et al. The E3 Ligase MuRF1 Degrades Myosin Heavy Chain Protein in Dexamethasone-Treated Skeletal Muscle. Cell Metab. 2007, 6, 376–385. [Google Scholar] [CrossRef]
- Castillero, E.; Alamdari, N.; Lecker, S.H.; Hasselgren, P.-O. Suppression of Atrogin-1 and MuRF1 Prevents Dexamethasone-Induced Atrophy of Cultured Myotubes. Metabolism 2013, 62, 1495–1502. [Google Scholar] [CrossRef]
- Dedieu, S.; Mazères, G.; Cottin, P.; Brustis, J.-J. Involvement of Myogenic Regulator Factors during Fusion in the Cell Line C2C12. Int. J. Dev. Biol. 2002, 46, 235–241. [Google Scholar]
- Ferri, P.; Barbieri, E.; Burattini, S.; Guescini, M.; D’Emilio, A.; Biagiotti, L.; Del Grande, P.; De Luca, A.; Stocchi, V.; Falcieri, E. Expression and Subcellular Localization of Myogenic Regulatory Factors during the Differentiation of Skeletal Muscle C2C12 Myoblasts. J. Cell. Biochem. 2009, 108, 1302–1317. [Google Scholar] [CrossRef]
- Hahn-Windgassen, A.; Nogueira, V.; Chen, C.-C.; Skeen, J.E.; Sonenberg, N.; Hay, N. Akt Activates the Mammalian Target of Rapamycin by Regulating Cellular ATP Level and AMPK Activity. J. Biol. Chem. 2005, 280, 32081–32089. [Google Scholar] [CrossRef]
- Milan, G.; Romanello, V.; Pescatore, F.; Armani, A.; Paik, J.-H.; Frasson, L.; Seydel, A.; Zhao, J.; Abraham, R.; Goldberg, A.L.; et al. Regulation of Autophagy and the Ubiquitin–Proteasome System by the FoxO Transcriptional Network during Muscle Atrophy. Nat. Commun. 2015, 6, 6670. [Google Scholar] [CrossRef]
- Sandri, M.; Sandri, C.; Gilbert, A.; Skurk, C.; Calabria, E.; Picard, A.; Walsh, K.; Schiaffino, S.; Lecker, S.H.; Goldberg, A.L. Foxo Transcription Factors Induce the Atrophy-Related Ubiquitin Ligase Atrogin-1 and Cause Skeletal Muscle Atrophy. Cell 2004, 117, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Nomura, K.; Kimira, Y.; Osawa, Y.; Kataoka-Matsushita, A.; Takao, K.; Sugita, Y.; Shimizu, J.; Wada, M.; Mano, H. Stimulation of the Runx2 P1 Promoter by Collagen-Derived Dipeptide Prolyl-Hydroxyproline Bound to Foxg1 and Foxo1 in Osteoblasts. Biosci. Rep. 2021, 41, BSR20210304. [Google Scholar] [CrossRef] [PubMed]
- Sandri, M. Protein Breakdown in Muscle Wasting: Role of Autophagy-Lysosome and Ubiquitin-Proteasome. Int. J. Biochem. Cell Biol. 2013, 45, 2121–2129. [Google Scholar] [CrossRef]
- Sun, H.; Gong, Y.; Qiu, J.; Chen, Y.; Ding, F.; Zhao, Q. TRAF6 Inhibition Rescues Dexamethasone-Induced Muscle Atrophy. Int. J. Mol. Sci. 2014, 15, 11126–11141. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kimira, Y.; Osawa, K.; Osawa, Y.; Mano, H. Preventive Effects of Collagen-Derived Dipeptide Prolyl-Hydroxyproline against Dexamethasone-Induced Muscle Atrophy in Mouse C2C12 Skeletal Myotubes. Biomolecules 2023, 13, 1617. https://doi.org/10.3390/biom13111617
Kimira Y, Osawa K, Osawa Y, Mano H. Preventive Effects of Collagen-Derived Dipeptide Prolyl-Hydroxyproline against Dexamethasone-Induced Muscle Atrophy in Mouse C2C12 Skeletal Myotubes. Biomolecules. 2023; 13(11):1617. https://doi.org/10.3390/biom13111617
Chicago/Turabian StyleKimira, Yoshifumi, Konosuke Osawa, Yoshihiro Osawa, and Hiroshi Mano. 2023. "Preventive Effects of Collagen-Derived Dipeptide Prolyl-Hydroxyproline against Dexamethasone-Induced Muscle Atrophy in Mouse C2C12 Skeletal Myotubes" Biomolecules 13, no. 11: 1617. https://doi.org/10.3390/biom13111617
APA StyleKimira, Y., Osawa, K., Osawa, Y., & Mano, H. (2023). Preventive Effects of Collagen-Derived Dipeptide Prolyl-Hydroxyproline against Dexamethasone-Induced Muscle Atrophy in Mouse C2C12 Skeletal Myotubes. Biomolecules, 13(11), 1617. https://doi.org/10.3390/biom13111617





