Supplementing L-Citrulline Can Extend Lifespan in C. elegans and Attenuate the Development of Aging-Related Impairments of Glucose Tolerance and Intestinal Barrier in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Lifespan in C. elegans
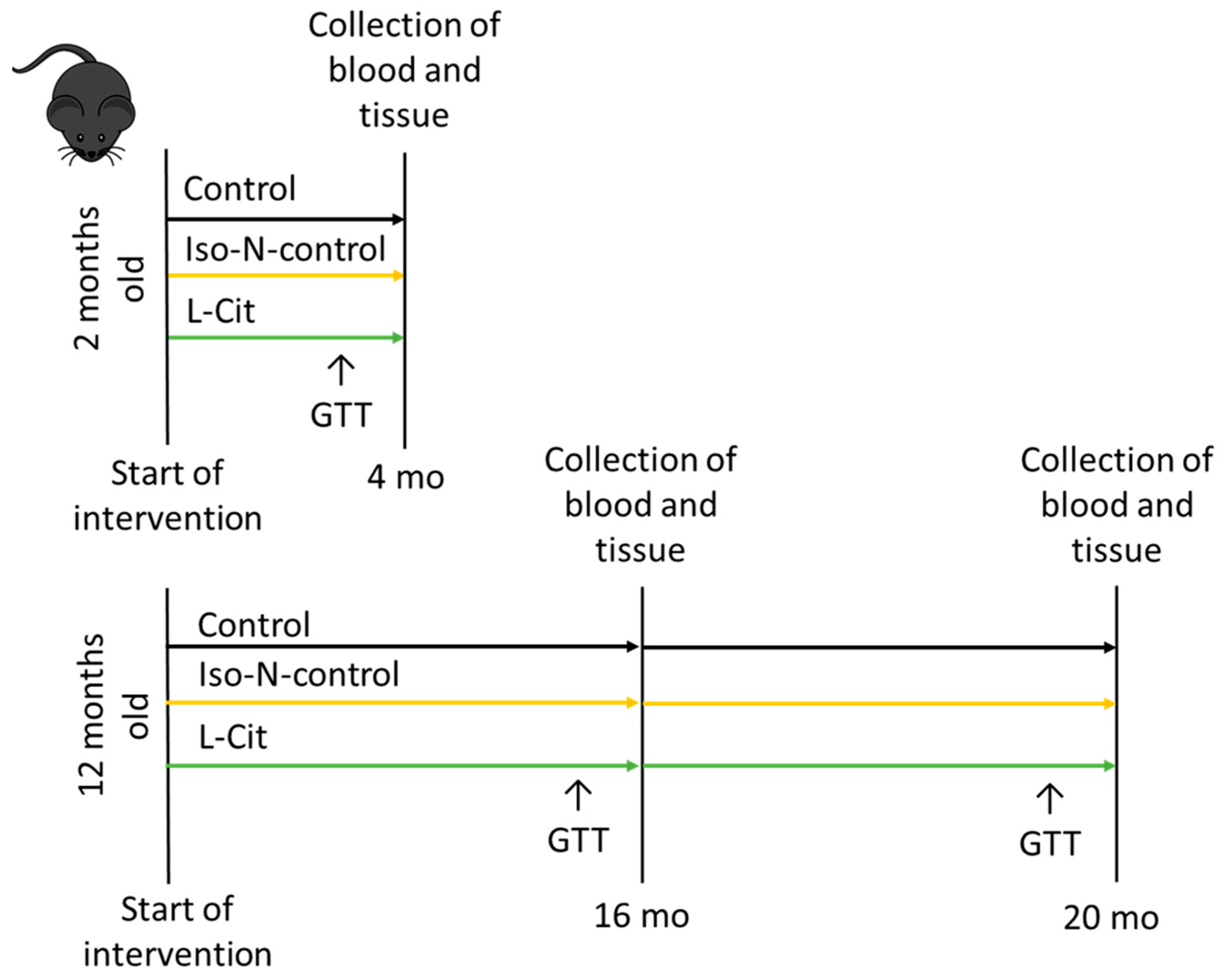
2.2. Animals and Treatment
2.3. Measurement of Intestinal Permeability
2.4. Detection of Nitrite (NO2−)
2.5. Western Blot
2.6. Measurement of Total PAI-1
2.7. Measurement of TLR2 and TLR4 Ligands
2.8. Microbiota Analysis
2.9. Statistical Analysis
3. Results
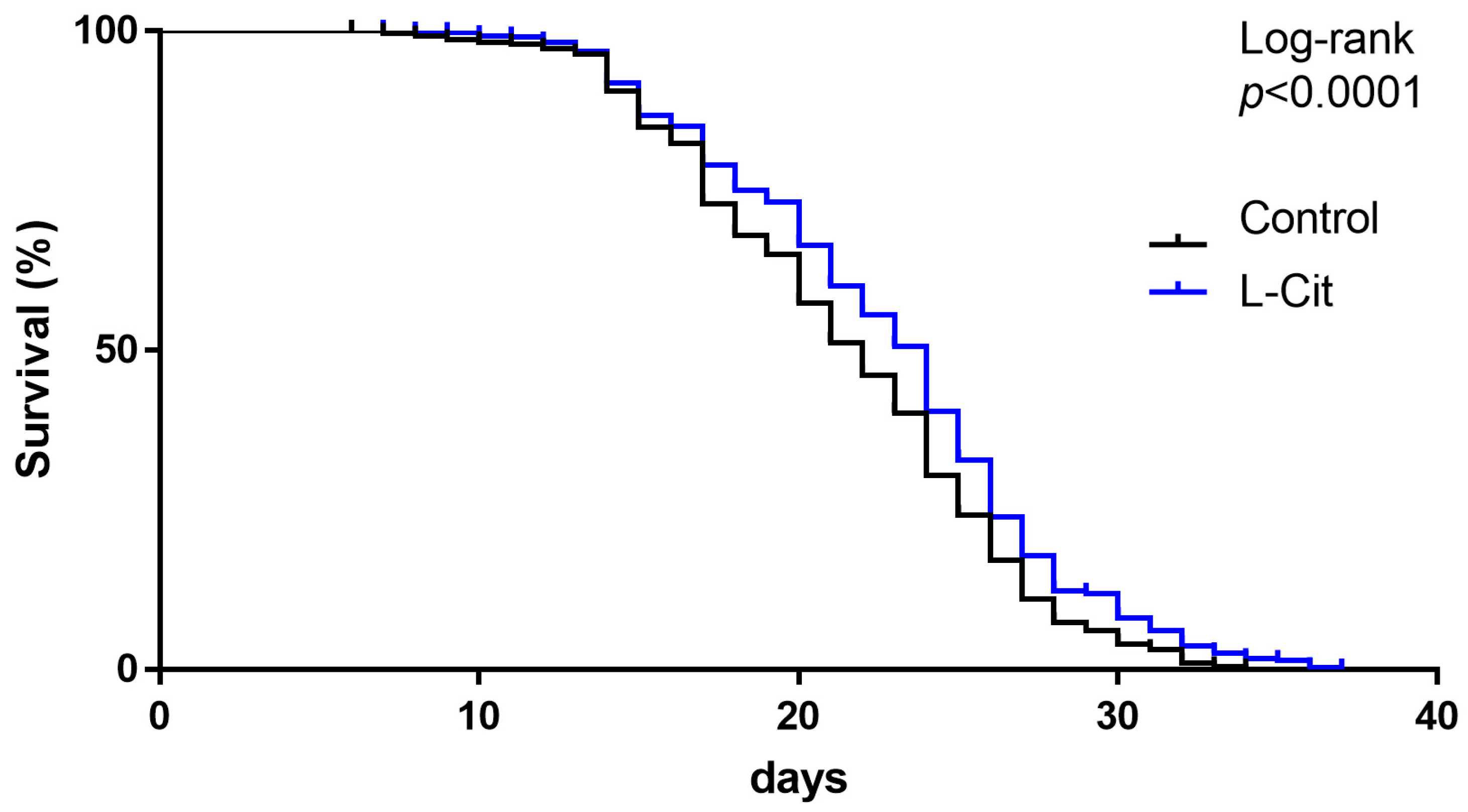
3.1. Effect of L-Cit on Lifespan in C. elegans
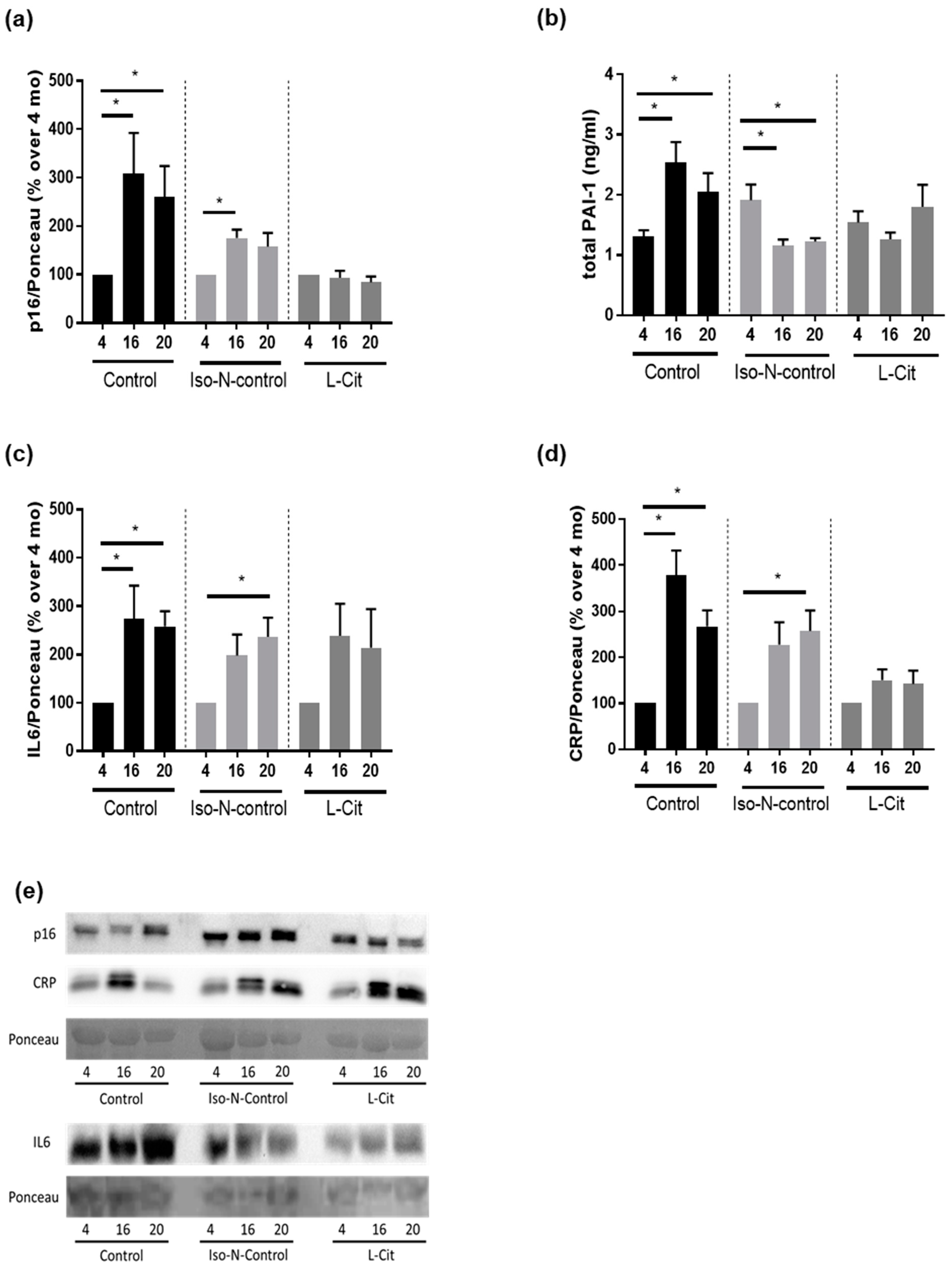
3.2. Effect of L-Cit on Markers of Senescence in C57BL/6J Mice
3.3. Effect of L-Cit on Markers of Glucose Metabolism
3.4. Effect of L-Cit on Intestinal Microbiota Composition in Small Intestines
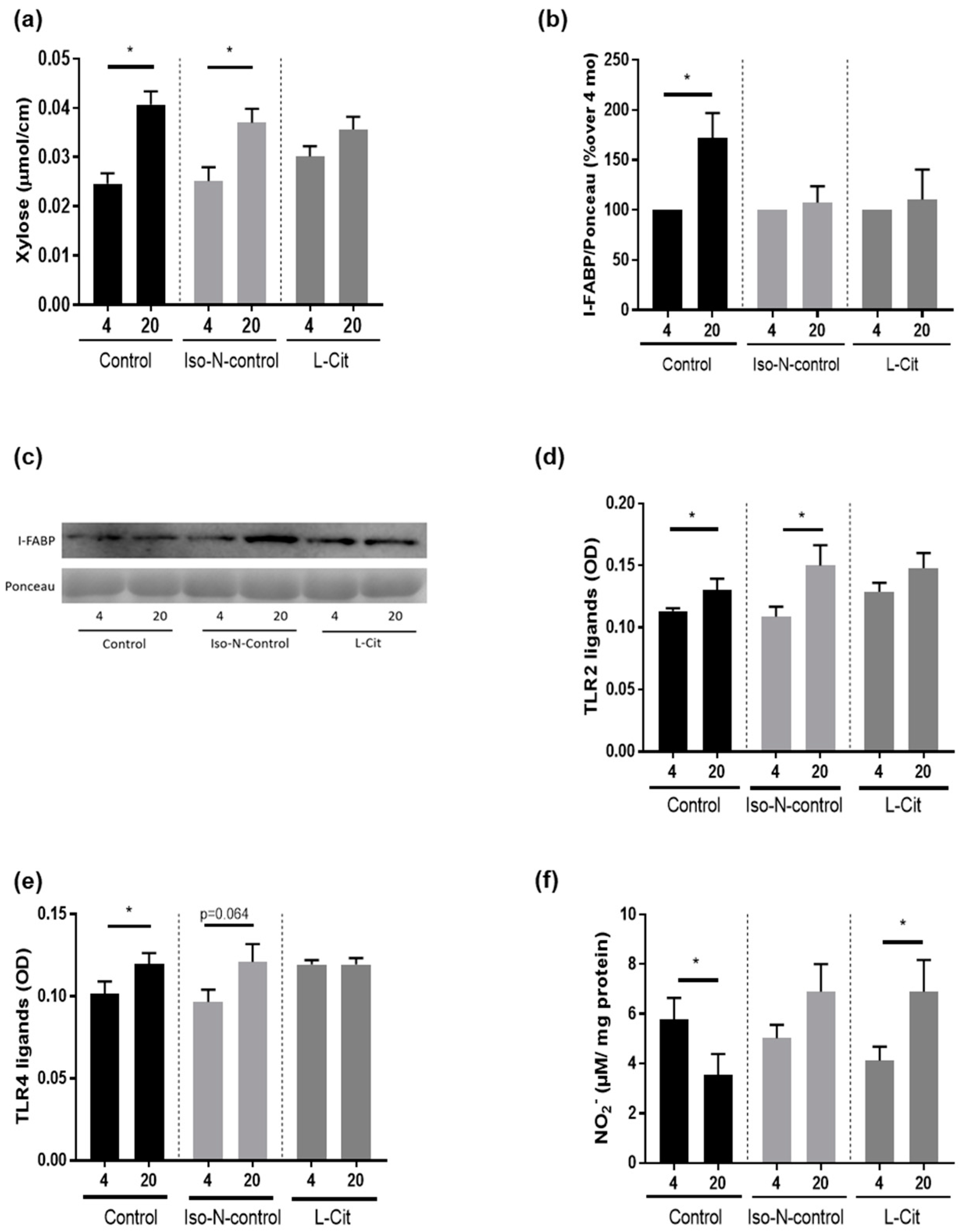
3.5. Effect of L-Cit on Markers of Intestinal Barrier Function
4. Discussion
The Protective Effects of L-Cit Are Associated with a Protection against the Aging-Associated Loss of Intestinal Integrity but Not with Changes of Intestinal Microbiota Composition
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geneva: World Health Organization. Decade of Healthy Ageing: Baseline Report. 2020. Available online: https://www.who.int/publications/i/item/9789240017900 (accessed on 14 March 2023).
- GBD 2019 Demographics Collaborators. Global age-sex-specific fertility, mortality, healthy life expectancy (HALE), and population estimates in 204 countries and territories, 1950-2019: A comprehensive demographic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1160–1203. [Google Scholar] [CrossRef] [PubMed]
- Niccoli, T.; Partridge, L. Ageing as a risk factor for disease. Curr. Biol. 2012, 22, R741–R752. [Google Scholar] [CrossRef]
- Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.G.; Croteau, D.L.; Bohr, V.A. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 2019, 15, 565–581. [Google Scholar] [CrossRef] [PubMed]
- Geneva: World Health Organization. World Health Statistics 2022. Available online: https://www.who.int/news/item/20-05-2022-world-health-statistics-2022 (accessed on 2 March 2023).
- Brivio, P.; Paladini, M.S.; Racagni, G.; Riva, M.A.; Calabrese, F.; Molteni, R. From Healthy Aging to Frailty: In Search of the Underlying Mechanisms. Curr. Med. Chem. 2019, 26, 3685–3701. [Google Scholar] [CrossRef]
