A Combined Biomarker That Includes Plasma Fibroblast Growth Factor 23, Erythropoietin, and Klotho Predicts Short- and Long-Term Morbimortality and Development of Chronic Kidney Disease in Critical Care Patients with Sepsis: A Prospective Cohort
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
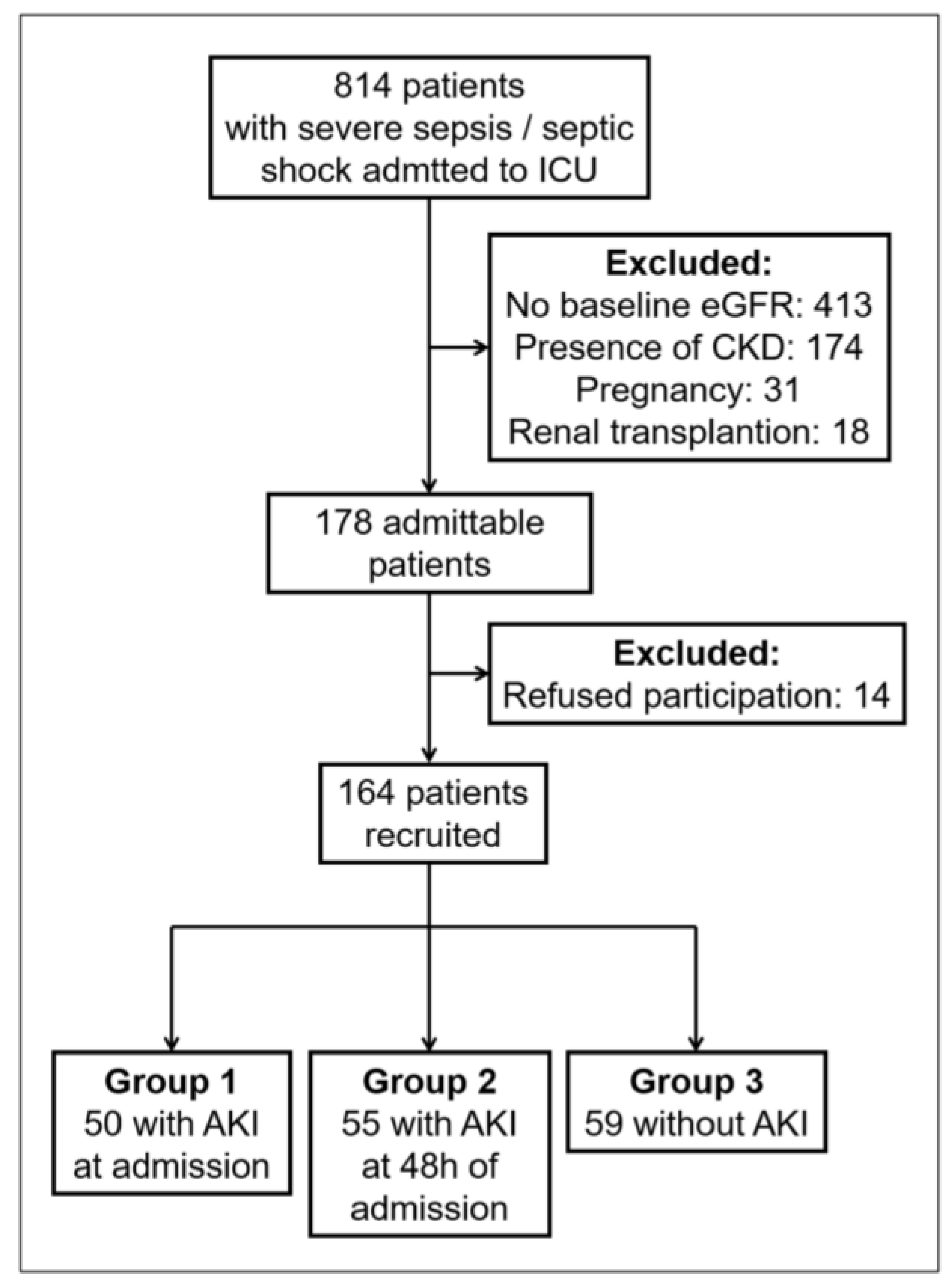
2.2. Cohort Characteristics
2.3. Outcomes
2.4. Data Collection and Plasma Measurements
2.5. Design of Combined Biomarker
2.6. Sample Size Calculation
2.7. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. FEK Indicator and Prediction of AKI
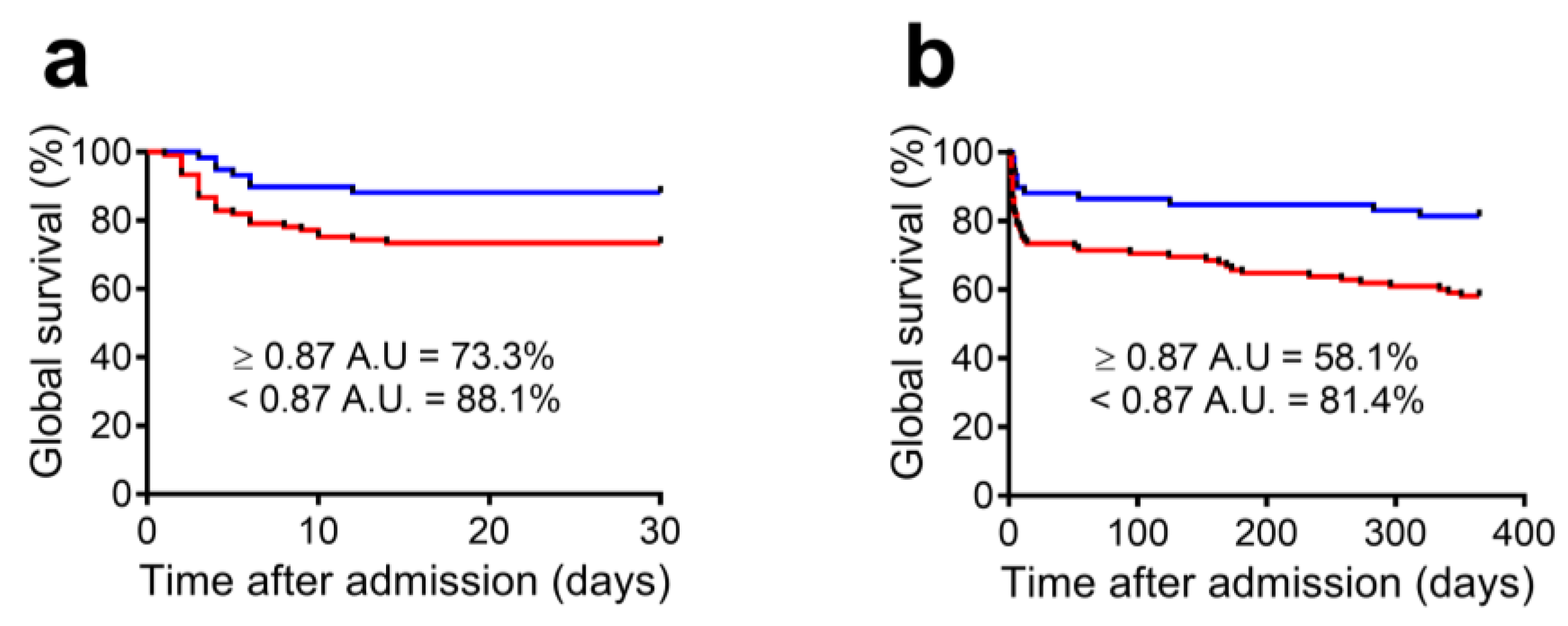
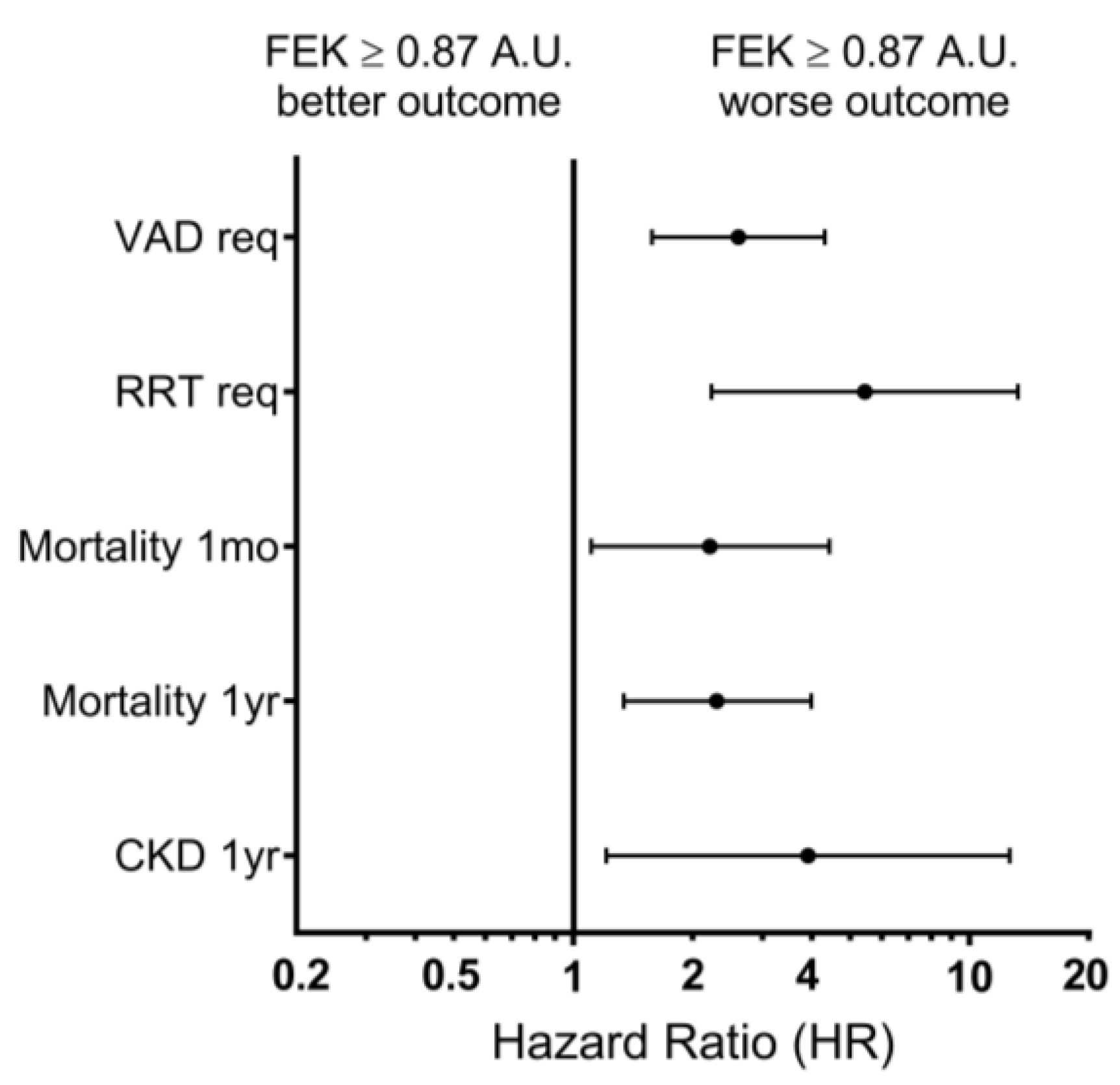
3.3. FEK Indicator and Outcome Prediction
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Verma, S.; Kellum, J.A. Defining Acute Kidney Injury. Crit. Care Clin. 2021, 37, 251–266. [Google Scholar] [CrossRef] [PubMed]
- Søvik, S.; Isachsen, M.S.; Nordhuus, K.M.; Tveiten, C.K.; Eken, T.; Sunde, K.; Brurberg, K.G.; Beitland, S. Acute Kidney Injury in Trauma Patients Admitted to the ICU: A Systematic Review and Meta-Analysis. Intensive Care Med. 2019, 45, 407–419. [Google Scholar] [CrossRef]
- Lewington, A.J.P.; Cerdá, J.; Mehta, R.L. Raising Awareness of Acute Kidney Injury: A Global Perspective of a Silent Killer. Kidney Int. 2013, 84, 457–467. [Google Scholar] [CrossRef]
- Ostermann, M.; Zarbock, A.; Goldstein, S.; Kashani, K.; Macedo, E.; Murugan, R.; Bell, M.; Forni, L.; Guzzi, L.; Joannidis, M.; et al. Recommendations on Acute Kidney Injury Biomarkers from the Acute Disease Quality Initiative Consensus Conference: A Consensus Statement: A Consensus Statement. JAMA Netw. Open 2020, 3, e2019209. [Google Scholar] [CrossRef]
- Vaara, S.T.; Pettilä, V.; Kaukonen, K.-M.; Bendel, S.; Korhonen, A.-M.; Bellomo, R.; Reinikainen, M. The Attributable Mortality of Acute Kidney Injury: A Sequentially Matched Analysis. Crit. Care Med. 2014, 42, 878–885. [Google Scholar] [CrossRef] [PubMed]
- Silver, S.A.; Wald, R. Improving Outcomes of Acute Kidney Injury Survivors. Curr. Opin. Crit. Care 2015, 21, 500–505. [Google Scholar] [CrossRef]
- An, J.N.; Hwang, J.H.; Kim, D.K.; Lee, H.; Ahn, S.Y.; Kim, S.; Park, J.T.; Kang, S.-W.; Oh, Y.K.; Kim, Y.S.; et al. Chronic Kidney Disease after Acute Kidney Injury Requiring Continuous Renal Replacement Therapy and Its Impact on Long-Term Outcomes: A Multicenter Retrospective Cohort Study in Korea: A Multicenter Retrospective Cohort Study in Korea. Crit. Care Med. 2017, 45, 47–57. [Google Scholar] [CrossRef]
- Neyra, J.A.; Chawla, L.S. Acute Kidney Disease to Chronic Kidney Disease. Crit. Care Clin. 2021, 37, 453–474. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Bagasha, P.; Nakwagala, F.; Kwizera, A.; Ssekasanvu, E.; Kalyesubula, R. Acute Kidney Injury among Adult Patients with Sepsis in a Low-Income Country: Clinical Patterns and Short-Term Outcomes. BMC Nephrol. 2015, 16, 4. [Google Scholar] [CrossRef]
- Peerapornratana, S.; Manrique-Caballero, C.L.; Gómez, H.; Kellum, J.A. Acute Kidney Injury from Sepsis: Current Concepts, Epidemiology, Pathophysiology, Prevention and Treatment. Kidney Int. 2019, 96, 1083–1099. [Google Scholar] [CrossRef] [PubMed]
