IL-33/ST2 Axis: A Potential Therapeutic Target in Neurodegenerative Diseases
Abstract
1. Introduction
2. IL-33 Distribution in Human and Mice Tissues and Cells
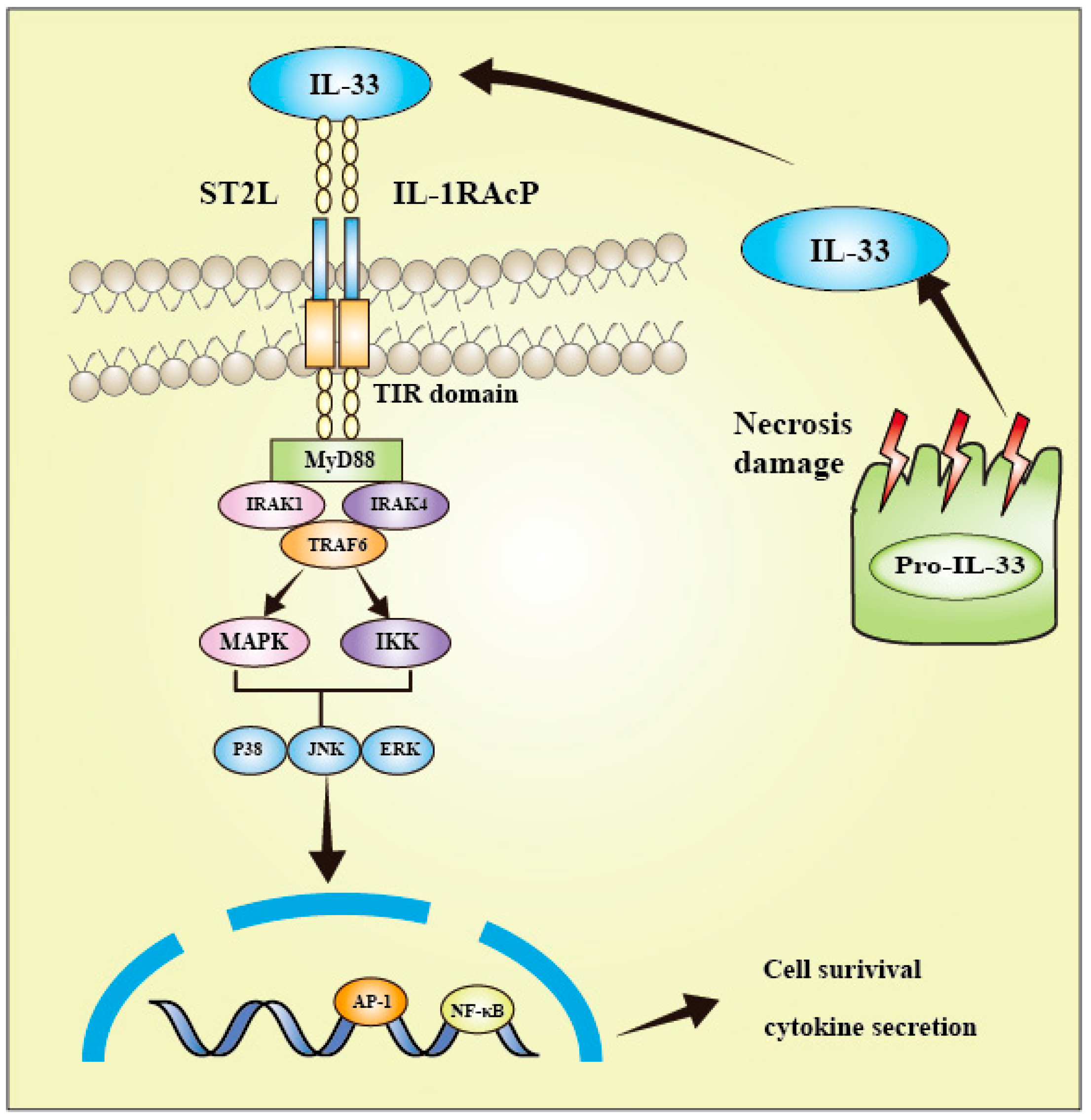
3. IL-33 Signaling Pathway
4. IL-33 and Immune Cells
5. Role of IL-33 in Neurodegenerative Disorders
5.1. AD
5.2. MS
5.3. PD
5.4. ALS
5.5. Other Types of Neurodegenerative Diseases

6. Clinical Trials on IL-33/ST2
7. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Macleod, T.; Berekmeri, A.; Bridgewood, C.; Stacey, M.; McGonagle, D.; Wittmann, M. The Immunological Impact of IL-1 Family Cytokines on the Epidermal Barrier. Front. Immunol. 2021, 12, 808012. [Google Scholar]
- Sun, Y.; Wen, Y.; Wang, L.; Wen, L.; You, W.; Wei, S.; Mao, L.; Wang, H.; Chen, Z.; Yang, X. Therapeutic Opportunities of Interleukin-33 in the Central Nervous System. Front. Immunol. 2021, 12, 654626. [Google Scholar] [CrossRef]
- Cayrol, C.; Girard, J.-P. IL-33: An alarmin cytokine with crucial roles in innate immunity, inflammation and allergy. Curr. Opin. Immunol. 2014, 31, 31–37. [Google Scholar] [CrossRef]
- Bertheloot, D.; Latz, E. HMGB1, IL-1α, IL-33 and S100 proteins: Dual-function alarmins. Cell. Mol. Immunol. 2016, 14, 43–64. [Google Scholar]
- Abd Rachman Isnadi, M.F.; Chin, V.K.; Abd Majid, R.; Lee, T.Y.; Atmadini Abdullah, M.; Bello Omenesa, R.; Osamah Ibraheem, Z.; Basir, R. Critical Roles of IL-33/ST2 Pathway in Neurological Disorders. Mediat. Inflamm. 2018, 2018, 5346413. [Google Scholar] [CrossRef]
- Onda, H.; Kasuya, H.; Takakura, K.; Hori, T.; Imaizumi, T.A.; Takeuchi, T.; Inoue, I.; Takeda, J. Identification of genes differentially expressed in canine vasospastic cerebral arteries after subarachnoid hemorrhage. J. Cereb. Blood Flow Metab. 1999, 19, 1279–1288. [Google Scholar] [CrossRef]
- Kakkar, R.; Lee, R.T. The IL-33/ST2 pathway: Therapeutic target and novel biomarker. Nat. Rev. Drug Discov. 2008, 7, 827–840. [Google Scholar]
- Schmitz, J.; Owyang, A.; Oldham, E.; Song, Y.; Murphy, E.; McClanahan, T.K.; Zurawski, G.; Moshrefi, M.; Qin, J.; Li, X.; et al. IL-33, an Interleukin-1-like Cytokine that Signals via the IL-1 Receptor-Related Protein ST2 and Induces T Helper Type 2-Associated Cytokines. Immunity 2005, 23, 479–490. [Google Scholar] [CrossRef]
- Liew, F.Y.; Girard, J.-P.; Turnquist, H.R. Interleukin-33 in health and disease. Nat. Rev. Immunol. 2016, 16, 676–689. [Google Scholar]
- Garlanda, C.; Dinarello, C.A.; Mantovani, A. The Interleukin-1 Family: Back to the Future. Immunity 2013, 39, 1003–1018. [Google Scholar]
- Baekkevold, E.S.; Roussigné, M.; Yamanaka, T.; Johansen, F.-E.; Jahnsen, F.L.; Amalric, F.; Brandtzaeg, P.; Erard, M.; Haraldsen, G.; Girard, J.-P. Molecular Characterization of NF-HEV, a Nuclear Factor Preferentially Expressed in Human High Endothelial Venules. Am. J. Pathol. 2003, 163, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Cayrol, C.; Girard, J.P. The IL-1-like cytokine IL-33 is inactivated after maturation by caspase-1. Proc. Natl. Acad. Sci. USA 2009, 106, 9021–9026. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.C.L.; Lam, C.W.K.; Tam, L.-S.; Wong, C.K. IL33: Roles in Allergic Inflammation and Therapeutic Perspectives. Front. Immunol. 2019, 10, 364. [Google Scholar] [PubMed]
- Choi, Y.-S.; Park, J.A.; Kim, J.; Rho, S.-S.; Park, H.; Kim, Y.-M.; Kwon, Y.-G. Nuclear IL-33 is a transcriptional regulator of NF-κB p65 and induces endothelial cell activation. Biochem. Biophys. Res. Commun. 2012, 421, 305–311. [Google Scholar] [CrossRef]
- Park, J.H.; Ameri, A.H.; Dempsey, K.E.; Conrad, D.N.; Kem, M.; Mino-Kenudson, M.; Demehri, S. Nuclear IL-33/SMAD signaling axis promotes cancer development in chronic inflammation. EMBO J. 2021, 40, e106151. [Google Scholar] [CrossRef]