- Lopez-Otin, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- Franceschi, C.; Campisi, J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69 (Suppl. 1), S4–S9. [Google Scholar] [CrossRef]
- Yamamoto, K.; Takeshita, K.; Saito, H. Plasminogen activator inhibitor-1 in aging. Semin. Thromb. Hemost. 2014, 40, 652–659. [Google Scholar] [CrossRef]
- Chung, H.Y.; Cesari, M.; Anton, S.; Marzetti, E.; Giovannini, S.; Seo, A.Y.; Carter, C.; Yu, B.P.; Leeuwenburgh, C. Molecular inflammation: Underpinnings of aging and age-related diseases. Ageing Res. Rev. 2009, 8, 18–30. [Google Scholar] [CrossRef]
- Rea, I.M.; Gibson, D.S.; McGilligan, V.; McNerlan, S.E.; Alexander, H.D.; Ross, O.A. Age and Age-Related Diseases: Role of Inflammation Triggers and Cytokines. Front. Immunol. 2018, 9, 586. [Google Scholar] [CrossRef] [PubMed]
- Baumann, A.; Hernandez-Arriaga, A.; Brandt, A.; Sanchez, V.; Nier, A.; Jung, F.; Kehm, R.; Hohn, A.; Grune, T.; Frahm, C.; et al. Microbiota profiling in aging-associated inflammation and liver degeneration. Int. J. Med. Microbiol. 2021, 311, 151500. [Google Scholar] [CrossRef]
- Odamaki, T.; Kato, K.; Sugahara, H.; Hashikura, N.; Takahashi, S.; Xiao, J.Z.; Abe, F.; Osawa, R. Age-related changes in gut microbiota composition from newborn to centenarian: A cross-sectional study. BMC Microbiol. 2016, 16, 90. [Google Scholar] [CrossRef] [PubMed]
- Brandt, A.; Baumann, A.; Hernandez-Arriaga, A.; Jung, F.; Nier, A.; Staltner, R.; Rajcic, D.; Schmeer, C.; Witte, O.W.; Wessner, B.; et al. Impairments of intestinal arginine and NO metabolisms trigger aging-associated intestinal barrier dysfunction and ‘inflammaging’. Redox Biol. 2022, 58, 102528. [Google Scholar] [CrossRef]
- Kuhn, F.; Adiliaghdam, F.; Cavallaro, P.M.; Hamarneh, S.R.; Tsurumi, A.; Hoda, R.S.; Munoz, A.R.; Dhole, Y.; Ramirez, J.M.; Liu, E.; et al. Intestinal alkaline phosphatase targets the gut barrier to prevent aging. JCI Insight 2020, 5, 134049. [Google Scholar] [CrossRef]
- Jin, C.J.; Baumann, A.; Brandt, A.; Engstler, A.J.; Nier, A.; Hege, M.; Schmeer, C.; Kehm, R.; Hohn, A.; Grune, T.; et al. Aging-related liver degeneration is associated with increased bacterial endotoxin and lipopolysaccharide binding protein levels. Am. J. Physiol. Gastrointest Liver Physiol. 2020, 318, G736–G747. [Google Scholar] [CrossRef]
- Goto, T.; Eden, S.; Nordenstam, G.; Sundh, V.; Svanborg-Eden, C.; Mattsby-Baltzer, I. Endotoxin levels in sera of elderly individuals. Clin. Diagn. Lab. Immunol. 1994, 1, 684–688. [Google Scholar] [CrossRef]