- International Society of Nephrology. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Summary of Recommendation Statements. Kidney Int. Suppl. 2012, 2, 8–12. [Google Scholar] [CrossRef]
- Kulvichit, W.; Kellum, J.A.; Srisawat, N. Biomarkers in Acute Kidney Injury. Crit. Care Clin. 2021, 37, 385–398. [Google Scholar] [CrossRef] [PubMed]
- Kane-Gill, S.L.; Meersch, M.; Bell, M. Biomarker-Guided Management of Acute Kidney Injury. Curr. Opin. Crit. Care 2020, 26, 556–562. [Google Scholar] [CrossRef]
- Molinari, L.; Heskia, F.; Peerapornratana, S.; Ronco, C.; Guzzi, L.; Toback, S.; Birch, R.; Beyhaghi, H.; Kwan, T.; Kampf, J.P.; et al. Limiting Acute Kidney Injury Progression in Sepsis: Study Protocol and Trial Simulation: Study Protocol and Trial Simulation. Crit. Care Med. 2021, 49, 1706–1716. [Google Scholar] [CrossRef]
- Engelman, D.T.; Crisafi, C.; Germain, M.; Greco, B.; Nathanson, B.H.; Engelman, R.M.; Schwann, T.A. Using Urinary Biomarkers to Reduce Acute Kidney Injury following Cardiac Surgery. J. Thorac. Cardiovasc. Surg. 2020, 160, 1235–1246.e2. [Google Scholar] [CrossRef]
- Kane-Gill, S.L.; Peerapornratana, S.; Wong, A.; Murugan, R.; Groetzinger, L.M.; Kim, C.; Smithburger, P.L.; Then, J.; Kellum, J.A. Use of Tissue Inhibitor of Metalloproteinase 2 and Insulin-like Growth Factor Binding Protein 7 [TIMP2]•[IGFBP7] as an AKI Risk Screening Tool to Manage Patients in the Real-World Setting. J. Crit. Care 2020, 57, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Rizo-Topete, L.M.; Rosner, M.H.; Ronco, C. Acute Kidney Injury Risk Assessment and the Nephrology Rapid Response Team. Blood Purif. 2017, 43, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.-P.; Bao, J.-F.; Li, A. Roles for Fibroblast Growth Factor-23 and α-Klotho in Acute Kidney Injury. Metabolism 2021, 116, 154435. [Google Scholar] [CrossRef]
- Christov, M.; Neyra, J.A.; Gupta, S.; Leaf, D.E. Fibroblast Growth Factor 23 and Klotho in AKI. Semin. Nephrol. 2019, 39, 57–75. [Google Scholar] [CrossRef]
- Neyra, J.A.; Hu, M.C.; Moe, O.W. Fibroblast Growth Factor 23 and AKlotho in Acute Kidney Injury: Current Status in Diagnostic and Therapeutic Applications. Nephron 2020, 144, 665–672. [Google Scholar] [CrossRef]
- Erben, R.G.; Andrukhova, O. FGF23-Klotho Signaling Axis in the Kidney. Bone 2017, 100, 62–68. [Google Scholar] [CrossRef]
- Ho, B.B.; Bergwitz, C. FGF23 Signalling and Physiology. J. Mol. Endocrinol. 2021, 66, R23–R32. [Google Scholar] [CrossRef]
- Kuro-O, M. The Klotho Proteins in Health and Disease. Nat. Rev. Nephrol. 2019, 15, 27–44. [Google Scholar] [CrossRef] [PubMed]
- Leaf, D.E.; Jacob, K.A.; Srivastava, A.; Chen, M.E.; Christov, M.; Jüppner, H.; Sabbisetti, V.S.; Martin, A.; Wolf, M.; Waikar, S.S. Fibroblast Growth Factor 23 Levels Associate with AKI and Death in Critical Illness. J. Am. Soc. Nephrol. 2017, 28, 1877–1885. [Google Scholar] [CrossRef] [PubMed]