- Wang, Y.; Fu, W.-Y.; Cheung, K.; Hung, K.-W.; Chen, C.; Geng, H.; Yung, W.-H.; Qu, J.Y.; Fu, A.K.Y.; Ip, N.Y. Astrocyte-secreted IL-33 mediates homeostatic synaptic plasticity in the adult hippocampus. Proc. Natl. Acad. Sci. USA 2020, 118, e2020810118. [Google Scholar] [CrossRef]
- Peine, M.; Marek, R.M.; Löhning, M. IL-33 in T Cell Differentiation, Function, and Immune Homeostasis. Trends Immunol. 2016, 37, 321–333. [Google Scholar]
- Hu, Z.Q.; Zhao, W.H. The IL-33/ST2 axis is specifically required for development of adipose tissue-resident regulatory T cells. Cell. Mol. Immunol. 2015, 12, 521–524. [Google Scholar] [CrossRef][Green Version]
- Li, D.; Guabiraba, R.; Besnard, A.-G.; Komai-Koma, M.; Jabir, M.S.; Zhang, L.; Graham, G.J.; Kurowska-Stolarska, M.; Liew, F.Y.; McSharry, C.; et al. IL-33 promotes ST2-dependent lung fibrosis by the induction of alternatively activated macrophages and innate lymphoid cells in mice. J. Allergy Clin. Immunol. 2014, 134, 1422–1432.e11. [Google Scholar]
- Wang, J.-X.; Kaieda, S.; Ameri, S.; Fishgal, N.; Dwyer, D.; Dellinger, A.; Kepley, C.L.; Gurish, M.F.; Nigrovic, P.A. IL-33/ST2 axis promotes mast cell survival via BCLXL. Proc. Natl. Acad. Sci. USA 2014, 111, 10281–10286. [Google Scholar] [CrossRef]
- Bergers, G.; Reikerstorfer, A.; Braselmann, S.; Graninger, P.; Busslinger, M. Alternative promoter usage of the Fos-responsive gene Fit-1 generates mRNA isoforms coding for either secreted or membrane-bound proteins related to the IL-1 receptor. EMBO J. 1994, 13, 1176–1188. [Google Scholar] [CrossRef] [PubMed]
- Liew, F.Y.; Pitman, N.I.; McInnes, I.B. Disease-associated functions of IL-33: The new kid in the IL-1 family. Nat. Rev. Immunol. 2010, 10, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Griesenauer, B.; Paczesny, S. The ST2/IL-33 Axis in Immune Cells during Inflammatory Diseases. Front. Immunol. 2017, 8, 475. [Google Scholar] [PubMed]
- Du, L.-X.; Wang, Y.-Q.; Hua, G.-Q.; Mi, W.-L. IL-33/ST2 Pathway as a Rational Therapeutic Target for CNS Diseases. Neuroscience 2018, 369, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Huang, M.; Yao, Y.-m. Autophagy and proinflammatory cytokines: Interactions and clinical implications. Cytokine Growth Factor Rev. 2018, 43, 38–46. [Google Scholar] [PubMed]
- Cayrol, C.; Girard, J.P. Interleukin-33 (IL-33): A nuclear cytokine from the IL-1 family. Immunol. Rev. 2017, 281, 154–168. [Google Scholar] [CrossRef]
- Unutmaz, D.; Moussion, C.; Ortega, N.; Girard, J.-P. The IL-1-Like Cytokine IL-33 Is Constitutively Expressed in the Nucleus of Endothelial Cells and Epithelial Cells In Vivo: A Novel ‘Alarmin’? PLoS ONE 2008, 3, e3331. [Google Scholar]
- Pichery, M.; Mirey, E.; Mercier, P.; Lefrancais, E.; Dujardin, A.; Ortega, N.; Girard, J.-P. Endogenous IL-33 Is Highly Expressed in Mouse Epithelial Barrier Tissues, Lymphoid Organs, Brain, Embryos, and Inflamed Tissues: In Situ Analysis Using a Novel Il-33–LacZ Gene Trap Reporter Strain. J. Immunol. 2012, 188, 3488–3495. [Google Scholar] [CrossRef]
- Oboki, K.; Ohno, T.; Kajiwara, N.; Arae, K.; Morita, H.; Ishii, A.; Nambu, A.; Abe, T.; Kiyonari, H.; Matsumoto, K.; et al. IL-33 is a crucial amplifier of innate rather than acquired immunity. Proc. Natl. Acad. Sci. USA 2010, 107, 18581–18586. [Google Scholar] [CrossRef]
- Wu, J.; Chen, Z.; Wickström, S.L.; Gao, J.; He, X.; Jing, X.; Wu, J.; Du, Q.; Yang, M.; Chen, Y.; et al. Interleukin-33 is a Novel Immunosuppressor that Protects Cancer Cells from TIL Killing by a Macrophage-Mediated Shedding Mechanism. Adv. Sci. 2021, 8, 2101029. [Google Scholar] [CrossRef]