- Buckinx, F.; Carvalho, L.P.; Marcangeli, V.; Dulac, M.; Hajj Boutros, G.; Gouspillou, G.; Gaudreau, P.; Noirez, P.; Aubertin-Leheudre, M. High intensity interval training combined with L-citrulline supplementation: Effects on physical performance in healthy older adults. Exp. Gerontol. 2020, 140, 111036. [Google Scholar] [CrossRef]
- Thibault, R.; Flet, L.; Vavasseur, F.; Lemerle, M.; Ferchaud-Roucher, V.; Picot, D.; Darmaun, D. Oral citrulline does not affect whole body protein metabolism in healthy human volunteers: Results of a prospective, randomized, double-blind, cross-over study. Clin. Nutr. 2011, 30, 807–811. [Google Scholar] [CrossRef]
- Churchward-Venne, T.A.; Cotie, L.M.; MacDonald, M.J.; Mitchell, C.J.; Prior, T.; Baker, S.K.; Phillips, S.M. Citrulline does not enhance blood flow, microvascular circulation, or myofibrillar protein synthesis in elderly men at rest or following exercise. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E71–E83. [Google Scholar] [CrossRef]
- Moinard, C.; Le Plenier, S.; Noirez, P.; Morio, B.; Bonnefont-Rousselot, D.; Kharchi, C.; Ferry, A.; Neveux, N.; Cynober, L.; Raynaud-Simon, A. Citrulline Supplementation Induces Changes in Body Composition and Limits Age-Related Metabolic Changes in Healthy Male Rats. J. Nutr. 2015, 145, 1429–1437. [Google Scholar] [CrossRef] [PubMed]
- Breuillard, C.; Curis, E.; Le Plenier, S.; Cynober, L.; Moinard, C. Nitric oxide production by peritoneal macrophages from aged rats: A short term and direct modulation by citrulline. Biochimie 2017, 133, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Maric, S.; Restin, T.; Muff, J.L.; Camargo, S.M.; Guglielmetti, L.C.; Holland-Cunz, S.G.; Crenn, P.; Vuille-Dit-Bille, R.N. Citrulline, Biomarker of Enterocyte Functional Mass and Dietary Supplement. Metabolism, Transport, and Current Evidence for Clinical Use. Nutrients 2021, 13, 2794. [Google Scholar] [CrossRef]
- Sellmann, C.; Jin, C.J.; Engstler, A.J.; De Bandt, J.P.; Bergheim, I. Oral citrulline supplementation protects female mice from the development of non-alcoholic fatty liver disease (NAFLD). Eur. J. Nutr. 2017, 56, 2519–2527. [Google Scholar] [CrossRef]