- Toro, L.; Barrientos, V.; León, P.; Rojas, M.; Gonzalez, M.; González-Ibáñez, A.; Illanes, S.; Sugikawa, K.; Abarzúa, N.; Bascuñán, C.; et al. Erythropoietin Induces Bone Marrow and Plasma Fibroblast Growth Factor 23 during Acute Kidney Injury. Kidney Int. 2018, 93, 1131–1141. [Google Scholar] [CrossRef]
- Neyra, J.A.; Li, X.; Mescia, F.; Ortiz-Soriano, V.; Adams-Huet, B.; Pastor, J.; Hu, M.-C.; Toto, R.D.; Moe, O.W.; Klotho and Acute Kidney Injury (KLAKI) Study Group. Urine Klotho Is Lower in Critically Ill Patients with versus without Acute Kidney Injury and Associates with Major Adverse Kidney Events. Crit. Care Explor. 2019, 1, e0016. [Google Scholar] [CrossRef]
- Seo, M.Y.; Yang, J.; Lee, J.Y.; Kim, K.; Kim, S.C.; Chang, H.; Won, N.H.; Kim, M.-G.; Jo, S.-K.; Cho, W.; et al. Renal Klotho Expression in Patients with Acute Kidney Injury Is Associated with the Severity of the Injury. Korean J. Intern. Med. 2015, 30, 489–495. [Google Scholar] [CrossRef]
- Jou-Valencia, D.; Molema, G.; Popa, E.; Aslan, A.; van Dijk, F.; Mencke, R.; Hillebrands, J.-L.; Heeringa, P.; Hoenderop, J.G.; Zijlstra, J.G.; et al. Renal Klotho Is Reduced in Septic Patients and Pretreatment with Recombinant Klotho Attenuates Organ Injury in Lipopolysaccharide-Challenged Mice. Crit. Care Med. 2018, 46, e1196–e1203. [Google Scholar] [CrossRef]
- Hu, M.-C.; Shi, M.; Zhang, J.; Quiñones, H.; Kuro-o, M.; Moe, O.W. Klotho Deficiency Is an Early Biomarker of Renal Ischemia-Reperfusion Injury and Its Replacement Is Protective. Kidney Int. 2010, 78, 1240–1251. [Google Scholar] [CrossRef] [PubMed]
- Nolan, K.A.; Wenger, R.H. Source and Microenvironmental Regulation of Erythropoietin in the Kidney. Curr. Opin. Nephrol. Hypertens. 2018, 27, 277–282. [Google Scholar] [CrossRef] [PubMed]
- van Vuren, A.J.; Gaillard, C.A.J.M.; Eisenga, M.F.; van Wijk, R.; van Beers, E.J. The EPO-FGF23 Signaling Pathway in Erythroid Progenitor Cells: Opening a New Area of Research. Front. Physiol. 2019, 10, 304. [Google Scholar] [CrossRef]
- Virankabutra, T.; Jones, S.; Tanudsintum, S.; Lipkin, G.; Todd, S.; Bion, J. Erythropoietin Mimics the Acute Phase Re-Sponse in Critical Illness. Crit Care 2003, 7, R35–R40. [Google Scholar]
- Clinkenbeard, E.L.; Hanudel, M.R.; Stayrook, K.R.; Appaiah, H.N.; Farrow, E.G.; Cass, T.A.; Summers, L.J.; Ip, C.S.; Hum, J.M.; Thomas, J.C.; et al. Erythropoietin Stimulates Murine and Human Fibroblast Growth Factor-23, Revealing Novel Roles for Bone and Bone Marrow. Haematologica 2017, 102, e427–e430. [Google Scholar] [CrossRef] [PubMed]
- Dellinger, R.P.; Levy, M.M.; Rhodes, A.; Annane, D.; Gerlach, H.; Opal, S.M.; Sevransky, J.E.; Sprung, C.L.; Douglas, I.S.; Jaeschke, R.; et al. Surviving Sepsis Campaign Guidelines Committee including The Pediatric Subgroup. Surviving Sepsis Campaign: International Guidelines for Management of Severe Sepsis and Septic Shock, 2012. Intensive Care Med. 2013, 39, 165–228. [Google Scholar] [CrossRef] [PubMed]
- Leaf, D.E.; Wolf, M.; Waikar, S.S.; Chase, H.; Christov, M.; Cremers, S.; Stern, L. FGF-23 Levels in Patients with AKI and Risk of Adverse Outcomes. Clin. J. Am. Soc. Nephrol. 2012, 7, 1217–1223. [Google Scholar] [CrossRef] [PubMed]