- Kearley, J.; Silver, J.S.; Sanden, C.; Liu, Z.; Berlin, A.A.; White, N.; Mori, M.; Pham, T.-H.; Ward, C.K.; Criner, G.J.; et al. Cigarette Smoke Silences Innate Lymphoid Cell Function and Facilitates an Exacerbated Type I Interleukin-33-Dependent Response to Infection. Immunity 2015, 42, 566–579. [Google Scholar] [CrossRef] [PubMed]
- Sanada, S.; Hakuno, D.; Higgins, L.J.; Schreiter, E.R.; McKenzie, A.N.J.; Lee, R.T. IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. J. Clin. Investig. 2007, 117, 1538–1549. [Google Scholar] [PubMed]
- Nguyen, P.T.; Dorman, L.C.; Pan, S.; Vainchtein, I.D.; Han, R.T.; Nakao-Inoue, H.; Taloma, S.E.; Barron, J.J.; Molofsky, A.B.; Kheirbek, M.A.; et al. Microglial Remodeling of the Extracellular Matrix Promotes Synapse Plasticity. Cell 2020, 182, 388–403.e15. [Google Scholar] [PubMed]
- Fairlie-Clarke, K.; Barbour, M.; Wilson, C.; Hridi, S.U.; Allan, D.; Jiang, H.-R. Expression and Function of IL-33/ST2 Axis in the Central Nervous System Under Normal and Diseased Conditions. Front. Immunol. 2018, 9, 2596. [Google Scholar] [PubMed]
- Gadani, S.P.; Walsh, J.T.; Smirnov, I.; Zheng, J.; Kipnis, J. The Glia-Derived Alarmin IL-33 Orchestrates the Immune Response and Promotes Recovery following CNS Injury. Neuron 2015, 85, 703–709. [Google Scholar] [CrossRef]
- Lingel, A.; Weiss, T.M.; Niebuhr, M.; Pan, B.; Appleton, B.A.; Wiesmann, C.; Bazan, J.F.; Fairbrother, W.J. Structure of IL-33 and Its Interaction with the ST2 and IL-1RAcP Receptors—Insight into Heterotrimeric IL-1 Signaling Complexes. Structure 2009, 17, 1398–1410. [Google Scholar] [CrossRef]
- Akira, S.; Takeda, K.; Kaisho, T. Toll-like receptors: Critical proteins linking innate and acquired immunity. Nat. Immunol. 2001, 2, 675–680. [Google Scholar]
- Lin, J.; Liu, J.; Ma, R.; Hao, J.; Liang, Y.; Zhao, J.; Zhang, A.; Meng, H.; Lu, J. Interleukin-33: Metabolic checkpoints, metabolic processes, and epigenetic regulation in immune cells. Front. Immunol. 2022, 13, 900826. [Google Scholar]
- Molofsky, A.B.; Savage, A.K.; Locksley, R.M. Interleukin-33 in Tissue Homeostasis, Injury, and Inflammation. Immunity 2015, 42, 1005–1019. [Google Scholar]
- Kabata, H.; Moro, K.; Koyasu, S. The group 2 innate lymphoid cell (ILC2) regulatory network and its underlying mechanisms. Immunol. Rev. 2018, 286, 37–52. [Google Scholar] [CrossRef]
- Burzyn, D.; Benoist, C.; Mathis, D. Regulatory T cells in nonlymphoid tissues. Nat. Immunol. 2013, 14, 1007–1013. [Google Scholar] [PubMed]
- Josefowicz, S.Z.; Lu, L.-F.; Rudensky, A.Y. Regulatory T Cells: Mechanisms of Differentiation and Function. Annu. Rev. Immunol. 2012, 30, 531–564. [Google Scholar] [CrossRef] [PubMed]
- Baumann, C.; Fröhlich, A.; Brunner, T.M.; Holecska, V.; Pinschewer, D.D.; Löhning, M. Memory CD8+ T Cell Protection from Viral Reinfection Depends on Interleukin-33 Alarmin Signals. Front. Immunol. 2019, 10, 1833. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Li, G.; Zhu, Y.; Liu, L.; Chen, E.; Turnquist, H.; Zhang, X.; Finn, O.J.; Chen, X.; Lu, B. IL-33 synergizes with TCR and IL-12 signaling to promote the effector function of CD8+T cells. Eur. J. Immunol. 2011, 41, 3351–3360. [Google Scholar] [CrossRef]
- Smithgall, M.D.; Comeau, M.R.; Park Yoon, B.R.; Kaufman, D.; Armitage, R.; Smith, D.E. IL-33 amplifies both Th1- and Th2-type responses through its activity on human basophils, allergen-reactive Th2 cells, iNKT and NK Cells. Int. Immunol. 2008, 20, 1019–1030. [Google Scholar] [CrossRef]
- Suzukawa, M.; Koketsu, R.; Iikura, M.; Nakae, S.; Matsumoto, K.; Nagase, H.; Saito, H.; Matsushima, K.; Ohta, K.; Yamamoto, K.; et al. Interleukin-33 enhances adhesion, CD11b expression and survival in human eosinophils. Lab. Investig. 2008, 88, 1245–1253. [Google Scholar] [CrossRef]