- Jegatheesan, P.; Beutheu, S.; Freese, K.; Waligora-Dupriet, A.J.; Nubret, E.; Butel, M.J.; Bergheim, I.; De Bandt, J.P. Preventive effects of citrulline on Western diet-induced non-alcoholic fatty liver disease in rats. Br. J. Nutr. 2016, 116, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Edwards, C.; Canfield, J.; Copes, N.; Brito, A.; Rehan, M.; Lipps, D.; Brunquell, J.; Westerheide, S.D.; Bradshaw, P.C. Mechanisms of amino acid-mediated lifespan extension in Caenorhabditis elegans. BMC Genet. 2015, 16, 8. [Google Scholar] [CrossRef]
- Baumann, A.; Burger, K.; Brandt, A.; Staltner, R.; Jung, F.; Rajcic, D.; Lorenzo Pisarello, M.J.; Bergheim, I. GW9662, a peroxisome proliferator-activated receptor gamma antagonist, attenuates the development of non-alcoholic fatty liver disease. Metabolism 2022, 133, 155233. [Google Scholar] [CrossRef]
- Rajcic, D.; Baumann, A.; Hernandez-Arriaga, A.; Brandt, A.; Nier, A.; Jin, C.J.; Sanchez, V.; Jung, F.; Camarinha-Silva, A.; Bergheim, I. Citrulline supplementation attenuates the development of non-alcoholic steatohepatitis in female mice through mechanisms involving intestinal arginase. Redox Biol. 2021, 41, 101879. [Google Scholar] [CrossRef] [PubMed]
- Spruss, A.; Kanuri, G.; Stahl, C.; Bischoff, S.C.; Bergheim, I. Metformin protects against the development of fructose-induced steatosis in mice: Role of the intestinal barrier function. Lab. Investig. 2012, 92, 1020–1032. [Google Scholar] [CrossRef]
- Wang, Q.; Han, W.; Ma, C.; Wang, T.; Zhong, J. Western blot normalization: Time to choose a proper loading control seriously. Electrophoresis 2023, 44, 854–863. [Google Scholar] [CrossRef]
- Jung, F.; Burger, K.; Staltner, R.; Brandt, A.; Mueller, S.; Bergheim, I. Markers of Intestinal Permeability Are Rapidly Improved by Alcohol Withdrawal in Patients with Alcohol-Related Liver Disease. Nutrients 2021, 13, 1659. [Google Scholar] [CrossRef]
- Brandt, A.; Hernandez-Arriaga, A.; Kehm, R.; Sanchez, V.; Jin, C.J.; Nier, A.; Baumann, A.; Camarinha-Silva, A.; Bergheim, I. Metformin attenuates the onset of non-alcoholic fatty liver disease and affects intestinal microbiota and barrier in small intestine. Sci. Rep. 2019, 9, 6668. [Google Scholar] [CrossRef] [PubMed]
- Kozich, J.J.; Westcott, S.L.; Baxter, N.T.; Highlander, S.K.; Schloss, P.D. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl. Environ. Microbiol. 2013, 79, 5112–5120. [Google Scholar] [CrossRef] [PubMed]
- Clarke, K.; Gorley, R. PRIMER version 7: User manual/tutorial. In PRIMER-E192; PRIMER-E Ltd.: Auckland, New Zealand, 2015. [Google Scholar]
- Vaughan, D.E.; Rai, R.; Khan, S.S.; Eren, M.; Ghosh, A.K. Plasminogen Activator Inhibitor-1 Is a Marker and a Mediator of Senescence. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1446–1452. [Google Scholar] [CrossRef]