- Leaf, D.E.; Christov, M.; Jüppner, H.; Siew, E.; Ikizler, T.A.; Bian, A.; Chen, G.; Sabbisetti, V.S.; Bonventre, J.V.; Cai, X.; et al. Fibroblast Growth Factor 23 Levels Are Elevated and Associated with Severe Acute Kidney Injury and Death Following Cardiac Surgery. Kidney Int. 2016, 89, 939–948. [Google Scholar] [CrossRef]
- de Oliveira Neves, F.M.; Araújo, C.B.; de Freitas, D.F.; Arruda, B.F.T.; de Macêdo Filho, L.J.M.; Salles, V.B.; Meneses, G.C.; Martins, A.M.C.; Libório, A.B. Fibroblast Growth Factor 23, Endothelium Biomarkers and Acute Kidney Injury in Critically-Ill Patients. J. Transl. Med. 2019, 17, 121. [Google Scholar] [CrossRef]
- Radhakrishnan, K.; Kim, Y.-H.; Jung, Y.S.; Kim, D.-K.; Na, S.-Y.; Lim, D.; Kim, D.H.; Kim, J.; Kim, H.-S.; Choy, H.E.; et al. Orphan Nuclear Receptor ERR-γ Regulates Hepatic FGF23 Production in Acute Kidney Injury. Proc. Natl. Acad. Sci. USA 2021, 118, e2022841118. [Google Scholar] [CrossRef]
- Simic, P.; Kim, W.; Zhou, W.; Pierce, K.A.; Chang, W.; Sykes, D.B.; Aziz, N.B.; Elmariah, S.; Ngo, D.; Pajevic, P.D.; et al. Glycerol-3-Phosphate Is an FGF23 Regulator Derived from the Injured Kidney. J. Clin. Investig. 2020, 130, 1513–1526. [Google Scholar] [CrossRef]
- Mace, M.L.; Gravesen, E.; Hofman-Bang, J.; Olgaard, K.; Lewin, E. Key Role of the Kidney in the Regulation of Fibroblast Growth Factor 23. Kidney Int. 2015, 88, 1304–1313. [Google Scholar] [CrossRef] [PubMed]
- Ito, N.; Wijenayaka, A.R.; Prideaux, M.; Kogawa, M.; Ormsby, R.T.; Evdokiou, A.; Bonewald, L.F.; Findlay, D.M.; Atkins, G.J. Regulation of FGF23 Expression in IDG-SW3 Osteocytes and Human Bone by pro-Inflammatory Stimuli. Mol. Cell Endocrinol. 2015, 399, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Rabadi, S.; Udo, I.; Leaf, D.E.; Waikar, S.S.; Christov, M. Acute Blood Loss Stimulates Fibroblast Growth Factor 23 Production. Am. J. Physiol. Renal Physiol. 2018, 314, F132–F139. [Google Scholar] [CrossRef]
- Flamme, I.; Ellinghaus, P.; Urrego, D.; Krüger, T. FGF23 Expression in Rodents Is Directly Induced via Erythropoietin after Inhibition of Hypoxia Inducible Factor Proline Hydroxylase. PLoS ONE 2017, 12, e0186979. [Google Scholar] [CrossRef] [PubMed]
- Daryadel, A.; Bettoni, C.; Haider, T.; Imenez Silva, P.H.; Schnitzbauer, U.; Pastor-Arroyo, E.M.; Wenger, R.H.; Gassmann, M.; Wagner, C.A. Erythropoietin Stimulates Fibroblast Growth Factor 23 (FGF23) in Mice and Men. Pflugers Arch. 2018, 470, 1569–1582. [Google Scholar] [CrossRef]
- Hanudel, M.R.; Eisenga, M.F.; Rappaport, M.; Chua, K.; Qiao, B.; Jung, G.; Gabayan, V.; Gales, B.; Ramos, G.; de Jong, M.A.; et al. Effects of Erythropoietin on Fibroblast Growth Factor 23 in Mice and Humans. Nephrol. Dial. Transplant. 2019, 34, 2057–2065. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Noiri, E.; Hamasaki, Y.; Matsubara, T.; Ishii, T.; Yahagi, N.; Nangaku, M.; Doi, K. Erythropoietin Concentration in Acute Kidney Injury Is Associated with Insulin-like Growth Factor-Binding Protein-1: Erythropoietin in Acute Kidney Injury. Nephrology (Carlton) 2016, 21, 693–699. [Google Scholar] [CrossRef]