- Halim, T.Y.F.; Rana, B.M.J.; Walker, J.A.; Kerscher, B.; Knolle, M.D.; Jolin, H.E.; Serrao, E.M.; Haim-Vilmovsky, L.; Teichmann, S.A.; Rodewald, H.-R.; et al. Tissue-Restricted Adaptive Type 2 Immunity Is Orchestrated by Expression of the Costimulatory Molecule OX40L on Group 2 Innate Lymphoid Cells. Immunity 2018, 48, 1195–1207.e6. [Google Scholar] [CrossRef]
- Kempuraj, D.; Thangavel, R.; Selvakumar, G.P.; Ahmed, M.E.; Zaheer, S.; Raikwar, S.P.; Zahoor, H.; Saeed, D.; Dubova, I.; Giler, G.; et al. Mast Cell Proteases Activate Astrocytes and Glia-Neurons and Release Interleukin-33 by Activating p38 and ERK1/2 MAPKs and NF-κB. Mol. Neurobiol. 2018, 56, 1681–1693. [Google Scholar]
- Wernersson, S.; Pejler, G. Mast cell secretory granules: Armed for battle. Nat. Rev. Immunol. 2014, 14, 478–494. [Google Scholar]
- Yehya, M.; Torbey, M.T. The Role of Mast Cells in Intracerebral Hemorrhage. Neurocrit. Care 2017, 28, 288–295. [Google Scholar] [CrossRef]
- Göpfert, C.; Andreas, N.; Weber, F.; Häfner, N.; Yakovleva, T.; Gaestel, M.; Kamradt, T.; Drube, S. The p38-MK2/3 Module Is Critical for IL-33–Induced Signaling and Cytokine Production in Dendritic Cells. J. Immunol. 2018, 200, 1198–1206. [Google Scholar] [CrossRef] [PubMed]
- De Kleer, I.M.; Kool, M.; de Bruijn, M.J.W.; Willart, M.; van Moorleghem, J.; Schuijs, M.J.; Plantinga, M.; Beyaert, R.; Hams, E.; Fallon, P.G.; et al. Perinatal Activation of the Interleukin-33 Pathway Promotes Type 2 Immunity in the Developing Lung. Immunity 2016, 45, 1285–1298. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.M.; Cookson, M.R.; Van Den Bosch, L.; Zetterberg, H.; Holtzman, D.M.; Dewachter, I. Hallmarks of neurodegenerative diseases. Cell 2023, 186, 693–714. [Google Scholar]
- Kempuraj, D.; Khan, M.M.; Thangavel, R.; Xiong, Z.; Yang, E.; Zaheer, A. Glia Maturation Factor Induces Interleukin-33 Release from Astrocytes: Implications for Neurodegenerative Diseases. J. Neuroimmune Pharmacol. 2013, 8, 643–650. [Google Scholar] [CrossRef]
- Zaheer, A.; Zaheer, S.; Sahu, S.K.; Knight, S.; Khosravi, H.; Mathur, S.N.; Lim, R. A novel role of glia maturation factor: Induction of granulocyte-macrophage colony-stimulating factor and pro-inflammatory cytokines. J. Neur. 2007, 101, 364–376. [Google Scholar] [CrossRef] [PubMed]
- Kempuraj, D.; Thangavel, R.; Selvakumar, G.P.; Zaheer, S.; Ahmed, M.E.; Raikwar, S.P.; Zahoor, H.; Saeed, D.; Natteru, P.A.; Iyer, S.; et al. Brain and Peripheral Atypical Inflammatory Mediators Potentiate Neuroinflammation and Neurodegeneration. Front. Cell Neurosci. 2017, 11, 216. [Google Scholar]
- Hudson, C.A.; Christophi, G.P.; Gruber, R.C.; Wilmore, J.R.; Lawrence, D.A.; Massa, P.T. Induction of IL-33 expression and activity in central nervous system glia. J. Leukoc. Biol. 2008, 84, 631–643. [Google Scholar] [CrossRef]
- Saresella, M.; Marventano, I.; Piancone, F.; La Rosa, F.; Galimberti, D.; Fenoglio, C.; Scarpini, E.; Clerici, M. IL-33 and its decoy sST2 in patients with Alzheimer’s disease and mild cognitive impairment. J. Neuroinflamm. 2020, 17, 174. [Google Scholar] [CrossRef]
- Italiani, P.; Puxeddu, I.; Napoletano, S.; Scala, E.; Melillo, D.; Manocchio, S.; Angiolillo, A.; Migliorini, P.; Boraschi, D.; Vitale, E.; et al. Circulating levels of IL-1 family cytokines and receptors in Alzheimer’s disease: New markers of disease progression? J. Neuroinflamm. 2018, 15, 342. [Google Scholar] [CrossRef]
- Carlock, C.; Wu, J.; Shim, J.; Moreno-Gonzalez, I.; Pitcher, M.R.; Hicks, J.; Suzuki, A.; Iwata, J.; Quevado, J.; Lou, Y. Interleukin33 deficiency causes tau abnormality and neurodegeneration with Alzheimer-like symptoms in aged mice. Transl. Psychiatry 2017, 7, e1164. [Google Scholar] [CrossRef]