- Holloszy, J.O.; Greiwe, J.S. Overview of glucose metabolism and aging. Int. J. Sport Nutr. Exerc. Metab. 2001, 11 (Suppl. 1), S58–S63. [Google Scholar] [CrossRef] [PubMed]
- Brandt, A.; Kromm, F.; Hernandez-Arriaga, A.; Martinez Sanchez, I.; Bozkir, H.O.; Staltner, R.; Baumann, A.; Camarinha-Silva, A.; Heijtz, R.D.; Bergheim, I. Cognitive Alterations in Old Mice Are Associated with Intestinal Barrier Dysfunction and Induced Toll-like Receptor 2 and 4 Signaling in Different Brain Regions. Cells 2023, 12, 2153. [Google Scholar] [CrossRef]
- Uyanga, V.A.; Amevor, F.K.; Liu, M.; Cui, Z.; Zhao, X.; Lin, H. Potential Implications of Citrulline and Quercetin on Gut Functioning of Monogastric Animals and Humans: A Comprehensive Review. Nutrients 2021, 13, 3782. [Google Scholar] [CrossRef]
- Kitada, M.; Ogura, Y.; Monno, I.; Koya, D. The impact of dietary protein intake on longevity and metabolic health. EBioMedicine 2019, 43, 632–640. [Google Scholar] [CrossRef] [PubMed]
- Pervin, M.; Unno, K.; Konishi, T.; Nakamura, Y. L-Arginine Exerts Excellent Anti-Stress Effects on Stress-Induced Shortened Lifespan, Cognitive Decline and Depression. Int. J. Mol. Sci. 2021, 22, 508. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Ladeiras, D.; Yu, Y.; Ming, X.F.; Yang, Z. Detrimental Effects of Chronic L-Arginine Rich Food on Aging Kidney. Front. Pharmacol. 2020, 11, 582155. [Google Scholar] [CrossRef]
- Dumenci, O.E.; U, A.M.; Khan, S.A.; Holmes, E.; Taylor-Robinson, S.D. Exploring Metabolic Consequences of CPS1 and CAD Dysregulation in Hepatocellular Carcinoma by Network Reconstruction. J. Hepatocell. Carcinoma 2020, 7, 1–9. [Google Scholar] [CrossRef]
- Agarwal, U.; Didelija, I.C.; Yuan, Y.; Wang, X.; Marini, J.C. Supplemental Citrulline Is More Efficient Than Arginine in Increasing Systemic Arginine Availability in Mice. J. Nutr. 2017, 147, 596–602. [Google Scholar] [CrossRef]
- Nyawose, S.; Naidoo, R.; Naumovski, N.; McKune, A.J. The Effects of Consuming Amino Acids L-Arginine, L-Citrulline (and Their Combination) as a Beverage or Powder, on Athletic and Physical Performance: A Systematic Review. Beverages 2022, 8, 48. [Google Scholar] [CrossRef]
- Khalaf, D.; Kruger, M.; Wehland, M.; Infanger, M.; Grimm, D. The Effects of Oral l-Arginine and l-Citrulline Supplementation on Blood Pressure. Nutrients 2019, 11, 1679. [Google Scholar] [CrossRef]