- Post, E.H.; Kellum, J.A.; Bellomo, R.; Vincent, J.-L. Renal Perfusion in Sepsis: From Macro- to Microcirculation. Kidney Int. 2017, 91, 45–60. [Google Scholar] [CrossRef]
- Skytte Larsson, J.; Krumbholz, V.; Enskog, A.; Bragadottir, G.; Redfors, B.; Ricksten, S.-E. Renal Blood Flow, Glomerular Filtration Rate, and Renal Oxygenation in Early Clinical Septic Shock. Crit. Care Med. 2018, 46, e560–e566. [Google Scholar] [CrossRef]
- Liu, Y.-J.; Sun, H.-D.; Chen, J.; Chen, M.-Y.; Ouyang, B.; Guan, X.-D. Klotho: A Novel and Early Biomarker of Acute Kidney Injury after Cardiac Valve Replacement Surgery in Adults. Int. J. Clin. Exp. Med. 2015, 8, 7351–7358. [Google Scholar]
- Jorge, L.B.; Coelho, F.O.; Sanches, T.R.; Malheiros, D.M.A.C.; Ezaquiel de Souza, L.; Dos Santos, F.; de Sá Lima, L.; Scavone, C.; Irigoyen, M.; Kuro-O, M.; et al. Klotho Deficiency Aggravates Sepsis-Related Multiple Organ Dysfunction. Am. J. Physiol. Renal Physiol. 2019, 316, F438–F448. [Google Scholar] [CrossRef]
- Liu, X.; Niu, Y.; Zhang, X.; Zhang, Y.; Yu, Y.; Huang, J.; Li, J.; Yu, C. Recombinant α-Klotho Protein Alleviated Acute Cardiorenal Injury in a Mouse Model of Lipopolysaccharide-Induced Septic Cardiorenal Syndrome Type 5. Anal. Cell. Pathol. (Amst.) 2019, 2019, 5853426. [Google Scholar] [CrossRef] [PubMed]
- Mitobe, M.; Yoshida, T.; Sugiura, H.; Shirota, S.; Tsuchiya, K.; Nihei, H. Oxidative Stress Decreases Klotho Expression in a Mouse Kidney Cell Line. Nephron Exp. Nephrol. 2005, 101, e67–e74. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.A.; Izquierdo, M.C.; Sanchez-Niño, M.D.; Suárez-Alvarez, B.; Lopez-Larrea, C.; Jakubowski, A.; Blanco, J.; Ramirez, R.; Selgas, R.; Ruiz-Ortega, M.; et al. The Inflammatory Cytokines TWEAK and TNFα Reduce Renal Klotho Expression through NFκB. J. Am. Soc. Nephrol. 2011, 22, 1315–1325. [Google Scholar] [CrossRef]
- Morii, K.; Yamasaki, S.; Doi, S.; Irifuku, T.; Sasaki, K.; Doi, T.; Nakashima, A.; Arihiro, K.; Masaki, T. MicroRNA-200c Regulates KLOTHO Expression in Human Kidney Cells under Oxidative Stress. PLoS ONE 2019, 14, e0218468. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Fan, H.; Le, J.; Chen, G.; Chen, H.; Li, J.; Zhu, J. Protective effects of Klotho protein on acute kidney injury in septic mice and its mechanism. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2019, 31, 160–164. [Google Scholar] [CrossRef]
- Li, Y.; Lerman, L.O. Cellular Senescence: A New Player in Kidney Injury. Hypertension 2020, 76, 1069–1075. [Google Scholar] [CrossRef]
- Castellano, G.; Intini, A.; Stasi, A.; Divella, C.; Gigante, M.; Pontrelli, P.; Franzin, R.; Accetturo, M.; Zito, A.; Fiorentino, M.; et al. Complement Modulation of Anti-Aging Factor Klotho in Ischemia/Reperfusion Injury and Delayed Graft Function: Complement Modulation of Klotho. Am. J. Transplant. 2016, 16, 325–333. [Google Scholar] [CrossRef]
- Abdel-Rahman, E.M.; Turgut, F.; Gautam, J.K.; Gautam, S.C. Determinants of Outcomes of Acute Kidney Injury: Clinical Predictors and Beyond. J. Clin. Med. 2021, 10, 1175. [Google Scholar] [CrossRef]