- Fu, A.K.Y.; Hung, K.-W.; Yuen, M.Y.F.; Zhou, X.; Mak, D.S.Y.; Chan, I.C.W.; Cheung, T.H.; Zhang, B.; Fu, W.-Y.; Liew, F.Y.; et al. IL-33 ameliorates Alzheimer’s disease-like pathology and cognitive decline. Proc. Natl. Acad. Sci. USA 2016, 113, E2705–E2713. [Google Scholar] [CrossRef] [PubMed]
- Khodadadi, H.; Salles, É.L.; Jarrahi, A.; Costigliola, V.; Khan, M.B.; Yu, J.C.; Morgan, J.C.; Hess, D.C.; Vaibhav, K.; Dhandapani, K.M.; et al. Cannabidiol Ameliorates Cognitive Function via Regulation of IL-33 and TREM2 Upregulation in a Murine Model of Alzheimer’s Disease. J. Alzheimer’s Dis. 2021, 80, 973–977. [Google Scholar] [CrossRef] [PubMed]
- Chapuis, J.; Hot, D.; Hansmannel, F.; Kerdraon, O.; Ferreira, S.; Hubans, C.; Maurage, C.A.; Huot, L.; Bensemain, F.; Laumet, G.; et al. Transcriptomic and genetic studies identify IL-33 as a candidate gene for Alzheimer’s disease. Mol. Psychiatry 2009, 14, 1004–1016. [Google Scholar] [CrossRef] [PubMed]
- Lau, S.-F.; Chen, C.; Fu, W.-Y.; Qu, J.Y.; Cheung, T.H.; Fu, A.K.Y.; Ip, N.Y. IL-33-PU.1 Transcriptome Reprogramming Drives Functional State Transition and Clearance Activity of Microglia in Alzheimer’s Disease. Cell Rep. 2020, 31, 107530. [Google Scholar] [CrossRef]
- Allan, D.; Fairlie-Clarke, K.J.; Elliott, C.; Schuh, C.; Barnett, S.C.; Lassmann, H.; Linnington, C.; Jiang, H.-R. Role of IL-33 and ST2 signalling pathway in multiple sclerosis: Expression by oligodendrocytes and inhibition of myelination in central nervous system. Acta Neuropathol. Commun. 2016, 4, 75. [Google Scholar] [CrossRef]
- Christophi, G.P.; Gruber, R.C.; Panos, M.; Christophi, R.L.; Jubelt, B.; Massa, P.T. Interleukin-33 upregulation in peripheral leukocytes and CNS of multiple sclerosis patients. Clin. Immunol. 2012, 142, 308–319. [Google Scholar]
- Jiang, H.-R.; Milovanović, M.; Allan, D.; Niedbala, W.; Besnard, A.-G.; Fukada, S.Y.; Alves-Filho, J.C.; Togbe, D.; Goodyear, C.S.; Linington, C.; et al. IL-33 attenuates EAE by suppressing IL-17 and IFN-γ production and inducing alternatively activated macrophages. Eur. J. Immunol. 2012, 42, 1804–1814. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, X.; Lv, Y.; Xu, Y.; Li, M.; Pan, Q.; Chu, Y.; Liu, N.; Zhang, G.-X.; Zhu, L. Matrine downregulates IL-33/ST2 expression in the central nervous system of rats with experimental autoimmune encephalomyelitis. Immunol. Lett. 2016, 178, 97–104. [Google Scholar] [CrossRef]
- Xiong, Z.; Thangavel, R.; Kempuraj, D.; Yang, E.; Zaheer, S.; Zaheer, A. Alzheimer’s Disease: Evidence for the Expression of Interleukin-33 and Its Receptor ST2 in the Brain. J. Alzheimer’s Dis. 2014, 40, 297–308. [Google Scholar] [CrossRef]
- Peng, J.; Pan, J.; Mo, J.; Peng, Y.; Rai, S.N. MPO/HOCl Facilitates Apoptosis and Ferroptosis in the SOD1G93A Motor Neuron of Amyotrophic Lateral Sclerosis. Oxid. Med. Cell. Longev. 2022, 2022, 8217663. [Google Scholar] [CrossRef]
- Korhonen, P.; Pollari, E.; Kanninen, K.M.; Savchenko, E.; Lehtonen, Š.; Wojciechowski, S.; Pomeshchik, Y.; Van Den Bosch, L.; Goldsteins, G.; Koistinaho, J.; et al. Long-term interleukin-33 treatment delays disease onset and alleviates astrocytic activation in a transgenic mouse model of amyotrophic lateral sclerosis. IBRO Rep. 2019, 6, 74–86. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Pfluger, C.M.; Henderson, R.D.; McCombe, P.A. Reduced levels of interleukin 33 and increased levels of soluble ST2 in subjects with amyotrophic lateral sclerosis. J. Neuroimmunol. 2012, 249, 93–95. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-E.; Li, S.; Li, X.-J. Lack of interleukin-1 type 1 receptor enhances the accumulation of mutant huntingtin in the striatum and exacerbates the neurological phenotypes of Huntington’s disease mice. Mol. Brain 2010, 3, 33. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Corey-Bloom, J.; Fischer, R.S.; Kim, A.; Snell, C.; Parkin, G.M.; Granger, D.A.; Granger, S.W.; Thomas, E.A. Levels of Interleukin-6 in Saliva, but Not Plasma, Correlate with Clinical Metrics in Huntington’s Disease Patients and Healthy Control Subjects. Int. J. Mol. Sci. 2020, 21, 6363. [Google Scholar] [PubMed]