- Vigne, P.; Frelin, C. The role of polyamines in protein-dependent hypoxic tolerance of Drosophila. BMC Physiol. 2008, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Vigne, P.; Frelin, C. Diet dependent longevity and hypoxic tolerance of adult Drosophila melanogaster. Mech. Ageing Dev. 2007, 128, 401–406. [Google Scholar] [CrossRef]
- Goron, A.; Lamarche, F.; Blanchet, S.; Delangle, P.; Schlattner, U.; Fontaine, E.; Moinard, C. Citrulline stimulates muscle protein synthesis, by reallocating ATP consumption to muscle protein synthesis. J. Cachexia Sarcopenia Muscle 2019, 10, 919–928. [Google Scholar] [CrossRef]
- Engstler, A.J.; Frahnow, T.; Kruse, M.; Pfeiffer, A.F.H.; Bergheim, I. Plasminogen Activator Inhibitor-1 is Regulated through Dietary Fat Intake and Heritability: Studies in Twins. Twin Res. Hum. Genet. 2017, 20, 338–348. [Google Scholar] [CrossRef]
- Kristensen, M.; Bugel, S. A diet rich in oat bran improves blood lipids and hemostatic factors, and reduces apparent energy digestibility in young healthy volunteers. Eur. J. Clin. Nutr. 2011, 65, 1053–1058. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Garcia, M.A.; Moncayo, S.; Insenser, M.; Alvarez-Blasco, F.; Luque-Ramirez, M.; Escobar-Morreale, H.F. Metabolic Cytokines at Fasting and During Macronutrient Challenges: Influence of Obesity, Female Androgen Excess and Sex. Nutrients 2019, 11, 2566. [Google Scholar] [CrossRef] [PubMed]
- Puzianowska-Kuznicka, M.; Owczarz, M.; Wieczorowska-Tobis, K.; Nadrowski, P.; Chudek, J.; Slusarczyk, P.; Skalska, A.; Jonas, M.; Franek, E.; Mossakowska, M. Interleukin-6 and C-reactive protein, successful aging, and mortality: The PolSenior study. Immun. Ageing 2016, 13, 21. [Google Scholar] [CrossRef]
- Liu, Z.; Fuentes, N.L.; Jones, S.A.; Hagood, J.S.; Fuller, G.M. A unique transcription factor for the A alpha fibrinogen gene is related to the mitochondrial single-stranded DNA binding protein P16. Biochemistry 1997, 36, 14799–14806. [Google Scholar] [CrossRef]
- Goodenough, C.G.; Wogksch, M.D.; Kundu, M.; Lear, M.; Thomas, P.G.; Srivastava, D.K.; Wang, Z.; Armstrong, G.T.; Hudson, M.M.; Robison, L.L.; et al. Associations between exercise capacity, p16(INK4a) expression and inflammation among adult survivors of childhood cancer. Front. Oncol. 2022, 12, 1014661. [Google Scholar] [CrossRef]
- Marmentini, C.; Soares, G.M.; Bronczek, G.A.; Piovan, S.; Mareze-Costa, C.E.; Carneiro, E.M.; Boschero, A.C.; Kurauti, M.A. Aging Reduces Insulin Clearance in Mice. Front. Endocrinol. 2021, 12, 679492. [Google Scholar] [CrossRef]