- Hoste, E.; Bihorac, A.; Al-Khafaji, A.; Ortega, L.M.; Ostermann, M.; Haase, M.; Zacharowski, K.; Wunderink, R.; Heung, M.; Lissauer, M.; et al. Identifi-Cation and Validation of Biomarkers of Persistent Acute Kidney Injury: The RUBY Study. Intensive Care Med. 2020, 46, 943–953. [Google Scholar] [CrossRef]
- Blanco-Gozalo, V.; Casanova, A.G.; Sancho-Martínez, S.M.; Prieto, M.; Quiros, Y.; Morales, A.I.; Martínez-Salgado, C.; Agüeros-Blanco, C.; Benito-Hernández, A.; Ramos-Barron, M.A.; et al. Combined Use of GM2AP and TCP1-Eta Urinary Levels Predicts Recovery from Intrinsic Acute Kidney Injury. Sci. Rep. 2020, 10, 11599. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, J.; Eriksson, M.; Brattström, O.; Hellgren, E.; Friman, O.; Gidlöf, A.; Larsson, E.; Oldner, A. Comparison of the Sepsis-2 and Sepsis-3 Definitions in Severely Injured Trauma Patients. J. Crit. Care 2019, 54, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Engoren, M.; Seelhammer, T.; Freundlich, R.E.; Maile, M.D.; Sigakis, M.J.G.; Schwann, T.A. A Comparison of Sepsis-2 (Systemic Inflammatory Response Syndrome Based) to Sepsis-3 (Sequential Organ Failure Assessment Based) Definitions-A Multicenter Retrospective Study. Crit. Care Med. 2020, 48, 1258–1264. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | All Patients | Patients with AKI at Admission | Patients with AKI at Day 1–2 | Patients without AKI | |
|---|---|---|---|---|---|
| Demographic | |||||
| Patients, n (%) | 164 (100%) | 50 (30.5%) | 55 (33.5%) | 59 (36.0%) | |
| Mean age ± SD, yr | 63.4 ± 14.3 | 64.7 ± 16.6 | 63.7 ± 13.9 | 62.1 ± 12.6 | |
| Men, n (%) | 103 (62.8%) | 33 (66.0%) | 38 (69.1%) | 32 (54.2%) | |
| Hispanic race, n (%) | 164 (100%) | 50 (100%) | 55 (100%) | 59 (100%) | |
| Hypertension, n (%) | 92 (56.1%) | 27 (54.0%) | 32 (58.2%) | 33 (55.9%) | |
| Diabetes, n (%) | 35 (21.3%) | 13 (26.0%) | 11 (20.0%) | 11 (18.6%) | |
| Coronary heart disease, n (%) | 20 (12.2%) | 7 (14.0%) | 8 (14.5%) | 5 (8.5%) | |
| Baseline renal function | |||||
| Serum creatinine ± SD, mg/dL | 0.85 ± 0.16 | 0.86 ± 0.16 | 0.85 ± 0.18 | 0.84 ± 0.15 | |
| eGFR # ± SD, mL/min/1.73m2 | 85.2 ± 15.5 | 83.9 ± 13.9 | 86.5 ± 15.8 | 85.1 ± 16.7 | |
| Infectious foci, n (%) | |||||
| Pulmonary | 60 (36.6%) | 22 (44.0%) | 20 (36.4%) | 18 (30.5%) | |
| Urinary | 26 (15.9%) | 8 (16.0%) | 10 (18.2%) | 8 (13.6%) | |
| Abdominal | 78 (47.6%) | 20 (40.0%) | 25 (45.5%) | 33 (55.9%) | |
| Emergency surgery required | 68 (41.5%) | 19 (38.0%) | 23 (41.8%) | 26 (44.1%) | |
| Biochemical parameters at admission | |||||
| Hematocrit ± SD, g/dL | 9.8 ± 2.1 | 9.3 ± 1.6 | 9.9 ± 1.8 | 10.9 ± 1.8 | |
| Blood ureic nitrogen ± SD, mg/dL | 24.6 ± 9.8 | 33.1 ± 10.4 *** | 21.7 ± 7.1 | 20.1 ± 6.5 | |
| Serum calcium ± SD, mg/dL | 8.8 ± 1.1 | 8.5 ± 1.1 * | 8.8 ± 0.9 | 9.0 ± 1.1 | |
| Serum phosphate ± SD, mg/dL | 3.9 ± 0.9 | 4.0 ± 0.9 | 4.0 ± 0.9 | 3.8 ± 0.9 | |
| Serum magnesium ± SD, mg/dL | 1.9 ± 0.2 | 1.8 ± 0.2 | 1.9 ± 0.2 | 2.0 ± 0.2 | |