- Bhardwaj, S.; Kesari, K.K.; Rachamalla, M.; Mani, S.; Ashraf, G.M.; Jha, S.K.; Kumar, P.; Ambasta, R.K.; Dureja, H.; Devkota, H.P.; et al. CRISPR/Cas9 gene editing: New hope for Alzheimer’s disease therapeutics. J. Adv. Res. 2022, 40, 207–221. [Google Scholar] [PubMed]
- Jia, L.; Quan, M.; Fu, Y.; Zhao, T.; Li, Y.; Wei, C.; Tang, Y.; Qin, Q.; Wang, F.; Qiao, Y.; et al. Dementia in China: Epidemiology, clinical management, and research advances. Lancet Neurol. 2020, 19, 81–92. [Google Scholar] [CrossRef]
- Pei, J.-J.; Giron, M.S.T.; Jia, J.; Wang, H.-X. Dementia studies in Chinese populations. Neurosci. Bull. 2014, 30, 207–216. [Google Scholar] [CrossRef]
- Liang, J.-H.; Jia, J.-P. Dysfunctional autophagy in Alzheimer’s disease: Pathogenic roles and therapeutic implications. Neurosci. Bull. 2014, 30, 308–316. [Google Scholar] [CrossRef]
- 2021 Alzheimer’s disease facts and figures. Alzheimers Dement. 2021, 17, 327–406.
- Liang, C.-S.; Su, K.-P.; Tsai, C.-L.; Lee, J.-T.; Chu, C.-S.; Yeh, T.-C.; Su, M.-W.; Lin, G.-Y.; Lin, Y.-K.; Chu, H.-T.; et al. The role of interleukin-33 in patients with mild cognitive impairment and Alzheimer’s disease. Alzheimers Res. Ther. 2020, 12, 86. [Google Scholar] [CrossRef]
- Pennisi, M.; Crupi, R.; Di Paola, R.; Ontario, M.L.; Bella, R.; Calabrese, E.J.; Crea, R.; Cuzzocrea, S.; Calabrese, V. Inflammasomes, hormesis, and antioxidants in neuroinflammation: Role of NRLP3 in Alzheimer disease. J. Neurosci. Res. 2016, 95, 1360–1372. [Google Scholar] [CrossRef] [PubMed]
- La Rosa, F.; Saresella, M.; Baglio, F.; Piancone, F.; Marventano, I.; Calabrese, E.; Nemni, R.; Ripamonti, E.; Cabinio, M.; Clerici, M. Immune and Imaging Correlates of Mild Cognitive Impairment Conversion to Alzheimer’s Disease. Sci. Rep. 2017, 7, 16760. [Google Scholar] [CrossRef] [PubMed]
- Dhib-Jalbut, S. Friend or foe? Targeting microglia in Alzheimer’s disease. Cytokine 2016, 86, 4–5. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, N.; Yokoyama, H.; Yashiro, T.; Nakano, N.; Nishiyama, M.; Kanada, S.; Fukai, T.; Hara, M.; Ikeda, S.; Ogawa, H.; et al. Role of PU.1 in MHC class II expression through transcriptional regulation of class II transactivator pI in dendritic cells. J. Allergy Clin. Immunol. 2012, 129, 814–824.e6. [Google Scholar] [CrossRef]
- Lau, S.-F.; Fu, A.K.Y.; Ip, N.Y. Cytokine signaling convergence regulates the microglial state transition in Alzheimer’s disease. Cell Mol. Life Sci. 2021, 78, 4703–4712. [Google Scholar] [CrossRef]
- Wu, J.; Carlock, C.; Shim, J.; Moreno-Gonzalez, I.; Glass, W.; Ross, A.; Barichello, T.; Quevedo, J.; Lou, Y. Requirement of brain interleukin33 for aquaporin4 expression in astrocytes and glymphatic drainage of abnormal tau. Mol. Psychiatry 2021, 26, 5912–5924. [Google Scholar] [CrossRef]
- Amini, M.; Abdolmaleki, Z. The Effect of Cannabidiol Coated by Nano-Chitosan on Learning and Memory, Hippocampal CB1 and CB2 Levels, and Amyloid Plaques in an Alzheimer’s Disease Rat Model. Neuropsychobiology 2022, 81, 171–183. [Google Scholar] [CrossRef]
- Pintér, A.; Cseh, D.; Sárközi, A.; Illigens, B.; Siepmann, T. Autonomic Dysregulation in Multiple Sclerosis. Int. J. Mol. Sci. 2015, 16, 16920–16952. [Google Scholar]
- Merkelbach, S.; Haensch, C.-A.; Hemmer, B.; Koehler, J.; König, N.H.; Ziemssen, T. Multiple sclerosis and the autonomic nervous system. J. Neurol. 2006, 253, i21–i25. [Google Scholar] [CrossRef]