- Leiter, E.H.; Premdas, F.; Harrison, D.E.; Lipson, L.G. Aging and glucose homeostasis in C57BL/6J male mice. FASEB J. 1988, 2, 2807–2811. [Google Scholar] [CrossRef] [PubMed]
- Eshreif, A.; Al Batran, R.; Jamieson, K.L.; Darwesh, A.M.; Gopal, K.; Greenwell, A.A.; Zlobine, I.; Aburasayn, H.; Eaton, F.; Mulvihill, E.E.; et al. l-Citrulline supplementation improves glucose and exercise tolerance in obese male mice. Exp. Physiol. 2020, 105, 270–281. [Google Scholar] [CrossRef]
- Abbaszadeh, F.; Azizi, S.; Mobasseri, M.; Ebrahimi-Mameghani, M. The effects of citrulline supplementation on meta-inflammation and insulin sensitivity in type 2 diabetes: A randomized, double-blind, placebo-controlled trial. Diabetol. Metab. Syndr. 2021, 13, 52. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.I.; Salazar, A.; Yamada, R.; Fitz-Gibbon, S.; Morselli, M.; Alcaraz, J.; Rana, A.; Rera, M.; Pellegrini, M.; Ja, W.W.; et al. Distinct Shifts in Microbiota Composition during Drosophila Aging Impair Intestinal Function and Drive Mortality. Cell Rep. 2015, 12, 1656–1667. [Google Scholar] [CrossRef]
- Mangiola, F.; Nicoletti, A.; Gasbarrini, A.; Ponziani, F.R. Gut microbiota and aging. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 7404–7413. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Lv, H.; Wang, H.; Yang, H.; Li, Y.; Qian, J. Aging Increases the Severity of Colitis and the Related Changes to the Gut Barrier and Gut Microbiota in Humans and Mice. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 1284–1292. [Google Scholar] [CrossRef]
- Parker, A.; Romano, S.; Ansorge, R.; Aboelnour, A.; Le Gall, G.; Savva, G.M.; Pontifex, M.G.; Telatin, A.; Baker, D.; Jones, E.; et al. Fecal microbiota transfer between young and aged mice reverses hallmarks of the aging gut, eye, and brain. Microbiome 2022, 10, 68. [Google Scholar] [CrossRef]
- Batista, M.A.; Nicoli, J.R.; Martins Fdos, S.; Machado, J.A.; Arantes, R.M.; Quirino, I.E.; Correia, M.I.; Cardoso, V.N. Pretreatment with citrulline improves gut barrier after intestinal obstruction in mice. J. Parenter. Enteral. Nutr. 2012, 36, 69–76. [Google Scholar] [CrossRef]
- March, D.S.; Marchbank, T.; Playford, R.J.; Jones, A.W.; Thatcher, R.; Davison, G. Intestinal fatty acid-binding protein and gut permeability responses to exercise. Eur. J. Appl. Physiol. 2017, 117, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Bahadoran, Z.; Mirmiran, P.; Kashfi, K.; Ghasemi, A. Endogenous flux of nitric oxide: Citrulline is preferred to Arginine. Acta Physiol. 2021, 231, e13572. [Google Scholar] [CrossRef] [PubMed]