| Serum creatinine ± SD, mg/dL | 1.35 ± 0.90 | 2.35 ± 1.07 *** | 0.94 ± 0.21 | 0.89 ± 0.16 | |
| Maximum serum creatinine ± SD, mg/dL | 1.91 ± 1.08 | 2.69 ± 1.09 *** | 2.22 ± 0.86 *** | 0.95 ± 0.14 | |
| Clinical parameters at admission | |||||
| Systolic arterial pressure ± SD, mmHg | 104.5 ± 21.1 | 100.2 ± 20.5 * | 103.9 ± 18.6 | 108.7 ± 23.2 | |
| Diastolic arterial pressure ± SD, mmHg | 55.9 ± 13.1 | 54.5 ± 13.3 | 54.8 ± 13.0 | 58.2 ± 12.8 | |
| Mean arterial pressure ± SD, mmHg | 72.1 ± 13.0 | 69.8 ± 13.5 * | 71.1 ± 12.1 | 75.1 ± 13.0 | |
| Total SOFA score | 5 [3–7] | 8 [6–10] *** | 5 [4–7] ** | 4 [3–5] | |
| Non-renal SOFA score | 5 [3–6] | 6 [4–7] *** | 5 [3–6] ** | 4 [3–5] | |
| Renal SOFA score | 0 [0–1] | 2 [1–3] *** | 0 [0–0] | 0 [0–0] | |
| AKI severity (KDIGO criteria)—no. (%) | |||||
| KDIGO 1 | 33 (20.1%) | 15 (30.0%) | 18 (32.7%) | 0 (0.0%) | |
| KDIGO 2 | 38 (23.2%) | 19 (38.0%) | 19 (34.5%) | 0 (0.0%) | |
| KDIGO 3 | 34 (20.7%) | 16 (32.0%) | 18 (32.7%) | 0 (0.0%) | |
| FGF23 (pg/mL) | EPO (mIU/mL) | Klotho (mIU/mL) | F.E.K. (A.U.) | ||
|---|---|---|---|---|---|
| All patients (n = 164) | 37.2 [24.0–56.8] | 16.6 [12.3–21.5] | 422.8 [284.4–504.6] | 1.52 [0.71–3.47] | |
| AKI (+) at admission (n = 50) | 63.3 [48.5–83.0] | 20.1 [15.9–25.5] | 289.3 [194.4–368.7] | 4.33 [2.46–9.61] | |
| KDIGO 1 (n = 15) | 45.2 [36.8–52.5] | 17.5 [14.1–20.4] | 369.9 [312.8–437.6] | 2.33 [1.29–3.16] | |
| KDIGO 2 (n = 19) | 61.5 [49.9–73.3] | 17.9 [14.9–20.6] | 284.5 [236.7–353.6] | 3.50 [2.54–5.22] | |
| KDIGO 3 (n = 16) | 88.7 [75.5–96.6] | 27.9 [24.1–32.0] | 186.7 [152.2–199.2] | 14.04 [9.61–17.26] | |
| AKI (+) at day 1–2 (n = 55) | 43.2 [34.6–54.1] | 17.6 [14.6–23.8] | 392.2 [275.3–505.2] | 2.01 [1.21–3.03] | |
| KDIGO 1 (n = 18) | 34.6 [32.0–37.1] | 15.4 [12.1–19.9] | 424.1 [375.4–563.7] | 1.29 [1.05–1.57] | |
| KDIGO 2 (n = 19) | 42.6 [36.5–49.6] | 17.1 [14.8–22.4] | 416.4 [301.1–487.7] | 2.25 [1.51–2.90] | |
| KDIGO 3 (n = 18) | 57.4 [44.7–79.3] | 19.7 [15.6–34.9] | 272.0 [197.4–490.2] | 4.86 [2.44–11.54] | |
| AKI (−) (n = 59) | 21.3 [14.9–28.1] | 12.3 [8.9–17.0] | 493.1 [442.6–593.7] | 0.58 [0.25–0.78] | |
| Characteristic | All Patients | FEK < 0.87 A.U. | FEK ≥ 0.87 A.U. | |
|---|---|---|---|---|
| Demographic | ||||
| Patients, n (%) | 164 (100%) | 59 (36.0%) | 105 (64.0%) | |
| Mean age ± SD, yr | 63.4 ± 14.3 | 61.9 ± 11.4 | 64.3 ± 15.7 | |
| Men, n (%) | 103 (62.8%) | 35 (59.3%) | 68 (64.8%) | |
| Hypertension, n (%) | 92 (56.1%) | 34 (57.6%) | 58 (55.2%) | |
| Diabetes, n (%) | 35 (21.3%) | 10 (16.9%) | 25 (23.8%) | |
| Coronary heart disease, n (%) | 20 (12.2%) | 6 (10.2%) | 14 (13.3%) | |
| Baseline renal function | ||||
| Serum creatinine ± SD, mg/dL | 0.85 ± 0.16 | 0.84 ± 0.16 | 0.85 ± 0.17 | |
| eGFR # ± SD, mL/min/1.73m2 | 85.2 ± 15.5 | 85.9 ± 16.4 | 84.9 ± 15.1 | |
| Infectious foci, n (%) | ||||
| Pulmonary | 60 (36.6%) | 20 (33.9%) | 40 (38.1%) | |
| Urinary | 26 (15.9%) | 8 (13.6%) | 18 (17.1%) | |
| Abdominal | 78 (47.6%) | 31 (52.5%) | 47 (44.8%) | |
| Emergency surgery required | 68 (41.5%) | 27 (45.8%) | 41 (39.0%) | |
| Biochemical parameters at admission | ||||