- Fowler, C.J.; Panicker, J.N.; Drake, M.; Harris, C.; Harrison, S.C.W.; Kirby, M.; Lucas, M.; Macleod, N.; Mangnall, J.; North, A.; et al. A UK consensus on the management of the bladder in multiple sclerosis. Postgrad. Med. J. 2009, 85, 552–559. [Google Scholar] [CrossRef]
- Alsahebfosoul, F.; Rahimmanesh, I.; Shajarian, M.; Etemadifar, M.; Sedaghat, N.; Hejazi, Z.; Naderi, S. Interleukin-33 plasma levels in patients with relapsing-remitting multiple sclerosis. Biomol. Concepts 2017, 8, 55–60. [Google Scholar] [CrossRef]
- Jafarzadeh, A.; Mahdavi, R.; Jamali, M.; Hajghani, H.; Nemati, M.; Ebrahimi, H.-A. Increased Concentrations of Interleukin-33 in the Serum and Cerebrospinal Fluid of Patients with Multiple Sclerosis. Oman Med. J. 2016, 31, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Fujinami, R.S.; Natarajan, C.; Yao, S.-Y.; Sriram, S. TLR3 Agonist Poly-IC Induces IL-33 and Promotes Myelin Repair. PLoS ONE 2016, 11, e0152163. [Google Scholar]
- Li, M.; Li, Y.; Liu, X.; Gao, X.; Wang, Y. IL-33 blockade suppresses the development of experimental autoimmune encephalomyelitis in C57BL/6 mice. J. Neuroimmunol. 2012, 247, 25–31. [Google Scholar] [CrossRef]
- Che, D.N.; Cho, B.O.; Kim, J.-S.; Shin, J.Y.; Kang, H.J.; Jang, S.I. Effect of Luteolin and Apigenin on the Production of Il-31 and Il-33 in Lipopolysaccharides-Activated Microglia Cells and Their Mechanism of Action. Nutrients 2020, 12, 811. [Google Scholar] [CrossRef] [PubMed]
- Ljunggren-Rose, Å.; Natarajan, C.; Matta, P.; Pandey, A.; Upender, I.; Sriram, S. Anacardic acid induces IL-33 and promotes remyelination in CNS. Proc. Natl. Acad. Sci. USA 2020, 117, 21527–21535. [Google Scholar] [CrossRef] [PubMed]
- Bessa, J.; Meyer, C.A.; de Vera Mudry, M.C.; Schlicht, S.; Smith, S.H.; Iglesias, A.; Cote-Sierra, J. Altered subcellular localization of IL-33 leads to non-resolving lethal inflammation. J. Autoimmun. 2014, 55, 33–41. [Google Scholar] [CrossRef]
- Holowka, D.; Kempuraj, D.; Thangavel, R.; Yang, E.; Pattani, S.; Zaheer, S.; Santillan, D.A.; Santillan, M.K.; Zaheer, A. Dopaminergic Toxin 1-Methyl-4-Phenylpyridinium, Proteins α-Synuclein and Glia Maturation Factor Activate Mast Cells and Release Inflammatory Mediators. PLoS ONE 2015, 10, e0135776. [Google Scholar]
- Do, H.-A.; Baek, K.-H. Cellular functions regulated by deubiquitinating enzymes in neurodegenerative diseases. Ageing Res. Rev. 2021, 69, 101367. [Google Scholar]
- Liguori, F.; Amadio, S.; Volonté, C. Fly for ALS: Drosophila modeling on the route to amyotrophic lateral sclerosis modifiers. Cell Mol. Life Sci. 2021, 78, 6143–6160. [Google Scholar]
- Abati, E.; Bresolin, N.; Comi, G.; Corti, S. Silence superoxide dismutase 1 (SOD1): A promising therapeutic target for amyotrophic lateral sclerosis (ALS). Expert Opin. Ther. Targets 2020, 24, 295–310. [Google Scholar] [CrossRef] [PubMed]
- Burk, K.; Pasterkamp, R.J. Disrupted neuronal trafficking in amyotrophic lateral sclerosis. Acta Neuropathol. 2019, 137, 859–877. [Google Scholar] [PubMed]
- Zubiri, I.; Lombardi, V.; Bremang, M.; Mitra, V.; Nardo, G.; Adiutori, R.; Lu, C.-H.; Leoni, E.; Yip, P.; Yildiz, O.; et al. Tissue-enhanced plasma proteomic analysis for disease stratification in amyotrophic lateral sclerosis. Mol. Neurodegener. 2018, 13, 60. [Google Scholar] [CrossRef] [PubMed]
- Rolfes, L.; Schulte-Mecklenbeck, A.; Schreiber, S.; Vielhaber, S.; Herty, M.; Marten, A.; Pfeuffer, S.; Ruck, T.; Wiendl, H.; Gross, C.C.; et al. Amyotrophic lateral sclerosis patients show increased peripheral and intrathecal T-cell activation. Brain Commun. 2021, 3, fcab157. [Google Scholar] [CrossRef]