- Wijnands, K.A.; Vink, H.; Briede, J.J.; van Faassen, E.E.; Lamers, W.H.; Buurman, W.A.; Poeze, M. Citrulline a more suitable substrate than arginine to restore NO production and the microcirculation during endotoxemia. PLoS ONE 2012, 7, e37439. [Google Scholar] [CrossRef] [PubMed]


| Groups | Age (Months) | |||
|---|---|---|---|---|
| 4 | 16 | 20 | ||
| Absolute end body weight (g) | Control | 29.2 ± 0.6 | 34.3 ± 1.0 * | 33.8 ± 0.7 * |
| Iso-N-control | 29.5 ± 0.6 | 34.6 ± 0.8 * | 36.2 ± 1.3 * | |
| L-Cit | 30.3 ± 0.7 | 32.5 ± 1.0 | 34.5 ± 1.2 * | |
| Absolute weight gain (g) | Control | 5.7 ± 0.3 | 2.2 ± 0.6 * | 2.0 ± 0.7 * |
| Iso-N-control | 5.9 ± 0.5 | 2.0 ± 0.2 * | 3.0 ± 0.4 * | |
| L-Cit | 6.3 ± 0.5 | 1.6 ± 0.2 * | 3.5 ± 0.7 * | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajcic, D.; Kromm, F.; Hernández-Arriaga, A.; Brandt, A.; Baumann, A.; Staltner, R.; Camarinha-Silva, A.; Bergheim, I. Supplementing L-Citrulline Can Extend Lifespan in C. elegans and Attenuate the Development of Aging-Related Impairments of Glucose Tolerance and Intestinal Barrier in Mice. Biomolecules 2023, 13, 1579. https://doi.org/10.3390/biom13111579
Rajcic D, Kromm F, Hernández-Arriaga A, Brandt A, Baumann A, Staltner R, Camarinha-Silva A, Bergheim I. Supplementing L-Citrulline Can Extend Lifespan in C. elegans and Attenuate the Development of Aging-Related Impairments of Glucose Tolerance and Intestinal Barrier in Mice. Biomolecules. 2023; 13(11):1579. https://doi.org/10.3390/biom13111579
Chicago/Turabian StyleRajcic, Dragana, Franziska Kromm, Angélica Hernández-Arriaga, Annette Brandt, Anja Baumann, Raphaela Staltner, Amélia Camarinha-Silva, and Ina Bergheim. 2023. "Supplementing L-Citrulline Can Extend Lifespan in C. elegans and Attenuate the Development of Aging-Related Impairments of Glucose Tolerance and Intestinal Barrier in Mice" Biomolecules 13, no. 11: 1579. https://doi.org/10.3390/biom13111579
APA StyleRajcic, D., Kromm, F., Hernández-Arriaga, A., Brandt, A., Baumann, A., Staltner, R., Camarinha-Silva, A., & Bergheim, I. (2023). Supplementing L-Citrulline Can Extend Lifespan in C. elegans and Attenuate the Development of Aging-Related Impairments of Glucose Tolerance and Intestinal Barrier in Mice. Biomolecules, 13(11), 1579. https://doi.org/10.3390/biom13111579