| Blood ureic nitrogen ± SD, mg/dL | 24.6 ± 9.8 | 20.6 ± 6.2 | 26.9 ± 10.7 *** | |
| Serum calcium ± SD, mg/dL | 8.8 ± 1.1 | 8.9 ± 1.1 | 8.7 ± 1.0 | |
| Serum phosphate ± SD, mg/dL | 3.9 ± 0.9 | 3.8 ± 0.9 | 4.0 ± 0.9 | |
| Serum creatinine ± SD, mg/dL | 1.35 ± 0.90 | 0.90 ± 0.17 | 1.60 ± 1.04 *** | |
| Maximum serum creatinine ± SD, mg/dL | 1.91 ± 1.08 | 1.07 ± 0.49 | 2.38 ± 1.03 *** | |
| Clinical parameters at admission | ||||
| Systolic arterial pressure ± SD, mmHg | 104.5 ± 21.1 | 109.1 ± 23.0 | 101.9 ± 19.6 * | |
| Diastolic arterial pressure ± SD, mmHg | 55.9 ± 13.1 | 58.7 ± 12.8 | 54.4 ± 13.0 * | |
| Mean arterial pressure ± SD, mmHg | 72.1 ± 13.0 | 75.5 ± 13.0 | 70.2 ± 12.7 * | |
| Total SOFA score | 5 [3–7] | 4 [3–5] | 6 [4–9] *** | |
| Non-renal SOFA score | 5 [3–6] | 4 [3–5] | 5 [4–6] *** | |
| Renal SOFA score | 0 [0–1] | 0 [0–0] | 1 [0–2] *** | |
| AKI development—no. (%) | ||||
| No AKI (−) | 59 (36.0%) | 53 (89.8%) | 6 (5.7%) *** | |
| AKI at admission | 50 (30.5%) | 0 (0%) | 50 (47.6%) *** | |
| AKI at day 1–2 | 55 (33.5%) | 6 (10.2%) | 49 (46.7%) *** | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toro, L.; Rojas, V.; Conejeros, C.; Ayala, P.; Parra-Lucares, A.; Ahumada, F.; Almeida, P.; Silva, M.F.; Bravo, K.; Pumarino, C.; et al. A Combined Biomarker That Includes Plasma Fibroblast Growth Factor 23, Erythropoietin, and Klotho Predicts Short- and Long-Term Morbimortality and Development of Chronic Kidney Disease in Critical Care Patients with Sepsis: A Prospective Cohort. Biomolecules 2023, 13, 1481. https://doi.org/10.3390/biom13101481
Toro L, Rojas V, Conejeros C, Ayala P, Parra-Lucares A, Ahumada F, Almeida P, Silva MF, Bravo K, Pumarino C, et al. A Combined Biomarker That Includes Plasma Fibroblast Growth Factor 23, Erythropoietin, and Klotho Predicts Short- and Long-Term Morbimortality and Development of Chronic Kidney Disease in Critical Care Patients with Sepsis: A Prospective Cohort. Biomolecules. 2023; 13(10):1481. https://doi.org/10.3390/biom13101481
Chicago/Turabian StyleToro, Luis, Verónica Rojas, Carolina Conejeros, Patricia Ayala, Alfredo Parra-Lucares, Francisca Ahumada, Paula Almeida, María Fernanda Silva, Karin Bravo, Catalina Pumarino, and et al. 2023. "A Combined Biomarker That Includes Plasma Fibroblast Growth Factor 23, Erythropoietin, and Klotho Predicts Short- and Long-Term Morbimortality and Development of Chronic Kidney Disease in Critical Care Patients with Sepsis: A Prospective Cohort" Biomolecules 13, no. 10: 1481. https://doi.org/10.3390/biom13101481
APA StyleToro, L., Rojas, V., Conejeros, C., Ayala, P., Parra-Lucares, A., Ahumada, F., Almeida, P., Silva, M. F., Bravo, K., Pumarino, C., Tong, A. M., Pinto, M. E., Romero, C., & Michea, L. (2023). A Combined Biomarker That Includes Plasma Fibroblast Growth Factor 23, Erythropoietin, and Klotho Predicts Short- and Long-Term Morbimortality and Development of Chronic Kidney Disease in Critical Care Patients with Sepsis: A Prospective Cohort. Biomolecules, 13(10), 1481. https://doi.org/10.3390/biom13101481