- Coque, E.; Salsac, C.; Espinosa-Carrasco, G.; Varga, B.; Degauque, N.; Cadoux, M.; Crabé, R.; Virenque, A.; Soulard, C.; Fierle, J.K.; et al. Cytotoxic CD8+T lymphocytes expressing ALS-causing SOD1 mutant selectively trigger death of spinal motoneurons. Proc. Natl. Acad. Sci. USA 2019, 116, 2312–2317. [Google Scholar] [CrossRef]
- Kempuraj, D.; Ahmed, M.E.; Selvakumar, G.P.; Thangavel, R.; Dhaliwal, A.S.; Dubova, I.; Mentor, S.; Premkumar, K.; Saeed, D.; Zahoor, H.; et al. Brain Injury—Mediated Neuroinflammatory Response and Alzheimer’s Disease. Neuroscientist 2019, 26, 134–155. [Google Scholar] [CrossRef]
- Smith, D.H.; Johnson, V.E.; Trojanowski, J.Q.; Stewart, W. Chronic traumatic encephalopathy—Confusion and controversies. Nat. Rev. Neurol. 2019, 15, 179–183. [Google Scholar]
- Fienko, S.; Landles, C.; Sathasivam, K.; McAteer, S.J.; Milton, R.E.; Osborne, G.F.; Smith, E.J.; Jones, S.T.; Bondulich, M.K.; Danby, E.C.E.; et al. Alternative processing of human HTT mRNA with implications for Huntington’s disease therapeutics. Brain 2022, 145, 4409–4424. [Google Scholar] [CrossRef]
- Wechsler, M.E.; Ruddy, M.K.; Pavord, I.D.; Israel, E.; Rabe, K.F.; Ford, L.B.; Maspero, J.F.; Abdulai, R.M.; Hu, C.-C.; Martincova, R.; et al. Efficacy and Safety of Itepekimab in Patients with Moderate-to-Severe Asthma. New Engl. J. Med. 2021, 385, 1656–1668. [Google Scholar] [CrossRef]
- Kelsen, S.G.; Agache, I.O.; Soong, W.; Israel, E.; Chupp, G.L.; Cheung, D.S.; Theess, W.; Yang, X.; Staton, T.L.; Choy, D.F.; et al. Astegolimab (anti-ST2) efficacy and safety in adults with severe asthma: A randomized clinical trial. J. Allergy Clin. Immunol. 2021, 148, 790–798. [Google Scholar] [CrossRef]

| Species | Conditions | Organs or Tissues | Cell Types | Refs. |
|---|---|---|---|---|
| human | Normal | brain | neuron, microglia, astrocyte, oligodendrocyte | [33,35] |
| stomach | epithelium cell | [27] | ||
| skin | keratinocytes | [27] | ||
| lymph node and tonsil | endothelial cell, fibroblastic reticular cell | [27] | ||
| colon, lung, cervix | endothelial cell | [28] | ||
| blood vessels | endothelial cell | [28] | ||
| mouse | Normal | skin | keratinocytes | [27] |
| lymph nodes, spleen, appendix | fibroblastic reticular cell | [28] | ||
| adipose tissue | endothelial cell | [28] | ||
| vagina, skin, lung, stomach, and salivary glands | epithelial cell | [27] | ||
| eye (exception of optic nerve) | glial cells (the retinal inner nuclear layer) | [28] | ||
| brain | neuron, microglia, astrocyte, oligodendrocyte | [34] | ||
| Disease | ||||
| liver | endothelial cell | [28] | |
| lung | epithelial cell | [28] | |
| colon | endothelial cell | [29] |
| Neurodegenerative Disease | Role of IL-33 | Refs. |
|---|---|---|
| AD |
| [58,59] |
| [60] | |
| [61] | |
| [62] | |
| [63] | |
| [64] | |
| MS |
| [65] |
| [66] | |
| [67] | |
| [68] | |
| PD |
| [59] |
| [69] | |
| ALS |
| [70] |
| [71] | |
| [72] | |
| CTE |
| [48] |
| HD |
| [73] |
| [74] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, Z.; Guo, M.; Ge, X.; Chen, F.; Lei, P. IL-33/ST2 Axis: A Potential Therapeutic Target in Neurodegenerative Diseases. Biomolecules 2023, 13, 1494. https://doi.org/10.3390/biom13101494
Jia Z, Guo M, Ge X, Chen F, Lei P. IL-33/ST2 Axis: A Potential Therapeutic Target in Neurodegenerative Diseases. Biomolecules. 2023; 13(10):1494. https://doi.org/10.3390/biom13101494
Chicago/Turabian StyleJia, Zexi, Mengtian Guo, Xintong Ge, Fanglian Chen, and Ping Lei. 2023. "IL-33/ST2 Axis: A Potential Therapeutic Target in Neurodegenerative Diseases" Biomolecules 13, no. 10: 1494. https://doi.org/10.3390/biom13101494
APA StyleJia, Z., Guo, M., Ge, X., Chen, F., & Lei, P. (2023). IL-33/ST2 Axis: A Potential Therapeutic Target in Neurodegenerative Diseases. Biomolecules, 13(10), 1494. https://doi.org/10.3390/biom13101494







